Diversity and Distribution of 18 Cephalopod Species, and Their Link with Some Environmental Factors in the NW Pacific
Abstract
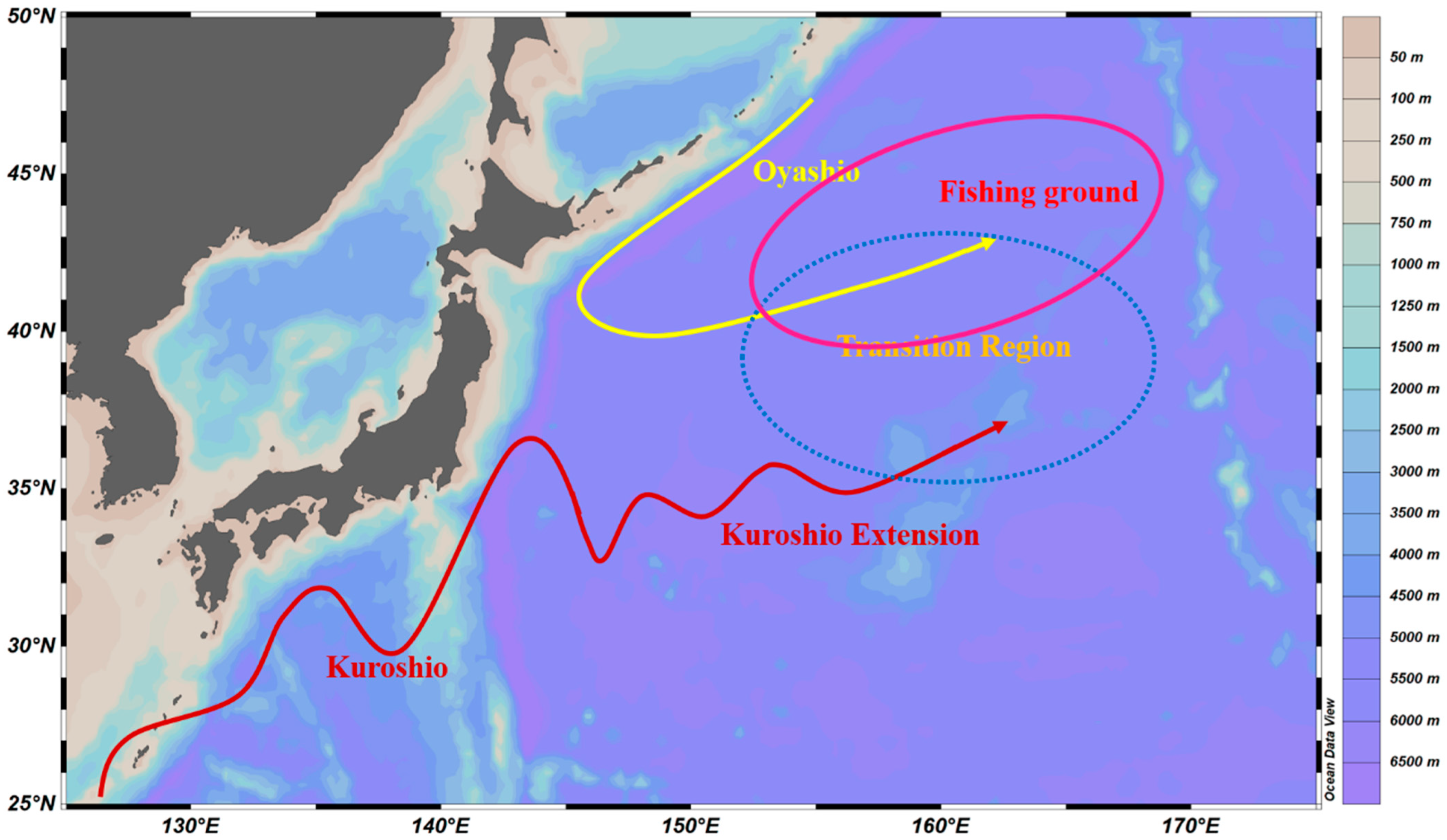
:1. Introduction
2. Material and Methods
2.1. Material
2.1.1. Resource Sampling Method
2.1.2. Environmental Data Acquisition Method
2.2. Methods
2.2.1. Catch (Weight) and Catch (Individual)
2.2.2. Diversity
2.2.3. Main Contributing Species
2.2.4. Relationship with Environmental Factors
3. Results
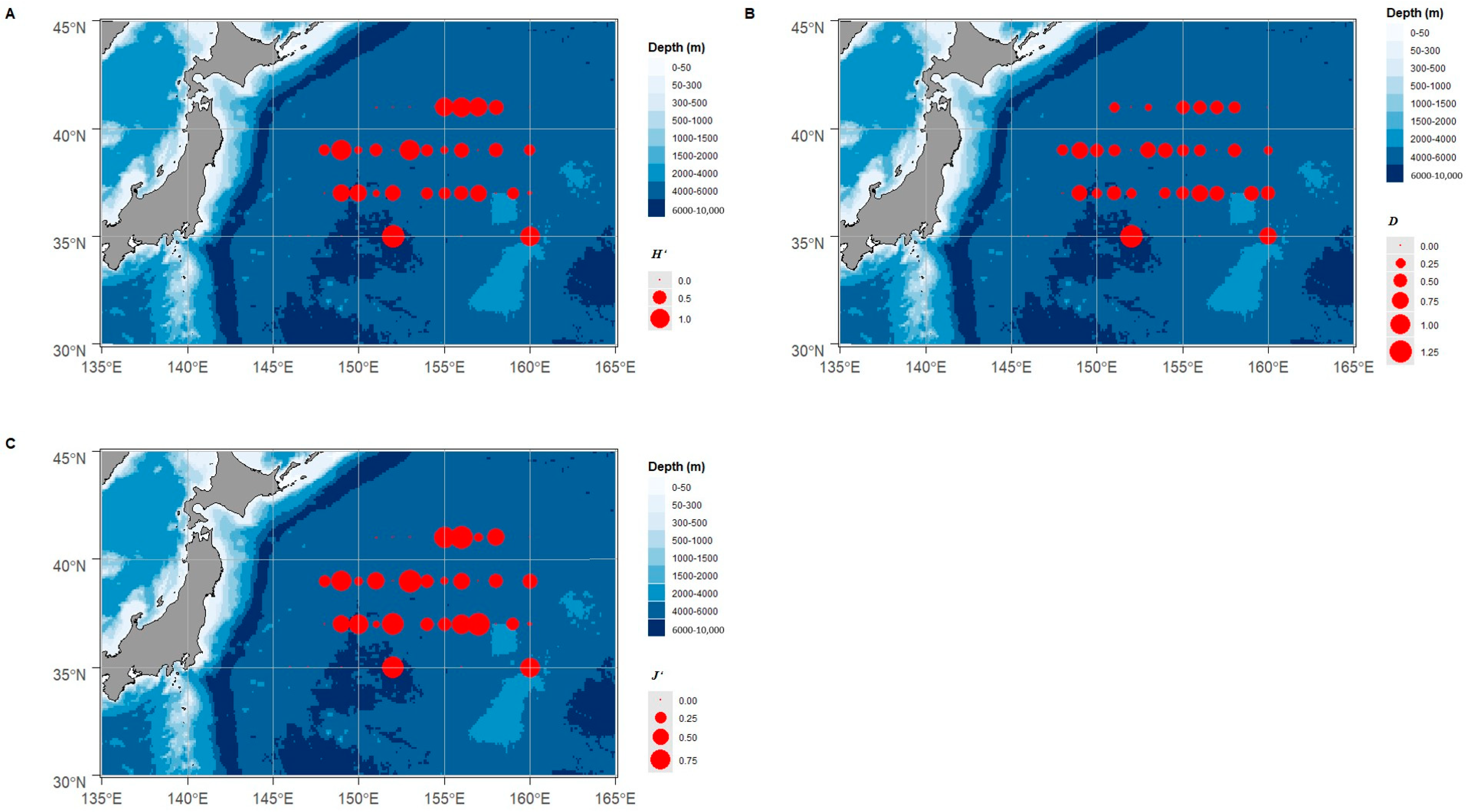
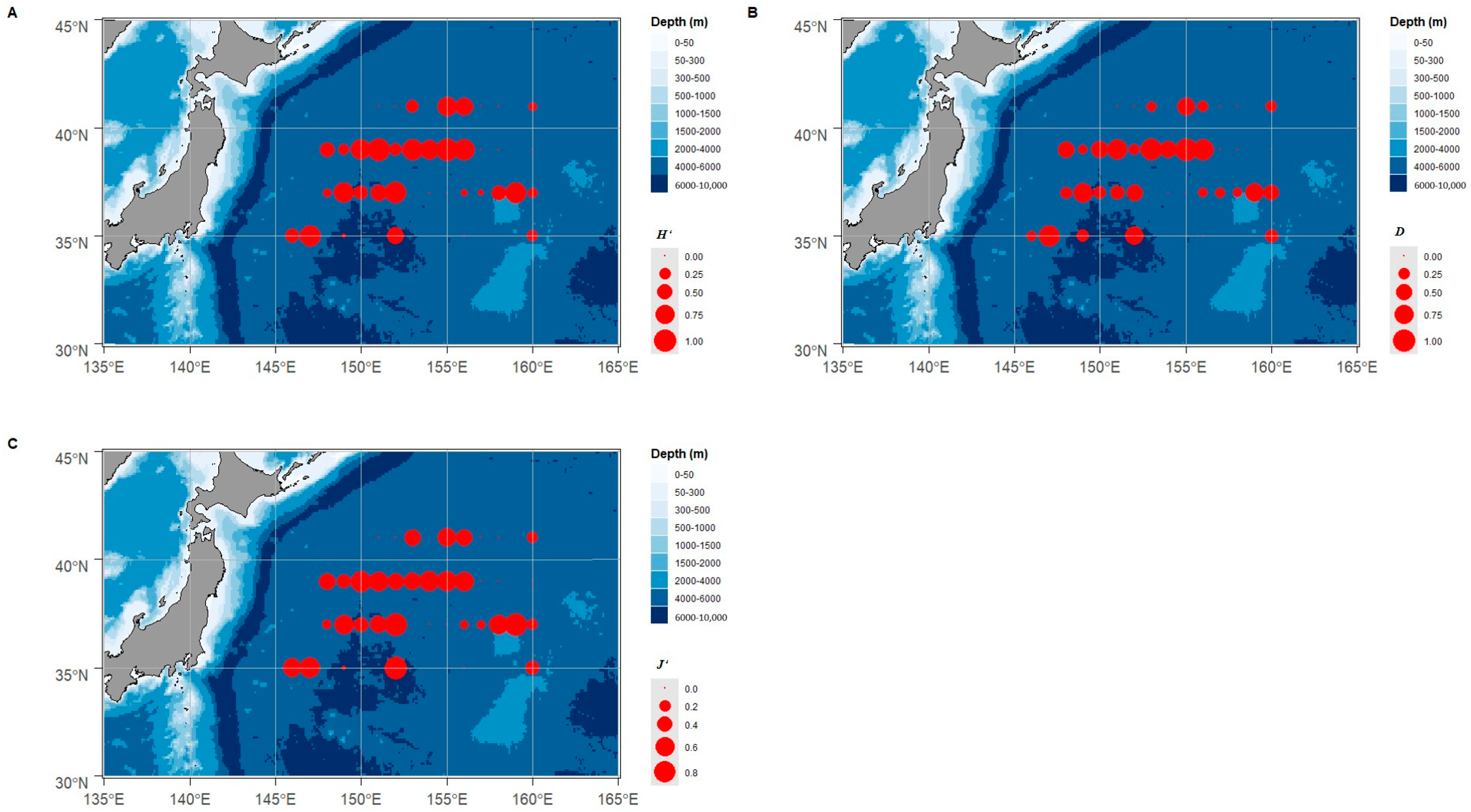
3.1. Cephalopods Species and Diversity
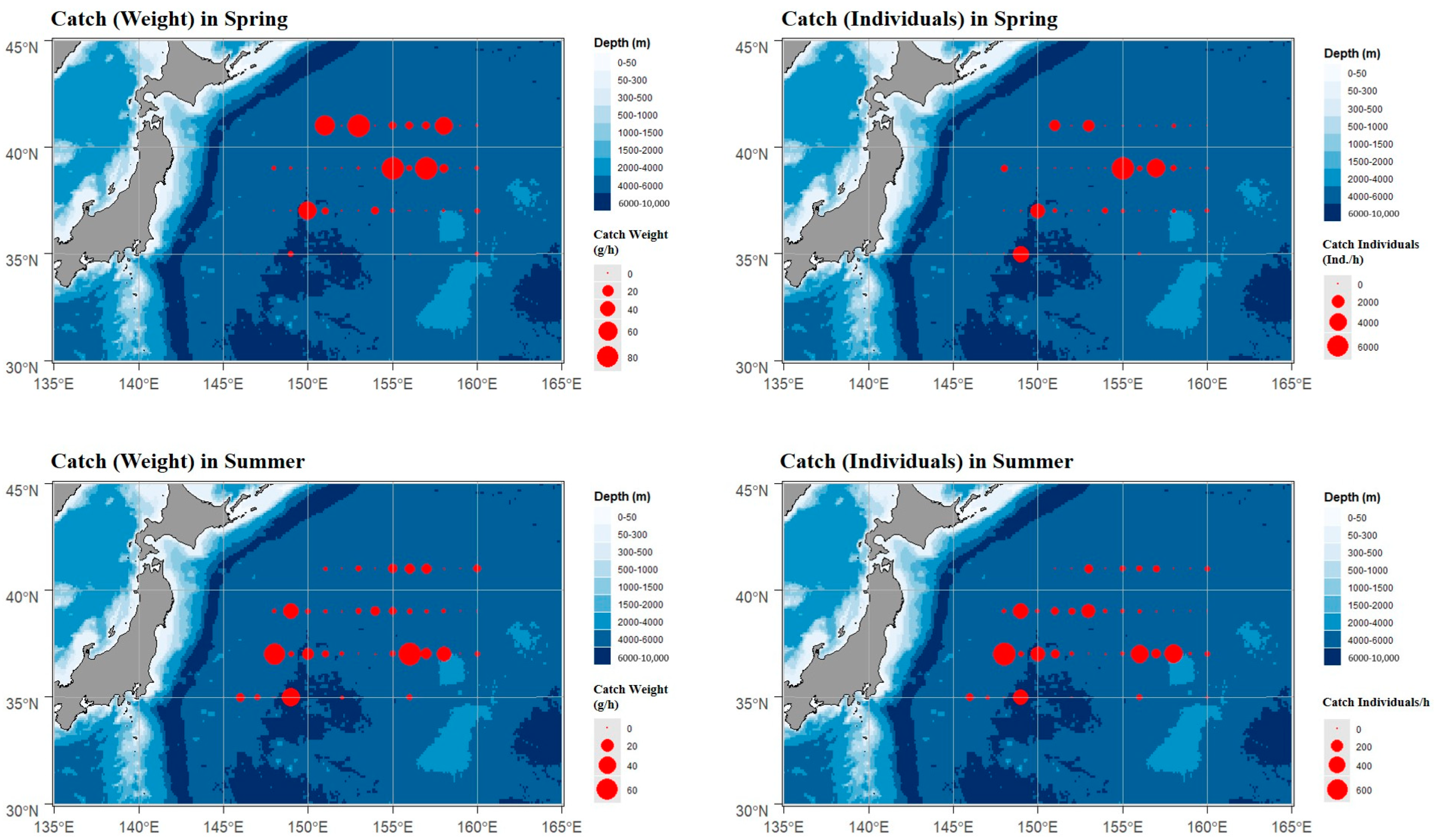
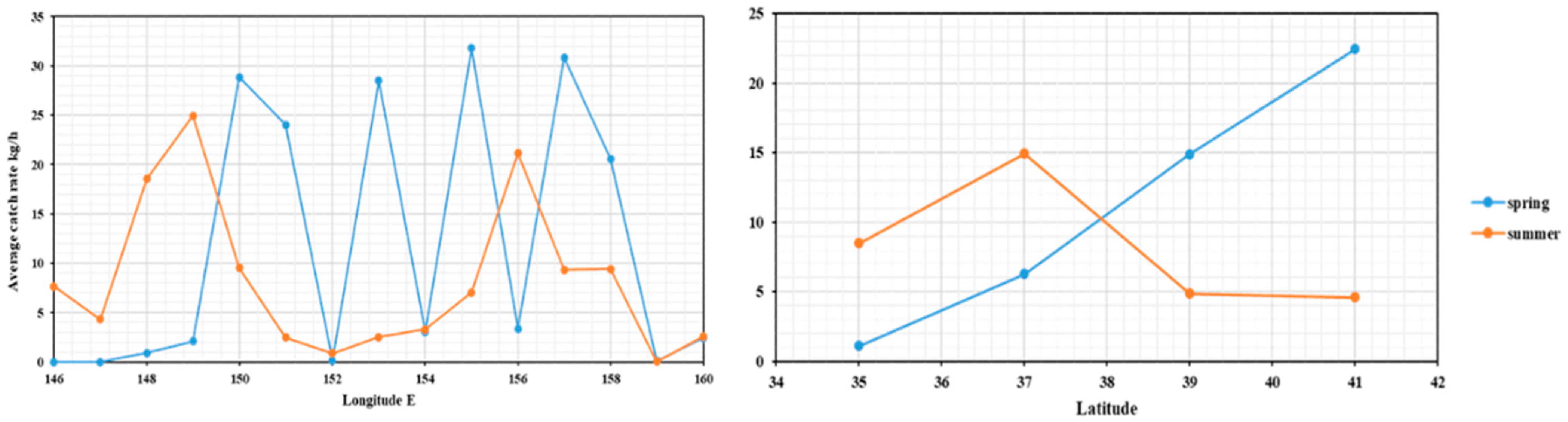
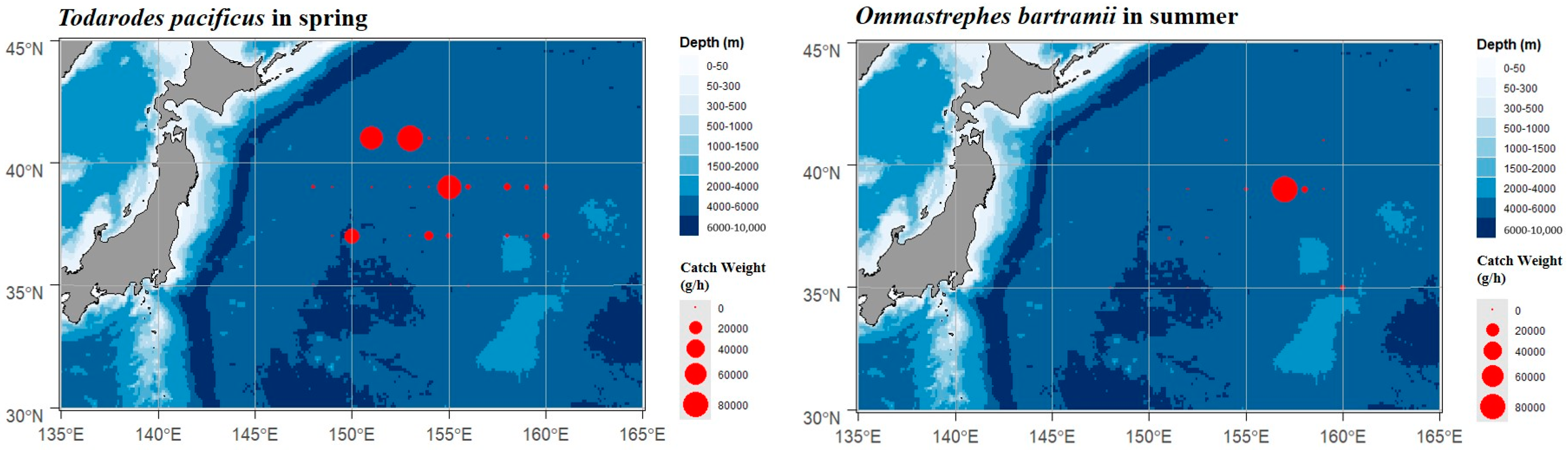
3.2. Resource of Cephalopods Distribution
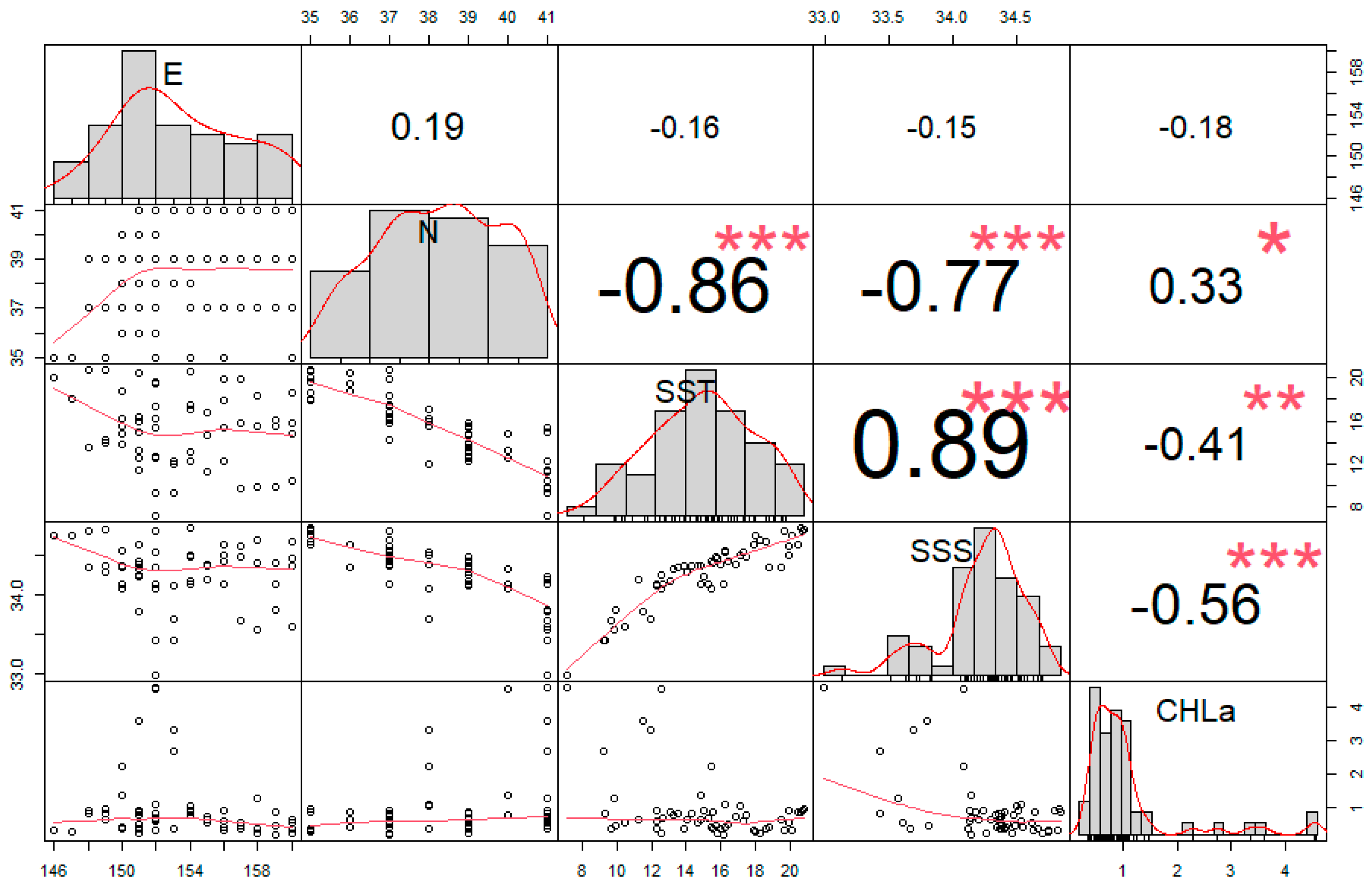
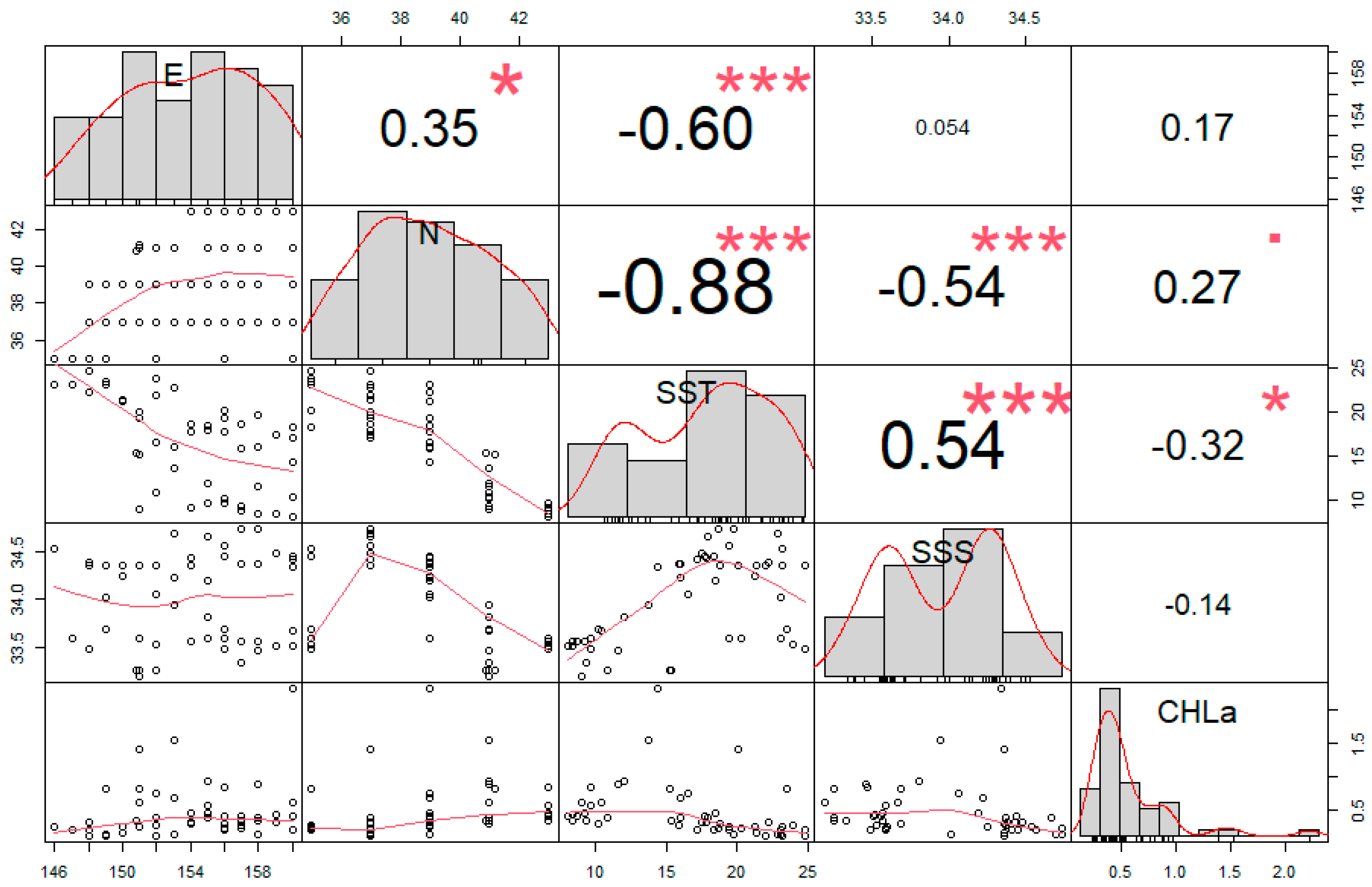
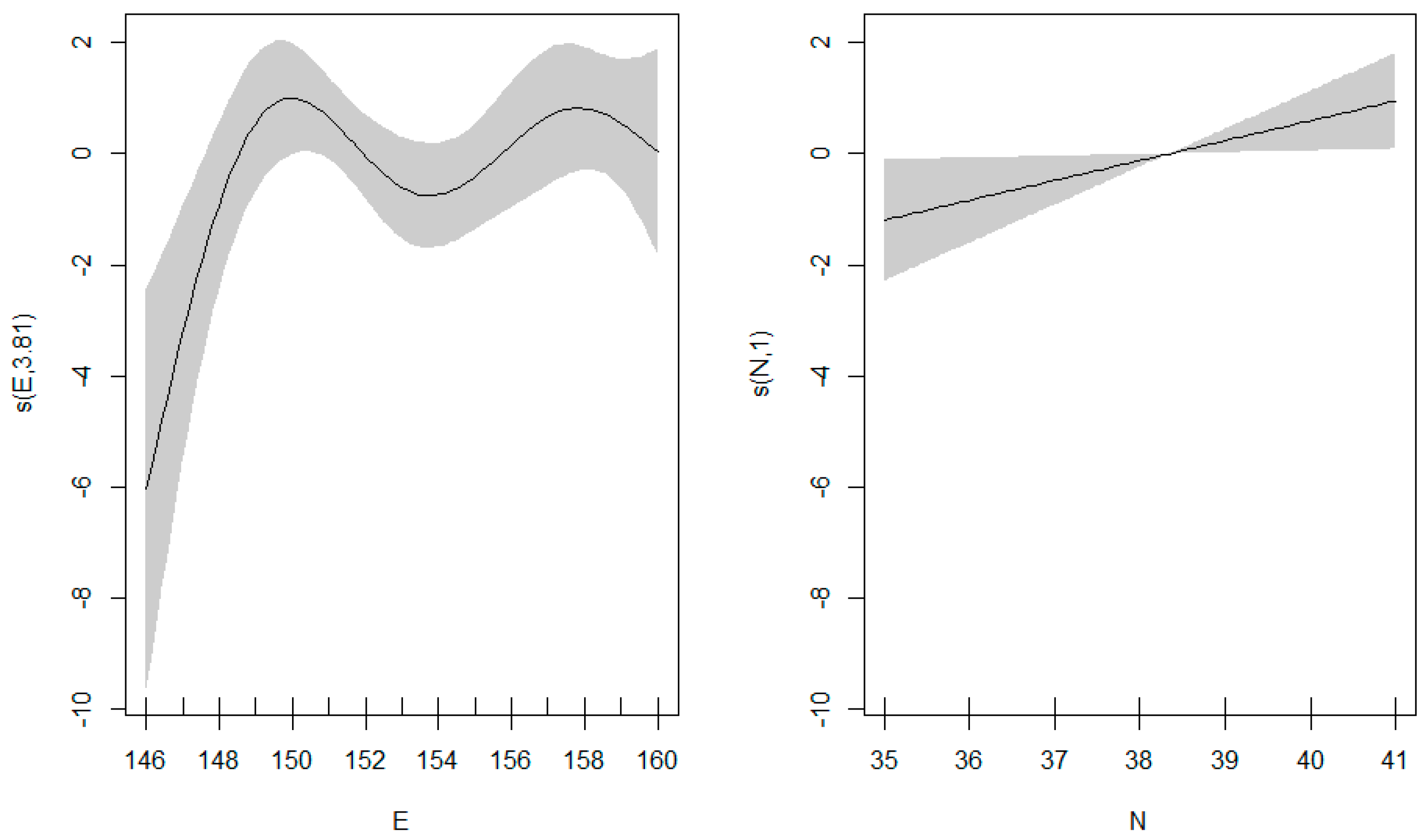
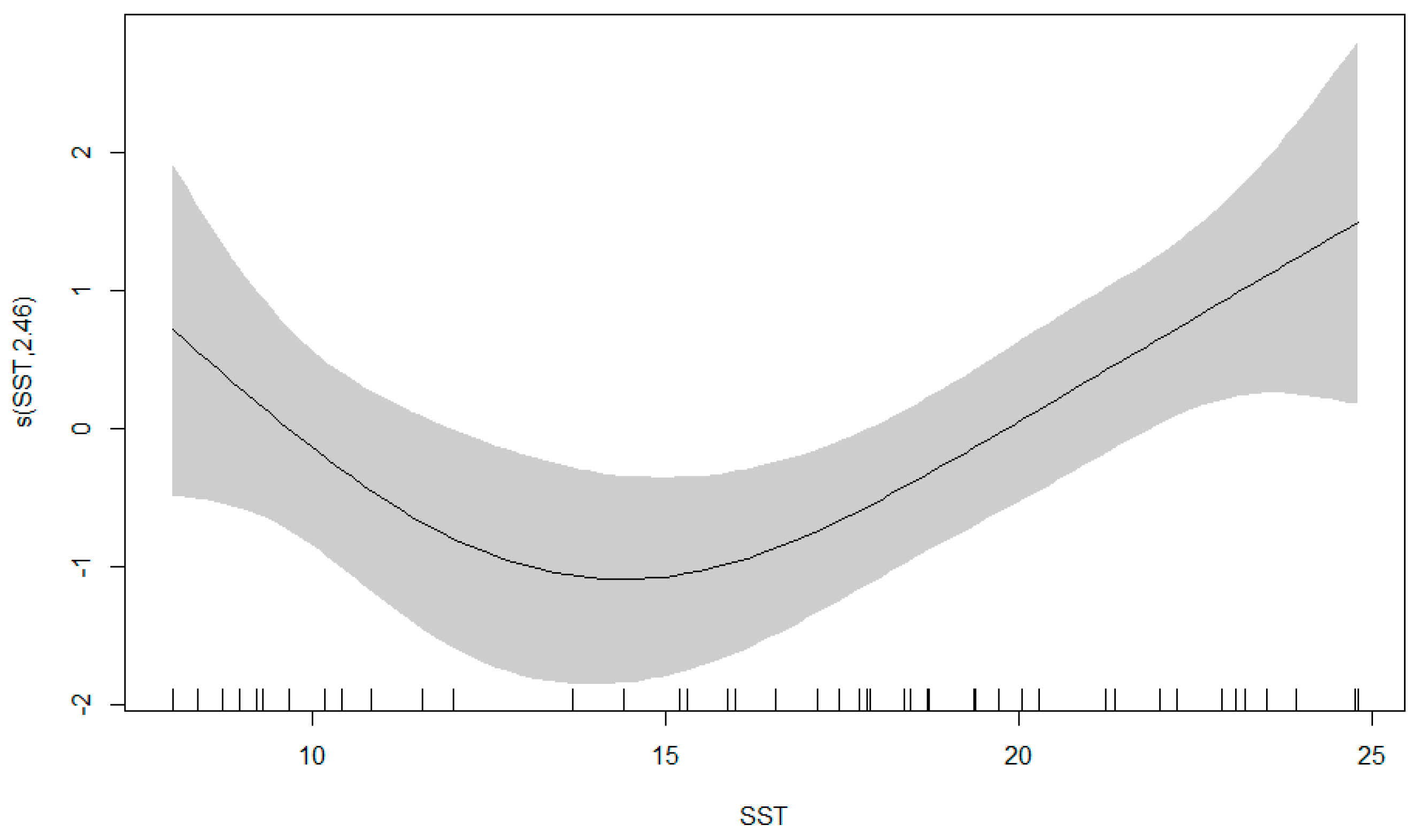
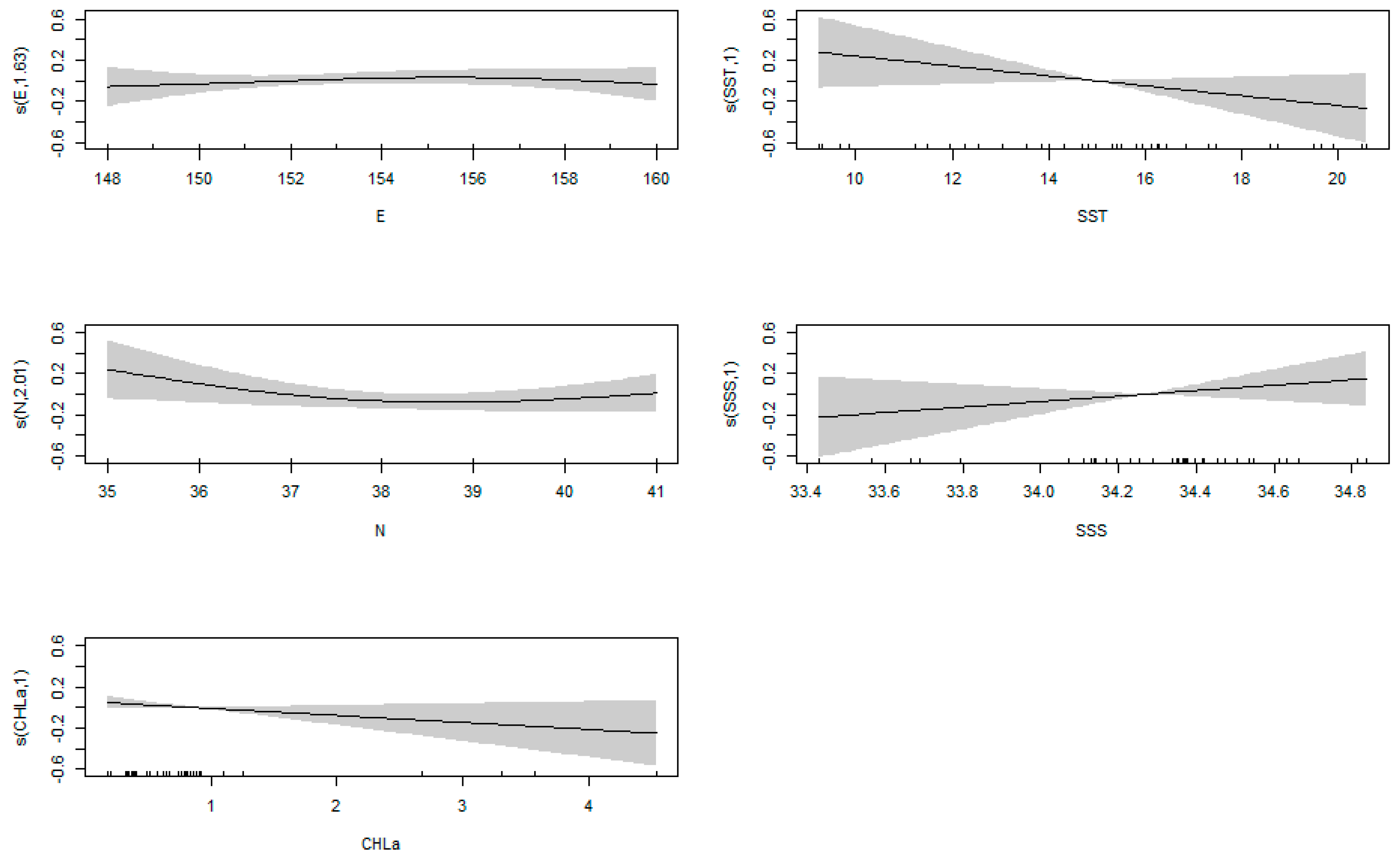
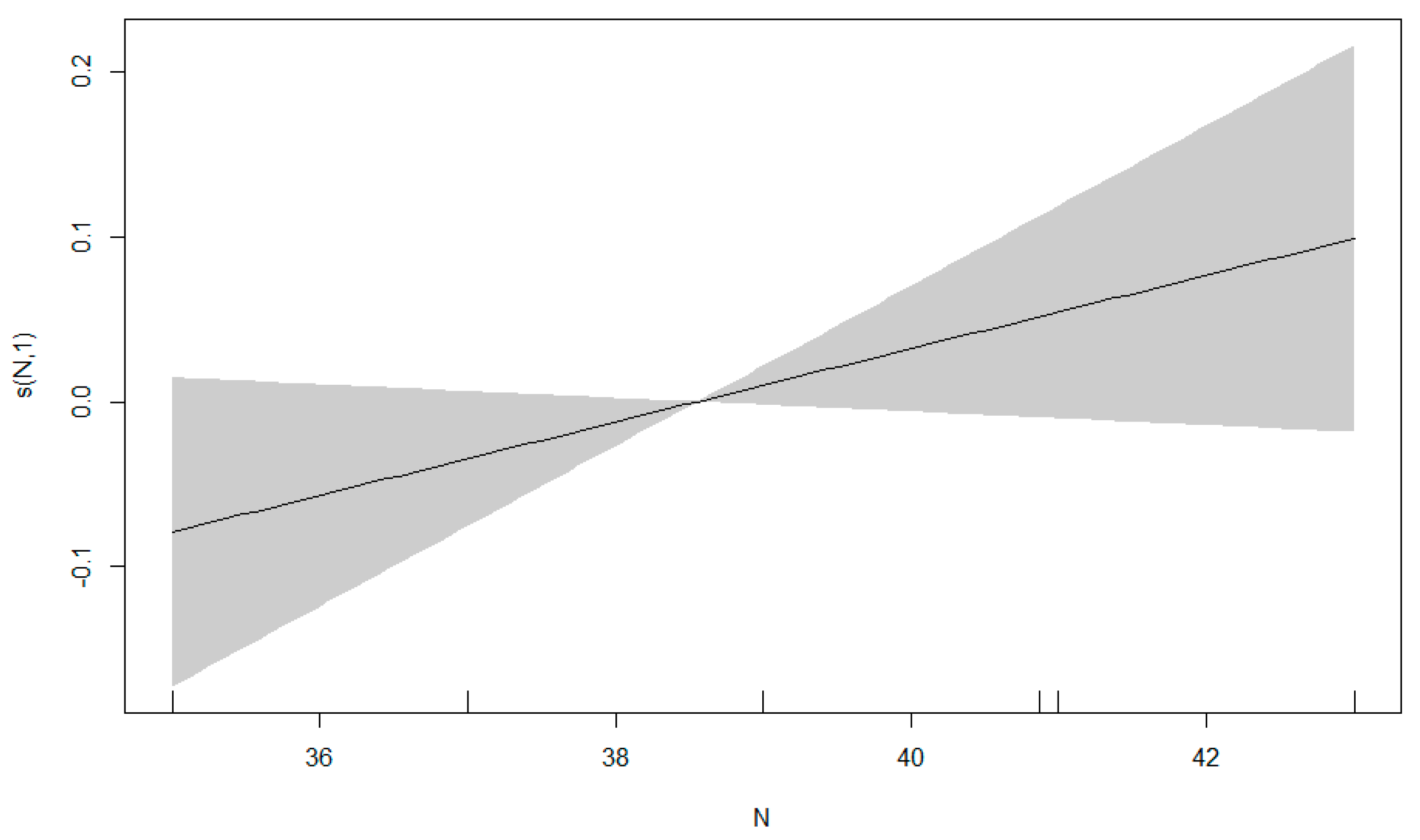
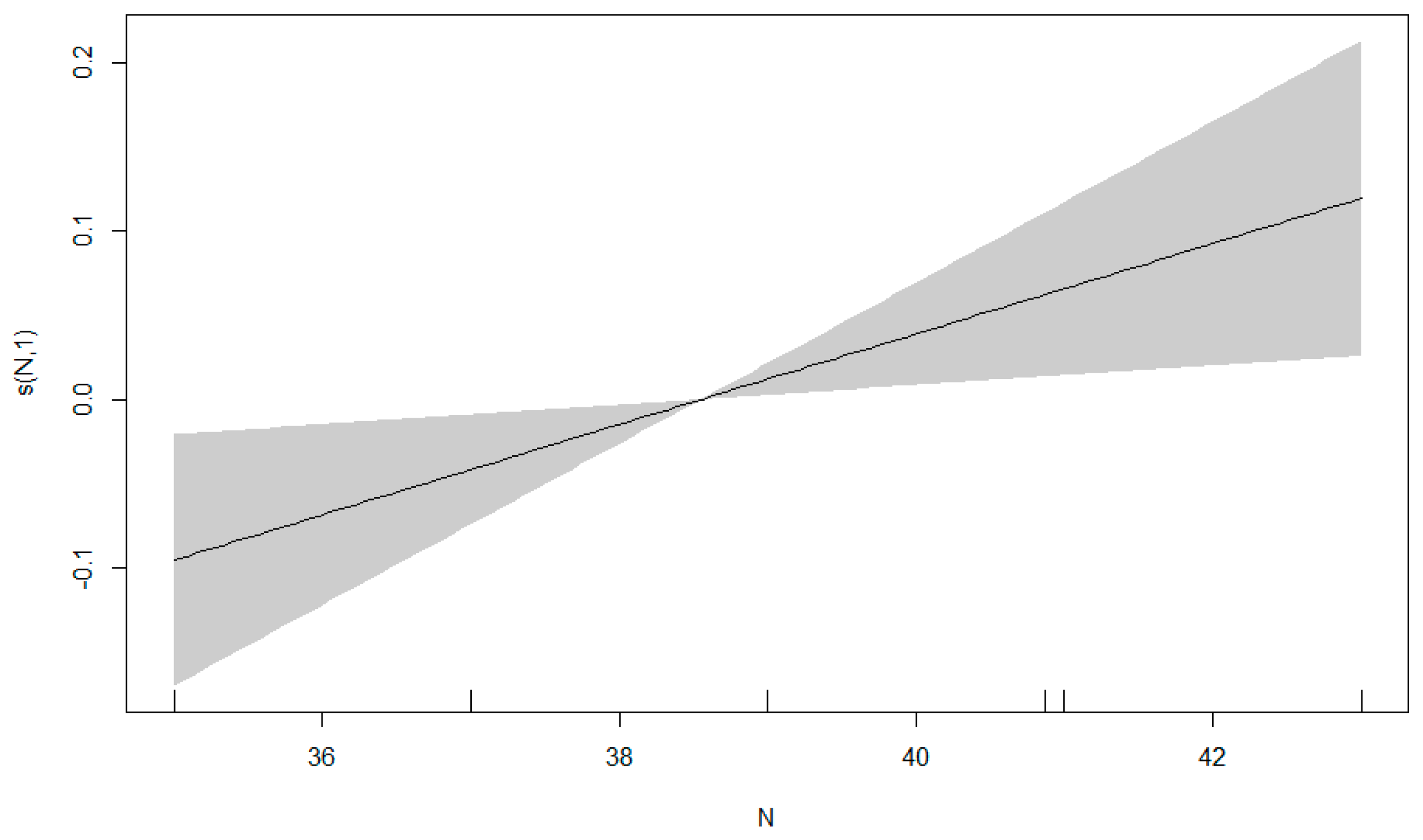
3.3. GAM Results
4. Discussion
4.1. Cephalopods Species Composition and Resource Distribution in the Northwest Pacific
4.2. Relationship between Cephalopod Resources and Environmental Factors in the Northwest Pacific
4.3. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kawabe, M. Variations of Current Path, Velocity, and Volume Transport of the Kuroshio in Relation with the Large Meander. J. Phys. Oceanogr. 1995, 25, 3103–3117. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kurita, Y.; Noto, M.; Oozeki, Y.; Kitagawa, D. Growth and survival of Pacific saury Cololabis saira in the Kuroshio-Oyashio transitional waters. J. Oceanogr. 2003, 59, 403–414. [Google Scholar] [CrossRef]
- Argüelles, J.; Rodhouse, P.G.; Villegas, P.; Castillo, G. Age, growth and population structure of the jumbo flying squid Dosidicus gigas in Peruvian waters. Fish. Res. 2001, 54, 51–61. [Google Scholar] [CrossRef]
