Euendolithic Infestation of Mussel Shells Indirectly Improves the Thermal Buffering Offered by Mussel Beds to Associated Molluscs, but One Size Does Not Fit All
Abstract
:1. Introduction
2. Materials and Methods
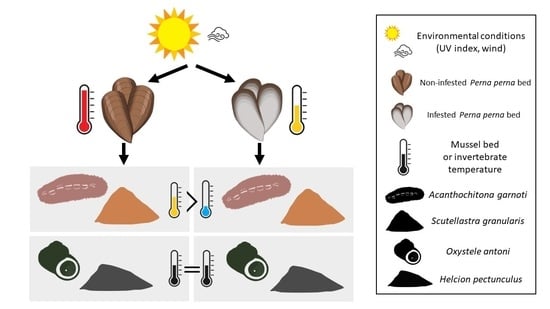
2.1. Focal Species and Experimental Setting
2.2. Body Temperature Assessment
2.3. Desiccation Assessment
2.4. Data Analyses
3. Results
3.1. Body Temperature Assessment
3.2. Dessication Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliver, E.C.J.; Benthuysen, J.A.; Darmaraki, S.; Donat, M.G.; Hobday, A.J.; Holbrook, N.J.; Schlegel, R.W.; Sen Gupta, A. Marine Heatwaves. Annu. Rev. Mar. Sci. 2021, 13, 313–342. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.; Feng, M.; Phillips, H.E.; Bindoff, N.L. A Global, Multiproduct Analysis of Coastal Marine Heatwaves: Distribution, Characteristics, and Long-Term Trends. J. Geophys. Res. Oceans 2021, 126, e2020JC016708. [Google Scholar] [CrossRef]
- Meehl, G.A.; Tebaldi, C. More Intense, More Frequent, and Longer Lasting Heat Waves in the 21st Century. Science 2004, 305, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.C.J.; Donat, M.G.; Burrows, M.T.; Moore, P.J.; Smale, D.A.; Alexander, L.V.; Benthuysen, J.A.; Feng, M.; Sen Gupta, A.; Hobday, A.J.; et al. Longer and More Frequent Marine Heatwaves over the Past Century. Nat. Commun. 2018, 9, 1324. [Google Scholar] [CrossRef]
- Frölicher, T.L.; Fischer, E.M.; Gruber, N. Marine Heatwaves under Global Warming. Nature 2018, 560, 360–364. [Google Scholar] [CrossRef]
- Mbokodo, I.; Bopape, M.-J.; Chikoore, H.; Engelbrecht, F.; Nethengwe, N. Heatwaves in the Future Warmer Climate of South Africa. Atmosphere 2020, 11, 712. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Burrows, M.T.; Donat, M.G.; Sen Gupta, A.; Alexander, L.V.; Perkins-Kirkpatrick, S.E.; Benthuysen, J.A.; Hobday, A.J.; Holbrook, N.J.; Moore, P.J.; et al. Projected Marine Heatwaves in the 21st Century and the Potential for Ecological Impact. Front. Mar. Sci. 2019, 6, 734. [Google Scholar] [CrossRef]
- Gupta, A.S.; Thomsen, M.; Benthuysen, J.A.; Hobday, A.J.; Oliver, E.; Alexander, L.V.; Burrows, M.T.; Donat, M.G.; Feng, M.; Holbrook, N.J.; et al. Drivers and Impacts of the Most Extreme Marine Heatwave Events. Sci. Rep. 2020, 10, 19359. [Google Scholar] [CrossRef]
