DNA vs. Morphology in Delineating Species Boundaries of Endemic Mongolian Eodorcadion Taxa (Coleoptera: Cerambycidae)
Abstract
:1. Introduction
2. Materials and Methods
| Gene | Primer Name | Direction | Sequence | Reference |
|---|---|---|---|---|
| COI | LCO1490 | F | GGTCAACAAATCATAAAGATATTGG | [9] |
| COI | HCO2198 | R | TAAACTTCAGGGTGACCAAAAATCA | [9] |
| COI | bycF | F | TTTCAACWAACCAYAAAGATATTGG | unpublished data |
| COI | bycR | R | TAAACTTCWGGATGWCCAAAAAATC | unpublished data |
| CAD | CD338F | F | ATGAARTAYGGYAATCGTGGHCAYAA | [27] |
| CAD | CD668R | R | ACGACTTCATAYTCNACYTCYTTCCA | [28] |
| CAD | CD688R | R | TGTATACCTAGAGGATCDACRTTYTCCATRTTRCA | [28] |
| ITS2 | 5.8S_cbgp_F | F | TCGATGAARRMCGCAGYDAAHTG | [19] |
| ITS2 | 28S_cbgp_R1 | R | GATATGYTTAAATTCRGSGGGT | [29] |
| Histone 3 | H3F | F | ATGGCTCGTACCAAGCAGACVGC | [30,31] |
| Histone 3 | H3R | R | ATATCCTTRGGCATRATRGTGAC | [30,31] |
3. Results
3.1. Molecular Analysis
3.2. Morphology
3.3. Phylogenetic Analysis
4. Discussion
4.1. Molecular Insight into Dorcadionini
4.2. Morphological Turmoil as a Result of Ecological Adaptation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danilevsky, M.L. Volume 6/1: Chrysomeloidea I (Vesperidae, Disteniidae, Cerambycidae). In Catalogue of Palaearctic Coleoptera, 2nd ed.; Brill: Leiden, The Netherlands; Boston, MA, USA, 2020; p. 712. [Google Scholar]
- Tavakilian, G.; Chevillotte, H. Titan Database about Longhorns or Timber-Beetles (Cerambycidae). Available online: http://titan.gbif.fr (accessed on 20 February 2023).
- Souza, D.S.; Marinoni, L.; Monné, M.L.; Gómez-Zurita, J. Molecular phylogenetic assessment of the tribal classification of Lamiinae (Coleoptera: Cerambycidae). Mol. Phylogenet. Evol. 2020, 145, 106736. [Google Scholar] [CrossRef] [PubMed]
- Danilevsky, M.L. Collection systematique. In Revision of the genus Eodorcadion Breuning, 1947 (Coleoptera, Cerambycidae); Magellanes: Andrésy, France, 2007; Volume 16, p. 230. [Google Scholar]
- Danilevsky, M.L. Review of Eodorcadion Breuning, 1946 of “intermedium-group” from Mongolia and China with a description of a new species (Coleoptera, Cerambycidae). Les Cah. Magellanes 2004, 33, 1–18. [Google Scholar]
- Karpiński, L.; Enkhnasan, D.; Boldgiv, B.; Kruszelnicki, L.; Iderzorig, B.; Gantulga, T.; Dorjsuren, A.; Szczepański, W.T. Longhorned beetles (Coleoptera: Cerambycidae) of southeastern Mongolia with particular emphasis on the genus Anoplistes Audinet-Serville, 1833 (Cerambycinae: Trachyderini). Zootaxa 2021, 5081, 451–482. [Google Scholar] [CrossRef]
- Karpiński, L.; Szczepański, W.T.; Boldgiv, B.; Walczak, M. New data on the longhorn beetles of Mongolia with particular emphasis on the genus Eodorcadion Breuning, 1947 (Coleoptera, Cerambycidae). ZooKeys 2018, 739, 107–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MNET (Ministry of Nature, Environment and Tourism of Mongolia). Sixth National Report to the Convention on Biological Diversity (2015–2018); Government of Mongolia: Ulaanbaatar, Mongolia, 2019; p. 168.