- Zheng, P.; Wu, D.; Lin, X.; Li, X.-T. Interannual variability of Kuroshio current and its effect on the nearshore branch in Japan/east sea. J. Hydrodyn. Ser. B 2010, 22, 305–311. [Google Scholar] [CrossRef]
- FAO and Agriculture. FAO Yearbook of Fisheries Statistics 2022; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Rosa, R.; Pissarra, V.; Borges, F.O.; Xavier, J.; Gleadall, I.G.; Golikov, A.; Bello, G.; Morais, L.; Lishchenko, F.; Roura, Á.; et al. Global patterns of species richness in coastal cephalopods. Front. Mar. Sci. 2019, 6, 469. [Google Scholar] [CrossRef]
- FAO and Agriculture. FAO Yearbook of Fisheries Statistics 2007; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007. [Google Scholar]
- Zhou, J.G.; Chen, X.J.; Liu, B.L. Notes on the present status of exploitation and potential of cephalopod resources on the world. Mar. Fish. 2008, 30, 268–275. [Google Scholar]
- Rodhouse, P.G.K.; Pierce, G.J.; Nichols, O.C.; Sauer, W.H.H.; Arkhipkin, A.I.; Laptikhovsky, V.V.; Lipiński, M.R.; Ramos, J.E.; Gras, M.; Kidokoro, H.; et al. Environmental effects on cephalopod population dynamics: Implications for management of fisheries. Adv. Mar. Biol. 2014, 67, 99–233. [Google Scholar]
- Lipiński, M.R. Cephalopod life cycles: Patterns and exceptions. S. Afr. J. Mar. Sci. 1998, 20, 439–447. [Google Scholar] [CrossRef]
- Pierce, G.J.; Valavanis, V.D.; Guerra, A.; Jereb, P.; Orsi-Relini, L.; Bellido-Millán, J.; Katara, I.; Piatkowski, U.; Pereira, J.; Balguerias, E.; et al. A review of cephalopod-environment interactions in European Seas. Hydrobiologia 2008, 612, 49–70. [Google Scholar] [CrossRef]
- Chen, X.; Tian, S.; Chen, Y.; Liu, B. A modeling approach to identify optimal habitat and suitable fishing grounds for neon flying squid (Ommostrephes bartramii) in the Northwest Pacific Ocean. Fish. Bull. 2010, 108, 1–14. [Google Scholar]
- Laptikhovsky, V. Latitudinal and bathymetric trends in egg size variation: A new look at Thorson’s and Rass’s rules. Mar. Ecol. 2006, 27, 7–14. [Google Scholar] [CrossRef]
