The Influence of Salinity Gradient and Island Isolation on Fauna Composition and Structure of Aquatic Invertebrate Communities of the Shantar Islands (Khabarovsk Krai)
Abstract
:1. Introduction
2. Materials and Methods
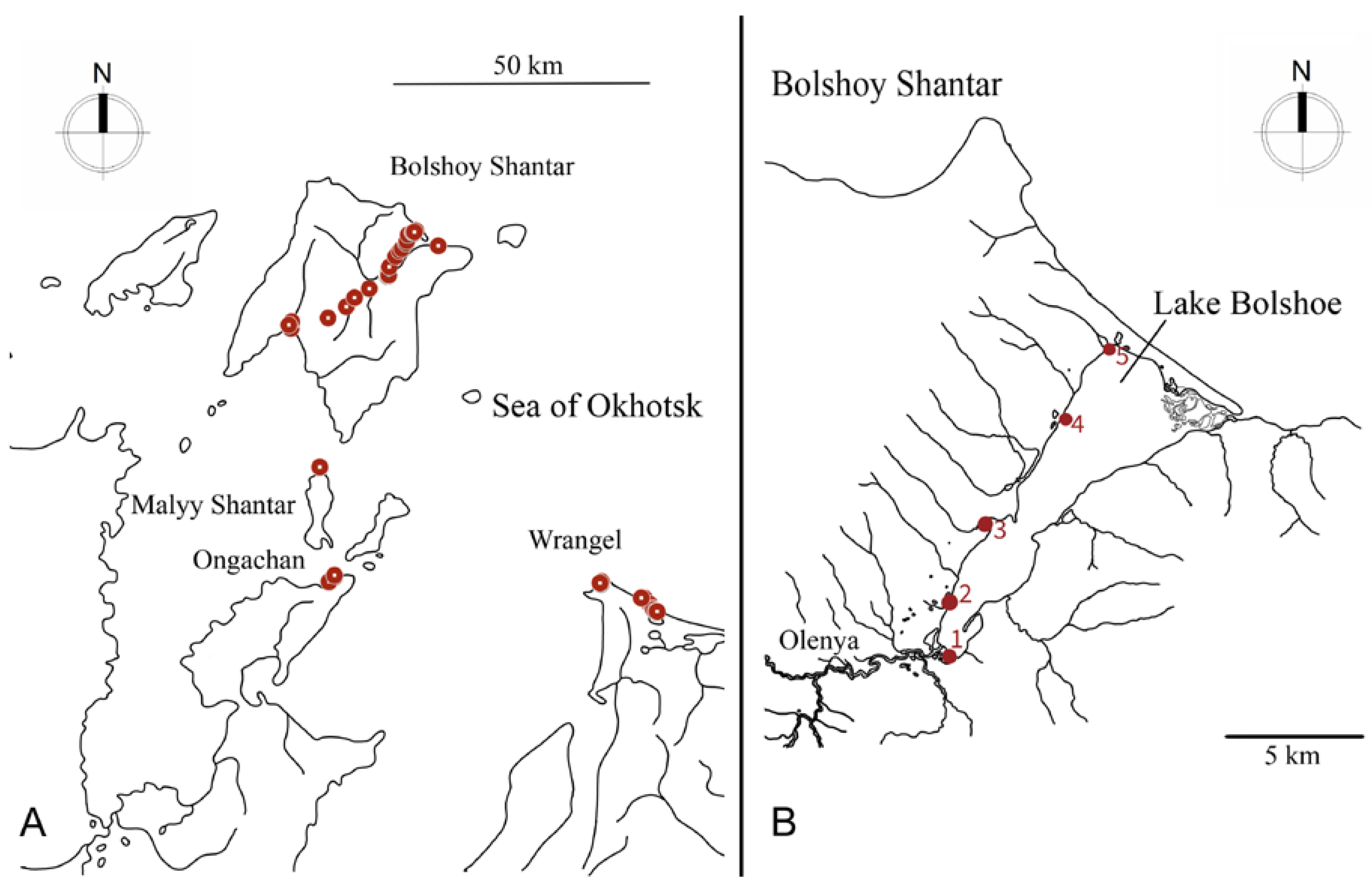
2.1. Study Area
2.2. Field Sampling and Laboratory Analysis
2.3. Statistical Analysis
3. Results
3.1. General Characteristics of Planktonic and Benthic Assemblages
3.2. Variation in the Integral Characteristics of the Fauna along the Salinity Gradient
3.3. The Comparison of Water Bodies of Different Hydrological Type and Localization
4. Discussion
4.1. Assemblages of Hydrobionts and Biogeographic Structure of Fauna
4.2. Salinity Gradient and Assemblage Structure
4.3. Effects of Insularity and Latitudinal Patterns in Species Richness
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barabanshchikov, E.I. Zooplankton of Ussury River basin (Primorye Territory). Levanidov’s Bienn. Memor. Meet. 2014, 6, 78–87. (In Russian) [Google Scholar]
- Barabanshchikov, E.I.; Kojevnikov, B.P. Dynamics of abundance and biomass of zooplankton in the open water of the Khanka Lake. News Paif. Rus. Res. Inst. Fish. Ocean. 1998, 123, 362–374. (In Russian) [Google Scholar]
- Garibian, P.G.; Chertoptud, E.S.; Sinev, A.Y.; Korovchinsky, N.M.; Kotov, A.A. Cladocera and Copepoda (Crustacea) of the Lake Bolon basin (Far East of Russia). Arthropoda Sel. 2019, 28, 37–63. [Google Scholar] [CrossRef]
- Garibian, P.G.; Chertoprud, E.S. First records of Cladocera and Copepoda from Chukchagir Lake and its basin (Khabarovsk Territory, Far East of Russia). Arthropoda Sel. 2022, 31, 10–18. [Google Scholar] [CrossRef]
- Levanidova, I.M. Amphibiotic Insects of the Mountainous Regions of the Far East of the USSR. Faunistics, Ecology; Nauka: Leningrad, Russia, 1982; p. 215. (In Russian) [Google Scholar]
- Teslenko, V.A. To the Fauna of stoneflies (Insecta, Plecoptera) of the Lower Amur Region. Levanidov’s Bienn. Memor. Meet. 2011, 5, 501–521. (In Russian) [Google Scholar]
- Tiunova, T.M.; Gorovaya, E.A. Fauna of mayflies (Insecta:Ephemeroptera) of the Lower Amur and its left-bank tributaries. Levanidov’s Bienn. Memor. Meet. 2011, 5, 522–539. (In Russian) [Google Scholar]
- Chernova, O.A. Mayflies (Ephemeroptera) of the Amur Basin and Adjacent Waters and Their Role in the Nutrition of Amur Fish. In Proceedings of the Amur Ichthyological Expedition of 1945–1949; MOIP: Moscow, Russia, 1952; Volume 3, pp. 229–360. [Google Scholar]
- Yavorskaya, N.M. Community structure of benthic invertebrates of the Levaya River (Amur River basin) (Khabarovsk territory). Amurian Zool. J. 2015, 71, 14–19. (In Russian) [Google Scholar] [CrossRef]
- Yavorskaya, N.M. Zoobenthos structure of the left-bank tributies of the Lower Amur (Khabarovsk territory). Reg. Probl. 2016, 19, 40–45. (In Russian) [Google Scholar]
- Yavorskaya, N.M. Zoobenthos of salmon rivers in the Anyuysky National Park (Khabarovsky Region, Russia). Amurian Zool. J. 2021, 13, 183–201. (In Russian) [Google Scholar] [CrossRef]
