Evaluation of the Presence of Arbuscular Mycorrhizae and Cadmium Content in the Plants and Soils of Cocoa Plantations in San Martin, Peru
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Area of Study
2.2. Sampling Description: Rhizosphere and Plant Analysis
2.3. Soil Analysis
2.4. Installation of Trap Pots
2.5. Vital Root Staining and Quantification of AMF Colonization
2.6. Determination of Glomalin-Related Soil Proteins and Bradford Method
2.7. DNA Extraction of Glomeromycota Fungi
2.8. PCR, Cloning and Sanger Sequencing of Glomeromycota Fungi
2.9. Phylogenetics of Glomeromycota Fungi
2.10. Analysis of Spore Morphotypes
2.11. Statistical Analysis
3. Results
3.1. Soil Chemical Analysis
3.2. Cd in Soil, Cocoa Leaves, and Seeds
3.3. Quantification of Glomeromycota Spores and Arbuscular Mycorrhizal Fungi Root Colonization
3.4. About Easily Extractable GSRP and Total GSRP
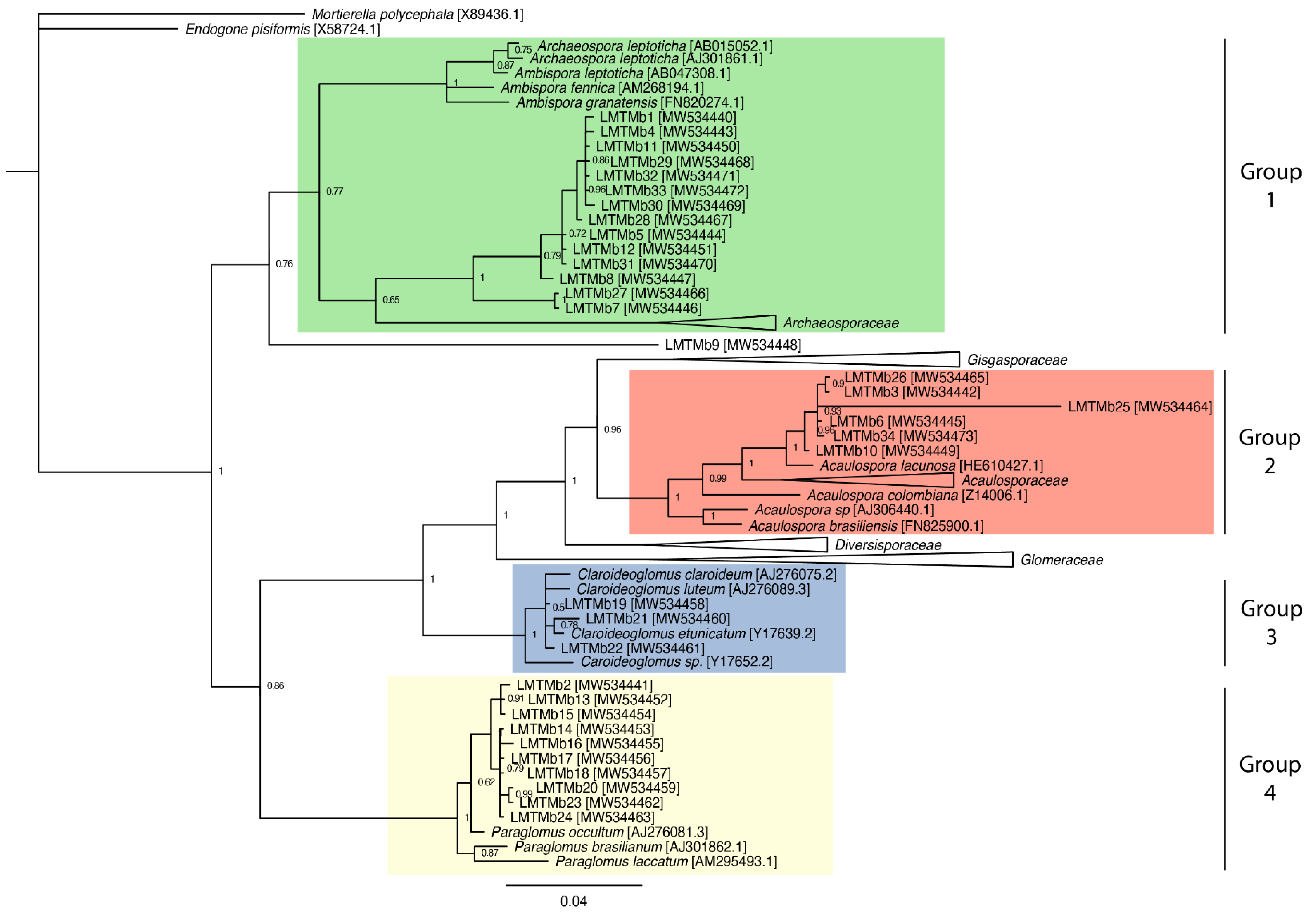
3.5. Phylogenetic Analysis of the Glomeromycota Fungi Found in the Cocoa Plantations Studied
3.6. Glomeromycota Morphotypes in Cocoa Plantations
3.7. Correlations between Biological Variables Measured
4. Discussion
4.1. Soil Cd Content
4.2. Foliar and Seeds Cd Content
4.3. Arbuscular Mycorrhizal Fungi Root Colonization
4.4. Glomalin Related Soil Proteins
4.5. Phylogenetic Analysis of Glomeromycota Fungi Found in the Cocoa Plantations Studied
4.6. Glomeromycota Morphotypes in Cocoa Plantations
4.7. Correlations between Biological Variables
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Cocoa Field | Lab Sequence | Closest Molecular Identification Using 18S rDNA Gene | Accession Number | Similarity (%) |
|---|---|---|---|---|
| 10 years | LMTMb1 | Glomeromycotina spp. | MW534440 | 98.75% |
| 10 years | LMTMb2 | Uncultured Glomus | MW534441 | 99.62% |
| 10 years | LMTMb3 | Uncultured Acaulospora | MW534442 | 99.62% |
| 10 years | LMTMb4 | Glomeromycotina spp. | MW534443 | 98.62% |
| 10 years | LMTMb5 | Glomeromycotina spp. | MW534444 | 98.62% |
| 10 years | LMTMb6 | Uncultured Acaulospora | MW534445 | 99.75% |
| 10 years | LMTMb7 | Glomeromycotina spp. | MW534446 | 97.60% |
| 10 years | LMTMb8 | Glomeromycotina spp. | MW534447 | 98.24% |
