Response of the Coastal Phytoplankton Community to the Runoff from Small Rivers in the Northeastern Black Sea
Abstract
:1. Introduction
2. Materials and Methods
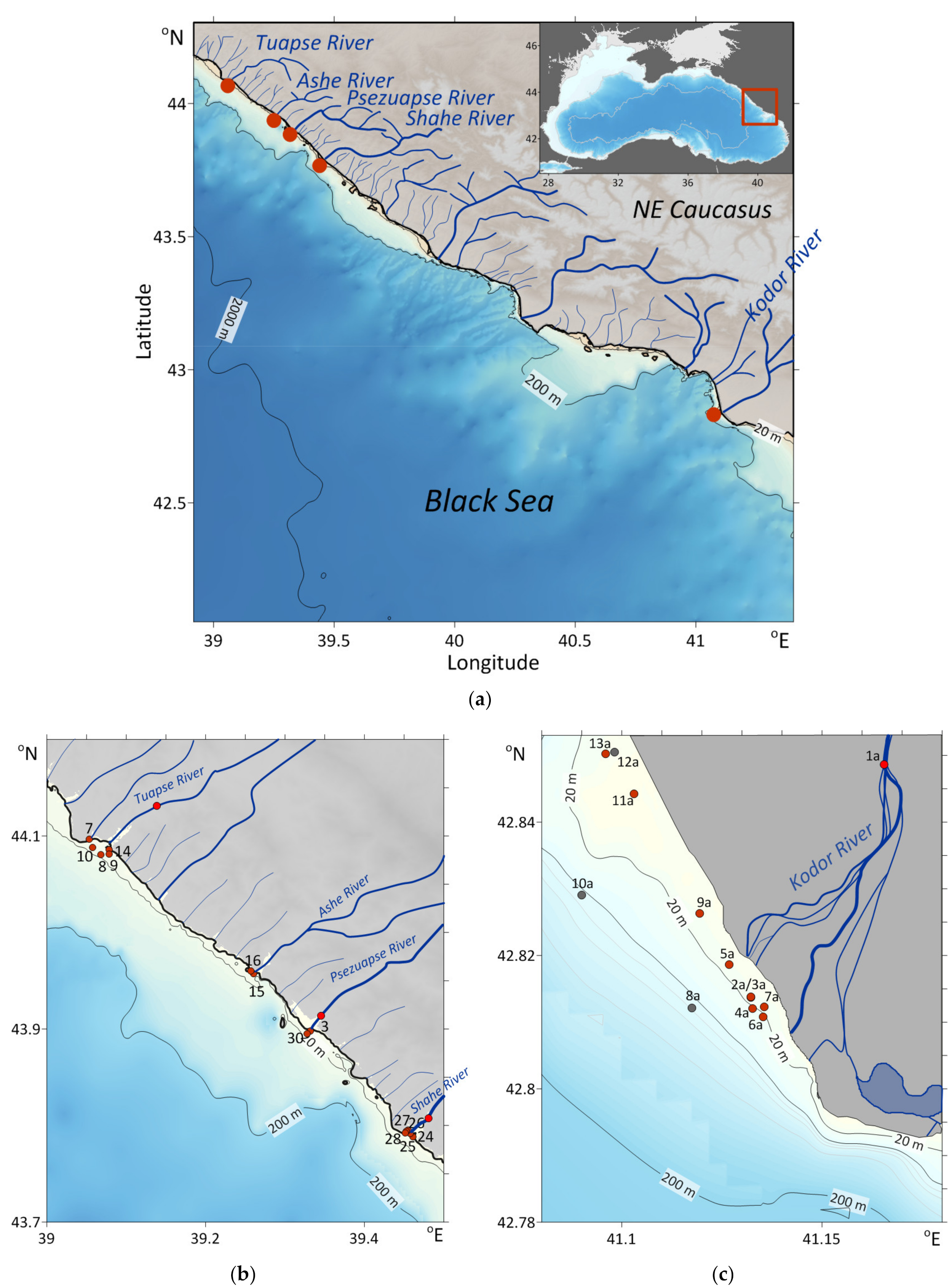
2.1. Research Area
2.2. Sampling
2.3. Phytoplankton
2.4. Primary Production (PP) and Chlorophyll a (Chl a)
2.5. Analysis of Nutrients, DOC, and n-Alkanes
3. Results