- Yavorskaya, N.M. Quantitative zoobenthos characteristics of the Udyl Lake basin (Udyl Nature Reserve, Khabarovsky Region). Amurian Zool. J. 2022, 14, 594–615. (In Russian) [Google Scholar] [CrossRef]
- Chertoprud, E.S.; Seleznev, D.G.; Garibian, P.G.; Kotov, A.A. Microcrustaceans (Cladocera and Copepoda) of three large lakes in the transitional zone between Boreal and Tropical faunas at Russian Far East: Case study of species richness and association structure. Diversity 2023, 15, 338. [Google Scholar] [CrossRef]
- Borutsky, E.V.; Klyuchareva, O.A.; Nikolsky, G.V. Benthic Invertebrates (zoobenthos) of the Amur River and their role in the nutrition of Amur fishes. In Proceedings of the Amur Ichthyological Expedition; Moscow Society of Nature Explorers Publ.: Moscow, Russia, 1952; Volume 3, pp. 5–39. (In Russian) [Google Scholar]
- Sirotsky, S.E.; Makarchenko, E.A.; Makarchenko, M.A. of the Amur River Basin by the Composition of Zoobenthos. Fish Iss. 2009, 10, 453–467. (In Russian) [Google Scholar]
- Chertoprud, M.V.; Chertoprud, E.S.; Vorobjeva, L.V.; Palatov, D.M.; Tsyganov, A.N.; Reshetov, I.S.; Kovacheva, N.P. Macrozoobenthic communities of the piedmont and lowland watercourses of the lower Amur Region. Inland Water Biol. 2020, 13, 51–61. [Google Scholar] [CrossRef]
- Kocharina, S.L.; Makarchenko, E.A.; Makarchenko, M.A.; Nikolaeva, E.A.; Tiunova, T.M.; Teslenko, V.A. Benthic Invertebrates in the Ecosystem of the Salmon River of the South of the Far East of the USSR. In Fauna, Systematics and Biology of Freshwater Invertebrates; Dalnauka: Vladivostok, Russia, 1988; pp. 86–108. [Google Scholar]
- Tiunova, T.M. Mayflies of the Kedrovaya River and Their Ecological and Physiological Characteristics; Dalnauka: Vladivostok, Russia, 1993; p. 196. (In Russian) [Google Scholar]
- Bogatov, V.V. Ecology of River Communities in the Russian Far East; Dalnauka: Vladivostok, Russia, 1994; p. 210. (In Russian) [Google Scholar]
- Sergeev, M.A. The Shantar Islands. In Soviet Islands of the Pacific Ocean; OGIZ: Leningrad, Russia, 1938; pp. 197–260. [Google Scholar]
- Alekseev, S.S.; Gruzdeva, M.A.; Skopets, M.B. Ichthyofauna of the Shantar Islands. Voprosy Ichthyol. 2004, 44, 42–58. (In Russian) [Google Scholar]
- Schlotgauer, S.D. Botanical and geographical features of the coastal and aquatic flora of the Shantar Islands National Park (Khabarovsk territory). Proc. Mordovia State Nat. Reserve. 2020, 25, 393–402. (In Russian) [Google Scholar]
- Lindbergh, G.W.; Dulkeit, G.D. Data on the fishes of the Shantar Sea. Izvestiya TONS 1929, 3, 138. (In Russian) [Google Scholar]
- Kolesnikov, B.P. Essay of Vegetation of the Far East; Khabarovskoe Knizhnoe Izd-vo: Khabarovsk, Russia, 1955; p. 141. (In Russian) [Google Scholar]
- Razzhigaeva, N.G.; Grebennikova, T.A.; Ganzey, L.A.; Chakov, V.V.; Klimin, M.A.; Mokhova, L.M.; Zakharchenko, E.N. Stratigraphy of the watershed peat bog and development of the natural environment of Bolshoi Shantar Island in the Late Glacial-Holocene. Pacific Geol. 2021, 40, 85–102. (In Russian) [Google Scholar]