- Schlegel, R.W.; Oliver, E.C.J.; Perkins-Kirkpatrick, S.; Kruger, A.; Smit, A.J. Predominant Atmospheric and Oceanic Patterns during Coastal Marine Heatwaves. Front. Mar. Sci. 2017, 4, 323. [Google Scholar] [CrossRef]
- Gaines, S.D.; Denny, M.W. The Largest, Smallest, Highest, Lowest, Longest, and Shortest: Extremes in Ecology. Ecology 1993, 74, 1677–1692. [Google Scholar] [CrossRef]
- Helmuth, B.; Harley, C.D.G.; Halpin, P.M.; O’Donnell, M.; Hofmann, G.E.; Blanchette, C.A. Climate Change and Latitudinal Patterns of Intertidal Thermal Stress. Science 2002, 298, 1015–1017. [Google Scholar] [CrossRef]
- Lima, F.P.; Ribeiro, P.A.; Queiroz, N.; Hawkins, S.J.; Santos, A.M. Do Distributional Shifts of Northern and Southern Species of Algae Match the Warming Pattern? Glob. Chang. Biol. 2007, 13, 2592–2604. [Google Scholar] [CrossRef]
- Sanford, E.; Sones, J.L.; García-Reyes, M.; Goddard, J.H.R.; Largier, J.L. Widespread Shifts in the Coastal Biota of Northern California during the 2014–2016 Marine Heatwaves. Sci. Rep. 2019, 9, 4216. [Google Scholar] [CrossRef]
- White, R.; Anderson, S.; Booth, J.; Braich, G.; Draeger, C.; Fei, C.; Harley, C.; Henderson, S.; Jakob, M.; Lau, C.-A.; et al. The Unprecedented Pacific Northwest Heatwave of June 2021; Faculty Research and Publications; In Review; University of British Columbia: Vancouver, BC, Canada, 2022. [Google Scholar]
- Helmuth, B.; Yamane, L.; Lalwani, S.; Matzelle, A.; Tockstein, A.; Gao, N. Hidden Signals of Climate Change in Intertidal Ecosystems: What (Not) to Expect When You Are Expecting. J. Exp. Mar. Biol. Ecol. 2011, 400, 191–199. [Google Scholar] [CrossRef]
- Helmuth, B.; Mieszkowska, N.; Moore, P.; Hawkins, S.J. Living on the Edge of Two Changing Worlds: Forecasting the Responses of Rocky Intertidal Ecosystems to Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 373–404. [Google Scholar] [CrossRef]
- Lathlean, J.A.; Seuront, L.; Ng, T.P.T. On the Edge: The Use of Infrared Thermography in Monitoring Responses of Intertidal Organisms to Heat Stress. Ecol. Indic. 2017, 81, 567–577. [Google Scholar] [CrossRef]
- Mislan, K.A.S.; Wethey, D.S.; Helmuth, B. When to Worry about the Weather: Role of Tidal Cycle in Determining Patterns of Risk in Intertidal Ecosystems. Glob. Chang. Biol. 2009, 15, 3056–3065. [Google Scholar] [CrossRef]
- Somero, G.N. The Physiology of Climate Change: How Potentials for Acclimatization and Genetic Adaptation Will Determine ‘Winners’ and ‘Losers’. J. Exp. Biol. 2010, 213, 912–920. [Google Scholar] [CrossRef]
