3.2. Descriptions
Order ACTINIARIA Hertwig, 1882.
Suborder ANENTHEMONAE Rodríguez and Daly, 2014.
Superfamily ACTINERNOIDEA Stephenson, 1922.
(Japanese name: yatsuba-kawari-ginchaku-jouka [
37].)
Diagnosis. Anenthemonae with a well-developed
basal disc but without basilar muscles. Column smooth, or with nematocyst batteries, nearly always with spirocysts. Margin tentaculate. Sphincter absent or weak mesogleal. Tentacles in variable number, with
or without thickening aboral side, either in two alternating cycles or, although usually arranged in cycles, in a peculiar way related to the development of mesenteries. Longitudinal muscles of tentacles and radial muscles of oral disc ectodermal, with a slight mesogleal tendency. Oral disc sometimes lobed. One or two siphonoglyphs. Usually more mesenteries than directives attached to siphonoglyphs. Unique mesenterial arrangement: after the first twelve mesenteries (six couples) are developed, all subsequent pairs appear in lateral endocoels with longitudinal muscles oriented as in directives. Cnidom: spirocysts, basitrichs, holotrichs, and microbasic
p-mastigophores. (Revised points from Rodríguez et al. (2014) [
13] are indicated in
bold.)
Remarks. Actinernidae Verrill, 1879, and Halcuriidae Carlgren, 1918, have peculiar mesenterial arrangement [
7,
38]. Despite that almost all sea anemones develop their second cycle of mesenteries out of first cycle of mesenterial pairs (at exocoels), only species belonging to these two families develop them in the first cycle pairs (at endocoels). So, in the classification system of Carlgren [
4], they were accommodated in the suborder Endocoelantheae Carlgren, 1925 [
9]. However, the phylogenetic research of Rodríguez et al. (2014) revealed that species of Endocoelantheae were related to Edwardsiidae, and thus, they established a new suborder, Anenthemonae Rodríguez and Daly, 2014, for Actinernidae, Halcuriidae, and Edwardsiidae [
13]. Thus, Endocoelantheae disappeared, and they used the superfamily Actinernoidea Stephenson, 1922, instead to accommodate those two families.
There are two families and six genera of actinernoideans confirmed from Japan. According to our phylogenetic and morphological analyses (
Figure 3 and
Figure 4), the monophyly was strongly supported as a superfamily, and the new family Isactinernidae and a new genus
Isohalcurias were established. See
Table 5 for comparison of Isactinernidae fam. nov. and the other families of Actinernoidea. The new taxonomic key of this superfamily to each genus, including
Isohalcurias gen. nov., is as below.
| Taxonomic key of the suborder Actinernoidea |
| 1A. Mesenteries distinguished macrocnemes and microcnemes | See 2. |
| 2A. Base of tentacles thickening in aboral side | Actinernus Verrill, 1879 |
| 2B. Tentacle simple, no thickening | See 3. |
| 3A. Edge of oral disc developed into lobes | Synactinernus Carlgren, 1918 |
| 3B. Edge of oral disc simple, not lobed | See 4. |
| 4A. 12 macrocnemes in column | Carlgrenia Stephenson, 1918 |
| 4B. 20 macrocnemes in column | See 5. |
| 5A. Spirocysts absent on column. Muscular processes simple and unified | Halcurias McMurrich, 1893 |
| 5B. Spirocysts present on column. Part of retractor muscles clustered | Isohalcurias gen. nov. |
| 1B. All mesenteries perfect and macrocnemes | See 6. |
| 6A. Edge of oral disc developing into lobes | Isactinernus Carlgren, 1918 |
| 6B. Edge of oral disc simple, not lobed | Synhalcurias Carlgren, 1914 |
Family ACTINERNIDAE Stephenson, 1922.
(Japanese name: yatsuba-kawari-ginchaku-ka [
39].)
Actinernidae Stephenson, 1922: 258; Carlgren, 1949: 20.
Diagnosis. Actinernoidea with an elongated body. Distal margin of column usually expanded and drawn into lobes but sometimes not expanded nor lobed. Column with or without nematocyst batteries. Tentacles numerous, with basal thickening on their aboral side. With two siphonoglyphs. Mesenteries numerous, divisible into macro- and microcnemes, and develop bilaterally. Retractors rather weak. All stronger mesenteries fertile. Cnidom: basitrichs, spirocysts, and microbasic p-mastigophores.
(The revised points from Uchida (2007) [
12] are indicated in
bold.)
Type genus. Actinernus Verrill, 1879.
Remarks. This family until now contained four genera:
Actinernus Verrill, 1879,
Synhalcurias Carlgren, 1914,
Isactinernus Carlgren, 1918, and
Synactinernus Carlgren, 1918, and have been characterized by having two siphonoglyphs on their actinopharynx and lacking the deviation between macrocnemes and microcnemes [
7,
39,
40]. However, though there was apparent deviation between macrocnemes and microcnemes only in
Actinernus (see Uchida, 2007 [
12];
Figure 5) and
Synactinernus (see Figure 4 in Izumi et al. (2019) [
14]), no apparent deviation was observed in
Isactinernus (see Figure 8) and
Synhalcurias (see Figure 3 in Izumi and Yanagi (2021) [
15]), and it is doubtful that these four genera should be accommodated in same family.
In this study, our phylogenetic analyses (
Figure 3 and
Figure 4) revealed that
Synactinernus was not monophyletic with the other three genera of Actinernidae but instead within the clade of Halcuriidae. Thus, it was necessary to remove this genus from Actinernidae. Moreover, there was no synapomorphy between
Actinernus and the other two genera, and rather, there were apparent differences between these genera in mesenterial arrangement and cnidom (see remarks part of Isactinernidae fam. nov.). These are the reasons why we have established a new family for
Isactinernus and
Synhalcurias and removed them from Actinernidae.
Finally, this family became monotypic to type genus Actinernus. The most characteristic feature of this genus is developing their mesenteries laterally: this arrangement is only observed in the species of Actinernus in Actinernoidea. In addition, the species of this family are distinguished from the species of the other two families by a combination of “lacking spirocysts in tentacle and column” and “thickened aboral ends of tentacles”.
Table 5.
Comparison of Isactinernidae fam. nov. and the other two families in superfamily Actinernoidea.
Table 5.
Comparison of Isactinernidae fam. nov. and the other two families in superfamily Actinernoidea.
| | Isactinernidae fam. nov. | Actinernidae Stephenson, 1922 | Halcuriidae Carlgren, 1918 |
|---|
| Type genus | Isactinernus Carlgren, 1918 | Actinernus Verrill, 1879 | Halcurias McMurrich, 1893 |
| The other genera | Synhalcurias Carlgren, 1914 | none | Synactinernus Carlgren, 1918
Carlgrenia Stephenson, 1918
Isohalcurias gen. nov. |
| Characters | | | |
| Mesenterial arrangement | Cyclic | Bilateral | Cyclic |
| Microcnemes | Absent | Present | Present |
| Number of macrocnemes | Unfixed (more than 68) | Unfixed (more than 20) | Fixed (12, 20, or 36) |
| References | [7,12,40]
The present study | [8,12] | [7,8,11,12]
The present study |
Genus Actinernus Verrill, 1879.
(Japanese name: Yatsuba-kawari-ginchaku-zoku.)
Actinernus Verrill, 1879: 474; McMurrich, 1893: 165; Carlgren, 1914: 62; Carlgren, 1918: 31; Carlgren, 1921: 14, 184; Stephenson, 1922: 259; Carlgren, 1949: 20.
Porponia Hertwig, 1882, p. 111; Carlgren, 1914, p. 61.
Diagnosis. Column cylindrical, the upper part expanded and forms more or less distinctly eight lobes. Tentacles numerous, with basal thickening on their aboral side. Mesenteries many, consist of 24–52 perfect mesenteries and a half number of imperfect mesenteries. After the formation of ten pairs, first and second cycles, further mesenterial formation takes place at the middle point of each eight lateral endocoel. Then, the formation is not in a radial but in a bilateral way. Furthermore, the size in higher cycles of mesenteries is much different to its partner in each mesenterial pair. Cnidom: basitrichs (all tissues), spirocysts (actinopharynx and filaments), and microbasic p-mastigophores (actinopharynx and filaments).
(The revised points from Uchida (2007) [
12] are indicated in
bold.)
Type species. Actinernus elongatus (Hertwig, 1882).
Actinernus robustus (Hertwig, 1882).
(Japanese name: yatsuba-kawari-ginchaku.)
Porponia robusta Hertwig, 1882: 107, pl. 1 Figure 10a; Carlgren, 1914: 61.
Actinernus robustus: Carlgren, 1918: 12, 34, pl. 1 Figure 9; Stephenson, 1922: 259; Carlgren, 1949: 21.
Material examined. CMNH–ZG 09735 (
Figure 5 and
Figure 6): specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared, corrected on 28 April 2002, from Okinawa Trough (27°02.34′ N, 126°58.24′ E; St. D-2), 1550 m in depth, during research cruise of R/V Tansei-Maru (KT02-3 reg. 2), by ORE beam trawl, by Kensuke Yanagi; CMNH–ZG 09736: specimen dissected, tissues embedded in paraffin, histological sections prepared, same collector, locality, and method as for CMNH–ZG 09735; CMNH–ZG 09737, 09738: whole specimens, same collector, locality, and method as for CMNH–ZG 09735; CMNH 10211: specimen dissected, corrected on 23 September 2001, from Pacific off Kushiro (42°11.02′ N, 146°17.02′ E; St. XR-11), 5346–5473 m in depth, collected during research cruise of R/V Hakuho-Maru (KH01-2), by ORE beam trawl, by Kensuke Yanagi; BM 89–11–25–30 (holotype) (
Figure 7A,B): dissected specimen, collected on 17 June 1875 southeast off the Boso Peninsula (34°37′ N, 140°32′ E), depth 1875 fathoms (ca. 3500 m) by trawl, St. 237 of the Challenger Expedition.
