Modeling Current and Future Distribution of Cochlodina laminata in Eastern Europe under Climate Change
Abstract
:1. Introduction
2. Materials and Methods
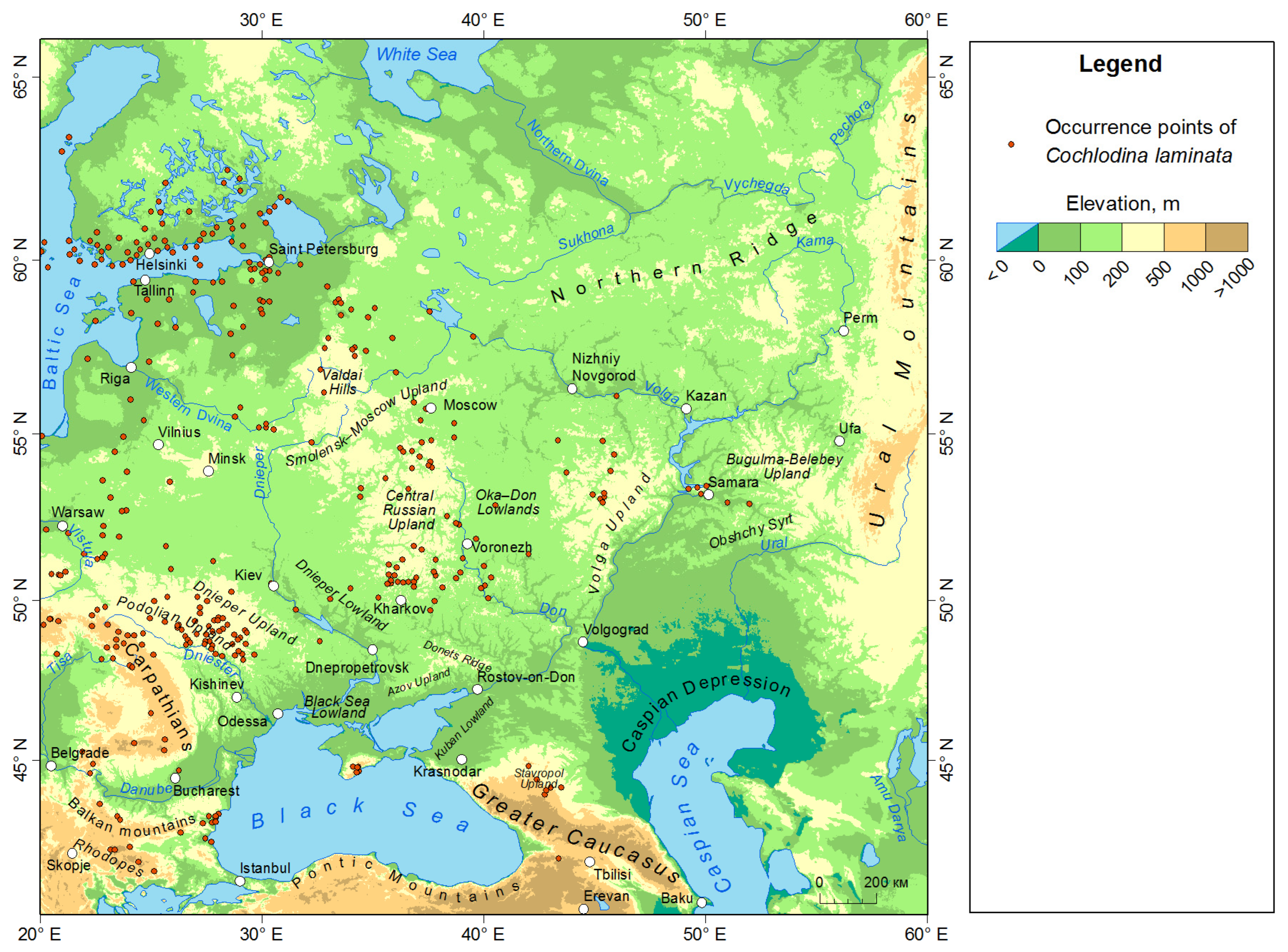
2.1. Species Data and Study Area
2.2. Environmental Predictors
2.3. Species Distribution Modeling
3. Results
3.1. Current Distribution Modeling
3.2. Future Distribution Modeling
3.3. Variable Importance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huntley, B.; Allen, J.R.M.; Forrest, M.; Hickler, T.; Ohlemüller, R.; Singarayer, J.S.; Valdes, P.J.; Williams, J. Projected Climatic Changes Lead to Biome Changes in Areas of Previously Constant Biome. J. Biogeogr. 2021, 48, 2418–2428. [Google Scholar] [CrossRef]
- Ortega, J.C.G.; Machado, N.; Diniz-Filho, J.A.F.; Rangel, T.F.; Araújo, M.B.; Loyola, R.; Bini, L.M. Meta-analyzing the Likely Cross-species Responses to Climate Change. Ecol. Evol. 2019, 9, 11136–11144. [Google Scholar] [CrossRef]
- Barlow, M.M.; Johnson, C.N.; McDowell, M.C.; Fielding, M.W.; Amin, R.J.; Brewster, R. Species Distribution Models for Conservation: Identifying Translocation Sites for Eastern Quolls under Climate Change. Glob. Ecol. Conserv. 2021, 29, e01735. [Google Scholar] [CrossRef]
- Seo, C.; Thorne, J.H.; Hannah, L.; Thuiller, W. Scale Effects in Species Distribution Models: Implications for Conservation Planning under Climate Change. Biol. Lett. 2009, 5, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Hällfors, M.H.; Liao, J.; Dzurisin, J.; Grundel, R.; Hyvärinen, M.; Towle, K.; Wu, G.C.; Hellmann, J.J. Addressing Potential Local Adaptation in Species Distribution Models: Implications for Conservation under Climate Change. Ecol. Appl. 2016, 26, 1154–1169. [Google Scholar] [CrossRef] [PubMed]
- Beltramino, A.A.; Vogler, R.E.; Gutiérrez Gregoric, D.E.; Rumi, A. Impact of Climate Change on the Distribution of a Giant Land Snail from South America: Predicting Future Trends for Setting Conservation Priorities on Native Malacofauna. Clim. Chang. 2015, 131, 621–633. [Google Scholar] [CrossRef]
- Lei, J.; Chen, L.; Li, H. Using Ensemble Forecasting to Examine How Climate Change Promotes Worldwide Invasion of the Golden Apple Snail (Pomacea Canaliculata). Environ. Monit. Assess. 2017, 189, 404. [Google Scholar] [CrossRef]
- Zemanova, M.A.; Broennimann, O.; Guisan, A.; Knop, E.; Heckel, G. Slimy Invasion: Climatic Niche and Current and Future Biogeography of Arion Slug Invaders. Divers. Distrib. 2018, 24, 1627–1640. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Steinmann, P.; Yang, G.-J.; Yang, K.; Zhou, X.-N.; Utzinger, J. The Emergence of Angiostrongyliasis in the People’s Republic of China: The Interplay between Invasive Snails, Climate Change and Transmission Dynamics: Emerging Angiostrongyliasis in China. Freshw. Biol. 2011, 56, 717–734. [Google Scholar] [CrossRef]
