Diversity, Distribution, and Habitat Association of Anuran Species from Keffa, Southwest Ethiopia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design
2.3. Data Collection
2.4. DNA Barcoding
2.5. Data Analyses
3. Results
3.1. Anuran Abundance in the Keffa Area
3.2. Diversity Indices of Anuran Species among the Habitat Types
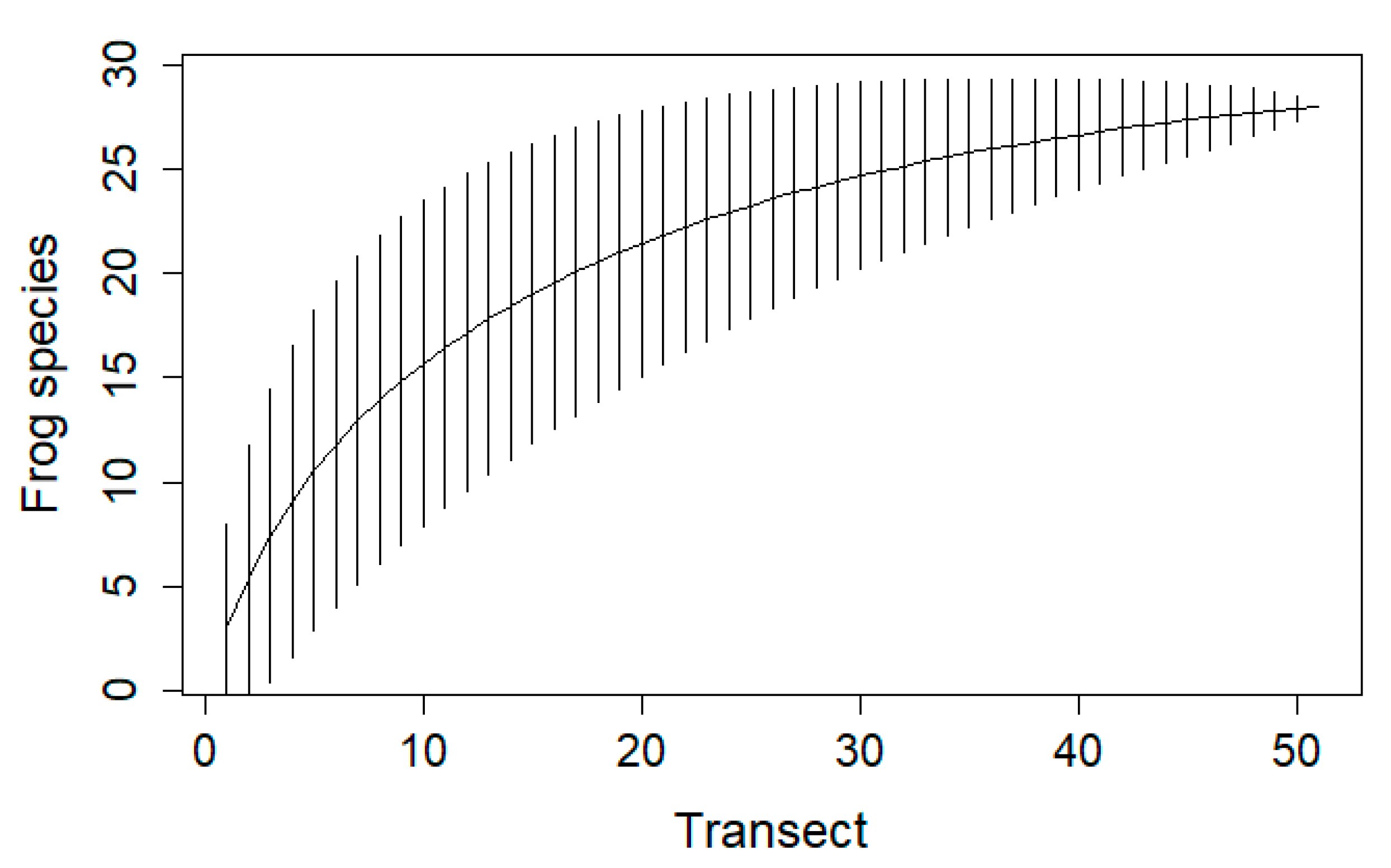
3.3. Accumulation Curve
3.4. Species Richness Estimation
3.5. Similarity Indices of Frog Species between Habitats
3.6. Seasonal Variation in Frog Species
3.7. Effects of Environmental Variables on Frog Species Abundance
4. Discussion
4.1. Anuran Diversity in the Keffa Area
4.2. Differences in Diversity of Anuran Species among Three Habitat Types of the Study Area
4.3. Accumulation Curve and Species Richness Estimation
4.4. Similarity of the Frog Assemblages in the Keffa Area
4.5. Seasonal Variation in Frog Species
4.6. Effects of Environmental Variables on Frog Species Abundance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AmphibiaWeb. University of California: Berkeley, CA, USA, 2022. Available online: https://amphibiaweb.org (accessed on 21 December 2022).