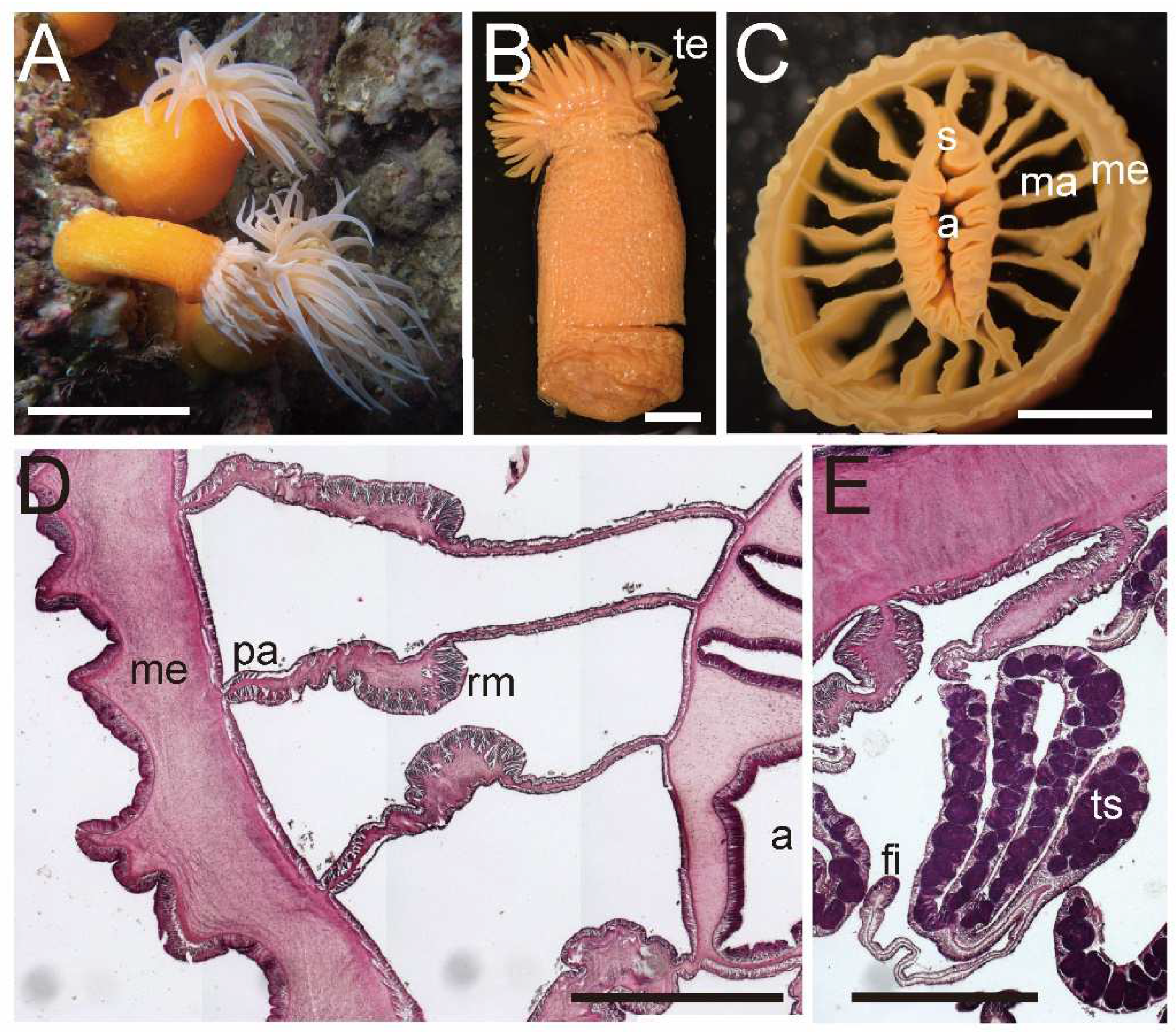
Diagnosis. External anatomy. Body cylindrical (
Figure 5A), up to ca. 2–6 cm in length and ca. 2.5–5 cm in width on preserved specimens. Column surface smooth, without any structures. Ectoderm of column easily peeled off, without nematocyst batteries, and nematocysts very sparsely distributed. The naked column is opaque milky white in color (
Figure 5A). Basal disk in aboral end, thin and fragile but adhesive (
Figure 5A). Upper part of column widely expanded and thrown into eight lobes, four larger and four smaller ones (
Figure 5B,C). Upper margin continued to tentacle bases, and the bases have a spine-like shape (
Figure 5A). Tentacles ca. 60–90 in number on oral disk, all marginal. All tentacles ca. 7–10 mm in length, pale white to brown in color, simple shape but with strong thickenings at their aboral base. Oral disc pale brown, with radial streaks corresponding to every tentacle. Mouth at center of oral disk, apparently swelled, lip-like, brown in color.
Internal anatomy. Circa 24–52 macrocnemes (
Figure 5D) on actinopharynx: 12, including 4 directives, in the 1st cycle; 8 in the 2nd cycle; maximum 16 in the 3rd cycle; and maximum 16 in the 4th cycle. Macrocnemes in the second cycle born in the endocoel of the first cycle mesenteries, and fourth ones in the endocoels of third ones (bilateral arrangement). Judged by the numbers of tentacles, 20–40 more microcnemes on distal end. Each tentacle either exo- or endocoelic. Tentacular longitudinal muscle and tentacular circular muscle both too weak to observe in histological sections. On aboral ends of tentacles, mesoglea apparently thickened. Retractor muscles extremely weak and diffused (
Figure 5D). Muscle processes very short, simple or a little branched, around 30–50 in each muscle pennon. Parietal muscles of macrocnemes very weak and indistinct, with a few muscle fibers. Mesoglea thickest in body wall and actinopharynx (
Figure 5D), reaching 2–2.5 mm in thickness, and far thicker than the ectoderm and endoderm. However, mesoglea thinner in mesenteries and basal disc (
Figure 5D,E) and thinnest in tentacles. Actinopharynx, with siphonoglyphs on dorsal and ventral sides (
Figure 5D), always connected to actinopharynx, and with ca. 8–12 longitudinal grooves as deep as siphonoglyphs. Sphincter muscle absent. On the aboral end, basilar muscle absent (
Figure 5E). Dioecious.
Cnidom. Basitrichs, spirocysts, and microbasic
p-mastigophores (
Figure 6,
Table 6).
Remarks. Actinernus robustus is the only species of this genus in Japan. Our specimens completely correspond to the description of Hertwig (1882) and Uchida (2007) [
12,
41]. In contrast to all Japanese Actinernoidea species, which live between 20 and 500 m, this species inhabits waters deeper than 1000 m.
The approximate morphology and cnidom of our specimens corresponded to the descriptions of Hertwig (1882) and Uchida (2007), and these specimens can be identified as
A. robustus. However, there were no holotrichs in filaments, though Uchida (2007) [
12] observed a few.
Table 6.
Cnidoms of the species of Actinernus and Isactinernus.
Table 6.
Cnidoms of the species of Actinernus and Isactinernus.
| | Actinernus robustus | Isactinernus quadrilobatus |
|---|
| | CMNH–ZG 9735 | NSMT–Co 1662 |
|---|
| | Length × Width (µm) | frequency | N | Length × Width (µm) | frequency | N |
|---|
| Tentacle | | | | | | |
| basitrichs | S | 18.2–28.8 × 3.7–5.9 | numerous | 53 | 9.1–21.5 × 2.0–3.5 | numerous | 15 |
| L | 40.0–53.6 × 4.3–4.8 | few | 8 | 31.1–42.3 × 3.0–4.4 | numerous | 24 |
| spirocysts | | 25.2–54.7 × 5.2–8.7 | few | 5 | 20.9–49.5 × 3.1–9.8 | numerous | 78 |
| microbasic p-mastigophores | | | | | | | |
| microbasic b-mastigophores | | | | | 29.9–31.7 × 4.4–5.2 | rare | 3 |
| Actinopharynx | | | | | | | |
| basitrichs | S | 20.2–25.1 × 4.0–5.5 | numerous | 10 | 8.3–13.0 × 2.2–3.3 | numerous | 36 |
| L | 38.8–49.3 × 3.9–5.4 | few | 9 | 36.1–43.4 × 3.5–5.2 | numerous | 27 |
| spirocysts | | | | | 25.4–36.4 × 4.7–6.3 | few | 4 |
| microbasic p-mastigophores | | 11.7–14.0 × 3.9–5.6 | few | 5 | 34.9–44.5 × 6.3–8.6 | numerous | 23 |
| Column | | | | | Column damaged | |
| basitrichs | | 20.4–22.5 × 4.3–5.4 | rare | 3 | |
| Filament | | | | | | | |
| basitrichs | | 15.8–23.8 × 3.8–5.6 | numerous | 35 | 17.8–47.2 × 2.9–4.2 | few | 9 |
| | | 27.7–52.8 × 3.4–5.4 | numerous | 27 |
| spirocysts | | 41.6 × 7.2 | rare | 1 | 28.1–42.5 × 4.8–7.6 | numerous | 55 |
| microbasic p-mastigophores | | 38.8–42.3 × 8.1–9.8 | rare | 4 | 35.3–42.4 × 6.0–7.2 | numerous | 25 |
Figure 5.
External and internal morphology of Actinernus robustus (CMNH–ZG 9735). (A) Lateral view of a fixed specimen; (B) oral view; (C) bared oral disc; (D) transverse section of column; (E) longitudinal section of basal disc. Basilar muscle absent; abbreviations: a, actinopharynx; bd, basal disc; ma, macrocneme; me, mesoglea; pa, parietal muscle; rm, retractor muscle; s, siphonoglyph; te, tentacle. Scale bars indicate 1 cm in (A–C), 1 mm in (B,E), and 500 µm in (D,E).
Figure 5.
External and internal morphology of Actinernus robustus (CMNH–ZG 9735). (A) Lateral view of a fixed specimen; (B) oral view; (C) bared oral disc; (D) transverse section of column; (E) longitudinal section of basal disc. Basilar muscle absent; abbreviations: a, actinopharynx; bd, basal disc; ma, macrocneme; me, mesoglea; pa, parietal muscle; rm, retractor muscle; s, siphonoglyph; te, tentacle. Scale bars indicate 1 cm in (A–C), 1 mm in (B,E), and 500 µm in (D,E).
Figure 6.
Cnidoms of Actinernus robustus and Isactinernus quadrilobatus. A–K: A. robustus. A–C: tentacle; A, small basitrich; B, large basitrich; C, spirocyst. D–F: actinopharynx; D, small basitrich; E, large basitrich; F, microbasic p-mastigophore. G, basitrich in column. H–K: filament; H, small basitrich; I, large basitrich; J, spirocyst; K, microbasic p-mastigophore. L–V: I. quadrilobatus. L–O: tentacle; L, small basitrich; M, large basitrich; N, spirocyst; O, microbasic b-mastigophore. P–S: actinopharynx; P, small basitrich; Q, large basitrich; R, spirocyst; S, microbasic p-mastigophore. T–V: filament; T, small basitrich; U, large basitrich; V, microbasic p-mastigophore.
Figure 6.
Cnidoms of Actinernus robustus and Isactinernus quadrilobatus. A–K: A. robustus. A–C: tentacle; A, small basitrich; B, large basitrich; C, spirocyst. D–F: actinopharynx; D, small basitrich; E, large basitrich; F, microbasic p-mastigophore. G, basitrich in column. H–K: filament; H, small basitrich; I, large basitrich; J, spirocyst; K, microbasic p-mastigophore. L–V: I. quadrilobatus. L–O: tentacle; L, small basitrich; M, large basitrich; N, spirocyst; O, microbasic b-mastigophore. P–S: actinopharynx; P, small basitrich; Q, large basitrich; R, spirocyst; S, microbasic p-mastigophore. T–V: filament; T, small basitrich; U, large basitrich; V, microbasic p-mastigophore.
Figure 7.
Type specimens of actinernoidean anemones. The type specimen of Actinernus robustus ((A,B): BM 89-11-25-30, holotype), Isactinernus quadrilobatus ((C,D): ZMUC–ANT–000098, one of the syntypes), and Isohalcurias carlgreni ((E): UUZM 705b (Anthozoa), one of the syntypes, (F): SMNH–TYPE–1200, one of the syntypes).
