Taxonomy
Loureedia Miller, Griswold, Scharff, Řezáč, Szűts and Marhabaie, 2012.
Loureedia Miller et al., 2012: 81 [
1] (original description).
Loureedia: Henriques et al. 2018: 5 [
3]; Zamani and Marusik 2020: 239 [
6].
Type species: Eresus annulipes Lucas, 1857, Patria ignota (unknown site).
Diagnosis. The most diagnostic character of the genus is the bifid conductor of the male palp (
Figure 4). See also Miller et al. [
1].
Figure 4.
Conductors of six species of
Loureedia (ventral view). (
A)
L. phoenixi; (
B)
L. jerbae; (
C)
L. annulipes; (
D)
L. lucasi; (
E)
L. maroccana; (
F)
L. colleni. A, D, and F are based on Zamani and Marusik [
6]. Line drawings by Mahla Pourcheraghi.
Figure 4.
Conductors of six species of
Loureedia (ventral view). (
A)
L. phoenixi; (
B)
L. jerbae; (
C)
L. annulipes; (
D)
L. lucasi; (
E)
L. maroccana; (
F)
L. colleni. A, D, and F are based on Zamani and Marusik [
6]. Line drawings by Mahla Pourcheraghi.
Composition. Six species: L. annulipes, L. colleni, L. jerbae, L. lucasi, L. maroccana, and L. phoenixi.
Distribution. Spain, North Africa, and the Middle East (east to Iran) (see
Figure 5).
Figure 5.
Distribution records of species of
Loureedia. Color codes: yellow—
L. colleni, blue—
L. maroccana, white—
L. lucasi, green—
L. jerbae, khaki—
L. annulipes (SAJ264 not shown), magenta—
L. phoenixi, black dot—unidentified
Loureedia specimen from Jordan [
7].
Figure 5.
Distribution records of species of
Loureedia. Color codes: yellow—
L. colleni, blue—
L. maroccana, white—
L. lucasi, green—
L. jerbae, khaki—
L. annulipes (SAJ264 not shown), magenta—
L. phoenixi, black dot—unidentified
Loureedia specimen from Jordan [
7].
Loureedia annulipes (Lucas, 1857).
Eresus annulipes Lucas, 1857: 21 (♂) [
4] (original description).
Eresus semicanus Simon, 1908: 83 (♂) [
9].
Eresus semicanus: Simon 1911: 294, Fig 5 (♂) [
24]; El-Hennawy 2004: 28, Figs 2A,B, 3A–C and 4A,B (♂♀) [
10].
Stegodyphus annulipes: Kraus & Kraus 1992: 15, 19 [
5].
Loureedia annulipes: Miller et al. 2012: 88, Figs 1G–H, 4I, 9I–L, 13G–I, 18A, D, 62A–J, 63A–F, 64A–D, 65A–F, 66A–F, and 67A–F (♂♀) [
1]; Henriques et al. 2018: 5, Fig 2a–h (♂♀) [
3]; Zamani and Marusik 2020: 242, Fig 3g (♂) [
6].
Type material. Holotype: male (AR5391, NMHN), Patria Ignota (unknown site) (not examined).
Other examined material. One male (HUJ Ara 16551), ISRAEL: Southern District: Negev desert, 1 km north of Kibbutz Retamim, 29.X.2016 (leg. Reut A. Ein-Gil).
Figure 6.
Male palps of five species of Loureedia (retrolateral view). (A) L. phoenixi; (B) L. jerbae; (C) L. annulipes; (D) L. maroccana, holotype (after conductor was broken); (E) L. maroccana, holotype (conductor still intact); (F) L. colleni. Scale bar: 0.25 mm.
Figure 6.
Male palps of five species of Loureedia (retrolateral view). (A) L. phoenixi; (B) L. jerbae; (C) L. annulipes; (D) L. maroccana, holotype (after conductor was broken); (E) L. maroccana, holotype (conductor still intact); (F) L. colleni. Scale bar: 0.25 mm.
Diagnosis. The male palp of
L. annulipes (
Figure 4C,
Figure 6C,
Figure 7C and
Figure 8C) is most similar to that of
L. colleni (
Figure 4F,
Figure 6F,
Figure 7F and
Figure 8F), with the retrolateral arm of the conductor being much longer than the prolateral arm and bearing a gradual curvature and a blunt tip (see
Figure 4C,F). The male of
L. annulipes can be distinguished from that of
L. colleni by the wider stem of the conductor (
Figure 4C), bearing a distinct concavity on the mesal margin (
Figure 4C) vs. a narrower stem (
Figure 4F) with an almost straight mesal margin (
Figure 4F), the curved tip of the retrolateral arm (
Figure 4C,
Figure 6C and
Figure 7C) of the conductor, and the abdominal coloration pattern consisting of numerous white spots (
Figure 1A,B and
Figure 2E) and two longitudinal, interrupted yellowish stripes (
Figure 1B and
Figure 2E) vs. one or two white semi-foliate patterns in some individuals in the form of two large patches (
Figure 1E and
Figure 2A). The females of the two species can be differentiated by the epigynal fovea (i.e., the median lobe described by Miller et al. [
1]), which is almost as long as it is wide in
L. annulipes (see Miller et al. [
1]: Fig 18A) vs. longer than wide in
L. colleni (see Henriques et al. [
3]: Fig 9C).