| 10 years | LMTMb9 | Glomeromycotina spp. | MW534448 | 98.12% |
| 10 years | LMTMb10 | Uncultured Glomeromycotina | MW534449 | 99.62% |
| 10 years | LMTMb11 | Glomeromycotina spp. | MW534450 | 98.74% |
| 10 years | LMTMb12 | Glomeromycotina spp. | MW534451 | 98.62% |
| 3 years | LMTMb13 | Uncultured Glomus | MW534452 | 99.75% |
| 3 years | LMTMb14 | Uncultured Glomus | MW534453 | 100.00% |
| 3 years | LMTMb15 | Uncultured Glomus | MW534454 | 99.75% |
| 3 years | LMTMb16 | Uncultured Glomus | MW534455 | 100.00% |
| 3 years | LMTMb17 | Uncultured Glomus | MW534456 | 100.00% |
| 3 years | LMTMb18 | Uncultured Glomus | MW534457 | 100.00% |
| 3 years | LMTMb19 | Uncultured Claroideoglomus | MW534458 | 100.00% |
| 3 years | LMTMb20 | Uncultured Glomus | MW534459 | 99.87% |
| 3 years | LMTMb21 | Uncultured Claroideoglomus | MW534460 | 100.00% |
| 3 years | LMTMb22 | Uncultured Glomus | MW534461 | 100.00% |
| 3 years | LMTMb23 | Uncultured Glomus | MW534462 | 99.87% |
| 3 years | LMTMb24 | Uncultured Glomus | MW534463 | 100.00% |
| 10 years | LMTMb25 | Uncultured Glomus | MW534464 | 100.00% |
| 10 years | LMTMb26 | Uncultured Acaulospora | MW534465 | 99.62% |
| 10 years | LMTMb27 | Glomeromycotina spp. | MW534466 | 97.60% |
| 10 years | LMTMb28 | Glomeromycotina spp. | MW534467 | 98.87% |
| 10 years | LMTMb29 | Glomeromycotina spp. | MW534468 | 98.74% |
| 10 years | LMTMb30 | Glomeromycotina spp. | MW534469 | 98.62% |
| 10 years | LMTMb31 | Glomeromycotina spp. | MW534470 | 98.73% |
| 10 years | LMTMb32 | Glomeromycotina spp. | MW534471 | 98.85% |
| 10 years | LMTMb33 | Glomeromycotina spp. | MW534472 | 98.85% |
| 10 years | LMTMb34 | Uncultured Acaulospora | MW534473 | 99.62% |
References
- Arévalo-Gardini, E.; Arévalo-Hernández, C.; Baligar, V.; He, Z. Heavy metal accumulation in leaves and beans of cacao (Theobroma cacao L.) in major cacao growing regions in Peru. Sci. Total Environ. 2017, 605, 792–800. [Google Scholar] [CrossRef]
- MIDAGRI. Ministerio de Desarrollo Agrario y Riego. 2022. Available online: https://www.gob.pe/institucion/midagri/noticias/305143-produccion-nacional-de-cacao-en-grano-crecio-en-la-ultima-decada-a-un-promedio-de-12-6-al-ano (accessed on 7 October 2022).