- Yavorskaya, N.M.; Teslenko, V.A.; Gorovaya, E.A. Bottom invertebrates in streams of the Tugur Peninsula (Khabarovsk Territory). Levanidov’s Bienn. Memor. Meet. 2023, 10, 300–316. (In Russian) [Google Scholar] [CrossRef]
- The Expedition of the Far Eastern Branch of the Russian Academy of Sciences to the Shantar Islands Has Ended. Available online: https://www.poi.dvo.ru/ru/node/1882 (accessed on 19 September 2023). (In Russian).
- Kussakin, O.G. Marine and Brackish-Water Isopod Crustaceans (Isopoda) of Cold and Temperate Waters of the Northern Hemisphere. I. Suborder Flabellifera; Nauka: Leningrad, Russia, 1979; p. 472. (In Russian) [Google Scholar]
- Scarlato, O.A. Bivalve Mollusks of Temperate Waters of the Northwestern Pacific; Nauka: Leningrad, Russia, 1981; p. 212. (In Russian) [Google Scholar]
- Kafanov, A.I. Bivalve Mollusks and Faunistic Biogeography of the Northern Pacific; Far East Branch of Academy of Sciences of the USSR: Vladivostok, Russia, 1991; p. 192. [Google Scholar]
- Garibyan, P.G.; Neretina, A.N.; Korovchinsky, N.M.; Sinev, A.Y.; Chabovsky, A.V.; Kotov, A.A.; Smirnov, N.N. The South of the Russian Far East and Korea as a Transition Zone between the Boreal and Subtropical Faunas of Cladocera, Crustacea. Zool. J. 2020, 99, 1094–1109. [Google Scholar] [CrossRef]
- Novichkova, A.A.; Azovsky, A.I. Factors affecting regional diversity and distribution of freshwater microcrustaceans (Cladocera, Copepoda) at high latitudes. Polar Biol. 2017, 40, 185–198. [Google Scholar] [CrossRef]
- Shurin, J.B.; Havel, J.E.; Leibold, M.A.; Pinel-Alloul, B. Local and regional zooplankton species richness: A scale-independent test for saturation. Ecology 2000, 81, 3062–3073. [Google Scholar] [CrossRef]
- Getz, E.; Eckert, C. Effects of Salinity on Species Richness and Community Composition in a Hypersaline Estuary. Estuaries Coasts 2023, 46, 2175–2189. [Google Scholar] [CrossRef]
- Josefson, A.B. Species sorting of benthic invertebrates in a salinity gradient—Importance of dispersal limitation. PLoS ONE 2016, 11, e0168908. [Google Scholar] [CrossRef] [PubMed]
- Khlebovich, V.V. Critical Salinity of Biological Processes; Nauka Publisher: Leningrad, Russia, 1974; p. 236. (In Russian) [Google Scholar]
- Udalov, A.A.; Burkovsky, I.V.; Mokievsky, V.O.; Stolyarov, A.P.; Mazey, Y.A.; Saburova, M.A.; Chertoprud, M.V.; Chertoprud, E.S.; Ilyinsky, V.V.; Kolobov, M.Y.; et al. Changes in the general characteristics of micro-, meio-, and macrobenthos along the Salinity gradient in the White Sea estuary. Oceanology 2004, 44, 514–525. [Google Scholar]
- Zinchenko, T.D.; Shitikov, V.K.; Golovatyuk, L.V.; Gusakov, V.A.; Lazareva, V.I. Analysis of relations between communities of hydrobionts in saline rivers by multidimensional block ordination. Inland Water Biol. 2019, 12, 104–110. [Google Scholar] [CrossRef]
- Alimov, A.F.; Bogatov, V.V.; Golubkov, S.M. Production Hydrobiology; Nauka: St. Petersburg, Russia, 2013; p. 342. (In Russian) [Google Scholar]