- Rekha Sarma, R.; Munsi, M.; Neelavara Ananthram, A. Effect of Climate Change on Invasion Risk of Giant African Snail (Achatina Fulica Férussac, 1821: Achatinidae) in India. PLoS ONE 2015, 10, e0143724. [Google Scholar] [CrossRef]
- Pedersen, U.B.; Stendel, M.; Midzi, N.; Mduluza, T.; Soko, W.; Stensgaard, A.-S.; Vennervald, B.J.; Mukaratirwa, S.; Kristensen, T.K. Modelling Climate Change Impact on the Spatial Distribution of Fresh Water Snails Hosting Trematodes in Zimbabwe. Parasites Vectors 2014, 7, 536. [Google Scholar] [CrossRef]
- Betts, M.G.; Yang, Z.; Hadley, A.S.; Smith, A.C.; Rousseau, J.S.; Northrup, J.M.; Nocera, J.J.; Gorelick, N.; Gerber, B.D. Forest Degradation Drives Widespread Avian Habitat and Population Declines. Nat. Ecol. Evol. 2022, 6, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, L.G.; Kunin, W.E.; Keil, P.; Aguirre-Gutiérrez, J.; Ellis, W.N.; Fox, R.; Groom, Q.; Hennekens, S.; Landuyt, W.; Maes, D.; et al. Species Richness Declines and Biotic Homogenisation Have Slowed down for NW -European Pollinators and Plants. Ecol. Lett. 2013, 16, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Boettger, C.R. Systematic and Geographical Notes on Clausiliidae. Arch. Für Naturgeschichte Ser. A 1926, 91, 1–18. [Google Scholar]
- Nordsieck, H. Annotated Check-List of the Genera of Fossil Land Snails (Gastropoda: Stylommatophora) of Western and Central Europe (Cretaceous–Pliocene), with Description of New Taxa. Arch. Für Molluskenkd. 2014, 143, 153–185. [Google Scholar]
- Nordsieck, H. Worldwide Door Snails (Clausiliidae), Recent and Fossil; ConchBooks: Hackenheim, Germany, 2007; 214p. [Google Scholar]
- Likharev, I.M. Clausiliidae. In Fauna SSSR, New Series, Mollusca. Vol. III, N 4; The Academy of Sciences of the Soviet Union: Moscow-Leningrad, Russia, 1962; 317p. (In Russian) [Google Scholar]
- Mamatkulov, A.L. Breeding biology of some East European Clausiliidae species (Mollusca, Pulmonata). Zool. Zhurnal 2007, 86, 403–414. (In Russian) [Google Scholar]
- Kantor, Y.I.; Sysoev, A.V. Land Snails and Slugs of Russia and Adjacent Countries; KMK: Moscow, Russia, 2005; 627p. (In Russian) [Google Scholar]
- Balashov, I. Fauna of Ukraine, Molluscs, Stylommatophora; Naukova Dumka: Kiev, Ukraine, 2016; Volume 29, 591p. (In Russian) [Google Scholar]
- Szybiak, K.; Leśniewska, M.; Taborska, M. Terrestrial Gastropods of the Carpathian Beech Forest in the Magura National Park (SE. Poland). Folia Malacol. 2005, 13, 97–103. [Google Scholar] [CrossRef]
- Sulikowska-Drozd, A. Distribution and Habitat Preferences of Clausiliids (Gastropoda: Pulmonata: Clausiliidae) in the Eastern Part of the Polish Carpathians. Folia Malacol. 2005, 13, 49–89. [Google Scholar] [CrossRef]