- Ramtahal, G.; Yen, I.C.; Bekele, I.; Bekele, F.; Wilson, L.; Maharaj, K.; Harrynanan, L. Relationships between cadmium in tissues of cacao trees and soils in plantations of Trinidad and Tobago. Food Nutr. Sci. 2016, 7, 37–43 . [Google Scholar] [CrossRef]
- Manton, W.I. Nonnutritive constituents in chocolate and cocoa. In Chocolate in Health and Nutrition; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Humana Press: New York, NY, USA, 2013; pp. 73–87. [Google Scholar] [CrossRef]
- Rojas Briceño, N.B.; Oliva-Cruz, M.; Rascón, J. Idoneidad del territorio para el cultivo sostenible de cacao (Theobroma cacao L.) según presencia de cadmio en suelos de Amazonas. Rev. Investig. Agroproducción Sustentable 2021, 5, 77–85 . [Google Scholar] [CrossRef]
- The European Commission. Commission regulation (EU) No 488/2014 of 12 May 2014 amending regulation (EC) No 1881/2006 as regards maximum levels of cadmium in foodstuffs. Off. J. Eur. Union 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R0488 (accessed on 1 May 2020).
- Florida Rofner, N. Revisión sobre límites máximos de cadmio en cacao (Theobroma cacao L.). Granja. Rev. Cienc. Vida 2021, 34, 117–130. [Google Scholar] [CrossRef]
- Chavez, E.; He, Z.L.; Stoffella, P.J.; Mylavarapu, R.S.; Li, Y.C.; Moyano, B.; Baligar, V.C. Concentration of cadmium in cacao beans and its relationship with soil cadmium in southern Ecuador. Sci. Total Environ. 2015, 533, 205–214 . [Google Scholar] [CrossRef]
- Huamani-Yupanqui, H.A.; Huauya-Rojas, M.A.; Mansilla-Minaya, L.G.; Florida-Rofner, N.; Meira-Trujillo, G.M. Presencia de metales pesados en cultivo de cacao (Theobroma cacao L.) orgánico. Acta Agronómica 2012, 61, 339–344. [Google Scholar]
- Oliva, M.; Rubio, K.; Epquin, M.; Marlo, G.; Leiva, S. Cadmium Uptake in Native Cacao Trees in Agricultural Lands of Bagua, Peru. Agronomy 2020, 10, 1551 . [Google Scholar] [CrossRef]
- Arévalo-Gardini, E.; Obando-Cerpa, M.E.; Zúñiga-Cernades, L.B.; Arévalo-Hernández, C.O.; Baligar, V.; He, Z. Metales pesados en suelos de plantaciones de cacao (Theobroma cacao L.) en tres regiones del Perú. Ecol. Apl. 2016, 15, 81–89. [Google Scholar] [CrossRef]
- Meier, S.; Borie, F.; Bolan, N.; Cornejo, P. Phytoremediation of metal-polluted soils by arbuscular mycorrhizal fungi. Crit. Rev. Environ. Sci. Technol. 2012, 42, 741–775 . [Google Scholar] [CrossRef]
- Audet, P. Arbuscular mycorrhizal fungi and metal phytoremediation: Ecophisiological complementarity in relation to environmental stress. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 133–159. [Google Scholar] [CrossRef]
- Hristozkova, M.; Geneva, M.; Stancheva, I.; Iliev, I.; Azcon-Aguilar, C. Symbiotic association between golden berry (Physalis peruviana) and arbuscular mycorrhizal fungi in heavy metal-contaminated soil. J. Plant Prot. Res. 2017, 57, 173–184. [Google Scholar] [CrossRef]
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265. [Google Scholar] [CrossRef]
- Toro, M.; Gamarra, R.; López, L.; Infante, C. Arbuscular mycorrhizal fungi and the remediation of soils contaminated with hydrocarbons. In Chemical Pollution Control via Microorganisms; Anjum, N., Ed.; Nova Science Publishers: New York, NY, USA, 2017; pp. 79–96. [Google Scholar]
- Janeeshma, E.; Puthur, J.T. Direct and indirect influence of arbuscular mycorrhizae on enhancing metal tolerance of plants. Arch. Microbiol. 2020, 202, 1–16. [Google Scholar] [CrossRef]
- Vlček, V.; Pohanka, M. Glomalin—An interesting protein part of the soil organic matter. Soil Water Res. 2020, 15, 67–74 . [Google Scholar] [CrossRef]
- Landa-Acuña, D.; Acosta, R.A.S.; Hualpa-Cutipa, E.; Vargas de la Cruz, C.; Luis-Alaya, B. Bioremediation: A Low-Cost and Clean-Green Technology for Environmental Management. In Microbial Bioremediation & Biodegradation; Shah, M., Ed.; Springer: Singapore; Bharuch, Gujarat, India, 2020; pp. 153–171. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Panda, S.K.; Bhattacharjee, M.K.; Dutta, S. Role of arbuscular mycorrhiza in heavy metal tolerance in plants: Prospects for phytoremediation. J. Phytol. 2010, 2, 16–27. [Google Scholar]