3.1. Phytoplankton
3.1.1. Taxonomic Structure
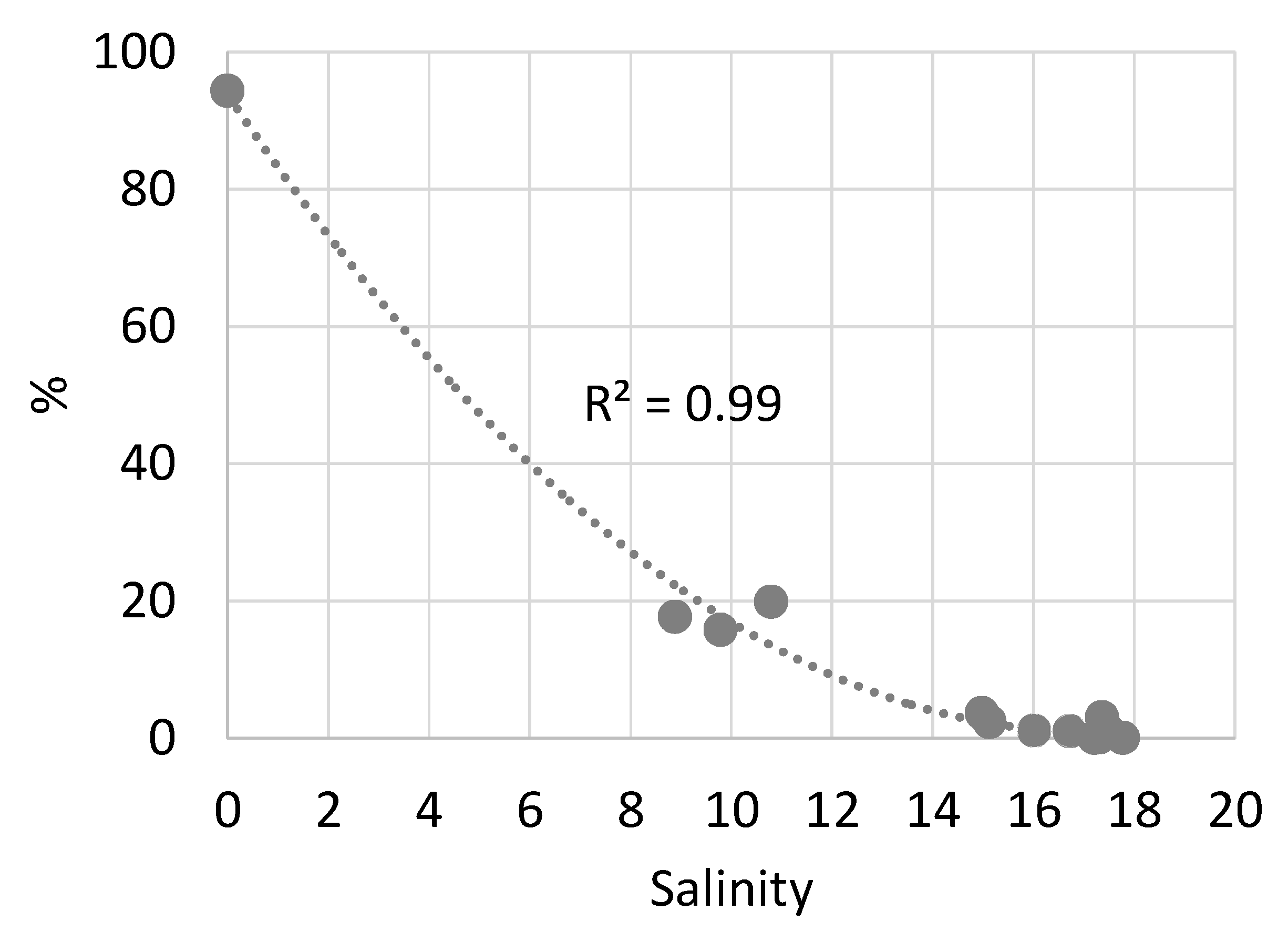
3.1.2. Freshwater Phytoplankton
3.2. Influence of Oligotrophic Rivers
3.2.1. Total Abundance of Cells and Carbon Biomass
3.2.2. Spatial Distribution of Diatoms, Dinoflagellates, and Coccolithophores
3.2.3. Active Phototrophs in Phytoplankton Community
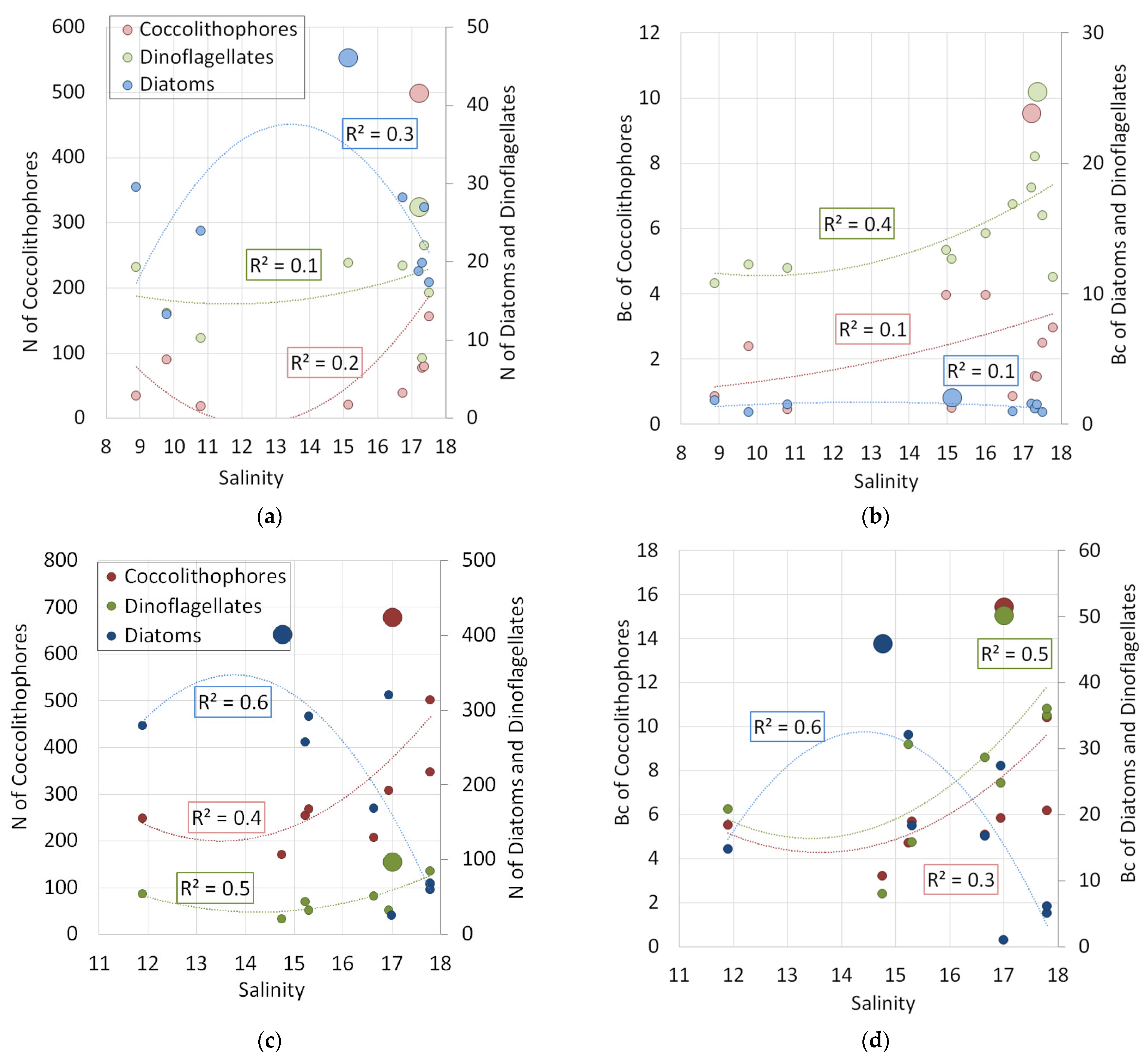