- Ceríaco, L.M.P.; Blackburn, D.C.; Marques, M.P.; Calado, F.M. Catalogue of the amphibian and reptile type specimens of the Museu de História Natural da Universidade do Porto in Portugal, with some comments on problematic taxa. Alytes 2014, 31, 13–36. [Google Scholar]
- Hocking, D.J.; Babbitt, K.J. Amphibian contributions to ecosystem services. Herpetol. Conserv. Biol. 2014, 9, 1–17. [Google Scholar]
- Jongsma Gregory, F.M.; Hedley, R.W.; Durães, R.; Karubian, J. Amphibian Diversity and Species Composition in Relation to Habitat Type and Alteration in the Mache-Chindul Reserve, Northwest Ecuador. Herpetologica 2014, 70, 34–46. [Google Scholar] [CrossRef]
- Archer, E.; Dziba, L.; Mulongoy, K.; Maoela, M.A.; Walters, M.; Biggs, R.; Cormier-Salem, M.-C.; DeClerck, F.; Diaw, M.C.; Dunham, A.E.; et al. Summary for Policymakers of the Regional Assessment Report on Biodiversity and Ecosystem Services for Africa of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2018. [Google Scholar]
- Thompson, M.E.; Donnelly, M.A. Effects of secondary forest succession on amphibians and reptiles: A review and meta-analysis. Copeia 2018, 106, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Zimkus, B.M.; Hassapakis, C.L.; Houck, M.L. Integrating current methods for the preservation of amphibian genetic resources and living tissues to achieve best practices for species conservation. Amphib. Reptile Conserv. 2018, 12, e165. [Google Scholar]
- Beebee, T.J.C.; Griffiths, R.A. The amphibian decline crisis: A watershed for conservation biology? Biol. Conserv. 2005, 125, 271–285. [Google Scholar] [CrossRef]
- Hirschfeld, M.; Blackburn, D.C.; Doherty-Bone, T.M.; Gonwouo, L.N.; Rödel, M.O. Dramatic decline of montane frog species in a Central African biodiversity hotspot. PLoS ONE 2016, 11, e0155129. [Google Scholar] [CrossRef] [Green Version]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; Garcia, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, el400253. [Google Scholar] [CrossRef] [Green Version]
- Daszak; Cunningham, A.A.; Hyatt, A.D. Infectious disease and amphibian population declines. Divers. Distrib. 2003, 9, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [Green Version]
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A review from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.L.; Rovito, S.M.; Wake, D.B.; Vredenburg, V.T. Coincident mass extirpation of neotropical amphibians with the emergence of the infection fungal pathogen Batrachochytrium dendrobatidis. Proc. Natl. Acad. Sci. USA 2011, 108, 9502–9507. [Google Scholar] [CrossRef] [Green Version]
- Dukes, J.S.; Mooney, H.A. Disruption of ecosystem processes in western North America by invasive species. Rev. Chil. Hist. Nat. 2004, 77, 411–437. [Google Scholar] [CrossRef]
- Runting, R.K.; Bryan, B.A.; Dee, L.E.; Maseyk, F.J.F.; Mandle, L.; Hamel, P.; Wilson, K.A.; Yetka, K.; Possingham, H.P.; Rhodes, J.R. Incorporating climate change into ecosystem service assessments and decisions: A review. Glob. Chang. Biol. 2017, 23, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Ficetola, G.F.; Rondinini, C.; Bonardi, A.; Baisero, D.; Padoa-Schioppa, E. Habitat availability for amphibians and extinction threat: A global analysis. Divers. Distrib. 2014, 21, 302–311. [Google Scholar] [CrossRef]
- Hof, C.; Araujo, M.B.; Jetz, W.; Rahbek, C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 2011, 480, 516–519. [Google Scholar] [CrossRef]
- Asefa, M.; Cao, M.; He, Y.; Mekonnen, E.; Song, X.; Yang, J. Ethiopian vegetation types, climate and topography. Plant Divers. 2020, 42, 302–311. [Google Scholar] [CrossRef]
- Estrada, A.; Garber, P.A.; Chaudhary, A. Current and future trends in socio-economic, demographic and governance factors affecting global primate conservation. PeerJ 2020, 8, e9816. [Google Scholar] [CrossRef]
- EBI. Ethiopia’s Fifth National Report to the Convention on Biological Diversity; Ethiopian Biodiversity Institute: Addis Ababa, Ethiopia, 2014. [Google Scholar]
- WCMC. Biodiversity Data Sourcebook; World Conservation Monitoring Centre, World Conservation Press: Cambridge, UK, 1994. [Google Scholar]
- Freilich, X.; Anadón, J.D.; Bukala, J.; Calderon, O.; Chakraborty, R.; Boissinot, S. Comparative Phylogeography of Ethiopian anurans: Impact of the Great Rift Valley and Pleistocene climate change. BMC Evol. Biol. 2016, 16, 206. [Google Scholar] [CrossRef] [Green Version]
- Olson, D.M.; Dinerstein, E. The Global 200: Priority Ecoregions for Global Conservation. Ann. Mo. Bot. Gard. 2002, 89, 199–224. [Google Scholar] [CrossRef]
- Reyes-Velasco, J.; Manthey, J.D.; Bourgeois, Y.; Freilich, X.; Boissinot, S. Revisiting the phylogeography, demography and taxonomy of the frog genus Ptychadena in the Ethiopian highlands with the use of genome-wide SNP data. PLoS ONE 2018, 13, e0190440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostin, D.S.; Kasso, M.; Komarova, V.A.; Martynov, A.A.; Gromov, A.R.; Alexandrov, D.Y.; Bekele, A.; Zewdie, C.; Bryja, J.; Lavrenchenko, L.A. Taxonomic and genetic diversity of rodents from the Arsi Mountains (Ethiopia). Mammalia 2018, 83, 237–247. [Google Scholar] [CrossRef]