- Loosjes, F.; Negrea, A. Contributions to the Distribution of the Clausiliidae (Gastropoda, Pulmonta) in the Karst Regions of Romania. Zool. Meded. 1968, 43, 41–55. [Google Scholar]
- Dedov, I. Annotated Check-List of the Bulgarian Terrestrial Snails (Mollusca, Gastropoda). Linzer biol. Beitr. 1998, 16, 197–205. [Google Scholar]
- Gural-Sverlova, N.V. Spatial distribution of land molluscs fauna of the steppe zone of Ukraine. Ruthenica Russ. Malacol. J. 2018, 28, 131–138. (In Russian) [Google Scholar] [CrossRef]
- Balashov, I.A.; Brusentsova, N.A. Terrestrial molluscs of the “Slobozhanskii” National Nature Park (Kharkiv region, Ukraine). Zool. Zhurnal 2015, 94, 1249–1256. (In Russian) [Google Scholar] [CrossRef]
- Balashov, I.A.; Krivohigaya, M.V. Distribution patterns of terrestrial mollusks in the chalk steppe and neighboring phytocenoses of the Oskol river valley in the Dvorichanskyi national nature park, Ukraine. Russ. J. Ecol. 2015, 46, 370–376. [Google Scholar] [CrossRef]
- Stoiko, T.G.; Komarova, E.V.; Bezina, O.V. Communities of terrestrial mollusks on chalky slopes in the foreststeppe zone (The Middle Volga River Region). Bull. Samara Sci. Cent. Russ. Acad. Sci. 2014, 16, 142–147. (In Russian) [Google Scholar]
- Stoiko, T.G.; Bulavkina, O.V.; Mazei, Y.A. Communities of terrestrial mollusks in aspen forests of the Middle Volga River basin. Zool. Zhurnal 2010, 89, 519–527. (In Russian) [Google Scholar]
- Puzanov, I.I. Materials for the knowledge of land mollusks in Crimea, Ch. 1: Mollusks of the mountainous Crimea. Bjulleten’ Mosk. Obs. Ispyt. Prir. 1925, 33, 48–104. (In Russian) [Google Scholar]
- Baidashnikov, A.A. East European lowland species of terrestrial snails in the fauna of Mountain Crimea. Vestn. Zool. 1990, 6, 8–70. (In Russian) [Google Scholar]
- Sharmina, M.; Anderson, K.; Bows-Larkin, A. Climate Change Regional Review: Russia: Climate Change Regional Review. WIREs Clim. Chang. 2013, 4, 373–396. [Google Scholar] [CrossRef]
- Araújo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of Species–Climate Impact Models under Climate Change. Glob. Change Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Gallardo, B.; Aldridge, D.C.; González-Moreno, P.; Pergl, J.; Pizarro, M.; Pyšek, P.; Thuiller, W.; Yesson, C.; Vilà, M. Protected Areas Offer Refuge from Invasive Species Spreading under Climate Change. Glob. Chang. Biol. 2017, 23, 5331–5343. [Google Scholar] [CrossRef]
