Prevalence, Molecular Characterization, and Ecological Associations of Filarioid Helminths in a Wild Population of Blue Tits (Cyanistes caeruleus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Blue Tit Sampling
2.2. DNA Extraction, Filarioid PCR Screening and Sequencing
2.3. Filarioid Phylogenetic Analysis
2.4. Statistical Analysis
3. Results
3.1. Prevalence and Probability of Infection by Filarioids
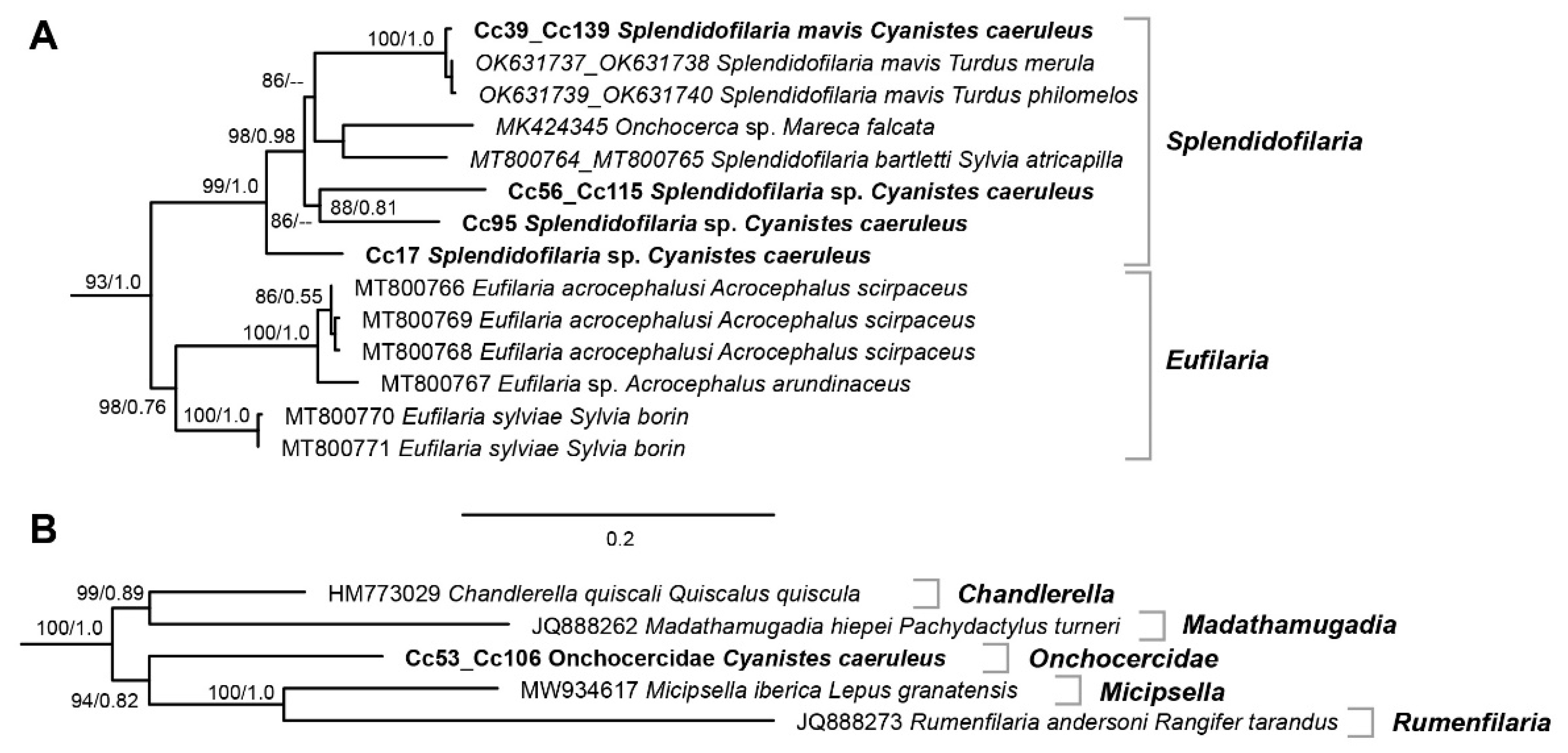
3.2. Filarioid Sequences and Phylogenetic Tree
4. Discussion
4.1. Host Body Condition and Infection by Filarioid Nematodes
4.2. Molecular Characterization of Filairoids Infecting Blue Tits
4.3. Prevalence of Filarioids Unaffected by Habitat Type and Host Sex
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCarthy, J.; Moore, T.A. Emerging Helminth Zoonoses. Int. J. Parasitol. 2000, 30, 1351–1360. [Google Scholar] [CrossRef]
- de Silva, N.R.; Brooker, S.; Hotez, P.J.; Montresor, A.; Engels, D.; Savioli, L. Soil-Transmitted Helminth Infections: Updating the Global Picture. Trends Parasitol. 2003, 19, 547–551. [Google Scholar] [CrossRef]
- Taylor, M.J.; Hoerauf, A.; Bockarie, M. Lymphatic Filariasis and Onchocerciasis. Lancet 2010, 376, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, R.; Khatri, V.; Chauhan, N. Advances in Vaccine Development for Human Lymphatic Filariasis. Trends Parasitol. 2020, 36, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Adjei, O.; Bain, O.; Hoerauf, A.; Hoffmann, W.H.; Makepeace, B.L.; Schulz-Key, H.; Tanya, V.N.; Trees, A.J.; Wanji, S.; et al. Of Mice, Cattle, and Humans: The Immunology and Treatment of River Blindness. PLoS Negl. Trop. Dis. 2008, 2, e217. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, D.H. Filaria Control and Elimination: Diagnostic, Monitoring and Surveillance Needs. Trans. Roy. Soc. Trop. Med. Hyg. 2009, 103, 338–341. [Google Scholar] [CrossRef]
- Dieki, R.; Nsi-Emvo, E.; Akue, J.P. The Human Filaria Loa loa: Update on Diagnostics and Immune Response. Res. Rep. Trop. Med. 2022, 13, 41–54. [Google Scholar] [CrossRef]
- Simón, F.; Siles-Lucas, M.; Morchón, R.; González-Miguel, J.; Mellado, I.; Carretón, E.; Montoya-Alonso, J.A. Human and Animal Dirofilariasis: The Emergence of a Zoonotic Mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Brianti, E.; Traversa, D.; Petrić, D.; Genchi, C.; Capelli, G. Vector-Borne Helminths of Dogs and Humans in Europe. Parasites Vectors 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Orihel, T.C.; Eberhard, M.L. Zoonotic Filariasis. Clin. Microbiol. Rev. 1998, 11, 366–381. [Google Scholar] [CrossRef]
- Bartlett, C.M. Filarioid Nematodes. In Parasitic Diseases of Wild Birds; Atkinson, C.T., Thomas, N.J., Hunter, D.B., Eds.; Wiley Blackwell: Oxford, UK, 2008; pp. 439–462. ISBN 978-0-8138-0462-0. [Google Scholar]
- Bain, O.; Babayan, S. Behaviour of Filariae: Morphological and Anatomical Signatures of Their Life Style within the Arthropod and Vertebrate Hosts. Filaria J. 2003, 2, 16. [Google Scholar] [CrossRef]