- Caballero-Alfonso, A.M.; Ganzedo, U.; Trujillo-Santana, A.; Polanco-Martínez, J.M.; del Pino, A.S.; Ibarra-Berastegi, G.; Hernandez, J.C. The role of climatic variability on the short-term fluctuations of octopus captures at the Canary Islands. Fish. Res. 2010, 102, 258–265. [Google Scholar] [CrossRef]
- Pierce, G.J.; Boyle, P.R. Empirical modelling of interannual trends in abundance of squid (Loligo forbesi) in Scottish waters. Fish. Res. 2003, 59, 305–326. [Google Scholar] [CrossRef]
- Vargas-Yáñez, M.; Moya, F.; García-Martínez, M.; Rey, J.; González, M.; Zunino, P. Relationships between Octopus vulgaris landings and environmental factors in the northern Alboran Sea (Southwestern Mediterranean). Fish. Res. 2009, 99, 159–167. [Google Scholar] [CrossRef]
- Jereb, P.; Roper, C.F.E. Cephalopods of the World—An Annotated and Illustrated Catalogue of Cephalopod Species Known to Date; Myopsid and oegopsid squids; FAO: Rome, Italy, 2010; Volume 2. [Google Scholar]
- Nabhitabhata, J.; Asawangkune, P.; Amornjaruchit, S.; Promboon, P. Tolerance of Eggs and Hatchlings of Neritic Cephalopods to Salinity Changes. Phuket Mar. Biol. Cent. Spec. Publ. 2001, 251, 91–99. [Google Scholar]
- Margalef, R. On certain unifying principles in ecology. Am. Nat. 1963, 97, 357–374. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Somerfield, P.J.; Clarke, K.R. Inverse analysis in non-parametric multivariate analyses: Distinguishing groups of associated species which covary coherently across samples. J. Exp. Mar. Biol. Ecol. 2013, 449, 261–273. [Google Scholar] [CrossRef]
- Chouldechova, A.; Hastie, T. Generalized additive model selection. arXiv 2015, arXiv:1506.03850. [Google Scholar]
- Volvenko, I.V.; Orlov, A.M.; Gebruk, A.V.; Katugin, O.N.; Vinogradov, G.M.; Maznikova, O.A. Species richness and taxonomic composition of trawl macrofauna of the North Pacific and its adjacent seas. Sci. Rep. 2018, 8, 16604. [Google Scholar] [CrossRef] [PubMed]
- Rathjen, W.F. Cephalopod capture methods: An overview. Bull. Mar. Sci. 1991, 49, 494–505. [Google Scholar]