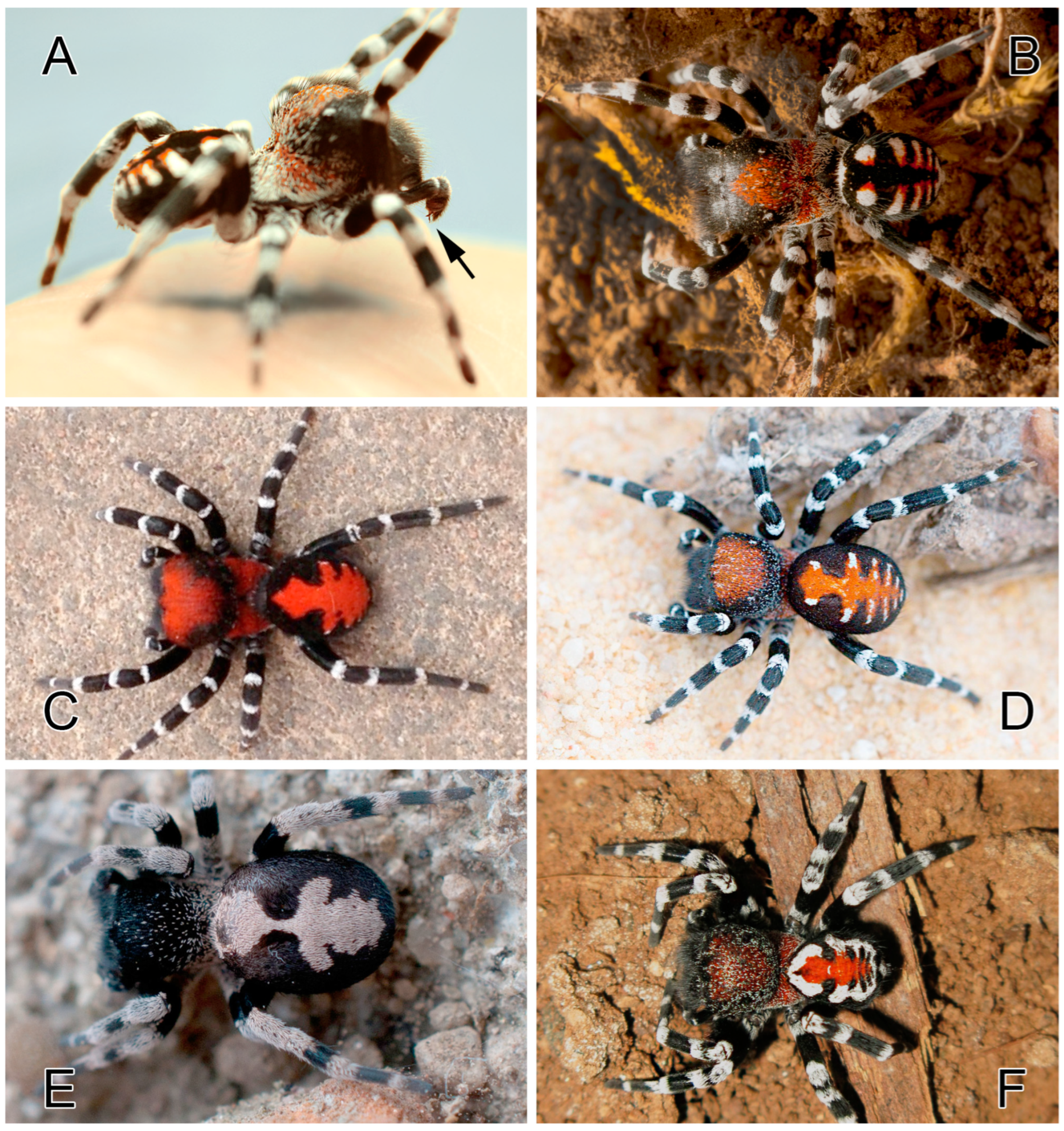
Description. Male. Habitus as in
Figure 1A,B and
Figure 2E. Total length: 8.01. Carapace: 4.40 long and 3.65 wide. Abdomen: 3.79 long and 3.20 wide. Eye sizes and inter-eye distances: AME 0.27, PME 0.23, ALE 0.12, PLE 0.12, AME–AME 0.34, and ALE–AME 0.93. The carapace, sternum, labium, chelicerae, and maxillae dark brown. The carapace mostly covered with fine, long, black and shorter white and orange setae. The pars cephalica in most individuals with a localized triangular patch of red scales (absent in some individuals, see Miller et al. [
1]: Fig 1G).