- Somero, G.N. Thermal Physiology and Vertical Zonation of Intertidal Animals: Optima, Limits, and Costs of Living. Integr. Comp. Biol. 2002, 42, 780–789. [Google Scholar] [CrossRef]
- Williams, G.; De Pirro, M.; Leung, K.; Morritt, D. Physiological Responses to Heat Stress on a Tropical Shore: The Benefits of Mushrooming Behaviour in the Limpet Cellana Grata. Mar. Ecol. Prog. Ser. 2005, 292, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, J.L.P.; Finke, G.R.; Camus, P.A.; Bozinovic, F. Thermoregulatory Behavior, Heat Gain and Thermal Tolerance in the Periwinkle Echinolittorina peruviana in Central Chile. Comp. Biochem. Physiol. 2005, 142, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, S.R.; Williams, G.A. How Hot for How Long? The Potential Role of Heat Intensity and Duration in Moderating the Beneficial Effects of an Ecosystem Engineer on Rocky Shores. Mar. Biol. 2014, 161, 2097–2105. [Google Scholar] [CrossRef]
- Lathlean, J.A.; Seuront, L.; McQuaid, C.D.; Ng, T.P.T.; Zardi, G.I.; Nicastro, K.R. Cheating the Locals: Invasive Mussels Steal and Benefit from the Cooling Effect of Indigenous Mussels. PLoS ONE 2016, 11, e0152556. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.; Bagur, M.; Palomo, M. Algal Epibionts as Co-Engineers in Mussel Beds: Effects on Abiotic Conditions and Mobile Interstitial Invertebrates. Diversity 2019, 11, 17. [Google Scholar] [CrossRef]
- Helmuth, B.S.T. Intertidal Mussel Microclimates: Predicting the Body Temperature of a Sessile Invertebrate. Ecol. Monogr. 1998, 68, 51–74. [Google Scholar] [CrossRef]
- Jurgens, L.J.; Gaylord, B. Physical Effects of Habitat-forming Species Override Latitudinal Trends in Temperature. Ecol. Lett. 2018, 21, 190–196. [Google Scholar] [CrossRef]
- Nicastro, K.R.; McQuaid, C.D.; Dievart, A.; Zardi, G.I. Intraspecific Diversity in an Ecological Engineer Functionally Trumps Interspecific Diversity in Shaping Community Structure. Sci. Total Environ. 2020, 743, 140723. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as Ecosystem Engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Lathlean, J.A.; Seuront, L.; McQuaid, C.D.; Ng, T.P.T.; Zardi, G.I.; Nicastro, K.R. Size and Position (Sometimes) Matter: Small-Scale Patterns of Heat Stress Associated with Two Co-Occurring Mussels with Different Thermoregulatory Behaviour. Mar. Biol. 2016, 163, 189. [Google Scholar] [CrossRef]