- Smith, M.L.; Noonan, B.P.; Colston, T.J. The role of climatic and geological events in generating diversity in Ethiopian grass frogs (genus ptychadena). R. Soc. Open Sci. 2017, 4, 17–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goutte, S.; Reyes-Velasco, J.; Kassie, A.; Boissinot, S. Genetic and morphometric analyses of historical type specimens clarify the taxonomy of the Ethiopian Leptopelis gramineus species complex (Anura, Arthroleptidae). ZooKeys 2022, 1128, 63–97. [Google Scholar] [CrossRef]
- Largen, M.J. Catalogue of the amphibians of Ethiopia, including a key for their identification. Trop. Zool. 2001, 14, 307–402. [Google Scholar] [CrossRef]
- Freilich, X.; Tollis, M.; Boissinot, S. Hiding in the highlands: Evolution of a frog species complex of the genus Ptychadena in the Ethiopian highlands. Mol. Phylogenetics Evol. 2014, 71, 157–169. [Google Scholar] [CrossRef]
- Goutte, S.; Reyes-Velasco, J.; Boissinot, S. A new species of puddle frog from an unexplored mountain in southwestern Ethiopia (Anura, Phrynobatrachidae, Phrynobatrachus). ZooKeys 2019, 824, 53–70. [Google Scholar] [CrossRef]
- Goutte, S.; Reyes-Velasco, J.; Freilich, X.; Kassie, A.; Boissinot, S. Taxonomic revision of grass frogs (Ptychadenidae, ptychadena) endemic to the Ethiopian highlands. ZooKeys 2021, 1016, 77–141. [Google Scholar] [CrossRef]
- Gower, D.J.; Doherty-Bone, T.M.; Kassahun, R.; Mengistu, A.; Menegon, M.; de Sá, R.; Saber, S.; Cunningham, A.A.; Loader, S.P. High prevalence of the amphibian chytrid fungus (Batrachochytrium dendrobatidis) across multiple taxa and localities in the highlands of Ethiopia. Herpetol. J. 2012, 22, 225–233. [Google Scholar]
- Teme, A.K.; Simegn, A.B.; Bogale, B.A. Species Composition and Distribution of Endemic Frog Species of Keffa, Southwest Ethiopia. Glob. Ecol. Conserv. 2022, 38, e02211. [Google Scholar] [CrossRef]
- Mengistu, A.A. Amphibian Diversity, Distribution and Conservation in the Ethiopian Highlands: Morphological, Molecular and Biogeographic Investigation on Leptopelis and Ptychadena (Anura). Ph.D. Thesis, Universität Basel, Basel, Switzerland, 2012. Available online: https://core.ac.uk/reader/18256268 (accessed on 21 December 2022).
- Reyes-Velasco, J.; Manthey, J.D.; Freilich, X.; Boissinot, S. Diversification of African tree frogs (genus Leptopelis) in the highlands of Ethiopia. Mol. Ecol. 2018, 27, 2256–2270. [Google Scholar] [CrossRef]
- Tiutenko, A.; Zinenko, O. A new species of leptopelis (Anura, arthroleptidae) from the south-eastern slope of the Ethiopian highlands, with notes on the leptopelis gramineus species complex and the revalidation of a previously synonymised species. ZooKeys 2021, 1023, 119–150. [Google Scholar] [CrossRef]
- Rödel, M.O.; Ernst, R. Measuring and monitoring amphibian diversity in tropical forests. I. An evaluation of methods with recommendations for standardization. Ecotropica 2004, 10, 1–14. [Google Scholar]
- Veith, M.; Lötters, S.; Andreone, F.; Rödel, M. Measuring and monitoring amphibian diversity in tropical forests. II. Estimating species richness from standardized transect censing. Ecotropica 2004, 10, 85–99. [Google Scholar]
- Heyer, W.R.; Donnelly, M.A.; McDiarmid, R.W.; Hayek, L.C.; Foster, M.S. Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians; Smithsonian Institution Press: Washington, DC, USA, 1994; p. 364. [Google Scholar]
- Channing, A.; Rödel, M.O.; Channing, J. Tadpoles of Africa—The Biology and Identification of All Known Tadpoles in Sub-Saharan Africa; Edition Chimaira: Frankfurt am Main, Germany, 2012; p. 402. [Google Scholar]
- Channing, A.; Rödel, M.O. Field Guide to the Frogs and Other Amphibians of Africa; Struik Nature: Cape Town, South Africa, 2019; p. 407. [Google Scholar]
- Largen, M.; Spawls, S. The Amphibians and Reptiles of Ethiopia and Eritrea; Chimaira Publications: Frankfurt am Main, Germany, 2010; p. 693. [Google Scholar]
- Tiutenko, A.; Zinenko, O. Additional diagnosis, observations of breeding biology and tadpole of a little known dwarf puddle frog, Phrynobatrachus inexpectatus Largen, 2001 (Anura: Phrynobatrachidae). Salamandra 2020, 56, 135–147. [Google Scholar]
- Stackhouse, P. NASA POWER | Data Access Viewer. Available online: https://power.larc.nasa.gov/data-access-viewer/ (accessed on 30 December 2021).
- Magurran, A.E. Ecological Diversity and its Measurement; Cambridge University Press: Cambridge, UK, 1988; p. 256. [Google Scholar]
- Shannon, C.L.; Weiner, W. The Mathematical Theory of Communication; Illinois Books: Urbana, IL, USA, 1949. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Paul, D.R. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 20 June 2022).