The center of the pars cephalica covered with orange setae, and the posterior part covered with fine white setae. Legs covered with thin black hairs, with distinct regions of white hairs at the joints of all segments, forming distinct white annulations. Abdomen velvet black; a foliate pattern with a black median elongated patch forming four pairs of elongated dots with orange markings on the inner parts and white markings on the outer parts. White patches unify at the posterior part of dorsum. Measurements of legs: I: 8.59 (2.99, 1.37, 1.71, 1.57, 0.94); II: 7.91 (2.47, 1.59, 1.41, 1.53, 0.89); III: 6.63 (2.31, 1.10, 1.21, 1.29, 0.70); IV: 9.56 (3.05, 1.82, 2.01, 1.77, 0.89).
Palp as in
Figure 4C,
Figure 6C,
Figure 7C and
Figure 8C. The stem of the conductor ca. 1.5 times longer than wide. The mesal and ectal margins of the conductor with slight curvatures. The retrolateral arm of the conductor ca. 2.5 times longer than the prolateral arm, and with blunt tip; prolateral arm with a pointed tip.
Female. Deciphering the identity of females of this species is still in progress. Miller et al. [
1] described the females based on both
L. jerbae and
L. annulipes specimens. The two females are indeed very similar, and comparative material is still being collected.
Variation. The number of white patches on the dorsal surface of the abdomen varies, typically from four to six pairs. They may be connected to each other at their inner margins in some specimens. There is also variation in the width of the median black stripe and the extent of the orange markings. Some specimens have a white band on the anterior portion of the abdomen.
Figure 7.
Male palps of five species of Loureedia (ventral view). (A) Loureedia phoenixi; (B) L. jerbae; (C) L. annulipes; (D) L. maroccana, holotype (after conductor was broken); (E) L. maroccana, holotype (conductor still intact); (F) L. colleni. Scale bar: 0.25 mm.
Figure 7.
Male palps of five species of Loureedia (ventral view). (A) Loureedia phoenixi; (B) L. jerbae; (C) L. annulipes; (D) L. maroccana, holotype (after conductor was broken); (E) L. maroccana, holotype (conductor still intact); (F) L. colleni. Scale bar: 0.25 mm.
Natural history. Known from the sandy dunes of the Negev desert (
Figure 9A,B).
Phenology. The males are active during October–November.
Distribution. Reliably known only from Israel (Southern District) (see
Figure 5).
Figure 8.
Male palps of five species of Loureedia (prolateral view). (A) Loureedia phoenixi; (B) L. jerbae; (C) L. annulipes; (D) L. maroccana, holotype (after conductor was broken); (E) L. maroccana, holotype (conductor still intact); (F) L. colleni. Scale bar: 0.25 mm.
Figure 8.
Male palps of five species of Loureedia (prolateral view). (A) Loureedia phoenixi; (B) L. jerbae; (C) L. annulipes; (D) L. maroccana, holotype (after conductor was broken); (E) L. maroccana, holotype (conductor still intact); (F) L. colleni. Scale bar: 0.25 mm.
Loureedia colleni Henriques, Miñano and Pérez-Zarcos, 2018.
Loureedia colleni Henriques, Miñano and Pérez-Zarcos in Henriques et al., 2018: 8, Figs 3, 4–8a–c, 9a–d, 13b and S–S12 (♂♀) [
3] (original description).
Loureedia colleni: Zamani and Marusik 2020: 242, Fig 3i (♂) [
6].
Type material. Holotype: male (MNCN), SPAIN: Andalucía: Granada province, 820 m a.s.l., 10.X.2010 (leg. Carlos Jerez del Valle) (not examined).
Other examined material. Two males and one female (HNHM 9207, 9209, and 9215), SPAIN: Andalucía: Almería Province, Sierra de Gádor, Vícar, 36°49′03.0″ N, 2°39′14.1″ W, 820 m a.s.l., 10.IX. 2017 (leg. Magali Fabregat).
Figure 9.
Habitats of three species of Loureedia. (A,B) L. annulipes from Israel (Reut Ein-Gil); (C) L. phoenixi from Iran (photo: Alireza Zamani); (D) L. colleni from Spain (photo: Magali Fabregat).
Figure 9.