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Miller, K.B.; Alarie, Y.; Wolfe, G.W.; Whiting, M.F. Association of insect life stages using DNA sequences: The larvae of Philodytes umbrinus (Motschulsky) (Coleoptera: Dytiscidae). Syst. Entomol. 2005, 30, 499–509. [Google Scholar] [CrossRef]
- Schmidt, S.; Schmid-Egger, C.; Morinière, J.; Haszprunar, G.; Hebert, P.D.N. DNA barcoding largely supports 250 years of classical taxonomy: Identifications for Central European bees (Hymenoptera, Apoidea partim). Mol. Ecol. Resour. 2015, 15, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Grebennikov, V.V.; Jendek, E.; Smirnov, M.E. Diagnostic and phylogenetic utility of the first DNA barcode library for long-horn beetles (Coleoptera: Cerambycidae) from the Russian FarEast. Zootaxa 2017, 4276, 441–445. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, X.; Li, G.; Mu, R.R.; Zhang, H.J. With DNA barcoding revealing sexual dimorphism in a water mite: The first description of male Sperchon fuxiensis (Acari: Hydrachnidia: Sperchontidae). Zootaxa 2019, 4560, 385–392. [Google Scholar] [CrossRef]
- Karpiński, L.; Gorring, P.; Kruszelnicki, L.; Kasatkin, D.G.; Szczepański, W.T. A fine line between species and ecotype: A case study of Anoplistes halodendri and A. kozlovi (Coleoptera: Cerambycidae) occurring sympatrically in Mongolia. Arthropod. Syst. Phylogeny 2021, 79, 1–23. [Google Scholar] [CrossRef]
- Karpiński, L.; Szczepański, W.T.; Plewa, R.; Kruszelnicki, L.; Koszela, K.; Hilszczański, J. The first molecular insight into the genus Turanium Baeckmann, 1922 (Coleoptera: Cerambycidae: Callidiini) with a description of a new species from Middle Asia. Arthropod. Syst. Phylogeny 2021, 79, 465–484. [Google Scholar] [CrossRef]
- Raupach, M.J.; Hannig, K.; Morinière, J.; Hendrich, L. A DNA barcode library for ground beetles of Germany: The genus Agonum Bonelli, 1810 (Insecta, Coleoptera, Carabidae). Dtsch. Entomol. Z. 2020, 67, 197–207. [Google Scholar] [CrossRef]
- Gorring, P.S.; Farrell, B.D. Evaluating species boundaries using coalescent delimitation in pine-killing Monochamus (Coleoptera: Cerambycidae) sawyer beetles. Mol. Phylogenet. Evol. 2023, 107777, in press. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, S. Multigene phylogeny uncovers oviposition-related evolutionary history of Cerambycinae (Coleoptera: Cerambycidae). Mol. Phylogenet. Evol. 2020, 145, 106707. [Google Scholar] [CrossRef]
- Dascălu, M.M.; Caba, F.G.; Fusu, L. DNA barcoding in Dorcadionini (Coleoptera, Cerambycidae) uncovers mitochondrial-morphological discordance and the hybridogenic origin of several subspecies. Org. Divers. Evol. 2021, 22, 205–229. [Google Scholar] [CrossRef]
- Karpiński, L.; Gorring, P.; Hilszczański, J.; Szczepański, W.T.; Plewa, R.; Łoś, K.; Cognato, A.I. Integrative taxonomy tests possible hybridisation between Central Asian cerambycids (Coleoptera). Zool. Scr. 2022, 52, 70–85. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.70. Available online: http://www.mesquiteproject.org (accessed on 20 December 2022).