- Mutinda, M.; Otiende, M.; Gakuya, F.; Kariuki, L.; Obanda, V.; Ndeere, D.; Ndambiri, E.; Kariuki, E.; Lekolool, I.; Soriguer, R.C.; et al. Putative Filariosis Outbreak in White and Black Rhinoceros at Meru National Park in Kenya. Parasites Vectors 2012, 5, 206. [Google Scholar] [CrossRef]
- Laaksonen, S.; Solismaa, M.; Orro, T.; Kuusela, J.; Saari, S.; Kortet, R.; Nikander, S.; Oksanen, A.; Sukura, A. Setaria tundra Microfilariae in Reindeer and Other Cervids in Finland. Parasitol. Res. 2009, 104, 257–265. [Google Scholar] [CrossRef]
- Bain, O. Evolutionary Relationships among Filarial Nematodes. In The Filaria; Klei, T.R., Rajan, T.V., Eds.; World Class Parasites; Springer: New York, NY, USA, 2002; Volume 5, pp. 21–29. ISBN 978-0-306-47661-7. [Google Scholar]
- Readel, A.M.; Goldberg, T.L. Blood Parasites of Frogs from an Equatorial African Montane Forest in Western Uganda. J. Parasitol. 2010, 96, 448–450. [Google Scholar] [CrossRef]
- Sehgal, R.N.M.; Jones, H.I.; Smith, T.B. Blood Parasites of Some West African Rainforest Birds. J. Vet. Med. Sci. 2005, 67, 295–301. [Google Scholar] [CrossRef]
- Savage, A.F.; Robert, V.; Goodman, S.M.; Raharimanga, V.; Raherilalao, M.J.; Andrianarimisa, A.; Ariey, F.; Greiner, E.C. Blood Parasites in Birds from Madagascar. J. Wildl. Dis. 2009, 45, 907–920. [Google Scholar] [CrossRef]
- Sehgal, R.N.M.; Jones, H.I.; Smith, T.B. Molecular Evidence for Host Specificity of Parasitic Nematode Microfilariae in Some African Rainforest Birds. Mol. Ecol. 2005, 14, 3977–3988. [Google Scholar] [CrossRef] [PubMed]
- Elahi, R.; Islam, A.; Hossain, M.S.; Mohiuddin, K.; Mikolon, A.; Paul, S.K.; Hosseini, P.R.; Daszak, P.; Alam, M.S. Prevalence and Diversity of Avian Haematozoan Parasites in Wetlands of Bangladesh. J. Parasitol. Res. 2014, 2014, 493754. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Iezhova, T.A. A Comparison of the Blood Parasites in Three Subspecies of the Yellow Wagtail Motacilla flava. J. Parasitol. 2001, 87, 930–934. [Google Scholar] [CrossRef]
- Silveira, P.; Belo, N.O.; Rodello, D.; Pinheiro, R.T.; Braga, É.M. Microfilariae Infection in Wild Birds from the Brazilian Cerrado. J. Wildl. Dis. 2010, 46, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Sebaio, F.; Braga, É.M.; Branquinho, F.; Fecchio, A.; Marini, M.Â. Blood Parasites in Passerine Birds from the Brazilian Atlantic Forest. Rev. Bras. Parasitol. Vet. 2012, 21, 7–15. [Google Scholar] [CrossRef]
- Bennett, G.F.; Garvin, M.; Bates, J.M. Avian Hematozoa from West-Central Bolivia. J. Parasitol. 1991, 77, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Matta, N.E.; Basto, N.; Gutierrez, R.; Rodríguez, O.A.; Greiner, E.C. Prevalence of Blood Parasites in Tyrannidae (Flycatchers) in the Eastern Plains of Colombia. Mem. Inst. Oswaldo Cruz 2004, 99, 271–274. [Google Scholar] [CrossRef]
- Londoño, A.; Pulgarin-R, P.C.; Blair, S. Blood Parasites in Birds from the Lowlands of Northern Colombia. Caribb. J. Sci. 2007, 43, 87–93. [Google Scholar] [CrossRef]
