Feeding Behaviour of the Mite Blattisocius mali on Eggs of the Fruit Flies Drosophila melanogaster and D. hydei
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Materials and General Methodology
2.2. Assay for Feeding Rates of B. mali on Fruit Fly Eggs
2.3. Observations of B. mali Feeding Behaviour on Fruit Fly Eggs
3. Results
3.1. Assay for Feeding Rates of B. mali on Fruit Fly Eggs
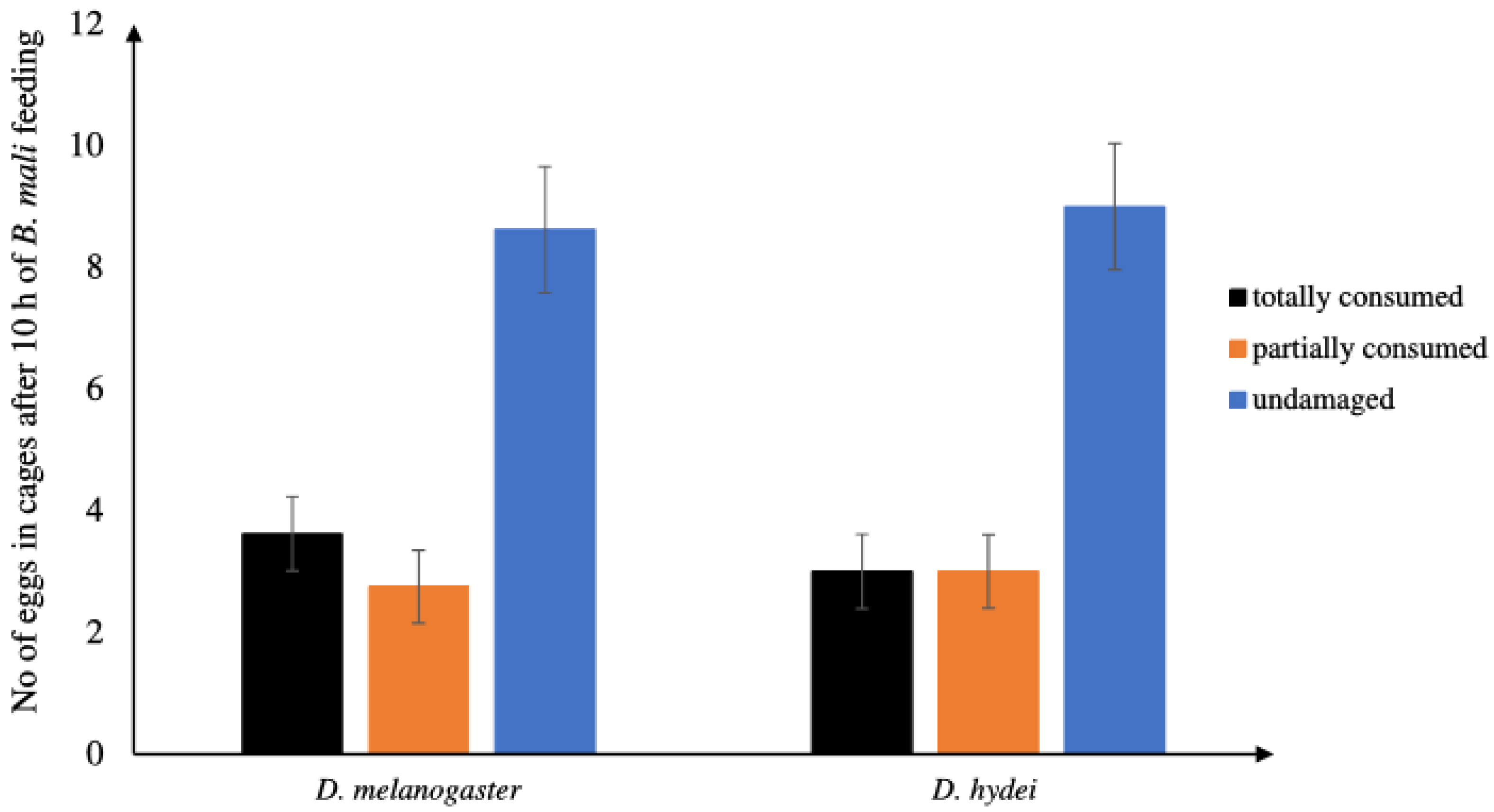
3.1.1. Feeding Rates of B. mali Females on Eggs of D. hydei and D. melanogaster
3.1.2. Hatching of D. melanogaster Larvae from Partially Consumed or Undamaged Eggs
3.1.3. Effect of the Age of D. hydei Eggs on the Feeding Rate of B. mali Females
3.2. Behavioural Observations on the Feeding of B. mali Females on Fruit Fly Eggs
3.2.1. Repertoire of Feeding Behaviours
3.2.2. The Site of the Insertion of Chelicerae into an Egg
3.2.3. Time of Attack and Consumption of an Egg, Partial Egg Consumptions, and Pauses before and after Attack and Egg Consumptions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, P.E.; Rosario, R.M.T. Associations of Mesostigmata with Other Arthropods. Annu. Rev. Entomol. 1988, 33, 393–417. [Google Scholar] [CrossRef]
- Szymkowiak, P.; Gorski, G.; Bajerlein, D. Passive dispersal in arachnids. Biol. Lett. 2007, 44, 75–101. [Google Scholar]
- Huck, K.; Schwarz, H.H.; Schmid-Hempel, P. Host choice in the phoretic mite Parasitellus fucorum (Mesostigmata: Parasitidae): Which bumblebee caste is the best? Oecologia 1998, 115, 385–390. [Google Scholar] [CrossRef]
