Vegetation History in Central Croatia from ~10,000 Cal BC to the Beginning of Common Era—Filling the Palaeoecological Gap for the Western Part of South-Eastern Europe (Western Balkans)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods
2.2.1. Core Extraction
2.2.2. Lithological Description
2.2.3. Carbon–Nitrogen Contents and Mineralogical Composition
2.2.4. Pollen, Non-Pollen Palynomorphs (NPPs) and Charcoal Extraction and Determination
2.2.5. Statistical Analysis
2.2.6. Chronology
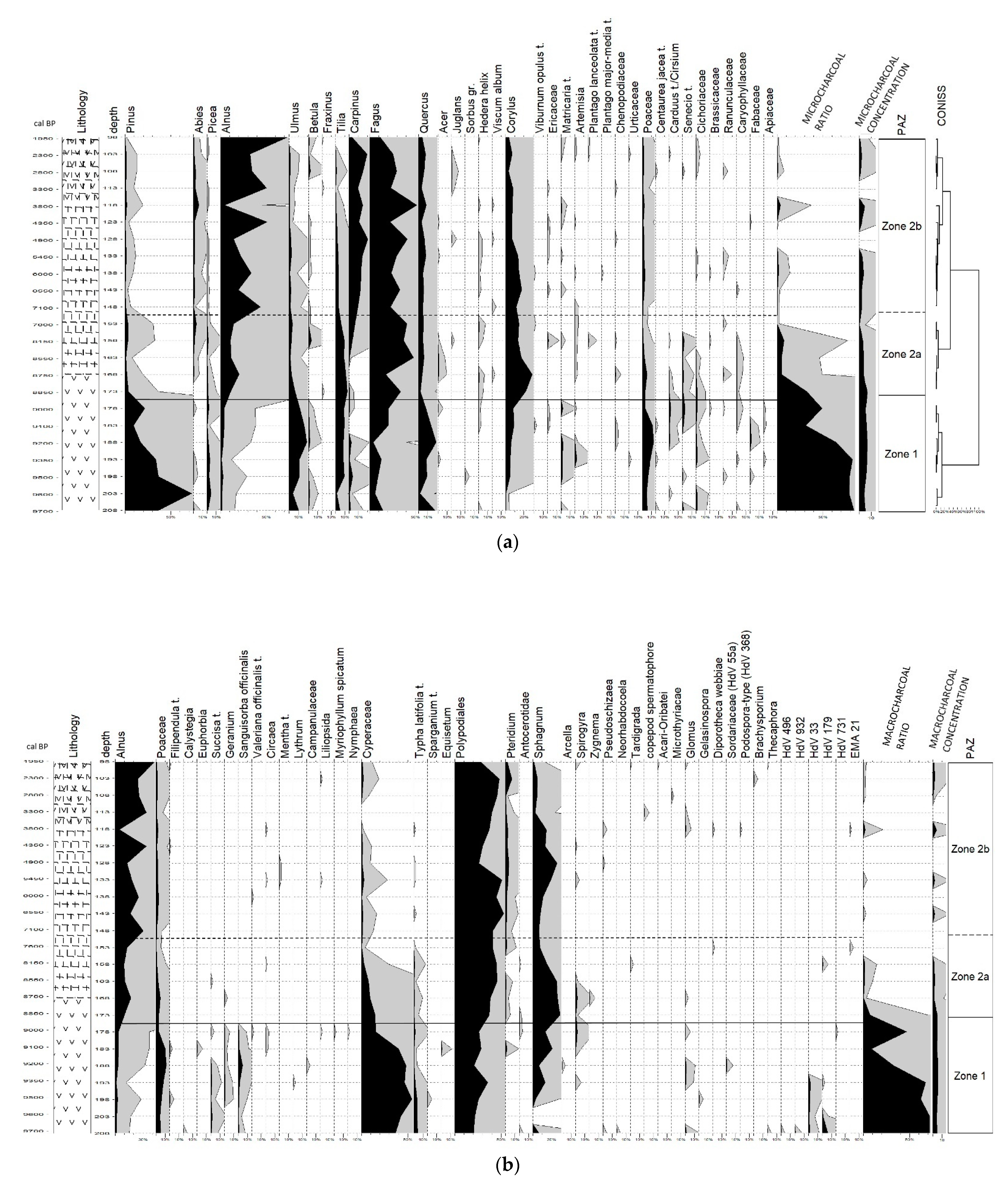
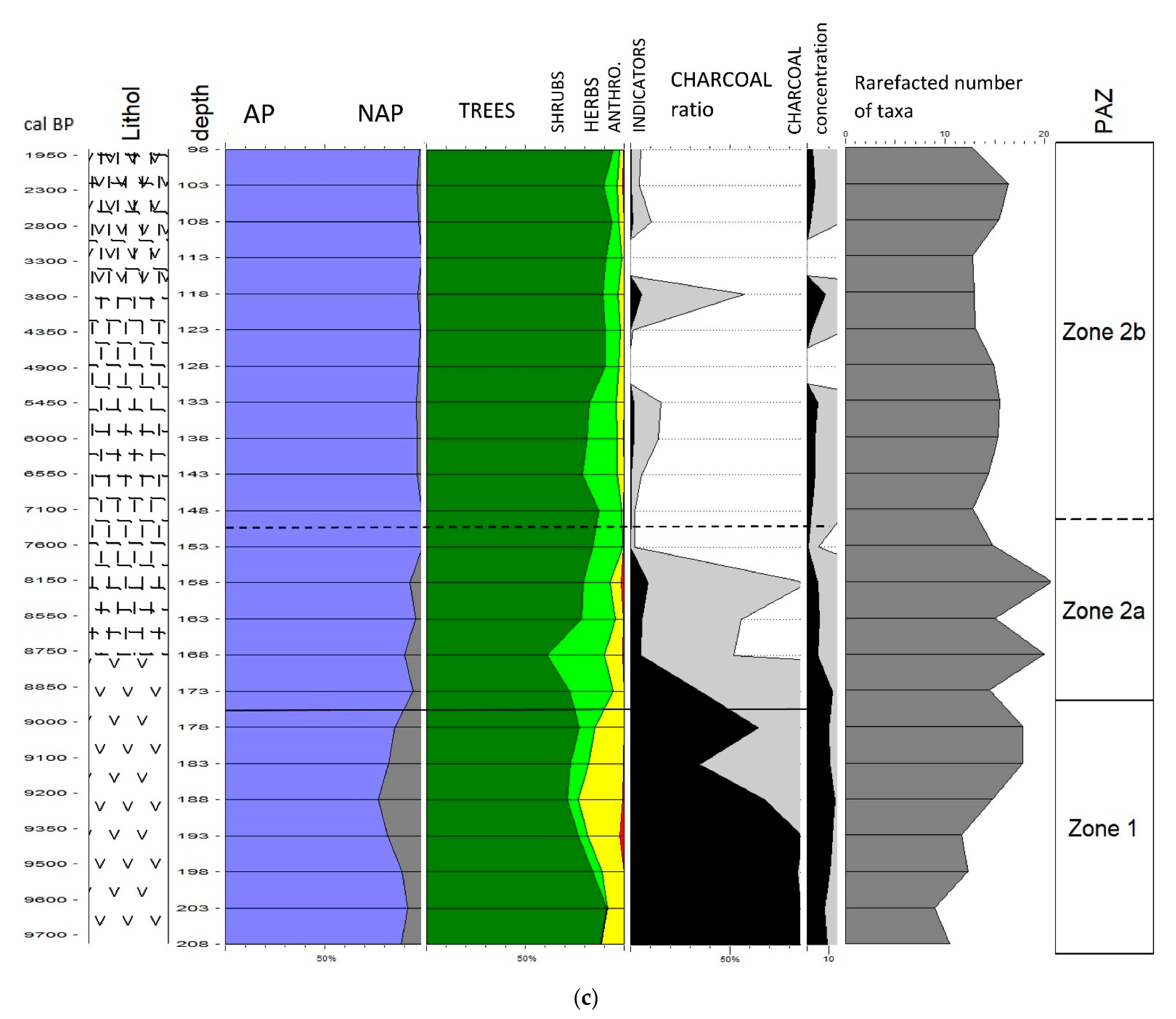
3. Results
3.1. Sediment Description and Chronology
3.2. Pollen-Based Vegetation, NPPs and Fire History
3.2.1. Zone 1
3.2.2. Zone 2a
3.2.3. Zone 2b
4. Discussion
4.1. Interpretation of Geochemical and Mineralogical Analyses
4.2. Vegetation, Fire and Hydrology Changes during the Preboreal Chronozone (from ~9800 to 9000 cal yr BP)
4.2.1. Regional Vegetation Changes and Fire History
4.2.2. Local Vegetation History, Fire and Hydrological Changes
4.3. Vegetation, Fire and Hydrology Changes during the Boreal Chronozone (from ~9000 to ~7000 cal yr BP)
4.3.1. Regional Vegetation Changes and Fire History
4.3.2. Local Vegetation History, Fire and Hydrological Changes
4.4. Vegetation, Fire and Hydrology Changes during the Atlantic, Subboreal and Older Subatlantic Chronozone (from ~7000 to ~1800 cal yr BP)
4.4.1. Regional Vegetation Changes and Fire History
4.4.2. Local Vegetation History, Fire and Hydrological Changes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Bellen, S.; Garneau, M.; Ali, A.A.; Lamarre, A.; Robert, É.C.; Magnan, G.; Asnong, H.; Pratte, S. Poor fen succession over ombrotrophic peat related to late Holocene increased surface wetness in subarctic Quebec, Canada. J. Quat. Sci. 2013, 28, 748–760. [Google Scholar] [CrossRef]
- Spitzer, K.; Danks, H.V. Insect biodiversity of boreal peat bogs. Annu. Rev. Entomol. 2006, 51, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Godwin, H. The Archives of the Peat Bogs; Cambridge University Press: Cambridge, UK, 1981. [Google Scholar]
- Barber, K.E. Peatlands as scientific archives of past biodiversity. Biodivers Conserv. 1993, 2, 474–489. [Google Scholar] [CrossRef]
- Lisitsyna, O.V.; Hicks, S.; Huusko, A. Do moss samples, pollen traps and modern lake sediments all collect pollen in the same way? A comparison from the forest limit area of northernmost Europe. Veget. Hist. Archaeobot. 2012, 21, 187–199. [Google Scholar] [CrossRef]
- Chevalier, M.; Davis, B.A.S.; Heiri, O.; Seppä, H.; Chase, B.M.; Gajewski, K.; Lacourse, T.; Telford, R.J.; Finsinger, W.; Guioti, J.; et al. Pollen-based climate reconstruction techniques for late Quaternary studies. Earth. Sci. Rev. 2020, 210, 103384. [Google Scholar] [CrossRef]
- Wojewódka, M.; Hruševar, D. The role of paleolimnology in climate and environment reconstruction and lake restoration in light of research on selected bioindicators. Holistic. Approach. Environ. 2020, 10, 16–28. [Google Scholar] [CrossRef]
- Noryśkiewicz, A.M. Postglacial vegetation changes in the development of raised mires in Poland. Monogr. Bot. 2005, 94, 119–133. [Google Scholar]
- Bałaga, K. Transformation of lake ecosystem into peat bog and vegetation history based on durne Bagno mire (Lublin Polesie, E Poland). Geochronometria 2007, 29, 23–43. [Google Scholar] [CrossRef]
- Andrič, M.; Kroflič, B.; Toman, M.J.; Ogrinc, N.; Dolenec, T.; Dobnikar, M.; Čermelj, B. Late quaternary vegetation and hydrological change at Ljubljansko barje (Slovenia). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 270, 150–165. [Google Scholar] [CrossRef]
- Muller, S.D.; Miramont, C.; Bruneton, H.; Carré, M.; Sottocornola, M.; Court-Picon, M.; Beaulieu, J.L.; Nakagawa, T.; Schevin, P. A palaeoecological perspective for the conservation and restoration of wetland plant communities in the central French Alps, with particular emphasis on alder carr vegetation. Rev. Palaeobot. Palynol. 2012, 171, 124–139. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Kulesza, P.; Łojek, J.; Pidek, I.A. Origin and evolution of the Bezedna lake–mire complex in the Lublin area (East Poland): A case study for permafrost lakes in karstic regions. J. Paleolimnol. 2015, 53, 191–213. [Google Scholar] [CrossRef] [Green Version]
- Byun, E.; Cowling, S.A.; Finkelstein, S.A. Holocene regional climate change and formation of southern Ontario’s largest swamp inferred from a kettle-lake pollen record. Quat. Res. 2021, 103, 1–19. [Google Scholar] [CrossRef]
- Herzschuh, U.; Kürschner, H.; Ma, Y. The surface pollen and relative pollen production of the desert vegetation of the Alashan Plateau, western Inner Mongolia. Sci. Bull. 2003, 48, 1488–1493. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, H.; Wang, H.; Hao, Q.; Han, Y.; Duan, K.; Dong, Z. Climate-Driven Holocene Migration of Forest-Steppe Ecotone in the Tien Mountains. Forests 2020, 11, 1139. [Google Scholar] [CrossRef]
- Behre, K.E. The interpretation of anthropogenic indicators in pollen diagrams. Pollen Spores 1981, 23, 225–245. [Google Scholar]
- Court-Picon, M.; Buttler, A.; de Beaulieu, J.-L. Modern pollen/vegetation/land-use relationships in mountain environments: An example from the Champsaur valley (French Alps). Veget. Hist. Archaeobot. 2006, 15, 151–168. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, L.; Cui, H. Pollen indicators of human activity. Chin. Sci. Bull. 2008, 53, 1281–1293. [Google Scholar] [CrossRef]
- Brun, C. Anthropogenic indicators in pollen diagrams in eastern France: A critical review. Veget. Hist. Archaeobot. 2011, 20, 135–142. [Google Scholar] [CrossRef]
- Florenzano, A. The History of Pastoral Activities in S Italy Inferred from Palynology: A Long-Term Perspective to Support Biodiversity Awareness. Sustainability 2019, 11, 404. [Google Scholar] [CrossRef]
- Beyens, L.; Meisterfeld, R. Protozoa: Testate Amoebae. In Tracking Environmental Change Using Lake Sediments: Terrestrial, Algal, and Siliceous Indicators; Smol, J.P., Birks, J.B., Last, W.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; Volume 3, pp. 121–153. [Google Scholar] [CrossRef]
- Van Geel, B. Non-pollen palynomorphs. In Tracking Environmental Change Using Lake Sediments: Terrestrial, Algal, and Siliceous Indicators; Smol, J.P., Birks, J.B., Last, W.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; Volume 3, pp. 99–120. [Google Scholar] [CrossRef]
- Shumilovskikh, L.S.; van Geel, B. 2020, Non-Pollen Palynomorphs. In Handbook for the Analysis of Micro-Particles in Archaeological Samples; Henry, A.G., Ed.; Springer Nature Switzerland: Cham, Switzerland, 2020; pp. 65–94. [Google Scholar]