- Kriegler, E.; O’Neill, B.C.; Hallegatte, S.; Kram, T.; Lempert, R.J.; Moss, R.H.; Wilbanks, T. The Need for and Use of Socio-Economic Scenarios for Climate Change Analysis: A New Approach Based on Shared Socio-Economic Pathways. Glob. Environ. Chang. 2012, 22, 807–822. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Riahi, K.; Ebi, K.L.; Hallegatte, S.; Carter, T.R.; Mathur, R.; van Vuuren, D.P. A New Scenario Framework for Climate Change Research: The Concept of Shared Socioeconomic Pathways. Clim. Chang. 2014, 122, 387–400. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Tebaldi, C.; van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.-F.; Lowe, J.; et al. The Scenario Model Intercomparison Project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef]
- Cook, B.I.; Mankin, J.S.; Marvel, K.; Williams, A.P.; Smerdon, J.E.; Anchukaitis, K.J. Twenty-First Century Drought Projections in the CMIP6 Forcing Scenarios. Earth’s Future 2020, 8, e2019EF001461. [Google Scholar] [CrossRef]
- Chen, H.; Sun, J.; Lin, W.; Xu, H. Comparison of CMIP6 and CMIP5 Models in Simulating Climate Extremes. Sci. Bull. 2020, 65, 1415–1418. [Google Scholar] [CrossRef]
- Li, C.; Zwiers, F.; Zhang, X.; Li, G.; Sun, Y.; Wehner, M. Changes in Annual Extremes of Daily Temperature and Precipitation in CMIP6 Models. J. Clim. 2021, 34, 3441–3460. [Google Scholar] [CrossRef]
- Lepore, C.; Abernathey, R.; Henderson, N.; Allen, J.T.; Tippett, M.K. Future Global Convective Environments in CMIP6 Models. Earth’s Future 2021, 9, e2021EF002277. [Google Scholar] [CrossRef]
- Aleksanov, V.V.; Ruleva, O.A.; Galemina, I.E. Inventory of the terrestrial mollusks of Kaluga City. In Research of Biological Diversity of the Kaluga region: Collection of Scientific Articles, Inventory and Monitoring Studies of Biological Diversity in the Kaluga Region; OOO “TPS”: Tambov, Russia, 2019; Volume 4, pp. 73–95. (In Russian) [Google Scholar]
- Kotsur, V.M. Biotope distribution of Mollusca, Gastropoda of the City of Vitebsk. Vesnik VDU 2013, 6, 60–65. (In Russian) [Google Scholar]
- Kotsur, V.M. Terrestrial molluscs (Mollusca, Gastropoda) of gray alder forests of the Belarusian Lakeland. Vesn. MDPU Im. I. P. Shamjakina 2015, 6, 26–32. (In Russian) [Google Scholar]
- Kowalczyk-Pecka, D.; Czepiel-Mil, K. The effect of accumulation of metals on selected physiological biomarkers in Cochlodina (Cochlodina) laminata (Pulmonata: Clausiliidae) inhabiting urban biocenoses. Environ. Prot. Nat. Resour. 2013, 24, 45–49. [Google Scholar] [CrossRef]
- Shipchina, M.A. To the fauna of terrestrial snails of the forest-steppe zone of the Samara region. In Bulletin of the Mordovian University; Series “Biologicheskie nauki”; Biologicheskie nauki: Moscow, Russia, 2008; Volume 2, pp. 148–149. (In Russian) [Google Scholar]
- Sachkova, Y.V. Terrestrial molluscs complexes of forest-steppe Zavolzhye. Bull. Samara Sci. Cent. Russ. Acad. Sci. 2009, 11, 650–653. (In Russian) [Google Scholar]
