Comparing Physical Collection and Environmental DNA Methods for Determining Abundance Patterns of Gammarus Species along an Estuarine Gradient
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Location and Sampling Design
2.2. Physical Capture Equipment and Processing
2.3. Sediment Collection and Analysis
2.4. Species-Specific Assay Design
2.5. qPCR
2.6. Statistical Analysis
3. Results
3.1. Habitat Conditions
3.2. Physical Sampling Summary and Occupancy
3.3. Absolute Abundance Gradients and Comparison of Physical Methods to eDNA
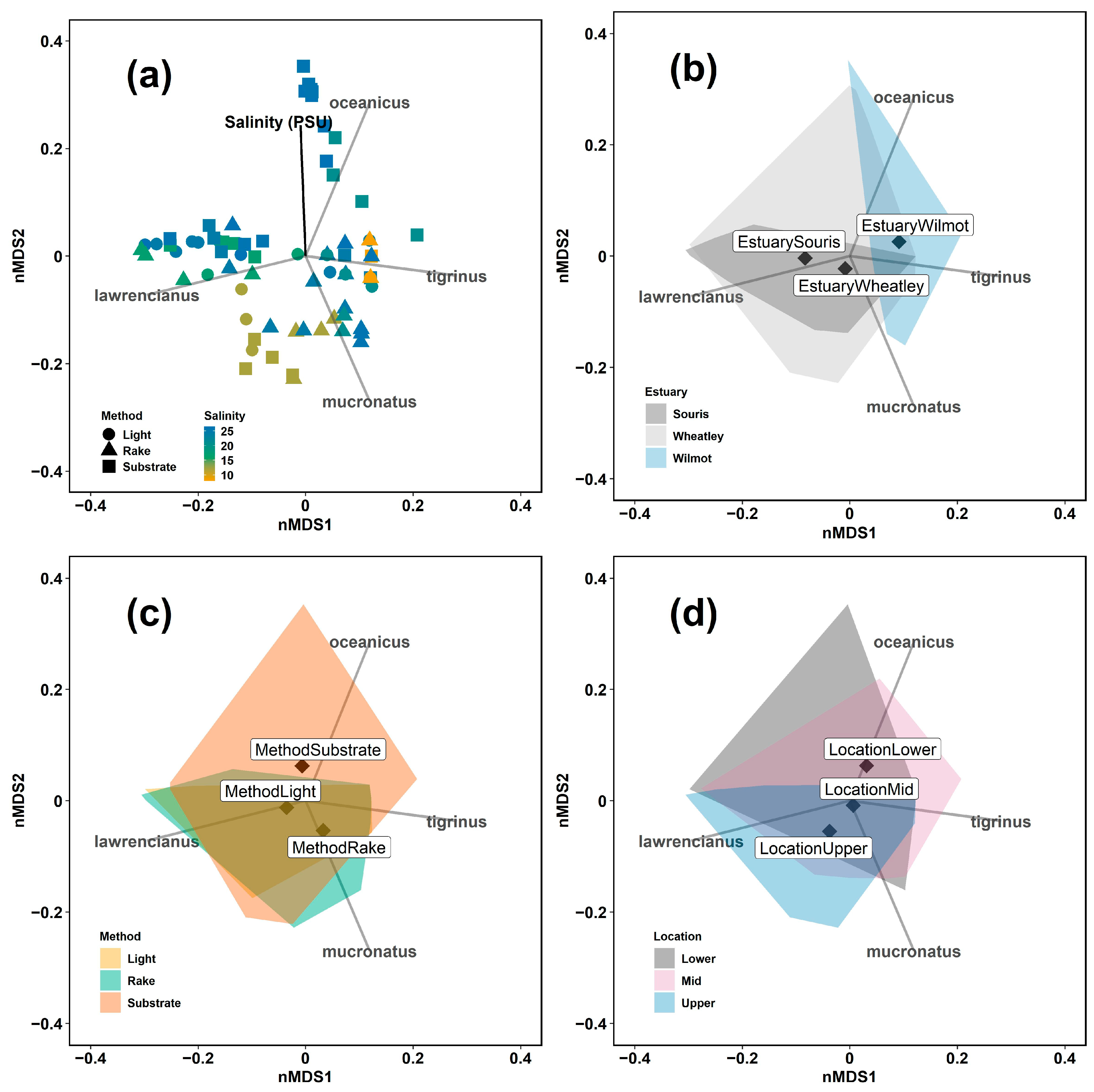
3.4. Multivariate Examination of Gammarus Assemblage between Zones and Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coffin, M.R.S.; Courtenay, S.C.; Knysh, K.M.; Pater, C.C.; van den Heuvel, M.R. Impacts of Hypoxia on Estuarine Macroinvertebrate Assemblages across a Regional Nutrient Gradient. Facets 2018, 3, 23–44. [Google Scholar] [CrossRef]
- Kraufvelin, P.; Salovius, S.; Christie, H.; Moy, F.E.; Karez, R.; Pedersen, M.F. Eutrophication-Induced Changes in Benthic Algae Affect the Behaviour and Fitness of the Marine Amphipod. Gammarus Locusta. Aquat. Bot. 2006, 84, 199–209. [Google Scholar] [CrossRef]
- Elliott, M.; Quintino, V. The Estuarine Quality Paradox, Environmental Homeostasis and the Difficulty of Detecting Anthropogenic Stress in Naturally Stressed Areas. Mar. Pollut. Bull. 2007, 54, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, A.K.; Elliott, M.; Basset, A.; Blaber, S.J.M.; West, R.J. Paradigms in Estuarine Ecology—A Review of the Remane Diagram with a Suggested Revised Model for Estuaries. Estuar. Coast. Shelf Sci. 2012, 97, 78–90. [Google Scholar] [CrossRef]
- Quintino, V.; Sangiorgio, F.; Mamede, R.; Ricardo, F.; Sampaio, L.; Martins, R.; Freitas, R.; Rodrigues, A.M.; Basset, A. The Leaf-Bag and the Sediment Sample: Two Sides of the Same Ecological Quality Story? Estuar. Coast. Shelf Sci. 2011, 95, 326–337. [Google Scholar] [CrossRef]
- van den Heuvel, M.R.; Hitchcock, J.K.; Coffin, M.R.S.; Pater, C.C.; Courtenay, S.C. Inorganic Nitrogen Has a Dominant Impact on Estuarine Eelgrass Distribution in the Southern Gulf of St. Lawrence, Canada. Limnol. Oceanogr. 2019, 64, 2313–2327. [Google Scholar] [CrossRef]
- Schein, A.; Courtenay, S.C.; Kidd, K.A.; Campbell, K.A.; van den Heuvel, M.R. Food Web Structure within an Estuary of the Southern Gulf of St. Lawrence Undergoing Eutrophication. Can. J. Fish. Aquat. Sci. 2013, 70, 1805–1812. [Google Scholar] [CrossRef]
