Nutrient Supplementation to Arboreal Ants: Effects on Trophic Position, Thermal Tolerance, Community Structure and the Interaction with the Host-Tree
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Supplementation Experiment
2.3. Critical Thermal Maximum (CTmax)
2.4. Stable Isotope Analyses
2.5. Sampling of the Ant Fauna
2.6. Artificial Nests
2.7. Herbivory Measurements
2.8. Statistical Analyses
3. Results
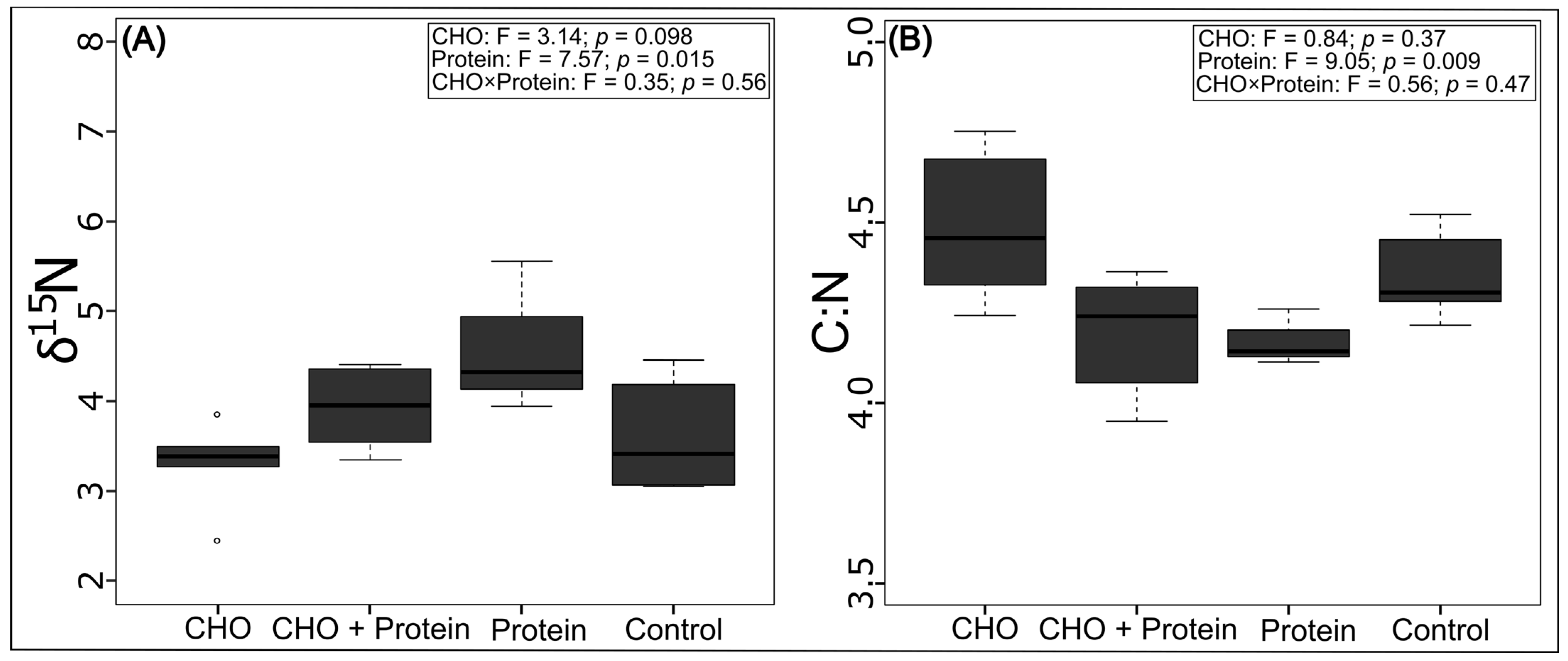
3.1. Effects on the Trophic Position and Thermal Tolerance of Azteca
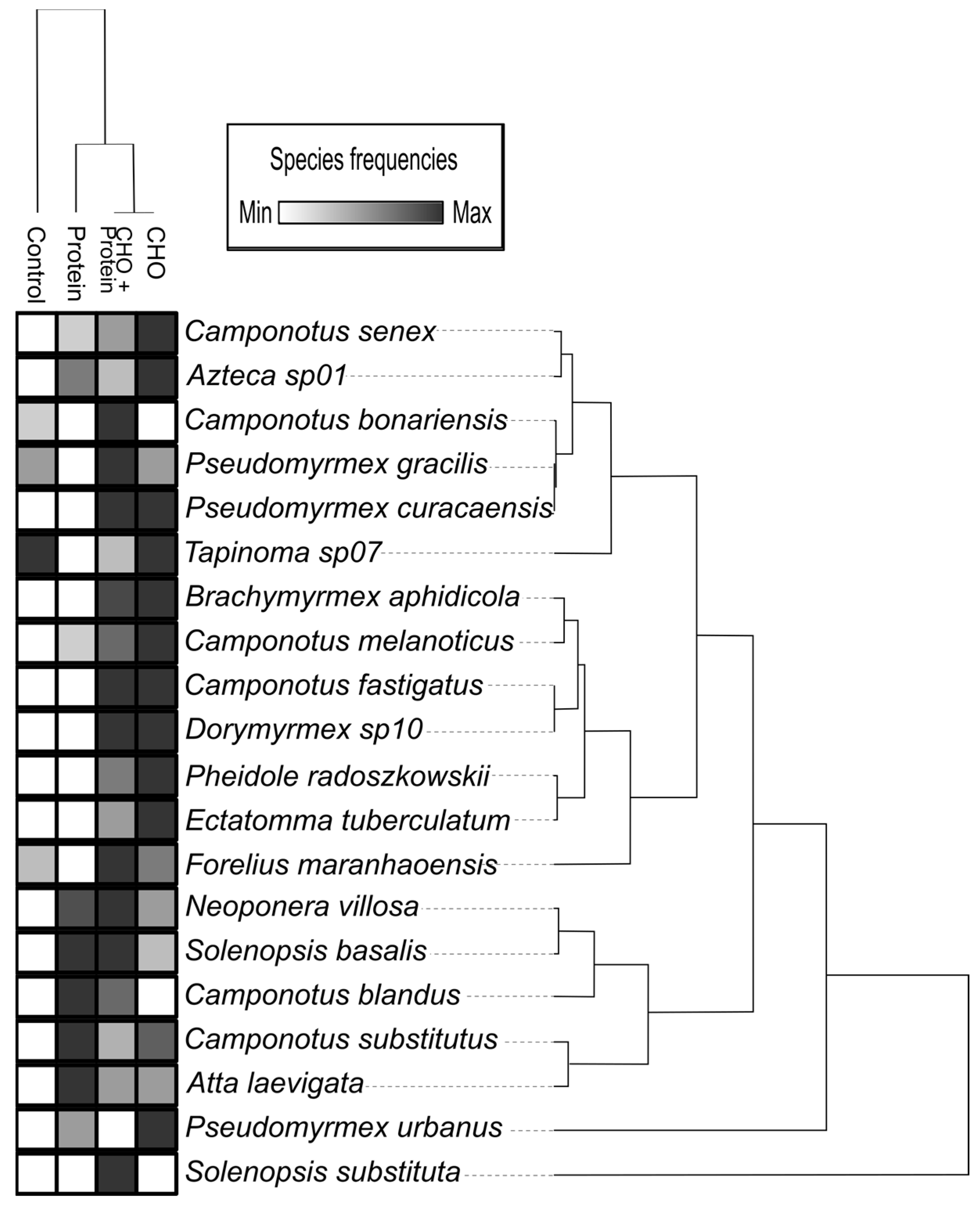
3.2. Effects on Overall Ant Abundance, Species Richness and Composition
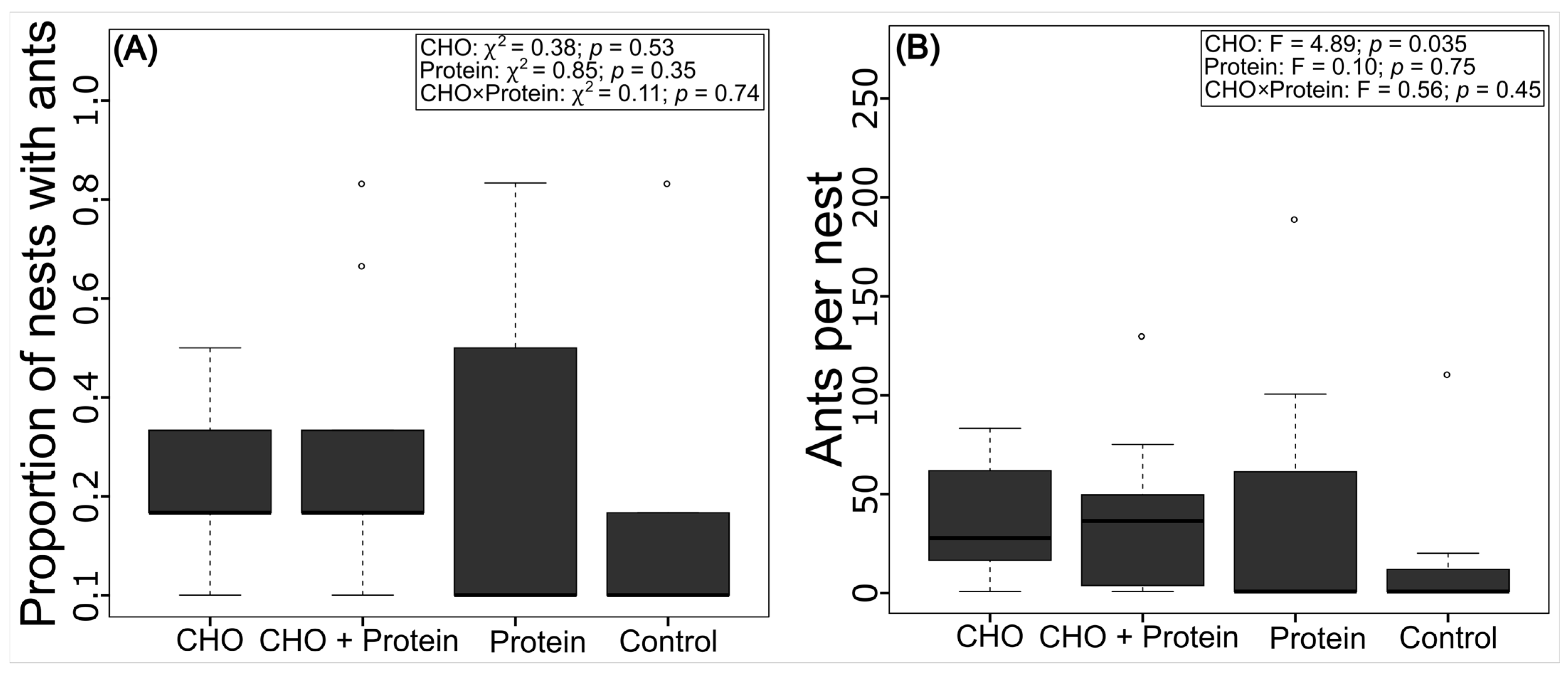
3.3. Colonization of the Artificial Nests
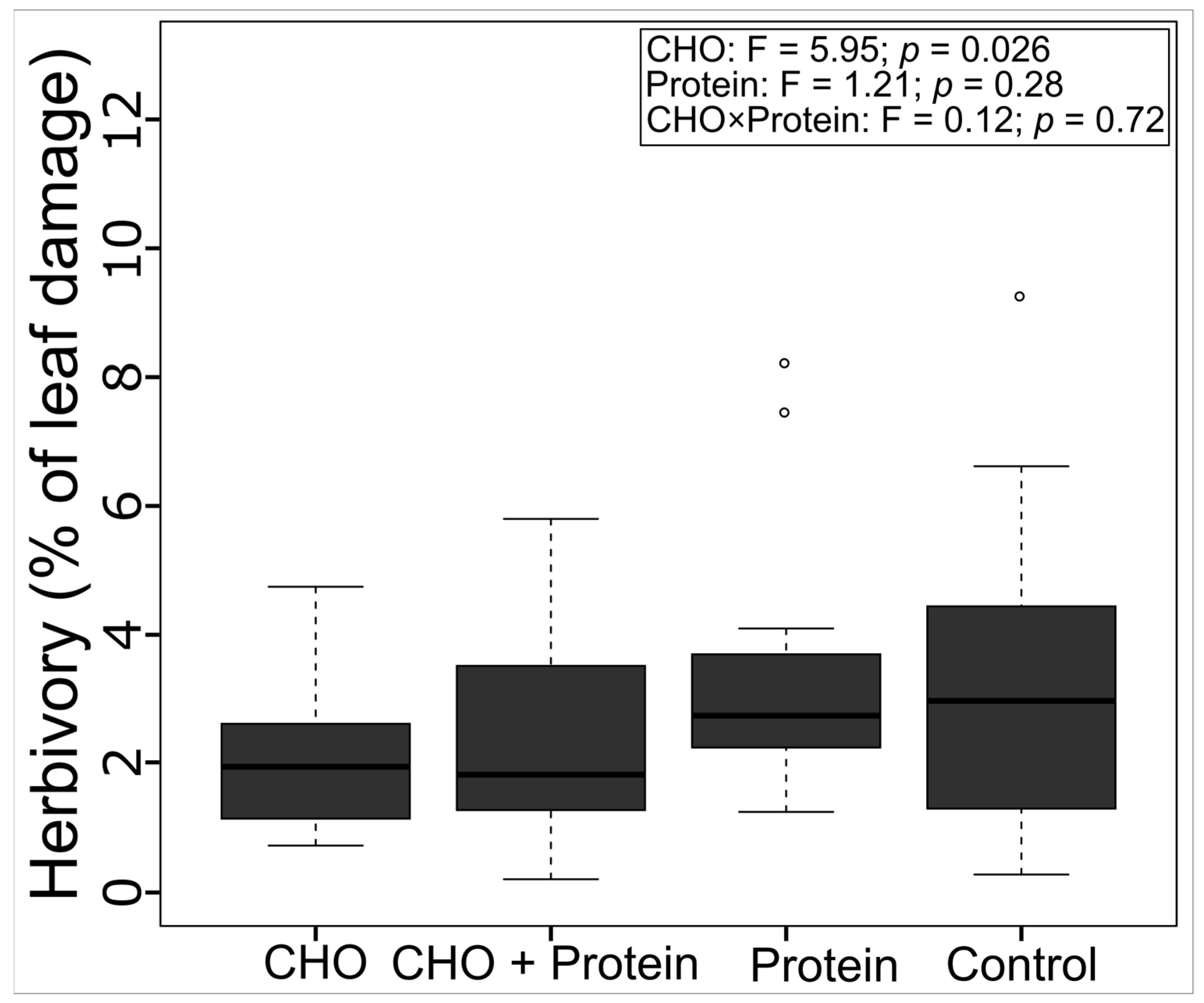
3.4. Leaf Herbivory
4. Discussion
4.1. Colony Level Effects
4.2. Community Level Effects
4.3. Effect on the Host Tree
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simpson, S.J.; Raubenheimer, D. The nature of nutrition: A unifying framework. Aust. J. Zool. 2012, 59, 350–368. [Google Scholar] [CrossRef] [Green Version]
- Simpson, S.J.; Raubenheimer, D. The hungry locust. Adv. Study Behav. 2000, 29, 1–44. [Google Scholar] [CrossRef]
- Feldhaar, H. Ant Nutritional Ecology: Linking the Nutritional Niche Plasticity on Individual and Colony-Level to Community Ecology. Curr. Opin. Insect Sci. 2014, 5, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Sterner, R. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, UK, 2002; pp. 262–369. [Google Scholar]
- Raubenheimer, D.; Simpson, S.J.; Mayntz, D. Nutrition, Ecology and Nutritional Ecology: Toward an Integrated Framework. Funct. Ecol. 2009, 23, 4–16. [Google Scholar] [CrossRef]
- Bujan, J.; Kaspari, M. Nutrition Modifies Critical Thermal Maximum of a Dominant Canopy Ant. J. Insect Physiol. 2017, 102, 1–6. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Simpson, S.J. Nutritional Ecology and Foraging Theory. Curr. Opin. Insect Sci. 2018, 27, 38–45. [Google Scholar] [CrossRef]