- Lourenço, C.R.; Nicastro, K.R.; McQuaid, C.D.; Sabour, B.; Zardi, G.I. Latitudinal Incidence of Phototrophic Shell-Degrading Endoliths and Their Effects on Mussel Bed Microclimates. Mar. Biol. 2017, 164, 129–139. [Google Scholar] [CrossRef]
- Thomas, F.; Renaud, F.; de Meeûs, T.; Poulin, R. Manipulation of Host Behaviour by Parasites: Ecosystem Engineering in the Intertidal Zone? Proc. R. Soc. Lond. B Biol. Sci. 1998, 265, 1091–1096. [Google Scholar] [CrossRef]
- Kaehler, S. Incidence and Distribution of Phototrophic Shell-Degrading Endoliths of the Brown Mussel Perna perna. Mar. Biol. 1999, 135, 505–514. [Google Scholar] [CrossRef]
- Zardi, G.I.; Nicastro, K.R.; McQuaid, C.D.; Ng, T.P.T.; Lathlean, J.; Seuront, L. Enemies with Benefits: Parasitic Endoliths Protect Mussels against Heat Stress. Sci. Rep. 2016, 6, 31413. [Google Scholar] [CrossRef]
- Golubic, S.; Friedmann, I.; Schneider, J. The Lithobiontic Ecological Niche, with Special Reference to Microorganisms. J. Sediment. Petrol. 1981, 51, 475–478. [Google Scholar]
- Golubic, S.; Perkins, R.D.; Lukas, K.J. Boring Microorganisms and Microborings in Carbonate Substrates. In The Study of Trace Fossils; Frey, R.W., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 1975; pp. 229–259. ISBN 978-3-642-65925-6. [Google Scholar]
- Couradeau, E.; Roush, D.; Guida, B.S.; Garcia-Pichel, F. Diversity and Mineral Substrate Preference in Endolithic Microbial Communities from Marine Intertidal Outcrops (Isla de Mona, Puerto Rico). Biogeosciences 2017, 14, 311–324. [Google Scholar] [CrossRef]
- Dievart, A.M.; McQuaid, C.D.; Zardi, G.I.; Nicastro, K.R.; Froneman, P.W. Photoautotrophic Euendoliths and Their Complex Ecological Effects in Marine Bioengineered Ecosystems. Diversity 2022, 14, 737. [Google Scholar] [CrossRef]
- Kaehler, S.; McQuaid, C.D. Lethal and Sub-Lethal Effects of Phototrophic Endoliths Attacking the Shell of the Intertidal Mussel Perna perna. Mar. Biol. 1999, 135, 497–503. [Google Scholar] [CrossRef]
- Zardi, G.I.; Nicastro, K.R.; McQuaid, C.D.; Gektidis, M. Effects of Endolithic Parasitism on Invasive and Indigenous Mussels in a Variable Physical Environment. PLoS ONE 2009, 4, e6560. [Google Scholar] [CrossRef]