- Herkül, K.; Lauringson, V.; Kotta, J. Specialization among Amphipods: The Invasive Gammarus tigrinus Has Narrower Niche Space Compared to Native Gammarids. Ecosphere 2016, 7, e01306. [Google Scholar] [CrossRef]
- MacNeil, C.; Dick, J.T.A. Intraguild Predation May Reinforce a Species-Environment Gradient. Acta Oecologica 2012, 41, 90–94. [Google Scholar] [CrossRef]
- Paiva, F.; Barco, A.; Chen, Y.; Mirzajani, A.; Chan, F.T.; Lauringson, V.; Baltazar-Soares, M.; Zhan, A.; Bailey, S.A.; Javidpour, J.; et al. Is Salinity an Obstacle for Biological Invasions? Glob. Chang. Biol. 2018, 24, 2708–2720. [Google Scholar] [CrossRef]
- Hunte, W.; Myers, R.A. Phototaxis and Cannibalism in Gammaridean Amphipods. Mar. Biol. 1984, 81, 75–79. [Google Scholar] [CrossRef]
- Wildish, D.J.; Radulovici, A.E. Amphipods in Estuaries: The Sibling Species Low Salinity Switch Hypothesis. Zoosyst. Evol. 2020, 96, 797–805. [Google Scholar] [CrossRef]
- Coffin, M.; Knysh, K.; Roloson, S.; Pater, C.; Theriaul, E.; Cormier, J.; Courtenay, S.; van den Heuvel, M. Influence of Nutrient Enrichment on Temporal and Spatial Dynamics of Dissolved Oxygen within Northern Temperate Estuaries. Environ. Monit. Assess. 2021, 193, 804. [Google Scholar] [CrossRef] [PubMed]
- Pascal, L.; Bernatchez, P.; Chaillou, G.; Nozais, C.; Lapointe Saint-Pierre, M.; Archambault, P. Sea Ice Increases Benthic Community Heterogeneity in a Seagrass Landscape. Estuar. Coast. Shelf Sci. 2020, 243, 106898. [Google Scholar] [CrossRef]
- Stearns, D.E.; Dardeau, M.R. Nocturnal and Tidal Vertical Migrations of “benthic” Crustaceans in an Estuarine System with Diurnal Tides. Northeast. Gulf Sci. 1990, 11, 93–104. [Google Scholar] [CrossRef]
- Coffin, M.R.S.; Knysh, K.M.; Theriault, E.F.; Pater, C.C.; Courtenay, S.C.; van den Heuvel, M.R. Are Floating Algal Mats a Refuge from Hypoxia for Estuarine Invertebrates? PeerJ 2017, 5, e3080. [Google Scholar] [CrossRef]
- Tummon Flynn, P.; Lynn, K.; Cairns, D.; Quijón, P. Mesograzer Interactions with a Unique Strain of Irish Moss Chondrus crispus: Colonization, Feeding, and Algal Condition-Related Effects. Mar. Ecol. Prog. Ser. 2021, 669, 83–96. [Google Scholar] [CrossRef]
- McLeod, L.E.; Costello, M.J. Light Traps for Sampling Marine Biodiversity. Helgol. Mar. Res. 2017, 71, 2. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Irazabal, A.; Moreira, J. Demersal Amphipod Migrations: Spatial Patterns in Marine Shallow Waters. J. Mar. Biol. Assoc. U. K. 2020, 100, 239–249. [Google Scholar] [CrossRef]
- Hungerford, H.B.; Spangler, P.J.; Walker, N.A. Subaquatic Light Traps for Insects and Other Animal Organisms. Trans. Kansas Acad. Sci. 1955, 58, 387–407. [Google Scholar] [CrossRef]