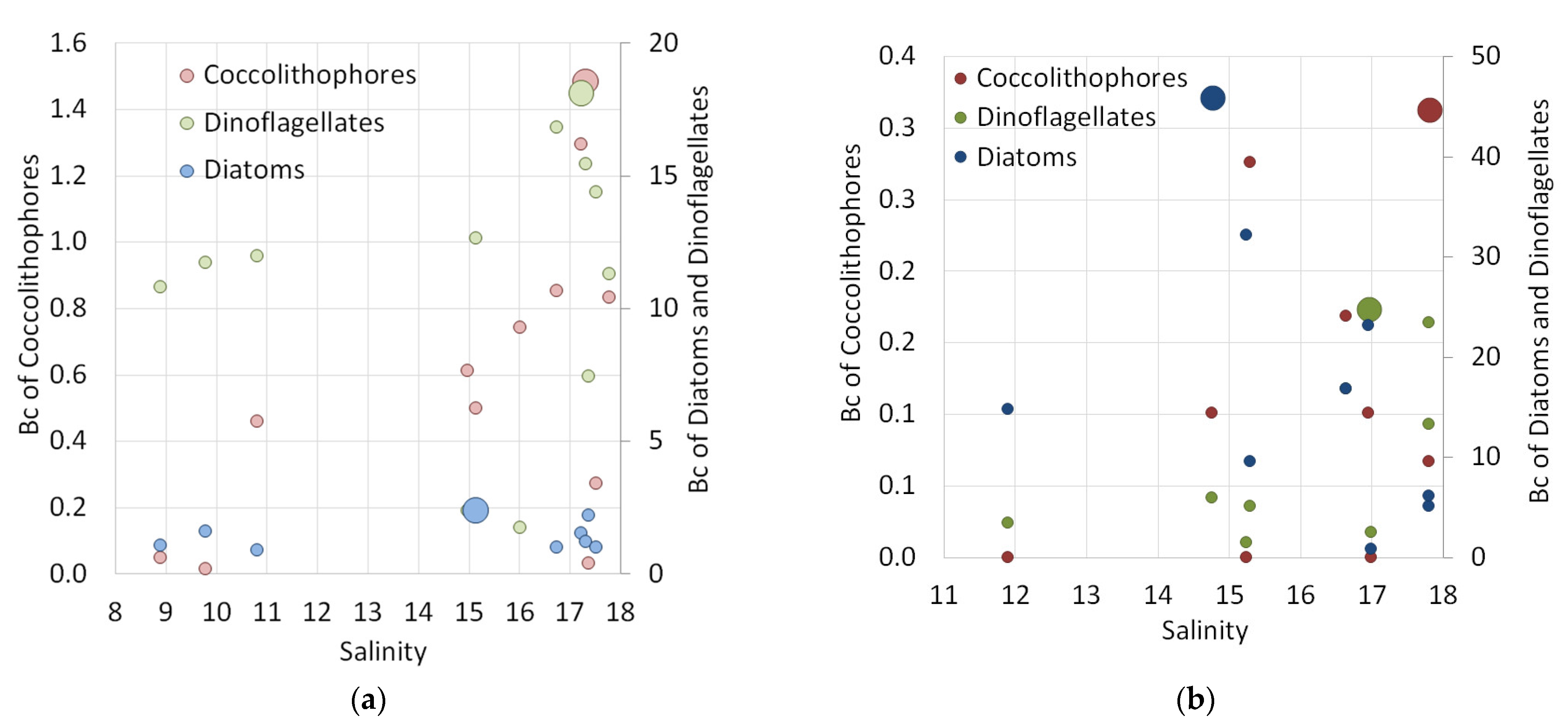
3.2.4. Distribution Patterns of Different Groups
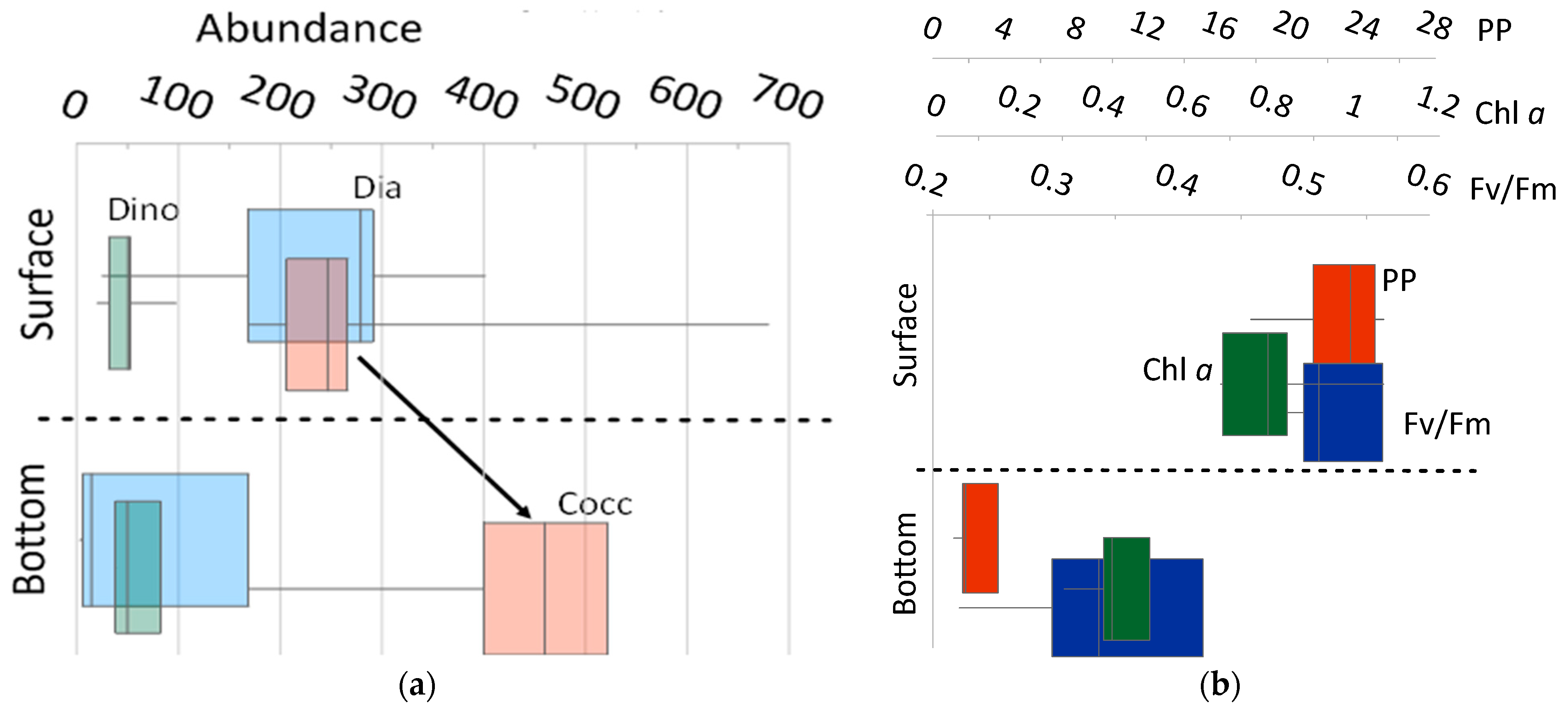
3.2.5. Features of Vertical Distribution of Phytoplankton
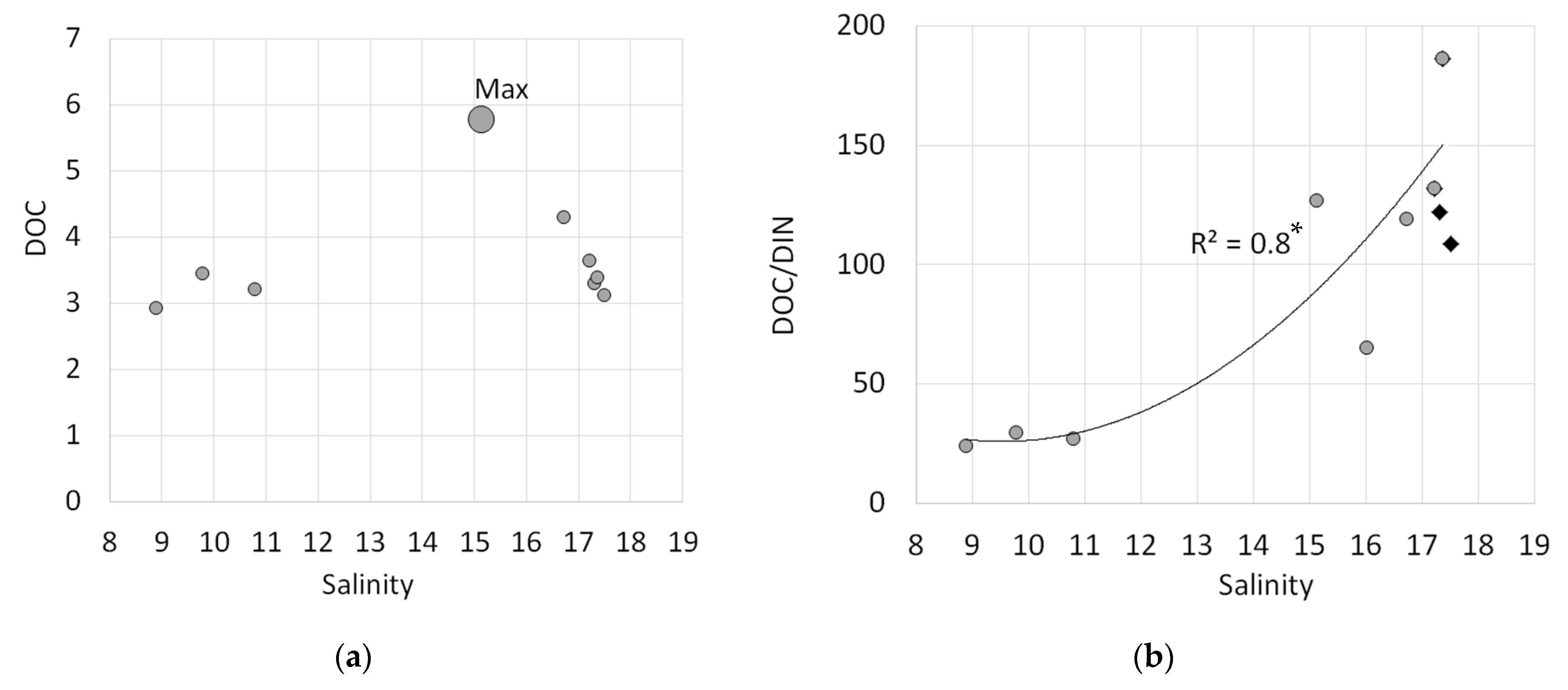
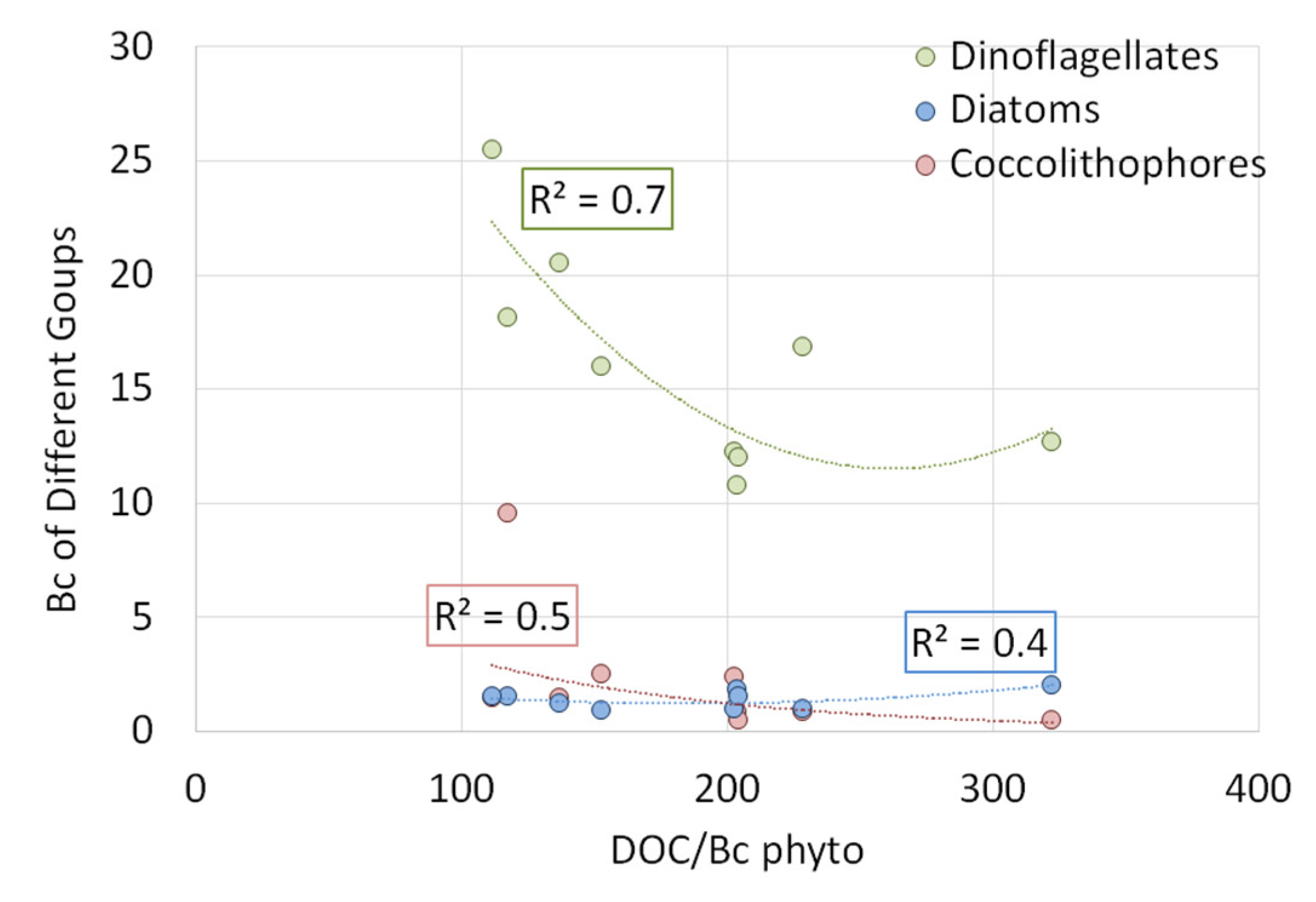
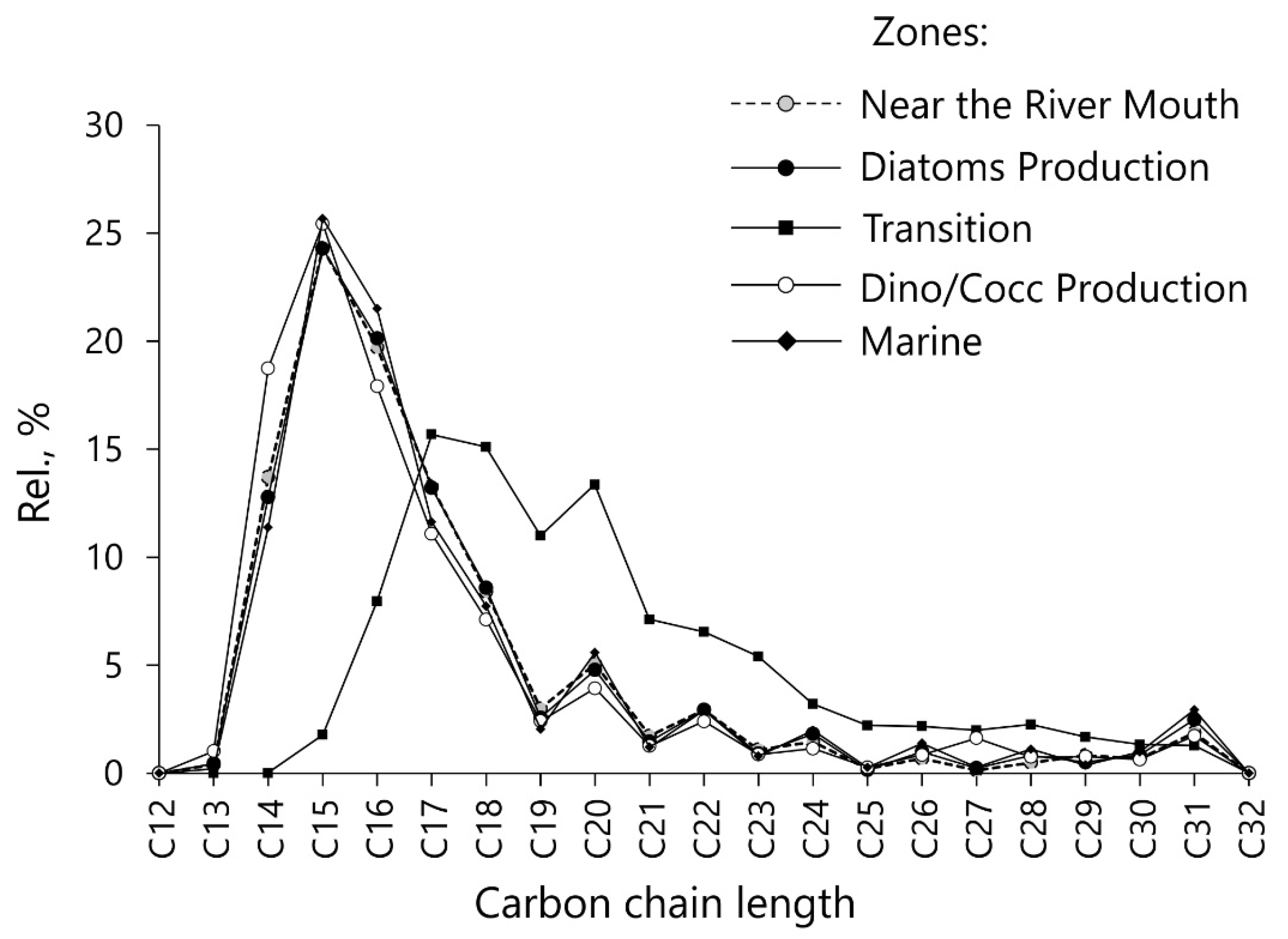
3.2.6. Distribution of DOC and n-Alkanes