- Sachkova, Y.V. Research of ground molluscs on Samara Luka. Samar. Luka Probl. Reg. Glob. Ecol. 2009, 18, 138–145. (In Russian) [Google Scholar]
- Stoiko, T.G.; Bulavkina, O.V. Data on the fauna of the terrestrial mollusks from Penza region (Part II). Izv. PGPU Im. V. G. Belinskogo 2008, 10, 66–71. (In Russian) [Google Scholar]
- Balashov, I.A.; Baidashnikov, A.A. Terrestrial mollusks (Gastropoda) of the Vinnytsia oblast and their biotopical preferences. Vestn. Zool. 2012, 46, 19–28. (In Russian) [Google Scholar]
- Balashov, I.A.; Baidashnikov, A.A.; Romanov, G.A.; Gural-Sverlova, N.V. Terrestrial molluscs of Khmelnitsky region (the Podolian Upland, Ukraine). Zool. Zhurnal 2013, 92, 154–166. (In Russian) [Google Scholar] [CrossRef]
- Baidashnikov, A.A. Terrestrial molluscs (Gastropoda, Pulmonata) of the Reserve “Medobory” (Podolsk Upland). Vestn. Zool. 2002, 36, 73–76. (In Russian) [Google Scholar]
- Baidashnikov, A.A. Terrestrial malacofauna of Ukrainian Polissya. Vestn. Zool. 1996, 36, 3–12. (In Russian) [Google Scholar]
- GBIF Academy of Natural Sciences. MAL. Occurrence Dataset. Available online: https://www.gbif.org/dataset/86b50d88-f762-11e1-a439-00145eb45e9a (accessed on 25 April 2022).
- GBIF Natural History Museum. Natural History Museum (London) Collection Specimens. 2022. Available online: https://www.gbif.org/dataset/7e380070-f762-11e1-a439-00145eb45e9a (accessed on 25 April 2022).
- GBIF Roasto, R. Estonian Nature Observations Database. Version 87.15. 2019. Estonian Environment Information Centre. 2019. Available online: https://cdn.gbif.org/dataset/c6bbb6ef-ad16-4f3c-99e2-f693760173e0 (accessed on 25 April 2022).
- GBIFHarvard University M; Morris, P.J. Museum of Comparative Zoology, Harvard University. Version 162.311. Museum of Comparative Zoology, Harvard University. 2022. Available online: https://www.gbif.org/dataset/4bfac3ea-8763-4f4b-a71a-76a6f5f243d3 (accessed on 25 April 2022).
- GBIF Estonian Naturalists’ Society. Estonian Naturalists’ Society. Available online: https://www.gbif.org/dataset/f1c4df18-12d6-40cb-ab51-5bb0d7f08d6e (accessed on 25 April 2022).
- GBIF Finnish Biodiversity Information Facility. Lajitietokeskus/FinBIF-Notebook, General Observations. 2022. Available online: https://www.gbif.org/dataset/df12ca07-f133-4550-ab3b-fde13f0e76ba (accessed on 25 April 2022).
- GBIF Finnish Biodiversity Information Facility. Hatikka.fi Observations. 2022. Available online: https://www.gbif.org/occurrence/download/0029067-180508205500799 (accessed on 25 April 2022).
- GBIF Finnish Biodiversity Information Facility. Mollusca (Luomus). 2022. Available online: https://www.gbif.org/dataset/e5d2aed2-a177-46ba-9ea8-c349160a4c2d (accessed on 25 April 2022).