- Hutcheson, K. A test for comparing diversities based on the shannon formula. J. Theor. Biol. 1970, 29, 151–154. [Google Scholar] [CrossRef]
- Sørensen, T.A. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content, and its application to analyses of the vegetation on Danish commons. K. Dan. Vidensk. Selsk. Biol. Skr. 1948, 5, 1–34. [Google Scholar]
- Jaccard, P. The distribution of the flora in the alpine zone. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; Version 2.5-6; The Comprehensive R Archive Network; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Ma, K.H.; Hsieh, T.C. iNEXT Online: Software for Interpolation and Extrapolation of Species Diversity. 2016. Program and User’s Guide. Available online: http://chao.stat.nthu.edu.tw/wordpress/software_download/inextonline/ (accessed on 25 June 2022).
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd edition; Chapman & Hall: London, UK, 1989. [Google Scholar]
- Pinheiro, J.; Bates, D.; Debroy, S.; Sarkar, D. R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 2012, 3.1-109. Available online: http://cran.r-project.org/package=nlme (accessed on 20 July 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 14 October 2022).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed] [Green Version]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- NABU. NABU’s Follow-Up Biodiversity Assessment at the Kafa Biosphere Reserve, Ethiopia; The Nature and Biodiversity Conservation Union: Berlin, Germany, 2020. [Google Scholar]
- Manthey, J.D.; Reyes-Velasco, J.; Freilich, X.; Boissinot, S. Diversification in a biodiversity hotspot: Genomic variation in the river frog Amietia nutti across the Ethiopian Highlands. Biol. J. Linn. Soc. 2017, 122, 801–813. [Google Scholar] [CrossRef]
- Drayer, A.N.; Richter, S.C. Physical Wetland Characteristics Influence Amphibian Community Compostion Differently in Constructed Wetlands and Natural Wetlands. Ecol. Eng. 2016, 93, 166–174. [Google Scholar] [CrossRef]
- Nneji, L.M.; Adeola, A.C.; Okeyoyin, A.; Oladipo, O.C.; Saidu, Y.; Samuel, D.; Usongo, J.Y.; Adedeji, B.; Omotoso, O.; Adeyi, A.O.; et al. Diversity and Distribution of Amphibians and Reptiles in Gashaka Gumti National Park, Nigeria. Herpetol. Notes 2019, 12, 543–559. [Google Scholar]
- Muro-Torres, V.M.; Amezcua, F.; Soto-Jiménez, M.; Balart, E.F.; Serviere-Zaragoza, E.; Green, L.; Rajnohova, J. Primary sources and food web structure of a tropical wetland with high density of mangrove forest. Water 2020, 12, 3105. [Google Scholar] [CrossRef]
- Onadeko, A.B. Distribution, diversity and abundance of anuran species in three different vegetation habitats in south-western Nigeria. Eth. J. Environ. Stud. Manag. 2016, 9, 22–34. [Google Scholar]
- Pearman, P.B. Correlates of Amphibian Diversity in an Altered Landscape of Amazonian Ecuador. Conserv. Biol. 1997, 11, 1211–1225. [Google Scholar] [CrossRef]
- Gouveia, S.F.; Faria, R.G. Effects of Habitat Size and Heterogeneity on Anuran Breeding Assemblages in the Brazilian Dry Forest. J. Herpetol. 2015, 49, 442–446. [Google Scholar] [CrossRef]
- Silva, R.A.; Martins, I.A.; Rossa-Feres, D.d.C. Environmental Heterogeneity: Anuran Diversity in Homogeneous Environments. Zoologia 2011, 28, 610–618. [Google Scholar] [CrossRef] [Green Version]