Figure 7.
Type specimens of actinernoidean anemones. The type specimen of Actinernus robustus ((A,B): BM 89-11-25-30, holotype), Isactinernus quadrilobatus ((C,D): ZMUC–ANT–000098, one of the syntypes), and Isohalcurias carlgreni ((E): UUZM 705b (Anthozoa), one of the syntypes, (F): SMNH–TYPE–1200, one of the syntypes).
Family ISACTINERNIDAE fam. nov.
(New Japanese name: yotsuba-kawari-ginchaku-ka.)
urn:lsid:zoobank.org:act:E3DEAB20-FDE6-4E96-A2E1-A0E892DD485A
Diagnosis. Actinernoidea with an elongated body. Distal margin of column usually expanded and drawn into lobes but sometimes not expanded nor lobed. Column with or without nematocyst batteries. Tentacles numerous, simple or with basal thickening or on their aboral side. With two siphonoglyphs. Mesenteries all macrocnemes, numerous, and developing cyclically. Retractors rather weak. All mesenteries fertile. Cnidom: basitrichs, spirocysts, and microbasic p-mastigophores (each in all tissue).
Type genus. Isactinernus Carlgren, 1918.
Etymology. Derived from the name of type genus, Isactinernus Carlgren, 1918.
Remarks. In this study, our phylogenetic analyses (
Figure 4) revealed that
Isactinernus and
Synhalcurias were the most closely related genera and formed a monophyly with
Actinernus. However, there were no synapomorphies between
Actinernus and
Isactinernus/Synhalcurias:
Synactinernus has almost all the general features of Actinernidae, and we could not find any accurate common feature shared among the remaining three families. In addition, there are apparent differences between
Actinernus and
Isactinernus/Synhalcurias in mesenterial arrangement and cnidom: in Actinernoidea, only the latter two genera have microbasic
p-mastigophores in their tentacles and column, and their mesenteries are all macrocnemes, the same in number as tentacles [
10,
12,
15]. For these reasons, we have established this new family for
Isactinernus and
Synhalcurias and removed them from Actinernidae (
Figure 4). See
Table 5 for the comparison with the other families of Actinernoidea. This family is characterized by possessing microbasic
p-mastigophores in their tentacles and column and all mesenteries being perfect and fertile.
Genus Isactinernus Carlgren, 1918.
(Japanese name: yotsuba-kawari-ginchaku-zoku.)
Isactinernus Carlgren, 1918: 29; Stephenson, 1922: 260; Carlgren, 1949: 20; Fautin and den Hartog, 2003: 107.
Diagnosis. Column cylindrical, the upper part expanded, and forms four large lobes. With many tentacles, thickening on aboral side. Mesenteries ca. 34–70 pairs of large and many small ones, all mesenteries perfect and almost all fertile. After the formation of ten pairs of the oldest mesenteries, in the first and second cycles, further mesenterial formation by cyclic in each eight lateral endocoel, in the same manner as those of Halcurias. With two siphonoglyphs. Column ectoderm with minute nematocyst batteries. Cnidom: basitrichs, spirocysts, and microbasic p-mastigophores (each in all tissue).
(The revised points from Uchida (2007) [
12] are indicated in
bold.)
Type species. Isactinernus quadrilobatus Carlgren, 1918.
Remarks. This genus had been monotypic with
I. quadrilobatus Carlgren, 1918, for a century. However, accompanied by Fautin and Hartog (2003), the work which synonymized
Synactinernus flavus Carlgren, 1918, into
I. quadrilobatus, genus
Synactinernus became a junior synonym of this genus. However, our phylogenetic analyses indicated that these genera are less related phylogenetically (
Figure 3):
I. quadrilobatus and
S. flavus are polyphyletic, not closely related in the phylogenetic tree, and thus, the species should be accommodated into a different family (see also remarks of
Synactinernus).
Thus,
Isactinernus should remain as a monotypic genus, the same status as Carlgren (1918) [
7]. However, it turned out to be appropriate that this genus should not be accommodated into Actinernidae, but into a new family (see the remarks of Isactinernidae).
Isactinernus quadrilobatus Carlgren, 1918.
(Japanese name: yotsuba-kawari-ginchaku.)
Isactinernus 4-lobatus Carlgren, 1918: 7, 29, textfigs. 5 and 6, pl. 1 Figures 4 and 5; Carlgren, 1940: 22; Carlgren, 1949: 20.
Isactinernus quadrilobatus: Stephenson, 1922: 260; Fautin and den Hartog, 2003: 108–113, Figures 1–5.
Material examined. NSMT–Co 1662: specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared and collected on 8 March 2016, from the Kumano-nada Sea off Hamajima Island, Mie Prefecture, around a depth of 350 m, by trawling of fishing boat
Kiei-Maru, kept in Toba Aquarium for a while, preserved on 19 March 2016 by Takeya Moritaki in 70% ethanol, and re-preserved on 19 April 2016 by Takuma Fujii in 10% formalin–seawater solution (
v/
v) for histological section; CMNH–ZG 09734: specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared and collected on 18 April 2018, from the East China Sea off Koshikijima Island, Kagoshima Prefecture, around a depth of 380 m, by the fishing boat
Koei-Maru, kept in Kagoshima City Aquarium for a while, and preserved on 6 May 2018 by Takato Izumi. ZMUC–ANT–000098 (syntypes): three specimens, 14 May 1914, off the Goto Islands (32°17′ N, 128°11′ E), depth 207 m, collected by Theodor Mortensen (
Figure 7C,D). MZLU–L14/3033 (syntype): collection data same as above syntypes.
Description. External anatomy. Body cylindrical (
Figure 8A), up to ca. 4.5–7 cm in length and ca. 5 cm in width on preserved specimens. Column surface comparatively smooth, without any structures or with small tenaculi. Ectoderm of column is pale white (
Figure 8A), with small nematocyst batteries. The naked column is opaque milky white in color. Basal disk in aboral end, robust and adhesive (
Figure 8A). Upper part of column widely expanded and thrown into four large lobes (
Figure 8B). Upper margin continued to tentacle bases, and the bases have numerous spine-like shapes (
Figure 8B). Tentacles ca. 72–140 in number on oral disk, all marginal. All tentacles ca. 3–5 mm in length, pale white in color, simple shape, but with strong thickenings at their aboral base. Oral disc is pale white, with radial streaks corresponding to every tentacle. Mouth at center of oral disk, apparently swelled, lip-like, cross-like shape, white in color.
Internal anatomy. Circa 72–140 macrocnemes on actinopharynx; 12, including 4 directives, in the 1st cycle; 8 in the 2nd cycle; 16 in the 3rd cycle; and 32 in 4th cycle. Judged by the numbers of tentacles, the mesenterial cycle reaching to sixth. Macrocnemes in the second cycle born in the endocoel of the first cycle mesenteries, same as the general arrangement of Actinernoidea. Microcnemes absent (
Figure 8C). Each tentacle either exo- or endocoelic. Tentacular longitudinal muscle and tentacular circular muscle both too weak to observe in histological sections. On aboral ends of tentacles, mesoglea apparently thickened. Retractor muscles extremely weak and diffused (
Figure 8C,D). Muscle processes very short, simple or a little branched, around 40–70 in each muscle pennon. Parietal muscles of macrocnemes very weak and indistinct, with a few muscle fibers. Mesoglea thickest in body wall and actinopharynx (
Figure 8C,D), reaching 2.5–3 mm in thickness, and far thicker than the ectoderm and endoderm. Mesoglea evenly thick in mesenteries (
Figure 8C) and basal disc but far thinner in tentacles. Actinopharynx, with siphonoglyphs on dorsal and ventral sides, always connected to actinopharynx, and with 10 longitudinal grooves as deep as siphonoglyphs. Sphincter muscle absent. On the aboral end, basilar muscle absent. Dioecious: almost all mesenteries fertile and matured oocytes in CMNH–ZG 09734.
Cnidom. Basitrichs, spirocysts, and microbasic
p-mastigophores (
Figure 6,
Table 6).
Remarks. This species has been the only species of genus
Isactinernus for a century. According to our research,
Synactinernus flavus Carlgren, 1918, once synonymized into
I. quadrilobatus by Fautin and den Hartog (2003), is not only a different species but also a species in a different family (see remarks of genus
Synactinernus [
14]).
Figure 8.
External and internal morphology of Isactinernus quadrilobatus (CMNH–ZG 9734). (A) Lateral view of a fixed specimen; (B) oral view; (C) enlarged transverse section of upper column; (D) enlarged transverse section of lower column. Abbreviations: a, actinopharynx; fi, filament; ma, macrocneme; me, mesoglea; ov, ovary; pa, parietal muscle; rm, retractor muscle; te, tentacle. Scale bars indicate 1 cm in (A,B) and 1 mm in (C,D).
Figure 8.
External and internal morphology of Isactinernus quadrilobatus (CMNH–ZG 9734). (A) Lateral view of a fixed specimen; (B) oral view; (C) enlarged transverse section of upper column; (D) enlarged transverse section of lower column. Abbreviations: a, actinopharynx; fi, filament; ma, macrocneme; me, mesoglea; ov, ovary; pa, parietal muscle; rm, retractor muscle; te, tentacle. Scale bars indicate 1 cm in (A,B) and 1 mm in (C,D).
Genus Synhalcurias Carlgren, 1914.
(Japanese name: seitaka-kawari-ginchaku-zoku.)
Synhalcurias Carlgren, 1914: 53; Carlgren, 1918: 27; Stephenson, 1922: 260; Carlgren, 1949: 19.
Diagnosis. Column cylindrical, the upper part not expanded nor forms lobes. With many tentacles in a simple shape. Mesenteries number 68 to ca. 100, and all mesenteries are perfect and fertile. After the formation of ten pairs of the oldest mesenteries, in the first and second cycles, further mesenterial formation by cyclic in each eight lateral endocoel in the same manner as those in Halcurias. With two siphonoglyphs. Column ectoderm with or without small nematocyst batteries. Cnidom: basitrichs, spirocysts, and microbasic p-mastigophores (each in all tissue).
(The revised points from Uchida (2007) [
12] are indicated in
bold.)
Type species. Synactinernus elegans (Wassilieff, 1908).
Remarks. Different from
Isactinernus,
Synhalcurias has been continuously monotypic since the foundation of the genus. However, a different species of
Synhalcurias were discovered recently: they are apparently smaller than
S. elegans, and there were several differences between
S. elegans and these recently discovered specimens, although their morphological features satisfied the diagnosis of
Synhalcurias. Based on this, Izumi and Yanagi (2021) [
15] determined these specimens as a new species of
Synhalcurias and described them as
Synhalcurias kahakui Izumi and Yanagi, 2021.
Synhalcurias has long belonged to Actinernidae, but it is appropriate that Synhalcurias and Isactinernus should not be accommodated into the family by our morphological and phylogenetic analyses, and so, we have established a new family for these genera (see the remarks of family Isactinernidae).
Synhalcurias elegans (Wassilieff, 1908).
(Japanese name: seitaka-kawari-ginchaku.)
Figures 2–4 and Table 1 in Izumi and Yanagi (2021) [
15].