- Dudová, L.; Hájková, P.; Buchtová, H.; Opravilová, V. Formation, succcession and landscape history of Central-European summit raised bogs: A multiproxy study from the Hrubý Jeseník Mountains. Holocene 2013, 23, 230–242. [Google Scholar] [CrossRef]
- Krąpiec, M.; Margielewski, W.; Korzeń, K. Late-Holocene palaeoclimate variability: The significance of bog pine dendrochronology related to peat stratigraphy. The Puścizna Wielka raised bog case study (Orawa-Nowy Targ Basin, Polish Inner Carpathians). Quat. Sci. Rev. 2016, 148, 192–208. [Google Scholar] [CrossRef]
- Glais, A.; López-Sáez, J.A.; Lespez, L.; Davidson, R. Climate and human-environment relationships on the edge of the Tenaghi-Philippon marsh (Northern Greece) during the Neolithization proces. Quat. Int. 2016, 403, 237–250. [Google Scholar] [CrossRef]
- Kołaczek, P.; Karpińska-Kołaczek, M.; Marcisz, K.; Gałka, M.; Lamentowicz, M. Palaeohydrology and the human impact on one of the largest raised bogs complex in the Western Carpathians (Central Europe) during the last two millennia. Holocene 2017, 28, 595–608. [Google Scholar] [CrossRef]
- Karpińska-Kołaczek, M.; Woszczyk, M.; Stachowicz-Rybka, R.; Obidowicz, A.; Kołaczek, P. The impact of climate changes during the last 6000 years on a small peatland in North-Eastern Poland: A multi-proxy study. Rev. Palaeobot. Palynol. 2018, 259, 81–92. [Google Scholar] [CrossRef]
- Bakels, C. Pollen and Archaeology. In Handbook for the Analysis of Micro-Particles in Archaeological Samples; Henry, A.G., Ed.; Springer Nature Switzerland: Cham, Switzerland, 2020; pp. 203–224. [Google Scholar]
- Gałka, M.; Miotk-Szpiganowicz, G.; Goslar, T.; Jęśko, M.; van der Knaap, W.O.; Lamentowicz, M. Palaeohydrology, fires and vegetation succession in the southern Baltic during the last 7500 years reconstructed from a raised bog based on multi-proxy data. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 370, 209–221. [Google Scholar] [CrossRef]
- López-Sáez, J.A.; Abel-Schaad, D.; Pérez-Díaz, S.; Blanco-González, A.; Alba-Sánchez, F.; Dorado, M.; Ruiz-Zapata, B.; Gil-García, M.J.; Gómez-González, C.; Franco-Múgica, F. Vegetation history, climate and human impact in the Spanish Central System over the last 9000 years. Quat. Int. 2014, 353, 98–122. [Google Scholar] [CrossRef]
- Magyari, E.K.; Veres, D.; Wennrich, V.; Wagner, B.; Braun, M.; Jakab, G.; Karátson, D.; Pál, Z.; Ferenczy, G.; St-Onge, G.; et al. Vegetation and environmental responses to climate forcing during the Last Glacial Maximum and deglaciation in the East Carpathians: Attenuated response to maximum cooling and increased biomass burning. Quat. Sci. Rev. 2014, 106, 278–298. [Google Scholar] [CrossRef]
- Lamentowicz, M.; Słowiński, M.; Marcisz, K.; Zielińska, M.; Kaliszan, K.; Lapshina, E.; Gilbert, D.; Buttler, A.; Fiałkiewicz-Kozieł, B.; Jassey, V.E.J.; et al. Hydrological dynamics and fire history of the last 1300 years in western Siberia reconstructed from a high-resolution, ombrotrophic peat archive. Quat. Res. 2015, 84, 312–325. [Google Scholar] [CrossRef]
- Mensing, S.A.; Tunno, I.; Sagnotti, L.; Florindo, F.; Noble, P.; Archer, C.; Zimmerman, S.; Pavon-Carrasco, F.J.; Cifani, G.; Passigli, S.; et al. 2700 years of Mediterranean environmental change in central Italy: A synthesis of sedimentary and cultural records to interpret past impacts of climate on society. Quat. Sci. Rev. 2015, 116, 72–94. [Google Scholar] [CrossRef]
- Monegato, G.; Ravazzi, C.; Culiberg, M.; Pini, R.; Bavec, M.; Calderoni, G.; Jež, J.; Perego, R. Sedimentary evolution and persistence of open forests between the south-eastern Alpine fringe and the Northern Dinarides during the Last Glacial Maximum. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 436, 23–40. [Google Scholar] [CrossRef]
- Blaus, A.; Reitalu, T.; Amon, L.; Vassiljev, J.; Alliksaar, T.; Veski, S. From bog to fen: Palaeoecological reconstruction of the development of a calcareous spring fen on Saaremaa, Estonia. Veget. Hist. Archaeobot. 2019, 29, 373–391. [Google Scholar] [CrossRef]
- Dendievel, A.-M.; Dietre, B.; Cubizolle, H.; Hajdas, I.; Kofler, W.; Oberlin, C.; Haas, J.N. Holocene palaeoecological changes and agro-pastoral impact on the La Narce du Béage mire (Massif Central, France). Holocene 2019, 29, 992–1010. [Google Scholar] [CrossRef]
- Marcisz, K.; Lamentowicz, M.; Gałka, M.; Colombaroli, D.; Adolf, C.; Tinner, W. Responses of vegetation and testate amoeba trait composition to fire disturbances in and around a bog in central European lowlands (northern Poland). Quat. Sci. Rev. 2019, 208, 129–139. [Google Scholar] [CrossRef]
- Dendievel, A.-M.; Jouffroy-Bapicot, I.; Argant, J.; Scholtès, A.; Tourman, A.; de Beaulieu, J.-L.; Cubizolle, H. From natural to cultural mires during the last 15 ka years: An integrated approachcomparing 14C ages on basal peat layers with geomorphological, palaeoecological and archaeological data (Eastern Massif Central, France). Quat. Sci. Rev. 2020, 233, 106219. [Google Scholar] [CrossRef]
- Hruševar, D.; Bakrač, K.; Miko, S.; Ilijanić, N.; Hasan, O.; Mamić, M.; Puljak, T.; Vucić, A.; Husnjak Malovec, K.; Weber, M.; et al. Environmental history in Central Croatia for the last two millennia—Vegetation, fire and hydrological changes under climate and human impact. Pril. Inst. arheol. Zagrebu 2020, 37, 117–164. [Google Scholar] [CrossRef]
- Tanneberger, F.; Tegetmeyer, C.; Busse, S.; Barthelmes, A.; Shumka, S.; Moles Mariné, A.; Jenderedjian, K.; Steiner, G.M.; Essl, F.; Etzold, J.; et al. The peatland map of Europe. Mires Peat 2017, 19, 1–17. [Google Scholar] [CrossRef]
- Šoštarić, R. The development of vegetation in the inland area of Croatia during the Postglacial period. Nat. Croat. 2004, 13, 357–369. [Google Scholar]
- Šoštarić, R. The development of postglacial vegetation in coastal Croatia. Acta Bot. Croat. 2005, 64, 383–390. [Google Scholar]
- Beug, H.-J. Über die ersten anthropogenen Vegetationsveränderungen in Süddalmatien an Hand eines neuen Pollendiagrammes vom Malo Jezero auf Mljet. Veröff. Geobot. Inst. Stiftung Rübel. 1962, 37, 9–15. [Google Scholar]
- Beug, H.-J. Vegetationsgeschichtliche Untersuchungen im Küstenbereich von Istrien (Jugoslawien). Flora 1977, 166, 357–381. [Google Scholar]
- Brande, A. Untersuchungen zur postglazialen Vegetationsgeschichte imGebiet der Neretva-Niederung (Dalmatien, Herzegowina). Flora 1973, 162, 1–44. [Google Scholar]
- Grüger, E. Vegetational change. In The Changing Face of Dalmatia, Archaeological and Ecological Studies in a Mediterranean Landscape; Chapman, J., Shiel, R., Batović, Eds.; Leichester Univ. Press: Leichester, UK, 1996; pp. 33–43. [Google Scholar]
- Jahns, S.; Bogaard van den, C. New palynological and tephrostratigraphical investigations of two salt lagoons on the island of Mljet, south Dalmatia, Croatia. Veget. Hist. Archaeobot. 1998, 7, 219–234. [Google Scholar]
