Abstract
Horticulture is a major pathway of introduction of aquatic plants. Among traded aquatic plants, we found two species belonging to the genus Lagarosiphon Harv. (Hydrocharitaceae), native to South and Central Africa, L. major (Ridl.) Moss and L. cordofanus Casp. L. major is the main representative of the genus, having already been introduced via horticulture sale beyond its native range, and often becoming invasive. In contrast, L. cordofanus is a lesser-known congener that could be potentially sold as an alternative to L. major. It is relatively understudied, and has yet to be recorded in the wild outside its native range. Many factors can promote the invasiveness of an alien plant; among them, increased nutrient availability often facilitates opportunistic alien species such as L. major. In a horizon-scanning perspective, a manipulative experiment under controlled conditions was performed to test the response of L. cordofanus to different trophy levels using L. major as the tolerant alternative species. According to our results, the naturalization of L. cordofanus in temperate shallow waters does not seem likely, especially if considered in comparison to L. major.
1. Introduction
Horticulture is considered a major pathway of introduction of aquatic plants [1], and the strategic importance of regulating the sector to prevent the introduction and spread of alien organisms is globally recognized [2].
Among traded aquatic plants, we found species of the genus Lagarosiphon Harv. (Hydrocharitaceae), native to South and Central Africa, namely, L. major (Ridl.) Moss and L. cordofanus Casp. Traditionally, the former, as easy-to-grow plant, is the main representative of the genus for sale, while among congeners, the latter is one of the few “alternatives” to L. major on the market, even if it is not so frequently traded.
Lagarosiphon major has been introduced via horticultural trade to multiple countries outside its native range, usually sold as an ornamental aquarium plant [3]. It has been accidentally released in the wild in different areas of the globe, and it often becomes invasive in lakes, dams and rivers. There, it can form thick mats that reduce light penetration and often oxygen availability [4]. Moreover, it can successfully outcompete native aquatic macrophyte species [4,5] and alter habitat conditions for indigenous macroinvertebrates and fish communities [5,6]. In heavy infestations, the presence of L. major can even negatively impact human activities, posing a serious threat to the functioning of irrigation and hydroelectric systems [7,8,9]. The costs of management of this aquatic weed are elevated [10] and, once established, the chances of eradication are very low (e.g., [11,12]).
On the other side, L. cordofanus is a quite understudied aquatic species, native to Central and Southern Africa, whose native range encompasses a much wider area than that of L. major, but it is not currently naturalized in other parts of the world [13]. Data about its ecology and potential impacts are still lacking.
One of the main and most-implemented measures to avoid or, at least, reduce problems relating to alien species introduction is trade embargos, complementary to banning the cultivation and release of invasive organisms in nature [14,15]. Indeed, due to its high invasive potential, embargos for L. major are currently active in the Member States of the European Union (Regulation EU n.1143/2014), in the United Kingdom (Invasive Alien Species—Enforcement and Permitting—Order 2019, which retains in UK law the Regulation EU n.1143/2014) and in New Zealand (National Pest Plant Accord), where it is a major water weed. In other countries, other biosecurity measures to prevent its introduction are implemented in several regions (e.g., the National Priority List of Exotic Environmental Pests, Weeds and Diseases in Australia, and the APHIS Federal noxious weed program in US).
While bans on trade may be effective for reducing invasion risks associated with L. major, they may also shift the attention of buyers to closely related species, such as L. cordofanus, as alternatives. In a horizon-scanning perspective [16], it would be prudent to forecast the likelihood of the establishment and possible invasiveness of L. cordofanus in case of its release in the wild. Unfortunately, this is not currently feasible based on the available data [17].
Factors promoting the invasiveness of an alien plant can be multiple, and they are also closely tied to the invasibility of an ecosystem [18]. Changes in ecosystem chemistry and resource availability (e.g., eutrophication and pollution), in particular, are expected to be the major drivers of potential future impacts of alien species [19]. Indeed, species composition can dramatically change in response to increasing levels of nutrient availability, and in many cases, opportunistic species, including many alien species, can take advantage of the situation [20]. Nitrogen and phosphorus are key elements in this process, especially in case of over-enrichment, resulting from increased agricultural intensification and urbanization [21,22]. Under such conditions, nutrient imbalance usually enhances high-biomass-forming microalgae and triggers the shift from macrophytes to phytoplankton communities [21]. Negative effects to submerged macrophytes are mainly due to alterations of light availability for photosynthesis, direct competition for resources and overgrowing phenomena (uncontrolled periphyton growth and mucilage directly on macrophytes) [23]. The latter effect can be highly detrimental for sensitive freshwater plants and their dispersal units due to mechanical damages and photosynthetic activity reductions [24]. These conditions can play a key role in alien species proliferation, as they provide an advantage to ruderal, pioneer plants compared to native species, which are less prone to rapid adaptation and resistance [25].
Therefore, the response of alien species to different trophic conditions could be an important element to consider in assessing the risk of naturalization of traded plants potentially escaping from confined environments, as in the case of L. major and other aquatic plants. Thanks to its broad ecological tolerance, L. major usually thrives in water bodies characterized by different levels of trophy [26]. No information is available in the scientific literature about the response to different trophic conditions of L. cordofanus.
To understand how the trophic conditions of water bodies can influence the growth of L. cordofanus and therefore the risk of naturalization, a manipulative experiment under controlled conditions was performed. The congener L. major was selected to be the alternative because of its well-known resistance to a wide spectrum of nutrient concentrations and its invasiveness in freshwater ecosystems [27].
Along a gradient of trophy, under controlled conditions, viable fragments of L. major and L. cordofanus were transplanted in water-filled tanks enriched with nitrogen (N) and phosphorus (P), simulating four different trophic conditions found in temperate water basins already invaded by L. major in Italy (Italian prealpine lakes). The experiment was conducted by simulating the typical climatic conditions of summer months in temperate areas, where peaks of trophy can easily occur in shallow, slow freshwaters. The response variables considered to evaluate the tolerance of species along the water eutrophication gradient related to plant survival and growth, root emission and plant architecture.
2. Materials and Methods
2.1. Target Species
Lagarosiphon major or curly leaved waterweed is an invasive aquatic perennial plant whose native range lies in southern Africa, in an area between southern Zimbabwe, Botswana and South Africa [28]. L. major thrives in clear, slow, sunny water with temperatures between 10–25 °C, with an optimal growth between 18–23 °C. Photosynthetic activity can increase at higher temperatures (20–25 °C), but it is reduced above 30 °C [29]. Cold temperatures do not represent a substantial limit to L. major persistence in nature, as winter dormancy allows the species to overcome cold winters [7]. It preferentially grows on sandy soils in shallow waters (generally less than 6 m of depth, even with exception in relation to turbidity), as light and increased pressure constrain its growth at greater depths [5,8]. L. major has adaptive capacities that promote its survival in stressful environments, such as those characterized by high-nutrient levels or low inorganic carbon concentration [7,27]. Carbon, nitrogen and phosphorus, together with a significant fine sediment portion in soils, seem to be the main factors influencing L. major growth and competitiveness [8,27,30]. The plant is dioecious, but in its invasive range, sexual reproduction has never been recorded due to lack of male individuals. Successful propagation is vegetative, via the release of viable fragments of stem able to generate new clones [8]. These propagative units are very resistant to desiccation, and even tiny fragments root rapidly to originate new clones, especially in shallow water and unvegetated substrata [31] They are the main unit of dispersal within and between water bodies, via overland transport by human-mediated vectors [32,33];
As L. major and other Lagarosiphon species, L. cordofanus is a perennial dioecious aquatic plant, but its native range is wider than that of L. major, and covers tropical and southern Africa (Angola, Botswana, Cameroon, Democratic Republic of the Congo, Ethiopia, Kenya, Malawi, Mozambique, Namibia, Rwanda, Somalia, South Africa, South Sudan, Sudan, United Republic of Tanzania, Uganda, Zambia, and Zimbabwe) [13,34]. In contrast to L. major, L. cordofanus is not naturalized elsewhere in the world. The information about its ecology and biology is very scarce; however, based on available descriptions, similarities with L. major are evident in relation to colonized habitats, at least from an ecological point of view: it occurs in lakes, dams and ponds, in permanent or temporary water on floodplains, in still or slow flowing freshwaters up to a depth of 2 m, and it prefers sheltered areas protected from wind, waves and currents with high light intensity [13,35]. It can vegetatively spread [34].
2.2. Experimental Setup
Shoots of Lagarosiphon major were harvested in the wild in 2017, in two different sites nearby Pavia and Varese, along the Ticino river (Lombardy region, Italy) in shallow waters (at a depth not exceeding 50 cm). Shoots of L. cordofanus were bought from online vendors (eBay, Aquasabi).
Shoots of both species were briefly maintained in water-filled tanks to be acclimated to the new conditions. After acclimation, shoots were cut, obtaining 108 viable fragments, each 3 cm long (9 fragments for each replicate) and with no roots. Fragments were placed in experimental tanks, maintaining L. major and L. cordofanus separated.