- Hamzaraj, E.; Lazo, P.; Paparisto, A.; Parllaku, B. Using bacteria and benthic macroinvertebrates as water quality parameters in Mat River, Albania. AQUA Water Infrastruct. Ecosyst. Soc. 2023, 72, 1852–1866. [Google Scholar] [CrossRef]
- Steward, A.L.; Datry, T.; Langhans, S.D. The terrestrial and semi-aquatic invertebrates of intermittent rivers and ephemeral streams. Biol. Rev. 2022, 97, 1408–1425. [Google Scholar] [CrossRef]
- Upadhyay, K.D.; Mainali, J.; Ghimire, N.P. Diversity of aquatic plants and macroinvertebrates and their spatial patterns in a Himalayan Watershed, Central Nepal. Aquat. Bot. 2022, 180, 103529. [Google Scholar] [CrossRef]
- Alimov, A.F. Studies on biodiversity in the plankton, benthos, and fish communities, and the ecosystems of freshwater bodies differing in productivity. Biol. Bull. Rus. Acad. Sci. 2001, 28, 75–83. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: New York, NY, USA, 2001. [Google Scholar]
- Kalff, J. Limnology: Inland Water Ecosystems; Prentice Hall: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Padmanabha, B. Comparative study on the hydrographical status in the lentic and lotic ecosystems. Global J. Ecol. 2017, 2, 15–18. [Google Scholar] [CrossRef]
- Wan Maznah, W.O.; Intan, S.; Sharifah, R.; Lim, C.C. Lentic and lotic assemblages of zooplankton in a tropical reservoir, and their association with water quality conditions. Int. J. Environ. Sci. Tech. 2017, 15, 533–542. [Google Scholar] [CrossRef]
- Brüchner-Hüttemann, H.; Ptatscheck, C.; Traunspurger, W. Meiofauna in stream habitats: Temporal dynamics of abundance, biomass and secondary production in different substrate microhabitats in a first-order stream. Aquat. Ecol. 2020, 54, 1079–1095. [Google Scholar] [CrossRef]
- Eramma, N.; Lalita, H.M.; Satishgouda, S.; Jyothi, S.R.; Venkatesh, C.N.; Patil, S.J. Zooplankton Productivity Evaluation of Lentic and Lotic Ecosystem. In Limnology—The Importance of Monitoring and Correlations of Lentic and Lotic Waters; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Fedorov, V.D.; Kapkov, V.I. Practical Hydrobiology of Freshwater Ecosystems; MGU: Moscow, Russia, 2006; p. 365. (In Russian) [Google Scholar]
- Schwoerbel, J. Methods of Hydrobiology: (Freshwater Biology); Elsevier: Amsterdam, The Netherlands, 2016; p. 210. [Google Scholar]
- Chertoprud, M.V.; Chertoprud, E.S. A Brief Guide to Freshwater Invertebrates in the Center of European Russia, 3rd ed.; KMK: Moscow, Russia, 2011; p. 219. (In Russian) [Google Scholar]
- Mare, M.F. A study of a marine benthic community with special reference to the micro-organisms. J. Mar. Biol. Assoc. UK 1942, 25, 517–554. [Google Scholar] [CrossRef]
- Meyer, J.L. A blackwater perspective on riverine ecosystems. BioScience 1990, 40, 643–651. [Google Scholar] [CrossRef]
- Hakenkamp, C.C.; Palmer, M.A. The ecology of hyporheic meiofauna. In Streams and Ground Waters; Academic Press: Cambridge, MA, USA, 2000; pp. 307–336. [Google Scholar]