- Czarnecka, M.; Grubisic, M.; Kakareko, T. Disruptive Effect of Artificial Light Light at Night on Leaf Litter Consumption, Growth and Activity of Freshwater Shredders. Sci. Total Environ. 2021, 786, 147407. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Nakajima, F.; Tobino, T. Variation of Environmental DNA in Sediment at Different Temporal Scales in Nearshore Area of Tokyo Bay. J. Water Environ. Technol. 2019, 17, 153–162. [Google Scholar] [CrossRef]
- Rice, C.J.; Larson, E.R.; Taylor, C.A. Environmental DNA Detects a Rare Large River Crayfish but with Little Relation to Local Abundance. Freshw. Biol. 2018, 63, 443–455. [Google Scholar] [CrossRef]
- Wei, N.; Nakajima, F.; Tobino, T. Effects of Treated Sample Weight and DNA Marker Length on Sediment eDNA Based Detection of a Benthic Invertebrate. Ecol. Indic. 2018, 93, 267–273. [Google Scholar] [CrossRef]
- Crane, L.C.; Goldstein, J.S.; Thomas, D.W.; Rexroth, K.S.; Watts, A.W. Effects of Life Stage on eDNA Detection of the Invasive European Green Crab (Carcinus maenas) in Estuarine Systems. Ecol. Indic. 2021, 124, 107412. [Google Scholar] [CrossRef]
- Trimbos, K.B.; Cieraad, E.; Schrama, M.; Saarloos, A.I.; Musters, K.J.M.; Bertola, L.D.; Bodegom, P.M. Stirring up the Relationship between Quantified Environmental DNA Concentrations and Exoskeleton-shedding Invertebrate Densities. Environ. DNA 2020, 3, 605–618. [Google Scholar] [CrossRef]
- Andruszkiewicz Allan, E.; Zhang, W.G.; Lavery, A.; Govindarajan, A. Environmental DNA Shedding and Decay Rates from Diverse Animal Forms and Thermal Regimes. Environ. DNA 2021, 3, 492–514. [Google Scholar] [CrossRef]
- Wei, N.; Nakajima, F.; Tobino, T. A Microcosm Study of Surface Sediment Environmental DNA: Decay Observation, Abundance Estimation, and Fragment Length Comparison. Environ. Sci. Technol. 2018, 52, 12428–12435. [Google Scholar] [CrossRef]
- Bousfield, E.L. Shallow-Water Gammaridean Amphipoda of New England; Comstock Publishing Associates Cornell University Press: Ithaca, NY, USA, 1973; p. 312. [Google Scholar]
- Steele, D.H.; Steele, V.J. The Biology of Gammarus (Crustacea, Amphipoda) in the Northwestern Atlantic. XI. Comparison and Discussion. Can. J. Zool. 1975, 53, 1116–1126. [Google Scholar] [CrossRef]
- Bousfield, E.L.; Thomas, M.L.H. Postglacial Changes in Distribution of Littoral Marine Invertebrates in the Canadian Atlanic Region. Proc. Nov. Scotian Inst. Scicene 1975, 27 (Suppl. S3), 47–60. [Google Scholar]