- Tilman, D.; Kilham, S.S.; Kilham, P. Phytoplankton Community Ecology: The Role of Limiting Nutrients. Annu. Rev. Ecol. Syst. 1982, 13, 349–372. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. A multi-level analysis of feeding behaviour: The geometry of nutritional decisions. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 1993, 342, 381–402. [Google Scholar] [CrossRef]
- Yanoviak, S.P.; Kaspari, M. Community Structure and the Habitat Templet: Ants in the Tropical Forest Canopy and Litter. Oikos 2000, 89, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Kaspari, M.; Yanoviak, S.P. Bait Use in Tropical Litter and Canopy Ants—Evidence of Differences in Nutrient Limitation. Biotropica 2001, 33, 207–211. [Google Scholar] [CrossRef]
- Davidson, D.W. Ecological Stoichiometry of Ants in a New World Rain Forest. Oecologia 2005, 142, 221–231. [Google Scholar] [CrossRef]
- Takahashi, M.Q.; Rothman, J.M.; Raubenheimer, D.; Cords, M. Dietary Generalists and Nutritional Specialists: Feeding Strategies of Adult Female Blue Monkeys (Cercopithecus mitis) in the Kakamega Forest, Kenya. Am. J. Primatol. 2019, 81, e23016. [Google Scholar] [CrossRef]
- Law, S.J.; Parr, C. Numerically Dominant Species Drive Patterns in Resource Use along a Vertical Gradient in Tropical Ant Assemblages. Biotropica 2020, 52, 101–112. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, UK, 1990. [Google Scholar] [CrossRef]
- Blüthgen, N.; Gebauer, G.; Fiedler, K. Disentangling a Rainforest Food Web Using Stable Isotopes: Dietary Diversity in a Species-Rich Ant Community. Oecologia 2003, 137, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.W.; Cook, S.C.; Snelling, R.R.; Chua, T.H. Explaining the Abundance of Ants in Lowland Tropical Rainforest Canopies. Science 2003, 300, 969–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rico-Gray, V.; Oliveira, P.S. The Ecology and Evolution of Ant-Plant Interactions; University of Chicago Press: Chicago, IL, USA, 2007; pp. 99–193. [Google Scholar]