- De Gasparini, O.; Kilner, R.M. Friend or foe: Inter-specific interactions and conflicts of interest within the family. Ecol. Entomol. 2015, 40, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Kvifte, G.M.; Kaczmarek, S.; Marquardt, T.; Seniczak, A. Linked seasonality between a phoretic mite and its moth fly host (Parasitiformes: Mesostigmata and Diptera: Psychodidae). Acarologia 2022, 62, 956–964. [Google Scholar] [CrossRef]
- Polak, M.; Markow, T.A. Effect of Ectoparasitic Mites on Sexual Selection in a Sonoran Desert Fruit Fly. Evolution 1995, 49, 660–669. [Google Scholar] [CrossRef]
- Horn, C.J.; Luong, L.T. Proximity to parasites reduces host fitness independent of infection in a Drosophila—Macrocheles system. Parasitology 2018, 145, 1564–1569. [Google Scholar] [CrossRef]
- Durkin, E.S.; Proctor, H.; Luong, L.T. Life history of Macrocheles muscaedomesticae (Parasitiformes: Macrochelidae): New insights on life history and evidence of facultative parasitism on Drosophila. Exp. Appl. Acarol. 2019, 79, 309–321. [Google Scholar] [CrossRef]
- Michalska, K.; Mrowińska, A.; Studnicki, M. Ectoparasitism of the Flightless Drosophila melanogaster and D. hydei by the Mite Blattisocius mali (Acari: Blattisociidae). Insects 2023, 14, 146. [Google Scholar] [CrossRef]
- Flanders, S.E.; Badgley, M.E. Prey-predator interactions in self-balanced laboratory populations. Hilgardia 1963, 35, 145–183. [Google Scholar] [CrossRef]
- Toda, M.J. DrosWLD: Taxonomic Information Database for World Species of Drosophilidae. Available online: https://bioinfo.museum.hokudai.ac.jp/db/index.php (accessed on 20 March 2023).