- Slaymaker, O.; Catto, N.; Kovanen, D.J. Eastern Canadian Landscapes as a Function of Structure, Relief and Process. In Landscapes and Landforms of Eastern Canada; World Geomorphological Landscapes; Slaymaker, O., Catto, N., Eds.; Springer: Cham, Switzerland, 2020; pp. 3–48. [Google Scholar] [CrossRef]
- Dalton, A.S.; Margold, M.; Stokes, C.R.; Tarasov, L.; Dyke, A.S.; Adams, R.S.; Allard, S.; Arends, H.E.; Atkinson, N.; Attig, J.W.; et al. An Updated Radiocarbon-Based Ice Margin Chronology for the Last Deglaciation of the North American Ice Sheet Complex. Quat. Sci. Rev. 2020, 234, 106223. [Google Scholar] [CrossRef]
- Catto, N. Atlantic Canada’s Tidal Coastlines: Geomorphology and Multiple Resources. In Landscapes and Landforms of Eastern Canada; World Geomorphological Landscapes; Slaymaker, O., Catto, N., Eds.; Springer: Cham, Switzerland, 2020; pp. 401–430. [Google Scholar] [CrossRef]
- Lavaud, R.; Filgueira, R.; Nadeau, A.; Steeves, L.; Guyondet, T. A Dynamic Energy Budget Model for the Macroalga Ulva lactuca. Ecol. Modell. 2020, 418, 108922. [Google Scholar] [CrossRef]
- Cheney, D.; Logan, J.M.; Gardner, K.; Sly, E.; Wysor, B.; Greenwood, S. Bioaccumulation of PCBs by a Seaweed Bloom (Ulva rigida) and Transfer to Higher Trophic Levels in an Estuarine Food Web. Mar. Ecol. Prog. Ser. 2019, 611, 75–93. [Google Scholar] [CrossRef]
- Douglass, J.G.; France, K.E.; Richardson, J.P.; Duffy, J.E. Seasonal and Interannual Change in a Chesapeake Bay Eelgrass Community: Insights into Biotic and Abiotic Control of Community Structure. Limnol. Oceanogr. 2010, 55, 1499–1520. [Google Scholar] [CrossRef]
- Vassallo, L.; Steele, D.H. Survival and Growth of Young Gammarus lawrencianus Bousfield, 1956, on Different Diets. Crustac. Suppl. 1980, 6, 118–125. [Google Scholar]
- Węsławski, J.M.; Dragańska-Deja, K.; Legeżyńska, J.; Walczowski, W. Range Extension of a Boreal Amphipod Gammarus oceanicus in the Warming Arctic. Ecol. Evol. 2018, 8, 7624–7632. [Google Scholar] [CrossRef]
- Gaston, K.J.; Spicer, J.I. The Relationship between Range Size and Niche Breadth: A Test Using Five Species of Gammarus (Amphipoda). Glob. Ecol. Biogeogr. 2001, 10, 179–188. [Google Scholar] [CrossRef]
- Steele, D.H.; Steele, V.J. Effects of Salinity on the Survival, Growth Rate, and Reproductive Output of Gammarus lawrencianus (Crustacea, Amphipoda). Mar. Ecol. Prog. Ser. 1991, 78, 49–56. [Google Scholar] [CrossRef]
- Chaplin, G.I.; Valentine, J.F. Macroinvertebrate Production in the Submerged Aquatic Vegetation of the Mobile-Tensaw Delta: Effects of an Exotic Species at the Base of an Estuarine Food Web. Estuaries Coasts 2009, 32, 319–332. [Google Scholar] [CrossRef]