- Laycock, D.H. Preliminary investigations into the function of the endotrophic mycorrhiza of Theobroma cacao L. Trop. Agric. 1945, 22, 77–80. [Google Scholar]
- Kähkölä, A.H.; Nygren, P.; Leblanc, H.; Pennanen, T.; Pietikainen, J. Leaf and root litter of a legume tree as nitrogen sources for cacaos with different root colonisation by arbuscular mycorrhizae. Nutr. Cycl. Agroecosyst. 2012, 92, 51–65 . [Google Scholar] [CrossRef]
- Gramlich, A.; Tandy, S.; Andres, C.; Chincheros-Paniagua, J.; Armengot, L.; Schneider, M.; Schulin, R. Cadmium uptake by cocoa trees in agroforestry and monoculture systems under conventional and organic management. Sci. Total Environ. 2016, 580, 677–687. [Google Scholar] [CrossRef]
- Sandoval-Pineda, J.F.; Pérez-Moneada, U.A.; Rodriguez, A.; Torres-Rojas, E. Alta presencia de cadmio resulta en baja diversidad de hongos formadores de micorrizas arbusculares asociados a cacao (Theobroma cacao L.). Acta Biológica Colomb. 2020, 25, 333–344. [Google Scholar] [CrossRef]
- Suparno, A.; Yahya, S.; Sudradjat; Setiadi, Y.; Idris, K. Arbuscular Mycorrhizal Fungi Increase Growth of Cocoa Seedlings Applied with Papuan Crandallite Phosphate Rock. Eur. J. Sci. Res. 2014, 127, 260–268. [Google Scholar]
- ICAR-Central Plantation Crops Research Institute (CPCRI). Nursery Manual on Cocoa; CPCRI Technical Bulletin No. 109: Kasaragod, India, 2016; pp. 1–20. [Google Scholar]
- Iglesias, L.; Salas, E.; Leblanc, H.A.; Nygren, P. Response of Theobroma cacao and Inga edulis seedlings to cross-inoculated populations of arbuscular mycorrhizal fungi. Agroforest. Syst. 2011, 83, 63–73 . [Google Scholar] [CrossRef]
- Torres-Arias, Y.; Ortega Fors, R.; Nobre, C.; Furrazola Gómez, E.; Berbara, R. Production of native arbuscular mycorrhizal fungi inoculum under different environmental conditions. Braz. J. Microbiol. 2017, 48, 87–94 . [Google Scholar] [CrossRef] [PubMed]
- Cornejo, P.; Perez, T.J.; Meier, S.; Valderas, A.; Borie, F.; Azcon, A.C.; Ferrol, N. Copper compartmentalization in spores as a survival strategy of arbuscular mycorrhizal fungi in Cu-polluted environments. Soil. Biol. Biochem. 2013, 57, 925–928 . [Google Scholar] [CrossRef]
- Ferrol, N.; Gonzalez Guerrero, M.; Valderas, A.; Benabdellah, K.; Azcón-Aguilar, C. Survival strategies of arbuscular mycorrhizal fungi in Cu-polluted environments. Phytochem. Rev. 2009, 8, 551–559. [Google Scholar] [CrossRef]
- Acero, L.A.L. Sostenibilidad y cadenas agroproductivas de cacao en el Perú perspectivas desde las regiones Piura y San Martín. Nova Rev. Amaz. 2020, 8, 179–189. [Google Scholar] [CrossRef]
- Jaimez, R.E.; Barragan, L.; Fernández-Niño, M.; Wessjohann, L.A.; Cedeño-Garcia, G.; Sotomayor Cantos, I.; Arteaga, F. Theobroma cacao L. cultivar CCN-51: A comprehensive review on origin, genetics, sensory properties, production dynamics, and physiological aspects. PeerJ 2022, 10, e12676. [Google Scholar] [CrossRef]
- McNear, D.H., Jr. The Rhizosphere—Roots, Soil and Everything In Between. Nat. Educ. Knowl. 2013, 4, 1. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and proposed modification on the chromic acid titration method. Soil. Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis, 2nd ed.; Parallel Press: Madison, WI, USA, 1985. [Google Scholar]
- Yuan, T.L.; Fiskell, J.G.A. Aluminum studies. II. The extraction of aluminum from some Florida soils. Soil Science Society of America. Proceedings 1959, 23, 202–205. [Google Scholar] [CrossRef]
- EPA US. SW-846 Test Method 3052: Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; US EPA: Washington, DC, USA, 1996.