- Zardi, G.I.; Monsinjon, J.R.; McQuaid, C.D.; Seuront, L.; Orostica, M.; Want, A.; Firth, L.B.; Nicastro, K.R. Foul-weather Friends: Modelling Thermal Stress Mitigation by Symbiotic Endolithic Microbes in a Changing Environment. Glob. Chang. Biol. 2021, 27, 2549–2560. [Google Scholar] [CrossRef]
- Monsinjon, J.R.; McQuaid, C.D.; Nicastro, K.R.; Seuront, L.; Oróstica, M.H.; Zardi, G.I. Weather and Topography Regulate the Benefit of a Conditionally Helpful Parasite. Funct. Ecol. 2021, 35, 2691–2706. [Google Scholar] [CrossRef]
- Lathlean, J.A.; Ayre, D.J.; Minchinton, T.E. Rocky Intertidal Temperature Variability along the Southeast Coast of Australia: Comparing Data from in Situ Loggers, Satellite-Derived SST and Terrestrial Weather Stations. Mar. Ecol. Prog. Ser. 2011, 439, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Stobart, B.; Mayfield, S.; Mundy, C.; Hobday, A.J.; Hartog, J.R. Comparison of in Situ and Satellite Sea Surface-Temperature Data from South Australia and Tasmania: How Reliable Are Satellite Data as a Proxy for Coastal Temperatures in Temperate Southern Australia? Mar. Freshw. Res. 2016, 67, 612. [Google Scholar] [CrossRef]
- Lathlean, J.; Seuront, L. Infrared Thermography in Marine Ecology: Methods, Previous Applications and Future Challenges. Mar. Ecol. Prog. Ser. 2014, 514, 263–277. [Google Scholar] [CrossRef]
- Caddy-Retalic, S.; Benkendorff, K.; Faireweather, P.G. Visualizing Hotspots: Applying Thermal Imaging to Monitor Internal Temperatures in Intertidal Gastropods. Molluscan Res. 2011, 31, 106–113. [Google Scholar]
- Chapperon, C.; Seuront, L. Behavioral Thermoregulation in a Tropical Gastropod: Links to Climate Change Scenarios. Glob. Chang. Biol. 2011, 17, 1740–1749. [Google Scholar] [CrossRef]
- Chapperon, C.; Seuront, L. Space-Time Variability in Environmental Thermal Properties and Snail Thermoregulatory Behaviour. Funct. Ecol. 2011, 25, 1040–1050. [Google Scholar] [CrossRef]
- Seuront, L.; Ng, T.P.T.; Lathlean, J.A. A Review of the Thermal Biology and Ecology of Molluscs, and of the Use of Infrared Thermography in Molluscan Research. J. Molluscan Stud. 2018, 84, 203–232. [Google Scholar] [CrossRef]
- Denny, M.W.; Harley, C.D.G. Hot Limpets: Predicting Body Temperature in a Conductance-Mediated Thermal System. J. Exp. Biol. 2006, 209, 2409–2419. [Google Scholar] [CrossRef]
- Harley, C.D.G.; Denny, M.W.; Mach, K.J.; Miller, L.P. Thermal Stress and Morphological Adaptations in Limpets. Funct. Ecol. 2009, 23, 292–301. [Google Scholar] [CrossRef]