- Davidson, D.W.; Cook, S.C.; Snelling, R.R. Liquid-Feeding Performances of Ants (Formicidae): Ecological and Evolutionary Implications. Oecologia 2004, 139, 255–266. [Google Scholar] [CrossRef]
- Ribeiro, L.F.; Solar, R.R.C.; Sobrinho, T.G.; Muscardi, D.C.; Schoereder, J.H.; Andersen, A.N. Different Trophic Groups of Arboreal Ants Show Differential Responses to Resource Supplementation in a Neotropical Savanna. Oecologia 2019, 190, 433–443. [Google Scholar] [CrossRef]
- Tsang, T.P.N.; Guénard, B.; Bonebrake, T.C. Omnivorous Ants Are Less Carnivorous and More Protein-limited in Exotic Plantations. J. Anim. Ecol. 2020, 89, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Lasmar, C.J.; Bishop, T.R.; Parr, C.L.; Queiroz, A.C.M.; Wilker, I.; Feitosa, R.M.; Schmidt, F.A.; Ribas, C.R. Testing the Context Dependence of Ant Nutrient Preference across Habitat Strata and Trophic Levels in N Eotropical Biomes. Ecology 2023, 104, e3975. [Google Scholar] [CrossRef]
- Blüthgen, N.; Feldhaar, H. Food and shelter: How resources influence ant ecology. In Ant Ecology; Lach, L., Parr, C., Abbott, K., Eds.; Oxford University Press: Oxford, UK, 2010. [Google Scholar] [CrossRef]
- Rowles, A.D.; Silverman, J. Carbohydrate Supply Limits Invasion of Natural Communities by Argentine Ants. Oecologia 2009, 161, 161–171. [Google Scholar] [CrossRef]
- Kaspari, M.; Donoso, D.; Lucas, J.A.; Zumbusch, T.; Kay, A.D. Using Nutritional Ecology to Predict Community Structure: A Field Test in Neotropical Ants. Ecosphere 2012, 3, art93. [Google Scholar] [CrossRef]