- Tomé, R. Effects of Habitat Quality on the Abundance, Behaviour and Breeding Performance of Owls: Barn and Little Owls in Agro-Pastoral Landscapes of Southern Europe; University of Turku: Turku, Finland, 2011. [Google Scholar] [CrossRef]
- Neckel-Oliveira, S.; Magnusson, W.E.; Lima, A.P.; Albernaz, A.L.K. Diversity and Distribution of Frogs in an Amazonian Savanna in Brazil. Amphib. Reptil. 2000, 21, 317–326. [Google Scholar] [CrossRef]
- Auguste, R.J.; Hailey, A. Diversity and Species Composition of Amphibians of the Aripo Savannas Scientific Reserve, Trinidad, West Indies. J. Herpetol. 2018, 52, 86–93. [Google Scholar] [CrossRef]
- Dodd, C.K., Jr. Amphibian Ecology and Conservation; Oxford University Press Inc.: New York, NY, USA, 2010; p. 556. [Google Scholar] [CrossRef] [Green Version]
- Borrell, J.S.; Biswas, M.K.; Goodwin, M.; Blomme, G.; Schwarzacher, T.; Heslop-Harrison, J.S.; Wendawek, A.M.; Berhanu, A.; Kallow, S.; Janssens, S.; et al. Enset in Ethiopia: A poorly characterized but resilient starch staple. Ann. Bot. 2019, 123, 747–766. [Google Scholar] [CrossRef] [Green Version]
- Kidane, S.A.; Meressa, B.H.; Haukeland, S.; Hvoslef-Eide, A.K.; Coyne, D.L. The Ethiopian staple food crop enset (Ensete ventricosum) was assessed for the first time for resistance against the root-lesion nematode Pratylenchus goodeyi. Nematology 2021, 23, 771–779. [Google Scholar] [CrossRef]
- Tobiaw, D.C.; Bekele, E. Analysis of genetic diversity among cultivated enset (Ensete ventricosum) populations from Essera and Kefficho, southwestern part of Ethiopia using inter simple sequence repeats (ISSRs) marker. Afr. J. Biotechnol. 2011, 10, 15697–15709. [Google Scholar] [CrossRef]
- Ndriantsoa, S.H.; Riemann, J.C.; Raminosoa, N.; Rödel, M.O.; Glos, J.S. Amphibian Diversity in the Matrix of a Fragmented Landscape around Ranomafana in Madagascar Depends on Matrix Quality. Trop. Conserv. Sci. 2017, 10, 1940082916686065. [Google Scholar] [CrossRef] [Green Version]
- Costa-Campos, C.E.; Freire, E.M.X. Richness and composition of anuran assemblages from an Amazonian savanna. ZooKeys 2019, 843, 149–169. [Google Scholar] [CrossRef] [Green Version]
- Maritz, B.; Gavin, M.; Mackay, D.; Alexander, G. The effect of funnel trap type and size of pitfall trap on trap success: Implications for ecological field studies. Amphib. Reptil. 2007, 28, 321–328. [Google Scholar] [CrossRef]
- Ribeiro, M.A., Jr.; Gardner, T.A.; Ávila-Pires, T.C.S. Evaluating the effectiveness of herpetofaunal sampling techniques across a gradient of habitat change in a tropical forest landscape. J. Herpetol. 2008, 42, 733–749. [Google Scholar] [CrossRef]
- Akoto, S.D.; Asare, A.; Gyabaa, G. Natural regeneration diversity and composition of native tree species under monoculture, mixed culture plantation and natural forest. Int. Res. J. Nat. Sci. 2015, 3, 24–38. [Google Scholar]
- Hammond, M.E.; Pokornỳ, R. Diversity of Tree Species in Gap Regeneration under Tropical Moist Semi-Deciduous Forest: An Example from Bia Tano Forest Reserve. Diversity 2020, 12, 301. [Google Scholar] [CrossRef]
- Giaretta, A.A.; Menin, M. Reproduction, phenology and mortality sources of a species of Physalaemus (Anura: Leptodactylidae). J. Nat. Hist. 2004, 38, 1711–1722. [Google Scholar] [CrossRef]
- Giaretta, A.A.; Facure, K.G.; Sawaya, R.J.; Meyer, J.D.M.; Chemin, N. Diversity and abundance of litter frogs in a montane forest of southeastern Brazil: Seasonal and altitudinal changes. Biotropica 1999, 31, 669–674. [Google Scholar] [CrossRef]