For the experiment, 12 tanks (size: 39 cm × 28 cm × 28 cm) for each species (3 replicates for each treatment), were filled with commercial river sand (Vaga Ticino) and tap water (15 L each tank). Sand (3 kg for each tank), extracted along the same water basin where L. major was harvested (Ticino river), was characterized by sand granulometry between 0.1 mm and 0.9 mm; it was a wet, siliceous sand (SiO2 83.3%) with other components in minor percentages (Al2O3 6.6%, K2O and Fe2O3 2.1%, NaO2 2%, MgO 1.5%, CaO 1.2%).
Water was periodically added to the tanks to maintain a constant water level. Plants were subjected to a 12 h day–night cycle in a growth chamber, with the air temperature (monitored with MadgeTech TransiTempII Temperature Data Logger) maintained at an average of 25 °C with minimal fluctuations between day and night.
In order to give an adequate supply of nutrients to the plants, for each tank, 1.5 mL of a breeding medium containing all key elements to plants was added (H3BO3 2.6 g, MnCl2·4H2O 1.81 g, ZnSO4·7H2O 0.222 g, Na2MoO4·2H2O 0.390 g, CuSO4·5H2O 0.079 g, Co(NO3)2·6H2O 0.0494 g).
The experiment lasted 56 days.
2.3. Environmental Parameters
The pH, water conductivity and water temperature were monitored, once a week, with a Combo pH/Conductivity/TDS Tester. Initial (T0) and final concentrations (Tf) of total N and P in water were estimated by means of spectrophotometric analyses.
2.4. Experimental Treatments
Controlled amounts of NH4Cl and KH2PO4 salts were added to water in order to simulate four different conditions of trophy, based on different concentrations of total N and total P. Reference concentrations for each level of trophy were based on the classification of trophy status by the Organisation for Economic Co-operation and Development (OECD) [36] with a calibration based on the trophy status of different prealpine lakes (Lombardy region, Northern Italy) [37,38]. Reference values were established from water bodies where L. major is established and often invasive (e.g., Maggiore, Iseo, Monate, Garda lake) [39,40,41].
Accordingly, concentrations for the four treatments obtained are reported in Table 1. The pH values, monitored for the entire duration of the experiment, were initially similar to those characterizing shallow waters of the reference lakes (https://www.arpalombardia.it/Pages/Dati/2021/Dati-analitici-corpi-idrici-lacustri-2021.aspx?tipodati=0&tema=Tema%20ambientale&sottotema=Sottotema%20ambientale&ordine=1, accessed on 9 March 2023) and in accordance with previous experiments involving L. major (e.g., [9]).

Table 1.
Total N, Total P and trophy classification in treatments.
2.5. Functional Traits
For each species under all treatments, the following traits were measured:
- Plant survival percentage;
- Main shoot length (cm);
- Final dry weight (mg);
- Number of lateral shoots (n° of shoots per plant);
- Lateral shoot length (cm);
- Branching degree (m−1);
- Number of roots (n° of roots per plant);
- Root length (cm).
All traits were measured at the beginning (day 1) and at the end (day 56) of the experiment.
2.6. Periphyton and Microalgal Mucilage Cover
The mean periphyton and microalgal mucilage cover in each tank was estimated by image analysis. Specifically, the surface of each plant was photographed by a digital camera, and the mucilage and algal cover (% of the total algal surface) was estimated using the software Image J, a free public-domain software application developed by the US National Institutes of Health (NIH).
2.7. Statistical Analysis
Data regarding the environmental parameters and the functional traits were analyzed by means of univariate statistical analyses (Dependent Sample t-test, ANOVA and SNK tests) with the software applications GMAV5 and Past 4.11. All details of the performed analyses are available in the Supplementary Materials.
3. Results
3.1. Environmental Parameters
Total nitrogen and phosphorous concentrations varied significantly between the beginning and the end of the experiment in relation to the applied treatment (Figure 1).
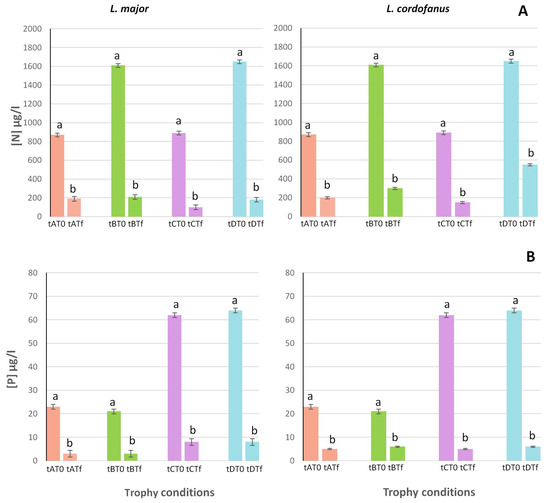
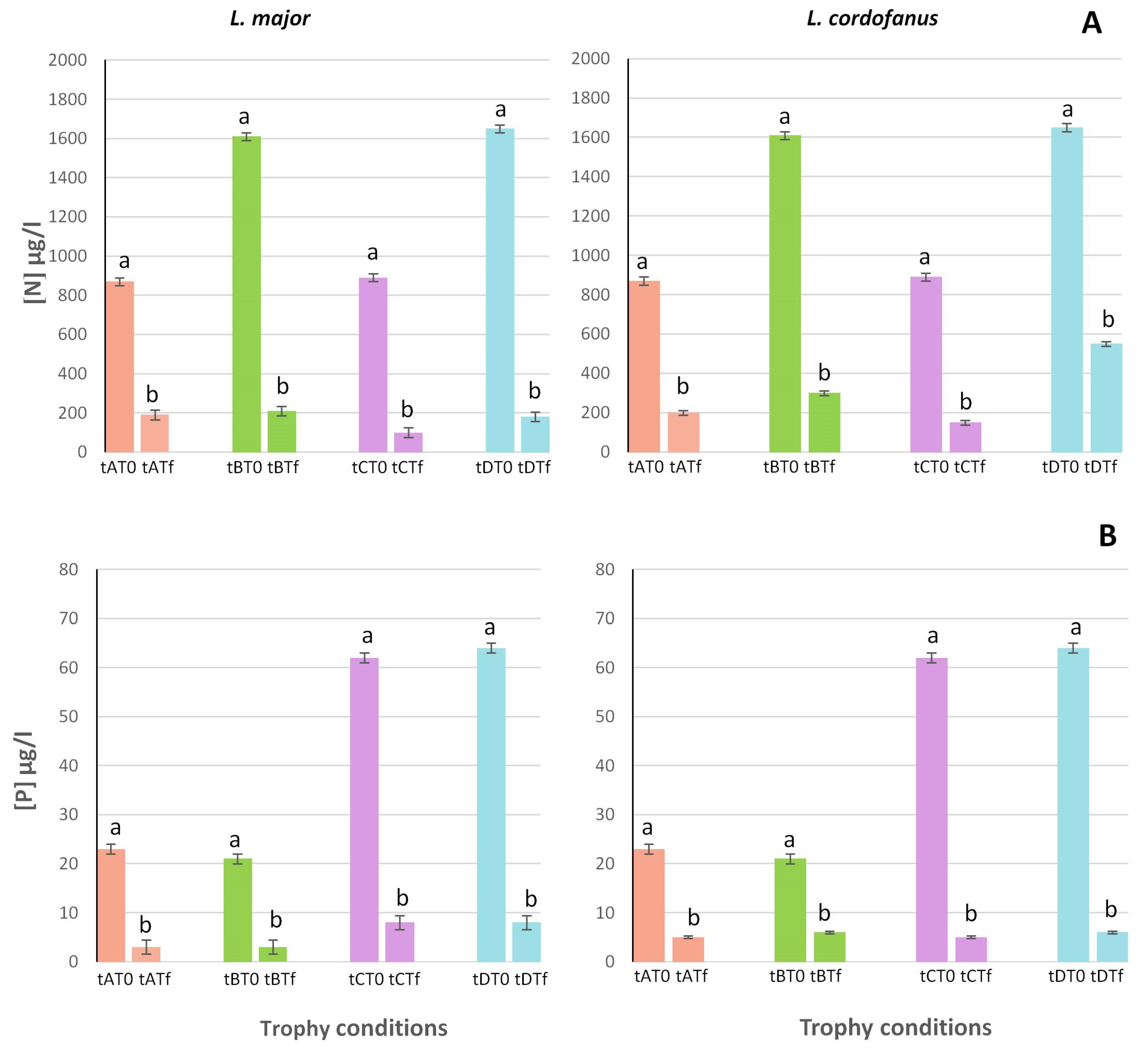
Figure 1.
Mean (+SE) concentrations of total N (A) and P (B) at the beginning (T0) and at the end (Tf) of the experiment for the two species in relation to the four treatments (tA, tB, tC and tD). Lowercase letters were used to indicate statistically significant differences between the beginning (T0) and the end (Tf) of the experiment for each treatment.
Both in tanks with L. major and those with L. cordofanus, N and P depletion was proportional to their initial concentration, meaning that significantly higher values of depletion were recorded in treatments with high initial concentrations of N and/or P (B and D for N, ~1600 µg/L; C and D for P, ~60 µg/L) than in treatments with low initial concentrations (A and C for N, ~870 µg/L; A and B for P, ~20 µg/L).