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. Partitionfinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z. Molecular Evolution: A Statistical Approach; Oxford University Press: Oxford, UK, 2014; p. 512. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Moulton, J.K.; Wiegmann, B.M. Evolution and phylogenetic utility of CAD (rudimentary) among Mesozoic-aged Eremoneuran Diptera (Insecta). Mol. Phylogenet. Evol. 2004, 31, 363–378. [Google Scholar] [CrossRef]
- Wild, A.L.; Maddison, D.R. Evaluating nuclear protein-coding genes for phylogenetic utility in beetles. Mol. Phylogenet. Evol. 2008, 48, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Germain, J.F.; Chatot, C.; Meusnier, I.; Artige, E.; Rasplus, J.Y.; Cruaud, A. Molecular identification of Epitrix potato flea beetles (Coleoptera: Chrysomelidae) in Europe and North America. Bull. Entom. Res. Lond. 2013, 103, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Colgan, D.J.; McLauchlan, A.; Wilson, G.D.F.; Livingston, S.P.; Edgecombe, G.D.; Macaranas, J.; Cassis, G.; Gray, M.R. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Aust. J. Zool. 1998, 46, 419–437. [Google Scholar] [CrossRef]
- Colgan, D.J.; Ponder, W.F.; Eggler, P.E. Gastropod evolutionary rates and phylogenetic relationships assessed using partial 28S rDNA and histone H3 sequences. Zool. Scr. 2000, 29, 29–63. [Google Scholar] [CrossRef]