- Andrič, M. The Holocene vegetation dynamics and the formation of Neolithic and present-day Slovenian landscape. Doc. Praehist. 2001, 28, 133–175. [Google Scholar] [CrossRef]
- Andrič, M. Paleookolje v Sloveniji in severnemu delu hrvaške Istre v pozni prazgodovini. Arh. Vest. 2004, 55, 509–525. [Google Scholar]
- Andrič, M. Prapoče pollen core and Holocene vegetation change in northern Istria. In Prehistoric Herders in Istria (Croatia): The Archaeology of Pupićina Cave; Miracle, P.T., Forenbaher, Š., Eds.; Archaeological Museum of Istria: Pula, Croatia, 2006; pp. 31–62. [Google Scholar]
- Balbo, A.L.; Andrič, M.; Rubinić, J.; Moscariello, A.; Miracle, P.T. Palaeoenvironmental and Archaeological Implications of a Sediment Core from Polje Čepić, Istria, Croatia. Geol. Croat. 2006, 59, 109–124. [Google Scholar]
- Gigov, A.; Nikolić, V. Rezultati analize polena na nekim tresavama u Hrvatskoj. Gl. Prir. Muz. Beogr. Ser. B 1960, 15, 3–26. [Google Scholar]
- Šercelj, A. Postglacijalni razvoj gorskih gozdov v Severozahodni Jugoslaviji. Razpr. SAZU 4. R 1971, 14, 267–294. [Google Scholar]
- Culiberg, M.; Šercelj, A. Pollen analyses of the sediments of Plitvička jezera (Lakes of Plitvice). Acta Bot. Croat. 1981, 40, 147–154. [Google Scholar]
- Culiberg, M.; Šercelj, A. Palynological Research in the Plitvice National Park. Razpr. SAZU 4. R 1994, 35, 177–185. [Google Scholar]
- Srdoč, D.; Obelić, B.; Horvatinčić, N.; Culiberg, M.; Šercelj, A.; Sliepčević, A. Radiocarbon dating and pollen analyses of two peat bogs in the Plitvice National Park. Acta Bot. Croat. 1985, 44, 41–46. [Google Scholar]
- Posavec-Vukelić, V.; Alegro, A.; Šegota, V. Đon močvar. In Botanički Važna Područja Hrvatske; Nikolić, T., Topić, J., Vuković, N., Eds.; Školska knjiga: Zagreb, Croatia, 2010; pp. 121–125. [Google Scholar]
- Reed, J.M. The physical geography of the Balkans and nomenclature of place names. In Balkan Biodiversity, Pattern and Process in the European Hotspot; Griffiths, H.I., Kryštufek, B., Reed, J.M., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 9–22. [Google Scholar]
- Anonymous 2021: Balkanski poluotok. Hrvatska Enciklopedija, Mrežno Izdanje; Leksikografski zavod Miroslav Krleža: Zagreb, Croatia, 2021. Available online: http://www.enciklopedija.hr/Natuknica.aspx?ID=5541 (accessed on 9 November 2021).
- Šegota, T.; Filipčić, A. Köppen’s Classification of Climates and the Problem of Corresponding Croatian Terminology. Geoadria 2003, 8, 17–37. [Google Scholar]
- Mesić, M. Značajke podneblja. In Agroekološka Studija Program Razvitka Poljoprivrede na Području Sisačko-Moslavačke Županije. Posebni dio Agroekologija; Sveučilište u Zagrebu: Agronomski fakultet, Zagreb, 2000. [Google Scholar]
- Korolija, B.; Živaljević, T.; Šimunić, A. Osnovna geološka karta SFRJ. 1:100000; Savezni geološki zavod: Beograd, Jugoslavija, 1979; List Slunj L33–104. [Google Scholar]
- Korolija, B.; Živaljević, T.; Šimunić, A. Osnovna geološka karta SFRJ. 1:100000; Savezni geološki zavod Beograd: Jugoslavija, 1981; Tumač za list Slunj L33–104. [Google Scholar]
- Velić, I.; Vlahović., I. Tumač geološke karte 1:300.000; Hrvatski geološki institut: Zagreb, Croatia, 2009; p. 147. [Google Scholar]
- Modrić Surina, Ž. Ecological gradients as determinants of different vegetation types on mires in Croatia. Ph.D. Thesis, University of Zagreb, Faculty of Science, Department of Biology, Zagreb, Croatia, 2011. [Google Scholar]
- Horvat, I. Vegetacija planina zapadne Hrvatske. Prirodosl. Istraživanja JAZU 1962, 30, 1–179. [Google Scholar]
- Alegro, A.; Šegota, V. Florističke i Vegetacijske Značajke Botaničkog Rezervata„ Đon Močvar“ u Blatuši; Državni zavod za zaštitu prirode: Zagreb, Croatia, 2008; pp. 1–33. [Google Scholar]
- Alegro, A.; Šegota, V. Mahovi Tresetari i Njihova Staništa u Hrvatskoj; Državni zavod za zaštitu prirode: Zagreb, Croatia, 2009; pp. 1–88. [Google Scholar]
- Komšo, D. The Mesolithic in Croatia. Op. Arch. 2007, 30, 55–92. [Google Scholar]
- Minichreiter, K.; Krajcar Bronić, I. New Radiocarbon Dates for the Early Starčevo Culture in Croatia. Pril. Inst. arheol. Zagrebu 2006, 23, 5–16. [Google Scholar]
- Botić, K. Neolithisation of Sava-Drava-Danube interfluve at the end of the 6600–6000 BC period of Rapid Climate Change—A new solution to an old problem. Doc. Praehist. 2016, 43, 183–207. [Google Scholar] [CrossRef]
- Botić, K. Climatic influences on appearance and development of Neolithic cultures in southern outskirts of Carpathian basin. Studia. Quat. 2016, 33, 11–26. [Google Scholar] [CrossRef]
- Težak-Gregl, T. Hrvatske Zemlje od Starijega Kamenog do Bakrenog Doba; Leykam international: Zagreb, Croatia, 2017. [Google Scholar]
- Dimitrijević, S. Problem neolita i eneolita u sjeverozapadnoj Jugoslaviji. Op. Arch. 1961, 5, 5–78. [Google Scholar]
- Dimitrijević, S. Lasinjska kultura. Akademija nauka i umjetnosti Bosne i Hercegovine: Centar za balkanološka ispitivanja, Sarajevo, Bosnia and Herzegovina. In Praistorija Jugoslavenskih Zemalja; Benac, A., Ed.; Eneolitsko doba, 1979; Volume 3, pp. 137–182. [Google Scholar]
- Škiljan, F. Kulturno—Historijski Spomenici Korduna, s Pregledom Povijesti Korduna od Prapovijesti do 1881; Srpsko narodno vijeće: Zagreb, Hrvatska, 2007. [Google Scholar]
- Ložnjak Dizdar, D.; Potrebica, H. Brončano Doba Hrvatske u Okviru Srednje i Jugoistočne Europe; Meridijani: Zagreb, Croatia, 2017. [Google Scholar]
- Birks, H.J.B.; Birks, H.H. Quaternary Palaeoecology; Arnold: London, UK, 1980. [Google Scholar]
- Schnurrenberger, D.; Russell, J.; Kerry, K. Classification of lacustrine sediments based on sedimentary components. J. Paleolimnol. 2003, 29, 141–154. [Google Scholar] [CrossRef]
- Kershaw, P.P. A modification of the Troels-Smith system of sediment description and portrayal. Quat. Australas. 1997, 15, 63–68. [Google Scholar]
- Anonymous. Munsell Soil Color Charts (Revised ed.); Munsell Color Co.: Baltimore, MD, USA, 1994. [Google Scholar]
- Moore, D.M.; Reynolds, R.C. X-Ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford Univ. Press: Oxford, UK, 1997; p. 378. [Google Scholar]
- Moore, P.D.; Webb, J.A.; Collinson, M.E. Pollen Analysis, 2nd ed.; Blackwell Science: Oxford, UK, 1991. [Google Scholar]
- Faegri, K.; Iversen, J.; Krzywinski, K.; Kaland, P.E. Textbook of Pollen Analysis, 4th ed.; John Wiley and Sons: Chichester, UK, 2000. [Google Scholar]
- Stockmarr, J. Tablets with Spores Used in Absolute Pollen Analysis. Pollen Spores 1971, 13, 615–621. [Google Scholar]
- Beug, J.-H. Leitfaden der Pollenbestimmung für Mitteleuropa und Angrenzende Gebiete; Verlag Dr. Friedrich Pfeil: München, Germany, 2015. [Google Scholar]
- Anonymous 2000–2022. PalDat—A palynological Database. Available online: https://www.paldat.org (accessed on 27 August 2022).