In particular, for total N (Figure 1A), a double depletion occurred in tanks for which the initial concentration was two times higher (for L. major and L. cordofanus, respectively: B = 1384.6 ± 0.77 µg/L and 1304.1 ± 0.65 µg/L; D = 1495.3 ± 0.67 µg/L and 1208.8 ± 1.19 µg/L) if compared to A (Lm: 678.6 ± 0.56 µg/L; Lc: 669.9 ± 0.78 µg/L) and C (Lm: 783.2 ± 0.79 µg/L; Lc: 738.7 ± 0.91 µg/L) treatments.
For P (Figure 1B) the same depletion trend was observed, with final values even three times lower for those treatments having the highest (3×) initial P concentration (C = 53.94 ± 0.92 µg/L and 56.42 ± 0.77 µg/L; D = 55.68 ± 0.89 µg/L and 57.6 ± 0.95 µg/L for L. major and L. cordofanus, respectively) if compared to the low concentration ones (A: Lm = 19.78 ± 0.89 µg/L; Lc = 17.94 ± 0.94 µg/L; B: Lm = 17.85 ± 0.81 µg/L; Lc = 15.91 ± 1.01 µg/L).
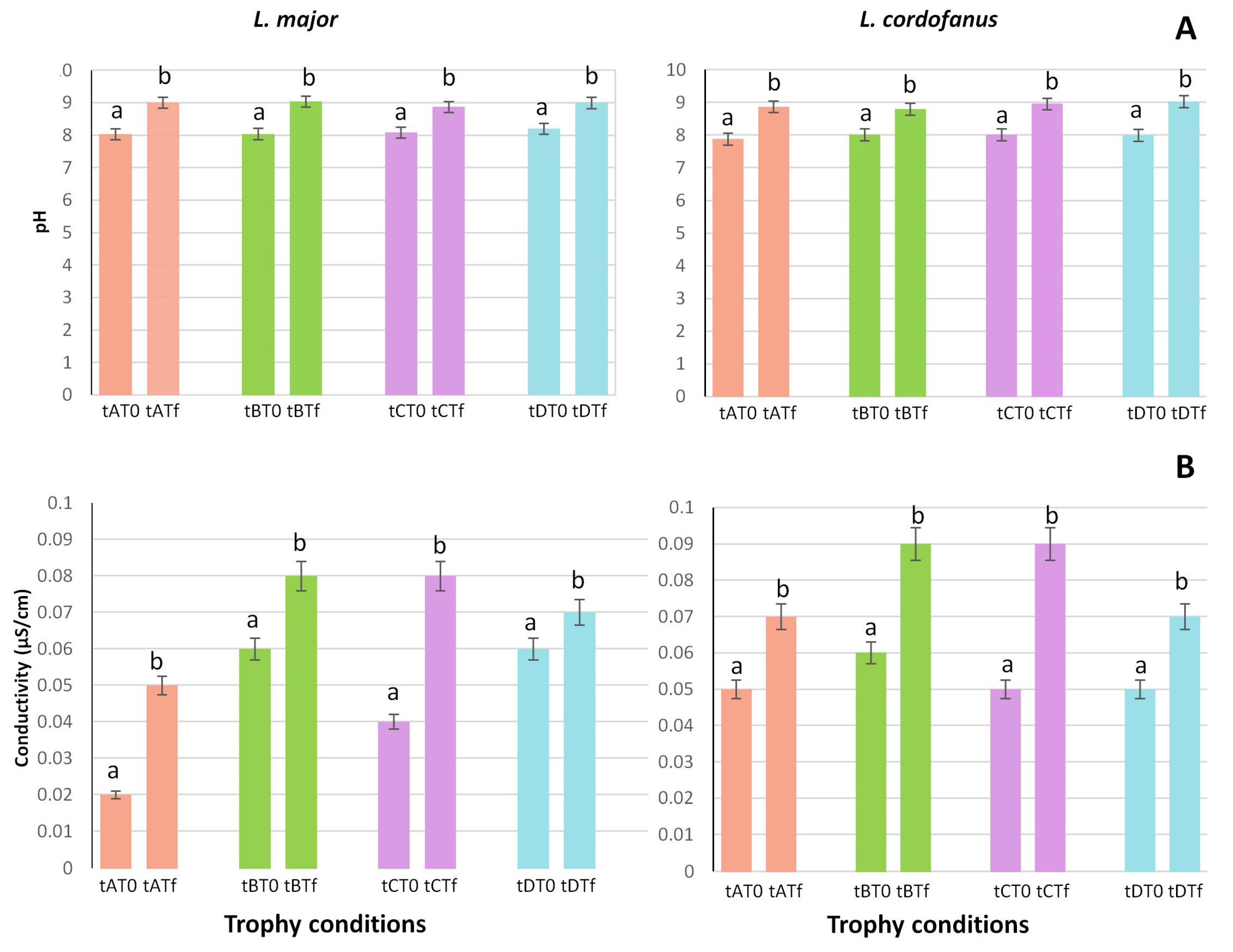
Regarding pH (Figure 2A) and conductivity (Figure 2B), the only significant differences observed were between the beginning and the end of the experiment, while no differences either between treatments or between species were recorded. For both L. major and L. cordofanus, indeed, a constant increase was observed during the experiment in all the tanks, independently of the applied treatment.
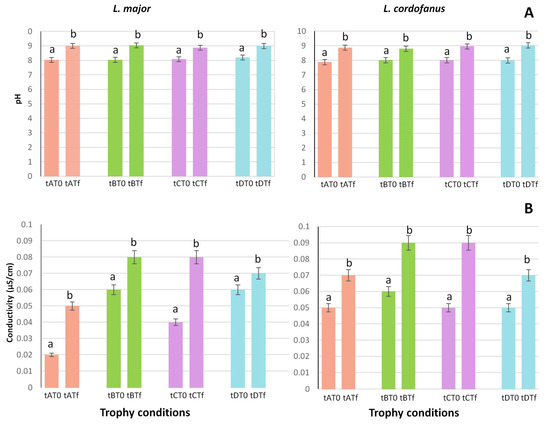
Figure 2.
Mean (+SE) of pH (A) and conductivity (B) values at the beginning (T0) and at the end (Tf) of the experiment for the two species in relation to the four treatments (tA, tB, tC and tD). Lowercase letters are used to indicate statistically significant differences between the beginning (T0) and the end (Tf) of the experiment for each treatment.
3.2. Functional Traits
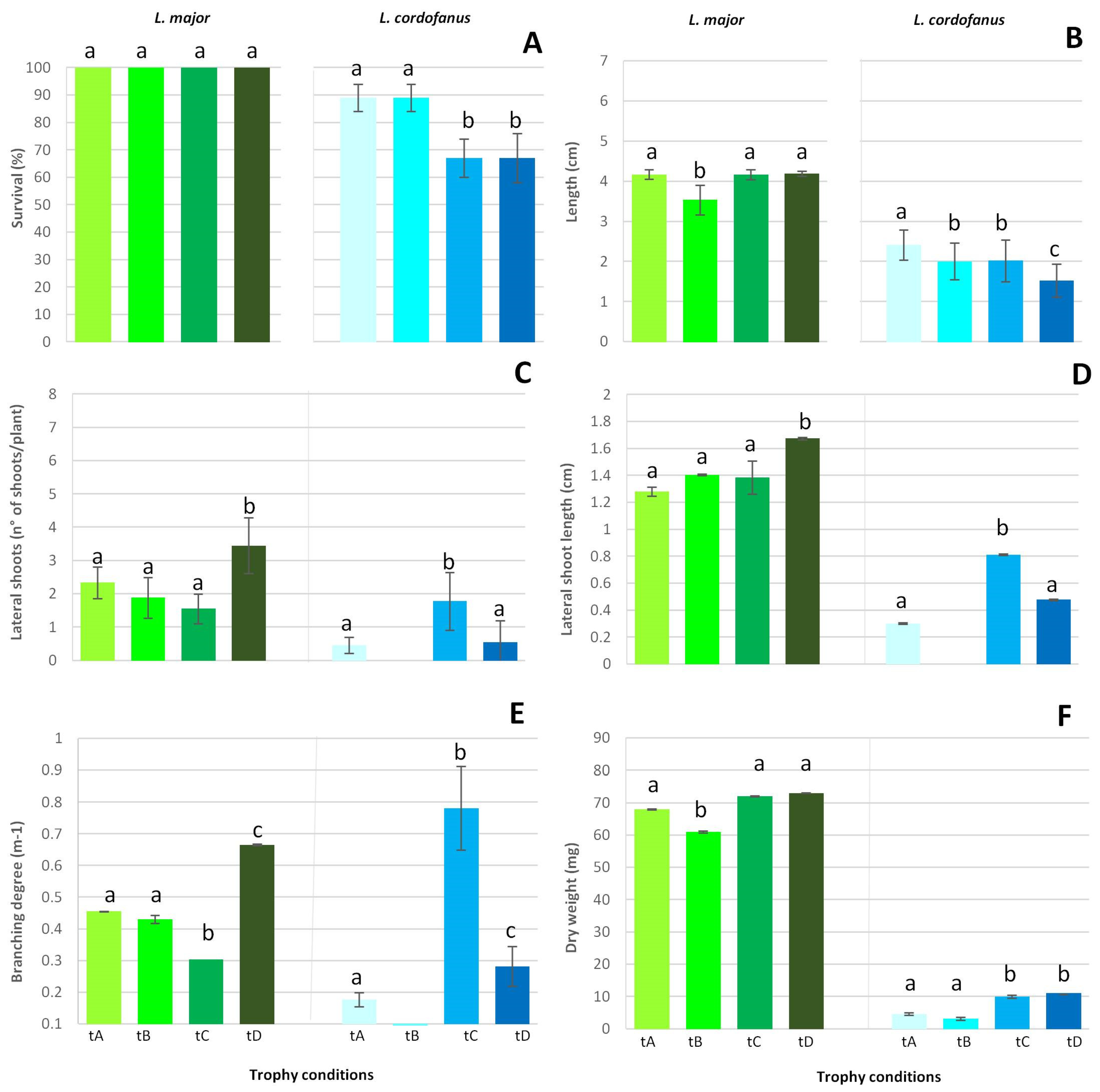
During the experiment, the health conditions of L. cordofanus plants overall worsened, with significant differences among treatments. On the contrary, L. major plants were healthy through the end of the experiment. These observations were confirmed by the different percentage of survival obtained for the two species at the end of the experiment (Figure 3A). For L. major, indeed, all the plants survived in the 4 treatments, while for L. cordofanus, a remarkably lower survival percentage (−35% of alive shoots) was recorded in treatments with high total P concentrations, independently of N.