- Marshall, D.J.; McQuaid, C.D.; Williams, G.A. Non-Climatic Thermal Adaptation: Implications for Species’ Responses to Climate Warming. Biol. Lett. 2010, 6, 669–673. [Google Scholar] [CrossRef]
- Wethey, D.S. Biogeography, Competition, and Microclimate: The Barnacle Chthamalus Fragilis in New England. Integr. Comp. Biol. 2002, 42, 872–880. [Google Scholar] [CrossRef]
- McMahon, R.F. Thermal Tolerance, Evaporative Water Loss, Air-Water Oxygen Consumption and Zonation of Intertidal Prosobranchs: A New Synthesis. Hydrobiologia 1990, 193, 241–260. [Google Scholar] [CrossRef]
- Bownes, S.J.; McQuaid, C.D. Will the Invasive Mussel Mytilus Galloprovincialis Lamarck Replace the Indigenous Perna perna L. on the South Coast of South Africa? J. Exp. Mar. Biol. Ecol. 2006, 338, 140–151. [Google Scholar] [CrossRef]
- Zardi, G.; McQuaid, C.; Teske, P.; Barker, N. Unexpected Genetic Structure of Mussel Populations in South Africa: Indigenous Perna perna and Invasive Mytilus Galloprovincialis. Mar. Ecol. Prog. Ser. 2007, 337, 135–144. [Google Scholar] [CrossRef]
- Ndhlovu, A.; McQuaid, C.D.; Nicastro, K.R.; Zardi, G.I. Community Succession in Phototrophic Shell-Degrading Endoliths Attacking Intertidal Mussels. J. Molluscan Stud. 2021, 87, eyaa036. [Google Scholar] [CrossRef]
- Helmuth, B.S.T.; Hofmann, G.E. Microhabitats, Thermal Heterogeneity, and Patterns of Physiological Stress in the Rocky Intertidal Zone. Biol. Bull. 2001, 201, 374–384. [Google Scholar] [CrossRef]
- Nicastro, K.R.; Zardi, G.I.; McQuaid, C.D.; Pearson, G.A.; Serrão, E.A. Love Thy Neighbour: Group Properties of Gaping Behaviour in Mussel Aggregations. PLoS ONE 2012, 7, e47382. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing 2022. R Core Team R: Vienna, Austria.
- Simpson, G.L. Gratia: Graceful Ggplot-Based Graphics and Other Functions for GAMs Fitted Using Mgcv. R package version 0.8.1.1. 2022. Available online: https://gavinsimpson.github.io/gratia/ (accessed on 12 February 2022).
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2017; ISBN 978-1-315-37027-9. [Google Scholar]
- Wood, S.N. On p-Values for Smooth Components of an Extended Generalized Additive Model. Biometrika 2013, 100, 221–228. [Google Scholar] [CrossRef]
- Zardi, G.I.; Seuront, L.; McQuaid, C.D.; Froneman, W.; Nicastro, K.R. Symbiont-Induced Phenotypic Variation in an Ecosystem Engineer Mediates Thermal Stress for the Associated Community. J. Therm. Biol. 2023, 112, 103428. [Google Scholar] [CrossRef]