Ilyanthopsis elegans Wassilieff, 1908: 8, textfigs. 2–5, pl. 1 Figure 2, pl. 3 Figure 38, pl. 4 Figures 39, 40a, and 40b.
Synhalcurias elegans: Carlgren, 1914: 50–53, pl. Figures 1–4; Carlgren, 1918: 6, 27, textfigs. 2–4; Stephenson, 1922: 260; Carlgren, 1940: 22; Carlgren, 1949: 20; Uchida, 1992: 129, pl. 29 Figure 5; Uchida and Soyama, 2001: 21.
Material examined/description. See Izumi and Yanagi (2021) [
15].
Synhalcurias kahakui Izumi, 2021.
(New Japanese name: kobito-seitaka-kawari-ginchaku.)
Figures 4 and 5 and Table 1 in Izumi and Yanagi (2021) [
15].
Synhalcurias kahakui Izumi and Yanagi, 2021: 567–573.
Material examined/description. See Izumi and Yanagi (2021) [
15].
Family HALCURIIDAE Carlgren, 1918 (1897).
(Japanese name: kawari-ginchaku-ka.)
Halcuriidae Carlgren, 1918: 24; Carlgren, 1921: 13; Stephenson, 1922: 257; Carlgren, 1938: 17; Carlgren, 1949: 18.
Diagnosis. (Revised parts are shown in bold). Actinernoidea with an elongated body. Distal margin of column lobed or not lobed. Column with or without nematocyst batteries. Tentacles without basal thickening on their aboral side. With a single or double siphonoglyph. Mesenteries divisible into macro- and microcnemes. Macrocnemes six, ten or eighteen pairs. Some of the microcnemes, however, are perfect in many species. Retractors of the macrocnemes restricted. Macrocnemes fertile with filaments but microcnemes sterile without filaments.
(The revised points from Uchida (2004) [
11] are indicated in
bold.)
Type genus. Halcurias McMurrich, 1893.
Remarks. Regarding the nomenclature of the family, refer to Sanamyan and Sanamyan (2020) [
42]. This family has accommodated two genera:
Halcurias McMurrich, 1893, and
Carlgrenia, Stephenson, 1918 (the latter genus never confirmed from Japan, including in our research) [
7,
43]. However, our phylogenetic analyses revealed that
Synactinernus Carlgren, 1918, one of the genera of Actinernidae, was in the clade of Halcuriidae (
Figure 3). However, species of this genus have almost the same morphology as Actinernidae, and there was no synapomorphy between
Halcurias and
Synactinernus. Thus, it was unnatural to classify the synactinernid species into
Halcurias.
Thus, we divided the previous
Halcurias into “true”
Halcurias and a new genus,
Isohalcurias gen. nov., following the phylogeny (
Figure 4). According to the tree, there were only two species from Japan,
Halcurias hiroomii sp. nov. and
Halcurias fragum sp. nov., in the same clade as
Halcurias pilatus McMurrich, 1893, the type species of the genus. On the other hand, the other three
Halcurias species were in the other clade, which was paraphyletic to “true”
Halcurias and
Synactinernus. Thus, these three species,
Isohalcurias carlgreni (McMurrich, 1901) comb. nov.,
Isohalcurias citreum sp. nov., and
Isohalcurias malum sp. nov., were accommodated into the new genus,
Isohalcurias. See
Table 7 for a comparison of the four genera included in the suborder Anenthemonae.
Genus Halcurias McMurrich, 1893.
(Japanese name: kawari-ginchaku-zoku.)
Halcurias McMurrich 1893: 142; McMurrich, 1901: 155, 158; Carlgren, 1914: 60; Carlgren, 1918: 25; Stephenson, 1918: 14; Carlgren 1938: 18; Carlgren, 1949: 18.
Halcuriopsis Carlgren 1921: 93.
Diagnosis. Mesenteries up to 34 pairs arranged in 4 cycles, 6 + 4 + 8 + 16. Macrocnemes, ten pairs, fertile, filamented and with restricted retractors. Microcnemes in only the upper part of the body, some of them perfect. Retractor muscles strong and diffused. Parietal muscles rather well developed to fairly weak. Tentacles, up to 68. Dioecious. Cnidom: basitrichs (in all tissues), spirocysts (in tentacles, actinopharynx, and filaments), and microbasic p-mastigophores (in actinopharynx and filaments).
(The revised points from Uchida (2004) [
11] are indicated in
bold.)
Type species. Halcurias pilatus McMurrich, 1893.
Remarks. Halcuriidae Carlgren, 1918, contained two genera,
Halcurias McMurrich, 1893, and
Carlgrenia Stephenson, 1918 [
7,
43]. The latter genus has been monotypic, so almost all species of this family have been accommodated in
Halcurias. However, according to our phylogenetic analyses,
Halcurias became paraphyletic (
Figure 3). Thus, we established the new genus
Isohalcurias gen. nov. and moved some species into this genus (
Figure 4). Compared to
Isohalcurias,
Halcurias is characterized by two features: lacking spirocysts in their column (compare
Halcurias species in
Table 8 and
Isohalcurias species in Table 10) and retractor muscles with comparatively simple, not clustered muscular processes (
Figure 9D and
Figure 11D;
Table 7).
The two species of Halcuriidae from Japan remain in
Halcurias:
Halcurias hiroomii sp. nov. and
Halcurias fragum sp. nov. Across the world,
Halcurias pilatus McMurrich, 1893, was confirmed to belong to this genus by phylogenetic analyses (
Figure 4). Concerning the other described species, classification was conjectured by the cnidom of the column, as below.
The following species possibly remain in
Halcurias:
Halcurias uchidai, Rodríguez and Lauretta, 2023 (according to Rodríguez et al. (2013); this species, described as
Halcurias macmurrich Uchida, 2004, does not contain spirocysts in its column [
44]; recently, Rodriguez and Lauretta (2023) gave a new name for this species [
45]);
Halcurias endocoelactis Stephenson, 1918 (it was described that spirocysts were absent in the body wall [
36]);
Halcurias capensis Carlgren, 1928 (same as
H. endocoelactis [
36]).
Species possibly assigned to
Isohalcurias:
Halcurias minimus Carlgren, 1928;
Halcurias sudanensis Riemann-Zürneck, 1983 (it was described that spirocysts were present on the body walls of both species [
46,
47]).
Halcurias hiroomii sp. nov.
(Japanese name: abata-kawari-ginchaku.)
urn:lsid:zoobank.org:act:806B2C63-878E-4569-86C4-E3F6F1AF2783
Halcurias japonicus (nomen nudum) Uchida, 2004: 13–15, Figure 2. pl. 1C–1E.
Material examined. Holotype. CMNH–ZG 10212: specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared, collected on 2016 (date unknown) from Toyama Bay, Toyama Prefecture, around a depth of 50–100 m, gill net of fishing boat Koei-Maru, provided by Itaru Kobayashi. Paratype. NSMT–Co 1824: specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared, collected from Toyama Bay, off Toyama Prefecture, by gill net (anonymous collector, and date unknown), kept in a tank of Uozu Aquarium by Tomoharu Kimura, and fixed on 28 February 2015 by Takato Izumi. Other specimens. CMNH–ZG 10213: whole specimen, same date, collector, and method as for CMNH–ZG 10212; NSMT–Co 1825–1827, same date, collector, and method as for NSMT–Co 1824: whole specimens; CMNH–ZG 10214: whole specimen; originally collected from Ise Bay by trawling of fishing boat Kiei-Maru, kept in Takeshima Aquarium, and fixed by Takato Izumi on 29 June 2017. CMNH–ZG 10215: whole specimen; originally collected in January 2018 at Uragami, Wakayama Prefecture, at 100–130 m by Isao Hirabayashi, and fixed by Takuma Fujii and Kensuke Yanagi from the tank at the Kushimoto Marine Park on 22 May 2018.
Description.
External anatomy. Body cylindrical (
Figure 9A,B), up to ca. 40 mm in height and ca. 15 mm in width in a living specimen and 15–30 mm in height and 5–22 mm in width in a preserved specimen. Column surface comparatively rough, reddish-orange ectoderm layer with white tenaculi-like nematocyst batteries. Nematocysts densely distributed, especially in nematocyst batteries. Upper part of column a little narrower, pale yellow in color, simple and not thrown into any lobes. Tentacles simple, conical, all marginal, 6–8 mm in length, no thickenings including their aboral base, pale yellow in color, and 68 in number on oral disk; inner and outer ones alternatingly bared (
Figure 9C). The tip of tentacles pointed. Basal disk in aboral end, opaque and mesenterial insertion invisible (
Figure 9A,B). Oral disk diameter as broad as column, hemi-transparent pale yellow. Mouth at center of oral disk, highly swelled, lip-like, smooth, bright yellow and orange (
Figure 9C).
Internal anatomy. Twenty macrocnemes on actinopharynx (our specimens, all twenty): twelve, including four directives, in the first cycle and eight in the second cycle. Macrocnemes in the second cycle born in the endocoel of the first cycle mesenteries, an arrangement obeying the rule of mesenterial arrangement of Actinernidae. Mesenteries in third and fourth cycle microcnemes. Each tentacle between either exo- or endocoelic. Tentacular longitudinal muscle exocoeletic and circular muscle too weak to observe in histological section; retractor muscles restricted at the center of mesenteries, comparatively weak but distinct (
Figure 9D). Muscle processes simple or slightly branched, around 25–41 in each muscle pennon (
Figure 9D). Parietal muscles of macrocnemes quite weak with 10–13 simple processes (
Figure 9D). Mesoglea thickest in body wall and actinopharynx, far thicker than ectoderm and endoderm (
Figure 9G). Mesoglea thinner in mesoglea (
Figure 9D,E). Actinopharynx, with siphonoglyphs on one side, always connected to actinopharynx, and with the other eleven longitudinal grooves less deep than siphonoglyphs. Sphincter muscle absent. On the aboral end, basilar muscle absent. Dioecious, immature testis in our specimen (
Figure 9E). Mesenteries in second cycle fertile.
Cnidom. Basitrichs, spirocysts, and microbasic
p-mastigophores. Spirocysts are absent in the column (
Figure 10,
Table 8).
Etymology. This name is derived from Hiro’omi Uchida, the author of Uchida (2004) [
11] in which
Halcurias hiroomii sp. nov. was originally described. He defined this anemone as a new species. Thus, we renamed this species to make it valid and used his first name.
Table 8.
Cnidoms of the species of Halcurias.
Table 8.
