Abstract
The ice fly Diamesa steinboecki Goetghebuer, 1933 (Diptera: Chironomidae: Diamesinae) is exclusive to glacier-fed streams in the East Palaearctic region and is threatened by extinction due to global warming and glacier retreat. To date, no data are available on its thermal tolerance or ability to develop a heat shock response (HSR) or involve other biomarkers when exposed to higher-than-natural temperatures (i.e., >4–6 °C). Our study aimed to investigate the warmth resistance of IV-instar larvae of D. steinboecki in terms of (1) ability to survive heat shock and (2) gene expression of four genes known to be involved in the detoxification/stress response (cytochrome p450 (Cyp450), heat shock protein 70 (hsp70), hsp70 with intron and heat shock protein cognate 70 (hsc70)). Larvae were exposed to short-term shocks for 1 h at increasing temperatures (26, 28, 30, 32, 34, 36, 38, and 40 °C) to estimate the lethal temperature, obtaining high values (LT10 = 38.1 °C, LT50 = 39.2 °C, LT99 = 40.3 °C), suggesting a strong heat resistance up to 38 °C and a very rapid decline in survival thereafter. Moreover, gene expression analysis by real-time PCR was performed on larvae from the control (at 2 °C) and larvae found alive after the previous treatment at 26, 28, 30, 32, 34, 36, and 38 °C. Modulation of the expression was observed only for hsc70 and hsp70 genes. Specifically, hsc70 resulted in constitutive overexpression, even at 26 °C when all larvae were found alive without evidence of suffering. By contrast, hsp70 showed up and downregulation according to the specific temperature, suggesting the activation of an HSR at 28 °C, when some larvae were found alive but suffering (almost paralyzed). The results suggest that, based on LTs, D. steinboecki is more thermally tolerant than other Diamesa species (e.g., D. tonsa) from cold freshwaters, but, as in these, hsp70 and hsc70 are involved in surviving short-term heat shock. This makes the ice fly from the Alps different from Belgica antarctica and other cold-adapted organisms living in extremely cold habitats that, constantly exposed to cold, have lost the ability to develop an HSR. Further research is needed to investigate the response to prolonged exposure to temperatures higher that the natural one, giving new insights into the biological response to climate change of alpine species threatened by extinction.
1. Introduction
Cold-adapted invertebrate species living in glacier-fed streams (i.e., kryal fauna) are threatened with extinction within the scenario of global warming and glacier retreat, documented for all glacialized areas of the planet [1,2].
In the European Alps, they are represented by chironomids (Diptera: Chironomidae) of the genus Diamesa, colonizing habitats fed by snow and icemelt (streams, springs, and ponds) with several species. They have evolved unique biochemical, physiological, morphological, and phenological traits, making them the sole colonizers of kryal habitats [3,4]. Adaptations within aquatic insects include melanism as a thermoregulation strategy and protection from UV rays, rapid egg development, hairiness to preserve heat, reduction of flight capability as in brachyptery or aptery to reduce energetic costs and avoid being transported away by the wind, parthenogenesis, cocoon building, temporary suspension of development, and cold hardiness [5,6,7]. Some species of Diamesinae (Chironomidae; Diptera) from glacier-fed streams have been shown to survive and maintain normal metabolism at 0–4 °C [8,9].
Such habitats are considered extreme for life, mainly because of low water temperature, with a yearly maximum <4 °C and mean around 0 °C, scarce food, high turbidity, high and highly variable discharge, and high channel and substrate instability.
Among kryal fauna, the non-biting midge Diamesa steinboecki, known as the ice fly, colonizes only glacier-fed streams in the East Palaearctic region and has been recognized as the main bioindicator of climate change in Alpine freshwaters [10]. Recently, Makarchenko et al., published a study focused on Diamesa and specifically on the subgroups steinboecki and longipes, emphasizing the division in geographical areas, presenting D. steinboecki as main colonizer in the European Alps [11]. Long-term ecological studies emphasized that it completes the life cycle at water temperature <4 °C and survives winter as diapausing larvae in streams frozen in the substrate. Its abundance declines where summer maximum water temperature exceeds 6 °C and the air maximum exceeds 12 °C [12].
There is evidence of water warming of glacier-fed streams and other cold habitats at high altitudes. As the atmospheric temperature continues to rise globally [13], the water temperature will adjust to the local air temperature, with incremental warming during the summer [14,15]. As a consequence of the rising water temperature, the composition and functional characteristics of the kryal community have been changing in alpine freshwaters [16,17], with water temperature being the main environmental disruptor influencing invertebrate communities [18,19,20].
To date, no information is available on the thermal tolerance of D. steinboecki, while some evidence of heat shock response (HSR) development was reported for other Diamesinae not exclusive to glacier-fed streams [21,22,23]. Laboratory experiments highlighted that other Diamesa species (e.g., D. tonsa) can survive higher temperature than the optimal one, exhibiting high thermal tolerance (LT50 = 30.9–32.8 °C and LT100 = 34.0–37.8 °C) by developing an HSR [23].