- Chown, S.L.; Nicholson, S.W. Insect Physiological Ecology: Mechanisms and Patterns, 1st ed.; Oxford University Press: Oxford, UK, 2004; pp. 115–153. [Google Scholar] [CrossRef]
- Gibbs, A.G.; Chippindale, A.K.; Rose, M.R. Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. J. Exp. Biol. 1997, 200, 1821–1832. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.H.; Kristensen, T.N.; Loeschcke, V.; Toft, S.; Mayntz, D. Protein and Carbohydrate Composition of Larval Food Affects Tolerance to Thermal Stress and Desiccation in Adult Drosophila Melanogaster. J. Insect Physiol. 2010, 56, 336–340. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.; MacRae, T.H. Insect Heat Shock Proteins During Stress and Diapause. Annu. Rev. Entomol. 2015, 60, 59–75. [Google Scholar] [CrossRef]
- Cerda, X.; Retana, J.; Cros, S. Thermal Disruption of Transitive Hierarchies in Mediterranean Ant Communities. J. Anim. Ecol. 1997, 66, 363. [Google Scholar] [CrossRef]
- Cerdá, X.; Retana, J.; Manzaneda, A. The Role of Competition by Dominants and Temperature in the Foraging of Subordinate Species in Mediterranean Ant Communities. Oecologia 1998, 117, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, G.; Lanan, M.C.; Bronstein, J.L. Thermal Tolerance Affects Mutualist Attendance in an Ant–Plant Protection Mutualism. Oecologia 2014, 176, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Carroll, C.R.; Janzen, D.H. Ecology of foraging by ants. Annu. Rev. Ecol. Syst. 1973, 4, 231–257. [Google Scholar] [CrossRef]
- Davidson, D.W. Resource Discovery versus Resource Domination in Ants: A Functional Mechanism for Breaking the Trade-off: Discovery/Dominance Trade-off in Ants. Ecological Entomology 1998, 23, 484–490. [Google Scholar] [CrossRef]
- Grover, C.D.; Kay, A.D.; Monson, J.A.; Marsh, T.C.; Holway, D.A. Linking Nutrition and Behavioural Dominance: Carbohydrate Scarcity Limits Aggression and Activity in Argentine Ants. Proc. R. Soc. B 2007, 274, 2951–2957. [Google Scholar] [CrossRef] [Green Version]