- Kelly, D.W.; MacIsaac, H.J.; Heath, D.D. Vicariance and Dispersal Effects on Phylogeographic Structure and Speciation in a Widespread Estuarine Invertebrate. Evolution 2006, 60, 257–267. [Google Scholar] [CrossRef]
- Hester, F.E.; Dendy, J.S. A Multiple-Plate Sampler for Aquatic Macroinvertebrates. Trans. Am. Fish. Soc. 1962, 91, 420–421. [Google Scholar] [CrossRef]
- Perkin, E.K.; Hölker, F.; Heller, S.; Berghahn, R. Artificial Light and Nocturnal Activity in Gammarids. PeerJ 2014, 2, e297. [Google Scholar] [CrossRef]
- Porter, M.L.; Cronin, T.W.; McClellan, D.A.; Crandall, K.A. Molecular Characterization of Crustacean Visual Pigments and the Evolution of Pancrustacean Opsins. Mol. Biol. Evol. 2007, 24, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on Ignition as a Method for Estimating Organic and Carbonate Content in Sediments: Reproducibility and Comparability of Results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- deWaard, J.R.; Ivanova, N.V.; Hajibabaei, M.; Hebert, P.D.N. Assembling DNA Barcodes-Environmental Genomics. In Methods in Molecular Biology; Martin, C.C., Martin, C.C., Eds.; Humana Press: Totowa, NJ, USA, 2008; Volume 410, pp. 275–294. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System: Barcoding. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- White, T.P.; Veit, R.R. Spatial Ecology of Long-Tailed Ducks and White-Winged Scoters Wintering on Nantucket Shoals. Ecosphere 2020, 11, e03002. [Google Scholar] [CrossRef]
- Costa, F.O.; Henzler, C.M.; Lunt, D.H.; Whiteley, N.M.; Rock, J. Probing Marine Gammarus (Amphipoda) Taxonomy with DNA Barcodes. Syst. Biodivers. 2009, 7, 365–379. [Google Scholar] [CrossRef]
- Walsh, P.S.; Metzger, D.A.; Higuchi, R. Chelex 100 as a Medium for Simple Extraction of DNA for PCR-Based Typing from Forensic Material. Biotechniques 1991, 10, 506–513. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 June 2022).
- Clarke, K.R.; Gorley, R.N. Getting Started with PRIMER V7; PRIMER-E Ltd.: Plymouth, UK, 2015; p. 18. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2006; p. 199. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using {lme4}. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference; R package version 1.46.0. 2022. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 1 July 2022).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package; R package version 2.6-2. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 June 2022).