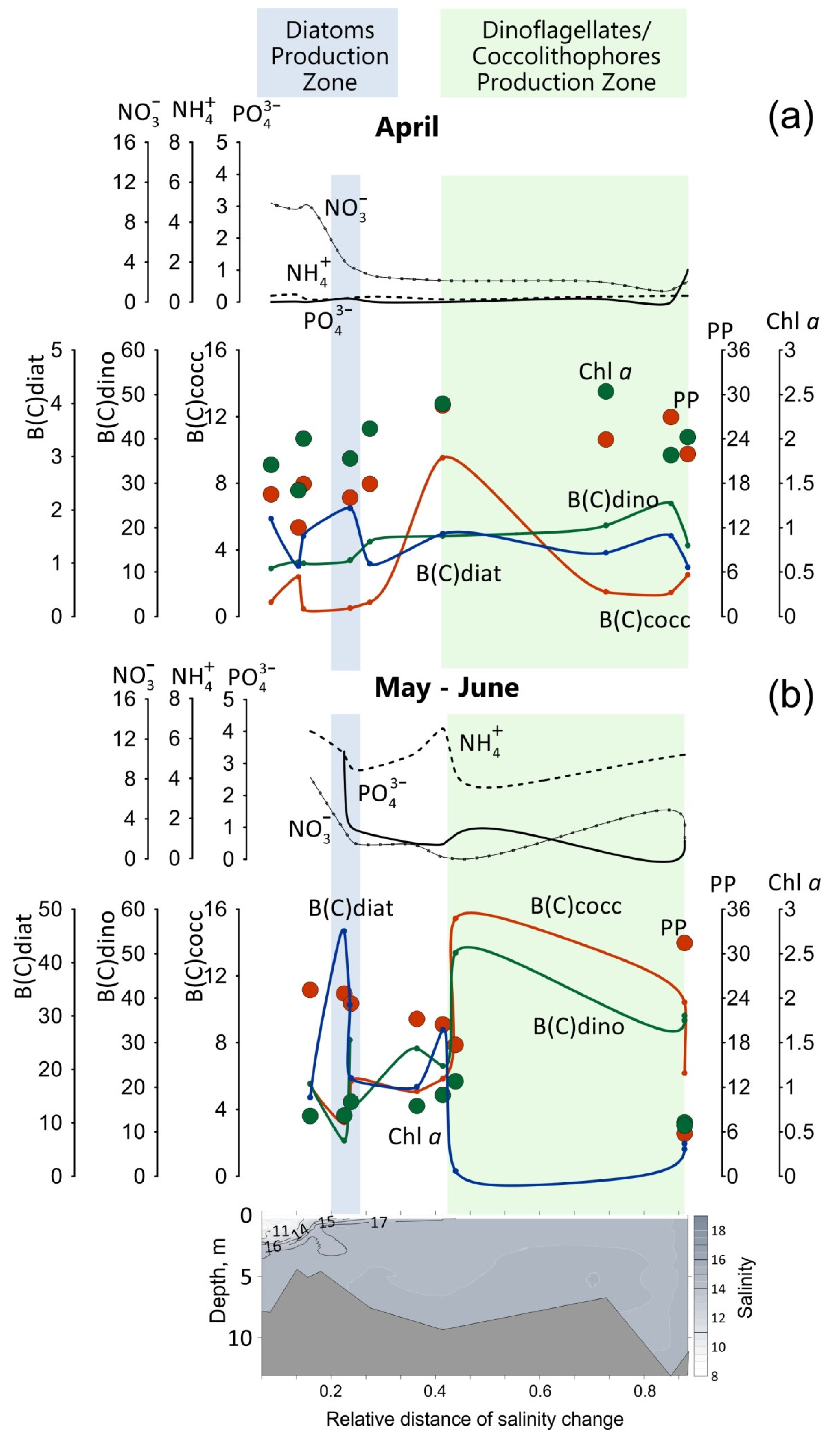
3.3. Influence of the Mesotrophic Tuapse River
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorokin, Y.I. The Black Sea Ecology and Oceanography; Backhuys Publishers: Leiden, The Netherlands, 2002; p. 875. [Google Scholar]
- Krivoguz, D.; Mal’ko, S.; Semenova, A. Spatial analysis of salinity distribution patterns in upper layers of the Black Sea. E3S Web Conf. 2020, 203, 03010. [Google Scholar] [CrossRef]
- Jaoshvili, S. The rivers of the Black Sea. In European Environmental Agency, Technical Report No. 71; Chomeriki, I., Gigineishvili, G., Kordzadze, A., Eds.; 2002; Available online: https://www.eea.europa.eu/publications/technical_report_2002_71/at_download/file (accessed on 25 April 2022).