- Fitzhenry, T.; Halpin, P.M.; Helmuth, B. Testing the Effects of Wave Exposure, Site, and Behavior on Intertidal Mussel Body Temperatures: Applications and Limits of Temperature Logger Design. Mar. Biol. 2004, 145, 339–349. [Google Scholar] [CrossRef]
- Helmuth, B.; Choi, F.; Matzelle, A.; Torossian, J.L.; Morello, S.L.; Mislan, K.A.S.; Yamane, L.; Strickland, D.; Szathmary, P.L.; Gilman, S.E.; et al. Long-Term, High Frequency in Situ Measurements of Intertidal Mussel Bed Temperatures Using Biomimetic Sensors. Sci. Data 2016, 3, 160087. [Google Scholar] [CrossRef] [PubMed]
- Gehman, A.M.; Harley, C.D.G. Symbiotic Endolithic Microbes Alter Host Morphology and Reduce Host Vulnerability to High Environmental Temperatures. Ecosphere 2019, 10, e02683. [Google Scholar] [CrossRef] [Green Version]
- McQuaid, C.D.; Branch, G.M.; Frost, P.G.H. Aestivation Behaviour and Thermal Relations of the Pulmonate Theba Pisana in a Semi-Arid Environment. J. Therm. Biol. 1979, 4, 47–55. [Google Scholar] [CrossRef]
- Vermeij, G.J. Morphological Patterns in High-Intertidal Gastropods: Adaptive Strategies and Their Limitations. Mar. Biol. 1973, 20, 319–346. [Google Scholar] [CrossRef]
- Etter, R.J. Physiological Stress and Color Polymorphism in the Intertidal Snail Nucella Lapillus. Evolution 1988, 42, 660–680. [Google Scholar] [CrossRef]
- Geen, M.R.S.; Johnston, G.R. Coloration Affects Heating and Cooling in Three Color Morphs of the Australian Bluetongue Lizard, Tiliqua Scincoides. J. Therm. Biol. 2014, 43, 54–60. [Google Scholar] [CrossRef]
- Hayworth, A.M.; Quinn, J.F. Temperature of Limpets in the Rocky Intertidal Zone: Effects of Caging and Substratum. Limnol. Oceanogr. 1990, 35, 967–970. [Google Scholar] [CrossRef]
- Harper, K.D.; Williams, G.A. Variation in Abundance and Distribution of the Chiton Acanthopleura Japonica and Associated Molluscs on a Seasonal, Tropical, Rocky Shore. J. Zool. 2001, 253, 293–300. [Google Scholar] [CrossRef]
- Chapperon, C.; Studerus, K.; Clavier, J. Mitigating Thermal Effect of Behaviour and Microhabitat on the Intertidal Snail Littorina Saxatilis (Olivi) over Summer. J. Therm. Biol. 2017, 67, 40–48. [Google Scholar] [CrossRef]