- Gerdemann, J.; Nicolson, T.H. Spores Of Mycorrhizal Endogone Species Extracted From Soil By Wet Sieving And Decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Marques, G.; Tampakaki, A.; Alsina, I. Working with Microbial Symbioses of Legumes: Handbook of Protocols; FP7 Research Project No. 613781; Eurolegume: Vila Real, Portugal, 2014. [Google Scholar]
- Schaffer, G.F.; Peterson, R.L. Modifications to clearing methods used in combination with vital staining of roots colonized with vesicular-arbuscular mycorrhizal fungi. Mycorrhiza 1993, 4, 29–35 . [Google Scholar] [CrossRef]
- Giovanetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular-arbuscular infection in roots. New Phytol. 1980, 84, 489–500 . [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A Survey of Soils for Aggregate Stability and Glomalin, a Glycoprotein Produced by Hyphae of Arbuscular Mycorrhizal Fungi. Plant Soil. 1998, 198, 97–107 . [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantification of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254 . [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, C.; Huang, L.; Ban, Y.; Tang, M. The effects of arbuscular mycorrhizal fungi on glomalin-related soil protein distribution, aggregate stability and their relationships with soil properties at different soil depths in lead-zinc contaminated area. PLoS ONE 2017, 12, e0182264. [Google Scholar] [CrossRef] [PubMed]
- Faggioli, V.S.; Cabello, M.N.; Grilli, G.; Vasar, M.; Covacevich, F.; Öpik, M. Root colonizing and soil borne communities of arbuscular mycorrhizal fungi differ among soybean fields with contrasting historical land use. Agric. Ecosyst. Environ. 2019, 269, 174–182. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Young, J.P. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 2008, 65, 339–349. [Google Scholar] [CrossRef]
- Opik, M.; Vanatoa, A.; Vanatoa, E.; Moora, M.; Davison, J.; Kalwij, J.M.; Reier, U.; Zobel, M. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 2010, 188, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molec. Biol. Evol. 2007, 24, 1596–1599 . [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574 . [Google Scholar] [CrossRef]
- Schenck, N.; Perez, Y. Manual for the Identification of VA Mycorrhizal Fungi, 3rd ed.; Sinergistic Publications: Gainesville, FL, USA, 1990; pp. 1–286. [Google Scholar]
- Ministerio de Agricultura. Programa Para el Desarrollo de la Amazonía. Caracterización de Las Zonas Productoras de Cacao en el Perú y su Competitividad. Informe Final, Lima, Perú. 2003. Available online: http://infocafes.com/portal/wp-content/uploads/2016/03/cacao_completo.pdf (accessed on 8 October 2022).
- MINAM. Ministerio del Ambiente. Decreto Supremo N° 011-2017-MINAM. Estándares de Calidad Ambiental (ECA) Para Suelo. 2017. Available online: https://sinia.minam.gob.pe/normas/aprueban-estandares-calidad-ambiental-eca-suelo-0 (accessed on 7 October 2022).
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: San Diego, CA, USA, 2008; pp. 42–90. [Google Scholar]
- Emran, M.; Gispert, M.; Pardini, G. Patterns of soil organic carbon, glomalin and structural stability in abandoned Mediterranean terraced lands. Eur. J. Soil Sci. 2012, 63, 637–649 . [Google Scholar] [CrossRef]
- Kumar, S.; Kumar Singh, A.; Ghosh, P. Distribution of soil organic carbon and glomalin related soil protein in reclaimed coal mine-land chronosequence under tropical condition. Sci. Total Environ. 2018, 625, 1341–1350 . [Google Scholar] [CrossRef] [PubMed]
- Zug, K.L.M.; Huamaní Yupanqui, H.A.; Meyberg, F.; Cierjacks, J.S.; Cierjacks, A.; Huamaní; Yupanqui, H.A.; Meyberg, F.; Cierjacks, J.S.; Cierjacks, A. Cadmium Accumulation in Peruvian Cacao (Theobroma cacao L.) and Opportunities for Mitigation. Water Air Soil Pollut. 2019, 230, 72. [Google Scholar] [CrossRef]