- Watanabe, S.; Nakanishi, N.; Izawa, M. Seasonal Abundance in the Floor-Dwelling Frog Fauna on Iriomote Island of the Ryukyu Archipelago, Japan. J. Trop. Ecol. 2005, 21, 85–91. [Google Scholar] [CrossRef]
- Vonesh, J.R. Patterns of richness and abundance in a tropical African leaf-litter herpetofauna. Biotropica 2001, 33, 502–510. [Google Scholar] [CrossRef]
- Blaustein, A.; Walls, S.; Bancroft, B.; Lawler, J.; Searle, C.; Gervasi, S. Direct and indirect effects of climate change on Amphibian populations. Diversity 2010, 2, 281–313. [Google Scholar] [CrossRef]
- Cortés-Gómez, A.M.; Castro-Herrera, F.; Urbina-Cardona, J.N. Small changes in vegetation structure create great changes in amphibian ensembles in the Colombian Pacific rainforest. Trop. Conserv. Sci. 2013, 6, 749–769. [Google Scholar] [CrossRef] [Green Version]
- Sirami, C.; Nespoulous, A.; Cheylan, J.P.; Marty, P.; Hvenegaard, G.T.; Geniez, P.; Schatz, B.; Martin, J.L. Long-term anthropogenic and ecological dynamics of a Mediterranean landscape: Impacts on multiple taxa. Landsc. Urban Plan. 2010, 96, 214–223. [Google Scholar] [CrossRef]
- Wanger, T.C.; Saro, A.; Iskandar, D.T.; Brook, B.W.; Sodhi, N.S.; Clough, Y.; Tscharntke, T. Conservation value of cacao agroforestry for amphibians and reptiles in South-East Asia: Combining correlative models with follow-up field experiments. J. Appl. Ecol. 2009, 46, 823–832. [Google Scholar] [CrossRef]
- Navas, C.A. Herpetological diversity along Andean elevational gradients: Links with physiological ecology and evolutionary physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 133, 469–485. [Google Scholar] [CrossRef]
- Matavelli, R.; Oliveira, J.M.; Soininen, J.; Ribeiro, M.C.; Bertoluci, J. Altitude and Temperature Drive Anuran Community Assembly in a Neotropical Mountain Region. Biotropica 2022, 54, 607–618. [Google Scholar] [CrossRef]
- Sanders, N.J.; Rahbek, C. The patterns and causes of elevational gradients. Ecography 2012, 35, 1–3. [Google Scholar] [CrossRef]



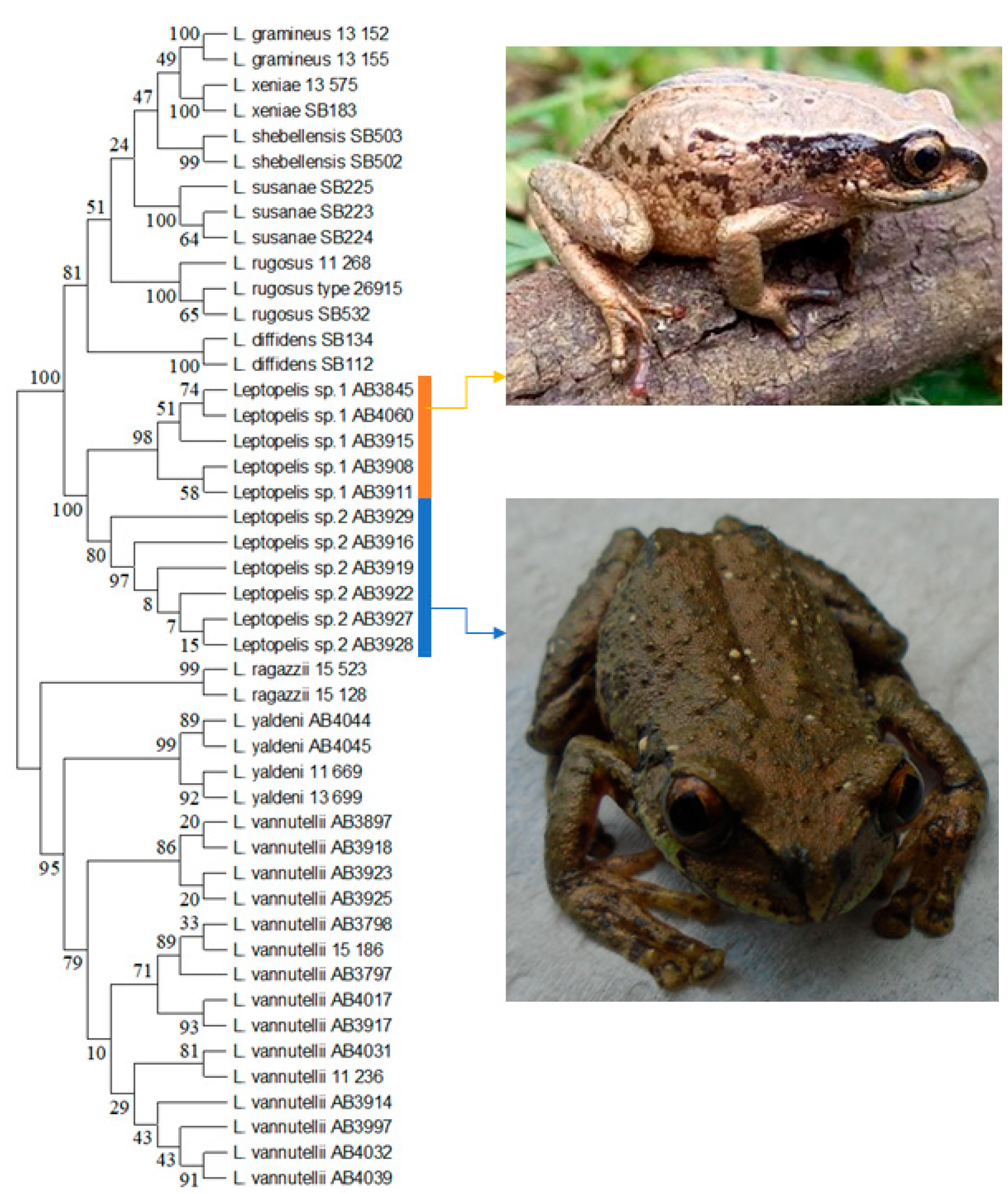





| Species | Collection no. | Gene Bank Accession No. | Collection Year | Locality | Lat. | Long. |
|---|---|---|---|---|---|---|
| Phrynobatrachus sp. 1 | AB3830 | OQ398465 | 2021 | Boqa, Ethiopia | 7.30 | 36.37 |
| Phrynobatrachus sp. 1 | AB3834 | OQ398466 | 2021 | Boqa, Ethiopia | 7.30 | 36.37 |
| Phrynobatrachus sp. 1 | AB3848 | OQ398467 | 2021 | Bita, Ethiopia | 7.27 | 35.78 |
| Phrynobatrachus sp. 1 | AB3862 | OQ398468 | 2021 | Nata, Ethiopia | 7.58 | 35.75 |
| Phrynobatrachus sp. 1 | AB3891 | OQ398469 | 2021 | Bariacho, Ethiopia | 7.82 | 35.83 |
| Phrynobatrachus sp. 1 | AB3910 | OQ398470 | 2022 | Ganeti, Ethiopia | 7.42 | 35.64 |
| P. natalensis | AB3949 | OQ398471 | 2022 | Bita, Ethiopia | 7.27 | 35.78 |
| Phrynobatrachus sp. 1 | AB3987 | OQ398472 | 2022 | Utali, Ethiopia | 7.83 | 35.82 |
| Phrynobatrachus sp. 1 | AB4024 | OQ398473 | 2022 | Shamali, Ethiopia | 7.13 | 36.19 |
| Phrynobatrachus sp. 2 | AB4047 | OQ398474 | 2022 | Chagni, Ethiopia | 10.92 | 36.57 |
| P. natalensis | AB4054 | OQ398475 | 2022 | Chagni, Ethiopia | 10.92 | 36.57 |
| Phrynobatrachus sp. 1 | AB3830 | OQ398465 | 2021 | Boqa, Ethiopia | 7.30 | 36.37 |
| Leptopelis sp. 1 | AB3845 | OQ413091 | 2020 | Boqa, Ethiopia | 7.30 | 36.37 |
| Leptopelis sp. 1 | AB3908 | OQ407700 | 2021 | Ganeti, Ethiopia | 7.42 | 35.64 |