- De La Torre, G.M.; Campião, K.M. Bird Habitat Preferences Drive Hemoparasite Infection in the Neotropical Region. Integr. Zool. 2021, 16, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Villalva-Pasillas, D.; Medina, J.P.; Soriano-Vargas, E.; Martínez-Hernández, D.A.; García-Conejo, M.; Galindo-Sánchez, K.P.; Sánchez-Jasso, J.M.; Talavera-Rojas, M.; Salgado-Miranda, C. Haemoparasites in Endemic and Non-Endemic Passerine Birds from Central Mexico Highlands. Int. J. Parasitol. 2020, 11, 88–92. [Google Scholar] [CrossRef]
- Benedikt, V.; Barus, V.; Capek, M.; Havlicek, M.; Literak, I. Blood Parasites (Haemoproteus and Microfilariae) in Birds from the Caribbean Slope of Costa Rica. Acta Parasitol. 2009, 54, 197–204. [Google Scholar] [CrossRef]
- Young, B.E.; Garvin, M.C.; McDonald, D.B. Blood Parasites in Birds from Monteverde, Costa Rica. J. Wildl. Dis. 1993, 29, 555–560. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T.A.; Brooks, D.R.; Hanelt, B.; Brant, S.V.; Sutherlin, M.E.; Causey, D. Additional Observations on Blood Parasites of Birds in Costa Rica. J. Wildl. Dis. 2004, 40, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Baruš, V.; Benedikt, V.; Literák, I. Microfilariae in Birds in the Czech Republic, Including a Note on Adult Nematodes Eufilaria delicata in a Song Thrush Turdus philomelos. Parasitol. Res. 2011, 109, 645–655. [Google Scholar] [CrossRef]
- Merino, S.; Potti, J.; Fargallo, J.A. Blood Parasites of Passerine Birds from Central Spain. J. Wildl. Dis. 1997, 33, 638–641. [Google Scholar] [CrossRef]
- Travis, E.K.; Vargas, F.H.; Merkel, J.; Gottdenker, N.; Miller, R.E.; Parker, P.G. Hematology, Serum Chemistry, and Serology of Galápagos Penguins (Spheniscus mendiculus) in the Galápagos Islands, Ecuador. J. Wildl. Dis. 2006, 42, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Travis, E.K.; Vargas, F.H.; Merkel, J.; Gottdenker, N.; Miller, R.E.; Parker, P.G. Hematology, Plasma Chemistry, and Serology of the Flightless Cormorant (Phalacrocorax harrisi) in the Galápagos Islands, Ecuador. J. Wildl. Dis. 2006, 42, 133–141. [Google Scholar] [CrossRef]
- Clark, N.J.; Wells, K.; Dimitrov, D.; Clegg, S.M. Co-Infections and Environmental Conditions Drive the Distributions of Blood Parasites in Wild Birds. J. Anim. Ecol. 2016, 85, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Merkel, J.; Jones, H.I.; Whiteman, N.K.; Gottdenker, N.; Vargas, H.; Travis, E.K.; Miller, R.E.; Parker, P.G. Microfilariae in Galápagos Penguins (Spheniscus mendiculus) and Flightless Cormorants (Phalacrocorax harrisi): Genetics, Morphology, and Prevalence. J. Parasitol. 2007, 93, 495–503. [Google Scholar] [CrossRef]
- Cardells-Peris, J.; Gonzálvez, M.; Ortega-Porcel, J.; Ruiz de Ybáñez, M.R.; Martínez-Herrero, M.C.; Garijo-Toledo, M.M. Parasitofauna Survey of Song Thrushes (Turdus philomelos) from the Eastern Part of Spain. Parasitol. Int. 2020, 79, 102176. [Google Scholar] [CrossRef]
- Deviche, P.; McGraw, K.; Greiner, E.C. Interspecific Differences in Hematozoan Infection in Sonoran Desert Aimophila Sparrows. J. Wildl. Dis. 2005, 41, 532–541. [Google Scholar] [CrossRef]