- Bolshakov, V.S. River Waters’ Transformation in the Black Sea; Naukova dumka: Kiev, Ukraine, 1970; p. 328. [Google Scholar]
- Sur, H.I.; Özsoy, E.; Ilyin, Y.P.; Ünlüata, Ü. Coastal/deep ocean interactions in the Black Sea and their ecological/environmental impacts. J. Mar. Syst. 1996, 7, 293–320. [Google Scholar] [CrossRef]
- Mihnea, P.E. Effect of pollution on phytoplankton species. Rapp. Commun. Int. Du Mer Meditteranee 1985, 29, 85–88. [Google Scholar]
- Zaitsev, Y.P. Impacts of eutrophication on the Black Sea fauna. In Fisheries and Environment Studies in the Black Sea System; Studies and Reviews—General Fisheries Council for the Mediterranean (FAO); No.64/FAO; General Fisheries Council for the Mediterranean: Rome, Italy, 1993; pp. 59–86. [Google Scholar]
- Zaitsev, Y.P.; Alexandrov, B.G. Recent man-made changes in the Black Sea ecosystem. In Sensitivity to Change: Black Sea, Baltic Sea and North Sea; Ozsoy, E., Mikaelyan, A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997; pp. 25–32. [Google Scholar] [CrossRef]
- Yunev, O.A.; Carstensen, J.; Moncheva, S.; Khaliulin, A.; Ærtebjerg, G.; Nixon, S. Nutrient and phytoplank-ton trends on the western Black Sea shelf in response tocultural eutrophication and climate changes. Estuar. Coast. Shelf Sci. 2007, 74, 63–76. [Google Scholar] [CrossRef]
- Bodeanu, N. Microalgal blooms in the Romanian area of the Black Sea and contemporary eutrophication conditions. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 203–209. [Google Scholar]
- Petranu, A.; Apas, M.; Bodeanu, N.; Bologa, A.S.; Dumitrache, C.; Moldoveanu, M.; Radu, G.; Tiganus, V. Status and evolution of the Romanian Black Sea coastal ecosystem. In NATO TU-Black Sea Project: Environmental Degradation of the Black Sea: Challenges and Remedies; Besiktepe, S.T., Unluata, U., Bologa, A.S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 175–195. [Google Scholar] [CrossRef]
- Moncheva, S.; Gotsis-Skretas, O.; Pagou, K.; Krastev, A. Phytoplankton blooms in the Black Sea and Mediterranean coastal ecosystem subjected to anthropogenic eutrophication: Similarities and differences. Estuar. Coast. Shelf Sci. 2001, 53, 281–295. [Google Scholar] [CrossRef]
- Moncheva, S.; Krastev, A. Some aspects of phytoplankton long-term alterations off Bulgarian Black Sea shelf. In Sensitivity to Change: Black Sea, Baltic Sea and North Sea; Ozsoy, E., Mikaelyan, A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997; pp. 79–94. [Google Scholar] [CrossRef]
- Mousing, E.A.; Andersen, T.J.; Ellegaard, M. Changes in the Abundance and Species Composition of Phytoplankton in the Last 150 Years in the Southern Black Sea. Estuaries Coasts 2013, 36, 1206–1218. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Yoshida, M.; Kim, C.-H. Morphological Variation of Lingulodinium polyedrum (Dinophyceae) in Culture Specimens and Reinterpretation of the Thecal Formula. Algae 2005, 20, 299–304. [Google Scholar] [CrossRef]
- Vershinin, A.O.; Orlova, T.Y. Toxic and harmful algae in the coastal waters of Russia. Oceanology 2008, 48, 524–537. [Google Scholar] [CrossRef]
- Mertens, K.N.; Carbonell-Moore, C. Introduction to Spiniferites Mantell 1850 special issue. Palynology 2018, 42, 1–9. [Google Scholar] [CrossRef]
- Zavialov, P.O.; Makkaveev, P.N.; Konovalov, B.V.; Osadchiev, A.A.; Khlebopashev, P.V.; Pelevin, V.V.; Grabovskiy, A.; Izhitskiy, A.S.; Goncharenko, I.V.; Soloviev, D.M.; et al. Hydrophysical and hydrochemical characteristics of the sea areas adjacent to the estuaries of small rivers if the Russian coast of the Black Sea. Oceanology 2014, 54, 265–280. [Google Scholar] [CrossRef]
- Alexeevsky, N.I.; Magritsky, D.V.; Koltermann, K.P.; Krylenko, I.N.; Toropov, P.A. Causes and Systematics of Inundations of the Krasnodar Territory on the Russian Black Sea Coast. Nat. Hazards Earth Syst. Sci. 2016, 16, 1289–1308. [Google Scholar] [CrossRef]
- Korshenko, E.; Panasenkova, I.; Osadchiev, A.; Belyakova, P.; Fomin, V. Synoptic and Seasonal Variability of Small River Plumes in the Northeastern Part of the Black Sea. Water 2023, 15, 721. [Google Scholar] [CrossRef]
- Lebedev, S.A.; Kostianoy, A.G.; Soloviev, D.M.; Kostianaia, E.A.; Ekba, Y.A. On a relationship between the river runoff and the river plume area in the northeastern Black Sea International. J. Remote Sens. 2020, 41, 5806–5818. [Google Scholar] [CrossRef]
- Kostyleva, A.V.; Podymov, O.I.; Makkaveev, P.N.; Polukhin, A.A. Influence of small rivers runoff on the hydrochemical structure of coastal waters of the north-eastern Black Sea. In Proceedings of the Coastal Engineering, Conference Proceedings, San-Diego, CA, USA, 21–24 August 2011; pp. 286–297, ISBN: 978-0-7844-1190-2. [Google Scholar]
- Kostyleva, A.V. Distribution of dissolved organic carbon in river mouth areas of the Greater Sochi region (North-Eastern part of the Black Sea). Oceanology 2015, 55, 200–206. [Google Scholar] [CrossRef]
- Polukhin, A.A.; Zagovenkova, A.D.; Khlebopashev, P.V.; Sergeeva, V.M.; Osadchiev, A.A.; Dbar, R. Hydrochemical Composition of the Runoff of Abkhazian Rivers and the Distinctive Features of Its Transformation in the Coastal Zone. Oceanology 2021, 61, 15–24. [Google Scholar] [CrossRef]
- Mikaelyan, A.S. Long-Term Variability of Phytoplankton Communities in Open Black Sea in Relation to Environmental Changes. In Sensitivity to Change: Black Sea, Baltic Sea and North Sea; NATO ASI Series; Ozsoy, E., Mikaelyan, A., Eds.; Springer: Dordrecht, The Netherlands, 1997; Volume 27. [Google Scholar] [CrossRef]
- Nezlin, N.P. The Black Sea Environment. The Handbook of Environmental Chemistry; Kostianoy, A.G., Kosarev, A.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 5Q. [Google Scholar] [CrossRef]
- Finenko, Z.Z. Biodiversity and Bioproductivity. In The Black Sea Environment. The Handbook of Environmental Chemistry; Kostianoy, A.G., Kosarev, A.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 5Q. [Google Scholar] [CrossRef]
- Nesterova, D.; Moncheva, S.; Mikaelyan, A.; Vershinin, A.; Akatov, V.; Boicenco, L.; Aktan, Y.; Sahin, F.; Gvarishvili, T. The state of phytoplankton. In State of the Environment of the Black Sea (2001–2006/7); Oğuz, T., Ed.; BSS: Istanbul, Turkey, 2008; pp. 173–192. Available online: https://www.researchgate.net/publication/312372456_The_state_of_phytoplankton (accessed on 30 April 2023).