- Meter, A.; Atkinson, R.J.; Laliberte, B. Cadmio en el Cacao de América Latina y el Caribe—Análisis de la Investigación y Soluciones Potenciales Para la Mitigación; Bioversity International: Rome, Italy, 2019. [Google Scholar]
- MacDonald, R.M.; Lewis, M. The occurrences of some acid phosphatases and dehydrogenases in the vesicular-arbuscular mycorrhizal fungus Glomus mosseae. New Phytol. 1978, 80, 135–141. [Google Scholar] [CrossRef]
- Cuenca, G.; Meneses, E. Diversity patterns of arbuscular mycorrhizal fungi associated with cacao in Venezuela. Plant Soil. 1996, 183, 315–322 . [Google Scholar] [CrossRef]
- Pérez Moncada, A.U.; Gómez Ramírez, M.; Ordoñez Serralde, D.P.; Peñaranda Rolón, M.A.; Wilches Ortiz, A.W.; Ramírez, L.; Rengifo Estrada, A.G. Arbuscular mycorrhizal fungi (AMF) as a strategy to reduce the absorption of cadmium in cocoa (Theobroma cacao) plants. Terra Latinoam. 2019, 37, 121–130 . [Google Scholar] [CrossRef]
- Gadkar, V.; Rillig, M. The arbuscular mycorrhizal fungal protein glomalin is a putative 16 homolog of heat shock protein 60. FEMS Microbiol. Lett. 2006, 263, 93–101. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, M.; Tripathi, B.N. Glomalin: An arbuscular mycorrhizal fungal soil protein. Protoplasma 2013, 250, 663–669. [Google Scholar] [CrossRef]
- Nichols, K. Characterization of Glomalin a Glycoprotein Produced by Arbuscular Mycorrhizal Fungi. Doctoral Dissertation, University of Maryland, College Park, MD, USA, 2003. [Google Scholar]
- Chern, E.C.; Tsai, D.W.; Ogunseitan, O.A. Deposition of glomalin-related soil protein and sequestered toxic metals into watersheds. Environ. Sci. Technol. 2007, 41, 3566–3572. [Google Scholar] [CrossRef]
- Vodnik, D.; Grcman, H.; Macek, I.; van Elteren, J.T.; Kovacevic, M. The contribution of glomalin related soil protein to Pb and Zn sequestration in polluted soil. Sci. Total Environ. 2008, 392, 130–136. [Google Scholar] [CrossRef]
- Cornejo, P.; Meier, S.; Borie, G.; Rillig, M.; Borie, F. Glomalin-related soil protein in a Mediterranean ecosystem affected by a copper smelter and its contribution to Cu and Zn sequestration. Sci. Total Environ. 2008, 406, 154–160 . [Google Scholar] [CrossRef]
- Gonzalez-Chávez, M.C.; Carrillo-González, R.; Gutierrez-Castorena, M.C. Natural attenuation in a slag heap contaminated with cadmium: The role of plants and arbuscular mycorrhizal fungi. J. Hazard. Mater. 2009, 161, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Zhu, X.; Chen, C.; Wu, J.; Yang, B.; Zakari, S.; Jiang, X.J.; Sing, N.; Liu, W. The role of glomalin in mitigation of multiple soil degradation problems. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1604–1638 . [Google Scholar] [CrossRef]
- Ramtahal, G.; Chang Yen, I.; Seegobin, D.; Bekele, I.; Bekele, F.; Wilson, L.; Harrynanan, L. Investigation of the effects of mycorrhizal fungi on cadmium accumulation in cacao. Proc. Caribb. Food Crops Soc. 2012, 48, 147–152 . [Google Scholar] [CrossRef]
- Khan, A.G. Mycorrhizoremediation—An enhanced form of phytoremediation. Zhejiang Univ. Sci. B 2006, 7, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.B.; Redecker, D. Two new families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 2001, 93, 181–195. [Google Scholar] [CrossRef]
- Schüßler, A.; Walker, C. Archaeospora ecuadoriana sp. nov. from a mountainous biodiversity hotspot area in Ecuador, and transfer of Palaeospora spainiae to Archaeospora, as A. spainiae comb. nov. Mycorrhiza 2019, 29, 435–443 . [Google Scholar] [CrossRef]
- Stürmer, S.L.; Kemmelmeier, K. The Glomeromycota in the Neotropics. Front. Microbiol. 2021, 11, 553679. [Google Scholar] [CrossRef]
- Rimington, W.R.; Pressel, S.; Duckett, J.; Field, K.; Read, D.J.; Bidartondo, M. Ancient plants with ancient fungi: Liverworts associate with early-diverging arbuscular mycorrhizal fungi. Proc. R. Soc. B 2018, 285, 20181600. [Google Scholar] [CrossRef]