- Sehgal, R.N.M. Manifold Habitat Effects on the Prevalence and Diversity of Avian Blood Parasites. Int. J. Parasitol. 2015, 4, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, M.; Martínez-de la Puente, J.; Bensch, S.; Roiz, D.; Ruiz, S.; Viana, D.S.; Soriguer, R.C.; Figuerola, J. Ecological Determinants of Avian Malaria Infections: An Integrative Analysis at Landscape, Mosquito and Vertebrate Community Levels. J. Anim. Ecol. 2018, 87, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Bautista, J.; Martínez-de la Puente, J.; Ros-Santaella, J.L.; Pintus, E.; Lopezosa, P.; Bernardo, N.; Comas, M.; Moreno-Rueda, G. Habitat-Dependent Culicoides Species Composition and Abundance in Blue Tit (Cyanistes caeruleus) Nests. Parasitology 2022, 149, 1119–1128. [Google Scholar] [CrossRef]
- Siers, S.; Merkel, J.; Bataille, A.; Vargas, F.H.; Parker, P.G. Ecological Correlates of Microfilariae Prevalence in Endangered Galápagos Birds. J. Parasitol. 2010, 96, 259–272. [Google Scholar] [CrossRef]
- González, A.D.; Matta, N.E.; Ellis, V.A.; Miller, E.T.; Ricklefs, R.E.; Gutiérrez, H.R. Mixed Species Flock, Nest Height, and Elevation Partially Explain Avian Haemoparasite Prevalence in Colombia. PLoS ONE 2014, 9, e100695. [Google Scholar] [CrossRef]
- Campbell, T.W.; Ellis, C.K. Avian and Exotic Animal Hematology and Citology, 3rd ed.; Blackwell Publishing Ltd.: Oxford, UK, 2007. [Google Scholar]
- De La Torre, G.M.; Freitas, F.F.; Fratoni, R.D.O.; Guaraldo, A.D.C.; Dutra, D.D.A.; Braga, M.; Manica, L.T. Hemoparasites and Their Relation to Body Condition and Plumage Coloration of the White-Necked Thrush (Turdus albicollis). Ethol. Ecol. Evol. 2020, 32, 509–526. [Google Scholar] [CrossRef]
- Atawal, A.F.; Mgbeahuruike, A.C.; Hammers, M. Microfilarial Infections Associated with Body Mass Loss of Village Weavers Ploceus cucullatus. Ostrich 2019, 90, 41–44. [Google Scholar] [CrossRef]
- Höglund, J.; Alatalo, R.V.; Lundberg, A. The Effects of Parasites on Male Ornaments and Female Choice in the Lek-Breeding Black Grouse (Tetrao tetrix). Behav. Ecol. Sociobiol. 1992, 30, 71–76. [Google Scholar] [CrossRef]
- Valera, F.; Hoi, H.; Krištín, A. Parasite Pressure and Its Effects on Blood Parameters in a Stable and Dense Population of the Endangered Lesser Grey Shrike. Biodivers. Conserv. 2006, 15, 2187–2195. [Google Scholar] [CrossRef]
- Davidar, P.; Morton, E.S. Are Multiple Infections More Severe for Purple Martins (Progne subis) than Single Infections? Auk 2006, 123, 141–147. [Google Scholar] [CrossRef]
- Larrat, S.; Dallaire, A.D.; Lair, S. Emaciation and Larval Filarioid Nematode Infection in Boreal Owls (Aegolius funereus). Avian Pathol. 2012, 41, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Binkienė, R.; Chagas, C.R.F.; Bernotienė, R.; Valkiūnas, G. Molecular and Morphological Characterization of Three New Species of Avian Onchocercidae (Nematoda) with Emphasis on Circulating Microfilariae. Parasites Vectors 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E.; Barbuto, M.; Bain, O.; Galimberti, A.; Uni, S.; Guerrero, R.; Ferté, H.; Bandi, C.; Martin, C.; Casiraghi, M. Integrated Taxonomy: Traditional Approach and DNA Barcoding for the Identification of Filarioid Worms and Related Parasites (Nematoda). Front. Zool. 2009, 6, 1. [Google Scholar] [CrossRef]