The heat shock proteins (HSPs) play an important role as chaperones for correct protein folding and recovery from stress [24]. Among HSPs, the 70 kDa family is the best known and studied in relation to thermal stress due to its rapid response against unfavourable conditions, making it a relevant biomarker of thermal stress [25,26]. The hsp70 gene is the most sensitive to diverse stressors, such as heat, chemicals, and hypoxia [27,28,29]. This protein family is traditionally divided into two groups based on their gene expression patterns: those with low or null expression under non-stress conditions, but which can be quickly induced under stress conditions (HSP70), and a second group of constitutive proteins expressed under non-stress conditions (HSC70). The heat shock response (HSR) is a reaction that involves the expression of the hsp70 gene in response to high temperature [26].
In this study, we investigated the effect of increasing temperature on survival and the gene expression of four candidate genes (one cytochrome P450 monooxygenases Cyp450 and three heat shock proteins; two genes for hsp70 (hsp70 and hsp70 no intron) and one for the heat shock protein cognate 70 (hsc70)) known to be involved in thermal, chemical, and physical stress response in insects including chironomids (e.g., [4,30,31]). Even genes encoding four antioxidant and detoxification enzymes (i.e., cytochrome P450 monooxygenase) are also elevated in response to dehydration, which often is associated with warm or cold resistance [32]. The upregulation of these genes during dehydration may enhance resistance to subsequent temperature stress, particularly the ability to resist and repair oxidative damage [32].
On the assumption that warming up represents a stress for the larvae, we expected that heat shock proteins and cytochrome P450 monooxygenases would be involved in the molecular response of the larvae to rising temperatures. Our expectations were that: 1. D. steinboecki being the best cold-adapted species among Diamesinae is more sensitive to rising temperature than the other species of Diamesa, i.e., it has lower LTs; 2. The molecular response to heat shock involves Cyp450 proteins as observed in Belgica. antarctica exposed to thermal stress [32]; 3. D. steinboecki does not develop an HSR as a marked adaptation to the low temperatures they constantly face. The HSR has been demonstrated in almost all organisms examined [26], except for some aquatic cold-stenothermal organisms (i.e., the larvae of the non-biting midge B. antarctica and several notothenioid fishes [33,34]. For this reason, we expected that it would not be developed in D. steinboecki.
2. Materials and Methods
2.1. Sampling of Larvae and Acclimatization
Fourth-instar larvae of D. steinboecki were collected in two glacier-fed streams, Presena and Amola (southern Alps, Trentino Province, northeast Italy), within 50 m downstream of the glacier snout (Presena: 2685 m a.s.l.; WGS84 GPS = 46°13.596′ N, 010°34.929′ E; Noce River catchment); Amola: 2540 m a.s.l.; WGS84 GPS = 46°13.025′ N, 010°41.677′ E; Sarca River catchment). Larvae were collected in Presena and Amola during September 2018 and September 2019, respectively. Sampling was conducted according to [35] in 2020 and was summarized in [36]. In summary, 30 × 30 cm pond nets (mesh size 100 µm) (Scubla SNC, Udine, Italy) were employed for the collection. Larvae were sorted in the field with tweezers in plastic vessels, transferred into 100 mL PVC bottles containing stream water, transported in cooling bags from the sampling site to the car and then to the laboratory by car, with a battery-operated freezer to keep the temperature <4 °C (as in natural habitat). Once the species was identified [37] (Rossaro & Lencioni, 2015), larvae were moved to aquaria and maintained in a thermostatic chamber (ISCO, model FTD250-plus; Teledyne Isco Inc., Lincoln, NE, USA) at a controlled temperature of 2 °C under aeration (>80% saturation) for 24 h to acclimatize the organisms before the start of exposures. The incubation temperature (2 °C) was selected to be approximate to the water temperature in these streams; it was 1.4 °C in Presena and 1.7 °C in Amola, measured with a Hydrolab Quanta multiparametric probe (Hydrolab Quanta, Hydrolab Corporation®, Austin, TX, USA).
After acclimatization, larvae were randomly selected from the rearing aquarium and transferred to a 500 mL beaker with 200 mL of soft-medium reconstituted water (RW) prepared according to [38,39]: 4.36 mg/L NaHCO3, 2.73 mg/L CaSO4·2H2O, 2.73 mg/L MgSO4 and 0.19 mg/L KCl (pH = 7.7). In both cases, the larvae were maintained at 2 ± 1 °C with aeration and were not fed during the exposure.