- GBIF Adam Mickiewicz University in Poznań. Natural History Collections of the Faculty of Biology AMU. Available online: https://cdn.gbif.org/dataset/84b18cce-083a-4464-bee8-25b2083a17cd (accessed on 25 April 2022).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Mansfield, E.R.; Helms, B.P. Detecting Multicollinearity. Am. Stat. 1982, 36, 158–160. [Google Scholar] [CrossRef]
- Goberville, E.; Beaugrand, G.; Hautekèete, N.-C.; Piquot, Y.; Luczak, C. Uncertainties in the Projection of Species Distributions Related to General Circulation Models. Ecol. Evol. 2015, 5, 1100–1116. [Google Scholar] [CrossRef] [PubMed]
- Puchałka, R.; Dyderski, M.K.; Vítková, M.; Sádlo, J.; Klisz, M.; Netsvetov, M.; Prokopuk, Y.; Matisons, R.; Mionskowski, M.; Wojda, T.; et al. Black Locust (Robinia Pseudoacacia L.) Range Contraction and Expansion in Europe under Changing Climate. Glob. Chang. Biol. 2021, 27, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Naujokaitis-Lewis, I.R.; Curtis, J.M.R.; Tischendorf, L.; Badzinski, D.; Lindsay, K.; Fortin, M.-J. Uncertainties in Coupled Species Distribution-Metapopulation Dynamics Models for Risk Assessments under Climate Change. Divers. Distrib. 2013, 19, 541–554. [Google Scholar] [CrossRef]
- Roberts, M. MOHC HadGEM3-GC31-LL Model Output Prepared for CMIP6 HighResMIP 2017. Available online: http://cera-www.dkrz.de/WDCC/meta/CMIP6/CMIP6.HighResMIP.MOHC.HadGEM3-GC31-LL (accessed on 25 April 2022).
- Ziehn, T.; Chamberlain, M.A.; Law, R.M.; Lenton, A.; Bodman, R.W.; Dix, M.; Stevens, L.; Wang, Y.-P.; Srbinovsky, J. The Australian Earth System Model: ACCESS-ESM1.5. J. South. Hemisph. Earth Syst. Sci. 2020, 70, 193–214. [Google Scholar] [CrossRef]
- Swart, N.C.; Cole, J.N.S.; Kharin, V.V.; Lazare, M.; Scinocca, J.F.; Gillett, N.P.; Anstey, J.; Arora, V.; Christian, J.R.; Hanna, S.; et al. The Canadian Earth System Model Version 5 (CanESM5.0.3). Geosci. Model. Dev. 2019, 12, 4823–4873. [Google Scholar] [CrossRef]
- Wu, T.; Yu, R.; Lu, Y.; Jie, W.; Fang, Y.; Zhang, J.; Zhang, L.; Xin, X.; Li, L.; Wang, Z.; et al. BCC-CSM2-HR: A High-Resolution Version of the Beijing Climate Center Climate System Model. Geosci. Model. Dev. 2021, 14, 2977–3006. [Google Scholar] [CrossRef]
- Abdelmoaty, H.M.; Papalexiou, S.M.; Rajulapati, C.R.; AghaKouchak, A. Biases Beyond the Mean in CMIP6 Extreme Precipitation: A Global Investigation. Earth’s Future 2021, 9, e2021EF002196. [Google Scholar] [CrossRef]
- Yazdandoost, F.; Moradian, S.; Izadi, A.; Aghakouchak, A. Evaluation of CMIP6 Precipitation Simulations across Different Climatic Zones: Uncertainty and Model Intercomparison. Atmos. Res. 2021, 250, 105369. [Google Scholar] [CrossRef]
- Flato, G.M. Earth System Models: An Overview. WIREs Clim. Chang. 2011, 2, 783–800. [Google Scholar] [CrossRef]
- Albert, C.H.; Rayfield, B.; Dumitru, M.; Gonzalez, A. Applying Network Theory to Prioritize Multispecies Habitat Networks That Are Robust to Climate and Land-Use Change: Prioritizing a Network for Biodiversity. Conserv. Biol. 2017, 31, 1383–1396. [Google Scholar] [CrossRef]
- Amini Tehrani, N.; Naimi, B.; Jaboyedoff, M. Modeling Current and Future Species Distribution of Breeding Birds as Regional Essential Biodiversity Variables (SD EBVs): A Bird Perspective in Swiss Alps. Glob. Ecol. Conserv. 2021, 27, e01596. [Google Scholar] [CrossRef]