Cnidoms of the species of Halcurias.
| | Halcurias hiroomii sp. nov. | Halcurias fragum sp. nov. |
|---|
| | CMNH–ZG 10212 | CMNH–ZG 10216 |
|---|
| | Length × Width (µm) | frequency | N | Length × Width (µm) | frequency | N |
|---|
| Tentacle |
| basitrichs | S | 10.3–12.4 × 2.0–3.0 | rare | 2 | 9.7–13.7 × 2.0–3.1 | numerous | 13 |
| | L | 24.0–34.5 × 2.3–4.6 | numerous | 60 | 21.1–27.5 × 2.5–4.8 | numerous | 44 |
| spirocysts | | 13.6–39.4 × 2.6–6.7 | numerous | 67 | 20.8–31.0 × 3.5–7.0 | numerous | 68 |
| Actinopharynx |
| basitrichs | S | 8.6–12.3 × 2.2–3.1 | few | 8 | 22.8–32.6 × 2.4–4.0 | numerous | 45 |
| | L | 20.3–31.5 × 2.3–4.2 | numerous | 61 |
| spirocysts | | 26.2–36.8 × 5.4–7.4 | numerous | 64 | 19.6–27.8 × 4.2–5.3 | few | 6 |
| microbasic p-mastigophores | | 23.8–31.9 × 4.5–6.7 | numerous | 63 | 20.4–29.2 × 4.5–7.7 | numerous | 43 |
| Column |
| basitrichs | | 26.7–36.1 × 2.0–4.2 | numerous | 84 | 31.2–39.9 × 2.3–4.2 | numerous | 70 |
| Filament | | | | | | | |
| basitrichs | | 25.3–33.6 × 2.9–4.2 | numerous | 31 | 23.9–33.5 × 2.4–4.3 | numerous | 28 |
| spirocysts | | 20.4–38.5 × 4.9–7.1 | numerous | 30 | 24.8 × 45.3 | rare | 1 |
| microbasic p-mastigophores | | 12.2–33.5 × 3.6–8.7 | numerous | 61 | 13.4–29.6 × 3.5–10.5 | numerous | 79 |
Figure 9.
External and internal morphology of Halcurias hiroomii sp. nov. (CMNH–ZG 10212). (A) Lateral view of a living specimen; (B) lateral view of a fixed specimen; (C) oral view of a living specimen; (D) enlarged transverse section of upper column; (E) enlarged transverse section of lower column. Abbreviations: fi, filament; go, gonad; ma, macrocneme; me, mesoglea; pa, parietal muscle; rm, retractor muscle; te, tentacle. Scale bars indicate 1 cm in (A–C) and 500 µm in (D,E).
Figure 9.
External and internal morphology of Halcurias hiroomii sp. nov. (CMNH–ZG 10212). (A) Lateral view of a living specimen; (B) lateral view of a fixed specimen; (C) oral view of a living specimen; (D) enlarged transverse section of upper column; (E) enlarged transverse section of lower column. Abbreviations: fi, filament; go, gonad; ma, macrocneme; me, mesoglea; pa, parietal muscle; rm, retractor muscle; te, tentacle. Scale bars indicate 1 cm in (A–C) and 500 µm in (D,E).
Figure 10.
Cnidoms of Halcurias hiroomii sp. nov. and H. fragum sp. nov. A–K: H. hiroomii. A–C: tentacle; A, small basitrich; B, large basitrich; C, spirocyst. D–F: actinopharynx; D, small basitrich; E, large basitrich; F, spirocyst. G, microbasic p-mastigophore. H, basitrich in column. I–K: filament; I, small basitrich; J, large basitrich; K, microbasic p-mastigophore. L–U: H. fragum. L–N: tentacle; L, small basitrich; M, large basitrich; N, spirocyst. O–Q: actinopharynx; O, basitrich; P, spirocyst; Q, microbasic p-mastigophore. R, basitrich in column. S–U: filament; S, basitrich; T, spirocyst; U, microbasic p-mastigophore.
Figure 10.
Cnidoms of Halcurias hiroomii sp. nov. and H. fragum sp. nov. A–K: H. hiroomii. A–C: tentacle; A, small basitrich; B, large basitrich; C, spirocyst. D–F: actinopharynx; D, small basitrich; E, large basitrich; F, spirocyst. G, microbasic p-mastigophore. H, basitrich in column. I–K: filament; I, small basitrich; J, large basitrich; K, microbasic p-mastigophore. L–U: H. fragum. L–N: tentacle; L, small basitrich; M, large basitrich; N, spirocyst. O–Q: actinopharynx; O, basitrich; P, spirocyst; Q, microbasic p-mastigophore. R, basitrich in column. S–U: filament; S, basitrich; T, spirocyst; U, microbasic p-mastigophore.
Remarks. See
Table 9 for comparison to the other species of Halcuriidae. Uchida (2004) [
11] described this species as
Halcurias japonicus. However, this species name is nomen nudum as Uchida’s description in 2004 did not designate any type specimens. Even though Uchida indicated a specimen as the type belatedly in Uchida (2007), there is no description together, and the species name is not valid currently [
48]. In the present study, we collected halcuriids which almost corresponded to the description of Uchida (2004) [
11]. He only reported this species in the Japanese Sea off the Hokuriku region, but we recorded this species also from Kumano-nada Sea, the Pacific Ocean.
According to our phylogenetic tree (
Figure 4), this halcuriid species was in the clade of
Halcurias and lacks spirocysts in their column, and
H. hiroomii should therefore remain in this genus.
Table 9.
Comparison of Halcurias fragum sp. Nov. and the other species of Halcurias, including species possibly assignable to the genus.
Table 9.
Comparison of Halcurias fragum sp. Nov. and the other species of Halcurias, including species possibly assignable to the genus.
| | Halcurias fragum
sp. nov. | Halcurias hiroomii
sp. nov. | Halcurias pilatus
McMurrich, 1893 | * Halcurias capensis
Carlgren, 1928 | * Halcurias endocoelactis
Stephenson, 1918 | * Halcurias uchidai
Uchida, 2004 |
|---|
| Characters | | | | | | |
| Size (height) | 9–10 mm | 15–30 mm | 14–15 mm | 12–22 mm | 48 mm | 40 mm |
| Basitrichs of tentacles | 2 types | 1 type | 1 type | 1 type | 1 type | 1 type |
| Spirocysts in actinopharynx | Present | Present | Present | Absent | Absent | Present |
| Long basitrichs in filaments | Absent | Absent | Present | Unknown | Unknown | Absent |
| References | The present study | [11]
The present study | [43,44] | [11,46] | [11,36] | [11,45] |
Halcurias fragum sp. nov.
(New Japanese name: ichigo-kawari-ginchaku.)
urn:lsid:zoobank.org:act:F32F94B6-06FF-4A69-8862-861F38D79C6E
Material examined. Holotype. CMNH–ZG 10216: specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared, collected by scuba diving on 22 March 2018 at Mushizaki, Sado Island, Niigata Pref., 15 m in depth, by Akihito Omori. Paratypes. NSMT–Co 1828–1829: whole specimen, same date, collector, and method as for CMNH–ZG 10216. Other specimens; CMNH–ZG 10217: whole specimen; originally collected in January 2018 at Uragami, Wakayama Prefecture, at 100–130 m, by Isao Hirabayashi, and fixed by Takuma Fujii and Kensuke Yanagi from the tank at the Kushimoto Marine Park on 22 May 2018.
Description. External anatomy. Body cylindrical (
Figure 11A,B), up to ca. 10 mm in height and ca. 5 mm in width in a living specimen and 9 mm in height and 4 mm in width in a preserved specimen. Column surface comparatively smooth, reddish-orange ectoderm layer and sometimes patchy pattern with dark red and light orange, and with white tenaculi-like nematocyst batteries. Nematocysts densely distributed, especially in nematocyst batteries. Upper part of column a little narrower, white in color, simple and not thrown into lobes. Tentacles simple, all marginal, 3–6 mm in length, no thickenings including their aboral base, bright red to orange in color and pale white in roots, and 68 in number on oral disk; inner and outer ones alternatingly bared (
Figure 11C). The tip of tentacles pointed. Basal disk in aboral end, opaque and mesenterial insertion invisible. Oral disk diameter broader than column, hemi-transparent, pale white. Mouth at center of oral disk, highly swelled, lip-like, groove on surface, bright orange (
Figure 11A).
Internal anatomy. Twenty (ten pairs of) macrocnemes on actinopharynx: twelve, including four directives, in the first cycle and eight in the second cycle (
Figure 11G,H). Macrocnemes in the second cycle born in the endocoel of the first cycle mesenteries, an arrangement obeying the rule of mesenterial arrangement of Actinernidae. Mesenteries in third and fourth cycle microcnemes. Each tentacle between either exo- or endocoelic. Tentacular longitudinal muscle exocoeletic (
Figure 11F) and circular muscle too weak to observe in histological section (
Figure 11F); retractor muscles restricted near actinopharynx or filaments, obviously developed (
Figure 11D). Muscle processes simple or slightly branched, around 15–25 in each muscle pennon (
Figure 11D). Parietal muscles of macrocnemes comparatively developed and distinct (
Figure 11D). Parietal muscle processes are simple, four to seven on each side. Mesoglea thickest in body wall and actinopharynx, generally thicker than ectoderm and endoderm (
Figure 11G). Mesoglea thinner than the other parts, especially in the tentacle: far thinner than ectoderm (
Figure 11E,F). Actinopharynx, with siphonoglyphs on one side, always connected to actinopharynx, and the other 11 longitudinal grooves are less deep than siphonoglyphs (
Figure 11G). Sphincter muscle absent. On the aboral end, basilar muscle absent. Dioecious, immature testis in our specimen. Mesenteries in second cycle fertile.
Cnidom. Basitrichs, spirocysts, and microbasic
p-mastigophores. Spirocysts are absent in the column (
Figure 10,
Table 8).
Etymology. Fragum means strawberry in Latin. This name is derived from the nematocyst batteries on the red surface of columns of this species, which look like the seeds of strawberries.
Remarks. See
Table 9 for comparison to the other species of
Halcurias. This species can be easily distinguished from any other species of
Halcurias as they possess two types of basitrichs in their tentacles.
This halcuriid most resembles
H. pilatus McMurrich, 1893, in external morphology, but there are no long basitrichs in the filaments of this species. The long basitrichs strongly characterize
H. pilatus [
44]. Moreover, phylogenetic analyses indicated that this halcuriid was monophyletic with
Halcurias hiroomii sp. nov., and this anemone and
H. pilatus were paraphyletic. Thus, we determined this sea anemone is not
H. pilatus but a new species described here.
Genus Synactinernus Carlgren, 1918.
(New Japanese name: Kuroba-kawari-ginchaku-zoku.)
Synactinernus Carlgren, 1918: 30; Carlgren, 1949: 21.
Diagnosis (revised parts are shown in bold). Actinernidae with a cylindrical body which distally is drawn out into eight distinct lobes; all eight are the same size or four larger and four smaller alternating. Column without papillae. No sphincter. Tentacles in at least two cycles without distinct mesogleal thickenings, all same length or largest at apices of the lobes, numerous. Longitudinal muscles of tentacles ectodermal, radial muscles of oral disc chiefly ectodermal, strong. Two broad siphonoglyphs on actinopharynx. In total, 36 macrocnemes cyclic in arrangement, beyond them weak mesenteries of unequal size in the upper part of the body. Retractors weak, parietal muscles weak or rather well developed.
Type species. Synactinernus flavus Carlgren, 1918.
Figure 11.