| Leptopelis sp. 1 | AB3911 | OQ413101 | 2021 | Ganeti, Ethiopia | 7.42 | 35.64 |
| Leptopelis sp. 1 | AB3915 | OQ413102 | 2021 | Ganeti, Ethiopia | 7.42 | 35.64 |
| Leptopelis sp. 1 | AB4060 | OQ413100 | 2022 | Boqa, Ethiopia | 7.30 | 36.37 |
| Leptopelis sp. 2 | AB3916 | OQ413180 | 2021 | Ganeti, Ethiopia | 7.43 | 35.65 |
| Leptopelis sp. 2 | AB3919 | OQ413183 | 2021 | Ganeti, Ethiopia | 7.43 | 35.65 |
| Leptopelis sp. 2 | AB3922 | OQ413184 | 2021 | Ganeti, Ethiopia | 7.43 | 35.65 |
| Leptopelis sp. 2 | AB3927 | OQ413187 | 2021 | Ganeti, Ethiopia | 7.43 | 35.65 |
| Leptopelis sp. 2 | AB3928 | OQ413188 | 2021 | Ganeti, Ethiopia | 7.43 | 35.65 |
| Leptopelis sp. 2 | AB3929 | OQ413189 | 2021 | Ganeti, Ethiopia | 7.43 | 35.65 |
| L. yaldeni | AB4044 | OQ413098 | 2022 | Chagni, Ethiopia | 10.93 | 36.69 |
| L. yaldeni | AB4045 | OQ413099 | 2022 | Chagni, Ethiopia | 10.93 | 36.69 |
| L. vannutellii | AB4031 | OQ413095 | 2022 | Oda, Ethiopia | 7.12 | 36.46 |
| L. vannutellii | AB4032 | OQ413096 | 2022 | Oda, Ethiopia | 7.12 | 36.46 |
| L. vannutellii | AB4039 | OQ413097 | 2022 | Oda, Ethiopia | 7.12 | 36.46 |
| L. vannutellii | AB3914 | OQ413103 | 2021 | Ganeti, Ethiopia | 7.43 | 35.65 |
| L. vannutellii | AB3997 | OQ413093 | 2022 | Sor, Ethiopia | 7.84 | 35.84 |
| L. vannutellii | AB3923 | OQ413185 | 2022 | Ganeti, Ethiopia | 7.43 | 35.65 |
| L. vannutellii | AB3925 | OQ413186 | 2022 | Ganeti, Ethiopia | 7.43 | 35.65 |
| L. vannutellii | AB3918 | OQ413182 | 2022 | Ganeti, Ethiopia | 7.43 | 35.65 |
| L. vannutellii | AB3897 | OQ413092 | 2021 | Utali (Saylem), Ethiopia | 7.83 | 35.82 |
| L. vannutellii | AB4017 | OQ413094 | 2022 | Shamali, Ethiopia | 7.20 | 36.28 |
| L. vannutellii | AB3917 | OQ413181 | 2020 | Ganeti, Ethiopia | 7.43 | 35.65 |
| L. vannutellii | AB3798 | OQ413090 | 2020 | Shuneti, Ethiopia | 7.38 | 35.73 |
| L. vannutellii | AB3797 | OQ413089 | 2020 | Shuneti, Ethiopia | 7.38 | 35.73 |
| Family | Species | Habitats | Total | Relative Abundance (Rank) | ||
|---|---|---|---|---|---|---|
| WL | AL | RF | ||||
| Hyperoliidae | Afrixalus clarkei Largen, 1974 | 784 | 115 | 25 | 924 | 16.27 (3) |
| Afrixalus enseticola Largen, 1974 | 99 | 148 | 0 | 247 | 4.35 (8) | |
| Hyperolius microps Günther, 1864 | 478 | 0 | 0 | 478 | 8.41 (4) | |
| Hyperolius kivuensis Ahl, 1931 | 260 | 0 | 0 | 260 | 4.58 (7) | |
| Hyperolius viridiflavus (Duméril and Bibron, 1841) | 247 | 0 | 13 | 260 | 4.58 (7) | |
| Kassina senegalensis (Duméril and Bibron, 1841) | 23 | 0 | 0 | 23 | 0.41 (15) | |
| Paracassina obscura (Boulenger, 1895) | 0 | 12 | 0 | 12 | 0.21 (20) | |
| Arthroleptidae | Leptopelis sp. 1 | 22 | 0 | 0 | 22 | 0.39 (16) |
| Leptopelis sp. 2 | 0 | 28 | 0 | 28 | 0.49 (14) | |
| Leptopelis cf. susanae Largen 1977 | 2 | 0 | 0 | 2 | 0.04 (25) | |
| Leptopelis vannutellii (Boulenger, 1898) | 0 | 167 | 289 | 456 | 8.03 (5) | |
| Pyxicephalidae | Amietia nutti (Boulenger, 1896) | 0 | 0 | 46 | 46 | 0.81 (12) |
| Conrauidae | Conraua beccarii (Boulenger, 1911) | 0 | 0 | 7 | 7 | 0.12 (23) |
| Hemisotidae | Hemisus marmoratus (Peters, 1854) | 0 | 0 | 3 | 3 | 0.05 (24) |
| Hemisus microscaphus Laurent, 1972 | 0 | 8 | 41 | 49 | 0.86 (11) | |
| Phrynobatrachidae | Phrynobatrachus sp. 1 | 883 | 0 | 274 | 1157 | 20.38 (1) |
| Phrynobatrachus natalensis (Smith, 1849) | 1044 | 0 | 0 | 1044 | 18.38 (2) | |