- Janzen, D.H. Allelopathy by Myrmecophytes: The Ant Azteca as an Allelopathic Agent of Cecropia. Ecology 1969, 50, 147–153. [Google Scholar] [CrossRef]
- Davidson, D.W.; Longino, J.T.; Snelling, R.R. Pruning of Host Plant Neighbors by Ants: An Experimental Approach. Ecology 1988, 69, 801–808. [Google Scholar] [CrossRef]
- Ness, J.H.; Morris, W.F.; Bronstein, J.L. For Ant-Protected Plants, the Best Defense Is a Hungry Offense. Ecology 2009, 90, 2823–2831. [Google Scholar] [CrossRef] [Green Version]
- Kay, A.D.; Zumbusch, T.; Heinen, J.L.; Marsh, T.C.; Holway, D.A. Nutrition and Interference Competition Have Interactive Effects on the Behavior and Performance of Argentine Ants. Ecology 2010, 91, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Pacelhe, F.T.; Costa, F.V.; Neves, F.S.; Bronstein, J.; Mello, M.A.R. Nectar Quality Affects Ant Aggressiveness and Biotic Defense Provided to Plants. Biotropica 2019, 51, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Kost, C.; Heil, M. Increased Availability of Extrafloral Nectar Reduces Herbivory in Lima Bean Plants (Phaseolus Lunatus, Fabaceae). Basic Appl. Ecol. 2005, 6, 237–248. [Google Scholar] [CrossRef]
- González-Teuber, M.; Silva Bueno, J.C.; Heil, M.; Boland, W. Increased Host Investment in Extrafloral Nectar (EFN) Improves the Efficiency of a Mutualistic Defensive Service. PLoS ONE 2012, 7, e46598. [Google Scholar] [CrossRef] [Green Version]
- Savolainen, R.; Vepsäläinen, K.; Vepsalainen, K. A Competition Hierarchy among Boreal Ants: Impact on Resource Partitioning and Community Structure. Oikos 1988, 51, 135. [Google Scholar] [CrossRef]
- Parr, C.L.; Gibb, H. Competition and the role of dominant ants. In Ant Ecology; Lach, L., Parr, C., Abbott, K., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 77–96. [Google Scholar]