External and internal morphology of Halcurias fragum sp. nov. (CMNH–ZG 10206). (A) Lateral view of a living specimen; (B) lateral view of a fixed specimen; (C) oral view of a fixed specimen; (D) enlarged transverse section of upper column; (E) enlarged longitudinal section of tentacle; (F) enlarged transverse section of tentacle; (G) enlarged transverse section of upper column; (H) enlarged transverse section of lower column. Abbreviations: a, actinopharynx; fi, filament; ma, macrocneme; me, mesoglea; oo, oocyte; pa, parietal muscle; rm, retractor muscle; s, siphonoglyph; te, tentacle. Scale bars indicate 1 cm in (A–C), 1 mm in (G,H), 500 µm in (D), and 100 µm in (E,F). Picture A was taken by Akihito Omori.
Figure 11.
External and internal morphology of Halcurias fragum sp. nov. (CMNH–ZG 10206). (A) Lateral view of a living specimen; (B) lateral view of a fixed specimen; (C) oral view of a fixed specimen; (D) enlarged transverse section of upper column; (E) enlarged longitudinal section of tentacle; (F) enlarged transverse section of tentacle; (G) enlarged transverse section of upper column; (H) enlarged transverse section of lower column. Abbreviations: a, actinopharynx; fi, filament; ma, macrocneme; me, mesoglea; oo, oocyte; pa, parietal muscle; rm, retractor muscle; s, siphonoglyph; te, tentacle. Scale bars indicate 1 cm in (A–C), 1 mm in (G,H), 500 µm in (D), and 100 µm in (E,F). Picture A was taken by Akihito Omori.
Remarks. The genus
Synactinernus was established in the family Actinernidae by Carlgren (1918) [
4] monotypically for
Synactinernus flavus Carlgren, 1918. Later, Carlgren (1949) [
7] transferred this genus to Family Actinernidae Stephenson, 1922, of suborder Endocoelantheae Carlgren, 1925. Recently, Fautin and den Hartog (2003) [
10] argued that
Synactinernus flavus, the only species of
Synactinernus, cannot be distinguished from
Isactinernus quadrilobatus Carlgren, 1918, and so, they synonymized
Synactinernus with
Isactinernus. However, other studies treated this genus as valid [
1]. Thus, the actual status of
S. flavus and the validity of
Synactinernus has remained unclear.
One century after the original description of Carlgren, Izumi et al. (2019) [
14] newly collected specimens of two
Synactinernus species and
Isactinernus quadrilobatus. According to their morphological and phylogenetic analyses, it was suggested that the
Synactinernus species can be distinguished from
Isactinernus quadrilobatus both by morphology and phylogeny—
Synactinernus species lack the thickening on the edges of their oral disc that
Isactinernus have, and they only have 36 macrocnemes, while
Isactinernus have over 100;
S.
flavus and
S.
churaumi formed a clade separate from
I. quadrilobatus [
14]. Thus, they redescribed
Synactinernus as a valid genus of the family Actinernidae and revised the diagnosis to accommodate
S. churaumi Izumi and Fujii, 2019.
However, the molecular phylogeny of the present study showed that the genus
Synactinernus is an independent clade from the other three genera of Actinernidae (
Figure 3). Moreover, two Halcuriidae clades were paraphyletic to
Synactinernus, so this genus should not be accommodated in Actinernidae but should be in Halcuriidae.
This genus is endemic in Japan and is distributed at around 300 m in depth in the East China Sea and the Pacific Ocean.
Synactinernus flavus Carlgren, 1918.
(Japanese name: Kuroba-kawari-ginchaku.)
Figures 2, 3, 5, and 6 in Izumi et al. (2019).
Synactinernus flavus Carlgren, 1918: 31.
Synactinernus flavus: Izumi et al., 2019b: 531–534, Figures 2, 3, 5, and 6.
Material examined/Description. See Izumi et al. (2019) [
14].
Synactinernus churaumi Izumi and Fujii, 2019.
(Japanese name: Churaumi-kawari-ginchaku.)
Figures 2, 5, and 7 in Izumi et al. (2019).
Synactinernus churaumi Izumi and Fujii, 2019: Izumi et al., 2019b: 534–537; Figures 2, 5, and 7.
Material examined/description. See Izumi et al. (2019) [
14].
Genus Isohalcurias gen. nov.
(New Japanese name: kawari-ginntyaku-modoki-zoku.)
urn:lsid:zoobank.org:act:E16FF13C-6711-4822-9F54-0803733AC956
Diagnosis. Mesenteries up to 34 pairs arranged in 4 cycles, 6 + 4 + 8 + 16. Macrocnemes, 10 pairs, fertile, filamented, and with restricted retractors. Microcnemes in only the upper part of the body, some of them perfect. Retractor muscles diffused and pinnate. Parietal muscles rather well developed to fairly weak. Tentacles, up to 68. Dioecious. Cnidom: spirocysts and basitrichs (in all tissues) and microbasic p-mastigophores (in actinopharynx and filaments).
Type species. Isohalcurias carlgreni (McMurrich, 1901).
Etymology. Isohalcurias is derived from “Iso (=Greek isos, equal)” + “halcurias (the divided genus)”. Isohalcurias species resemble species of Halcurias so much that they cannot be distinguished unless making sections or inspecting cnidae.
Remarks. According to our phylogenetic analyses (
Figure 9 and
Figure 10), the species which were originally identified as genus
Halcurias were paraphyletic. The outer clade, which contained
H. carlgreni, H. citreum sp. nov., and
H. malum sp. nov., did not contain the type species,
Halcurias pilatus. Thus, this group cannot be named
Halcurias. This is why we established the new genus
Isohalcurias gen. nov. for these three species.
See
Table 7 for a comparison with the other genera of Halcuriidae. This genus can be identified by having spirocysts in the column (comparison of
Isohalcurias gen. nov. in
Table 8 and
Table 10) and partly pinnate retractor muscles (e.g.,
Figure 12D, 14D, and 15C). In addition to the three species,
Halcurias minimus Carlgren, 1928, and
Halcurias sudanensis Riemann-Zürneck, 1983, are possibly in this new genus as these species have spirocysts in the body wall [
11,
46,
47]. It is hoped to obtain molecular information on these species for phylogenetic analyses in future.
Isohalcurias carlgreni (McMurrich, 1901) comb. nov.
(Japanese name: kawari-ginchaku.)
urn:lsid:zoobank.org:act:71D07E54-D6C4-4888-8FD5-4A3F5301CE7A
Halcurias Carlgreni McMurrich 1901: 159.
Halcurias carlgreni: Carlgren, 1914: 69; Uchida 2004: 9–13, Figure 1, pl. 1A, B.
Material examined. NSMT–Co 1697: specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared, collected on 19 September 2011, collected from Suo-Oshima, Yamaguchi Pref., Japan, at 10 m in depth, by scuba diving, by Takuma Fujii; NSMT–Co 1698: specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared, same collector, locality, and method as for NSMT–Co 1697; NSMT–Co 1830: whole specimen, same collector, locality, and method as for NSMT–Co 1697; CMNH–ZG 10218: specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared; collected by trawling of fishing boat Jinsho-Maru from Kumano-nada Sea off Mie Pref. (depth and date unknown), by Takeya Moritaki, kept in Toba Aquarium for a while, and preserved on 18 June 2017 by Takato Izumi; CMNH–ZG 10219: juvenile specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared, collected on 19 September 2015, collected from west-off Uki-shima, Chiba Pref., Japan, at 20 m in depth, by scuba diving, by Junji Okuno; CMNH–ZG 10220: whole specimen, same collector, locality, and method as for CMNH–ZG 10219; UUZM 705b, 705c (syntypes): on 14 March 1890, Hirado Strait (33°5′ N, 129°16 E); UUZM 705a (syntype): in 1893, off Hirado (33°15 N, 129°15 E), 45 fathoms (ca. 82 m) depth; UUZM 705d (syntype;
Figure 7E): Korea Strait 65 fathoms (ca. 120 m) depth; ZMNH–TYPE–4908 (syntype): in 1893, China sea, 30 fathoms (ca 55 m) depth; ZMNH–TYPE–1200 (syntype;
Figure 7F): in 1893, off Hirado (33°15 N, 129°15 E), 45 fathoms (ca, 82 m) depth.
Description. External anatomy. Body cylindrical (
Figure 12A,B), up to 17–70 mm in height and ca. 11–50 mm in width. Column surface comparatively smooth, with reddish-orange ectoderm layer and sparse small nematocyst batteries. Nematocysts densely distributed, especially in nematocyst batteries. Tentacles simple, all marginal, 6–40 mm in length, no thickenings including their aboral base, pale white in color, and 68 in number on oral disk; inner and outer ones alternatingly bared (
Figure 12A). The tip of tentacles pointed. Basal disc distinct and adhesive (
Figure 12A), opaque, and mesenterial insertion invisible. Oral disk diameter as broad as column, hemi-transparent, pale white. Mouth at center of oral disk, highly swelled, lip-like, groove on surface, white in color.
Figure 12.
External and internal morphology of Isohalcurias carlgreni comb. nov. (NSMT–Co 1697). (A) Living individuals in nature; (B) lateral view of a fixed specimen; (C) transverse section of upper column; (D) enlarged transverse section of upper column; (E) enlarged transverse section of lower column. Abbreviations: a, actinopharynx; fi, filament; ma, macrocneme; me, mesoglea; pa, parietal muscle; rm, retractor muscle; s, siphonoglyph; te, tentacle; ts, testis. Scale bars indicate 5 cm in (A), 1 cm in (B,C), 1 mm in (D), and 500 µm in (E). Picture A was taken by Takuma Fujii.
Figure 12.
External and internal morphology of Isohalcurias carlgreni comb. nov. (NSMT–Co 1697). (A) Living individuals in nature; (B) lateral view of a fixed specimen; (C) transverse section of upper column; (D) enlarged transverse section of upper column; (E) enlarged transverse section of lower column. Abbreviations: a, actinopharynx; fi, filament; ma, macrocneme; me, mesoglea; pa, parietal muscle; rm, retractor muscle; s, siphonoglyph; te, tentacle; ts, testis. Scale bars indicate 5 cm in (A), 1 cm in (B,C), 1 mm in (D), and 500 µm in (E). Picture A was taken by Takuma Fujii.
Internal anatomy. Twenty (ten pairs of) macrocnemes on actinopharynx: twelve, including four directives, in the first cycle and eight in the second cycle (
Figure 12C). Macrocnemes in the second cycle born in the endocoel of the first cycle mesenteries, an arrangement obeying the rule of mesenterial arrangement of Actinernidae. Mesenteries in third and fourth cycle microcnemes. Each tentacle between either exo- or endocoelic. Tentacular longitudinal muscle exocoeletic and circular muscle endocoeletic; retractor muscles restricted near parietal muscles, obviously developed and pinnate (
Figure 12D). Muscle processes well-branched, around 8–15 in each muscle pennon (
Figure 12D). Parietal muscles of macrocnemes comparatively developed and distinct with simple or slightly branched 4–11 processes in each side. Mesoglea thickest in body wall and actinopharynx, generally thicker than ectoderm and endoderm (
Figure 12D). Mesoglea thinner than the other parts, especially in the tentacle: far thinner than ectoderm. Actinopharynx, with siphonoglyphs on one side, always connected to actinopharynx, and the other 15–19 longitudinal grooves are less deep than siphonoglyphs (
Figure 12C). Sphincter muscle absent. On the aboral end, basilar muscle absent. Dioecious, immature testis in our specimen (
Figure 12E). Mesenteries in second cycle fertile.