Habitats of three species of Loureedia. (A,B) L. annulipes from Israel (Reut Ein-Gil); (C) L. phoenixi from Iran (photo: Alireza Zamani); (D) L. colleni from Spain (photo: Magali Fabregat).
Diagnosis. This species differs from all of its congeners by the black-and-white coloration pattern of the male (
Figure 1E and
Figure 2A) vs. having yellowish to scarlet red abdominal patterns (see
Figure 1A–D and
Figure 2B–F). The male palp of
L. colleni (
Figure 4F,
Figure 6F,
Figure 7F and
Figure 8F) is most similar to that of
L. annulipes (
Figure 4C), as the prolateral arm of the conductor is much shorter than the retrolateral arm (
Figure 4F), which bears a gradual curvature (7F). The male of
L. colleni can be diagnosed by the narrower stem of the conductor (
Figure 6F), with an almost straight mesal margin (
Figure 4F). The female can be recognized by an epigynal fovea that is longer than it is wide (see Henriques et al. [
3]: Fig 8a).
Description. Male. Habitus as in
Figure 1E and
Figure 2A. Total length: 6.43. Carapace: 3.35 long and 2.84 wide. Abdomen: 3.19 long and 2.55 wide. Eye sizes and inter-eye distances: AME 0.14, PME 0.16, ALE 0.04, PLE 0.08, AME–AME 0.30, and ALE–AME 0.76. The carapace, sternum, labium, chelicerae, and maxillae black. Carapace mostly covered with long black setae and scattered shorter white setae. White setae localized densely on the pars thoracica and form a triangle on the pars cephalica (
Figure 1E). Legs covered with thick white hairs. Abdomen velvet black with a longitudinal median white foliate pattern bearing a distinct mediolateral lobe; the most anterior part of the folium merging and forming a distinct white spot. Measurements of legs: I: 7.15 (2.07, 1.16, 1.45, 1.43, 1.01); II: 6.33 (1.94, 1.19, 1.18, 1.22, 0.78); III: 5.44 (1.85, 0.80, 1.08, 1.01, 0.68); IV: 7.29 (2.32, 1.28, 1.56, 1.39, 0.71).
Palp as in
Figure 4F,
Figure 6F,
Figure 7F and
Figure 8F. The stem of the conductor ca. 1.5 times longer than wide. The mesal margin of the conductor almost straight, and the ectal margin with an apical invagination. The retrolateral arm of the conductor ca. 2.5 times longer than the prolateral arm, and both arms with blunt tips.
Female. See Henriques et al. [
3].
Variation. A wide array of abdominal pattern variations has already been illustrated [
3]. The highest amount of variation occurs in the white foliate pattern, which may either be solid or form two large separate patches. Here, we examined two distinct color pattern forms (Figure 15A,B). Minor variations also occur on the male palp; these are considered intraspecific variations, as the COI sequences of the two males were identical, whereas they were slightly different (99.965% similarity) from that of the female.
Natural history. The species’ habitat preference has already been described [
3]. The examined specimens were collected on a hillside with south and south-east exposure in a semi-arid open area (
Figure 9D) with short, sparse vegetation. The vegetation in this area mainly consists of degraded bushes, tufts of thyme, thorny broom,
Launaea arborescens, and
Ononis natrix hispanica. The soil is mainly puddingstone, made up of the Alpujarride complex and Baetic/Penibaetic cordillera, covered with small flat stones.
Several webs have been observed in multiple similar biotopes in Andalusia. This singular web pattern turned out to be common, and adult and juvenile specimens both constructed it (including those who were kept alive in captivity). The very discreet webs are located under small stones on the ground. This structure provides the spider with protection against the elements: mainly intense heat but also wind and rare precipitation. Hunting canopies are very short and simple compared to those woven by species of
Eresus. The details of a retreat are illustrated in
Figure 10A–E. Sectional views of the canvas and lodge assembly (between the stone and the ground) are depicted in
Figure 10A,B. The main lodge, located below the surface of the stone, is the main living space. Females have been observed in captivity to consume their prey and sometimes molt or copulate (sharing this lodge with the male) in this area. Hunting behavior is mainly sit-and-wait. Vibration is received from the external radial silk lines. The periphery of this lodge consists of dense cribellate silk, mixed with small pieces of agglomerated soil, anchored both to the ground and under the stone. From the main lodge, two separate exits with two capture canopies exist. The silk retreat is covered with a trapdoor-like hatch made of thick cribellate silk with soil particles in it (
Figure 10C–E).
Figure 10.
Retreats of Loureedia colleni from Spain. (A,B) retreat layout, dorsal and lateral views (illustration: Thomas Romanoff); (C) female with the open hatch; (D) hatch being closed; (E) closed hatch. Legend (A,B): 1—main lodge; 2—external access tunnels; 3—hunting canopy and radial silk lines; 4—“retreat” hatch; 5—annex “retreat” lodge.