- Pautova, L.A.; Mikaelyan, A.S.; Silkin, V.A. Structure of plankton phytocoenoses in the shelf waters of the northeastern Black Sea during the Emiliania huxleyi bloom in 2002–2005. Oceanology 2007, 47, 377–385. [Google Scholar] [CrossRef]
- Mikaelyan, A.S.; Silkin, V.A.; Pautova, L.A. Coccolithophorids in the Black Sea: Their interannual and long-term changes. Oceanology 2011, 51, 39–48. [Google Scholar] [CrossRef]
- Silkin, V.A.; Pautova, L.A.; Pakhomova, S.V.; Lifanchuk, A.V.; Yakushev, E.V.; Chasovnikov, V.K. Environmental control on phytoplankton community structure in the NE Black Sea. J. Exp. Mar. Biol. Ecol. 2014, 461, 267–274. [Google Scholar] [CrossRef]
- Mikaelyan, A.S.; Pautova, L.A.; Chasovnikov, V.K.; Mosharov, S.A.; Silkin, V.A. Alternation of diatoms and coccolithophores in the north-eastern Black Sea: A response to nutrient changes. Hydrobiologia 2015, 755, 89–105. [Google Scholar] [CrossRef]
- Arashkevich, E.G.; Louppova, N.E.; Nikishina, A.B.; Pautova, L.A.; Chasovnikov, V.K.; Drits, A.V.; Podymov, O.I.; Romanova, N.D.; Stanichnaya, R.R.; Zatsepin, A.G.; et al. Marine environmental monitoring in the shelf zone of the Black Sea: Assessment of the current state of the pelagic ecosystem. Oceanology 2015, 55, 871–876. [Google Scholar] [CrossRef]
- Silkin, V.A.; Pautova, L.A.; Giordano, M.; Chasovnikov, V.K.; Vostokov, S.V.; Podymov, O.I.; Pakhomova, S.V.; Moskalenko, L.V. Drivers of phytoplankton blooms in the northeastern Black Sea. Mar. Pollut. Bull. 2019, 138, 274–284. [Google Scholar] [CrossRef]
- Silkin, V.; Mikaelyan, A.S.; Pautova, L.; Fedorov, A. Annual Dynamics of Phytoplankton in the Black Sea in Relation to Wind Exposure. J. Mar. Sci. Eng. 2021, 9, 1435. [Google Scholar] [CrossRef]
- Vershinin, A.O.; Moruchkov, A.A.; Sukhanova, I.N.; Kamnev, A.N.; Morton, S.L.; Pankov, S.L.; Ramsdell, J.S. Seasonal changes in phytoplankton in the area of cape Bolshoi Utrish off the Northern Caucasian coast in the Black Sea, 2001–2002. Oceanology 2004, 44, 372–378. [Google Scholar]
- Vershinin, A.; Moruchkov, A.; Leighfield, T.; Sukhanova, I.; Morton, S.; Pankov, S.; Ramsdell, J. Species composition and potentially toxic algae of northeast Black Sea coastal phytoplankton in 2000–2002. Oceanology 2005, 45, 224–232. [Google Scholar]
- Yasakova, O.N. New species of phytoplankton in the northeastern part of the Black Sea. Russ. J. Biol. Invasions 2011, 2, 63–67. [Google Scholar] [CrossRef]
- Yasakova, O.N. The seasonal dynamics of potentially toxic and harmful phytoplankton species in Novorossiysk Bay (Black Sea). Russ. J. Mar. Biol. 2013, 39, 107–115. [Google Scholar] [CrossRef]
- Selifonova, Z.; Makarevich, P.R.; Samyshev, E.; Bartsits, L. Study of Ecosystem of the Sukhum Bay with Emphasis Anthropogenic Impact, Abkhazian Black Sea Coast. Ecol. Montenegrina 2019, 22, 108–116. [Google Scholar] [CrossRef]
- Yasakova, O.N.; Makarevich, P.R.; Okolodkov, Y.B. Long-term Changes in Community of Planktonic Algae of the Northeastern Black Sea (2005–2011). KnE Life Sci. 2020, 5, 569–580. [Google Scholar] [CrossRef]
- Yasakova, O.N. The Status of Plankton Algocenosis in the NorthEast Part of the Black Sea (2011–2020). Ecol. Saf. Coast. Shelf Zones Sea 2022, 1, 82–103. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. (Eds.) Methods of Seawater Analysis, 3rd ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1999; p. 577. [Google Scholar] [CrossRef]
- Dmitrieva, A.G. Bioassay method for determining live and dead algae cells using a fluorescent microscope. In Water Biotesting Methods; Chernogolovka: Moscow, Russia, 1988; pp. 85–89. (In Russian) [Google Scholar]
- Sournia, A. (Ed.) Phytoplankton Manual; UNESCO: Paris, France, 1978; 337p, ISBN 93-3-101572-9. [Google Scholar]
- Sun, J.; Liu, D. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef]
- Bryantseva, Y.V.; Kurilov, A.V. Calculation of Biovolumes of the Black Sea Microalgae and Planktonic Ciliates; NAS Ukraine; Institute of Biology of the Southern Seas: Sevastopol, Russia, 2005; 20p, Available online: https://www.researchgate.net/publication/272790173_Calculation_of_biovolumes_and_surface_areas_of_Black_Sea_microalgae (accessed on 22 May 2023). (In Russian)
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Ahyong, S.; Boyko, C.B.; Bailly, N.; Bernot, J.; Bieler, R.; Brandão, S.N.; Daly, M.; De Grave, S.; Gofas, S.; Hernandez, F.; et al. World Register of Marine Species 2023. Available online: https://www.marinespecies.org (accessed on 26 June 2023). [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E: Plymouth, UK, 2001. [Google Scholar]
- Steemann-Nielsen, E. The use of radio-active carbon (C14) for measuring organic production in the sea. ICES J. Mar. Sci. 1952, 18, 117–140. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Riemann, B. Chlorophyll a determination: Improvements in methodology. Oikos 1978, 30, 438–447. Available online: https://www.researchgate.net/publication/239457237_Holm-Hansen_O_B_Riemann_Chlorophyll_a_determination_improvements_in_methodology_Oikos (accessed on 20 April 2023). [CrossRef]
- Mosharov, S.A.; Sergeeva, V.M.; Kremenetskiy, V.V.; Sazhin, A.F.; Stepanova, S.V. Assessment of phytoplankton photosynthetic efficiency based on measurement of fluorescence parameters and radiocarbon uptake in the Kara Sea. Estuar. Coast. Shelf Sci. 2019, 218, 59–69. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 313–341. [Google Scholar] [CrossRef]
- Suggett, D.J.; Moore, C.M.; Hickman, A.E.; Geide, R.J. Interpretation of fast repetition rate (FRR) fluorescence: Signatures of phytoplankton community structure versus physiological state. Mar. Ecol. Prog. Ser. 2009, 376, 1–19. [Google Scholar] [CrossRef]
- Belyaev, N.A.; Ponyaev, M.S.; Kiriutin, A.M. Organic carbon in water, particulate matter, and upper layer of bottom sediments of the central part of the Kara Sea. Oceanology 2015, 55, 508–520. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Bernhardt, S.E. Chapter 8—Inland Waters. In Biogeochemistry, 4th ed.; Schlesinger, W.H., Bernhardt, E.S., Eds.; Academic Press, Elsevier: Cambridge, MA, USA, 2020; pp. 293–360. [Google Scholar] [CrossRef]
- Adl, S.M.; Simpson, A.G.B.; Farmer, M.A.; Andersen, R.A.; Andersen, O.R.; Barta, J.R.; Bowser, S.S.; Brugerolle, G.; Fensome, R.A.; Fredericq, S.; et al. The New Higher Level Classification of Eukaryotes with Emphasis on the Taxonomy of Protists. J. Eukaryot. Microbiol. 2005, 52, 399–451. [Google Scholar] [CrossRef]
- Mosharov, S.A.; Sergeeva, V.M. Estimate of the marine phytoplankton state by fluorescence parameters and ratio of chlorophyll a and pheophytin concentrations. Voprosy sovremennoi algologii (Issues Mod. Algol.) 2018, 1, 16. Available online: http://www.algology.ru/1257 (accessed on 28 April 2023).