- Jiaxin, S. Analysis and Research on Fishing Capacity of Fishing Vessels Based on Data. Ph.D. Thesis, Shanghai Ocean University, Shanghai, China, 2021. [Google Scholar] [CrossRef]
- Goodwin, C.L.; Pease, B. Life histories and environmental requirements of coastal fishes and invertebrates (Pacific Northwest)-Pacific geoduck clam. U. S. Fish Wildl. Serv. Biol. Rep. 1989, 82, 14. [Google Scholar]
- Andre, J.; Haddon, M.; Pecl, G.T. Modelling climate-change-induced nonlinear thresholds in cephalopod population dynamics. Glob. Change Biol. 2010, 16, 2866–2875. [Google Scholar] [CrossRef]
- Boyle, P.R. Cephalopod biology in the fisheries context. Fish. Res. 1990, 8, 303–321. [Google Scholar] [CrossRef]
- Zheng, X.D.; Han, S.; Lin, X.Z.; Li, Q. Research progress in Cephalopod reproductive behaviors. J. Fish. Sci. China 2009, 16, 459–465. [Google Scholar]
- Rosa, A.L.; Yamamoto, J.; Sakurai, Y. Effects of environmental variability on the spawning areas, catch, and recruitment of the Japanese common squid, Todarodes pacificus (Cephalopoda: Ommastrephidae), from the 1970s to the 2000s. ICES 28. J. Mar. Sci. 2011, 68, 1114–1121. [Google Scholar] [CrossRef]
- Pierce, G.J.; Portela, J.; Robin, J.P. Foreword: Environmental effects on cephalopod life history and fisheries. Aquat. Living Resour. 2005, 18, 325–326. [Google Scholar] [CrossRef]
- Huang, W.B.; Huang, Y.C. Maturity characteristics of pacific saury during fishing season in the Northwest Pacific. J. Mar. Sci. Technol. 2015, 23, 819–826. [Google Scholar]
- Yu, W.; Chen, X.; Yi, Q.; Chen, Y. Spatio-temporal distributions and habitat hotspots of the winter–spring cohort of neon flying squid Ommastrephes bartramii in relation to oceanographic conditions in the Northwest Pacific Ocean. Fish. Res. 2016, 175, 103–115. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X.J.; Chen, Y.; Yi, Q.; Zhang, Y. Effects of environmental variations on the abundance of western winter-spring cohort of neon flying squid (Ommastrephes bartramii) in the Northwest Pacific Ocean. Acta Oceanol. Sin. 2015, 34, 43–51. [Google Scholar] [CrossRef]