- Desiro, A.; Duckett, J.G.; Pressel, S.; Villarreal, J.C.; Bidartondo, M.I. Fungal symbioses in hornworts: A chequered history. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130207. [Google Scholar] [CrossRef]
- Haug, I.; Setaro, S.; Suárez, J. Reforestation sites show similar and nested AMF communities to an adjacent pristine forest in a tropical mountain area of South Ecuador. PLoS ONE 2013, 8, e63524. [Google Scholar] [CrossRef]
- Corazon-Guivin, M.; Vallejos-Tapullima, A.; De la Sota-Ricaldi, A.; Vallejos-Torres, G.; Ruíz-Sánchez, M.; Santos, V.M.; Alves da Silva, G.; Oehl, F. Acaulospora flavopapillosa, a new fungus in the Glomeromycetes from a coffee plantation in Peru, with an updated key for the identification of Acaulosporaceae species. J. Appl. Bot. Food Qual. 2022, 95, 6–16. [Google Scholar] [CrossRef]
- Corazon-Guivin, M.A.; Cerna-Mendoza, A.; Guerrero, A.J.C.; Vallejos-Tapullima, A.; Ríos, O.; Vallejos Torres, G.; De la Sota-Ricaldi, A.M.; Santos, V.M.; Da Silva, G.A.; Oehl, F. Paraglomus occidentale, a new arbuscular mycorrhizal fungus from the sources of the Amazon river in Peru, with a key to the Paraglomeromycetes species. Sydowia 2020, 72, 85–94. [Google Scholar] [CrossRef]
- Simonoff-Smith, A. Changes in Arbuscular Mycorrhizal Diversity during Restoration of a Riparian Forest. Master’s Thesis., California State University, State of California, Long Beach, CA, USA, 2018. [Google Scholar]
- Alguacil, M.M.; Torrecillas, E.; Lozano, Z.; Roldán, A. Arbuscular mycorrhizal fungi communities in a coral cay system (Morrocoy, Venezuela) and their relationships with environmental variables. Sci. Total Environ. 2015, 505, 805–813. [Google Scholar] [CrossRef]
- Haug, I.; Setaro, S.; Suárez, J.P. Species Composition of Arbuscular Mycorrhizal Communities Changes with Elevation in the Andes of South Ecuador. PLoS ONE 2019, 14, e0221091 . [Google Scholar] [CrossRef]
- Bruns, T.D.; Corradi, N.; Redecker, D.; Taylor, J.W.; Öpik, M. Glomeromycotina: What is a species and why should we care? New Phytol. 2018, 220, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, A.A.; Gorbunova, A.O.; Machs, E.M.; Mikhaylova, Y.V.; Rodionov, A.V.; Zhurbenko, P.M.; Yurkov, A.P. Perspectives of using Illumina MiSeq for identification of arbuscular mycorrhizal fungi. Vavilov J. Genet. Breed. 2020, 24, 158. [Google Scholar] [CrossRef] [PubMed]
- Crossay, T.; Antheaume, C.; Redecker, D.; Bon, L.; Chedri, N.; Richert, C.; Guentas, L.; Cavaloc, Y.; Amir, H. New method for the identification of arbuscular mycorrhizal fungi by proteomic-based biotyping of spores using MALDI-TOF-MS. Sci. Rep. 2017, 7, 14306 . [Google Scholar] [CrossRef]
- Vestberg, M.V.; Assefa, F. Diversty and abundance of arbuscular mycorrhizal fungi across different land use types in a humid low land area of Ethiopia. Trop. Subtrop. Agroecosystems 2015, 18, 47–69. [Google Scholar]
- Pacheco Flores de Valgaz, A.; Naranjo-Morán, J.; Reyes Román, G.; Oviedo-Anchundia, J.; RattiTorres, M.; Barcos-Arias, M. Discovering the Diversity of Arbuscular Mycorrhizal Fungi Associated with Two Cultivation Practices of Theobroma cacao. Diversity 2022, 14, 651 . [Google Scholar] [CrossRef]
- Kramadibrata, K. Glomeromycota recovered from cacao soil. Reinwardtia 2009, 12, 357–371. [Google Scholar] [CrossRef]
- Edy, N.; Zakaria, E.K.; Lakani, I.; Hasriyanti. Forest conversion into cacao agroforestry and cacao plantation change the diversity of arbuscular mycorrhizal fungi. Earth Environ. Sci. 2019, 270, 012015. [Google Scholar] [CrossRef]
- Nurhalisyah, P.R.; Chainarong, R.C.; Kaewgrajang, T. Role of arbuscular mycorrhizal fungi (AMF) in cocoa (Theobroma cacao L.) seedlings growth. Khon Kaen Agr. J. 2020, 48, 923–932. [Google Scholar] [CrossRef]