- Hou, Z.; Sket, B.; Fisěr, C.; Lia, S. Eocene Habitat Shift from Saline to Freshwater Promoted Tethyan Amphipod Diversification. Proc. Natl. Acad. Sci. USA 2011, 108, 14533–14538. [Google Scholar] [CrossRef] [PubMed]
- Feeley, J.B.; Wass, M.L. The Distribution and Ecology of the Gammaridae (Crustacea: Amphipoda) of the Lower Chesapeake Estuaries. Va. Inst. Mar. Sci. Spec. Pap. Mar. Sci. 1971, 2, 1–58. [Google Scholar]
- Krebes, L.; Blank, M.; Bastrop, R. Phylogeography, Historical Demography and Postglacial Colonization Routes of Two Amphi-Atlantic Distributed Amphipods. Syst. Biodivers. 2011, 9, 259–273. [Google Scholar] [CrossRef]
- Normant, M.; Lamprecht, I. Does Scope for Growth Change as a Result of Salinity Stress in the Amphipod Gammarus oceanicus? J. Exp. Mar. Biol. Ecol. 2006, 334, 158–163. [Google Scholar] [CrossRef]
- Zajac, R.; Whitlatch, R. Responses of Estuarine Infauna to Disturbance. I. Spatial and Temporal Variation of Initial Recolonization. Mar. Ecol. Prog. Ser. 1982, 10, 1–14. [Google Scholar] [CrossRef]
- Guidone, M.; Thornber, C.S. Examination of Ulva Bloom Species Richness and Relative Abundance Reveals Two Cryptically Co-Occurring Bloom Species in Narragansett Bay, Rhode Island. Harmful Algae 2013, 24, 1–9. [Google Scholar] [CrossRef]
- Berke, S.K.; Keller, E.L.; Needham, C.N.; Salerno, C.R. Grazer Interactions with Invasive Agarophyton vermiculophyllum (Rhodophyta): Comparisons to Related versus Unrelated Native Algae. Biol. Bull. 2020, 238, 145–153. [Google Scholar] [CrossRef]
- Douglass, J.G.; Emmett Duffy, J.; Canuel, E.A. Food Web Structure in a Chesapeake Bay Eelgrass Bed as Determined through Gut Contents and 13C and 15N Isotope Analysis. Estuaries Coasts 2011, 34, 701–711. [Google Scholar] [CrossRef]
- Hansen, J.P.; Wikström, S.A.; Axemar, H.; Kautsky, L. Distribution Differences and Active Habitat Choices of Invertebrates between Macrophytes of Different Morphological Complexity. Aquat. Ecol. 2011, 45, 11–22. [Google Scholar] [CrossRef]
- Schneider, F.I.; Mann, K.H. Rapid Recovery of Fauna Following Simulated Ice Rafting in a Nova Scotian Seagrass Bed. Mar. Ecol. Prog. Ser. 1991, 78, 57–70. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. A Study of Unusual Intracellular Organelles and Ultrastructural Organisation of the Eye of Gammarus oceanicus (Segerstrale 1947) Fixed in the Midnight Sun of the Spitsbergen (Svalbard) Summer. Biomed. Res. 1985, 6, 353–365. [Google Scholar] [CrossRef]
- Perry, R.I.; Harding, G.C.; Loder, J.W.; Tremblay, M.J.; Sinclair, M.M.; Drinkwater, K.F. Zooplankton Distributions at the Georges Bank Frontal System: Retention or Dispersion? Cont. Shelf Res. 1993, 13, 357–383. [Google Scholar] [CrossRef]
- Macintyre, L. Comparing Environmental DNA (eDNA) Sample Mediums for Detecting Estuarine Macroinvertebrates on Prince Edward Island. Bachelor’s Thesis, University of Prince Edward Island, Charlottetown, PE, Canada, 2022. [Google Scholar]