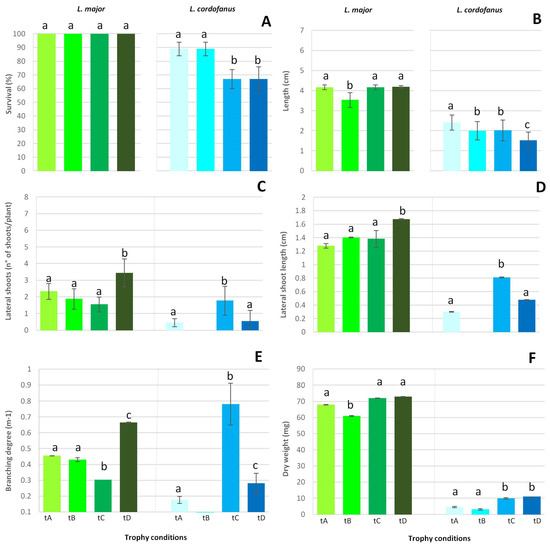
Figure 3.
Mean (+SE) survival percentage (A), main shoot length increase (B), lateral shoot number (C) and length increase (D), branching degree (E) and final dry weight increase (F) for the two species in relation to the four treatments (tA, tB, tC and tD). Lowercase letters were used to indicate statistically significant differences among treatments for each species.
Regarding the traits measured in alive individuals, according to the results of the statistical analyses (see Supplementary Materials), higher values were found for L. major in comparison to L. cordofanus for all the considered traits (Figure 3). The only exceptions observed were in the treatment C (N:P = 890:62 µg/L), where the number of lateral shoots did not differ between species (treatment C), but a significantly higher branching degree was recorded for L. cordofanus (treatment E).
Moreover, some significant differences among treatments were recorded for each species. Specifically, for L. major, the number of lateral shoots, as well as their length and the associated branching degree significantly increased at high concentrations of both N and P (treatment D, N:P = 1680:64 µg/L). A remarkably lower lateral shoot development was observed for the other treatments, especially at low N and high P (treatment C, N:P = 890:62 µg/L). For L. cordofanus, in contrast, the highest lateral shoot development was overall observed at low N and high P (treatment C). Moreover, for L. cordofanus, differently to L. major, almost no lateral shoots were present at high N and low P concentrations (treatment B, N:P = 1610:21 µg/L).
With regard to the main shoot length, the less remarkable increase occurred in tanks with a high N and low P concentration (treatment B, N:P = 1610:21 µg/L) for L. major. In all treatments, L. major doubled the length of the main shoot, but at high N and low P concentrations (treatment B, N:P = 1610:21 µg/L), the percentage increase was +116%, while for other treatments, it ranged from 139% (treatment C) to 141% (treatment D). Additionally, under not eutrophic conditions (treatment A), the percentage increase was greater than in treatment B (+140%). On the other hand, for L. cordofanus, the main shoot length increased less than for L. major (maximum +80% in treatment A), growing the least (+50%) when both N and P concentrations were high (treatment D, N:P = 1680:64 µg/L). For both species, no differences were observed for the other treatments. Growth determined by final dry weight increase, for L. major, differed significantly among treatments with high N and low P (treatment B, N:P = 1610:21 µg/L) where a lower increase was overall observed if compared to that of the other treatments, for which no differences were significant. Coherently with the percentage increase in the main shoot length, the dry weight increased slightly more than 100% (+103%) in treatment B, while in other treatments, it ranged from +130% (treatment A) to +140% (treatment D). For L. cordofanus, a significantly greater increase in dry weight occurred in tanks with high P concentrations, independently of N (treatments C and D, N:P = 890:62 µg/L and N:P = 1680:64 µg/L, respectively). Considering the weight increment in percentage, in treatments C and D, the increase ranged from +36% to +41%, respectively, much more than +9% in treatment B and +14% in treatment A. In spite of moderate growth for every trait, dry weight showed a relatively large increase in treatment D; this was probably due to both the intrinsic variability of plant fragments and abundance of leaves, a trait not analyzed by the present analysis.
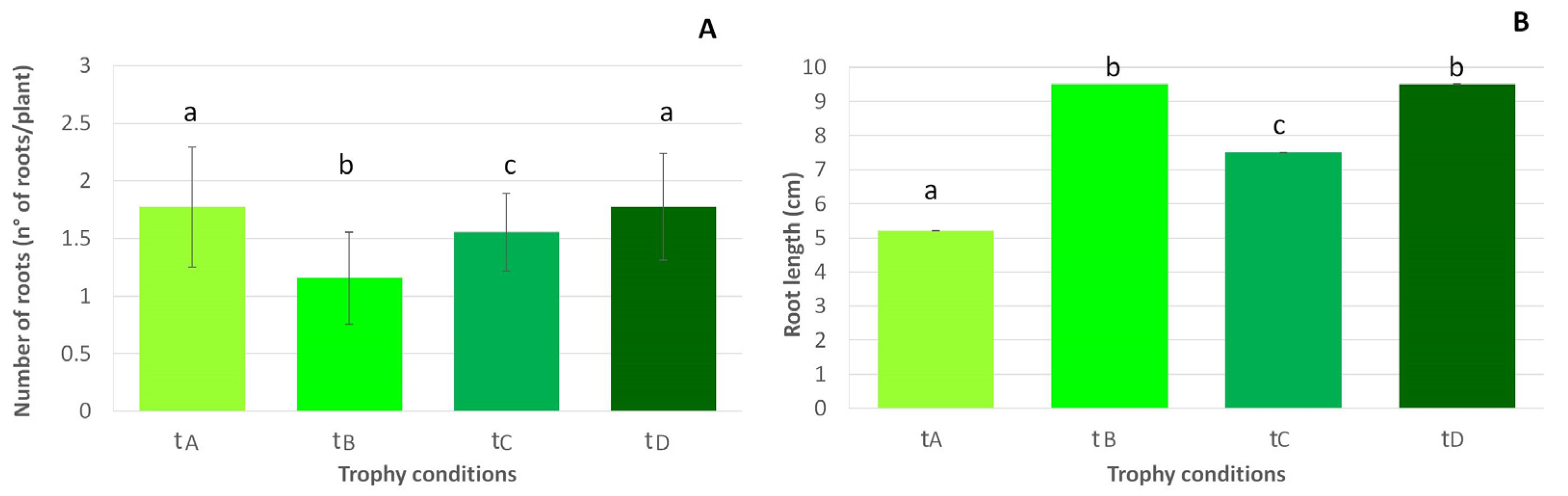
Finally, the development of roots (Figure 4) was not observed in L. cordofanus; however, in L. major, a maximum of four roots per plant were observed, and this root emission was accompanied by some significant differences among treatments. In particular, despite a significantly higher number of roots observed on plants in tanks with low and high concentrations for both N and P (A and D treatments, N:P = 870:23 µg/L and N:P = 1680:64 µg/L, respectively), they were longer where a high concentration of N occurred, independently of P (treatments B and D, N:P = 1610:21 µg/L and N:P = 1680:64 µg/L, respectively).
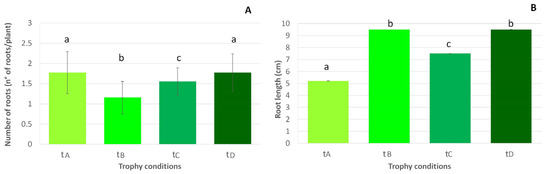
Figure 4.