- Máca, J.; Otranto, D. Drosophilidae feeding on animals and the inherent mystery of their parasitism. Parasit. Vectors 2014, 7, 516. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M. Drosophila: A Laboratory Handbook and Manual; Two Volumes; Cold Spring Harbor Laboratory: New York, NY, USA, 1989; ISBN 9780879693213. [Google Scholar]
- Poinar, G.O.; Grimaldi, D.A. Fossil and Extant Macrochelid Mites (Acari: Macrochelidae) Phoretic on Drosophilid Flies (Diptera: Drosophilidae). J. N. Y. Entomol. Soc. 1990, 98, 88–92. [Google Scholar]
- Lehtinen, P.T.; Aspi, J. Aspi, J. A Phytoseiid mite Paragarmania mali, associated with drosophilid flies. In Acari Physiological and Ecological Aspects of Acari-Host Relationships; European Association of Acarologists: Krynica, Poland, 1992; pp. 537–544. [Google Scholar]
- Perez-Leanos, A.; Loustalot-Laclette, M.R.; Nazario-Yepiz, N.; Markow, T.A. Ectoparasitic mites and their Drosophila hosts. Fly 2017, 11, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Kerezis, V.; Kiss, B.; Deucht, F.; Kontschan, J. First record of Blattisocius mali (Oudemans, 1929) in Hungary associated with the drosophilid fly Phortica semivirgo (Máca, 1977). Redia 2019, 102, 69–72. [Google Scholar] [CrossRef]
- Polak, M. Effects of Ectoparasitism on Host Condition in the Drosophila–Macrocheles System. Ecology 1998, 79, 1807–1817. [Google Scholar] [CrossRef]
- Luong, L.T.; Penoni, L.R.; Horn, C.J.; Polak, M. Physical and physiological costs of ectoparasitic mites on host flight endurance. Ecol. Entomol. 2015, 40, 518–524. [Google Scholar] [CrossRef]
- Polak, M. Ectoparasitic Effects on Host Survival and Reproduction: The Drosophila–Macrocheles Association. Ecology 1996, 77, 1379–1389. [Google Scholar] [CrossRef]
- Axtell, R.C. New Records of North American Macrochelidae (Acarina: Mesostigmata) and Their Predation Rates on the House Fly. Ann. Entomol. Soc. Am. 1961, 54, 748. [Google Scholar] [CrossRef]
- de Moraes, G.J.; Venancio, R.; dos Santos, V.L.V.; Paschoal, A.D. Potential of Ascidae, Blattisociidae and Melicharidae (Acari: Mesostigmata) as Biological Control Agents of Pest Organisms. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Springer International Publishing: Cham, Switzerland, 2015; pp. 33–75. [Google Scholar]
- Gallego, J.R.; Gamez, M.; Cabello, T. Potential of the Blattisocius mali Mite (Acari: Blattisociidae) as a Biological Control Agent of Potato Tubermoth (Lepidoptera: Gelechiidae) in Stored Potatoes. Potato Res. 2020, 63, 241–251. [Google Scholar] [CrossRef]
- Pirayeshfar, F.; Moayeri, H.R.S.; Da Silva, G.L.; Ueckermann, E.A. Comparison of biological characteristics of the predatory mite Blattisocius mali (Acari: Blattisocidae) reared on frozen eggs of Tyrophagus putrescentiae (Acari: Acaridae) alone and in combination with cattail and olive pollens. Syst. Appl. Acarol. 2022, 27, 399–409. [Google Scholar] [CrossRef]