Cnidom. Basitrichs, spirocysts, and microbasic
p-mastigophores. Spirocysts are numerous in the column (
Figure 13,
Table 10).
Figure 13.
Cnidoms of Isohalcurias carlgreni comb. nov., I. citreum sp. nov., and I. malum sp. nov. A–N: I. carlgreni. A–B: tentacle; A, basitrich; B, spirocyst. C–G: actinopharynx; C, small basitrich; D, large basitrich; E, spirocyst; F, microbasic p-mastigophore; G, microbasic b-mastigophore. H–J: column; H, small basitrich; I, large basitrich; J, spirocyst. K–N: filament; K, small basitrich; L, large basitrich; M, microbasic p-mastigophore; N, microbasic b-mastigophore. O–a: I. carlgreni. O–Q: tentacle; O, small basitrich; P, large basitrich; Q, spirocyst. R–U: actinopharynx; R, basitrich; S, spirocyst; T, microbasic p-mastigophore; U, microbasic b-mastigophore. V–W: column; V, basitrich; W, spirocyst. X–a: filament; X, small basitrich; Y, large basitrich; Z, spirocyst; a, microbasic p-mastigophore. b–o: I. malum. b–e: tentacle; b, small basitrich; c, small basitrich; d, spirocyst; e, microbasic b-mastigophore. f–i: actinopharynx; f, basitrich; g, spirocyst; h, microbasic p-mastigophore; i, microbasic b-mastigophore. j–k: column; j, basitrich; k, spirocyst. l–o: filament; l, small basitrich; m, large basitrich; n, microbasic p-mastigophore; o, microbasic p-mastigophore.
Figure 13.
Cnidoms of Isohalcurias carlgreni comb. nov., I. citreum sp. nov., and I. malum sp. nov. A–N: I. carlgreni. A–B: tentacle; A, basitrich; B, spirocyst. C–G: actinopharynx; C, small basitrich; D, large basitrich; E, spirocyst; F, microbasic p-mastigophore; G, microbasic b-mastigophore. H–J: column; H, small basitrich; I, large basitrich; J, spirocyst. K–N: filament; K, small basitrich; L, large basitrich; M, microbasic p-mastigophore; N, microbasic b-mastigophore. O–a: I. carlgreni. O–Q: tentacle; O, small basitrich; P, large basitrich; Q, spirocyst. R–U: actinopharynx; R, basitrich; S, spirocyst; T, microbasic p-mastigophore; U, microbasic b-mastigophore. V–W: column; V, basitrich; W, spirocyst. X–a: filament; X, small basitrich; Y, large basitrich; Z, spirocyst; a, microbasic p-mastigophore. b–o: I. malum. b–e: tentacle; b, small basitrich; c, small basitrich; d, spirocyst; e, microbasic b-mastigophore. f–i: actinopharynx; f, basitrich; g, spirocyst; h, microbasic p-mastigophore; i, microbasic b-mastigophore. j–k: column; j, basitrich; k, spirocyst. l–o: filament; l, small basitrich; m, large basitrich; n, microbasic p-mastigophore; o, microbasic p-mastigophore.
![Diversity 15 00773 g013]()
Table 10.
Cnidoms of the species of Isohalcurias gen. nov.
Table 10.
Cnidoms of the species of Isohalcurias gen. nov.
| | Isohalcurias carlgreni comb. nov. | Isohalcurias citreum sp. nov. | Isohalcurias malum sp. nov. |
|---|
| | NSMT–Co 1697 | CMNH–ZG 10221 | NSMT–Co 1699 |
|---|
| | Length × Width (µm) | frequency | N | Length × Width (µm) | frequency | N | Length × Width (µm) | frequency | N |
| Tentacle |
| basitrichs | | 13.1–38.4 × 2.4–7.8 | numerous | 30 | 27.0–34.8 × 2.6–4.1 | numerous | 61 | 15.8–19.9 × 3.0–3.5 | rare | 3 |
| 47.4 × 5.2 | rare | 1 | 28.9–39.1 × 2.7–4.4 | numerous | 37 |
| spirocysts | | 20.6–26.3 × 2.6–3.7 | numerous | 101 | 22.5–42.9 × 2.6–5.9 | numerous | 43 | 21.8–41.2 × 4.0–7.3 | numerous | 25 |
| microbasic b-mastigophores | | | | | | | | 30.1–36.3 × 3.4–6.0 | numerous | 11 |
| Actinopharynx |
| basitrichs | S | 8.4–12.9 × 1.5–2.4 | numerous | 19 | 22.6–30.8 × 3.0–4.3 | numerous | 62 | 25.7–36.2 × 3.2–4.5 | numerous | 37 |
| | L | 20.6–27.8 × 2.7–3.9 | numerous | 13 |
| spirocysts | | 21.0–25.5 × 4.3–5.9 | few | 5 | 22.6–33.9 × 4.1–7.9 | numerous | 51 | 31.5–52.0 × 5.8–9.6 | numerous | 64 |
| microbasic p-mastigophores | | 19.3–23.7 × 4.5–6.3 | numerous | 13 | 23.4–29.2 × 5.2–7.0 | numerous | 39 | 29.0–35.2 × 5.3–7.2 | numerous | 16 |
| microbasic b-mastigophores | | 20.6–23.9 × 5.4–6.4 | rare | 3 | 25.6–28.6 × 4.3–5.4 | rare | 4 | 30.1–37.1 × 5.6–7.4 | few | 6 |
| Column |
| basitrichs | S | 7.3–11.3 × 1.5–2.6 | numerous | 23 | 23.6–34.1 × 2.3–3.5 | numerous | 24 | 23.8–34.2 × 2.7–4.1 | numerous | 37 |
| | L | 23.4–31.6 × 2.2–3.8 | numerous | 57 |
| spirocysts | | 15.7–27.5 × 3.9–5.2 | numerous | 13 | 24.7–32.3 × 4.2–7.0 | numerous | 69 | 27.8–37.1 × 4.2–6.1 | numerous | 27 |
| Filament |
| basitrichs | S | 7.3–11.9 × 1.5–2.8 | numerous | 38 | 7.0–12.5 × 1.5–2.8 | numerous | 47 | 9.9–19.8 × 1.7–4.4 | numerous | 48 |
| | L | 24.5–25.3 × 3.9–4.2 | rare | 2 | 25.9–30.1 × 2.7–4.2 | numerous | 24 | 26.7–28.0 × 3.8–5.0 | rare | 2 |
| spirocysts | | | | | 7.5–31.4 × 1.6–6.9 | numerous | 71 | 28.3–33.3 × 5.4–7.1 | rare | 4 |
| microbasic p-mastigophores | | 14.4–26.3 × 5.0–8.4 | numerous | 41 | 23.2–27.7 × 5.4–7.6 | numerous | 28 | 25.2–34.8 × 5.6–9.2 | numerous | 52 |
| microbasic b-mastigophores | | 23.6–25.3 × 6.2–7.8 | rare | 3 | | | | | | |
Remarks. This species was described as
Halcurias carlgreni in McMurrich (1901) [
49] and reported from Japan in 2004 [
11].
H. carlgreni was characterized by the ectoderm of the column containing numerous spirocysts [
11]. Our specimens corresponded well with these descriptions, so we identified them as
H. carlgreni. However, as mentioned above,
Halcurias became paraphyletic to
Synactinernus. The clade, including
Halcurias carlgreni, H. citreum sp. nov., and
H. malum sp. nov., did not contain
Halcurias pilatus, the type species (
Figure 4). Thus, these species should be moved to the new genus
Isohalcurias, and thus,
H. carlgreni becomes
Isohalcurias carlgreni comb. nov.
Isohalcurias citreum sp. nov.
(Japanese name: oo-kawari-ginchaku.)
urn:lsid:zoobank.org:act:AB02934A-BFDF-400B-8432-E33092D96DE9
Halcurias levis (nomen nudum) Uchida, 2004: 16, Figures 3 and 4, pl. 1F.
Material examined. Holotype. NSMT–Co 1831: specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared, collected on 28 January 2017 from Kuju-kushima (off Kuroshima), Nagasaki Pref., 35 m in depth, bycatch with fishes by gill net, kept in the tank of Saikai National Park Kuju-kushima Aquarium, and preserved on 14 April 2017 by Takato Izumi. Paratype. CMNH 10221: specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared, collected on 7 October 2008 from Aishima Island, Hagi, Yamaguchi Pref., 47 m in depth, collected by scuba diving by Kensuke Yanagi. Other specimen. CMNH–ZG10222: specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared, collected on 17 May 2018 off Yaku-shima Island (30°09.39′ N, 130°38.03′ E; St. 3) at 200–255 m in depth, using beam trawl, during research cruise of R/V Toyoshio-Maru, by Itaru Kobayashi.
Description. External anatomy. Body cylindrical (
Figure 14A,B), up to ca. 150 mm in height and ca. 100 mm in width in a living specimen and 34–90 mm in height and 20–35 mm in width in a preserved specimen. Column surface smooth and without nematocysts batteries. Whole body bright yellow in color. Nematocysts sparsely distributed on column. Upper part of column narrower, same color as column, simple and not thrown into any lobes. Tentacles simple, all marginal, 30–50 mm in length, no thickenings including their aboral base, bright yellow in color, and 68 in number; inner and outer ones alternatingly bared (
Figure 14A). The tips of tentacles pointed. Basal disk in aboral end (
Figure 14A), yellowish opaque and mesenterial insertion inapparently visible. Oral disk diameter narrower than column, hemi-transparent, bright yellow. Mouth at center of oral disk, highly swelled, lip-like, groove on surface, bright yellow.
Internal anatomy. Twenty (ten pairs of) macrocnemes on actinopharynx: twelve, including four directives, in the first cycle and eight in the second cycle (
Figure 14C). Macrocnemes in the second cycle born in the endocoel of the first cycle mesenteries, an arrangement obeying the rule of mesenterial arrangement of Actinernidae. Mesenteries in third and fourth cycle microcnemes. Each tentacle between either exo- or endocoelic. Tentacular longitudinal muscle and circular muscle too weak to observe in histological section (
Figure 14E); retractor muscles diffused, weak, and pinnate (
Figure 14D,F). Muscle processes well branched, around 9–17 in each muscle pennon (
Figure 14D). Parietal muscles of macrocnemes well developed and distinct. Parietal muscle weak, with simple or slightly branched processes, around 10 in each side (
Figure 14D,F). Mesoglea thickest in body wall, and even thick in mesenteries and actinopharynx, far thicker than ectoderm and endoderm (
Figure 14C,D). Actinopharynx, with siphonoglyphs on one side, always connected to actinopharynx, and the other 19 longitudinal grooves are as deep as siphonoglyphs (
Figure 14C). Sphincter muscle absent. On the aboral end, basilar muscle absent. Dioecious, matured ovaries in NSMT–Co 1831 (
Figure 14F). Mesenteries in second to third cycle fertile.