- Sakurai, Y.; Kiyofuji, H.; Saitoh, S.; Goto, T.; Hiyama, Y. Changes in inferred spawning areas of Todarodes pacificus (Cephalopoda: Ommastrephidae) due to changing environmental conditions. ICES J. Mar. Sci. 2000, 57, 24–30. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, Y.; Chen, X.; Yi, Q.; Qian, W. Response of winter cohort abundance of Japanese common squid Todarodes pacificus to the ENSO events. Acta Oceanol. Sin. 2018, 37, 61–71. [Google Scholar] [CrossRef]
- Rosa, R.; González, L.; Dierssen, H.M.; Seibel, B.A. Environmental determinants of latitudinal size-trends in cephalopods. Mar. Ecol. Prog. Ser. 2012, 464, 153–165. [Google Scholar] [CrossRef]
- Rosa, R.; Dierssen, H.M.; González, L.; Seibel, B.A. Large-scale diversity patterns of cephalopods in the Atlantic open ocean and deep sea. Ecology 2008, 89, 3449–3461. [Google Scholar] [CrossRef]
- Pecl, G.T.; Jackson, G.D. The potential impacts of climate change on inshore squid: Biology, ecology and fisheries. Rev. Fish Biol. Fish. 2008, 18, 373–385. [Google Scholar] [CrossRef]
- Xavier, J.C.; Allcock, A.L.; Cherel, Y.; Lipinski, M.R.; Pierce, G.J.; Rodhouse, P.G.K.; Rosa, R.; Shea, E.K.; Strugnell, J.M.; Vidal, E.A.G.; et al. Future challenges in cephalopod research. J. Mar. Biol. Assoc. United Kingd. 2015, 95, 999–1015. [Google Scholar] [CrossRef]
- Rosa, R.; Seibel, B.A. Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator. Proc. Natl. Acad. Sci. USA 2008, 105, 20776–20780. [Google Scholar] [CrossRef]
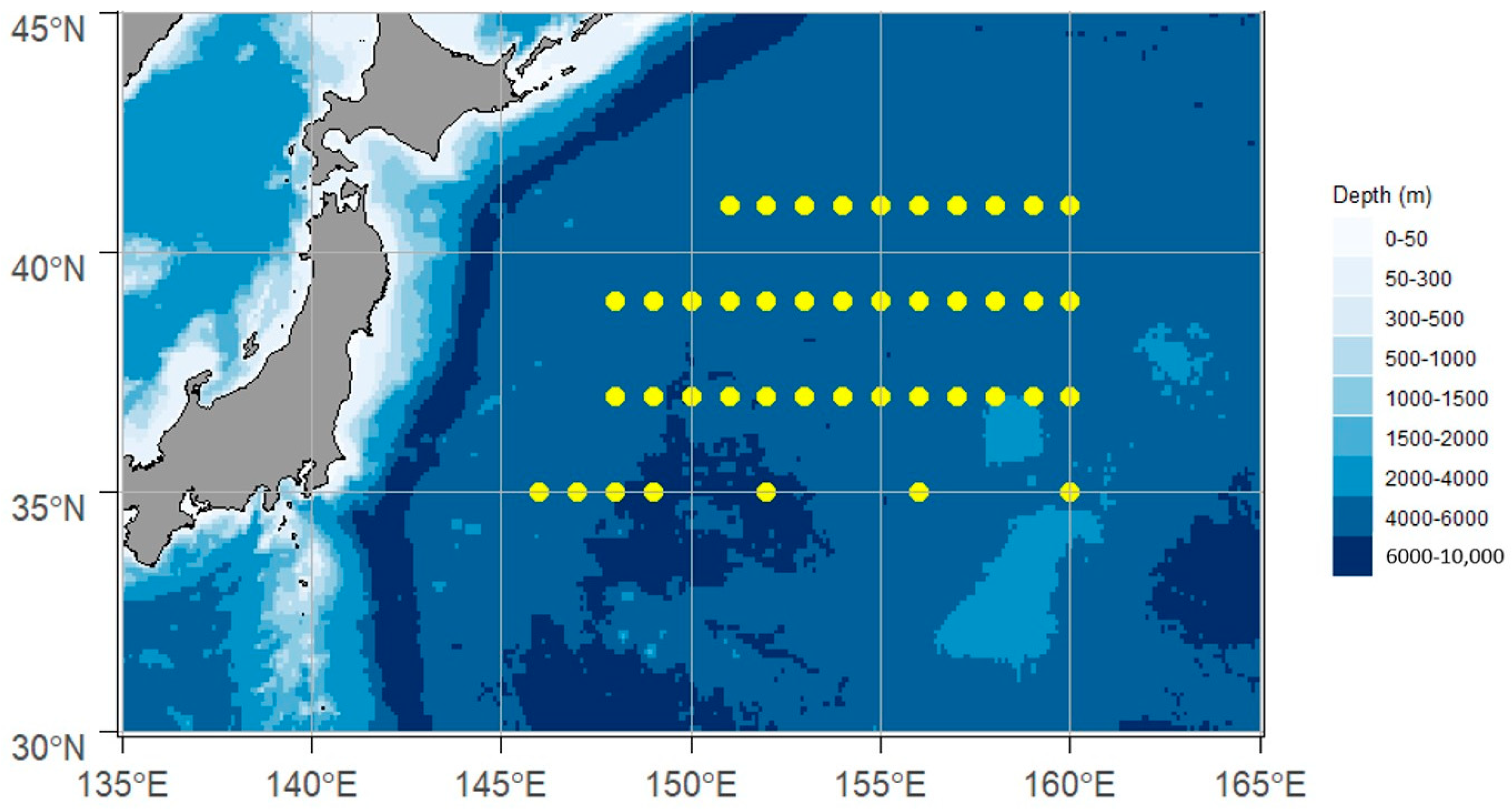



| Season | Time | Number of Stations | Major Data Field |
|---|---|---|---|
| Spring | 16 April 2015–25 June 2015 | 45 | Latitude/longitude/catch/SST/SSS/Chl a |
| Summer | 21 May 2016–23 July 2016 | 45 | Latitude/longitude/catch/SST/SSS/Chl a |
| Class | Order | Family | Genus | Species | Catch in Spring | Catch in Summer |
|---|---|---|---|---|---|---|
| Cephalopoda | Oegopsida | Ommastrephidae | Ommastrephes | Ommastrephes bartramii | 3084.99 | 5885.86 |
| Todarodes | Todarodes pacificus | 9141.30 | 220.15 | |||