2.2. Survival Following Heat Shock
The 50% and 100% lethal temperature values (LT50 and LT100) for D. steinboecki larvae were calculated by performing short-term shocks (1 h) at 10 different temperatures from 26 °C (upper experimental thermal at with 100% larvae were found alive after 1 h of shock) to 40 °C (experimental lethal temperature) and checking for larvae survival at 26, 28, 30, 32, 34, 36, 38, 39, and 40 °C. Those numbers were used to evaluate their thermotolerance.
All experiments were conducted in a thermostatic chamber. For exposure to different temperatures and times, three groups (replicates) of 3 larvae were transferred into three different 25 mL plastic bottles (Kartell, Italy) filled with 10 mL of preheated filtered stream water (on Whatman GF/C, particle retention 1.2 µm) under aeration to avoid mortality due to oxygen depletion, and without a food supply. Three replicates of three larvae were maintained at 2 °C for each temperature treatment as a control. The mortality in the control was used as a correction factor [40].
After the treatments, the larvae were immediately placed at 2 °C (stabulation temperature) and examined for survival 2 h later. The larvae that moved spontaneously were considered active, whereas those that moved only following a tactile stimulus were considered suffering. Survival was determined by the sum of active and suffering larvae. The immobile larvae were considered dead.
Overall, including controls, 30 replicates (for a total of 90 larvae) were analysed. Finally, three alive individual larvae per shock temperature between 26 to 38 °C were transferred into individual Eppendorf vials and frozen at −80 °C for subsequent RNA extraction.
2.3. RNA Extraction and Complementary DNA (cDNA) Synthesis
Each larva was homogenized with 200 µL of TRIzol and frozen again in dry ice following the manufacturer’s protocol, and then chloroform was added and centrifuged for 15 min at 10,000 rpm at 4 °C. The supernatant was collected to a new tube. For precipitation, 2-propanol alcohol was used, and then the pellet was washed with ethanol and centrifuged. The supernatant was discarded. The pellet was treated with RNAse-free DNAase I (Roche, Basel, Switzerland). Finally, the RNA was resuspended in 25 µL of diethylpyrocarbonate (DEPC) water and stored at −80 °C.
RNA was employed as template for cDNA synthesis by retrotranscription using the First Strand cDNA Synthesis kit (Thermo Fisher, Monza, Italy) according to the manufacturer’s instructions. To summarize, 1 µg of RNA, 1 µL of Primer Oligo (dT)18 primer, and nuclease-free water to a volume of 12µL were placed in a new tube, mixed gently, incubated at 65 °C for 5 min, and placed on ice. After, 4 µL of 5× Reaction Buffer, 1 µL of RiboLock RNase Inhibitor (20 U/µL), 2 µL of 10 mM dNTP Mix and finally 1 µL of RevertAid M-Mul V RT (20 U/µL) were mixed until a final volume of 20 µL was reached. This mix was incubated for 60 min at 42 °C, and then the reaction was stopped by heating at 70 °C for 5 min. The cDNA product was stored at −20 °C.
2.4. cDNA Amplification by Real-Time PCR (RT-PCR)
RT-PCR was used to evaluate the response at the molecular level by mRNA levels. Amplification was performed using 2× qPCRBIO SyGreen Blue Mix Separate-ROX (PCR BIOSYSTEMS, Barcelona, Spain) and Mic Real Time PCR cycling (Bio molecular systems (bms), London, UK). RT-PCR was performed using 1 µL of product from cDNA synthesis, 0.4 µL of each primer (forward and reverse), 5 µL RT-PCR Mastermix, and 3.2 µL of DNA-free water, to a total volume of 10 µL. The PCR conditions were: 95 °C for 2 min, 45 cycles each of 5 s at 95 °C, 20 s at 60 °C, and 20 s at 72 °C, with a final melting curve analysis (from 72 °C to 95 °C, increment of 0.3 °C for each second). The specificity of the amplification for each transcript was verified by gel electrophoresis and melting curve analysis.
The actin gene was employed as the endogenous reference. Each sample was run in two different tubes, and two independent replicates were used from each sample (technical replicates). Primer sequences and their corresponding information are listed in Table S1. The mRNA levels were determined by relative quantification according to 2−ΔΔCt [41].
2.5. Statistical Analysis and Figure Design
The data were analyzed employing the SPSS 25 software (IBM, Armonk, NY, USA). Normality was tested with the Shapiro-Wilk test. The data did not follow a normal distribution and, thus, the non-parametric Kruskal-Wallis test was used, and the Mann-Whitney-Wilcoxon test was employed for post-hoc analysis. In all cases, the significance level was fixed at α = 0.05. Probit analysis was used to estimate the upper lethal temperature at which 50% (LT50) and 100% (LT100) mortality occurred. For figure construction and design, GraphPad Prism 8 was employed.