Mean (+SE) L. major main root number (A) and length increase (B), in relation to the four treatments (tA, tB, tC and tD). Lowercase letters were used to indicate statistically significant differences among treatments. Data about L. cordofanus roots are not shown since the values are zero in all of the treatments.
3.3. Periphyton and Microalgal Mucilage Cover
Periphyton and microalgal mucilage appeared in every tank, but with different cover. From the visual analysis, plants in treatment B, C and D were the most affected (Table 2).

Table 2.
Periphyton and microalgal mucilage cover (mean%) for each species in each treatment.
4. Discussion
Our work gives a preliminary overview of the potential of L. cordofanus to thrive under different levels of nutrient enrichment as a proxy of the likelihood that the species may become naturalized in water bodies characterized by different trophy conditions. As counter-altar species, its congener L. major, a highly tolerant species invasive in different parts of the globe, was subjected to the same treatments. Treatments simulated a gradient of trophy conditions with regard to enrichment of N and P up to levels found in different temperate natural water bodies of the Italian prealpine belt where L. major already occurs and it is often invasive.
In terms of both survival and growth (Figure 3 and Figure 4), under all treatments, the performance of L. cordofanus was inferior in comparison to L. major. In fact, the mortality of plants was indicative of this, ranging from 10 to 35% of total shoots depending on the treatment. In contrast, L. major shoots experienced no mortality. (Figure 3A). Moreover, L. cordofanus did not invest in adventitious roots at all, while L. major was quite productive in this regard (Figure 4), as well as in shoot growth. During the experiment, L. cordofanus invested in the development and elongation of shoots (both main and lateral) differing from L. major and among treatments (Figure 3B–E). L. cordofanus showed the best vegetative performance, especially under high concentrations of total P (treatment C). When high P and N were combined (treatment D), L. cordofanus mainly invested in new shoots, while in low N and P conditions (treatment A), energies were channeled to the elongation of the main shoot. L. cordofanus seemed to suffer the imbalance of N and P in favor of N (treatment B) as it had a weak vegetative response. Basically, the increase in dry weight (Figure 3F) followed the investment in new lateral shoots, although the relevant increment at high N and P concentrations (treatment D) was likely to relate to a major leaf emission, a trait not specifically not recorded in the present analysis. In contrast, L. major responded vegetatively more strongly when both P and N were at high concentration (treatment D), while a weaker production of lateral shoots and roots occurred when P was at a high concentration (treatment C) and when N was at a high concentration (treatment B), respectively. However, differences among treatments were slight for L. major, confirming its broad tolerance to different nutrient concentration.
4.1. Species Response to Nutrient Enrichment
The response of L. cordofanus to treatments in our experiment was in agreement with studies that highlighted that elevated dissolved phosphorus levels promote the biomass formation in submerged macrophytes and that additional phosphorus supplies can be invested in stem elongation or in the formation of lateral branches [42,43,44]. Thiébaut [45] indicated that primarily phosphorus and, when phosphorus is in excess, nitrogen, plays an important role in controlling the abundance and development of aquatic plants. Nutrient-use efficiencies depend both on the plant P metabolism and on its ability to assimilate this nutrient within its vegetative structures [46]. Interestingly, the vegetative response to nitrogen at a high concentration did not have the same magnitude as that at a high P concentration for L. cordofanus. In fact, in this condition, L. cordofanus had a good survival percentage and growth, both comparable to other treatments, but it did not invest in lateral shoots at all. Although its primary role together with phosphorus is beyond question [47,48], this may indicate that, when in excess, nitrogen had an inhibiting effect on L. cordofanus, an effect also observed under the condition of high nitrogen combined with high phosphorus concentrations. Even if it is often difficult to distinguish the effects of nitrogen from those of phosphorus due to the close nitrogen and phosphorus relationship [49], in the case of L. cordofanus, this seems to be quite evident. This situation can be partially explained considering the potential toxic effect of nitrogen to more sensitive species, when it is at critical concentrations (e.g., around 2 mg N L−1), together with the effect of the increase in both phytoplankton and algae due to nitrogen loading [50,51,52]. Macrophytes are partially resilient to abrupt increases in nitrogen loading at moderate phosphorus concentrations; however, especially after prolonged exposure, a complete collapse often occurs, not only in relation to competition for resources, but also for the increase in turbidity and the inhibition of photosynthesis [53].
L. major seemed not to suffer excess of either nitrogen or phosphorus; nevertheless, concentrations in favor of one or the other did not promote its vegetative response in the same way as when both nutrients were simultaneously at a high concentration.
4.2. Strategic Investment into Shoots and Roots
Particularly for canopy-forming species, the investment in shoot biomass, often rather than roots, is considered key for their competitiveness. This has been shown to be valid for L. major: especially during the first phase of colonization of new sites, this strategy allows the plant to rapidly increase the leaf surface, forming dense beds and quickly establishing itself shading out the other species [26]. Increasing the leaf surface area has direct benefits for photosynthesis and nutrient uptake. In fact, increasing the shoot biomass facilitates enhanced nutrient uptake from the water column: in rooted submerged aquatic plants, leaves as roots contribute to nutrient absorption, and biomass is strategically invested into shoots or roots, depending on whether nutrients are more abundant in the water column or in the sediment, respectively [48,54]. Mainly investing in shoot biomass, L. cordofanus seemed to follow the same strategy as L. major. Even so, differences from L. major were evident, and they mainly related to a complete absence of roots and a definitely lower growth of shoot for L. cordofanus, together with a lower survival percentage.
Regarding the first point, L. major emitted both new shoots and adventitious roots, while for L. cordofanus, no roots developed at all. A low production of roots has already been recorded for L. cordofanus, even if conducted under different experimental conditions [55]. In fact, testing the ability of coping with the scarce availability of CO2 and using HCO3− as a source of carbon, Hussner et al. [55] already found that L. cordofanus had a lower investment into roots with respect to L. major (and other macrophytes). In submerged aquatic plants, beyond their role in nutrient storage and anchorage, adventitious roots can facilitate the absorption of nutrients directly from the water column [8,56,57]. However, rooted submerged macrophytes can thrive even without roots, if the nutrient concentration in the water is sufficient to satisfy their requirements [58]. This could indicate that the leaf uptake is the preferential pathway for nutrient absorption of L. cordofanus. Nonetheless, the exclusive emission of new shoots likely did not ensure L. cordofanus an alternative pathway of absorption in case of inhibition of leaf uptake when the plants were covered by mucilage and algae, as happened with L. major [59].
The investment in shoots rather than in roots can be also seen in an ecological perspective as a different strategy of establishment of species. In this regard, it is important to underline that the experiment analyzed the response to nutrients of fragments of L. cordofanus and L. major (not fully acclimated rooted plants) simulating conditions typical of the initial phases of colonization, when viable vegetative units of dispersal “drifted to a new environment”. Initially, aquatic plants can invest differently in shoots and/or adventitious roots depending on species–specific strategies of regeneration and colonization [31]: a preference for early formation of new shoots emphasizes a higher potential for the production of new propagules, which in turn may be further dispersed, while fast root formation indicates an increased likelihood of rapid colonization [60]. According to this, along the experimental gradient of trophy, the vegetative response of L. cordofanus would seem to be more prone to develop and “potentially” disperse vegetative units moving away to new conditions, rather than to colonize the “new” environment. On the contrary, L. major excelled in both the strategies (regeneration and colonization), as already tested by [31].
4.3. Factors Influencing the Growth and Survival
Considering the dramatically lower growth of shoots and the loss of viable fragments for L. cordofanus with respect to L. major, different factors might have played a role during the experiment—both intrinsic to the species and external—mainly relating to processes of simulated eutrophication. An intrinsic factor may relate to the fact that we compared two diverse species, congeners, but they are likely to be different in morphology and metabolism. For example, even if it is a quite variable species, L. cordofanus has a filiform stem and thin, narrow, almost-transparent leaves, while L. major is characterized by a thicker stem and broad, firm, mostly opaque leaves [34]; plant and leaf morphology can influence nutrient uptake in some species [61]. Then, the size of fragments used as a starting material and their position (apical, central, etc.) might have influenced the vegetative response by macrophytes. Due to limitations in the availability of plants of L. cordofanus, the length of fragments was quite short (3 cm) with respect to more recent experiments in which the species was used (10 cm; [55]), even if it was retained sufficient based on data regarding L. major, whose fragments can remain viable and produce new shoots and roots when ≥10 mm [32]. However, the size of clonal fragments under which species retain the viability can be a sensitive species–specific element [62], and a full vegetative response of L. cordofanus could have been prevented or inhibited if fragments were too small.