Figure 10.
Retreats of Loureedia colleni from Spain. (A,B) retreat layout, dorsal and lateral views (illustration: Thomas Romanoff); (C) female with the open hatch; (D) hatch being closed; (E) closed hatch. Legend (A,B): 1—main lodge; 2—external access tunnels; 3—hunting canopy and radial silk lines; 4—“retreat” hatch; 5—annex “retreat” lodge.
Phenology. Males are active during February–November.
Distribution. Spain (Albacete, Alicante, Almería, Ciudad Real, Granada, Madrid, and Murcia provinces) (see
Figure 5).
Loureedia jerbae (El-Hennawy, 2005).
Eresus jerbae El-Hennawy, 2005: 88, Figs 1–4 (♀) [
11] (original description).
Loureedia annulipes Miller et al. 2012: 88 [
1] (synonymy with
L.
annulipes;
rejected here).
Type material. Holotype: female (MNHN 471/AR 835), TUNISIA: Djerba; misidentified as Eresus petagnae (not examined).
Other examined material. One male (HNHM), TUNISIA: Djerba, Djerba Midun, 33°48′36.2″ N, 11°02′38.3″ E, X. 2019 (leg. S. Macík).
Diagnosis. The male palp of
L. jerbae (
Figure 4B,
Figure 6B,
Figure 7B and
Figure 8B) is most similar to that of
L. phoenixi (
Figure 4A,
Figure 6A,
Figure 7A and
Figure 8A), as the arms of the conductor are almost the same length and bear pointed tips and the terminal portion of the prolateral arm curves retrolaterally (
Figure 4A,B). The male palp of
L. jerbae differs from that of
L. phoenixi, in that the longer stem of the conductor bears only a slight curvature along its ectal margin (
Figure 4B and
Figure 7B) vs. a shorter stem with an abrupt invagination on the ectal margin (
Figure 4A and
Figure 7A), the retrolateral arm of the conductor is slightly longer than the prolateral one (
Figure 4B) vs. both arms of the same length (
Figure 4A), and the base of the prolateral arm of the conductor is wider (
Figure 4B and
Figure 7B). The male coloration pattern of
L. jerbae (
Figure 1D and
Figure 2D) is similar to those of
L. maroccana (
Figure 1C and
Figure 2B) and
L. lucasi (Fig 2C, Henriques et al. [
3]: Fig 1d); it differs from both species by having numerous white spots and short stripes at the tips of the lateral branches of the median abdominal foliate pattern (
Figure 1D and
Figure 2D) vs. no white spots (Fig 2B, Gál et al. [
2]: Fig 1) or only a few very small spots (Fig 2C, Henriques et al. [
3]: Fig 1d). It also differs from
L. lucasi by having a reddish posterior part on the carapace (
Figure 2D) vs. dark (
Figure 2C). The female of
L. jerbae differs from that of
L. lucasi by its longer than wide epigynal windows (see El-Hennawy [
11]: Figs 1–4) vs. round (see Henriques et al. [
3]: Fig 1e,f).
Description. Male. Habitus as in
Figure 1D,
Figure 2D and
Figure 11A,B. Total length: ca. 8.00. Carapace: 4.61 long and 3.61 wide. Abdomen: 4.09 long and 3.49 wide. Eye sizes and inter-eye distances: AME 0.12, PME 1.89, ALE 0.03, PLE 0.03, AME–AME 0.09, and ALE–AME 0.30. The carapace, sternum, labium, chelicerae, and maxillae dark brown. Carapace mostly covered with long black setae and scattered short crimson and white scales. Scale patches of short red setae present mostly on the sides of the pars thoracica and the center of the pars cephalica, with two white spots next to the PLE. Legs covered with thin black hairs, with distinct regions of white hairs at the joints of all segments, forming distinct white annulation (
Figure 2D). Abdomen with a crimson red longitudinal foliate pattern with white lines at its lateral extensions. The most anterior part of the median globular pattern with three lobes: white lateral lobes and a crimson red anterior lobe. Measurements of legs: I: 9.11 (3.04, 1.55, 1.86, 1.58, 1.06); II: 8.85 (2.76, 1.62, 1.72, 1.63, 1.11); III: 7.57 (2.63, 1.39, 1.50, 1.32, 0.71); IV: 10.2 (3.25, 1.60, 2.25, 2.03, 1.03).
Figure 11.
Frontal views and habitus of males of Loureedia jerbae and Loureedia maroccana (holotype). (A) L. jerbae, prosoma, frontal view; (B) L. jerbae, habitus, dorsal view; (C) L. maroccana, frontal view; (D,E) L. maroccana, slightly pushed down, showing changes in the perception of the carapace. Scale bar: 1.0 mm.