Cnidom. Basitrichs, spirocysts, and microbasic
p-mastigophores. Spirocysts are numerou in the column (
Figure 13,
Table 10).
Etymology. Citreum means lemon in Latin. This name is derived from the fluorescent lemon-yellow color of this species.
Remarks. Uchida (2004) [
11] described this species as
Halcurias levis. However, this species name became nomen nudum for the same reason as
H. japonicus. The specimens we collected correspond well to the description of Uchida [
11] morphologically, so we report them as this species provisionally.
Figure 14.
External and internal morphology of Isohalcurias citreum sp. nov. (NSMT–Co 1831). (A) Lateral view of a living specimen; (B) lateral view of a fixed specimen; (C) transverse section of dissected specimen; (D) enlarged transverse section of upper column; (E) enlarged longitudinal section of lower column. (F) enlarged transverse section of lower column; Abbreviations: a, actinopharynx; fi, filament; ma, macrocneme; me, mesoglea; ov, ovary; pa, parietal muscle; rm, retractor muscle; s, siphonoglyph; te, tentacle. Scale bars indicate 1 cm in (A–C) and 1 mm in (D,E).
Figure 14.
External and internal morphology of Isohalcurias citreum sp. nov. (NSMT–Co 1831). (A) Lateral view of a living specimen; (B) lateral view of a fixed specimen; (C) transverse section of dissected specimen; (D) enlarged transverse section of upper column; (E) enlarged longitudinal section of lower column. (F) enlarged transverse section of lower column; Abbreviations: a, actinopharynx; fi, filament; ma, macrocneme; me, mesoglea; ov, ovary; pa, parietal muscle; rm, retractor muscle; s, siphonoglyph; te, tentacle. Scale bars indicate 1 cm in (A–C) and 1 mm in (D,E).
This species was accommodated in the clade of
Isohalcurias but cannot be obviously separated from
I. carlgreni comb. nov. in the phylogenetic tree (
Figure 10). However, there were several differences between these halcuriids, e.g., body sizes, presence or absence of nematocyst batteries, development of retractor muscles, and so forth (see
Table 11). Judging by the common view on phylogenetic analyses of sea anemones with the combination of several molecular markers [
13,
50,
51], it is probable that any simple DNA marker phylogenetic analysis method of the present day cannot distinguish
I. carlgreni and
I.
citreum sp. nov. in a phylogenetic tree. In conclusion, this study described this species as
Isohalcurias citreum sp. nov. by morphology.
This species had been discovered only in Syoga-se, off Wakayama Pref. [
11,
52,
53], but we newly confirmed it in the Sea of Japan (CMNH–ZG 10221), Goto-Nada Sea (NSMT–Co 1831), and the East China Sea (CMNH–ZG 10222).
Isohalcurias malum sp. nov.
(New Japanese name: ringo-kawari-ginchaku.)
urn:lsid:zoobank.org:act:6E2B09F5-3C94-4BAD-B690-E12C13E9711B
Material examined. Holotype. CMNH–ZG 10223: specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared, originally collected on 15 May 2005 from Ryukyu Trough off Kume-jima Island (25°30.96′ N, 126°29.21′ E; St. OT-14), at 372–375 m in depth, using beam trawl, during research cruise of R/V Hakuho-Maru by Kensuke Yanagi. Paratypes. NSMT–Co 1699: specimen dissected, tissues embedded in paraffin, histological sections prepared, nematocysts prepared, originally collected n 24 May 2015, west off Cape Sata (31°02.06′ N, 130°33.37′ E; St. 7), at 202 m in depth, using biological dredge, during research cruise of R/V Toyoshio-Maru by Mikihito Arai and Akito Ogawa; CMNH–ZG 10224: specimen dissected, tissues embedded in paraffin, nematocysts prepared, collected on 23 May 2019, west off Amami-Oshima Island (28°22.42′ N, 129°15.14′ E), at 315 m in depth, using beam trawl, during research cruise of R/V Toyoshio-Maru by Itaru Kobayashi. Other specimen: CMNH–ZG 10225: whole specimen, originally collected on January 2018 at Uragami, Wakayama Prefecture, at 100–130 m by Isao Hirabayashi, and fixed by Takuma Fujii and Kensuke Yanagi from the tank at the Kushimoto Marine Park on 22 May 2018.
Description. External anatomy. Body cylindrical (
Figure 15A), up to ca. 50 mm in height and ca. 30 mm in width in a living specimen and 25–40 mm in height and 10–20 mm in width in a preserved specimen. Column surface smooth and without nematocysts batteries, pale red to pale orange ectoderm layer, and sometimes fine dark red or orange patches on the middle column. Aboral end of column pale yellow. Nematocysts sparsely distributed on column. Upper part of column a little expanded, same color as column, simple and not thrown into lobes. Tentacles simple, all marginal, 10–20 mm in length, no thickenings including their aboral base, bright red to orange in color (more blight color on distal side), and 68 in number on oral disk; inner and outer ones alternatingly bared. The tips of tentacles pointed. Basal disk in aboral end (
Figure 15A), yellowish opaque and mesenterial insertion inapparently visible. Oral disk diameter broader than column, hemi-transparent, pale white. Mouth at center of oral disk, highly swelled, lip-like, groove on surface, bright orange.
Internal anatomy. Twenty (ten pairs of) macrocnemes on actinopharynx: twelve, including four directives, in the first cycle and eight in the second cycle (
Figure 15B). Macrocnemes in the second cycle born in the endocoel of the first cycle mesenteries, an arrangement obeying the rule of mesenterial arrangement of Actinernidae. Mesenteries in third and fourth cycle microcnemes (
Figure 15B). Each tentacle between either exo- or endocoelic. Tentacular longitudinal muscle and circular muscle too weak to observe in histological section (
Figure 15D); retractor muscles diffused but obviously developed and pinnate (
Figure 15C,E). Muscle processes well branched, around 10–20 in each muscle pennon (
Figure 15C). Parietal muscles of macrocnemes well developed and distinct. Parietal muscle processes well branched, around 10 in each side (
Figure 15E). Mesoglea thickest in body wall and actinopharynx (
Figure 15B,E), far thicker than ectoderm and endoderm. Mesoglea a little thinner than the other parts, but generally thicker than ectoderm and endoderm (
Figure 15C,D). Actinopharynx, with siphonoglyphs on one side, always connected to actinopharynx, and the other 11 longitudinal grooves are as deep as siphonoglyphs (
Figure 15B). Sphincter muscle absent. On the aboral end, basilar muscle absent (
Figure 15G). Dioecious, matured ovaries in NSMT–Co 1699 (
Figure 15F). Mesenteries in second to third cycle fertile.
Cnidom. Basitrichs, spirocysts, microbasic
p-mastigophores, and microbasic
b-mastigophores. Spirocysts are numerous in the column (
Figure 13,
Table 10).
Etymology. Malum means apple in Latin. This name is derived from the red and yellow color pattern of this species, which resembles apples.
Remarks. See
Table 11 for a comparison with the other species of
Isohalcurias and some species possibly assignable to this genus; since they have spirocysts on their columns [
46,
47], these species of
Halcurias are possibly assignable to the genus
Isohalcurias. However, molecular phylogenetic analyses of them have not been carried out. Thus, new combinations were not proposed for these species in this study.
Figure 15.
External and internal morphology of Isohalcurias malum sp. nov. (NSMT–Co 1699). (A) Lateral view of a living specimen (photographed by Mikihito Arai); (B) transverse section of dissected specimen; (C) enlarged view of retractor muscle; (D) transverse section of tentacle; (E) enlarged transverse section of upper column; (F) enlarged transverse section of lower column; (G) longitudinal section of basal disc. Abbreviations: a, actinopharynx; bd, basal disc; ma, macrocneme; me, mesoglea; mi, microcneme; ov, ovary; pa, parietal muscle; rm, retractor muscle; s, siphonoglyph; te, tentacle. Scale bars indicate 1 cm in (A,B), 1 mm in (G), 500 µm in (C,E,F), and 200 µm in (D).
Figure 15.
External and internal morphology of Isohalcurias malum sp. nov. (NSMT–Co 1699). (A) Lateral view of a living specimen (photographed by Mikihito Arai); (B) transverse section of dissected specimen; (C) enlarged view of retractor muscle; (D) transverse section of tentacle; (E) enlarged transverse section of upper column; (F) enlarged transverse section of lower column; (G) longitudinal section of basal disc. Abbreviations: a, actinopharynx; bd, basal disc; ma, macrocneme; me, mesoglea; mi, microcneme; ov, ovary; pa, parietal muscle; rm, retractor muscle; s, siphonoglyph; te, tentacle. Scale bars indicate 1 cm in (A,B), 1 mm in (G), 500 µm in (C,E,F), and 200 µm in (D).
According to the Uchida’s taxonomic key [
11], these sea anemones are most similar to
Halcurias minimus Carlgren, 1928, because they have a column without longitudinal muscles, an actinopharynx containing large spirocysts, a column containing spirocysts (though no nematocyst batteries), and tentacles with two types of basitrichs. However, there are several differences between these anemones and
H. minimus: there are no nematocyst batteries on this species, while they are sparsely distributed on the column of
H. minimus [
11,
46]; the size of
H. minimus is 0.8 cm [
46], approximately one-fourth of the size of our specimens. Moreover,
H. minimus only inhabits the deep sea of the circum-antiboreal region, far distant from the localities of this anemone, so it is unlikely that the same species lives in Japan, especially in the seas of the temperate zone. Thus, we concluded that these anemones represent a new species,
Isohalcurias malum sp. nov.
Table 11.
Comparison of Isohalcurias malum sp. nov. and the other species of Isohalcurias gen. nov., including a species possibly assignable to the genus.
Table 11.
Comparison of Isohalcurias malum sp. nov. and the other species of Isohalcurias gen. nov., including a species possibly assignable to the genus.
| | Isohalcurias malum sp. nov. | Isohalcurias carlgreni
(McMurrich, 1901) comb. nov. | Isohalcurias citreum sp. nov. | * Halcurias minimus
Carlgren, 1928 | * Halcurias sudanensis
Riemann-Zürneck, 1983 |
|---|
| Characters | | | | | |
| Size (height) | 25–40 mm | 11–50 mm | 34–90 mm | 8 mm | 30 mm |
| Retractor muscle | Distinct
Pinnate | Distinct
Strongly pinnate | Weak
Strongly pinnate | Distinct
Pinnate | Weak
Slightly Pinnate |
| Parietal muscle | Distinct
with well-branched muscular processes | Weak
with simple muscular processes | Weak
with simple muscular processes | Weak
with simple muscular processes | Weak
with simple muscular processes |
| Basitrichs of tentacles | 2 types | 1 type | 2 types | 2 types | 1 type |
| Spirocysts in actinopharynx | Present | Present | Present | Absent | Present |
| Spirocysts in column | Present | Present | Present | Present (but sparse) | Present |
| Long basitrichs in filaments | Absent | Absent | Absent | Unknown | Absent |
| References | The present study | [11,49]
The present study | [11]
The present study | [11,46] | [47] |