| Ptychadenidae | Ptychadena anchietae (Barboza du Bocage, 1868) | 48 | 7 | 0 | 55 | 0.97 (10) |
| Ptychadena beka Goutte, Reyes-Velasco, Freilich, Kassie, and Boissinot, 2021 | 12 | 10 | 0 | 22 | 0.39 (17) | |
| Ptychadena doro Goutte, Reyes-Velasco, Freilich, Kassie, and Boissinot, 2021 | 15 | 0 | 0 | 15 | 0.26 (19) | |
| Ptychadena erlangeri (Ahl, 1924) | 29 | 0 | 0 | 29 | 0.51 (13) | |
| Ptychadena neumanni (Ahl, 1924) | 16 | 0 | 0 | 16 | 0.28 (18) | |
| Ptychadena nilotica (Seetzen, 1855) | 382 | 6 | 5 | 393 | 6.92 (6) | |
| Ptychadena schillukorum (Werner, 1908) | 11 | 0 | 0 | 11 | 0.19 (21) | |
| Pipidae | Xenopus clivii Peracca, 1898 | 93 | 0 | 17 | 110 | 1.93 (9) |
| Xenopus largeni Tinsley, 1995 | 9 | 0 | 0 | 9 | 0.16 (22) | |
| Total | 4457 | 501 | 720 | 5678 | ||
| Family | Genus | Species | Abundance | Relative Abundance (%) |
|---|---|---|---|---|
| Arthroleptidae | 1 | 4 | 508 | 8.95 |
| Hyperoliidae | 4 | 7 | 2204 | 38.82 |
| Conrauidae | 1 | 1 | 7 | 0.12 |
| Hemisotidae | 1 | 2 | 52 | 0.92 |
| Phrynobatrachidae | 1 | 2 | 2201 | 38.76 |
| Pipidae | 1 | 2 | 119 | 2.10 |
| Ptychadenidae | 1 | 7 | 541 | 9.53 |
| Pyxicephalidae | 1 | 1 | 46 | 0.81 |
| Total | 11 | 26 | 5678 | 100.00 |
| Paired Habitats | Number of Species | Similarity Indices | ||||
|---|---|---|---|---|---|---|
| Unique to AL | Unique to RF | Unique to WL | Shared | SCSI | JCSI | |
| AL Vs RF | 5 | 6 | - | 3 | 0.40 | 0.25 |
| AL Vs WL | 4 | - | 14 | 5 | 0.34 | 0.21 |
| RF Vs WL | - | 5 | 14 | 5 | 0.39 | 0.24 |
| Dry Season | Rainy Season | Diversity t-Tests | |||
|---|---|---|---|---|---|
| t | df | p (Same) | |||
| Richness | 26 | 25 | |||
| Abundance | 2287 | 3391 | |||
| Simpson D | 0.87 | 0.88 | 2.32 | 4636.7 | 0.02 |
| Shannon H | 2.41 | 2.47 | −2.17 | 4791.2 | 0.03 |
| Abundance | ||||
|---|---|---|---|---|
| Predictors | Estimate | SE | z Value | p |
| Intercept | 1.132 × 1001 | 1.319 × 10+00 | 8.582 | <2 × 10−16 *** |
| Habitat (Riverine Forest) | 1.089 × 10−01 | 5.917 × 10−02 | 1.840 | 0.0657 |
| Habitat (Wetland) | 2.093 × 10+00 | 4.486 × 10−02 | 46.649 | <2 × 10−16 *** |
| Temperature | −5.106 × 10−01 | 4.449 × 10−02 | −11.477 | <2 × 10−16 *** |
| Precipitation | 4.507 × 10−04 | 2.775 × 10−04 | 1.624 | 0.1044 |
| Altitude | −9.363 × 10−05 | 5.672 × 10−05 | −1.651 | 0.0988 |
| Slope | −1.889 × 10−02 | 3.387 × 10−03 | −5.579 | 2.43 × 10−08 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassie, A.; Simegn, A.B.; Bogale, B.A.; Goutte, S.; Boissinot, S. Diversity, Distribution, and Habitat Association of Anuran Species from Keffa, Southwest Ethiopia. Diversity 2023, 15, 300. https://doi.org/10.3390/d15020300
Kassie A, Simegn AB, Bogale BA, Goutte S, Boissinot S. Diversity, Distribution, and Habitat Association of Anuran Species from Keffa, Southwest Ethiopia. Diversity. 2023; 15(2):300. https://doi.org/10.3390/d15020300
Chicago/Turabian StyleKassie, Abeje, Afework Bekele Simegn, Bezawork Afework Bogale, Sandra Goutte, and Stephane Boissinot. 2023. "Diversity, Distribution, and Habitat Association of Anuran Species from Keffa, Southwest Ethiopia" Diversity 15, no. 2: 300. https://doi.org/10.3390/d15020300
APA StyleKassie, A., Simegn, A. B., Bogale, B. A., Goutte, S., & Boissinot, S. (2023). Diversity, Distribution, and Habitat Association of Anuran Species from Keffa, Southwest Ethiopia. Diversity, 15(2), 300. https://doi.org/10.3390/d15020300