- Andersen, T.J.; Pejrup, M. Biological Influences on Sediment Behavior and Transport. In Treatise on Estuarine and Coastal Science; Academic Press: Cambridge, MA, USA, 2011; pp. 289–309. [Google Scholar]
- Watling, L. Macrofauna. In Encyclopedia of Ocean Sciences, 3rd ed.; Cochran, J.K., Bokuniewicz, H.J., Yager, P.L., Eds.; Academic Press: Oxford, UK, 2019; pp. 728–734. [Google Scholar]
- Giere, O. Meiobenthology: The Microscopis Fauna in Aquatic Sediments; Springer: Berlin, Germany, 1993; p. 328. [Google Scholar]
- Alekseev, V.R.; Tsalolikhin, S.Y. Guide of Freshwater Zooplankton and Zoobenthos of European Russia. Zooplankton, 1; KMK Press: Moscow, Russia, 2010; p. 495. (In Russian) [Google Scholar]
- Borutsky, E.V. Crustaceans Freshwater Harpacticoids; Fauna of USSR Crustacea, 3; AN USSR: Moscow, Russia, 1952; p. 425. (In Russian)
- Borutsky, E.V.; Stepanova, L.A.; Kos, M.S. Key to Identification of Calanoida from Fresh Waters; Nauka: St. Petersburg, Russia, 1991; p. 504. (In Russian) [Google Scholar]
- Dussart, B.H.; Defaye, D. Répertoire Mondial des Crustacés Copépodes des eaux Intérieures. Calanoïdes; CNRS Bordeaux: Paris, France, 1983; p. 224. [Google Scholar]
- Fefilova, E.B. Copepods (Copepoda). Fauna of the European North-East of Russia; KMK Scientific Press: Moscow, Russia, 2015; p. 319. (In Russian) [Google Scholar]
- Korovchinsky, N.M.; Kotov, A.A.; Sinev, A.Y.; Neretina, A.N.; Garibian, P.G. Cladocera (Crustacea) of North Eurasia; KMK Scientific Press Ltd.: Moscow, Russia, 2021; p. 544. (In Russian) [Google Scholar]
- Lieder, U. Crustacea: Cladocera/Bosminidae. In Süsswasserfauna von Mitteleuropa; Fischer Verlag: Stuttgart, Germany, 1996; p. 80. [Google Scholar]
- Sinev, A.Y. A key to identifying cladocerans of the genus Alona (Anomopoda, Chydoridae) from the Russian European part and Siberia. Russ. J. Zool. 2002, 81, 926–939. [Google Scholar]
- Smirnov, N.N. Chydoridae of the world fauna. In Fauna SSSR. Rakoobraznye (Crustacea), 1; Smirnov, N.N., Ed.; Nauka: Leningrad, Russia, 1971; p. 529. (In Russian) [Google Scholar]
- Tsalolikhin, S.J. (Ed.) Key to Freshwater Invertebrates of Russia and Adjacent Lands. Vol. 4. Dipteran Insects; Nauka: St. Petersburg, Russia, 2000; p. 998. (In Russian) [Google Scholar]
- Tsalolikhin, S.J. (Ed.) Key to Freshwater Invertebrates of Russia and Adjacent Lands. Vol. 5, Higher Insects; Nauka: St. Petersburg, 2001; p. 825. (In Russian) [Google Scholar]
- Tsalolikhin, S.J. (Ed.) Key to Freshwater Invertebrates of Russia and Adjacent Lands. Vol. 6, Mollusks. Polychaetes. Nemerteans; Nauka: St. Petersburg, Russia, 2004; p. 528. (In Russian) [Google Scholar]
- Leley, A.S. Key to the Insects of Russian Far East. Diptera and Siphonaptera; Dalnauka: Vladivostok, Russia, 2001; Volume 4, 936p. (In Russian) [Google Scholar]
- Teslenko, V.A.; Zhiltzova, L.A. Key to the Stoneflies (Insecta, Plecoptera) of Russia and Adjacent Countries. Imagines and Nymphs; Dalnauka: Vladivostok, Russia, 2009; p. 382. (In Russian) [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 11 April 2020).