3. Results
3.1. Survival and Lethal Temperature Determination
An extraordinarily high lethal temperature was estimated (LT10 = 38.1 °C, LT50 = 39.2 °C, LT99 = 40.3 °C), suggesting a strong warm resistance until 38 °C and a very rapid decline in survival for values above (Figure 1). Only at 26 °C were all larvae found alive without evidence of suffering. Only one larva molted into a pupa after 1 h of exposure, suggesting that at this temperature the metabolism increased. Between 28 and 30 °C, all larvae were found alive but some were suffering (almost paralyzed). Dead larvae were found at 34 °C, and above 38 °C survival decreased sharply with values of 10%, 50%, and 100% of mortality between 38 and 40 °C.
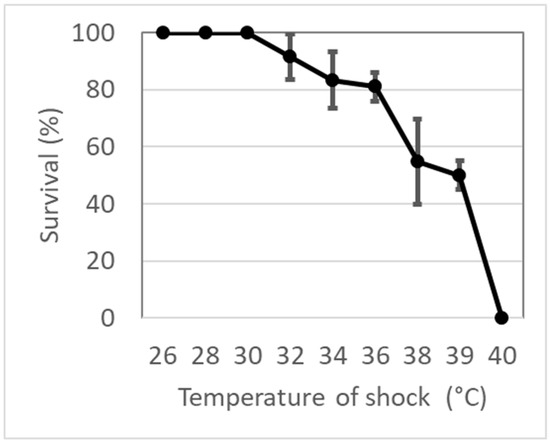
Figure 1.
Survival (%) of Diamesa steinboecki larvae under 1 h heat shock.
3.2. Gene Expression after Thermal Exposure
The results from the four genes evaluated are presented in Figure 2, showing modulation for hsp70 (A) and hsc70 (C). For the hsp70 gene, increased expression was detected at 28, 32 and 34 °C but decreased at 30, 36 and 38 °C. In the case of hsc70, all the temperatures provoked up-regulation except for 38 °C. No mRNA alteration was observed for the hsp70 no intron and cyp450 genes.
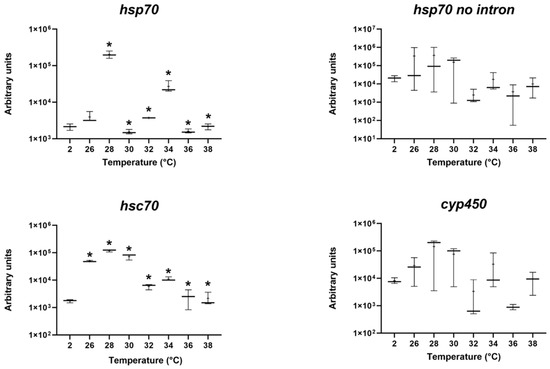
Figure 2.
Expression of hsp70, hsp70 no intron, hsc70, and Cyp450 in fourth instar Diamesa steinboecki larvae after temperature shock and recovery. Messenger RNA (mRNA) levels were normalized using actin as the reference gene. Values were compared with control (2 °C). Whisker boxes are shown. The number of larvae for each box is three. The horizontal line within the box indicates the median. The boundaries of the box indicate the 25th and 75th percentiles, and the whiskers denote the highest and lowest results. The mean is indicated by the small point inside the box. All significant differences are marked with an asterisk (*; p < 0.05).
4. Discussion
Climate change is currently threatening glaciers and the survival of the animals adapted to them. The melting of the glaciers caused by global warming is reducing the glacial streams from the Alpine environment, which is the habitat of the ice fly Diamesa steinboecki. For this reason, high sensitivity to temperature variation was expected. Along these lines, recent work has shown how the number of D. steinboecki specimens has decreased in these environments due to global warming, notable in summer, when the mean maximum water temperature has reached >6 °C throughout the years [11]. However, the results obtained in this work suggest that this species can survive short-term exposure to temperatures much higher than we expected. The analysis of survival at increased temperatures ranging from 26 to 40 °C showed that the LT50 is 39.2 °C, far from the average temperature of the freshwater streams where it lives, which is usually 2–4 °C. In addition, during heat shock, larvae can be affected, showing stress symptoms, especially starting at 28 °C. However, all the larvae showing suffering recovered after one hour at 4 °C, indicating that the larvae managed such as strong heat shock, at least in the short-term. On the other hand, the fact that the LT99 is 40.3 °C, only one degree from the LT50, indicates that the species is relatively homogenous in its ability to respond to heat shock, reaching the limit in a high percentage before dying. A similar study with D. cinerella, another cold stenothermal midge, found that the LT50 was 30.9–32.8 °C and the LT100 was 34–37.8 °C. However, for Pseudodiamesa branickii, the LT50 and LT100 were 32.2 and 36 °C, respectively [23]. The range tested was even lower (up to 35 and 38 °C for each species), showing the specific range for each species, although they share a similar environment. In addition, a comparison of the three species shows that the ability to manage climate change could be different since the range between LT50 and LT99/100 is narrower for D. steinboecki than for the other two. This difference could be relevant since climate change is expected to increase the frequency of extreme events such as heat waves. Therefore, this difference could allow more individuals in the population to survive extreme temperatures, providing a stronger base for the later recovery of the population after a heat wave. Along these lines, another study with larvae of Diamesa mendotae Muttkowski (Diptera: Chironomidae) determined an (LT)99 of –25.4 °C showing higher freeze tolerance in these larvae compared with adults of the same species [42]. The question that arises is: how can species that are adapted to the cold resist such high temperatures? For D. cinerella, it was shown that high levels of HSP70 due to the response to cold could also work to protect against high temperatures, while P. branickii showed an increase in hsp70 but not in hsc70 after one hour, but both were upregulated at 26 °C when longer exposure times were used [21].