- Van Geel, B. Palynology of a section from the raised peat bog ‘Wietmarsche moor‘, with special reference to fungal remains. Acta Bot. Neerl. 1972, 21, 261–284. [Google Scholar] [CrossRef]
- Van Geel, B. Palaeoecological study of Holocene peat bog sections in Germany and the Netherlands, based on the analysis of pollen, spores and macro- and microscopic remains of fungi, algae, cormophytes and animals. Rev. Palaeobot. Palynol. 1978, 25, 1–120. [Google Scholar] [CrossRef]
- Van Geel, B.; van der Hammen, T. Zygnemataceae in Quaternary Colombian sediments. Rev. Palaeobot. Palynol. 1978, 25, 377–392. [Google Scholar] [CrossRef]
- Van Geel, B.; Middeldorp, A.A. Vegetational history of Carbury Bog (Co. Kildare, Ireland) during the last 850 years and a test of the temperature indicator value of ^H/^H measurements of peat samples in relation to historical sources and meteorological data. New Phytol. 1988, 109, 377–392. [Google Scholar] [CrossRef]
- Van Geel, B.; Bohncke, S.J.P.; Dee, H. A palaeoecological study of an upper late glacial and holocene sequence from “de borchert”, The Netherlands. Rev. Palaeobot. Palynol. 1980, 31, 367–448. [Google Scholar] [CrossRef]
- Van Geel, B.; Hallewas, D.P.; Pals, J.P. Late holocene deposit under the Westfriese Zeedijk near Enkhuizen (prov. of Noord-Holland, The Netherlands): Palaeoecological and archaeological aspects. Rev. Palaeobot. Palynol. 1983, 38, 269–335. [Google Scholar] [CrossRef]
- Van Geel, B.; Coope, G.R.; Van der Hammen, T. Palaeoecology and stratigraphy of the lateglacial type section at Usselo (The Netherlands). Rev. Palaeobot. Palynol. 1989, 60, 25–129. [Google Scholar] [CrossRef]
- Van Geel, B.; Buurman, J.; Brinkkemper, O.; Schelvis, J.; Aptroot, A.; van Reenen, G.; Hakbijl, T. Environmental reconstruction of a Roman Period settlement site in Uitgeest (The Netherlands), with special reference to coprophilous fungi. J. Archaeol. Sci. 2003, 30, 873–883. [Google Scholar]
- Pals, J.P.; van Geel, B.; Delfos, A. Paleoecological studies in the Klokkeweel bog near Hoogkarspel (prov. of Noord-Holland). Rev. Palaeobot. Palynol. 1980, 30, 371–418. [Google Scholar] [CrossRef]
- Haas, J.N. Neorhabdocoela oocytes—Palaeoecological indicators found in pollen preparations from Holocene freshwater lake sediments. Rev. Palaeobot. Palynol. 1996, 91, 371–382. [Google Scholar] [CrossRef]
- Kuhry, P. The palaeoecology of a treed bog in western boreal Canada: A study based on microfossils, macrofossils and physicochemical properties. Rev. Palaeobot. Palynol. 1997, 96, 183–224. [Google Scholar] [CrossRef]
- Carrión, J.S.; Navarro, C. Cryptogam spores and other non-pollen microfossils as sources of paleoecological information: Case studies from Spain. Ann. Bot. Fenn. 2002, 39, 1–14. [Google Scholar] [CrossRef]
- Aptroot, A.; van Geel, B. Fungi of the colon of the Yukagir Mammoth and from stratigraphically related permafrost samples. Rev. Palaeobot. Palynol. 2006, 141, 225–230. [Google Scholar] [CrossRef]
- Barthelmes, A.; Prager, A.; Joosten, H. Palaeoecological analysis of Alnus wood peats with special attention to non-pollen palynomorphs. Rev. Palaeobot. Palynol. 2006, 141, 33–51. [Google Scholar] [CrossRef]
- Barthelmes, A.; de Klerk, P.; Prager, A.; Unterseher, M.; Joosten, M. Expanding the approach of NPP analysis to eutrophic and forested sites—Part II: Occurrence and significance of (surface sample) NPPs in a Holocene wood peat section. Rev. Palaeobot. Palynol. 2012, 186, 22–37. [Google Scholar] [CrossRef]
- Medeanic, S. Freshwater algal palynomorph records from Holocene deposits in the coastal plain of Rio Grande do Sul, Brazil. Rev. Palaeobot. Palynol. 2006, 141, 83–101. [Google Scholar] [CrossRef]
- Prager, A.; Barthelmes, A.; Theuerkauf, M.; Joosten, H. Non-pollen palynomorphs from modern Alder carrs and their potential for interpreting microfossil data from peat. Rev. Palaeobot. Palynol. 2006, 141, 7–31. [Google Scholar] [CrossRef]
- Prager, A.; Heuerkauf, M.; Couwenberg, J.; Barthelmes, A.; Aptroot, A.; Joosten, H. Pollen and non-pollen palynomorphs as tools for identifying alder carr deposits: A surface sample study from NE Germany. Rev. Palaeobot. Palynol. 2012, 186, 38–57. [Google Scholar] [CrossRef]
- Cugny, C.; Mazier, F.; Galop, D. Modern and fossil non-pollen palynomorphs from the Basque mountains (western Pyrenees, France): The use of coprophilous fungi to reconstruct pastoral activity. Veg. Hist. Archaeobot. 2010, 19, 391–408. [Google Scholar] [CrossRef]
- Montoya, E.; Rull, V.; van Geel, B. Non-pollen palynomorphs from surface sediments along an altitudinal transect of the Venezuelan Andes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 297, 169–183. [Google Scholar] [CrossRef]
- Montoya, E.; Rull, V.; Vegas-Vilarrúbia, T. Non-pollen palynomorph studies in the Neotropics: The case of Venezuela. Rev. Palaeobot. Palynol. 2012, 186, 102–130. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Gołdyn, B.; Prokop, Z.M.; Michalczyk, Ł. New records of Tardigrada from Bulgaria with the description of Macrobiotus binieki sp. nov. (Eutardigrada: Macrobiotidae) and a key to the species of the harmsworthi group. Zootaxa 2011, 2781, 29–39. [Google Scholar] [CrossRef]
- Dietre, B.; Gauthier, É.; Gillet, F. Modern pollen rain and fungal spore assemblages from pasture woodlands around Lake Saint-Point France. Rev. Palaeobot. Palynol. 2012, 186, 69–89. [Google Scholar] [CrossRef]
- Kołaczek, P.; Zubek, S.; Błaszkowski, J.; Mleczko, P.; Margielewski, W. Erosion or plant succession—How to interpret the presence of arbuscular mycorrhizal fungi (Glomeromycota) spores in pollen profiles collected from mires. Rev. Palaeobot. Palynol. 2013, 189, 29–37. [Google Scholar] [CrossRef]
- López-Vila, J.; Montoya, E.; Cañellas-Boltà, N.; Rull, V. Modern nonpollen palynomorphs sedimentation along an elevational gradient in the south-central Pyrenees (southwestern Europe) as a tool for Holocene palaeoecological reconstruction. Holocene 2014, 24, 327–345. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; van Geel, B.; Wiltshire, P.E.J. The enigma of the Diporotheca palynomorph. Rev. Palaeobot. Palynol. 2016, 235, 94–98. [Google Scholar] [CrossRef]
- Jankovská, V.; Roszkowska, M.; Kaczmarek, Ł. Remains of nonpollen-palynomorphs—Tardigrades from Spitsbergen found during pollen analyses. Polar Rec. 2016, 52, 450–463. [Google Scholar] [CrossRef]
- Miola, A. Tools for Non-Pollen Palynomorphs (NPPs) analysis: A list of Quaternary NPP types and reference literature in English language (1972–2011). Rev. Palaeobot. Palynol. 2012, 186, 142–161. [Google Scholar] [CrossRef]
- Whitlock, C.; Larsen, C. Charcoal as a fire proxy. In Tracking Environmental Change Using Lake Sediments: Terrestrial, Algal, and Siliceous Indicators; Smol, J.P., Birks, J.B., Last, W.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; Volume 3, pp. 75–97. [Google Scholar] [CrossRef]
- Mooney, S.D.; Tinner, W. The analysis of charcoal in peat and organic sediments. Mires Peat 2011, 7, 1–18. [Google Scholar]
- Tinner, W.; Conedera, M.; Ammann, B.; Gaggeler, H.W.; Gedye, S.; Jones, R.; Sagesser, B. Pollen and charcoal in lake sediments compared with historically documented forest fires in southern Switzerland since AD 1920. Holocene 1998, 8, 31–42. [Google Scholar] [CrossRef]
- Walanus, A.; Nalepka, D. PolPal. Program for counting pollen grains, diagrams plotting and numerical analysis. Acta Palaeobot. Suppl. 1999, 2, 659–661. [Google Scholar]
- Nalepka, D.; Walanus, A. Data processing in pollen analysis. Acta Palaeobot. 2003, 43, 125–134. [Google Scholar]
- Legendre, P.; Birks, H.J.B. Clustering and partitioning. In Tracking Environmental Change using Lake Sediments: Data Handling and Numerical Techniques; Birks, H.J.B., Lotter, A.F., Juggins, S., Smol, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 5, pp. 167–200. [Google Scholar] [CrossRef]
- Prentice, I.C. Multidimensional scaling as a research tool in Quaternary palynology: A review of theory and methods. Rev. Palaeobot. Palynol. 1980, 31, 71–104. [Google Scholar] [CrossRef]
- Birks, H.J.B.; Line, J.M. The use of Rarefaction Analysis for Estimating Palynological Richness from Quaternary Pollen-Analytical Data. Holoce 1992, 2, 1–10. [Google Scholar] [CrossRef]
- Birks, H.J.B.; Felde, V.A.; Bjune, A.E.; Grytnes, J.-A.; Seppä, H.; Giesecke, T. Does pollen-assemblage richness reflect floristic richness? A review of recent developments and future challenges. Rev. Palaeobot. Palynol. 2016, 228, 1–25. [Google Scholar] [CrossRef]
- Blaauw, M.; Christen, J.A. Flexible palaeoclimate age-depth models using an autoregressive gamma process. Bayesian Anal. 2011, 6, 457–474. [Google Scholar] [CrossRef]
- Reimer, P.J.; Reimer, R.W.; Blaauw, M. Calibration of the 14C record. In Encyclopedia of Quaternary Science, 2nd ed.; Elias, S.A., Mock, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 345–352. [Google Scholar] [CrossRef]
- Hua, Q.; Barbetti, M.; Rakowski, A.Z. Atmospheric radiocarbon for the period 1950–2010. Radiocarbon 2013, 55, 2059–2072. [Google Scholar] [CrossRef]