- Carton, Y.; David, J.R. Relation between the genetic variability of digging behavior of Drosophila larvae and their susceptibility to a parasitic wasp. Behav. Genet. 1985, 15, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, J.; Dominguez, A. Genetic analysis of Drosophila melanogaster egg insertion behavior. Behav. Genet. 1987, 17, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, B.; Godoy-Herrera, R.; Mora, W. The development of larval movement patterns in Drosophila. Heredity 1987, 58, 321–329. [Google Scholar] [CrossRef]
- Darst, P.H.; King, E.W. Biology of Melichares tarsalis in association with Plodia interpunctella. Ann. Entomol. Soc. Am. 1969, 62, 747–749. [Google Scholar] [CrossRef]
- Nielsen, S. The use of Blattisocius tarsalis (Acari: Ascidae) for biological control in flour mills. In Proceedings of the 7th Intertuuional Working Conference on Stored-Product Protection, Beijing, China, 14–19 October 1998; Volume 2, pp. 1265–1268. [Google Scholar]
- Nielsen, P.S. Predation by Blattisocius tarsalis (Berlese) (Acari: Ascidae) on eggs of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2003, 39, 395–400. [Google Scholar] [CrossRef]
- Osterfield, M.; Berg, C.A.; Shvartsman, S.Y. Epithelial Patterning, Morphogenesis, and Evolution: Drosophila Eggshell as a Model. Dev. Cell 2017, 41, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Horne-Badovinac, S. The Drosophila micropyle as a system to study how epithelia build complex extracellular structures. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190561. [Google Scholar] [CrossRef]
- Kopecký, J.; Nesvorná, M.; Hubert, J. Bartonella-like bacteria carried by domestic mite species. Exp. Appl. Acarol. 2014, 64, 21–32. [Google Scholar] [CrossRef]
- Lourenço, F.; Calado, R.; Medina, I.; Ameixa, O.M.C.C. The Potential Impacts by the Invasion of Insects Reared to Feed Livestock and Pet Animals in Europe and Other Regions: A Critical Review. Sustainability 2022, 14, 6361. [Google Scholar] [CrossRef]
- Dabert, J.; Mironov, S.V.; Dabert, M. The explosive radiation, intense host-shifts and long-term failure to speciate in the evolutionary history of the feather mite genus Analges (Acariformes: Analgidae) from European passerines. Zool. J. Linn. Soc. 2022, 195, 673–694. [Google Scholar] [CrossRef]
- Dabert, M.; Witalinski, W.; Kazmierski, A.; Olszanowski, Z.; Dabert, J. Molecular phylogeny of acariform mites (Acari, Arachnida): Strong conflict between phylogenetic signal and long-branch attraction artifacts. Mol. Phylogenet. Evol. 2010, 56, 222–241. [Google Scholar] [CrossRef] [PubMed]
- Mironov, S.V.; Dabert, J.; Dabert, M. A new feather mite species of the genus Proctophyllodes Robin, 1877 (Astigmata: Proctophyllodidae) from the Long-tailed Tit Aegithalos caudatus (Passeriformes: Aegithalidae)—Morphological description with DNA barcode data. Zootaxa 2012, 3253, 54. [Google Scholar] [CrossRef]
- Shaffer, C.D.; Wuller, J.M.; Elgin, S.C.R. Raising Large Quantities of Drosophila for Biochemical Experiments. Methods Cell Biol. 1994, 44, 99–108. [Google Scholar] [PubMed]
- R Core Team. Team, R.D.C. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Binns, E.S. Phoresy as Migration—Some Functional Aspects of Phoresy in Mites. Biol. Rev. 1982, 57, 571–620. [Google Scholar] [CrossRef]