The molecular basis for the thermal tolerance of D. steinboecki was investigated by analyzing the transcriptional activity of four genes related to heat shock. As stated in the results, two of them showed alterations. HSP70 is the traditional protein associated with heat shock, which responds quickly to diverse stressors, such as chemicals and UV radiation [43,44,45]. For this reason, modulation of its mRNA levels is expected. However, the heterogeneous pattern in response to different temperatures was not expected, mainly because there is up-regulation in some of them and a decrease in mRNA levels in others. The triggering temperature of the hsp70 response is 28 °C, since the increase is not detected below this temperature. However, it is relevant to remember that the response to stressors is usually gradual and not an all-or-nothing response. So, the trigging of up-regulation of hsp70 could require longer heat stress time at lower temperatures to reach the threshold of activation. On the other hand, the response of heat shock proteins frequently involves an initial increase and subsequent decrease, even below the normal mRNA levels, and then recovery to the average levels [46,47]. Therefore, it could explain the results for 28–30 °C, with 28 °C mRNA levels reaching their peak and 30 °C showing the post-response effect to recover normal levels. However, it does not fit the later temperatures unless a bimodal response occurs. A previous report on bovine aortic endothelial cells described a bimodal response for HSP70 in heat shock and recovery [48]. However, additional research is necessary, and observations at later times are required to define the kinetics of hsp70 activity in response to the different temperatures.
The results obtained for hsc70 are more consistent with that expected with a heat shock response. In this case, up-regulation at 26 °C is observed, suggesting that even lower temperatures could induce the activity of hsc70. For this gene, the maximum level is observed for 28 °C, and higher temperatures also show high activity levels but in a decreasing trend. It suggests that they are recovering their usual levels. It is coherent with the assumption that higher temperatures induce an earlier response favoring an earlier recovery, because the response is stronger and more effective. Heat shock damages the proteins producing their misfolding, which can induce an aggregation that is toxic for the cell. Consequently, the cells respond by elevating the synthesis of molecular chaperones and proteases [49] to restore proteostasis and prevent further damage. Therefore, higher temperatures can induce a more substantial effect by affecting more proteins and initiating the response earlier. Here, the differences observed for hsc70 would reflect that higher temperatures were recovering the normal levels of this protein. It is important to note that the levels were measured after one hour of recovery, so the stimulus ceased. The recovery observed at higher temperatures was probably faster because they were recovering the levels at the end of the hour of exposure reaching very high values, especially considering that these organisms have a temperature range of about 2–4 °C in their natural habitats. So, these organisms seem to have an extraordinary degree of tolerance to temperature.
The hsp70 no intron mRNA analysis showed that no significant changes could be detected. The variability of the results was higher than that for hsp70 and hsc70, meaning a more diverse expression in this organism. The absence of response cannot discard its implication in thermal tolerance since the heat shock response implies a diversity of gene expression patterns that are changing over time [46,47,50].
Concerning cyp450, it is a gene usually associated with chemical stress more than thermal stress. However, it has been described that some members of the cyp450 family, LtCYP4g1, LtCYP4g15, and LtCYP301A1, can be induced in the fly Liriomyza trifolii [51]. However, no statistically significant changes were observed in D. steinboecki with the cyp450 tested, although a difference in the data distribution could be observed. It can be because it is not involved in the response or because although it is involved, the stimulus was insufficient, or the modulation is produced later. In any case, it would not be unexpected since the heat shock response involves different elements that participate sequentially, as happens, for example, with the small heat shock proteins [52]. The involvement of many players in the response frequently means a complex response pattern depending on the species’ environment and other life traits.
5. Conclusions
The resistance of D. steinboecki to high temperatures is striking since it is a species that lives in glacial streams. The LT50 and LT99 show high resistance but a narrow margin before the population collapses. However, the upper limit is higher than that observed in other cold-stream species of chironomids. It suggests that in heat waves or similar catastrophic events, D. steinboecki could gain advantage through the survival of more individuals at higher temperatures, providing a base for the latter recovery of the population. In addition, it would provide a better perspective to respond to the extreme events associated with climate change.