- Schwentner, M.; Zahiri, R.; Yamamoto, S.; Husemann, M.; Kullmann, B.; Thiel, R. EDNA as a Tool for Non-Invasive Monitoring of the Fauna of a Turbid, Well-Mixed System, the Elbe Estuary in Germany. PLoS ONE 2021, 16, e0250452. [Google Scholar] [CrossRef]
- Deiner, K.; Altermatt, F. Transport Distance of Invertebrate Environmental DNA in a Natural River. PLoS ONE 2014, 9, e88786. [Google Scholar] [CrossRef]
- Jo, T.; Minamoto, T. Complex Interactions between Environmental DNA (eDNA) State and Water Chemistries on eDNA Persistence Suggested by Meta-Analyses. Mol. Ecol. Resour. 2021, 21, 1490–1503. [Google Scholar] [CrossRef]
- Knysh, K.M.; Courtenay, S.C.; Grove, C.M.; van den Heuvel, M.R. The Differential Effects of Salinity Level on Chlorpyrifos and Imidacloprid Toxicity to an Estuarine Amphipod. Bull. Environ. Contam. Toxicol. 2021, 106, 753–758. [Google Scholar] [CrossRef]
- Zhang, G.K.; Chain, F.J.J.; Abbott, C.L.; Cristescu, M.E. Metabarcoding Using Multiplexed Markers Increases Species Detection in Complex Zooplankton Communities. Evol. Appl. 2018, 11, 1901–1914. [Google Scholar] [CrossRef]





| Estuary | Wilmot | Souris | Wheatley |
|---|---|---|---|
| Tidal amplitude (m) 1 | 1.85 | 1.64 | 1.07 |
| Percent eelgrass cover of available habitat in estuary 2 | 17.7% | 4.0% | 11.5% |
| Estuary area (km2) 2 | 3.6 | 4.2 | 2.9 |
| Watershed area (km2) | 71.6 | 31.6 | 42.1 |
| Nitrogen loading (kg/d) 1 | 421 | 57 | 119 |
| Percent watershed agricultural intensity | 75% | 40% | 66% |
| Parameter | G. lawrencianus | G. mucronatus | G. tigrinus | G. oceanicus |
|---|---|---|---|---|
| Forward Primer | ATCGGAAGCCCTGACATAGC | TGCTTTTAATGAGAGGCATAGTTGA | CTCCCTCCTTCTCTTACTCTTCTAT | TGGTAACTGGCTAGTACCCTTAATA |
| Reverse Primer | AGCTACAGTGGAGGCTAAAGG | AGGCTAAAGATTGCCAAGTCTAC | GGGAAAAGATGGCTAGATCTACTG | CTGTACCCACACCTCTTTCTACTA |
| Amplicon (bp) | 153 | 118 | 136 | 140 |
| Annealing temperature (°C) | 57.8 | 58.6 | 60.0 | 62.4 |
| Quantification limit at 35 cycles (copies/reaction) | 11.0 | 1.2 | 8.2 | 37.9 |
| Parameter | Estuary and Zone | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Location | Wilmot | Souris | Wheatley | ||||||
| Zone | Upper | Mid | Lower | Upper | Mid | Lower | Upper | Mid | Lower |
| Site Selection: Benthic Salinity (PSU) | 13 | 19 | 25 | 16 | 21 | 25 | 7 | 23 | 25 |
| Mean Organic Content (LOI (%) ± SEM); n = 3 | 2.0 (0.19) | 4.7 (1.84) | 1.8 (0.34) | 8.5 (0.42) | 1.1 (0.04) | 0.8 (0.08) | 7.9 (1.45) | 7.7 (0.23) | 1.9 (0.39) |
| Mean Macrophyte Biomass (g/Rake ± SEM); n = 4 | 0.8 (0.49) | 3.1 (0.33) | 12.6 (4.09) | 18.0 (1.90) | 9.9 (2.16) | 1.0 (0.43) | 3.6 (1.28) | 18.7 (4.51) | 10.5 (3.19) |
| Dominant Vegetation | Ulva | Ulva | Ulva/Zostera | Ulva | Ulva | Ulva | Ulva | Ulva | Zostera/Ulva |
| Estuary | Species | Upper | Mid | Lower | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rake | Substrate | Light-Trap | Rake | Substrate | Light-Trap | Rake | Substrate | Light-Trap | ||