- Frey, W.; Lösch, R. Geobotanik—Pflanze und Vegetation in Raum und Zeit; Spektrum Akademischer Verlag: Heidelberg, Germany, 2010; Volume 3, Auflage. [Google Scholar]
- Reddy, K.R.; Patrick, W.H.; Broadbent, F.E. Nitrogen transformations and loss in flooded soils and sediments. CRC Crit. Rev. Environ. Control. 1984, 13, 273–309. [Google Scholar] [CrossRef]
- Hansen, K. Sediments from Danish lakes. J. Sediment. Petrol. 1959, 29, 38–46. [Google Scholar]
- Prusty, B.A.K.; Chandra, R.; Azeez, P.A. Distribution of carbon, nitrogen, phosphorus, and sulfur in the soil in a multiple habitat system in India. Aust. J. Soil Res. 2009, 47, 177–189. [Google Scholar] [CrossRef]
- Gąsiorowski, M.; Kupryjanowicz, M. Lake–peat bog transformation recorded in the sediments of the Stare Biele mire (Northeastern Poland). Hydrobiologia 2009, 631, 143–154. [Google Scholar] [CrossRef]
- Ho, E.S.; Meyers, P.A. Variability of early diagenesis in lake sediments: Evidence from the sedimentary geolipid record in an isolated tarn. Chem. Geol. 1994, 112, 309–324. [Google Scholar] [CrossRef] [Green Version]
- Meyers, P.A. Preservation of elemental and isotopic source identification of sedimentary organic matter. Chem. Geol. 1994, 144, 289–302. [Google Scholar] [CrossRef]
- Mackie, E.A.V.; Leng, M.J.; Lloyd, J.M.; Arrowsmith, C. Bulk organic 13C and C/N ratios as palaeosalinity indicators within a Scottish isolation basin. J. Quat. Sci. 2005, 20, 303–312. [Google Scholar] [CrossRef]
- Zong, Y.; Lloyd, J.M.; Leng, M.J.; Yim, W.W.S.; Huang, G. Reconstruction of Holocene monsoon history from the Pearl River Estuary, southern China, using diatoms and carbon isotope ratios. Holocene 2006, 16, 251–263. [Google Scholar] [CrossRef]
- Herzschuh, U.; Mischke, S.; Meyer, H.; Plessen, B.; Zhang, C. Lake nutrient variability inferred from elemental (C, N, S) and isotopic (δ13C, δ15N) analyses of aquatic plant macrofossils. Quat. Sci. Rev. 2010, 29, 2161–2172. [Google Scholar] [CrossRef]
- Cabezas, A.; Gelbrecht, J.; Zwirnmann, E.; Barth, M.; Zak, D. Effects of degree of peat decomposition, loading rate and temperature on dissolved nitrogen turnover in rewetted fens. Soil Biol. Biochem. 2012, 48, 182–191. [Google Scholar] [CrossRef]
- Säurich, A.; Tiemeyer, B.; Don, A.; Bechtold, M.; Amelung, W.; Freibauer, A. Vulnerability of soil organic matter of anthropogenically disturbed organic soils. Biogeosciences Discuss. 2017. [preprint]. [Google Scholar] [CrossRef]
- Eickenscheidt, T.; Heinichen, J.; Augustin, J.; Freibauer, A.; Drösler, M. Nitrogen mineralization and gaseous nitrogen losses from waterlogged and drained organic soils in a black alder (Alnus glutinosa (L.) Gaertn.) forest. Biogeosciences 2014, 11, 2961–2976. [Google Scholar] [CrossRef]
- Slezák, M.; Hrivnák, R.; Petrášová, A. Syntaxonomy and ecology of black alder vegetation in the southern part of central Slovakia. Hacquetia 2011, 10, 119–136. [Google Scholar] [CrossRef]
- Hrivnák, R.; Slezák, M.; Jarčuška, B.; Jarolímek, I.; Kochjarová, J. Native and Alien Plant Species Richness Response to Soil Nitrogen and Phosphorus in Temperate Floodplain and Swamp Forests. Forests 2015, 6, 3501–3513. [Google Scholar] [CrossRef]
- Kuhry, P.; Vitt, D.H. Fossil Carbon/Nitrogen Ratios as a Measure of Peat Decomposition. Ecology 1996, 77, 271–275. [Google Scholar] [CrossRef]
- Rydin, H.; Jeglum, J.K. The Biology of Peatlands; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Chanton, J.P.; Powelson, D.K.; Abichou, T.; Hater, G. Improved field methods to quantify methane oxidation in landfill cover materials using stable carbon isotopes. Environ. Sci. Technol. 2008, 42, 665–670. [Google Scholar] [CrossRef]
- Berger, S.; Gebauer, G.; Blodau, C.; Knorr, K.H. Peatlands in a eutrophic world—Assessing the state of a poor fen-bog transition in southern Ontario, Canada, after long term nutrient input and altered hydrological conditions. Soil Biol. Biochem. 2017, 114, 131–144. [Google Scholar] [CrossRef]
- Evans, M.E.; Heller, F. Environmental Magnetism: Principles and Applications of Enviromagnetics; Academic Press: San Diego, CA, USA, 2003; pp. 1–299. [Google Scholar]
- Dearing, J. Environmental Magnetic Susceptibility: Using the Bartington MS2 System, 2nd ed.; Chi Publishing: Keniloworth, UK, 1999. [Google Scholar]
- Maher, B.A. The magnetic properties of Quaternary aeolian dusts and sediments, and their palaeoclimatic significance. Aeolian Res. 2011, 3, 87–145. [Google Scholar] [CrossRef]
- Hejcman, M.; Hejcmanová, P.; Pavlů, V.; Beneš, J. Origin and history of grasslands in Central Europe—A review. Grass Forage Sci. 2013, 68, 345–363. [Google Scholar] [CrossRef]
- Andrieu-Ponel, V.; Ponel, P.; Jull, A.J.T.; de Beaulieu, J.-L.; Bruneton, H.; Leveau, P. Towards the Reconstruction of the Holocene Vegetation History of Lower Provence: Two New Pollen Profiles from Marais Des Baux. Veget. Hist. Archaeobot 2000, 9, 71–84. [Google Scholar] [CrossRef]
- Jankovská, V. Late Glacial and Holocene history of Plešné Lake and its surrounding landscape based on pollen and palaeoalgological analyses. Biologia 2006, 61, 371–385. [Google Scholar] [CrossRef]
- Baker, A.G.; Zimny, M.; Keczyński, A.; Bhagwat, S.A.; Willis, K.J.; Latałowa, M. Pollen productivity estimates from old-growth forest strongly differ from those obtained in cultural landscapes—Evidence from the Białowieża National Park, Poland. Holocene 2016, 26, 80–92. [Google Scholar] [CrossRef]
- Traverse, A. Palaeopalynology, 2nd ed.; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Dörfler, W. Prokoško Jezero: An environmental record from a subalpine lake in Bosnia-Herzegowina. In Okolište 1—Untersuchungen einer spätneolithischen Siedlungskammer in Zentralbosnien; Müller, J., Rassmann, K., Hofmann, R., Eds.; Universitätsforschungen zur prähistorischen Archäologie 228, Institut für Ur- und Frühgeschichte der Christian-Albrechts-Universität zu Kiel, Dr. Rudolf Habelt GmbH: Bonn, Germany, 2013; pp. 311–340. [Google Scholar]
- Margielewski, W.; Kołaczek, P.; Michczyński, A.; Obidowicz, A.; Pazdur, A. Record of the meso- and neoholocene palaeoenvironmental changes in the Jesionowa landslide peat bog (Beskid Sądecki Mts. Polish Outer Carpathians). Geochronometria 2011, 38, 138–154. [Google Scholar] [CrossRef]
- Connor, S.E.; Thomas, I.; Kvavadze, E.V.; Arabuli, G.J.; Avakov, H.S.; Sagona, A. A survey of modern pollen and vegetation along an altitudinal transect in southern Georgia, Caucasus region. Rev. Palaeobot. Palynol. 2004, 129, 229–250. [Google Scholar] [CrossRef]
- Paal, J.; Jürjendal, I.; Suija, A.; Kull, A. Impact of drainage on vegetation of transitional mires in Estonia. Mires Peat 2016, 18, 1–19. [Google Scholar] [CrossRef]
- Panagiotopoulos, K.; Aufgebauer, A.; Schäbitz, F.; Wagner, B. Vegetation and climate history of the Lake Prespa region since the Lateglacial. Quat. Int. 2013, 293, 157–169. [Google Scholar] [CrossRef]
- Kuneš, P.; Svobodová-Svitavská, H.; Kolář, J.; Hajnalová, M.; Abraham, V.; Macek, M.; Tkáč, P.; Szabób, P. The origin of grasslands in the temperate forest zone of east-central Europe: Long-term legacy of climate and human impact. Quat. Sci. Rev. 2015, 116, 15–27. [Google Scholar] [CrossRef]
- Sykes, M.T.; Prentice, I.C.; Cramer, W. A bioclimatic model for the potential distribution of northern European tree species under present and future climates. J. Biogeogr. 1996, 23, 203–233. [Google Scholar]
- Cheddadi, R.; Araújo, M.B.; Maiorano, L.; Edwards, M.; Guisan, A.; Carré, M.; Chevalier, M.; Pearman, P.B. Temperature Range Shifts for Three European Tree Species over the Last 10,000 Years. Front. Plant Sci. 2016, 7, 1581. [Google Scholar] [CrossRef] [PubMed]
- Magyari, E. Holocene biogeography of Fagus sylvatica L. and Carpinus betulus L. in the Carpathian-Alpine Region. Fol. Hist. Nat. Mus. Matr. 2002, 26, 15–35. [Google Scholar]
- Schmidt, R.; Müller, J.; Drescher-Schneider, R.; Krisai, R.; Szeroczynska, K.; Barić, A. Changes in lake level and trophy at Lake Vrana, a large karstic lake on the Island of Cres (Croatia), with respect to palaeoclimate and anthropogenic impacts during the last approx. 16,000 years. J. Limnol. 2000, 59, 113–130. [Google Scholar] [CrossRef]
- Tzedakis, P. The Balkans as prime glacial refugial territory of European temperatetrees. In Balkan Biodiversity, Pattern and Process in the European Hotspot; Griffiths, H.I., Kryštufek, B., Reed, J.M., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 49–68. [Google Scholar]
- Brus, R. Growing evidence for the existence of glacial refugia of European beech (Fagus sylvatica L.) in the south-eastern Alps and north-western Dinaric Alps. Period. Biol. 2010, 112, 239–246. [Google Scholar]
- Postolache, D.; Popescu, F.; Paule, L.; Ballian, D.; Zhelev, P.; Fărcaş, S.; Paule, J.; Badea, O. Unique postglacial evolution of the hornbeam (Carpinus betulus L.) in the Carpathians and the Balkan Peninsula revealed by chloroplast DNA. Sci. Total Environ. 2017, 599–600, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Magri, D. Patterns of post-glacial spread and the extent of glacial refugia of European beech (Fagus sylvatica). J. Biogeogr. 2008, 35, 450–463. [Google Scholar] [CrossRef]