The results obtained suggest that the short-term thermal tolerance of D. steinboecki to high temperatures could be due to the heat shock response. There is a modulation of hsp70 and hsc70, central genes in the heat shock response, with a suggested bimodal response for hsp70 that requires additional research. The other two genes analyzed did not show any alteration in the conditions tested, but that does not discard their implication because later modulation could be possible. Further research is needed to evaluate the resistance to prolonged exposure times to temperatures higher that the natural one by mimicking natural values, giving new insights into the biological response to climate change of alpine species threatened by extinction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15060708/s1, Table S1: Sequences of the primers and reference used in this study and their efficiencies obtained for D. steinboecki.
Author Contributions
Conceptualization, V.L.; methodology, V.L. and A.-B.M.-G.; formal analysis, A.-B.M.-G.; investigation, V.L. and A.-B.M.-G.; resources, V.L.; data curation, V.L. and A.-B.M.-G.; writing—original draft preparation, V.L., A.-B.M.-G. and J.-L.M.-G.; writing—review and editing, V.L., A.-B.M.-G. and J.-L.M.-G.; supervision, V.L. and J.-L.M.-G.; project administration, V.L.; funding acquisition, V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the MUSE—Science Museum of Trento and the Italian Foundation “Cassa di Risparmio di Trento e Rovereto” (CARITRO) within the RACE-TN project “Valutazione del rischio ambientale dei contaminanti emergenti nei fiumi trentini: effetti sulla vita selvatica e sull’uomo/Environmental Risk assessment of emerging contaminants in Trentino rivers: effects on wildlife and human health”, Grant n. 2015.0199; 2015–2018).
Data Availability Statement
All the results obtained are presented in Section 3 by graphical representation. Moreover, for more information or more details, the corresponding author can be contacted to obtain such information.
Acknowledgments
We are grateful to Francesca Paoli (MUSE) for her help with the field and laboratory work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Giersch, J.J.; Jordan, S.; Luikart, G.; Jones, L.A.; Hauer, F.R.; Muhlfeld, C.C. Climate-induced range contraction of a rare alpine aquatic invertebrate. Freshw. Sci. 2015, 34, 53–65. [Google Scholar] [CrossRef]
- Jacobsen, D.; Dangles, O. Environmental harshness and global richness patterns in glacier-fed streams. Glob. Ecol. Biogeogr. 2012, 21, 647–656. [Google Scholar] [CrossRef]
- Birrell, J.H.; Shah, A.A.; Hotaling, S.; Giersch, J.J.; Williamson, C.E.; Jacobsen, D.; Woods, H.A. Insects in high-elevation streams: Life in extreme environments imperiled by climate change. Glob. Change Biol. 2020, 26, 6667–6684. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, V.; Jousson, O.; Guella, G.; Bernabò, P. Cold adaptive potential of chironomids overwintering in a glacial stream. Physiol. Entomol. 2015, 40, 43–53. [Google Scholar] [CrossRef]
- Danks, H.V. How aquatic insects live in cold climates. BioOne 2007, 139, 443–471. [Google Scholar] [CrossRef]
- Lencioni, V. Survival strategies of freshwater insects in cold environments. J. Limnol. 2004, 63, 45–55. [Google Scholar] [CrossRef]
- Schütz, S.A.; Füreder, L. Unexpected patterns of chironomid larval size in an extreme environment: A highly glaciated, alpine stream. Hydrobiologia 2018, 820, 49–63. [Google Scholar] [CrossRef]
- Lencioni, V.; Bernabò, P.; Vanin, S.; Di Muro, P.; Beltramini, M. Respiration rate and oxy-regulatory capacity in cold stenothermal chironomids. J. Insect Physiol. 2008, 54, 1337–1342. [Google Scholar] [CrossRef]
- Lencioni, V.; Bernabò, P. Thermal survival limits of young and mature larvae of a cold stenothermal chironomid from the Alps (Diamesinae: Pseudodiamesa branickii [Nowicki, 1873]). Insect Sci. 2017, 24, 314–324. [Google Scholar] [CrossRef]
- Lencioni, V. Glacial influence and stream macroinvertebrate biodiversity under climate change: Lessons from the Southern Alps. Sci. Total. Environ. 2018, 622–623, 563–575. [Google Scholar] [CrossRef]