- Bowman, C.E. Studies on feeding in the soil predatory mite Pergamasus longicornis (Berlese) (Mesostigmata: Parasitidae) using dipteran and microarthropod prey. Exp. Appl. Acarol. 1987, 3, 201–206. [Google Scholar] [CrossRef]
- Houck, M.A.; Clark, J.B.; Peterson, K.R.; Kidwell, M.G. Possible Horizontal Transfer of Drosophila Genes by the Mite Proctolaelaps regalis. Science 1991, 253, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- Esteca, F.; de Cassia Neves, F. Environmental Management Strategies for Pest Control in Strawberry Crop. Ph.D. Thesis, Universidade de São Paulo, Piracicaba, Brazil, 2021. [Google Scholar]
- Nawar, M.S.; Rakha, M.A.; Ali, F.S. Laboratory studies on the predaceous mite, Lasioseius bispinosus Evans (Acari: Mesostigmata: Ascidae) on various kinds of food substances. Bull. Soc. Entomol. 1990, 69, 247–255. [Google Scholar]
- Margaritis, L.H.; Kafatos, F.C.; Petri, W.H. The eggshell of Drosophila melanogaster. I. Fine structure of the layers and regions of the wild-type eggshell. J. Cell Sci. 1980, 43, 1–35. [Google Scholar] [CrossRef]
- Papanikolaou, N.E.; Kypraios, T.; Moffat, H.; Fantinou, A.; Perdikis, D.P.; Drovandi, C. Predators’ Functional Response: Statistical Inference, Experimental Design, and Biological Interpretation of the Handling Time. Front. Ecol. Evol. 2021, 9, 749. [Google Scholar] [CrossRef]
- Wernz, J.G.; Krantz, G.W. Studies on the function of the tritosternum in selected Gamasida (Acari). Can. J. Zool. 1976, 54, 202–213. [Google Scholar] [CrossRef]
- Hoyt, S.C. Effect of Short Feeding Periods by Metaseiulus occidentalis on Fecundity and Mortality of Tetranychus mcdanieli. Ann. Entomol. Soc. Am. 1970, 63, 1382–1384. [Google Scholar] [CrossRef]
- Sandness, J.N.; McMurtry, J.A. Prey consumption behavior of Amblyseius largoensis in relation to hunger. Can. Entomol. 1972, 104, 461–470. [Google Scholar] [CrossRef]
- Flechtmann, C.H.W.; McMurtry, J.A. Studies on how Phytoseiid mites feed on spider mites and pollen. Int. J. Acarol. 1992, 18, 157–162. [Google Scholar] [CrossRef]
- Fantinou, A.A.; Perdikis, D.C.; Maselou, D.A.; Lambropoulos, P.D. Prey killing without consumption: Does Macrolophus pygmaeus show adaptive foraging behaviour? Biol. Control 2008, 47, 187–193. [Google Scholar] [CrossRef]
- Holling, C.S. The functional response of predators to prey density and its role in mimicry and population regulation. Mem. Entomol. Soc. Can. 1965, 45, 5–60. [Google Scholar] [CrossRef]
- Johnson, D.M.; Akre, B.G.; Crowley, P.H. Modeling arthropod predation: Wasteful killing by damselfly naiads. Ecology 1975, 56, 1081–1093. [Google Scholar] [CrossRef]
- Samu, F. Wolf spider feeding strategies: Optimality of prey consumption in Pardosa hortensis. Oecologia 1993, 94, 139–145. [Google Scholar] [CrossRef]
- Ballard, R.C. The Biology of the Predacious Mite Typhlodromus Fallacis (Garman) (Phytoseiidae) at 78 degrees F. Ohio J. Sci. 1954, 54, 175–179. [Google Scholar]