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Plymouth Marine Laboratory: Plymouth, UK, 2001. [Google Scholar]
- Holmquist, C. Taxonomy, distribution, and ecology of the three species Neomysis intermedia (Czerniavsky), N. awatschensis (Brandt) and N. mercedis Holmes (Crustacea, Mysidacea). Zool. Jb. Syst. 1973, 100, 197–222. [Google Scholar]
- Zavarzin, D.S. Some issues of seasonal dynamics of zooplankton of Lake Tunaycha (southern Sakhalin) at the present stage. Levanidov’s Bienn. Memor. Meet. 2005, 3, 95–105. (In Russian) [Google Scholar]
- Ermolaeva, N.I. Species composition and spatial distribution of zooplankton of the Ob Bay and Gydan Bay. In Water and Ecological Problems of Siberia and Central Asia; Institute of Water and Environmental Problems of the Siberian Branch of the Russian Academy of Sciences: Barnaul, Russia, 2017; pp. 91–99. [Google Scholar]
- Chertoprud, E.S.; Novichkova, A.A. Crustaceans in the meiobenthos and plankton of the thermokarst lakes and polygonal ponds in the Lena River Delta (Northern Yakutia, Russia): Species composition and factors regulating assemblage structures. Water 2021, 13, 1936. [Google Scholar] [CrossRef]
- Chertoprud, E.S.; Frenkel, S.E.; Novichkova, A.A.; Vodopyanov, S.S. Fauna and structure of taxocenes of Harpacticoida (Copepoda), brackish-water lagoons and estuaries of the Far East of Russia. Oceanology 2014, 54, 739–751. [Google Scholar] [CrossRef]
- Kotov, A.A. Adaptations of Anomopoda crustaceans (Cladocera) to the benthic mode of life. Entmol. Rev. 2006, 86, 210–225. [Google Scholar] [CrossRef]
- Kotov, A.A. Faunistic complexes of the Cladocera (Crustacea, Branchiopoda) of Eastern Siberia and Far East of Russia. Biol. Bull. 2016, 43, 970–987. [Google Scholar] [CrossRef]
- Chertoprud, E.S.; Novichkova, A.A.; Tsyganov, A.N.; Vorobjeva, L.V.; Esaulov, A.S.; Krylenko, S.V.; Mazei, Y.A. Species diversity and driving factors of benthic and zooplanktonic assemblages at different stages of thermokarst lake development: A case study in the Lena River delta (Middle Siberia). Diversity 2023, 15, 511. [Google Scholar] [CrossRef]
- Bogatov, V.V.; Fedorovsky, A.S. Fundamentals of River Hydrology and Hydrobiology; Dalnauka: Vladivostok, Russia, 2017; p. 384. (In Russian) [Google Scholar]
- Labay, V.S.; Novoselova, A.I.; Abramova, E.V.; Berezova, O.N.; Korneev, E.S.; Sharlay, O.B.; Shpilko, T.S. Macrobenthos of a typical small stream of the Southern Kuril Islands (example of nameless stream, Shikotan Island). Res. Aqua. Biol. Res. Kamchatka North-West Part Pac. Ocean 2020, 1, 107–119. (In Russian) [Google Scholar] [CrossRef]
- Williams, D.D.; Hamm, T. Insect Community Organisation in Estuaries: The Role of the Physical Environment. Ecography 2002, 25, 372–384. [Google Scholar] [CrossRef]
- Teslenko, V.A. Review of the fauna of Plecoptera and zoning of watercourses in the Russian Far East. Euras. Entomol. J. 2007, 6, 157–180. [Google Scholar]
- Chertoprud, M.V. Diversity and classification of rheophilic communities of macrozoobenthos in middle latitudes of European Russia. Biol. Bull. Rev. 2011, 1, 165–184. [Google Scholar] [CrossRef]
- Kromkamp, J.; Peene, J. Possibility of net primary production in the turbid Shelde estuary (SW Netherlands). Mar. Ecol. Progr. Ser. 1995, 121, 249–259. [Google Scholar] [CrossRef]
- Montagna, P.A.; Palmer, T.A.; Pollack, J.B. Hydrological Changes and Estuarine Dynamics; Springer: Berlin/Heidelberg, Germany, 2013; Volume 8, p. 94. [Google Scholar] [CrossRef]
- Drits, A.V.; Arashkevich, E.G.; Nedospasov, A.A.; Amelina, A.B.; Flint, M.V. Structural-functional characteristics of zooplankton of the Ob estuary and adjacent areas of the Kara Sea shelf in the summer period. Oceanology 2019, 59, 383–395. [Google Scholar] [CrossRef]