| Todarodes sp. | / | 22.70 | ||||
| Eucleoteuthis | Eucleoteuthis luminosa | 211.70 | 918.65 | |||
| Sthenoteuthis | Sthenoteuthis oualaniensis | / | 221.87 | |||
| Enoploteuthidae | Abralia | Abralia andamanica | 148.44 | 21.49 | ||
| Abralia similis | / | 1.92 | ||||
| Enoploteuthis | Enoploteuthis chunii | 6.33 | / | |||
| Onychoteuthidae | Onychoteuthis | Onychoteuthis banksii | / | 8.41 | ||
| Onychoteuthis borealijaponica | 3973.62 | 626.25 | ||||
| Onychoteuthis sp. | / | 3.47 | ||||
| Onykia | Onykia robusta | 8.60 | 10.70 | |||
| Cranchiidae | Cranchia | Cranchia scabra | 0.34 | / | ||
| Architeuthidae | Architeuthis | Architeuthis dux | / | 14.82 | ||
| Gonatidae | Gonatopsis | Gonatopsis borealis | 328.20 | 192.29 | ||
| Thysanoteuthidae | Thysanoteuthis | Thysanoteuthis rhombus | / | 104.65 | ||
| Octopoda | Tremoctopodidae | Tremoctopus | Tremoctopus violaceus | / | 13.13 | |
| Ocythoidae | Ocythoe | Ocythoe tuberculata | / | 3.05 |
| Season | H′ | D | J′ |
|---|---|---|---|
| Spring | 0.3691 ± 0.37 | 0.3176 ± 0.27 | 0.3498 ± 0.33 |
| Summer | 0.3919 ± 0.35 | 0.3237 ± 0.29 | 0.3824 ± 0.31 |
| Spring: | Average | Sd | Ratio | Cumsum |
|---|---|---|---|---|
| Species | ||||
| Todarodes pacificus | 0.382 | 0.3664 | 1.0425 | 0.4218 |
| Onychoteuthis borealijaponica | 0.234 | 0.2888 | 0.81 | 0.6801 |
| Ommastrephes bartramii | 0.1011 | 0.235 | 0.4303 | 0.7918 |
| Eucleoteuthis luminosa | 0.0915 | 0.2448 | 0.3737 | 0.8928 |
| Abralia andamanica | 0.0434 | 0.1442 | 0.3011 | 0.9407 |
| Gonatopsis borealis | 0.042 | 0.1045 | 0.4023 | 0.9871 |
| Onykia robusta | 0.006 | 0.0477 | 0.1267 | 0.9938 |
| Enoploteuthis chunii | 0.0052 | 0.0409 | 0.1263 | 0.9995 |
| Cranchia scabra | 0.0004 | 0.0036 | 0.1197 | 1 |
| Summer: | ||||
| Ommastrephes bartramii | 0.3719 | 0.3511 | 1.0594 | 0.4242 |
| Onychoteuthis borealijaponica | 0.2103 | 0.2922 | 0.7196 | 0.6641 |
| Eucleoteuthis luminosa | 0.0812 | 0.1848 | 0.4395 | 0.7567 |
| Gonatopsis borealis | 0.0626 | 0.1147 | 0.5457 | 0.8281 |