- Jeong, Y.E.; Chung, H.M.; Ahn, T.I. Utilization of a storage protein in the embryonic development of Drosophila and Xenopus. Korean J. Biol. Sci. 2001, 5, 85–90. [Google Scholar] [CrossRef]
- Bownes, M. A photographic study of development in the living embryo of Drosophila melanogaster. J. Embryol. Exp. Morphol. 1975, 33, 789–801. [Google Scholar] [CrossRef]
- Akyazi, R.; Altunc, Y.E.; Soysal, M. Amblyseius swirskii ve Neoseiulus californicus (Mesostigmata: Phytoseiidae)’un Tüketim Kapasitesine Avın Yumurta Yaşının Etkisi. Türk Tarım Doğa Bilim. Derg. 2018, 5, 628–633. [Google Scholar] [CrossRef]
- Cavalcante, A.C.C.; Borges, L.R.; Lourenção, A.L.; de Moraes, G.J. Potential of two populations of Amblyseius swirskii (Acari: Phytoseiidae) for the control of Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) in Brazil. Exp. Appl. Acarol. 2015, 67, 523–533. [Google Scholar] [CrossRef] [PubMed]
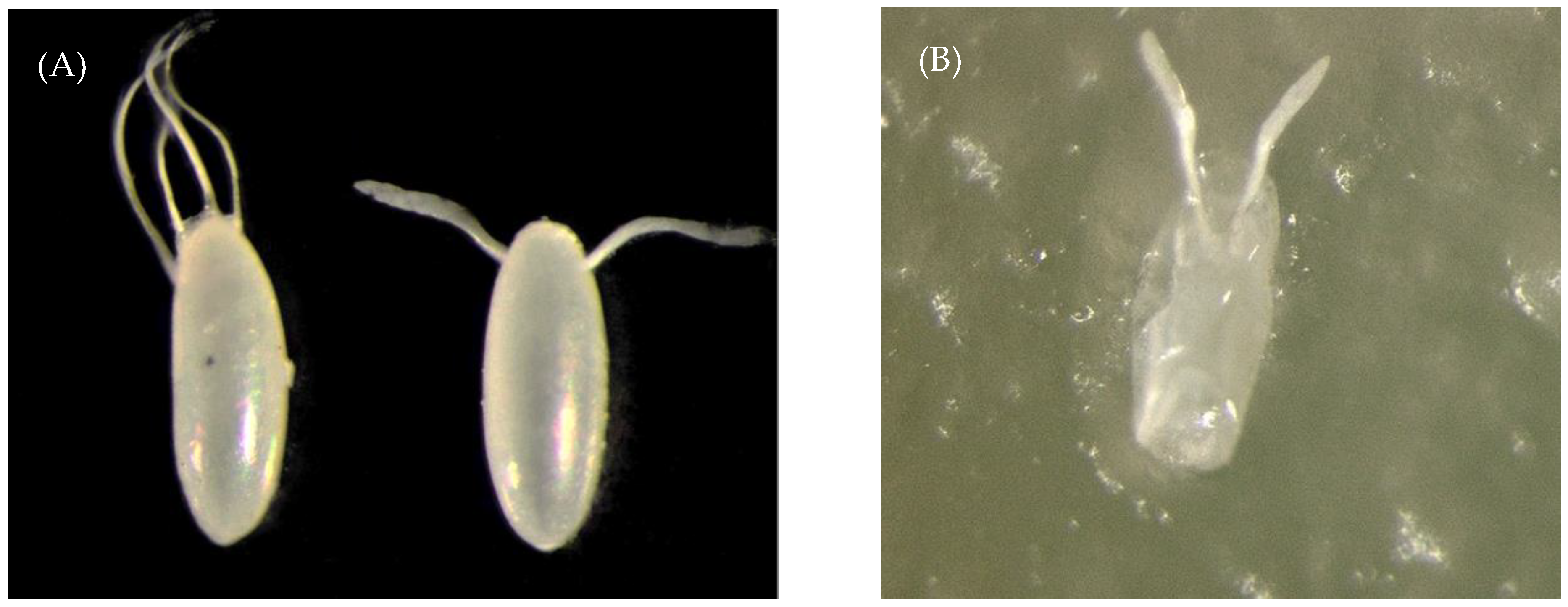



| Eggs Partially Consumed by a Predator | Eggs Undamaged by a Predator | Eggs from Control Cages | ||
|---|---|---|---|---|
| Transferred | Left | Transferred | Left | |
| 0 | 0 | 0.56 ± 0.095 | 0.58 ± 0.031 | 0.53 ± 0.022 |
| (N = 7) | (N = 7) | (N = 7) | (N = 7) | (N = 14) |
| Mean Time | Fruit Fly Species | Statistics | |||
|---|---|---|---|---|---|
| D. melanogaster | D. hydei | t Test | Degree of Fredom | p-Value | |
| Pause before attack and consumption of the first egg | 302.80 ± 49.36 (93–556) N = 10 | 429.63 ± 57.31 (159–689) N = 8 | −3.68 | 16 | 0.0115 |
| Attack and egg consumption | 89.41 ± 7.68 (6–286) N = 58 | 67.98 ± 6.34 (5–187) N = 65 | 2.15 | 121 | 0.0335 |
| First attack and partial egg consumption | 44.80 ± 8.45 (4–121) N = 22 | 21.36 ± 4.69 (5–120) N = 23 | 2.55 | 43 | 0.0143 |
| Second attack and partial egg consumption | 83.00 ± 20.91 (6–224) N = 11 | 57.88 ± 14.77 (15–137) N = 9 | 0.88 | 18 | 0.3394 |
| Pause after attack and egg consumption | 5218.43 ± 527.30 (88–16,445) N = 68 | 4447.59 ± 518.62 (21–18,883) N = 71 | 1.04 | 137 | 0.2991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalska, K.; Mrowińska, A.; Studnicki, M.; Jena, M.K. Feeding Behaviour of the Mite Blattisocius mali on Eggs of the Fruit Flies Drosophila melanogaster and D. hydei. Diversity 2023, 15, 652. https://doi.org/10.3390/d15050652
Michalska K, Mrowińska A, Studnicki M, Jena MK. Feeding Behaviour of the Mite Blattisocius mali on Eggs of the Fruit Flies Drosophila melanogaster and D. hydei. Diversity. 2023; 15(5):652. https://doi.org/10.3390/d15050652
Chicago/Turabian StyleMichalska, Katarzyna, Agnieszka Mrowińska, Marcin Studnicki, and Manoj Kumar Jena. 2023. "Feeding Behaviour of the Mite Blattisocius mali on Eggs of the Fruit Flies Drosophila melanogaster and D. hydei" Diversity 15, no. 5: 652. https://doi.org/10.3390/d15050652
APA StyleMichalska, K., Mrowińska, A., Studnicki, M., & Jena, M. K. (2023). Feeding Behaviour of the Mite Blattisocius mali on Eggs of the Fruit Flies Drosophila melanogaster and D. hydei. Diversity, 15(5), 652. https://doi.org/10.3390/d15050652