- Bain, O.; Casiraghi, M.; Martin, C.; Uni, S. The Nematoda Filarioidea: Critical Analysis Linking Molecular and Traditional Approaches. Parasite 2008, 15, 342–348. [Google Scholar] [CrossRef]
- Eamsobhana, P.; Lim, P.-E.; Yong, H.S. Molecular Phylogeny of Filarial Worms (Nematoda: Filarioidea). Raffles Bull. Zool. 2013, 29, 99–109. [Google Scholar]
- Chagas, C.R.F.; Binkienė, R.; Valkiūnas, G. Description and Molecular Characterization of Two Species of Avian Blood Parasites, with Remarks on Circadian Rhythms of Avian Haematozoa Infections. Animals 2021, 11, 3490. [Google Scholar] [CrossRef] [PubMed]
- Hamer, G.L.; Anderson, T.K.; Berry, G.E.; Makohon-Moore, A.P.; Crafton, J.C.; Brawn, J.D.; Dolinski, A.C.; Krebs, B.L.; Ruiz, M.O.; Muzzall, P.M.; et al. Prevalence of Filarioid Nematodes and Trypanosomes in American Robins and House Sparrows, Chicago USA. Int. J. Parasitol. 2013, 2, 42–49. [Google Scholar] [CrossRef]
- Garrido-Bautista, J.; Soria, A.; Trenzado, C.E.; Pérez-Jiménez, A.; Ros-Santaella, J.L.; Pintus, E.; Bernardo, N.; Comas, M.; Moreno-Rueda, G. Oxidative Status of Blue Tit Nestlings Varies with Habitat and Nestling Size. Comp. Biochem. Physiol. A 2021, 258, 110986. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Bautista, J.; Soria, A.; Trenzado, C.E.; Pérez-Jiménez, A.; Pintus, E.; Ros-Santaella, J.L.; Bernardo, N.; Comas, M.; Kolenčík, S.; Moreno-Rueda, G. Within-Brood Body Size and Immunological Differences in Blue Tit (Cyanistes caeruleus) Nestlings Relative to Ectoparasitism. Avian Res. 2022, 13, 100038. [Google Scholar] [CrossRef]
- Moreno-Rueda, G. Selección de Cajas-Nido Por Aves Insectívoras En Sierra Nevada. Zool. Baetica 2003, 13/14, 131–138. [Google Scholar]
- Schlicht, E.; Kempenaers, B. Immediate Effects of Capture on Nest Visits of Breeding Blue Tits, Cyanistes caeruleus, Are Substantial. Anim. Behav. 2015, 105, 63–78. [Google Scholar] [CrossRef]
- Perrins, C.M. British Tits; Collins: Glasgow, UK, 1979. [Google Scholar]
- Owen, J.C. Collecting, Processing, and Storing Avian Blood: A Review. J. Field Ornithol. 2011, 82, 339–354. [Google Scholar] [CrossRef]
- de Jong, A. Less Is Better. Avoiding Redundant Measurements in Studies on Wild Birds in Accordance to the Principles of the 3Rs. Front. Vet. Sci. 2019, 6, 195. [Google Scholar] [CrossRef]