| Wheatley | G. tigrinus | 1.3 | 0.4 | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| G. mucronatus | 77.0 | 62.1 | 37.0 | 71.4 | 50.0 | 0.6 | 0 | 0 | 0.2 | |
| G. lawrencianus | 20.4 | 37.1 | 61.0 | 28.6 | 50.0 | 98.9 | 89.1 | 0.7 | 99.2 | |
| G. oceanicus | 1.3 | 0.5 | 0.8 | 0 | 0 | 0.6 | 10.9 | 99.3 | 0.6 | |
| Total (n) | 152 (4) | 1388 (4) | 7125 (3) | 7(4) | 2 (4) | 175 (3) | 55 (4) | 291 (4) | 481 (3) | |
| Souris | G. tigrinus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| G. mucronatus | 3.1 | 0.8 | 6.0 | 33.8 | 1.3 | 0.7 | 0 | 0 | 0 | |
| G. lawrencianus | 96.7 | 99.2 | 94.0 | 66.2 | 94.6 | 99.3 | 0 | 99.0 | 100.0 | |
| G. oceanicus | 0.2 | 0 | 0 | 0 | 4.0 | 0 | 0 | 1.0 | 0 | |
| Total (n) | 2266 (4) | 253 (4) | 184 (3) | 91 (4) | 223 (4) | 430 (3) | 0 (4) | 210 (4) | 2 (3) | |
| Wilmot | G. tigrinus | 0 | 0 | 0 | 0 | 2.2 | 0 | 0 | 0 | 0 |
| G. mucronatus | 50.0 | 0 | 33.3 | 90.5 | 8.8 | 42.9 | 100.0 | 0 | 20.0 | |
| G. lawrencianus | 0 | 0 | 66.7 | 9.5 | 1.1 | 42.9 | 0 | 0 | 40.0 | |
| G. oceanicus | 50.0 | 0 | 0 | 0 | 87.9 | 14.3 | 0 | 100.0 | 40.0 | |
| Total (n) | 2 (4) | 0 (4) | 3 (3) | 17 (4) | 91 (4) | 7 (3) | 22 (4) | 540 (4) | 5 (3) | |
| G. mucronatus | G. lawrencianus | G. oceanicus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eDNA | Substrate | Light | Rake | eDNA | Substrate | Light | Rake | eDNA | Substrate | Light | Rake | |
| eDNA | ||||||||||||
| Substrate | 0.67 | −0.72 | 0.02 | |||||||||
| Light | 0.58 | 0.95 | −0.36 | 0.53 | 0.37 | 0.17 | ||||||
| Rake | 0.49 | 0.96 | 0.92 | −0.69 | 0.62 | 0.5 | 0.08 | −0.19 | 0.54 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knysh, K.M.; MacIntyre, L.P.; Cormier, J.M.; Grove, C.M.; Courtenay, S.C.; van den Heuvel, M.R. Comparing Physical Collection and Environmental DNA Methods for Determining Abundance Patterns of Gammarus Species along an Estuarine Gradient. Diversity 2023, 15, 714. https://doi.org/10.3390/d15060714
Knysh KM, MacIntyre LP, Cormier JM, Grove CM, Courtenay SC, van den Heuvel MR. Comparing Physical Collection and Environmental DNA Methods for Determining Abundance Patterns of Gammarus Species along an Estuarine Gradient. Diversity. 2023; 15(6):714. https://doi.org/10.3390/d15060714
Chicago/Turabian StyleKnysh, Kyle M., Leah P. MacIntyre, Jerrica M. Cormier, Carissa M. Grove, Simon C. Courtenay, and Michael R. van den Heuvel. 2023. "Comparing Physical Collection and Environmental DNA Methods for Determining Abundance Patterns of Gammarus Species along an Estuarine Gradient" Diversity 15, no. 6: 714. https://doi.org/10.3390/d15060714
APA StyleKnysh, K. M., MacIntyre, L. P., Cormier, J. M., Grove, C. M., Courtenay, S. C., & van den Heuvel, M. R. (2023). Comparing Physical Collection and Environmental DNA Methods for Determining Abundance Patterns of Gammarus Species along an Estuarine Gradient. Diversity, 15(6), 714. https://doi.org/10.3390/d15060714