Figure 11.
Frontal views and habitus of males of Loureedia jerbae and Loureedia maroccana (holotype). (A) L. jerbae, prosoma, frontal view; (B) L. jerbae, habitus, dorsal view; (C) L. maroccana, frontal view; (D,E) L. maroccana, slightly pushed down, showing changes in the perception of the carapace. Scale bar: 1.0 mm.
Palp as in
Figure 4B,
Figure 6B,
Figure 7B and
Figure 8B. The stem of the conductor ca. two times longer than wide. The mesal margin of the conductor almost straight. The ectal margin with a slight medial invagination. The retrolateral arm of the conductor slightly longer than the prolateral arm. The retrolateral arm curves centrally, and both arms with pointed tips.
Female. See El-Hennawy [
11], which is the only source regarding this species so far.
Variation. There are two observations of Loureedia from Tunisia on iNaturalist: one from Bizerte, with a very similar abdominal pattern to our specimen. The second specimen, although from Djerba, has noticeably larger white spots lateral to the median red band; two individuals with the same pattern have been photographed in northwestern Libya, not far from Djerba. Likely, these specimens belong to L. jerbae, although it is necessary to examine them to confirm this.
Natural history. No information.
Phenology. Males are active during October.
Distribution. Tunisia (Djerba) (see
Figure 5).
Loureedia maroccana Gál, Kovács, Bagyó, Vári and Prazsák, 2017.
Figure 1B,
Figure 2C,
Figure 3,
Figure 4E,
Figure 6D,E,
Figure 7D,E,
Figure 8D,E,
Figure 11C–E,
Figure 12,
Figure 13 and
Figure 14.
Loureedia maroccana Gál et al., 2017: 12, Figs 1, 2, 3A–C and 4A–D (♂) [
2] (original description).
L.
lucasi: Henriques et al. 2018: 5 [
3] (in part; synonymy with
L.
lucasi;
rejected here).
Type material. Holotype: male (HNHM Araneae-8869), MOROCCO: Khémisset Province: near Sidi Boukhalkhal, 04.XI.2013 (leg. J. Gál). Paratypes: two males (HNHM Araneae-9007), same data as for the holotype (examined).
Other examined material. One male (PCGJ), MOROCCO: Khémisset Province: near Sidi Boukhalkhal, 04.XI.2013 (leg. J. Gál), and one male (PCGJ), MOROCCO: Khémisset Province: near Sidi Boukhalkhal, XI.2021 (leg. J. Gál).
Remark: For a closer examination, the right palp of the holotype was removed and illustrated. However, the retrolateral arm of the conductor was accidentally damaged by the first author (see
Figure 12). One palp of one of the paratypes was removed for SEM, while the other one was also unintentionally damaged. These structures seemingly become fragile when deposited in 96% alcohol. Due to the relatively low number of specimens available, both the original (intact
Figure 6E,
Figure 7E and
Figure 8E and
Figure 12A) and damaged states (
Figure 6D,
Figure 7D,
Figure 8D and
Figure 12B,C) of the holotype’s palp were illustrated.
Figure 12.
Holotype of Loureedia maroccana Gál et al., 2017 (damaged male palp). (A) Palp, before the damage, retrolateral view; (B) palp, after the damage (ra disconnected), retrolateral view; (C) palp, after the damage (ra disconnected), ventral view. Scale bar: 0.25 mm.
Figure 12.
Holotype of Loureedia maroccana Gál et al., 2017 (damaged male palp). (A) Palp, before the damage, retrolateral view; (B) palp, after the damage (ra disconnected), retrolateral view; (C) palp, after the damage (ra disconnected), ventral view. Scale bar: 0.25 mm.
Diagnosis. The male palp of
L. maroccana (
Figure 3,
Figure 4E,
Figure 6E,
Figure 7E,
Figure 13 and
Figure 14A–N) is similar to that of
L. lucasi (
Figure 4D and
Figure 14O), as it has a relatively short stem of the conductor (
Figure 4D,E) and the retrolateral arm of the conductor is longer than the prolateral arm (
Figure 4D,E and
Figure 6E). It differs from it, as both arms of the conductor bear pointed tips (
Figure 4E,
Figure 6D,E,
Figure 7D,E and
Figure 8D,E) vs. blunt (
Figure 4D), there is a deeper concavity on the frontal margin of the conductor (
Figure 3 and
Figure 4E), and there is an almost straight ectal margin (
Figure 4E and
Figure 7D,E) at the stem of conductor (
Figure 3A) vs. with a distinct invagination apically (
Figure 4D). The basal margin of the conductor (
Figure 3A) is also wider in
L. maroccana, and the prolateral arm is wider and longer (
Figure 4E and
Figure 7E) than that of
L. lucasi (
Figure 4D). The two species are also very similar in the male coloration pattern (
Figure 2B,C) but differ due to the reddish posterior part of the carapace in
L. maroccana (
Figure 1C and
Figure 2B) vs. dark (Fig 2C, Henriques et al. [
3]: Fig 1d). Moreover, the male of
L. maroccana lacks minute white spots on the dorsal abdominal surface (
Figure 1C) which are present in
L.
lucasi (
Figure 2C).