- Nath, B.; Wang, Z.; Ge, Y.; Islam, K.; Singh, R.P.; Niu, Z. Land Use and Land Cover Change Modeling and Future Potential Landscape Risk Assessment Using Markov-CA Model and Analytical Hierarchy Process. IJGI 2020, 9, 134. [Google Scholar] [CrossRef]
- Gobeyn, S.; Mouton, A.M.; Cord, A.F.; Kaim, A.; Volk, M.; Goethals, P.L.M. Evolutionary Algorithms for Species Distribution Modelling: A Review in the Context of Machine Learning. Ecol. Model. 2019, 392, 179–195. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y. Applying Various Algorithms for Species Distribution Modelling. Integr. Zool. 2013, 8, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Pohjankukka, J.; Pahikkala, T.; Nevalainen, P.; Heikkonen, J. Estimating the Prediction Performance of Spatial Models via Spatial K-Fold Cross Validation. Int. J. Geogr. Inf. Sci. 2017, 31, 2001–2019. [Google Scholar] [CrossRef]
- Araujo, M.; New, M. Ensemble Forecasting of Species Distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting Thresholds of Occurrence in the Prediction of Species Distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Thuiller, W.; Guéguen, M.; Renaud, J.; Karger, D.N.; Zimmermann, N.E. Uncertainty in Ensembles of Global Biodiversity Scenarios. Nat. Commun. 2019, 10, 1446. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A.; Turner, R. Spatstat: An R Package for Analyzing Spatial Point Patterns. J. Stat. Soft. 2005, 12, 1–42. [Google Scholar] [CrossRef]
- Naimi, B.; Araújo, M.B. Sdm: A Reproducible and Extensible R Platform for Species Distribution Modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 25 April 2022).
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. SpThin: An R Package for Spatial Thinning of Species Occurrence Records for Use in Ecological Niche Models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Bivand, R.; Lewin-Koh, N. Maptools: Tools for Handling Spatial Objects. 2022. Available online: https://CRAN.R-project.org/package=maptools (accessed on 25 April 2022).
- Pebesma, E.J.; Bivand, R.S. Classes and Methods for Spatial Data in R. R News 5 (2). 2005. Available online: https://cran.r-project.org/doc/Rnews/ (accessed on 25 April 2022).
- Bivand, R.S.; Pebesma, E.; Gomez-Rubio, V. Applied Spatial Data Analysis with R, 2nd ed.; Springer: New York, NY, USA, 2013; Available online: https://asdar-book.org/ (accessed on 25 April 2022).
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. R Package Version 3.6-3. 2022. Available online: https://CRAN.R-project.org/package=raster (accessed on 25 April 2022).
- Chamberlain, S.; Barve, V.; Mcglinn, D.; Oldoni, D.; Desmet, P.; Geffert, L.; Ram, K. rgbif: Interface to the Global Biodiversity Information Facility API. R Package Version 3.7.3. 2023. Available online: https://CRAN.R-project.org/package=rgbif (accessed on 25 April 2022).
- Bivand, R.; Rundel, C. rgeos: Interface to Geometry Engine-Open Source (‘GEOS’). R Package Version 0.5-9. 2021. Available online: https://CRAN.R-project.org/package=rgeos (accessed on 25 April 2022).
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. dismo: Species Distribution Modeling. R Package Version 1.3-9. 2022. Available online: https://CRAN.R-project.org/package=dismo (accessed on 25 April 2022).
- Bivand, R.; Keitt, T.; Rowlingson, B. rgdal: Bindings for the ‘Geospatial’ Data Abstraction Library. R Package Version 1.5-32. 2022. Available online: https://CRAN.R-project.org/package=rgdal (accessed on 25 April 2022).