- Hendrich, L.; Morinière, J.; Haszprunar, G.; Hebert, P.D.N.; Hausmann, A.; Köhler, F.; Balke, M. A comprehensive DNA barcode database for Central European beetles with a focus on Germany: Adding more than 3500 identified species to BOLD. Mol. Ecol. Resour. 2015, 15, 795–818. [Google Scholar] [CrossRef]
- Pentinsaari, M.; Hebert, P.D.N.; Mutanen, M. Barcoding beetles: A regional survey of 1872 species reveals high identification success and unusually deep interspecific divergences. PLoS ONE 2014, 9, e108651. [Google Scholar] [CrossRef] [Green Version]
- Sama, G.; Dascălu, M.M.; Pesarini, C. Description of Dorcadion gashtarovi n. sp. (Coleoptera, Cerambycidae) from Romania and Bulgaria with review of the closely related species. N. West. J. Zool. 2010, 6, 286–293. [Google Scholar]
- Danilevsky, M.L.; Kasatkin, D.G.; Rubenyan, A.A. Revision of the taxonomic structure of the tribe Dorcadionini (Coleoptera: Cerambycidae) on the base of endophallic morphology. Russ. Entomol. J. 2005, 13, 127–149. [Google Scholar]


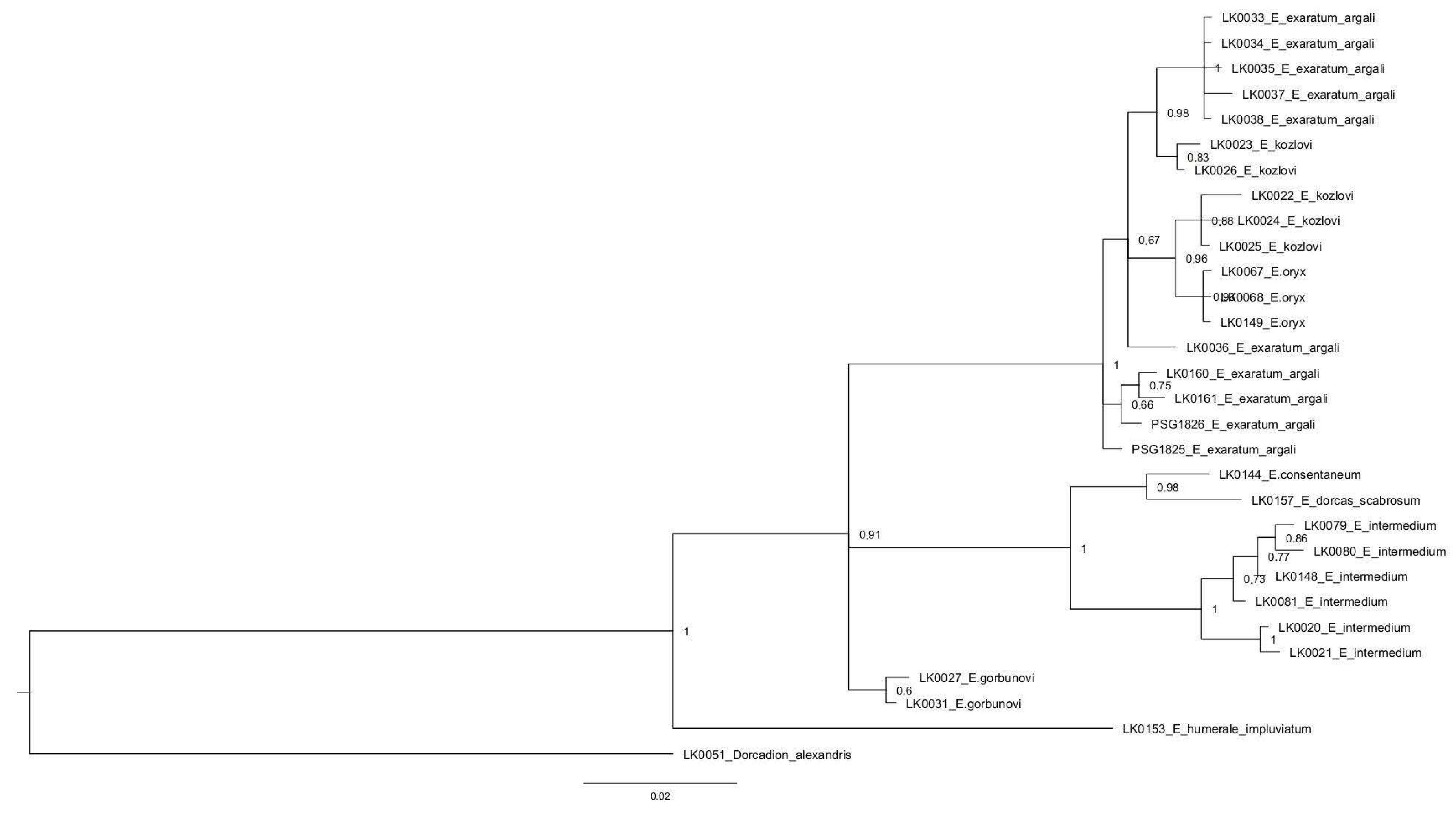
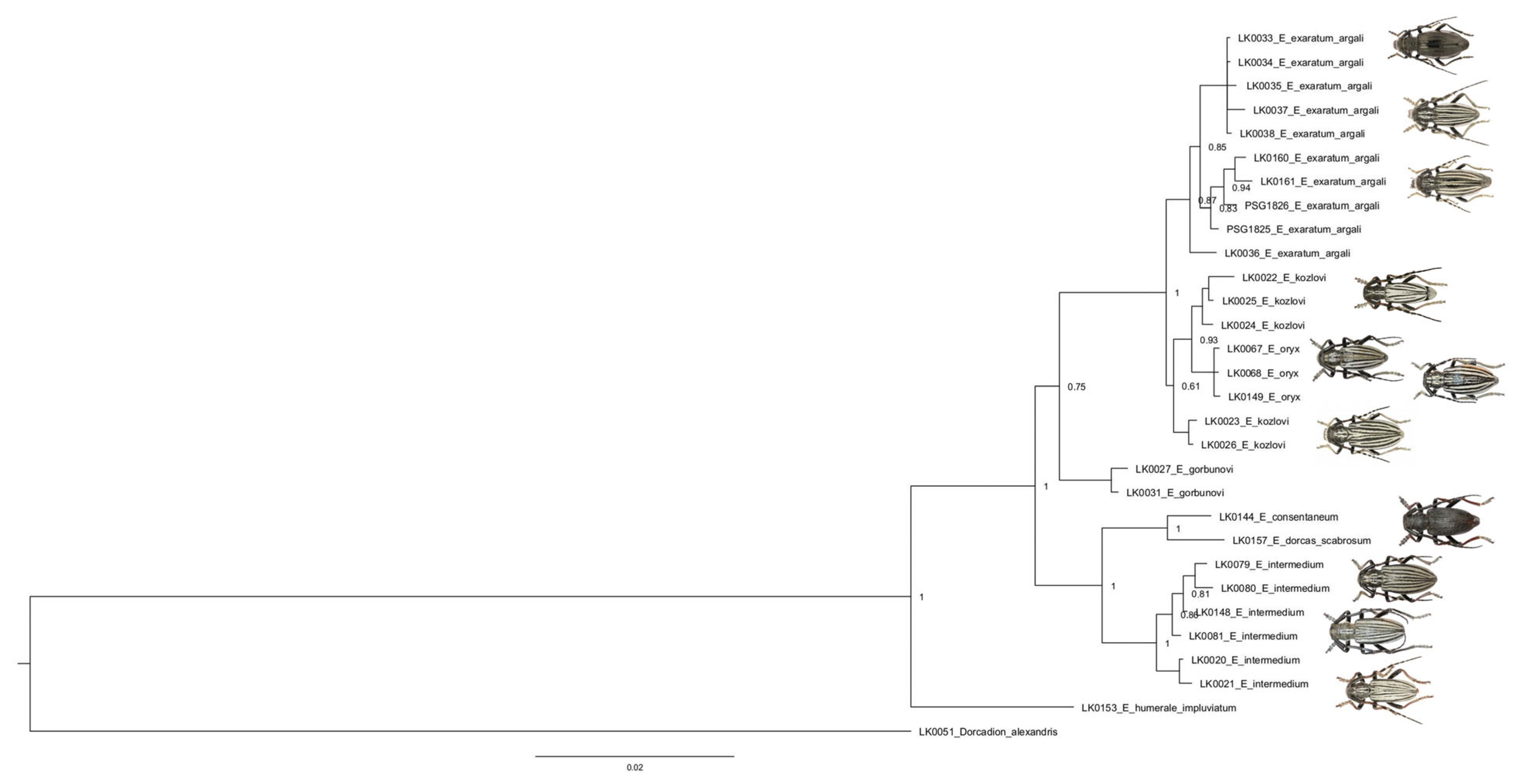
| Intraspecific Distances [%] | No of Spec. | Species | Interspecific Distances [%] | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| 0.0–2.3 | 10 | Eodorcadion exaratum | 1 | - | 1.2–2.3 | 0.6–2.3 | 6.2–7.2 |