- Makarchenko, E.A.; Semenchenko, A.A.; Palatov, D.M. Taxonomy of Diamesa steinboecki group (Diptera: Chironomidae: Diamesinae), with description and DNA barcoding of new species. I. Subgroups steinboecki and longipes. Zootaxa 2022, 5125, 483–512. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, V.; Stella, E.; Grazia, M.; Bellin, A. On the delay between water temperature and invertebrate community response to warming climate. Sci. Total. Environ. 2022, 837, 155759. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability ; Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; p. 3056. [Google Scholar]
- Niedrist, G.H.; Füreder, L. Real-time warming of Alpine streams: (Re)defining invertebrates temperature preferences. River Res. 2021, 37, 283–293. [Google Scholar] [CrossRef]
- Webb, B.W.; Hannah, D.M.; Moore, R.D.; Brown, L.E.; Nobilis, F. Recent advances in stream and river temperature research. Hydrol. Process. 2008, 22, 902–918. [Google Scholar] [CrossRef]
- Brown, L.E.; Khamis, K.; Wilkes, M.; Blaen, P.; Brittain, J.E.; Carrivick, J.L.; Fell, S.; Friberg, N.; Füreder, L.; Gislason, G.M. Functional diversity and community assembly of river invertebrates show globally consistent responses to decreasing glacier cover. Nat. Ecol. Evol. 2018, 2, 325–333. [Google Scholar] [CrossRef]
- Milner, A.M.; Brittain, J.E.; Castella, E.; Petts, G.E. Trends of macroinvertebrate community structure in glacial fed streams in relation to environmental conditions: A synthesis. Freshw. Biol. 2001, 46, 1833–1848. [Google Scholar] [CrossRef]
- Caissie, D. The thermal regime of rivers: A review. Freshw. Biol. 2006, 51, 1389–1406. [Google Scholar] [CrossRef]
- Dickson, N.E.; Carrivick, J.L.; Brown, L.E. Flow regulation alters alpine river thermal regimes. J. Hydrol. 2012, 464–465, 505–516. [Google Scholar] [CrossRef]
- Wilkes, M.A.; Carrivick, J.L.; Castella, E.; Ilg, C.; Cauvy-Fraunié, S.; Fell, S.; Füreder, L.; Huss, M.; James, W.; Lencioni, V.; et al. Glacier retreat reorganises river habitats leaving refugia for Alpine invertebrate biodiversity poorly protected. Nat. Ecol. Evol. 2023. [Google Scholar] [CrossRef]
- Bernabò, P.; Rebecchi, L.; Jousson, O.; Martinez-Guitarte, J.L.; Lencioni, V. Thermotolerance and hsp70 heat shock response in the cold-stenothermal chironomid Pseudodiamesa branickii (NE Italy). Cell Stress Chaperones 2011, 16, 403–410. [Google Scholar] [CrossRef]
- Bernabò, P.; Viero, G.; Lencioni, V. A long noncoding RNA acts as a post-transcriptional regulator of heat shock protein (HSP70) synthesis in the cold hardy Diamesa tonsa under heat shock. PLoS ONE 2020, 15, e0227172. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, V.; Bernabo, P.; Cesari, M.; Rebecchi, L. Thermal stress induces hsp70 proteins synthesis in larvae of the cold stream non-biting midge Diamesa cinerella Meigen. Arch. Insect Biochem. Physiol. 2013, 83, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Morrow, G.; Tanguay, R.M. Small heat shock protein expression and functions during development. Int. J. Biochem. Cell Biol. 2012, 44, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Morimoto, R.I.; Kline, M.P.; Bimston, D.N.; Cotto, J.J. The heat-shock response: Regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997, 32, 17–29. [Google Scholar]
- Muñiz-González, A.B.; Novo, M.; Martínez-Guitarte, J.L. Persistent pesticides: Effects of endosulfan at the molecular level on the aquatic invertebrate Chironomus riparius. Environ. Sci. Pollut. Res. 2021, 28, 31431–31446. [Google Scholar] [CrossRef]
- Nie, H.; Liu, L.; Wang, H.; Huo, Z.; Yan, X. Stress levels over time in Ruditapes philippinarum: The effects of hypoxia and cold stress on Hsp70 gene expression. Aquacult. Rep. 2018, 12, 1–4. [Google Scholar] [CrossRef]
- Xu, X.H.; Meng, X.; Gan, H.T.; Liu, T.H.; Yao, H.Y.; Zhu, X.Y.; Xu, J.T. Immune response, MT and HSP70 gene expression, and bioaccu-mulation induced by lead exposure of the marine crab, Charybdis japonica. Aquat. Toxicol. 2019, 210, 98–105. [Google Scholar] [CrossRef]
- Bernabò, P.; Gaglio, M.; Bellamoli, F.; Viero, G.; Lencioni, V. DNA damage and translational response during detoxification from copper exposure in a wild population of Chironomus riparius. Chemosphere 2017, 173, 235–244. [Google Scholar] [CrossRef]
- Lencioni, V.; Boschini, D.; Rebecchi, L. Expression of the 70 kDa Heat shock protein family in Alpine freshwater chironomids (Diptera, Chironomidae) under natural conditions. J. Limnol. 2009, 68, 251–256. [Google Scholar] [CrossRef]