- Cerda, X.; Arnan, X.; Retana, J. Is competition a significant hallmark of ant (Hymenoptera: Formicidae) ecology. Myrmecol. News 2013, 18, 131–147. [Google Scholar]
- Camarota, F.; Vasconcelos, H.L.; Koch, E.B.A.; Powell, S. Discovery and Defense Define the Social Foraging Strategy of Neotropical Arboreal Ants. Behav. Ecol. Sociobiol. 2018, 72, 110. [Google Scholar] [CrossRef]
- Eiten, G. The cerrado vegetation of Brazil. Bot. Rev. 1972, 38, 201–341. [Google Scholar] [CrossRef]
- Powell, S.; Costa, A.N.; Lopes, C.T.; Vasconcelos, H.L. Canopy Connectivity and the Availability of Diverse Nesting Resources Affect Species Coexistence in Arboreal Ants: Species Coexistence in Arboreal Ants. J. Anim. Ecol. 2011, 80, 352–360. [Google Scholar] [CrossRef]
- Bluthgen, N.; Gottsberger, G.; Fiedler, K. Sugar and Amino Acid Composition of Ant-Attended Nectar and Honeydew Sources from an Australian Rainforest. Austral. Ecol. 2004, 29, 418–429. [Google Scholar] [CrossRef]
- Dussutour, A.; Simpson, S.J. Description of a Simple Synthetic Diet for Studying Nutritional Responses in Ants. Insect. Soc. 2008, 55, 329–333. [Google Scholar] [CrossRef]
- Tillberg, C.V.; McCarthy, D.P.; Dolezal, A.G.; Suarez, A.V. Measuring the Trophic Ecology of Ants Using Stable Isotopes. Insect. Soc. 2006, 53, 65–69. [Google Scholar] [CrossRef]
- Rasband, W. Image J Documentation. 2013. Available online: http://rsb.info.nih.-gov/ij/docs/index.html (accessed on 12 January 2021).
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.R-project.org/ (accessed on 20 January 2021).
- Fox, J.; Weisberg, S. Using car and effects Functions in Other Functions. Using Car Eff. Funct. Other Funct. 2020, 3, 1–5. [Google Scholar]
- Russell, V.L. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.6.2-1. 2021. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 20 January 2021).