- Casiraghi, M.; Anderson, T.J.C.; Bandi, C.; Bazzocchi, C.; Genchi, C. A Phylogenetic Analysis of Filarial Nematodes: Comparison with the Phylogeny of Wolbachia Endosymbionts. Parasitology 2001, 122, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Bensch, S.; Inumaru, M.; Sato, Y.; Lee Cruz, L.; Cunningham, A.A.; Goodman, S.J.; Levin, I.I.; Parker, P.G.; Casanueva, P.; Hernández, M.; et al. Contaminations Contaminate Common Databases. Mol. Ecol. Resour. 2021, 21, 355–362. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucl. Acid. Sym. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. DAMBE7: New and Improved Tools for Data Analysis in Molecular Biology and Evolution. Mol. Biol. Evol. 2018, 35, 1550–1552. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Labocha, M.K.; Hayes, J.P. Morphometric Indices of Body Condition in Birds: A Review. J. Ornithol. 2012, 153, 1–22. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Symonds, M.R.E.; Moussalli, A. A Brief Guide to Model Selection, Multimodel Inference and Model Averaging in Behavioural Ecology Using Akaike’s Information Criterion. Behav. Ecol. Sociobiol. 2011, 65, 13–21. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A Protocol for Data Exploration to Avoid Common Statistical Problems: Data Exploration. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- R Core Team. Development Core Team R: A Language and Environment for Statistical Computing 2020; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Bartoń, K. MuMIn: Multi-Model Inference; R Package Version 1.43.17; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Astudillo, V.G.; Hernández, S.M.; Kistler, W.M.; Boone, S.L.; Lipp, E.K.; Shrestha, S.; Yabsley, M.J. Spatial, Temporal, Molecular, and Intraspecific Differences of Haemoparasite Infection and Relevant Selected Physiological Parameters of Wild Birds in Georgia, USA. Int. J. Parasitol. 2013, 2, 178–189. [Google Scholar] [CrossRef]
- Merino, S.; Moreno, J.; Sanz, J.J.; Arriero, E. Are Avian Blood Parasites Pathogenic in the Wild? A Medication Experiment in Blue Tits (Parus caeruleus). Proc. R. Soc. B Biol. Sci. 2000, 267, 2507–2510. [Google Scholar] [CrossRef]
- Marzal, A.; Lope, F.D.; Navarro, C.; Møller, A.P. Malarial Parasites Decrease Reproductive Success: An Experimental Study in a Passerine Bird. Oecologia 2005, 142, 541–545. [Google Scholar] [CrossRef]
- Christe, P.; Glaizot, O.; Strepparava, N.; Devevey, G.; Fumagalli, L. Twofold Cost of Reproduction: An Increase in Parental Effort Leads to Higher Malarial Parasitaemia and to a Decrease in Resistance to Oxidative Stress. Proc. R. Soc. B Biol. Sci. 2012, 279, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Druilhe, P.; Tall, A.; Sokhna, C. Worms Can Worsen Malaria: Towards a New Means to Roll Back Malaria? Trends Parasitol. 2005, 21, 359–362. [Google Scholar] [CrossRef]
- Merino, S.; Martínez, J.; Møller, A.P.; Barbosa, A.; De Lope, F.; Rodríguez-Caabeiro, F. Blood Stress Protein Levels in Relation to Sex and Parasitism of Barn Swallows (Hirundo rustica). Écoscience 2002, 9, 300–305. [Google Scholar] [CrossRef]
- Zamora-Vilchis, I.; Williams, S.E.; Johnson, C.N. Environmental Temperature Affects Prevalence of Blood Parasites of Birds on an Elevation Gradient: Implications for Disease in a Warming Climate. PLoS ONE 2012, 7, e39208. [Google Scholar] [CrossRef]