| Sthenoteuthis oualaniensis | 0.0549 | 0.1919 | 0.2862 | 0.8908 |
| Todarodes pacificus | 0.0463 | 0.1403 | 0.3303 | 0.9436 |
| Thysanoteuthis rhombus | 0.0154 | 0.0824 | 0.1866 | 0.9611 |
| Todarodes sp. | 0.0113 | 0.0778 | 0.1458 | 0.9741 |
| Abralia andamanica | 0.0055 | 0.0285 | 0.1948 | 0.9804 |
| Architeuthis dux | 0.0054 | 0.0324 | 0.168 | 0.9866 |
| Tremoctopus violaceus | 0.0038 | 0.023 | 0.1664 | 0.991 |
| Onychoteuthis sp. | 0.003 | 0.0251 | 0.1184 | 0.9944 |
| Onychoteuthis banksii | 0.0022 | 0.009 | 0.2466 | 0.9969 |
| Ocythoe tuberculata | 0.001 | 0.0048 | 0.2073 | 0.998 |
| Onykia robusta | 0.0009 | 0.0049 | 0.1926 | 0.9991 |
| Abralia similis | 0.0008 | 0.0047 | 0.1651 | 1 |
| Model | Covariates | Deviance Explanation | AIC | R2 |
|---|---|---|---|---|
| Catch in spring | ||||
| 1 | E | 27.30% | 261.434 | 0.219 |
| 2 | N | 14.40% | 264.98 | 0.13 |
| 3 | SST | 13.80% | 266.85 | 0.11 |
| 4 | SSS | 21.40% | 265.14 | 0.16 |
| 5 | CHLa | 7.24% | 271.73 | 0.03 |
| 6 | * E + N | 33.40% | 258.5 | 0.27 |
| 7 | E + N + SST | 33.90% | 260.4 | 0.26 |
| 8 | E + N + SSS + SST | 33.80% | 262.22 | 0.24 |
| 9 | E + N + SSS + SST + CHLa | 41.80% | 260.26 | 0.3 |
| Catch in summer | ||||
| 1 | E | 18% | 199.81 | 0.12 |
| 2 | N | 6.45% | 203.86 | 0.02 |
| 3 | * SST | 21.60% | 195.98 | 0.17 |
| 4 | SSS | 22.30% | 197.02 | 0.17 |
| 5 | CHLa | 12.10% | 198.67 | 0.1 |
| H′ in spring | ||||
| 1 | E | 0.64% | 2.38 | −0.0191 |
| 2 | N | 11.40% | −0.26 | 0.0668 |
| 3 | SST | 2.84% | 2.3 | −0.0073 |
| 4 | SSS | 14.30% | 0.32 | 0.0736 |
| 5 | CHLa | 5.85% | 0.17 | 0.0343 |
| 6 | E + N | 11.60% | 1.59 | 0.0444 |
| 7 | E + N + SST | 12.50% | 3.13 | 0.0283 |
| 8 | E + N + SST + SSS | 25.50% | 0.9 | 0.119 |
| 9 | * E + N + SST + SSS + CHLa | 28.50% | 0.16 | 0.143 |
| D in spring | ||||
| 1 | E | 0.06% | −33.34 | −0.025 |
| 2 | N | 21.40% | −40.46 | 0.165 |
| 3 | SST | 10.90% | −35.92 | 0.0606 |
| 4 | * SSS | 35.30% | −45.83 | 0.288 |
| 5 | CHLa | 2.21% | −34.23 | −0.003 |
| 6 | SSS + E | 35.20% | −43.85 | 0.267 |
| J′ in spring | ||||