- Power, M.J.; Marlon, J.; Ortiz, N.; Bartlein, P.J.; Harrison, S.P.; Mayle, F.E.; Ballouche, A.; Bradshaw, R.H.W.; Carcaillet, C.; Cordova, C.; et al. Changes in fire regimes since the Last Glacial Maximum: An assessment based on a global synthesis and analysis of charcoal data. Clim. Dyn. 2008, 30, 887–907. [Google Scholar] [CrossRef]
- Carter, V.; Moravcová, A.; Chiverrell, R.; Clear, J.; Finsinger, W.; Dreslerová, D.; Halsall, H.; Kuneš, P. Holocene scale fire dynamics of central European temperate spruce-beech forests. Quat. Sci. Rev. 2018, 191, 15–30. [Google Scholar] [CrossRef]
- Feurdean, A.; Tonkov, S.; Pfeifer, M.; Panait, A.; Warren, D.; Vannière, B.; Marinova, E. Fire frequency and intensity associated with functional traits of dominant forest type in the Balkans during the Holocene. Eur. J. For. Res. 2019, 1049–1066. [Google Scholar] [CrossRef]
- Feurdean, A.; Perşoiu, A.; Tanţău, I.; Stevens, T.; Magyari, E.K.; Onac, B.P.; Marković, S.; Andrič, M.; Connor, S.; Fărcaş, S.; et al. Climate variability and associated vegetation response throughout Central and Eastern Climate variability and associated vegetation response throughout Central and Eastern Europe (CEE) between 60 and 8 ka. Quat. Sci. Rev. 2014, 106, 206–224. [Google Scholar] [CrossRef] [Green Version]
- Heiri, O.; Ilyashuk, B.; Millet, L.; Samartin, S.; Lotter, A.F. Stacking of discontinuous regional palaeoclimate records: Chironomidbased summer temperatures from the Alpine region. Holocene 2015, 25, 137–149. [Google Scholar] [CrossRef]
- Mauri, A.; Davis, B.A.; Collins, P.M.; Kaplan, J.O. The climate of Europe during the Holocene: A gridded pollen-based reconstruction and its multi-proxy evaluation. Quat. Sci. Rev. 2015, 112, 109–127. [Google Scholar] [CrossRef]
- Furyaev, V.V.; Vaganov, E.A.; Tchebakova, N.M.; Valendik, E.N. Effects of Fire and Climate on Successions and Structural Changes in The Siberian Boreal Forest. Eur. J. For. Res. 2001, 2, 1–15. [Google Scholar]
- Isaev, A.P.; Protopopov, A.V.; Protopopova, V.V.; Egorova, A.A.; Timofeyev, P.A.; Nikolaev, A.N.; Shurduk, I.F.; Lytkina, L.P.; Ermakov, N.B.; Nikitina, N.V.; et al. Vegetation of Yakutia: Elements of Ecology and Plant Sociology. In The Far North: Plant Biodiversity and Ecology of Yakutia; Troeva, E.I., Isaev, A.P., Cherosov, M.M., Karpov, N.S., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 143–260. [Google Scholar]
- Rogers, B.M.; Soja, A.J.; Goulden, M.L.; Randerson, J.T. Influence of tree species oncontinental differences in boreal fires and climate feedbacks. Nature Geosci. 2015, 8, 228–234. [Google Scholar] [CrossRef]
- Tautenhahn, S.; Lichstein, J.W.; Jung, M.; Kattge, J.; Bohlman, S.A.; Heilmeier, H.; Prokushkin, A.; Kahl, A.; Wirth, C. Dispersal limitation drives successional pathways in Central Siberian forests under current and intensified fire regimes. Glob. Change Biol. 2016, 22, 2178–2197. [Google Scholar] [CrossRef]
- Xanthopoulos, G.; Calfapietra, C.; Fernandes, P. Fire Hazard and Flammability of European Forest Types. In Post-Fire Management and Restoration of Southern European Forests; Moreira, F., Arianoutsou, M., Corona, P., De las Heras, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 79–92. [Google Scholar] [CrossRef]
- Olsson, F.; Gaillard, M.-J.; Lemdah, G.; Greisman, A.; Lanos, P.; Marguerie, D.; Marcoux, N.; Skoglund, P.; Wäglind, J. A continuous record of fire covering the last 10,500 calendar years from southern Sweden—The role of climate and human activities fire activity. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 291, 128–141. [Google Scholar] [CrossRef]
- Adámek, M.; Bobek, P.; Hadincová, V.; Wild, J.; Kopecký, M. Forest fires within a temperate landscape: A decadal and millennial perspective from a sandstone region in Central Europe. For. Ecol. Manag. 2015, 336, 81–90. [Google Scholar] [CrossRef]
- Kołaczek, P.; Buczek, K.; Margielewski, W.; Gałka, M.; Rycerz, A.; Woszczyk, M.; Karpińska-Kołaczek, M.; Marcisz, K. Towards the understanding of fire impact on the lower montane forest in the Polish Western Carpathians during the Holocene. Quat. Sci. Rev. 2020, 229, 106–137. [Google Scholar] [CrossRef]
- Tinner, W.; Conedera, M.; Gobet, E.; Hubschmid, P.; Wehrli, M.; Ammann, B. A palaeoecological attempt to classify fire sensitivity of trees in the southern Alps. Holocene 2000, 10, 565–574. [Google Scholar] [CrossRef]
- Jamrichová, E.; Hédl, R.; Kolář, J.; Tóth, P.; Bobek, P.; Hajnalová, M.; Procházka, J.; Kadlec, J.; Szabó, P. Human impact on open temperate woodlands during the middle Holocene in Central Europe. Rev. Palaeobot. Palynol. 2017, 245, 55–68. [Google Scholar] [CrossRef]
- Bunting, M.J.; Warner, B.G. Late Quaternary vegetation dynamics and hydroseral development in a shrub swamp in southern Ontario, Canada. Can. J. Earth Sci. 1999, 36, 1603–1616. [Google Scholar] [CrossRef]
- Kuhry, P. Palsa and peat plateau development in the Hudson Bay Lowlands, Canada: Timing, pathways and causes. Boreas 2008, 37, 316–327. [Google Scholar] [CrossRef]
- Gałka, M.; Aunina, L.; Feurdean, A.; Hutchinson, S.; Kołaczek, P.; Apolinarska, K. Rich fen development in CE Europe, resilience to climate change and human impact over the last ca. 3500 years. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 473, 57–72. [Google Scholar] [CrossRef]
- Stallegger, M. Management of Natura 2000 Habitats. 7150 Depressions Onpeat Substrates of the Rhynchosporion; Technical Report 19/24; European Commission: Brusseles, Belgium, 2008. [Google Scholar]
- Hájková, P. Peucedano palustris-Caricetum lasiocarpae Tüxen ex Balátová-Tuláčková 1972. In Vegetace České republiky: Vodní a mokřadní vegetace; Chytrý, M., Ed.; Academia: Praha, Czech Republic, 2011; Volume 3, pp. 534–537. [Google Scholar]
- Hájek, M.; Hájková, P. Drosero anglicae-Rhynchosporetum albae Klika 1935. In Vegetace České Republiky: Vodní a Mokřadní Vegetace; Chytrý, M., Ed.; Academia: Praha, Czech Republic, 2011; Volume 3, pp. 665–668. [Google Scholar]
- Fernández-Pascual, E. Comparative seed germination traits in bog and fen mire wetlands. Aquat. Bot. 2016, 130, 21–26. [Google Scholar] [CrossRef]
- Frolking, S.; Roulet, N.T.; Tuittila, E.; Bubier, J.L.; Quillet, A.; Talbot, J.; Richard, P.J.H. A new model of Holocene peatland net primary production, decomposition, water balance, and peat accumulation. Earth Syst. Dynam. 2010, 1, 1–21. [Google Scholar] [CrossRef]
- Bufková, I.; Prach, K.; Bastl, M. Relationships between vegetation and environment within the montane floodplain of the Upper Vltava River (Šumava National Park, Czech Republic). Silva Gabreta 2005, 2, 5–76. [Google Scholar]
- Zaharescu, D.G.; Palanca-Soler, A.; Hooda, P.S.; Tanase, C.; Burghelea, C.I.; Lester, R.N. Riparian vegetation in the alpine connectome: Terrestrial-aquatic and terrestrial-terrestrial interactions. Sci. Total Environ. 2017, 601–602, 247–259. [Google Scholar] [CrossRef]
- Bush, M.B. An 11400 year palaeoecological history of a British chalk grassland. J. Veg. Sci. 1993, 4, 47–66. [Google Scholar] [CrossRef]
- Sádlo, J.; Chytrý, M.; Pyšek, P. Regional species pools of vascular plants in habitats of the Czech Republic. Preslia 2007, 79, 303–321. [Google Scholar]
- Klotz, S.; Kühn, I.; Durka, W. BIOLFLOR—Eine Datenbank zu biologisch-ökologischen Merkmalen der Gefäßpflanzen in Deutschland. Schr.Reihe Veg. 2002, 38, 1–334. [Google Scholar]
- Bühler, C.H.; Schmid, B. The influence of management regime and altitude on the population structure of Succisa pratensis: Implication for vegetation monitoring. J. Appl. Ecol. 2001, 38, 689–698. [Google Scholar]
- Overbeck, G.; Kiehl, K.; Abs, C. Seedling recruitment of Succisella inflexa in fen meadows: Importance of seed and microsite availability. Appl. Veg. Sci. 2003, 6, 97–104. [Google Scholar] [CrossRef]
- Topić, J.; Vukelić, J. 6510 Nizinske košanice (Alopecurus pratensis, Sanguisorba officinalis). In Priručnik za Određivanje Kopnenih Staništa u Hrvatskoj Prema Direktivi o Staništima EU; Topić, J., Vukelić, J., Eds.; Državni zavod za zaštitu prirode: Zagreb, Hrvatska, 2009; pp. 205–209. [Google Scholar]
- Innes, J.B.; Blackford, J.J. The Ecology of Late Mesolithic Woodland Disturbances: Model Testing with Fungal Spore Assemblage Data. J. Archaeol. Sci. 2003, 30, 185–194. [Google Scholar] [CrossRef]
- Ryan, P.A.; Blackford, J.J. Late Mesolithic environmental change at Black Heath, south Pennines, UK: A test of Mesolithic woodland management models using pollen, charcoal and non-pollen palynomorph data. Veget. Hist. Archaeobot. 2010, 19, 545–558. [Google Scholar] [CrossRef]
- Väliranta, M.; Korhola, A.; Seppä, H.; Tuittila, E.-S.; Sarmaja-Korjonen, K.; Laine, J.; Alm, J. High-resolution reconstruction of wetness dynamics in a southern boreal raised bog, Finland, during the late Holocene: A quantitative approach. Holocene 2007, 17, 1093–1107. [Google Scholar] [CrossRef]