- Rooyen, J.V.; Lalubin, F.; Glaizot, O.; Christe, P. Altitudinal Variation in Haemosporidian Parasite Distribution in Great Tit Populations. Parasites Vectors 2013, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Illera, J.C.; López, G.; García-Padilla, L.; Moreno, Á. Factors Governing the Prevalence and Richness of Avian Haemosporidian Communities within and between Temperate Mountains. PLoS ONE 2017, 12, e0184587. [Google Scholar] [CrossRef]
- Álvarez-Ruiz, L.; Megía-Palma, R.; Reguera, S.; Ruiz, S.; Zamora-Camacho, F.J.; Figuerola, J.; Moreno-Rueda, G. Opposed Elevational Variation in Prevalence and Intensity of Endoparasites and Their Vectors in a Lizard. Curr. Zool. 2018, 64, 197–204. [Google Scholar] [CrossRef]
- Moreno-Rueda, G. Elevational Patterns of Blowfly Parasitism in Two Hole Nesting Avian Species. Diversity 2021, 13, 591. [Google Scholar] [CrossRef]
- Møller, A.P.; Christe, P.; Lux, E. Parasitism, Host Immune Function, and Sexual Selection. Q. Rev. Biol. 1999, 74, 3–20. [Google Scholar] [CrossRef]
- Zuk, M.; McKean, K.A. Sex Differences in Parasite Infections: Patterns and Processes. Int. J. Parasitol. 1996, 26, 1009–1024. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.; Zuk, M. Heritable True Fitness and Bright Birds: A Role for Parasites? Science 1982, 218, 384–387. [Google Scholar] [CrossRef] [PubMed]

| Variable | AIC | ΔAIC |
|---|---|---|
| Body condition index models | ||
| Body condition index | 106.7 | 0.00 |
| Body condition index, laying date | 107.5 | 0.87 |
| Null model 1 | 107.9 | 1.23 |
| Body condition index, forest | 108.4 | 1.75 |
| Body condition index, sex | 108.6 | 1.94 |
| Body mass models | ||
| Body mass | 105.9 | 0.00 |
| Body mass, laying date | 106.6 | 0.76 |
| Body mass, forest | 107.6 | 1.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido-Bautista, J.; Harl, J.; Fuehrer, H.-P.; Comas, M.; Smith, S.; Penn, D.J.; Moreno-Rueda, G. Prevalence, Molecular Characterization, and Ecological Associations of Filarioid Helminths in a Wild Population of Blue Tits (Cyanistes caeruleus). Diversity 2023, 15, 609. https://doi.org/10.3390/d15050609
Garrido-Bautista J, Harl J, Fuehrer H-P, Comas M, Smith S, Penn DJ, Moreno-Rueda G. Prevalence, Molecular Characterization, and Ecological Associations of Filarioid Helminths in a Wild Population of Blue Tits (Cyanistes caeruleus). Diversity. 2023; 15(5):609. https://doi.org/10.3390/d15050609
Chicago/Turabian StyleGarrido-Bautista, Jorge, Josef Harl, Hans-Peter Fuehrer, Mar Comas, Steve Smith, Dustin J. Penn, and Gregorio Moreno-Rueda. 2023. "Prevalence, Molecular Characterization, and Ecological Associations of Filarioid Helminths in a Wild Population of Blue Tits (Cyanistes caeruleus)" Diversity 15, no. 5: 609. https://doi.org/10.3390/d15050609
APA StyleGarrido-Bautista, J., Harl, J., Fuehrer, H.-P., Comas, M., Smith, S., Penn, D. J., & Moreno-Rueda, G. (2023). Prevalence, Molecular Characterization, and Ecological Associations of Filarioid Helminths in a Wild Population of Blue Tits (Cyanistes caeruleus). Diversity, 15(5), 609. https://doi.org/10.3390/d15050609