An external factor influencing the growth of species was the proliferation of periphyton and mucilage, which affected both species, especially at high P and N concentrations. Due to non-sterile conditions and direct release of nutrients into the water column, during the experiment, periphyton and mucilage appeared in the tanks but particularly on the plants. Plants experienced the effect of eutrophication: the increase in plankton and algae is usually a consequence of nutrient enrichment [49,53], and in eutrophic water, it is a major cause of macrophyte decline and the shift to communities dominated by tolerant, fast-growing species or, in the more extreme cases, to planktonic communities [63,64]. Based on visual evidence and measured traits, L. major was less affected by mucilage and periphyton, while L. cordofanus suffered from a high cover of algae. To our understanding, at least at the initial stage of the microorganism colonization, a key factor that made the difference between the two species could relate to the rapidity of growth: considering the length increase at the end of the experiment, L. major had rapid and more abundant growth in shoots that likely improved plants’ photosynthesis and increased the surface area-to-volume ratio, enabling the plants to outpace algal growth and its effects [54]. On the other hand, a lower and more restrained growth such as that of L. cordofanus left the plant exposed to the rapid colonization of fast-growing mucilage and periphytic organisms, dramatically reducing photosynthesis and nutrient uptake through shoots [65,66]. Moreover, L. major can release allelopathic compounds which likely inhibit the growth of phytoplankton and possibly also epiphytic algae and microorganisms in general [67].
The aggressive proliferation of periphyton and mucilage seemed to have a direct effect on the mortality of L. cordofanus, which was higher in P- and P-and-N-rich treatments (C and D) than in low-nutrient (A) and N-rich (B) treatments. Paradoxically, the highest mortality of L. cordofanus occurred in those conditions in which the species (at least the surviving individuals) performed the best in terms of growth. In fact, in spite of high mortality rates, the surviving fragments grew and developed new shoots. This can be seen as a strategic response to stress (e.g., from periphyton). Considering the importance of investing in shoot biomass, as previously discussed, a resilient response of traits relating to this mechanism could be key in the face of novel environmental conditions. Despite this, for an alien species to succeed in a new environment is a complicated process, [68], especially when the mortality rate is high, as is the case for L. cordofanus. Even so, a resilient response in shoots development may not be enough if many clones die, and only repeated introductions of the plant would eventually compensate the loss, by increasing the propagule pressure [69].
4.4. Change in Environmental Parameters
At the end of the experiment, the concentrations of nitrogen and phosphorus were significantly lower than at the beginning, while both pH and conductivity had increased.
Both nitrogen and phosphorus loading and/or their combination can promote the proliferation of microorganisms (phytoplankton, algae, etc.). The depletion of nutrients was especially evident when their availability was high; in that case, the final concentration of both nitrogen and phosphorus was two or three times lower than the initial concentration. The depletion of nutrients can be attributed both to plant activity and to microorganisms that proliferated due to eutrophic conditions. In this regard, it can be supposed that the activity of microorganisms was highly relevant, especially in tanks with L. cordofanus. Assuming that L. cordofanus absorbed nutrients only via leaf uptake, we would expect a lower absorption with respect to L. major due to the former’s lower growth and lack of adventitious roots. However, the final concentration of nutrients was comparable between species. Therefore, unless L. cordofanus has an outstanding ability to assimilate nutrients via leaf uptake, microorganisms may have strongly contributed to nutrient absorption. It is likely that the scarce growth of L. cordofanus corresponded to a major availability of nutrients that promoted the increase in microorganisms.
Together with nutrient depletion, pH and conductivity increased during the experiment. Increased conductivity might relate to an increase in plant and microorganism debris (especially for L. cordofanus, due to plant death). Regarding pH, the experiment was conducted at quite a high pH (>7), though the values were compatible with values of natural water bodies where L. major was found (see Materials and Methods). Increasing pH values can relate to the activity of plants and microorganisms, as photosynthesis is an alkalinization process [70]. Specifically, at elevated pH (7–10), bicarbonate (HCO3−) is the dominant carbon form instead of CO2 [54,66,70], and the alkalinization of water relates to the release in OH− [70]. In relation to plant survival, these conditions can be detrimental for CO2 users; however, both L. cordofanus and L. major have an affinity to bicarbonate, and their persistence at high pH and low CO2 suggests that both species are relatively resilient to these conditions [55]. Even so, utilizing bicarbonate is an active uptake process that incurs energy costs and may require increased investments to process the bicarbonate (e.g., carboxylating enzymes) with a consequent increase in the costs of synthesis, maintenance and operation of the cells [54,71,72].
In conclusion, according to our findings, L. cordofanus responded well to nutrient enrichment, especially to phosphorus, but it had difficulty with stress, especially due to competition with periphyton and mucilage, both important factors in the eutrophication of waters. The low growth of the plants, as well as the lack of diversification of absorption pathways (shoots and roots), especially in comparison with L. major performance, were seen as limits to the potential naturalization of L. cordofanus under conditions that resemble those simulated during the experiment and those similar to natural areas already invaded by L. major.
5. Conclusions
In case of release in the wild of viable propagules, the naturalization of L. cordofanus in temperate shallow waters does not seem so obvious, especially if considered in comparison to L. major. In any case, further experiments are needed to define the naturalization potential of L. cordofanus under different environmental conditions. For this reason, from a prevention perspective, the species should be treated carefully, and any release in the wild, whether voluntary or accidental, should be avoided.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15050693/s1. Table S1. Results of dependent sample t-tests used to compare N, P, pH and conductivity between T0 and Tf for each species and treatment; Table S2. Results of the ANOVAs and SNKs to test for differences in N and P depletion among treatments (tA vs. tB vs. tC vs. tD) and species (L. major (Lm) vs. L. cordofanus (Lc)). Significant results are given in bold; Table S3. Results of the ANOVAs and SNKs to test for differences in each of the considered functional traits in relation to the species (L. major (Lm) vs. L. cordofanus (Lc)) and the applied treatment (tA vs. tB vs. tC vs. tD) at the end of the experiment. Significant results are given in bold; Table S4. Results of the ANOVAs and SNKs to test for differences in the root number and length for L. major (Lm) in relation to treatments (tA vs. tB vs. tC vs. tD) at the end of the experiment. Significant results are given in bold [73,74].
Author Contributions
Conceptualization, C.M. and S.C. (Sarah Caronni); methodology, C.M. and S.C. (Sarah Caronni); validation, C.M., S.C. (Sarah Caronni) and S.C. (Sandra Citterio); formal analysis C.M. and S.C. (Sarah Caronni); investigation C.M., S.C. (Sarah Caronni), L.A.Q., R.G. and S.C. (Sandra Citterio); resources, S.C. (Sandra Citterio); writing—original draft preparation, C.M.; writing—review and editing, C.M., S.C. (Sarah Caronni), L.A.Q., N.S., R.G. and S.C. (Sandra Citterio); visualization, S.C. (Sarah Caronni); supervision, S.C. (Sandra Citterio); project administration, S.C. (Sandra Citterio); funding acquisition, S.C. (Sandra Citterio). All authors have read and agreed to the published version of the manuscript.
Funding
Project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4—Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union—NextGenerationEU. Award Number: Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP, H43C22000530001 Project title “National Biodiversity Future Center—NBFC”.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All the research data are shareable by the authors if requested.