- Rincón, C.; Droh, G.; Villard, L.; Masclaux, F.G.; N’guetta, A.; Zeze, A.; Sanders, I.R. Hierarchical spatial sampling reveals factors influencing arbuscular mycorrhizal fungus diversity in Côte d’Ivoire cocoa plantations. Mycorrhiza 2020, 31, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Tchabi, A.; Coyne, D.; Hountondji, F.; Lawouin, L.; Wiemken, A.; Oehl, F. Arbuscular mycorrhizal fungal communities in sub-Saharan Savannas of Benin, West Africa, as affected by agricultural land use intensity and ecological zone. Mycorrhiza 2008, 18, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Bedini, S.; Pellegrino, E.; Avio, L.; Pellegrini, S.; Bazzoffi, P.; Argese, E.; Giovannetti, M. Changes in soil aggregation and glomalin-related soil protein content as affected by the arbuscular mycorrhizal fungal species Glomus mosseae and Glomus intraradices. Soil Biol. Biochem. 2009, 41, 491–1496. [Google Scholar] [CrossRef]
- Brundrett, M.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. Working with Mycorrhizas in Forestry and Agriculture. AClAR Monograph 1996, 32, 374. [Google Scholar] [CrossRef]
- Liang, S.; Zheng, F.; Fathi, A.E.; Muthuramalingam, P.; Wu, Q.; Hashem, A. Spatial changes of arbuscular mycorrhizal fungi in peach and their correlation with soil properties. Saudi J. Biol. Sci. 2021, 28, 6495–6499. [Google Scholar] [CrossRef]


| Cocoa Field | pH | Salinity | Organic Matter | P | K | Al | Pb | Cd | Cr | Cd Leaves | Cd Seeds |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (dS/m) | (%) | (mg/kg) | |||||||||
| 3 years | 3.88 | 0.18 | 2.32 ** | 2.3 * | 54 * | 1.2 | 17.64 | 0.86 | 12.24 | - | - |
| 9 years | 4.21 | 0.12 | 3.56 ** | 13.5 *** | 72 * | 0.65 | 14.18 | 1.09 | 12.96 | 1.58 | 0.3 |
| 10 years | 4.71 | 0.1 | 2.36 ** | 22.7 *** | 71 * | 0.2 | 15.69 | 0.37 | 10.04 | 2.00 | 0.63 |
| Cocoa Field | Number of Spores/100 g Dry Soil | Number of Inactive Hyphae | Number of Active Hyphae | Number of Coils | Number of Active Arbuscules | Number of Vesicles | Total Active Mycorrhizal Colonization (%) | Total Mycorrhizal Colonization (%) | GRSP-EE Content (mg/g Dry Soil) | GRSP-T Content (mg/g Dry Soil) |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 years | 131.8 a | 30 a | 13 a | 9 * | 19 a | 0 | 59 a | 77 a | 8.30 a | 9.58 a |
| 9 years | 65.6 b | 33 a | 18 a | 13 * | 33 a | 9 * | 61 a | 86 a | 8.70 a | 13.75 b |
| 10 years | 13.4 b | 21 a | 19 a | 4 * | 28 a | 0 | 63 a | 68 a | 7.67 a | 12.83 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luis-Alaya, B.; Toro, M.; Calsina, R.; Ogata-Gutiérrez, K.; Gil-Polo, A.; Ormeño-Orrillo, E.; Zúñiga-Dávila, D. Evaluation of the Presence of Arbuscular Mycorrhizae and Cadmium Content in the Plants and Soils of Cocoa Plantations in San Martin, Peru. Diversity 2023, 15, 246. https://doi.org/10.3390/d15020246
Luis-Alaya B, Toro M, Calsina R, Ogata-Gutiérrez K, Gil-Polo A, Ormeño-Orrillo E, Zúñiga-Dávila D. Evaluation of the Presence of Arbuscular Mycorrhizae and Cadmium Content in the Plants and Soils of Cocoa Plantations in San Martin, Peru. Diversity. 2023; 15(2):246. https://doi.org/10.3390/d15020246
Chicago/Turabian StyleLuis-Alaya, Bernabé, Marcia Toro, Rocío Calsina, Katty Ogata-Gutiérrez, Alejandra Gil-Polo, Ernesto Ormeño-Orrillo, and Doris Zúñiga-Dávila. 2023. "Evaluation of the Presence of Arbuscular Mycorrhizae and Cadmium Content in the Plants and Soils of Cocoa Plantations in San Martin, Peru" Diversity 15, no. 2: 246. https://doi.org/10.3390/d15020246
APA StyleLuis-Alaya, B., Toro, M., Calsina, R., Ogata-Gutiérrez, K., Gil-Polo, A., Ormeño-Orrillo, E., & Zúñiga-Dávila, D. (2023). Evaluation of the Presence of Arbuscular Mycorrhizae and Cadmium Content in the Plants and Soils of Cocoa Plantations in San Martin, Peru. Diversity, 15(2), 246. https://doi.org/10.3390/d15020246