- Curtis, J.T. The Vegetation of Wisconsin: An Ordination of Plant Communities; University of Wisconsin Press: Madison, WI, USA, 1959. [Google Scholar]
- Van Diggelen, J.M.; Bense, I.H.M.; Brouwer, E.; Limpens, J.; van Schie, J.M.M.; Smolders, A.J.P.; Lamers, L.P.M. Restoration of acidified and eutrophied rich fens: Long-term effects of traditional management and experimental liming. Ecol. Eng. 2015, 75, 208–216. [Google Scholar] [CrossRef]
- Tinner, W.; Hubschmid, P.; Wehrli, M.; Ammann, B.; Conedera, M. Long-term forest fire ecology and dynamics in southern Switzerland. J. Ecol. 1999, 87, 273–289. [Google Scholar] [CrossRef]
- Tinner, W.; Lotter, A.F. Holocene expansions of Fagus silvatica and Abies alba in Central Europe: Where are we after eight decades of debate? Quat. Sci. Rev. 2006, 25, 526–549. [Google Scholar] [CrossRef]
- Stivrins, N.; Kalnina, L.; Veski, S.; Zeimule, S. Local and regional Holocene vegetation dynamics at two sites in eastern Latvia. Boreal Environ. Res. 2014, 19, 310–322. [Google Scholar]
- Kłosowski, S.; Jabłońska, E. Aquatic and swamp plant communities as indicators of habitat properties of astatic water bodies in north-eastern Poland. Limnologica 2009, 39, 115–127. [Google Scholar] [CrossRef]
- Šumberová, K. Typhetum latifoliae Nowiński 1930. In Vegetace České Republiky: Vodní a Mokřadní Vegetace; Chytrý, M., Ed.; Academia: Praha, Czech Republic, 2011; Volume 3, pp. 401–405. [Google Scholar]
- Timmermann, T.; Margóczi, K.; Takács, G.; Vegelin, K. Restoration of Peat-Forming Vegetation by Rewetting Species-Poor Fen Grasslands. Appl. Veg. Sci. 2006, 9, 241–250. [Google Scholar] [CrossRef]
- Wolowski, K. Taxonomic and environmental studies on euglenophytes of the Kraków-Czestochowa Upland (Southern Poland). Fragm. Flor. Geobot. 1998, 6, 3–192. [Google Scholar]
- Bakker, M.; van Smeerdijk, D.G. A palaeoecological study of a Late Holocene section from “Het Ilperveld”, Western Netherlands. Rev. Palaeobot. Palynol. 1982, 36, 95–163. [Google Scholar] [CrossRef]
- López-Sáez, J.A.; Van Geel, B.; Martínez-Sánchez, M. Aplicación de los microfósiles no polínicos en Palinología Arqueológica. In Contributos das Ciências e das Technologias para a Arqueologia da Península Ibérica, Actas 3° Congresso de Arqueologia Peninsular, Vila-Real; Oliveira Jorge, V., Ed.; Adecap: Porto, Portugal, 2000; Volume 9, pp. 11–20. [Google Scholar]
- Florenzano, A.; Mercuri, A.M.; Carter, J.C. Economy and environment of the Greek colonial system in southern Italy: Pollen and npps evidence of grazing from the rural site of Fattoria Fabrizio (6th–4th cent. BC., Metaponto, Basilicata). Ann. Bot. 2013, 3, 173–181. [Google Scholar] [CrossRef]
- Ejarque, A.; Scott Anderson, R.; Simms, A.R.; Gentry, B.J. Prehistoric fires and the shaping of colonial transported landscapes in southern California: Dune Pond, Santa Barbara County. Quat. Sci. Rev. 2015, 112, 181–196. [Google Scholar] [CrossRef]
- Revelles, J.; van Geel, B. Human impact and ecological changes in lakeshore environments. The contribution of non-pollen palynomorphs in Lake Banyoles (NE Iberia). Rev. Palaeobot. Palynol. 2016, 232, 81–97. [Google Scholar]
- Dietre, B.; Walser, C.; Kofler, W.; Kothieringer, K.; Hajdas, I.; Lambers, K.; Reitmaier, T.; Haas, J.N. Neolithic to Bronze Age (4850–3450 cal. BP) fire management of the Alpine Lower Engadine landscape (Switzerland) to establish pastures and cereal fields. Holocene 2017, 27, 181–196. [Google Scholar] [CrossRef]
- Lundqvist, N. Nordic Sordariaceae sensu lato. Symb. Bot. Upsal. 1972, 20, 1–314. [Google Scholar]
- Krug, J.C.; Benny, G.L.; Keller, H.W. Coprophilous fungi. In Biodiversity of Fungi: Inventory and Monitoring Methods; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Elsevier Academic Press: Burlington, ON, Canada, 2004; pp. 467–499. [Google Scholar]
- Piasai, O.; Sudsanguan, M. Morphological study of Gelasinospora from dung and antagonistic effect against plant pathogenic fungi in vitro. Agric. Nat. Resour. 2018, 52, 407–411. [Google Scholar] [CrossRef]
- Gardner, A. Biotic response to early Holocene human activity: Results from palaeoenvironmental analyses of sediments from Podpeško Jezero. Poročilo O Raziskovanju Paleolit. Neolit. Eneolit. V Slov. 1997, 24, 63–77. [Google Scholar]
- Andrič, M. Holocene vegetation development in Bela krajina (Slovenia) and the impact of firs farmers on the landscape. Holocene 2007, 17, 763–776. [Google Scholar] [CrossRef]
- Finsinger, W.; Tinner, W.; van der Knaap, W.O.; Ammann, B. The expansion of hazel (Corylus avellana L.) in the southern Alps: A key for understanding its early Holocene history in Europe? Quat. Sci. Rev. 2006, 25, 612–631. [Google Scholar] [CrossRef]
- Godwin, H. History of the natural forests of Britain: Establishment, dominance and destruction. Phil. Trans. R. Soc. B Biol. Sci. 1975, 271, 47–67. [Google Scholar] [CrossRef]
- Holst, D. Hazelnut economy of early Holocene hunter–gatherers: A case study from Mesolithic Duvensee, northern Germany. J. Archaeol. Sci. 2010, 37, 2871–2880. [Google Scholar] [CrossRef]
- Mithen, S.; Finlay, N.; Carruthers, W.; Carter, S.; Ashmore, P. Plant use in the Mesolithic: Evidence from Staosnaig, Isle of Colonsay, Scotland. J. Archaeol. Sci. 2001, 28, 223–234. [Google Scholar] [CrossRef]
- Groß, D.; Lübke, H.; Schmölcke, U.; Zanon, M. Early Mesolithic activities at ancient Lake Duvensee, northern Germany. Holocene 2018, 29, 197–208. [Google Scholar] [CrossRef]
- Huntley, B. Rapid early-Holocene migration and high abundance of hazel (Corylus avellana L.): Alternative hypotheses. In Climate Change and Human Impact on the Landscape; Chambers, F.M., Ed.; Chapman & Hall: London, UK, 1993; pp. 205–216. [Google Scholar]
- Prentice, I.C.; Helmisaari, H. Silvics of north European trees: Compilation, comparisons and implications for forest succession modelling. For. Ecol. Manag. 1991, 42, 79–93. [Google Scholar] [CrossRef]
- Giesecke, T.; Miller, P.A.; Sykes, M.T.; Ojala, A.E.K.; Seppä, H.; Bradshaw, R.H.W. The effect of past changes in inter-annual temperature variability on tree distribution limits. J. Biogeogr. 2010, 37, 1394–1405. [Google Scholar] [CrossRef]
- Pál, I.; Magyari, E.K.; Braun, M.; Vincze, I.; Pálfy, J.; Molnár, M.; Finsinger, W.; Buczkó, K. Small-scale moisture availability increase during the 8.2-ka climatic event inferred from biotic proxy records in the South Carpathians (SE Romania). Holocene 2016, 26, 1382–1396. [Google Scholar] [CrossRef] [Green Version]
- Von Grafenstein, U.; Erlenkeuser, H.; Müller, J.; Jouzel, J.; Johnsen, S. The cold event 8200 years ago documented in oxygen isotope records of precipitation in Europe and Greenland. Clim. Dyn. 1998, 14, 73–81. [Google Scholar] [CrossRef]
- Budja, M. The 8200 calBP ‘climate event’ and the process of neolithisation in south-eastern Europe. Doc. Praehist. 2007, 34, 191–201. [Google Scholar]
- Razum, I.; Bajo, P.; Brunović, D.; Ilijanić, N.; Hasan, O.; Röhl, U.; Šparica Miko, M.; Miko, S. Past climate variations recorded in needle-like aragonites correlate with organic carbon burial efciency as revealed by lake sediments in Croatia. Sci. Rep. 2021, 11, 7568. [Google Scholar] [CrossRef]
- Iversen, J. Viscum, Hedera and Ilex as climate indicators. Geol. Fören. Stockh. Förh. 1944, 66, 463–483. [Google Scholar]
- Zagwijn, W.H. Reconstruction of climate change during the Holocene in western and central Europe based on pollen records of indicator species. Veg. Hist. Archaeobot. 1994, 3, 65–88. [Google Scholar] [CrossRef]
- Li, H.; Renssen, H.; Roche, D.M.; Miller, P.A. Modelling the vegetation response to the 8.2 ka BP cooling event in Europe and Northern Africa. J. Quat. Sci. 2019, 34, 650–661. [Google Scholar] [CrossRef]
- Čater, M.; Levanič, T. Beech and silver fir’s response along the Balkan’s latitudinal gradient. Sci. Rep. 2019, 9, 16269. [Google Scholar] [CrossRef]
- Huntley, B.; Birks, H.J.B. An Atlas of Past and Present Pollen Maps for Europe: 0–13000 Years Ago; Cambridge University Press: London, UK, 1983. [Google Scholar]
- Tallantire, P.A. The palaeohistory of the grey alder (Alnus incana (L.) Moench.) and black alder (A. glutinosa (L.) Gaertn.) in Fennoscandia. New Phytol. 1974, 73, 529–546. [Google Scholar]
- Montanari, C. Recent pollen deposition in alder woods and in other riverine plant comunities. Allionia 1996, 34, 309–323. [Google Scholar]
- Gałka, M.; Graeme, T.; Swindles, G.T.; Szal, M.; Fulweber, R.; Feurdeane, A. Response of plant communities to climate change during the late Holocene: Palaeoecological insights from peatlands in the Alaskan Arctic. Ecol. Indic. 2018, 85, 525–536. [Google Scholar] [CrossRef]
- Rabett, R.J.; Pryor, A.J.E.; Simpson, D.J.; Farr, L.R.; Pyne-O’Donnell, S.; Blaauw, M.; Crowhurst, S.; Mulligan, R.P.M.; Hunt, C.O.; Stevens, R.; et al. A Multi-Proxy Reconstruction of Environmental Change in the Vicinity of the North Bay Outlet of Pro-Glacial Lake Algonquin. Open Quat. 2019, 5, 1–27. [Google Scholar] [CrossRef]