| 0.0 | 3 | Eodorcadion oryx | 2 | 1.2–2.3 | - | 0.5–1.8 | 6.2–7.0 |
| 0.2–2.0 | 5 | Eodorcadion kozlovi | 3 | 0.6–2.3 | 0.5–1.8 | - | 5.8–7.0 |
| 0.2–1.2 | 6 | Eodorcadion intermedium | 4 | 6.2–7.2 | 6.2–7.0 | 5.8–7.0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpiński, L.; Gorring, P.; Cognato, A.I. DNA vs. Morphology in Delineating Species Boundaries of Endemic Mongolian Eodorcadion Taxa (Coleoptera: Cerambycidae). Diversity 2023, 15, 662. https://doi.org/10.3390/d15050662
Karpiński L, Gorring P, Cognato AI. DNA vs. Morphology in Delineating Species Boundaries of Endemic Mongolian Eodorcadion Taxa (Coleoptera: Cerambycidae). Diversity. 2023; 15(5):662. https://doi.org/10.3390/d15050662
Chicago/Turabian StyleKarpiński, Lech, Patrick Gorring, and Anthony I. Cognato. 2023. "DNA vs. Morphology in Delineating Species Boundaries of Endemic Mongolian Eodorcadion Taxa (Coleoptera: Cerambycidae)" Diversity 15, no. 5: 662. https://doi.org/10.3390/d15050662
APA StyleKarpiński, L., Gorring, P., & Cognato, A. I. (2023). DNA vs. Morphology in Delineating Species Boundaries of Endemic Mongolian Eodorcadion Taxa (Coleoptera: Cerambycidae). Diversity, 15(5), 662. https://doi.org/10.3390/d15050662