Description. Male. Habitus as in
Figure 1C,
Figure 2B, and
Figure 10C–E. Total length: 8.77. Carapace: 4.39 long and 4.01 wide. Abdomen: 4.89 long and 3.97 wide. Eye sizes and inter-eye distances: AME 0.22, PME 0.19, ALE 0.12, PLE 0.20, AME–AME 0.39, and ALE–AME 1.08. The Carapace, sternum, labium, chelicerae, and maxillae dark brown with tones of red. Carapace mostly covered with long black and scarlet setae (these become orange (
Figure 10C) when bleached in alcohol; see the specimen depicted in Gál et al. [
2]: Fig 2B) and with very few scattered short white setae on the posterior part of pars cephalica. Pars cephalica well covered with scarlet red scales. Legs covered with thin black hairs, with distinct regions of white hairs at the joints, forming distinct white annulations. Abdomen with a compact longitudinal median red stripe with lateral projections with tiny white spots at their tips. The most anterior pair of the crimson red pattern quadrangle with three lobes. The lateral lobes with white tips. Leg measurements: I: 10.2 (3.21, 1.73, 1.95, 1.97, 1.33); II: 9.28 (3.05, 1.56, 1.72, 1.83, 1.10); III: 8.28 (2.78, 1.72, 1.55, 1.50, 0.71); IV: 10.4 (3.28, 1.66, 2.31, 2.15, 0.98).
Female. Unknown.
Natural history. No information.
Phenology. The males are active during October–November.
Distribution. Morocco (Khémisset Province) (see
Figure 5).
Loureedia phoenixi Zamani and Marusik, 2020.
Loureedia sp.: Henriques et al. 2018: 7, Fig 2h (♂) [
3].
Loureedia phoenixi Zamani and Marusik, 2020: 240, Figs 1a–f, 2a–d and 3a–f (♂) [
6] (original description).
Loureedia phoenixi: Zamani et al. 2021: 282, Fig 2A–D (♂) [
22].
Type material. Holotype: male (MHNG), IRAN: Alborz Province: Karaj, Chenarak, 8.XI.2019 (leg. A. Beigi) (examined).
Other examined material. One male (ZMUT), IRAN: Tehran Province: Shemiranat County, Lavasan, 35°49′ N, 51°37′ E, 25.XI.2020 (leg. S. Bisadi).
Diagnosis. The male of
L. phoenixi (
Figure 4A,
Figure 6A,
Figure 7A and
Figure 8A) is similar to that of
L. jerbae (
Figure 4B,
Figure 6B,
Figure 7B and
Figure 8B), in the prolateral arm of the conductor being (almost) as long as the retrolateral arm. It can be readily distinguished from it by the shorter stem of the conductor (
Figure 4A and
Figure 7A), and by the abdominal pattern, which is consisted of numerous large white spots on both the lateral and anterior margins of the median reddish band (
Figure 1 and
Figure 2F).
Description. Male. Habitus as in
Figure 1 and
Figure 2F. Total length: 5.55. Carapace: 2.72 long and 2.26 wide. Abdomen: 3.08 long and 2.12 wide. Eye sizes and inter-eye distances: AME 0.18, PME 0.15, ALE 0.03, PLE 0.04, AME–AME 0.28, and ALE–AME 0.57. Carapace, sternum, labium, chelicerae, and maxillae dark brown with tones of red. The carapace mostly covered with long black setae and scattered short white setae, with localized patches of short red setae, mostly in the pars thoracica or the center of the pars cephalica. Legs covered with thin black hairs, with distinct regions of white hairs at the joints of all segments, forming distinct white annulations. Abdomen with a compact longitudinal median red stripe with lateral projections with compact white spots at their tips. The most anterior pair of white spots either contiguous or very close to each other, sometimes merging and forming a distinct white spot above the pedicel. Measurements of legs: I: 6.70 (2.11, 1.02, 1.28, 1.40, 0.88); II: 5.94 (1.87, 0.98, 1.09, 1.24, 0.75); III: 4.89 (1.79, 0.68, 0.89, 0.96, 0.55); IV: 6.95 (2.19, 1.00, 1.61, 1.43, 0.69).