| 1 | E | 7.50% | −4.51 | 0.0313 |
| 2 | N | 6.01% | −4.1 | 0.0187 |
| 3 | SST | 8.78% | −4.44 | 0.0365 |
| 4 | SSS | 11.70% | −4.47 | 0.0509 |
| 5 | CHLa | 7.36% | −6.06 | 0.0499 |
| 6 | * E + CHLa | 15.90% | −6.25 | 0.0934 |
| H′ in summer | ||||
| 1 | E | 3.83% | −6.96 | −0.0041 |
| 2 | * N | 7.72% | −9.4 | 0.0501 |
| 3 | SST | 4.27% | −8.08 | 0.0146 |
| 4 | SSS | 10.90% | −8.84 | 0.0574 |
| 5 | CHLa | 10.70% | −7.48 | 0.0367 |
| D in summer | ||||
| 1 | E | 0.33% | −18.37 | −0.026 |
| 2 | N | 1.08% | −18.64 | −0.0183 |
| 3 | SST | 1.66% | −18.81 | −0.013 |
| 4 | * SSS | 23.20% | −23.15 | 0.152 |
| 5 | CHLa | 3.86% | −18.7258 | −0.0036 |
| 6 | SSS + E | 23.10% | −21.2494 | 0.125 |
| 7 | SSS + CHLa | 5.91% | −17.1975 | −0.0171 |
| 8 | SSS + E + CHLa | 24.90% | −20.2225 | 0.119 |
| J′ in summer | ||||
| 1 | E | 8.48% | −20.9394 | 0.0343 |
| 2 | * N | 16.10% | −25.7326 | 0.136 |
| 3 | SST | 13.10% | −24.4847 | 0.106 |
| 4 | SSS | 11% | −23.611 | 0.0839 |
| 5 | CHLa | 17.60% | −22.2702 | 0.097 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Zhang, R.; Song, P.; Liu, S.; Li, Y.; Li, H. Diversity and Distribution of 18 Cephalopod Species, and Their Link with Some Environmental Factors in the NW Pacific. Diversity 2023, 15, 694. https://doi.org/10.3390/d15050694
Wang R, Zhang R, Song P, Liu S, Li Y, Li H. Diversity and Distribution of 18 Cephalopod Species, and Their Link with Some Environmental Factors in the NW Pacific. Diversity. 2023; 15(5):694. https://doi.org/10.3390/d15050694
Chicago/Turabian StyleWang, Rui, Ran Zhang, Puqing Song, Shigang Liu, Yuan Li, and Hai Li. 2023. "Diversity and Distribution of 18 Cephalopod Species, and Their Link with Some Environmental Factors in the NW Pacific" Diversity 15, no. 5: 694. https://doi.org/10.3390/d15050694
APA StyleWang, R., Zhang, R., Song, P., Liu, S., Li, Y., & Li, H. (2023). Diversity and Distribution of 18 Cephalopod Species, and Their Link with Some Environmental Factors in the NW Pacific. Diversity, 15(5), 694. https://doi.org/10.3390/d15050694