- WaC/Nik, A.; Tylmann, W.; Bonk, A.; Goslar, T.; Enters, D.; Meyer-Jacob, C.; Grosjean, M. Determining the responses of vegetation to natural processes and human impacts in north-eastern Poland during the last millennium. Veget. Hist. Archaeobot. 2016, 25, 479–498. [Google Scholar] [CrossRef]
- Veski, S.; Seppä, H.; Ojala, A.E.K. Cold event at 8200 yr B.P. recorded inannually laminated lake sediments in eastern Europe. Geology 2004, 32, 681–684. [Google Scholar] [CrossRef]
- Seppä, H.; Birks, H.J.B.; Giesecke, T.; Hammarlund, D.; Alenius, T.; Antonsson, K.; Bjune, A.E.; Heikkilä, M.; MacDonald, G.M.; Ojala, A.E.K.; et al. Spatial structure of the 8200 cal yr BP event in northern Europe. Clim.Past Discuss. 2007, 3, 165–195. [Google Scholar] [CrossRef] [Green Version]
- Barhoumi, C.; Vogel, M.; Dugerdil, L.; Limani, H.; Joannin, S.; Peyron, O.; Ali, A.A. Holocene Fire Regime Changes in the Southern Lake Baikal Region Influenced by Climate-Vegetation-Anthropogenic Activity Interactions. Forests 2021, 12, 978. [Google Scholar] [CrossRef]
- Binney, H.A.; Waller, M.P.; Bunting, M.J.; Armitage, R.A. The interpretation of fen carr pollen diagrams: The representation of the dry land vegetation. Rev.Palaeobot. Palynol. 2005, 134, 197–218. [Google Scholar] [CrossRef]
- Bunting, M.J.; Armitage, R.; Binney, H.A.; Waller, M. Estimates of ‘relative pollen productivity’ and ‘relevant source area of pollen’ for major tree taxa in two Norfolk (UK) woodlands. Holocene 2005, 15, 459–465. [Google Scholar] [CrossRef]
- Janssen, C.R. Recent pollen spectra from the deciduous and coniferous-deciduous forests of northwestern Minnesota: A study in pollen dispersal. Ecology 1996, 47, 804–825. [Google Scholar] [CrossRef]
- Janssen, C.R. Modern pollen assemblages and vegetation in the Myrtle Lake peatland, Minnesota. Ecol. Monogr. 1984, 54, 213–252. [Google Scholar] [CrossRef]
- Jacobson, G.L., Jr.; Bradshaw, R.H.W. The selection of sites for palaeovegetational studies. Quat. Res. 1981, 16, 80–96. [Google Scholar]
- Pokorný, P.; Klimešová, J.; Klimeš, L. Late Holocene history and vegetation dynamics of a floodplain alder carr: A case study from eastern Bohemia, Czech Republic. Folia Geobot. 2000, 35, 43–58. [Google Scholar]
- Marek, S. Biologia i stratygrafia torfowisk olszynowych w Polsce(in Polish with English summary). Zesz. Problemove Posteprw Nauk Roln. 1965, 57, 5–158. [Google Scholar]
- Natlandsmyr, B.; Hjelle, K.L. Long-term vegetation dynamics and land-use history: Providing a baseline for conservation strategies in protected Alnus glutinosa swamp woodlands. For. Ecol. Manag. 2016, 372, 78–92. [Google Scholar] [CrossRef]
- Houston Durrant, T.; de Rigo, D.; Caudullo, G. Alnus glutinosa in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 64–65. [Google Scholar] [CrossRef]
- Wiegers, J. Succession in Fen Woodland Ecosystems in the Dutch haf District: With Special Reference to Betula pubescens Ehrh. Dissertationes botanicae; Cramer: Vaduz, Liechtenstein, 1985; pp. 1–152. [Google Scholar]
- Brock, T.C.M.; Jongerhuis, R.; van der Molen, P.C.; Ran, E.T.H. A comparison of the history and present state of an Alnus glutinosa and Betula pubescens dominated patch of wetland forest in the nature reserve "Het Molenven", The Netherlands. Acta Bot. Neerl. 1989, 38, 425–437. [Google Scholar]
- Janssen, C.R.; Berendsen, H.J.A.; van Broekhuizen, A.J.D. Fluvial activity and vegetation development 4000-2000 BP in southwestern Utrecht, The Netherlands. Meded. Rijks Geol. Dienst. 1995, 52, 357–367. [Google Scholar]
- Darell, P.; Cronberg, N. Bryophytes in black alder swamps in south Sweden: Habitat classification, environmental factors and life-strategies. Lindbergia 2011, 34, 9–29. [Google Scholar]
- Gerdol, R.; Pontin, A.; Tomaselli, M.; Bombonato, L.; Brancaleoni, L.; Gualmini, M.; Petraglia, A.; Siffi, C.; Gargini, A. Hydrologic controls on water chemistry, vegetation and ecological patterns in twomires in the South-Eastern Alps (Italy). J. Maps. 2011, 86, 86–97. [Google Scholar]
- Skre, O.; Oechel, W.C. Moss functioning in different taiga ecosystems in interior Alaska. Oecologia 1981, 48, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Weltzin, J.F.; Harth, C.; Bridgham, S.D.; Pastor, J.; Vonderharr, M. Production and microtopography of bog bryophytes: Response to warming and water-table manipulations. Oecologia 2001, 128, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Hájek, T.; Beckett, R.P. Effect of Water Content Components on Desiccation and Recovery in Sphagnum Mosses. Ann. Bot. 2008, 101, 165–173. [Google Scholar] [CrossRef] [Green Version]
- McCarter, C.P.R.; Price, J.S. Ecohydrology of Sphagnum moss hummocks: Mechanisms of capitula water supply and simulated effects of evaporation. Ecohydrology 2014, 7, 33–44. [Google Scholar] [CrossRef]
- Carrión, J.S.; van Geel, B. Fine-resolution Upper Weichselian and Holocene palynological record from Navarrés (Valencia, Spain) and a discussion about factors of Mediterranean forest succession. Rev. Palaeobot. Palynol. 1999, 106, 209–236. [Google Scholar] [CrossRef]
- Beug, H.-J. Vegetation changes during the Slavic period, shown by a high resolution pollen diagram from the Maujahn peat bog near Dannenberg, Hanover Wendland, Germany. Veg. Hist. Archaeobot. 2011, 20, 199–206. [Google Scholar] [CrossRef]
- Schlütz, F.; Shumilovskikh, L.S. Non-pollen palynomorphs notes: 1. Type HdV-368 (Podospora-type), descriptions of associated species, and the first key to related spore types. Rev. Palaeobot. Palynol. 2017, 239, 47–54. [Google Scholar] [CrossRef]
- Graf, M.-T.; Chmura, G.L. Development of modern analogues for natural, mowed and grazed grasslands using pollen assemblages and coprophilous fungi. Rev. Palaeobot. Palynol. 2006, 141, 139–149. [Google Scholar]
- Cook, E.J.; van Geel, B.; van der Kaars, S.; van Arkel, J. A review of the use of non-pollen palynomorphs in palaeoecology with examples from Australia. Palynology 2011, 35, 155–178. [Google Scholar] [CrossRef]
- Eisner, W.R.; Peterson, K.M. High-resolution pollen analysis of tundra polygons from the North Slope of Alaska. J. Geophys. Res. Atmos 1998, 103, 28929–28937. [Google Scholar] [CrossRef]
- López-Merino, L.; Martínez Cortizas, A.; López-Sáez, J.A. Human-induced changes on wetlands: A study case from NW Iberia. Quat. Sci. Rev. 2011, 30, 2745–2754. [Google Scholar] [CrossRef]
- Riera, S.; López-Sáez, J.A.; Julià, R. Lake responses to historical land use changes in northern Spain: The contribution of nonpollen palynomorphs in a multiproxy study. Rev. Palaeobot. Palynol. 2006, 141, 127–137. [Google Scholar] [CrossRef]
- Kaal, J.; Criado-Boado, F.; Costa-Casais, M.; López-Sáez, J.A.; López-Merino, L.; Mighall, T.; Carrión, Y.; Silva Sánchez, N.; Martínez Cortizas, A. Prehistoric land use at an archaeological hot-spot (the rock art park of Campo Lameiro, NW Spain) inferred from charcoal, synanthropic pollen and non-pollen palynomorph proxies. J. Archaeol. Sci. 2013, 40, 1518–1527. [Google Scholar] [CrossRef]
- Mudie, P.J.; Lelièvre, M.A. Palynological study of a Mi’kmaw shell midden, Northeast Nova Scotia, Canada. J. Archaeol. Sci. 2013, 40, 2161–2175. [Google Scholar] [CrossRef]




| Depth (cm) | Sediment Description (Troels-Smith Classification) | Munsell Colour |
|---|---|---|
| 95–110 | Tb1 Tl2 Th1 | 10 YR 2/1 black |
| 110–155 | Tb + Tl2 Th1 Dl1 | |
| 155–160 | Tb + Tl1 Th1 Dl1 Lf + As1 | |
| 160–200 | Tb + Tl1 Th + Dl1Lf + As3 | 10 YR 3/1 very dark gray |
| 200–210 | 2.5 Y 3/1 very dark gray |
| Laboratory Code | Depth (cm) | Material | 14C Age (BP) | Calibrated Age Range | Callibrated Age Range |
|---|---|---|---|---|---|
| 68.2% Confidence Level | 95.4% Confidence Level | ||||
| GdA-5127 | 98 | Seeds | 1856 ± 65 | 83–232 cal AD | 19–266 cal AD (87.0%) |
| 269–332 cal AD (8.4%) | |||||
| GdA-5573 | 163 | Charcoal | 7800 ± 30 | 6650–6600 cal BC | 6690–6590 cal BC (93.0%) |
| 6580–6570 BC (1.5%) | |||||
| 6540–6535 BC (0.8%) | |||||
| GdA-5428 | 327,5 | Charcoal | 10,590 ± 35 | 10,686–10,598 cal BC | 10,730–10,572 cal BC (87.9%) |
| 10,526–10,481 cal BC (7.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hruševar, D.; Bakrač, K.; Miko, S.; Ilijanić, N.; Šparica Miko, M.; Hasan, O.; Mitić, B. Vegetation History in Central Croatia from ~10,000 Cal BC to the Beginning of Common Era—Filling the Palaeoecological Gap for the Western Part of South-Eastern Europe (Western Balkans). Diversity 2023, 15, 235. https://doi.org/10.3390/d15020235
Hruševar D, Bakrač K, Miko S, Ilijanić N, Šparica Miko M, Hasan O, Mitić B. Vegetation History in Central Croatia from ~10,000 Cal BC to the Beginning of Common Era—Filling the Palaeoecological Gap for the Western Part of South-Eastern Europe (Western Balkans). Diversity. 2023; 15(2):235. https://doi.org/10.3390/d15020235
Chicago/Turabian StyleHruševar, Dario, Koraljka Bakrač, Slobodan Miko, Nikolina Ilijanić, Martina Šparica Miko, Ozren Hasan, and Božena Mitić. 2023. "Vegetation History in Central Croatia from ~10,000 Cal BC to the Beginning of Common Era—Filling the Palaeoecological Gap for the Western Part of South-Eastern Europe (Western Balkans)" Diversity 15, no. 2: 235. https://doi.org/10.3390/d15020235