Acknowledgments
The authors would like to thank Giulia Salerno for her invaluable support, as well as Fabio Moia, Enrico Casati, Valentina Soler and Barbara Leoni for their technical help during the experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Turbelin, A.J.; Malamud, B.D.; Francis, R.A. Mapping the global state of invasive alien species: Patterns of invasion and policy responses. Glob. Ecol. Biogeogr. 2017, 26, 78–92. [Google Scholar] [CrossRef]
- Hulme, P.E.; Brundu, G.; Carboni, M.; Dehnen-Schmutz, K.; Dullinger, S.; Early, R.; Essl, F.; González-Moreno, P.; Groom, Q.J.; Kueffer, C.; et al. Integrating invasive species policies across ornamental horticulture supply chains to prevent plant invasions. J. Appl. Ecol. 2017, 55, 92–98. [Google Scholar] [CrossRef]
- Brunel, S. Pathway analysis: Aquatic plants imported in 10 EPPO countries. EPPO Bull. 2009, 39, 201–213. [Google Scholar] [CrossRef]
- Caffrey, J.M.; Acevedo, S. Lagarosiphon major in Lough Corrib—Management options. In Fish Stocks and Their Environment; Moriary, C., Rossell, R., Gargan, P., Eds.; Institute of Fisheries Management: Westport, Ireland, 2008; pp. 85–97. [Google Scholar]
- Caffrey, J.M.; Millane, M.; Evers, S.; Moran, H.; Butler, M.A. Novel approach to aquatic weed control and habitat restoration using biodegradable jute matting. Aquat. Invasions 2010, 5, 123–129. [Google Scholar] [CrossRef]
- Baars, J.R.; Keenan, E.A.; O’Callaghan, P.; Caffrey, J.M. Changes to the invertebrate fauna of littoral habitats induced by the alien invasive species Lagarosiphon major (Hydrocharitaceae). In Research and Control Programme for Lagarosiphon major in Lough Corrib; Central Fisheries Board: Dublin, Ireland, 2009; p. 159. [Google Scholar]
- Bickel, T.O. Lagarosiphon major. In A Handbook of Global Freshwater Invasive Species; Francis, R.A., Ed.; Routledge: London, UK; New York, NY, USA, 2012. [Google Scholar]
- Matthews, J.; Beringen, R.; Collas, F.P.L.; Koopman, K.R.; Odé, B.; Pot, R.; Sparrius, L.B.; van Valkenburg, J.L.C.H.; Verbrugge, L.N.H.; Leuven, R.S.E.W. Knowledge Document for Risk Analysis of the Non-Native Curly Waterweed (Lagarosiphon major) in The Netherlands; Reports Environmental Science, 414; Department of Environmental Science: Nijmegen, The Netherlands, 2012. [Google Scholar]
- Redekop, P.; Hofstra, D.; Hussner, A. Elodea canadensis shows a higher dispersal capacity via fragmentation than Egeria densa and Lagarosiphon major. Aquat. Bot. 2016, 130, 45–49. [Google Scholar] [CrossRef]
- Turbelin, A.J.; Diagne, C.; Hudgins, E.J.; Moodley, D.; Kourantidou, M.; Novoa, A.; Haubrock, P.J.; Bernery, C.; Gozlan, R.E.; Francis, R.A.; et al. Introduction pathways of economically costly invasive alien species. Biol. Invasions 2022, 24, 2061–2079. [Google Scholar] [CrossRef]
- McGregor, P.G.; Gourlay, H. Assessing the Prospects for Biological Control of Lagarosiphon (Lagarosiphon major (Hydrocharitaceae)); Department of Conservation: Wellington, New Zealand, 2002; p. 14. [Google Scholar]
- Caffrey, J.; Millane, M.; Evers, S.; Moran, H. Management of Lagarosiphon major (Ridley) Moss in Lough Corrib—A review. In Biology and Environment: Proceedings of the Royal Irish Academy; Royal Irish Academy: Dublin, Ireland, 2011; pp. 205–212. [Google Scholar]
- Lansdown, R.V. Lagarosiphon cordofanus. In The IUCN Red List of Threatened Species; IUCN Global Species Programme Red List Unit: Cambridge, UK, 2019; p. e.T185529A120203981. [Google Scholar]
- Migliorini, D.; Ghelardini, L.; Tondini, E.; Luchi, N.; Santini, A. The potential of symptomless potted plants for carrying invasive soilborne plant pathogens. Divers. Distrib. 2015, 21, 1218–1229. [Google Scholar] [CrossRef]
- Branquart, E.; Brundu, G.; Buholzer, S.; Chapman, D.; Ehret, P.; Fried, G.; Starfinger, U.; van Valkenburg, J.; Tanner, R. A prioritization process for invasive alien plant species incorporating the requirements of EU Regulation no. 1143/2014. EPPO Bull. 2016, 46, 603–617. [Google Scholar] [CrossRef]
- Roy, H.E.; Bacher, S.; Essl, F.; Adriaens, T.; Aldridge, D.C.; Bishop, J.D.D.; Blackburn, T.M.; Branquart, E.; Brodie, J.; Carboneras, C.; et al. Developing a list of invasive alien species likely to threaten biodiversity and ecosystems in the European Union. Glob. Chang. Biol. 2019, 25, 1032–1048. [Google Scholar] [CrossRef]
- Leung, B.; Roura-Pascual, N.; Bacher, S.; Heikkilä, J.; Brotons, L.; Burgman, M.A.; Dehnen-Schmutz, K.; Essl, F.; Hulme, P.E.; Richardson, D.M.; et al. TEASIng apart alien species risk assessments: A framework for best practices. Ecol. Lett. 2012, 15, 1475–1493. [Google Scholar] [CrossRef]
- Alpert, P.; Bone, E.; Holzapfel, C. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect. Plant Ecol. Evol. Syst. 2000, 3, 52–66. [Google Scholar] [CrossRef]
- Essl, F.; Lenzner, B.; Bacher, S.; Bailey, S.; Capinha, C.; Daehler, C.; Dullinger, S.; Genovesi, P.; Hui, C.; Hulme, P.E.; et al. Drivers of future alien species impacts: An expert-based assessment. Glob. Chang. Biol. 2020, 26, 4880–4893. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.L.; Hedman, H.D.; Johnson, P.T.J. Nutrient availability and invasive fish jointly drive community dynamics in an experimental aquatic system. Ecosphere 2018, 9, e02153. [Google Scholar] [CrossRef]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Paerl, H.W. Controlling eutrophication along the freshwater–marine continuum: Dual nutrient (N and P) reductions are essential. Estuaries Coasts 2009, 32, 593–601. [Google Scholar] [CrossRef]
- Egertson, C.J.; Kopaska, J.A.; Downing, J.A. A Century of Change in Macrophyte Abundance and Composition in Response to Agricultural Eutrophication. Hydrobiologia 2004, 524, 145–156. [Google Scholar] [CrossRef]
- Phillips, G.L.; Eminson, D.; Moss, B. A mechanism to account for macrophyte decline in progressively eutrophicated freshwaters. Aquat. Bot. 1978, 4, 103–126. [Google Scholar] [CrossRef]
- Bolpagni, R. Towards global dominance of invasive alien plants in freshwater ecosystems: The dawn of the Exocene? Hydrobiologia 2021, 848, 2259–2279. [Google Scholar] [CrossRef]
- Rattray, M.R.; Howard-Williams, C.; Brown, J.M.A. Rates of early growth of propagules of Lagarosiphon major and Myriophyllum triphyllum in lakes of differing trophic status. N. Z. J. Mar. Freshw. Res. 1994, 28, 235–241. [Google Scholar] [CrossRef]
- Martin, G.D.; Coetzee, J.A. Competition between two aquatic macrophytes, Lagarosiphon major (Ridley) Moss (Hydrocharitaceae) and Myriophyllum spicatum Linnaeus (Haloragaceae) as influenced by substrate sediment and nutrients. Aquat. Bot. 2014, 114, 1–11. [Google Scholar] [CrossRef]
- Cholo, F.; Foden, W. Lagarosiphon major. In The IUCN Red List of Threatened Species; IUCN Global Species Programme Red List Unit: Cambridge, UK, 2010; p. e.T185287A8382399. [Google Scholar]
- Riis, T.; Olesen, B.; Clayton, J.S.; Lambertini, C.; Brix, H.; Sorrell, B.K. Growth and morphology in relation to temperature and light availability during the establishment of three invasive aquatic plant species. Aquat. Bot. 2012, 102, 56–64. [Google Scholar] [CrossRef]
- Stiers, I.; Njambuya, J.; Triest, L. Competitive abilities of invasive Lagarosiphon major and native Ceratophyllum demersum in monocultures and mixed cultures in relation to experimental sediment dredging. Aquat. Bot. 2011, 95, 161–166. [Google Scholar] [CrossRef]
- Heidbüchel, P.; Hussner, A. Fragment type and water depth determine the regeneration and colonization success of submerged aquatic plants. Aquat. Sci. 2019, 81, 6. [Google Scholar] [CrossRef]
- Coughlan, N.E.; Armstrong, F.; Baker-Arney, C.; Crane, K.; Cuthbert, R.N.; Jansen, M.A.; Kregting, L.; Vong, G.Y.; Dick, J.T. Retention of viability by fragmented invasive Crassula helmsii, Elodea canadensis and Lagarosiphon major. River Res. Appl. 2022, 38, 1356–1361. [Google Scholar] [CrossRef]
- Hussner, A. Information on Measures and Related Costs in Relation to the Species Included on the Union List: Lagarosiphon major; Technical Note Prepared by IUCN for the European Commission; IUCN: Gland, Switzerland, 2019.
- Symoens, J.J.; Triest, L. Monograph of the african genus Lagarosiphon Harvey (Hydrocharitaceae). Bull. Du Jard. Bot. Natl. De Belg./Bull. Van De Natl. Plantentuin Van Belg. 1983, 53, 441–488. [Google Scholar] [CrossRef]
- Ngumbau, V.M.; Luke, Q.; Nyange, M.; Wanga, V.O.; Watuma, B.M.; Mbuni, Y.M.; Munyao, J.N.; Oulo, M.A.; Mkala, E.M.; Kipkoech, S.; et al. An annotated checklist of the coastal forests of Kenya, East Africa. PhytoKeys 2020, 147, 1–191. [Google Scholar] [CrossRef]
- Premazzi, G.; Chiaudani, G. Ecological Quality of Surface Waters: Quality Assessment Schemes for European Community Lakes; European Commission: Brussels, Luxembourg, 1992; Volume 14563.
- Monitoraggio PFAS in Lombardia. Available online: https://www.arpalombardia.it/Pages/Acque-Superficiali/Rapporti-Annuali.aspx (accessed on 20 February 2023).