- Sukhanova, I.N.; Flint, M.V.; Sakharova, E.G.; Fedorov, A.V.; Makkaveev, P.N.; Nedospasov, A.A. Structure of phytocenosises of the Yenisei Estuary and adjacent Kara Sea shelf in late spring. Oceanology 2020, 60, 858–875. (In Russian) [Google Scholar] [CrossRef]
- Cabrol, J.; Winkler, G.; Tremblay, R. Physiological condition and differential feeding behaviour in the cryptic species complex Eurytemora affinis in the St Lawrence estuary. J. Plank. Res. 2015, 37, 372–387. [Google Scholar] [CrossRef]
- Tackx, M.L.M.; Herman, P.J.M.; Gasparini, S.; Irigoien, X.; Billiones, R.; Daro, M.H. Selective feeding of Eurytemora affinis (Copepoda, Calanoida) in temperate estuaries: Model and field observations. Estuar. Coast Shelf Sci. 2003, 56, 305–311. [Google Scholar] [CrossRef]
- Murano, M. Fisheries biology of a marine relict mysid Neomysis intermedia Czerniawsky. Fish. Food 1964, 12, 109–117. (In Japanese) [Google Scholar]
- Pasternak, A.; Arashkevich, E.; Reigstad, M.; Wassmann, P.; Falk-Petersen, S. Dividing mesozooplankton into upper and lower size groups: Applications to the grazing impact in the Marginal Ice Zone of the Barents Sea. DeepSea Res. 2008, 55, 2245–2256. [Google Scholar] [CrossRef]
- Hirche, H.J.; Fetzer, I.; Graeve, M.; Kattner, G. Limnocalanus macrurus in the Kara Sea (Arctic Ocean): An opportunistic copepod as evident from distribution and lipid patterns. Polar Biol. 2003, 26, 720–726. [Google Scholar] [CrossRef]
- Sedova, L.G.; Budnikova, L.L. State of aggregations and biological characteristics for mysid Neomysis awatschensis in the northern Amur Bay (Peter the Great Bay, Japan Sea). Izv. TINRO 2020, 200, 101–117. (In Russian) [Google Scholar] [CrossRef]
- Labay, V.S.; Korneev, E.S.; Abramova, E.V.; Ushakov, A.A.; Akhmadeeva, E.S. Macrobenthos in the estuary of a typical «salmon» river of Sakhalin Island (on example of the Manuy River). Izvest. TINRO 2022, 202, 640–660. (In Russian) [Google Scholar] [CrossRef]
- Petryashev, V.V. Order Mysididae—Mysidacea Boas, 1883. In Crustaceans and Sea Spiders: Biota of the Russian Waters of the Sea of Japan; Dalnauka: Vladivostok, Russia, 2004; Volume 1, pp. 55–96. (In Russian) [Google Scholar]
- Shuntov, V.P. Biology of the Far Eastern Seas of Russia; TINRO–Center: Vladivostok, Russia, 2016; Volume 2, p. 603. (In Russian) [Google Scholar]
- McLusky, D.S. The Estuarine Ecosystem; Blackie: Glasgow, UK; London, UK, 1981; p. 150. [Google Scholar]
- Van Damme, D.; Herman, R.; Sharma, Y.; Holvoet, M.; Martens, P. Benthic studies of the Southern Bight of the North Sea and its adjacent continental estuaries. In Progress Report 2. Fluctuation of the Meiobenthic Communities in the Westerschelde Estuary; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1980; pp. 131–170. [Google Scholar]
- Chertoprud, E.S.; Chertoprud, M.V.; Kondar, D.V.; Kornev, P.N.; Udalov, A.A. Harpacticoida taxocene diversity in the sandy-silty littoral zone of Kandalaksha bay of the White Sea. Oceanology 2006, 46, 492–500. [Google Scholar] [CrossRef]
- Khlebovich, V.V. Applied aspects of the concept of critical salinity. Usp. Sovrem. Biol. 2015, 135, 272–278. (In Russian) [Google Scholar] [CrossRef]
- Lang, K. Monographi der Harpacticiden; Hakan Ohlssons Boktryckery: Lund, Sweden, 1948; p. 1682. [Google Scholar]
- Cesarini, G.; Gallitelli, L.; Traversetti, L.; Bandini, T.; Scalici, M. Hydromorphological discontinuities deeply modify the benthic multi-species assemblage diversity in a Mediterranean running river. Rend. Fis. Acc. Lincei. 2023, 34, 257–266. [Google Scholar] [CrossRef]