- Hirzel, A.H.; Le Lay, G. Habitat Suitability Modelling and Niche Theory. J. Appl. Ecol. 2008, 45, 1372–1381. [Google Scholar] [CrossRef]
- Lissovsky, A.A.; Dudov, S.V.; Obolenskaya, E.V. Species-Distribution Modeling: Advantages and Limitations of Its Application. 1. General Approaches. Biol. Bull. Rev. 2021, 11, 254–264. [Google Scholar] [CrossRef]
- Akramowski, N.N. Fauna Armyanskoy SSR. Mollyuski (Mollusca); Akademiya Nauk armyanskoy SSR: Erevan, USSR, 1976; 268p. (In Russian) [Google Scholar]
- Walther, F.; Kijashko, P.; Harutyunova, L.; Mumladze, L.; Neiber, M.T.; Hausdorf, B. Biogeography of the Land Snails of the Caucasus Region. Tentacle 2014, 22, 3–5. [Google Scholar]
- Koch, E.L.; Neiber, M.T.; Walther, F.; Hausdorf, B. Presumable Incipient Hybrid Speciation of Door Snails in Previously Glaciated Areas in the Caucasus. Mol. Phylogenetics Evol. 2016, 97, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Baidashnikov, A.A. The morphological reason for the stenobionticity of Clausiliidae (Gastropoda, Pulmonata). Vestn. Zool. 2003, 37, 49–63. (In Russian) [Google Scholar]
- Baidashnikov, A.A. The variability of the land snails from Crimean genus Mentissa (Gastropoda, Pulmonata, Clausiliidae. Vestn. Zool. 2006, 40, 297–310. (In Russian) [Google Scholar]
- Neiber, M.T.; Helfenrath, K.; Walther, F.; Hausdorf, B. Ecological Specialization Resulting in Restricted Gene Flow Promotes Differentiation in Door Snails. Mol. Phylogenetics Evol. 2019, 141, 106608. [Google Scholar] [CrossRef]
- Nikolaev, V.A. Terrestrial Molluscs of the Central Russian Uplandl; MSU: Moscow, USSR, 1973. (In Russian) [Google Scholar]
- Sales, L.P.; Galetti, M.; Pires, M.M. Climate and Land-use Change Will Lead to a Faunal “Savannization” on Tropical Rainforests. Glob. Chang. Biol. 2020, 26, 7036–7044. [Google Scholar] [CrossRef]
- Cordellier, M.; Pfenninger, A.; Streit, B.; Pfenninger, M. Assessing the Effects of Climate Change on the Distribution of Pulmonate Freshwater Snail Biodiversity. Mar. Biol. 2012, 159, 2519–2531. [Google Scholar] [CrossRef]
- Puchałka, R.; Paź-Dyderska, S.; Jagodziński, A.M.; Sádlo, J.; Vítková, M.; Klisz, M.; Koniakin, S.; Prokopuk, Y.; Netsvetov, M.; Nicolescu, V.-N.; et al. Predicted Range Shifts of Alien Tree Species in Europe. Agric. For. Meteorol. 2023, 341, 109650. [Google Scholar] [CrossRef]
- Baidashnikov, A.A. The Intraspecific Variability of the Some Species of Clausiliidae (Gastropoda, Pulmonata) under Influence of Habitat Conditions. Vestn. Zool. 2005, 39, 37–47. (In Russian) [Google Scholar]
- Maltz, T.K.; Sulikowska-Drozd, A. Life Cycles of Clausiliids of Poland—Knowns and Unknowns. Ann. Zool. 2008, 58, 857–880. [Google Scholar] [CrossRef]
- Muhammad, R.; Zhang, W.; Abbas, Z.; Guo, F.; Gwiazdzinski, L. Spatiotemporal Change Analysis and Prediction of Future Land Use and Land Cover Changes Using QGIS MOLUSCE Plugin and Remote Sensing Big Data: A Case Study of Linyi, China. Land 2022, 11, 419. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamova, V.V.; Ukrainskiy, P.A. Modeling Current and Future Distribution of Cochlodina laminata in Eastern Europe under Climate Change. Diversity 2023, 15, 1155. https://doi.org/10.3390/d15111155
Adamova VV, Ukrainskiy PA. Modeling Current and Future Distribution of Cochlodina laminata in Eastern Europe under Climate Change. Diversity. 2023; 15(11):1155. https://doi.org/10.3390/d15111155
Chicago/Turabian StyleAdamova, Valeria V., and Pavel A. Ukrainskiy. 2023. "Modeling Current and Future Distribution of Cochlodina laminata in Eastern Europe under Climate Change" Diversity 15, no. 11: 1155. https://doi.org/10.3390/d15111155