- Benoit, J.B.; Lopez-Martinez, G.; Elnitsky, M.A.; Lee, R.E., Jr.; Denlinger, D.L. Dehydration-induced cross tolerance of Belgica antarctica larvae to cold and heat is facilitated by trehalose accumulation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 152, 518–523. [Google Scholar] [CrossRef]
- Clark, M.; Fraser, K.; Burns, G.; Peck, L. The HSP70 heat shock response in the Antarctic fish Harpagifer antarcticus. Polar Biol. 2008, 31, 171–180. [Google Scholar] [CrossRef]
- Rinehart, J.P.; Hayward, S.A.; Elnitsky, M.A.; Sandro, L.H.; Lee, R.E.; Denlinger, D.L. Continuous up-regulation of heat shock proteins in larvae, but not adults, of a polar insect. Proc. Natl. Acad. Sci. USA 2006, 103, 14223–14227. [Google Scholar] [CrossRef]
- Di Nica, V.; González, A.B.M.; Lencioni, V.; Villa, S. Behavioural and biochemical alterations by chlorpyrifos in aquatic insects: An emerging environmental concern for pristine Alpine habitats. Environ. Sci. Pollut. Res. 2020, 27, 30918–30926. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-González, A.B.; Paoli, F.; Martínez-Guitarte, J.L.; Lencioni, V. Molecular biomarkers as tool for early warning by chlorpyrifos exposure on Alpine chironomids. Environ. Pollut. 2021, 290, 118061. [Google Scholar] [CrossRef] [PubMed]
- Rossaro, B.; Lencioni, V. A key to larvae of species belonging to the genus Diamesa from Alps and Apennines (Italy). Eur. J. Environ. Sci. 2015, 5, 62–79. [Google Scholar] [CrossRef]
- Holdway, D. Hydra Population Reproduction Toxicity Test Method. In Small-Scale Freshwater Toxicity Investigations, 1st ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 395–411. [Google Scholar]
- Lencioni, V.; Bellamoli, F.; Bernabó, P.; Miari, F.; Scotti, A. Response of Diamesa spp. (Diptera: Chironomidae) from alpine streams to newly emergent contaminants and pesticides. J. Limnol. 2018, 77, 131–140. [Google Scholar]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 16–21. [Google Scholar] [CrossRef]
- Bouchard, R.W.; Carrillo, M.A.; Keils, S.A.; Ferrington, L.C. Freeze tolerance in larvae of the winter-active Diamesa mendotae Muttkowski (Diptera: Chironomidae): A contrast to adult strategy for survival at low temperatures. Hydrobiologia 2006, 568, 403–416. [Google Scholar] [CrossRef]
- Kim, B.M.; Rhee, J.S.; Lee, K.W.; Kim, M.J.; Shin, K.H.; Lee, S.J.; Lee, Y.M.; Lee, J.S. UV-B radiation-induced oxidative stress and p38 signaling pathway involvement in the benthic copepod Tigriopus japonicus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 167, 15–23. [Google Scholar] [CrossRef]
- Muñiz-González, A.B. Ibuprofen as an emerging pollutant on non-target aquatic invertebrates: Effects on Chironomus riparius. Environ. Toxicol. Pharmacol. 2021, 81, 103537. [Google Scholar] [CrossRef] [PubMed]
- Tartarotti, B.; Torres, J.J. Sublethal stress: Impact of solar UV radiation on protein synthesis in the copepod Acartia tonsa. J. Exp. Mar. Biol. Ecol. 2009, 375, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, R.D. Stress Protein Expression Kinetics. Annu. Rev. Biomed. Eng. 2006, 8, 403–424. [Google Scholar]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell. 2010, 40, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Diller, K.R.; Aggarwal, S.J. Kinetics study of endogenous heat shock protein 70 expression. J. Biomech. Eng. 2003, 125, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Richard, I.M.; Sandy, D. Westerheide, Chapter 268—The Heat Shock Response and the Stress of Misfolded Proteins. In Handbook of Cell Signaling, 2nd ed.; Ralph, A.B., Edward, A.D., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 2231–2239. ISBN 9780123741455. [Google Scholar]
- Kim, J.; Joo, H.J.; Kim, Y.H.; Ahn, S.; Chang, J.; Hwang, B.; Lee, H.; Lee, J. Systemic Analysis of Heat Shock Response Induced by Heat Shock and a Proteasome Inhibitor MG132. PLoS ONE 2011, 6, e20252. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chang, Y.W.; Bai, J. High temperature stress induces expression of CYP450 genes and contributes to insecticide tolerance in Liriomyza trifolii. Pestic. Biochem. Physiol. 2021, 174, 104826. [Google Scholar] [CrossRef]
- Ruan, H.Y.; Meng, J.Y.; Yang, C.L.; Zhou, L.; Zhang, C.Y. Identification of Six Small Heat Shock Protein Genes in Ostrinia furnacalis (Lepidoptera: Pyralidae) and Analysis of Their Expression Patterns in Response to Environmental Stressors. J. Insect Sci. 2022, 22, 7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).