- Schönberg, C.H.L.; Wisshak, M. Marine Bioerosion. In The Mediterranean Sea; Goffredo, S., Dubinsky, Z., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 449–461. ISBN 978-94-007-6703-4. [Google Scholar]
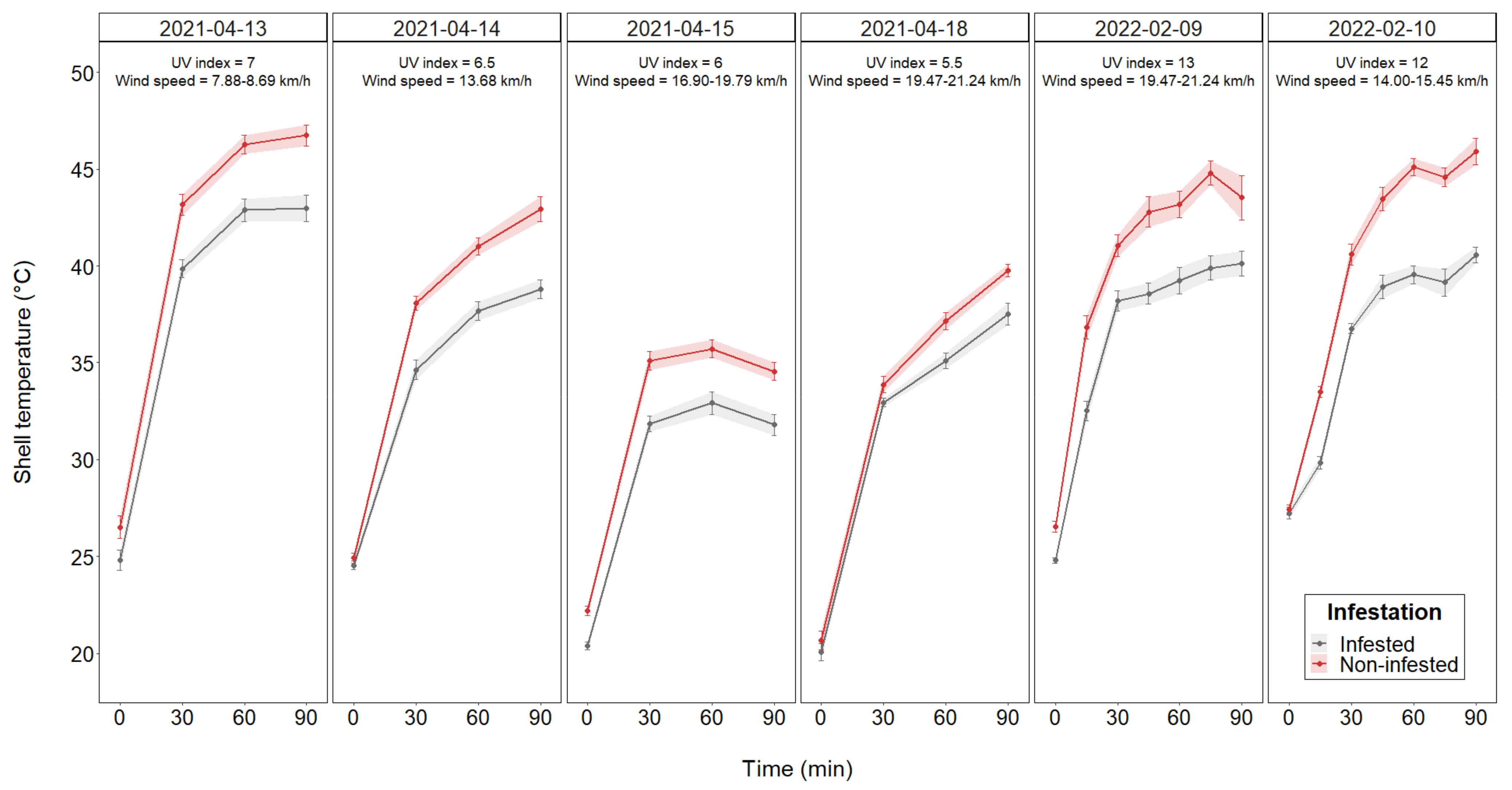
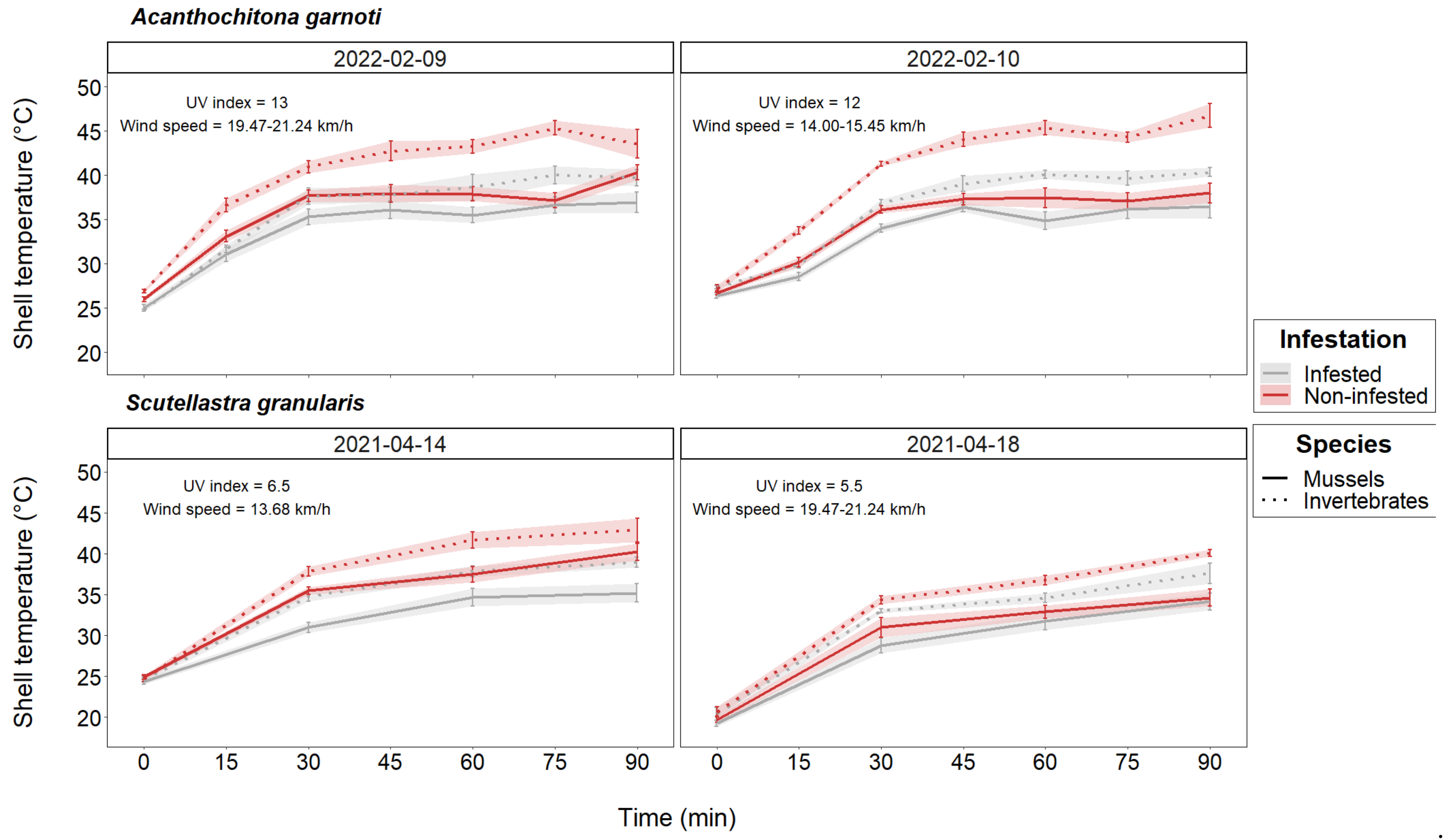

| Date | Infaunal Species | Size Range (mm) 1 | UV Index 2 | Air Temperature 3 (°C) | Humidity 3 (%RH) | Pressure 4 (×103 Pa) | Wind Speed 4 (km.h−1) | Wind Direction 4 |
|---|---|---|---|---|---|---|---|---|
| 13 April 2021 | Helcion pectunculus | 18–23 | 7 | 26.11–27.78 | 38–43 | 96.2–96.5 | 7.88–8.69 | S-E |
| Oxystele antoni | 13–16 | |||||||
| 14 April 2021 | Scutellastra granularis | 20–30 | 6.5 | 22.78–25.00 | 45–55 | 96.2–96.5 | 13.68 | S-W |
| 15 April 2021 | Helcion pectunculus | 18–25 | 6 | 22.22–22.78 | 41–44 | 95.8 | 16.90–19.79 | S-W |
| Oxystele antoni | 13–16 | |||||||
| 18 April 2021 | Scutellastra granularis | 20–30 | 5.5 | 25.00–27.78 | 11 | 95.8 | 19.47–21.24 | N-W |
| 9 February 2022 | Acanthochitona garnoti | 19–25 | 13 | 25.00 | 47–49 | 95.2 | 19.47–21.24 | S |
| 10 February 2022 | Acanthochitona garnoti | 19–25 | 12 | 25.00–26.11 | 43 | 95.5 | 14.00–15.45 | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dievart, A.M.; McQuaid, C.D.; Zardi, G.I.; Nicastro, K.R.; Froneman, P.W. Euendolithic Infestation of Mussel Shells Indirectly Improves the Thermal Buffering Offered by Mussel Beds to Associated Molluscs, but One Size Does Not Fit All. Diversity 2023, 15, 239. https://doi.org/10.3390/d15020239
Dievart AM, McQuaid CD, Zardi GI, Nicastro KR, Froneman PW. Euendolithic Infestation of Mussel Shells Indirectly Improves the Thermal Buffering Offered by Mussel Beds to Associated Molluscs, but One Size Does Not Fit All. Diversity. 2023; 15(2):239. https://doi.org/10.3390/d15020239
Chicago/Turabian StyleDievart, Alexia M., Christopher D. McQuaid, Gerardo I. Zardi, Katy R. Nicastro, and Pierre W. Froneman. 2023. "Euendolithic Infestation of Mussel Shells Indirectly Improves the Thermal Buffering Offered by Mussel Beds to Associated Molluscs, but One Size Does Not Fit All" Diversity 15, no. 2: 239. https://doi.org/10.3390/d15020239
APA StyleDievart, A. M., McQuaid, C. D., Zardi, G. I., Nicastro, K. R., & Froneman, P. W. (2023). Euendolithic Infestation of Mussel Shells Indirectly Improves the Thermal Buffering Offered by Mussel Beds to Associated Molluscs, but One Size Does Not Fit All. Diversity, 15(2), 239. https://doi.org/10.3390/d15020239