- Rossaro, B.; Marziali, L.; Boggero, A. Response of Chironomids to Key Environmental Factors: Perspective for Biomonitoring. Insects 2022, 13, 911. [Google Scholar] [CrossRef]
- Novichkova, A.A.; Chertoprud, E.S. The freshwater crustaceans (Cladocera: Copepoda) of Bering Island (Commander Islands, Russian Far East): Species richness and taxocene structure. J. Nat. Hist. 2016, 50, 1357–1368. [Google Scholar] [CrossRef]
- Pinel-Alloul, B.; André, A.; Legendre, P.; Cardille, J.A.; Patalas, K.; Salki, A. Large-scale geographic patterns of diversity and community structure of pelagic crustacean zooplankton in Canadian lakes. Glob. Ecol. Biogeogr. 2013, 22, 784–795. [Google Scholar] [CrossRef]
- Nekhaev, I.O. Freshwater gastropods of the western part of the Kola Peninsula and northern Karelia (northern Europe). Ruthenica 2021, 31, 147–175. [Google Scholar] [CrossRef] [PubMed]
- Vinarski, M.V.; Bolotov, I.N.; Aksenova, O.V.; Babushkin, E.S.; Bespalaya, Y.V.; Makhrov, A.A.; Nekhaev, I.O.; Vikhrev, I.V. Freshwater Mollusca of the Circumpolar Arctic: A review on their taxonomy, diversity and biogeography. Hydrobiologia 2021, 848, 2891–2918. [Google Scholar] [CrossRef]
- De Meester, L.; Gómez, A.; Okamura, B.; Schwenk, K. The Monopolization Hypothesis and the dispersal-gene flow paradox in aquatic organisms. Acta Oecol. 2002, 23, 121–135. [Google Scholar] [CrossRef]
- Novichkova, A.A.; Chertoprud, E.S. Crustaceans of Wrangel Island (Russia, Chukotka): Species composition, community structure and variability. Nat. Cons. Res. 2020, 5, 37–50. (In Russian) [Google Scholar] [CrossRef]
- Bonacina, L.; Fasano, F.; Mezzanotte, V.; Fornaroli, R. Effects of water temperature on freshwater macroinvertebrates: A systematic review. Biol. Rev. Camb. Philos. Soc. 2023, 98, 191–221. [Google Scholar] [CrossRef] [PubMed]
- Bespalaya, Y.V.; Travina, O.V.; Tomilova, A.A.; Khrebtova, I.S.; Aksenova, O.V.; Aksenov, A.S.; Vinarskii, M.V.; Kondakov, A.V.; Nekhaev, I.O.; Palatov, D.M.; et al. Species Diversity, Settlement Routes, and Ecology of Freshwater Mollusks of Kolguev Island (Barents Sea, Russia). Inland Water Biol. 2022, 15, 836–849. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novichkova, A.A.; Borisov, R.R.; Vorobjeva, L.V.; Palatov, D.M.; Chertoprud, M.V.; Chertoprud, E.S. The Influence of Salinity Gradient and Island Isolation on Fauna Composition and Structure of Aquatic Invertebrate Communities of the Shantar Islands (Khabarovsk Krai). Diversity 2023, 15, 1198. https://doi.org/10.3390/d15121198
Novichkova AA, Borisov RR, Vorobjeva LV, Palatov DM, Chertoprud MV, Chertoprud ES. The Influence of Salinity Gradient and Island Isolation on Fauna Composition and Structure of Aquatic Invertebrate Communities of the Shantar Islands (Khabarovsk Krai). Diversity. 2023; 15(12):1198. https://doi.org/10.3390/d15121198
Chicago/Turabian StyleNovichkova, Anna A., Rostislav R. Borisov, Lada V. Vorobjeva, Dmitry M. Palatov, Mikhail V. Chertoprud, and Elena S. Chertoprud. 2023. "The Influence of Salinity Gradient and Island Isolation on Fauna Composition and Structure of Aquatic Invertebrate Communities of the Shantar Islands (Khabarovsk Krai)" Diversity 15, no. 12: 1198. https://doi.org/10.3390/d15121198
APA StyleNovichkova, A. A., Borisov, R. R., Vorobjeva, L. V., Palatov, D. M., Chertoprud, M. V., & Chertoprud, E. S. (2023). The Influence of Salinity Gradient and Island Isolation on Fauna Composition and Structure of Aquatic Invertebrate Communities of the Shantar Islands (Khabarovsk Krai). Diversity, 15(12), 1198. https://doi.org/10.3390/d15121198