Palp as in
Figure 4A,
Figure 6A,
Figure 7A and
Figure 8A. The stem of the conductor ca. 1.2 times longer than it wide. The mesal margin of the conductor with a slight curvature. The ectal margin with a distinct apical invagination. The prolateral and retrolateral arms of the conductor subequal in length, and both with pointed tips.
Female. Unknown.
Variation. The extent of the white abdominal patches is variable: the lateral patches may be connected to each other in a few specimens (
Figure 1F), and the anterior patches are usually connected to each other and to the white plate above the pedicel (Fig 1a,d and f in Zamani and Marusik [
6]), although exceptions have been recorded (Fig 1c,e in Zamani and Marusik [
6]).
Natural history. Wandering males have primarily been collected in well-vegetated steppes but also in and around urban habitats (
Figure 9C).
Phenology. The males are active during October–November.
Distribution. Iran (Alborz, Chaharmahal and Bakhtiari, Fars, Kerman, Qom, Semnan, Tehran, and Yazd provinces) (see
Figure 5).
Loureedia lucasi (Simon, 1873).
Eresus lucasi Simon, 1873: 353, plate. 10, Figs 8 and 9 (♂♀) [
8] (original description).
Loureedia lucasi: Henriques et al. 2018: 5, Fig 1a–h (♂♀) [
3]; Zamani and Marusik 2020: 242, Fig 3h (♂) [
6].
Type material. Syntypes: male and female (NMHN), ALGERIA: Oran (leg. H. Lucas) (not examined).
Figure 13.
Intraspecific variation in the male palp of Loureedia maroccana Gál et al., 2017. (A–C) ventral view, (D–F) prolateral view. (A,D) non-type specimen; (B,E) paratype specimen; (C,F) fresh non-type specimen. Scale bar: 0.25 mm.
Figure 13.
Intraspecific variation in the male palp of Loureedia maroccana Gál et al., 2017. (A–C) ventral view, (D–F) prolateral view. (A,D) non-type specimen; (B,E) paratype specimen; (C,F) fresh non-type specimen. Scale bar: 0.25 mm.
Diagnosis. The male palp of
L. lucasi (
Figure 4D) is similar to that of
L. maroccana (
Figure 4E) in that it has a relatively short stem of conductor and the retrolateral arm of the conductor is longer than the prolateral arm, but differs from it, as both arms of the conductor bear blunt tips (
Figure 4D) vs. pointed (
Figure 4E), it has shallower concavity on the frontal margin of the conductor (
Figure 4D), and there is a distinct invagination apically on the ectal margin of the stem of the conductor (
Figure 4E). The two species are also very similar in the male coloration pattern but differ due to the dark posterior part of the carapace in
L. lucasi (
Figure 2C) vs. crimson red in
L. maroccana (
Figure 2B). Moreover, the male of
L. lucasi has more distinct white spots on the dorsal abdominal surface (
Figure 2C).
Figure 14.
Effect of the angle on the perceived shape of the conductor in
Loureedia maroccana Gál et al., 2017 (retrolateral views) (
A–
N). (
A,
D–
G) non-type specimen; (
B,
H–
K) paratype specimen; (
C,
L–
N) fresh specimen; cymbial tip gradually turned in (
D–
G,
H–
K,
L–
N). (
O) Male palp of
Loureedia lucasi (Simon, 1873) (retrolateral view), redrawn based on Henriques et al. [
3]. Scale bar: 0.25 mm.
Figure 14.
Effect of the angle on the perceived shape of the conductor in
Loureedia maroccana Gál et al., 2017 (retrolateral views) (
A–
N). (
A,
D–
G) non-type specimen; (
B,
H–
K) paratype specimen; (
C,
L–
N) fresh specimen; cymbial tip gradually turned in (
D–
G,
H–
K,
L–
N). (
O) Male palp of
Loureedia lucasi (Simon, 1873) (retrolateral view), redrawn based on Henriques et al. [
3]. Scale bar: 0.25 mm.
Description. Henriques et al. [
3] did not provide any description of the male, and the specimen was not available for us to examine.
Female. See Henriques et al. [
3].
Variation. No information.
Phenology. No information.
Distribution. Algeria (Oran Province) (see
Figure 5).
Figure 15.
Intraspecific variation in Loureedia colleni. (A–C) male color form 1, (D–F) color form 2. (A) male habitus, dorsal view; (B) bulb, retrolateral view; (C) same, prolateral view; (D) male habitus, dorsal view; (E) bulb, retrolateral view; (F) same, prolateral view. Scale bar: 0.25 mm.
Figure 15.
Intraspecific variation in Loureedia colleni. (A–C) male color form 1, (D–F) color form 2. (A) male habitus, dorsal view; (B) bulb, retrolateral view; (C) same, prolateral view; (D) male habitus, dorsal view; (E) bulb, retrolateral view; (F) same, prolateral view. Scale bar: 0.25 mm.