- Peck, J.E. Multivariate Analysis for Community Ecologists: Step-by-Step Using PC-ORD; MJM Software Design: Gleneden Beach, OR, USA, 2010; pp. 1–162. [Google Scholar]
- Duyck, P.-F.; Lavigne, A.; Vinatier, F.; Achard, R.; Okolle, J.N.; Tixier, P. Addition of a New Resource in Agroecosystems: Do Cover Crops Alter the Trophic Positions of Generalist Predators? Basic Appl. Ecol. 2011, 12, 47–55. [Google Scholar] [CrossRef]
- Dejean, A.; Grangier, J.; Leroy, C.; Orivel, J. Predation and Aggressiveness in Host Plant Protection: A Generalization Using Ants from the Genus Azteca. Naturwissenschaften 2009, 96, 57–63. [Google Scholar] [CrossRef]
- Koch, E.B.A.; Camarota, F.; Vasconcelos, H.L. Plant Ontogeny as a Conditionality Factor in the Protective Effect of Ants on a Neotropical Tree. Biotropica 2016, 48, 198–205. [Google Scholar] [CrossRef]
- Dejean, A.; Orivel, J.; Leponce, M.; Compin, A.; Delabie, J.H.C.; Azémar, F.; Corbara, B. Ant–Plant Relationships in the Canopy of an Amazonian Rainforest: The Presence of an Ant Mosaic. Biol. J. Linn. Soc. 2018, 125, 344–354. [Google Scholar] [CrossRef]
- Johnson, C.; Agosti, D.; Delabie, J.H.; Dumpert, K.; Williams, D.J.; Tschirnhaus, M.V.; Maschwitz, U. Acropyga and Azteca Ants (Hymenoptera: Formicidae) with Scale Insects (Sternorrhyncha: Coccoidea): 20 Million Years of Intimate Symbiosis. Am. Mus. Novit. 2001, 3335, 1–18. [Google Scholar] [CrossRef]
- Thompson, S.N. Trehalose—The insect ‘blood’sugar. Adv. Insect Physiol. 2003, 31, 205–285. [Google Scholar] [CrossRef]
- Suarez, R.K.; Lighton, J.R.; Joos, B.; Roberts, S.P.; Harrison, J.F. Energy Metabolism, Enzymatic Flux Capacities, and Metabolic Flux Rates in Flying Honeybees. Proc. Natl. Acad. Sci. USA 1996, 93, 12616–12620. [Google Scholar] [CrossRef] [Green Version]
- Sokolova, I.M. Energy-Limited Tolerance to Stress as a Conceptual Framework to Integrate the Effects of Multiple Stressors. Integr. Comp. Biol. 2013, 53, 597–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacktor, B. Regulation of Intermediary Metabolism, with Special Reference to the Control Mechanisms in Insect Flight Muscle. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 1970; Volume 7, pp. 267–347. ISBN 978-0-12-024207-8. [Google Scholar] [CrossRef]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and Reproduction in Drosophila: New Insights from Nutritional Geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef] [Green Version]
- Makalkov, A.A.; Simpson, S.J.; Zajitschek, F.; Hall, M.; Dessman, J.; Clissold, F.J.; Raubenheimer, D.; Bonduriansky, R.; Brooks, R.C. Sex specific fitness effects of nutrient intake on reproduction and lifespan. Curr. Biol. 2008, 14, 1062–1066. [Google Scholar] [CrossRef] [Green Version]
- Dussutour, A.; Simpson, S.J. Communal Nutrition in Ants. Curr. Biol. 2009, 19, 740–744. [Google Scholar] [CrossRef] [Green Version]
- Kay, A.D.; Shik, J.Z.; Van Alst, A.; Miller, K.A.; Kaspari, M. Diet Composition Does Not Affect Ant Colony Tempo: Diet Composition Does Not Affect Ant Colony Tempo. Funct. Ecol. 2012, 26, 317–323. [Google Scholar] [CrossRef]
- O’Donnell, M. Insect excretory mechanisms. Adv. Insect Physiol. 2008, 35, 1–122. [Google Scholar] [CrossRef]
- Zuanon, L.A. Niche partitioning and Thermal tolerance of arboreal ants in a Neotropical savanna. Master’s Thesis, Universidade Federal de Uberlândia, Uberlândia, Brazil, 2018. [Google Scholar]
- Byk, J.; Del-Claro, K. Ant–Plant Interaction in the Neotropical Savanna: Direct Beneficial Effects of Extrafloral Nectar on Ant Colony Fitness. Popul. Ecol. 2011, 53, 327–332. [Google Scholar] [CrossRef]