- Scibona, A.; Nizzoli, D.; Hupfer, M.; Valerio, G.; Pilotti, M.; Viaroli, P. Decoupling of silica, nitrogen and phosphorus cycling in a meromictic subalpine lake (Lake Iseo, Italy). Biogeochemistry 2022, 159, 371–392. [Google Scholar] [CrossRef]
- Bolpagni, R.; Cerabolini, B.E.L. Habitat Acquatici in Lombardia: Aggiornamento delle Conoscenze e Proposte per un Monitoraggio Integrato; Università degli Studi dell’Insubria—Fondazione Lombardia per l’Ambiente, Osservatorio Regionale per la Biodiversità di Regione Lombardia: Milan, Italy, 2016. [Google Scholar]
- Bolpagni, R.; Azzella, M.M.; Agostinelli, C.; Beghi, A.; Bettoni, E.; Brusa, G.; De Molli, C.; Formenti, R.; Galimberti, F.; Cerabolini, B.E. Integrating the Water Framework Directive into the Habitats Directive: Analysis of distribution patterns of lacustrine EU habitats in lakes of Lombardy (northern Italy). J. Limnol. 2017, 76, 75–83. [Google Scholar] [CrossRef]
- Ciutti, F.; Cappelletti, C. Invasioni biologiche: Il caso del Lago di Garda. Biol. Ambient. 2017, 31, 59–164. [Google Scholar]
- Eugelink, A.H. Phosphorus uptake and active growth of E. canadensis Michx. and Elodea nuttallii (Planch.) St. John. Water Sci. Technol. 1998, 37, 59–65. [Google Scholar] [CrossRef]
- Barrat-Segretain, M.H. Growth of E. canadensis and Elodea nuttallii in mono-cultures and mixture under different light and nutrient conditions. Arch. Hydrobiol. 2004, 161, 133–144. [Google Scholar] [CrossRef]
- Zehnsdorf, A.; Hussner, A.; Eismann, F.; Rönicke, H.; Melzer, A. Management options of invasive Elodea nuttallii and Elodea canadensis. Limnologica 2015, 51, 110–117. [Google Scholar] [CrossRef]
- Thiébaut, G. Phosphorus and aquatic plants. In The Ecophysiology of Plant-Phosphorus Interactions; White, P.J., Hammond, J.P., Eds.; Springer Science & Business Media: Dordrecht, The Netherlands, 2008; Volume 7, pp. 31–49. [Google Scholar]
- Baldy, V.; Thiebaut, G.; Fernandez, C.; Sagova-Mareckova, M.; Korboulewsky, N.; Monnier, Y.; Perez, T.; Tremolieres, M. Experimental assessment of the water quality influence on the phosphorus uptake of an invasive aquatic plant: Biological responses throughout its phenological stage. PLoS ONE 2015, 10, e0118844. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Interactions between Nitrogen and Phosphorus metabolism. In Annual Plant Reviews; Plaxton, W.C., Lambers, H., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; Volume 48. [Google Scholar]
- Rao, Q.; Su, H.; Deng, X.; Xia, W.; Wang, L.; Cui, W.; Ruan, L.; Chen, J.; Xie, P. Carbon, nitrogen, and phosphorus allocation strategy among organs in submerged macrophytes is altered by eutrophication. Front. Plant Sci. 2020, 11, 524450. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, H.Z.; Li, Y.; Shao, J.C.; Liang, X.M.; Jeppesen, E.; Wang, H.J. Effects of high nitrogen concentrations on the growth of submersed macrophytes at moderate phosphorus concentrations. Water Res. 2015, 83, 385–395. [Google Scholar] [CrossRef]
- Gonzalez Sagrario, M.A.; Jeppesen, E.; Gomà, J.; Søndergaard, M.; Jensen, J.P.; Lauridsen, T.; Landkildehus, F. Does high nitrogen loading prevent clear-water conditions in shallow lakes at moderately high phosphorus concentrations? Freshw. Biol. 2005, 50, 27–41. [Google Scholar] [CrossRef]
- Moss, B.; Jeppesen, E.; Søndergaard, M.; Lauridsen, T.L.; Liu, Z. Nitrogen, macrophytes, shallow lakes and nutrient limitation: Resolution of a current controversy? Hydrobiologia 2013, 710, 3–21. [Google Scholar] [CrossRef]
- Bornette, G.; Puijalon, S. Response of aquatic plants to abiotic factors: A review. Aquat. Sci. 2011, 73, 1–14. [Google Scholar] [CrossRef]
- Olsen, S.; Chan, F.; Li, W.; Zhao, S.; Søndergaard, M.; Jeppesen, E. Strong impact of nitrogen loading on submerged macrophytes and algae: A long-term mesocosm experiment in a shallow Chinese lake. Freshw. Biol. 2015, 60, 1525–1536. [Google Scholar] [CrossRef]
- Hussner, A.; Smith, R.; Mettler-Altmann, T.; Hill, M.P.; Coetzee, J. Simulated global increases in atmospheric CO2 alter the tissue composition, but not the growth of some submerged aquatic plant bicarbonate users growing in DIC rich waters. Aquat. Bot. 2019, 153, 44–50. [Google Scholar] [CrossRef]
- James, C.S.; Eaton, J.W.; Hardwick, K. Responses of three invasive aquatic macrophytes to nutrient enrichment do not explain their observed field displacements. Aquat. Bot. 2006, 84, 347–353. [Google Scholar] [CrossRef]
- Garbey, C.; Murphy, K.J.; Thiébaut, G.; Muller, S. Variation in P-content in aquatic plant tissues offers an efficient tool for determining plant growth strategies along a resource gradient. Freshw. Biol. 2004, 49, 346–356. [Google Scholar] [CrossRef]
- Wersal, R.M.; Madsen, J.D. Influences of water column nutrient loading on growth characteristics of the invasive aquatic macrophyte Myriophyllum aquaticum (Vell.). Verdc. Hydrobiol. 2011, 665, 93–105. [Google Scholar] [CrossRef]
- Madsen, T.V.; Cedergreen, N. Sources of nutrients to rooted submerged macrophytes growing in a nutrient-rich stream. Freshw. Biol. 2002, 47, 283–291. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Han, R.; Song, K.; Zhou, X.; Wang, G.; Wang, Q. Eutrophication triggers the shift of nutrient absorption pathway of submerged macrophytes: Implications for the phytoremediation of eutrophic waters. J. Environ. Manag. 2019, 239, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, K.; Heidbüchel, P.; Hussner, A. Effects of water nutrients on regeneration capacity of submerged aquatic plant fragments. Ann. Limnol.-Int. J. Lim. 2014, 50, 155–162. [Google Scholar] [CrossRef]
- Levi, P.S.; Riis, T.; Alnøe, A.B.; Peipoch, M.; Maetzke, K.; Bruus, C.; Baattrup-Pedersen, A. Macrophyte complexity controls nutrient uptake in lowland streams. Ecosystems 2015, 18, 914–931. [Google Scholar] [CrossRef]
- Heidbüchel, P.; Sachs, M.; Stanik, N.; Hussner, A. Species-specific fragmentation rate and colonization potential partly explain the successful spread of aquatic plants in lowland streams. Hydrobiologia 2019, 843, 107–123. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, J.; Ma, X.; Zhong, F.; Cui, N.; Cheng, S. Increasing phytoplankton-available phosphorus and inhibition of macrophyte on phytoplankton bloom. Sci. Total Environ. 2017, 579, 871–880. [Google Scholar] [CrossRef]
- Søndergaard, M.; Lauridsen, T.L.; Johansson, L.S.; Jeppesen, E. Nitrogen or phosphorus limitation in lakes and its impact on phytoplankton biomass and submerged macrophyte cover. Hydrobiologia 2017, 795, 35–48. [Google Scholar] [CrossRef]
- Qin, B.Q.; Gao, G.; Zhu, G.W.; Zhang, Y.L.; Song, Y.Z.; Tang, X.M.; Xu, H.; Deng, J.M. Lake eutrophication and its ecosystem response. Chin. Sci. Bull. 2013, 58, 961–970. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, H.; Zhang, J.; Yu, J.; Xie, P.; Chen, J. Physiological differences between free-floating and periphytic filamentous algae, and specific submerged macrophytes induce proliferation of filamentous algae: A novel implication for lake restoration. Chemosphere 2020, 239, 124702. [Google Scholar] [CrossRef] [PubMed]
- Hussner, A.; Heidbüchel, P.; Coetzee, J.; Gross, E.M. From introduction to nuisance growth: A review of traits of alien aquatic plants which contribute to their invasiveness. Hydrobiologia 2021, 848, 2119–2151. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P. Naturalization of introduced plants: Ecological drivers of biogeographical patterns. New Phytol. 2012, 196, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Zenni, R.D.; Nuñez, M.A. The elephant in the room: The role of failed invasions in understanding invasion biology. Oikos 2013, 122, 801–815. [Google Scholar] [CrossRef]
- Pedersen, O.; Colmer, T.D.; Sand-Jensen, K. Underwater photosynthesis of submerged plants–recent advances and methods. Front. Plant Sci. 2013, 4, 140. [Google Scholar] [CrossRef]
- Hussner, A.; Mettler-Altmann, T.; Weber, A.P.; Sand-Jensen, K. Acclimation of photosynthesis to supersaturated CO2 in aquatic plant bicarbonate users. Freshw. Biol. 2016, 61, 1720–1732. [Google Scholar] [CrossRef]
- Prins, H.B.A.; Elzenga, J.T.M. Bicarbonate utilization: Function and mechanism. Aquat. Bot. 1989, 34, 59–83. [Google Scholar] [CrossRef]
- Underwood, A. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Gerald, B. A brief review of independent, dependent and one sample t-test. Int. J. Appl. Math. Theor. Phys. 2018, 4, 50–54. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).