- Russell, J.A.; Moreau, C.S.; Goldman-Huertas, B.; Fujiwara, M.; Lohman, D.J.; Pierce, N.E. Bacterial Gut Symbionts Are Tightly Linked with the Evolution of Herbivory in Ants. Proc. Natl. Acad. Sci. USA 2009, 106, 21236–21241. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Sanders, J.G.; Łukasik, P.; D’Amelio, C.L.; Millar, J.S.; Vann, D.R.; Lan, Y.; Newton, J.A.; Schotanus, M.; Kronauer, D.J.C.; et al. Herbivorous Turtle Ants Obtain Essential Nutrients from a Conserved Nitrogen-Recycling Gut Microbiome. Nat. Commun. 2018, 9, 964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, C.F.D.; Bergallo, H.G. Bigger Ant Colonies Reduce Herbivory and Herbivore Residence Time on Leaves of an Ant-Plant: Azteca Muelleri vs. Coelomera Ruficornis on Cecropia Pachystachya. Oecologia 1992, 91, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Rico-Gray, V.; Thien, L.B. Effect of Different Ant Species on Reproductive Fitness of Schomburgkia Tibicinis (Orchidaceae). Oecologia 1989, 81, 487–489. [Google Scholar] [CrossRef]
- Del-Claro, K.; Marquis, R.J. Ant Species Identity Has a Greater Effect than Fire on the Outcome of an Ant Protection System in Brazilian Cerrado. Biotropica 2015, 47, 459–467. [Google Scholar] [CrossRef]
- Yachi, S.; Loreau, M. Biodiversity and Ecosystem Productivity in a Fluctuating Environment: The Insurance Hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1463–1468. [Google Scholar] [CrossRef] [Green Version]
- Fagundes, R.; Dáttilo, W.; Ribeiro, S.P.; Rico-Gray, V.; Jordano, P.; Del-Claro, K. Differences among Ant Species in Plant Protection Are Related to Production of Extrafloral Nectar and Degree of Leaf Herbivory. Biol. J. Linn. Soc. 2017, 122, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, H.L.; Koch, E.B.A.; Camarota, F.; Tito, R.; Zuanon, L.A.; Maravalhas, J. Severe Fires Alter the Outcome of the Mutualism between Ants and a Neotropical Savanna Tree. Biol. J. Linn. Soc. 2020, 131, 476–486. [Google Scholar] [CrossRef]
- Bronstein, J.L.; Alarcón, R.; Geber, M. The evolution of plant–insect mutualisms. New Phytol. 2006, 172, 412–428. [Google Scholar] [CrossRef]
- Blüthgen, N.; Fiedler, K. Competition for composition: Lessons from nectar-feeding ant communities. Ecology 2004, 85, 1479–1485. [Google Scholar] [CrossRef] [Green Version]
- Nepi, M.; Soligo, C.; Nocentini, D.; Abate, M.; Guarnieri, M.; Cai, G.; Bini, L.; Puglia, M.; Bianchi, L.; Pacini, E. Amino Acids and Protein Profile in Floral Nectar: Much More than a Simple Reward. Flora Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 475–481. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuanon, L.A.; Leão, R.E.O.S.; Quero, A.; Neves, K.C.; Vasconcelos, H.L. Nutrient Supplementation to Arboreal Ants: Effects on Trophic Position, Thermal Tolerance, Community Structure and the Interaction with the Host-Tree. Diversity 2023, 15, 786. https://doi.org/10.3390/d15060786
Zuanon LA, Leão REOS, Quero A, Neves KC, Vasconcelos HL. Nutrient Supplementation to Arboreal Ants: Effects on Trophic Position, Thermal Tolerance, Community Structure and the Interaction with the Host-Tree. Diversity. 2023; 15(6):786. https://doi.org/10.3390/d15060786
Chicago/Turabian StyleZuanon, Lino A., Ruthe E. O. S. Leão, Adilson Quero, Karen C. Neves, and Heraldo L. Vasconcelos. 2023. "Nutrient Supplementation to Arboreal Ants: Effects on Trophic Position, Thermal Tolerance, Community Structure and the Interaction with the Host-Tree" Diversity 15, no. 6: 786. https://doi.org/10.3390/d15060786
APA StyleZuanon, L. A., Leão, R. E. O. S., Quero, A., Neves, K. C., & Vasconcelos, H. L. (2023). Nutrient Supplementation to Arboreal Ants: Effects on Trophic Position, Thermal Tolerance, Community Structure and the Interaction with the Host-Tree. Diversity, 15(6), 786. https://doi.org/10.3390/d15060786