- Osadchiev, A.A.; Barymova, A.A.; Sedakov, R.O.; Rybin, A.V.; Tanurkov, A.G.; Krylov, A.A.; Kremenetskiy, V.V.; Mosharov, S.A.; Polukhin, A.A.; Ulyantsev, A.S.; et al. Hydrophysical Structure and Current Dynamics of the Kodor River Plume. Oceanology 2021, 61, 1–14. [Google Scholar] [CrossRef]
- Kinne, O. (Ed.) Environmental factors, Part 2. In Marine Ecology; Wiley Interscience: London, UK, 1971; Volume 1. [Google Scholar]
- Osadchiev, A.; Gordey, A.; Barymova, A.; Sedakov, R.; Rogozhin, V.; Zhiba, R.; Dbar, R. Lateral Border of a Small River Plume: Salinity Structure, Instabilities and Mass Transport. Remote Sens. 2022, 14, 3818. [Google Scholar] [CrossRef]
- Riegman, R.; Stolte, W.; Noordeloos, A.A.M.; Slezak, D. Nutrient uptake and alkaline phosphatase (EC 3:1:3:1) activity of Emiliania huxleyi (Prymnesiophyceae) during growth under N and P limitation in continuous cultures. J. Phycol. 2000, 36, 87–96. [Google Scholar] [CrossRef]
- Godrijan, J.; Drapeau, D.T.; Balch, W.M. Osmotrophy of dissolved organic carbon by coccolithophores in darkness. New Phytol. 2022, 233, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Killops, S.; Killops, V. Introduction to Organic Geochemistry; Blackwell Publishing Ltd.: Malden, MA, USA, 2004. [Google Scholar] [CrossRef]
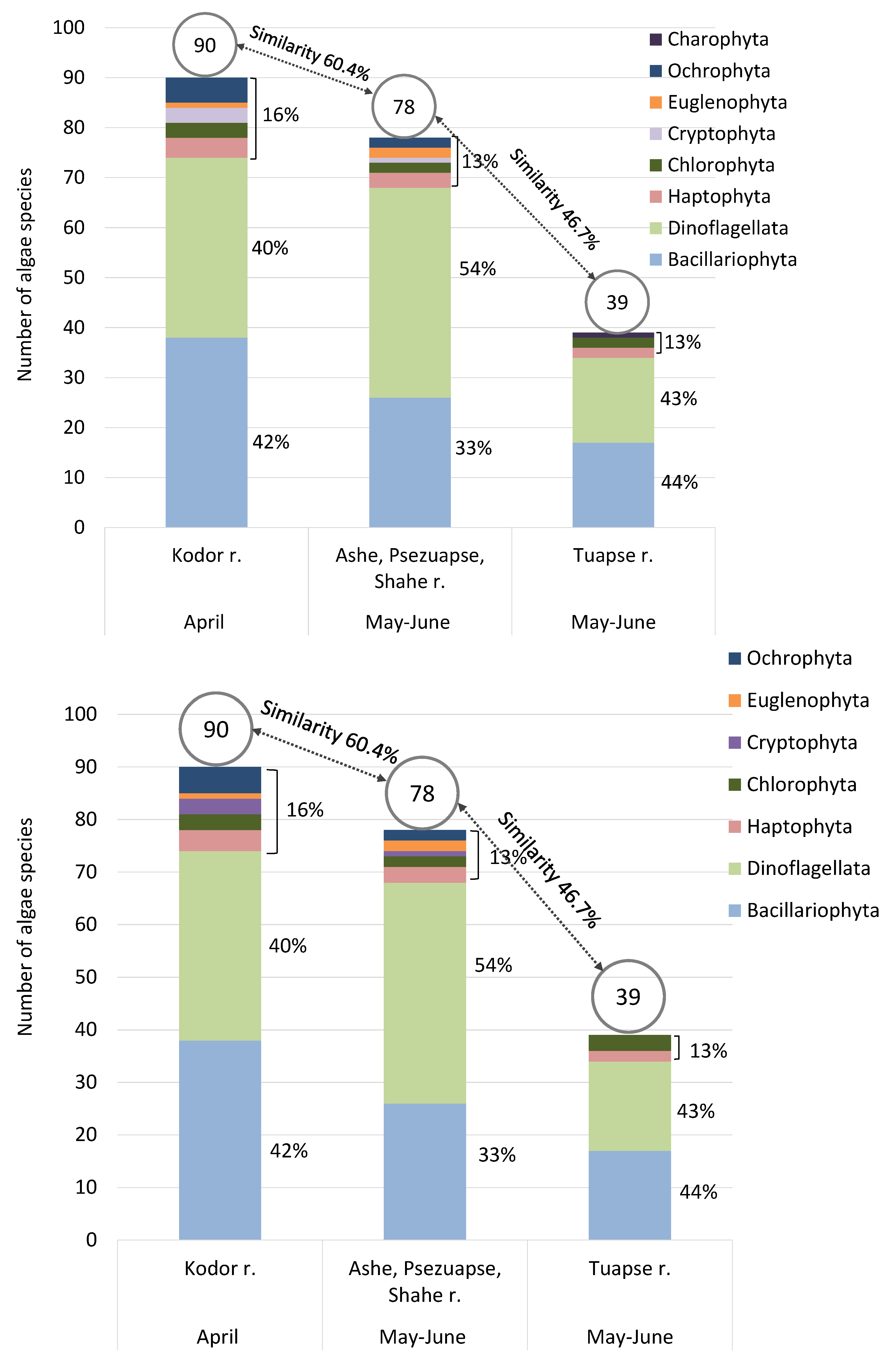
 —in April (under Kodor influence),
—in April (under Kodor influence),  —in May–June (under influence of Ashe, Psezuapse, and Shahe rivers).
—in May–June (under influence of Ashe, Psezuapse, and Shahe rivers).
 —in April (under Kodor influence),
—in April (under Kodor influence),  —in May–June (under influence of Ashe, Psezuapse, and Shahe rivers).
—in May–June (under influence of Ashe, Psezuapse, and Shahe rivers).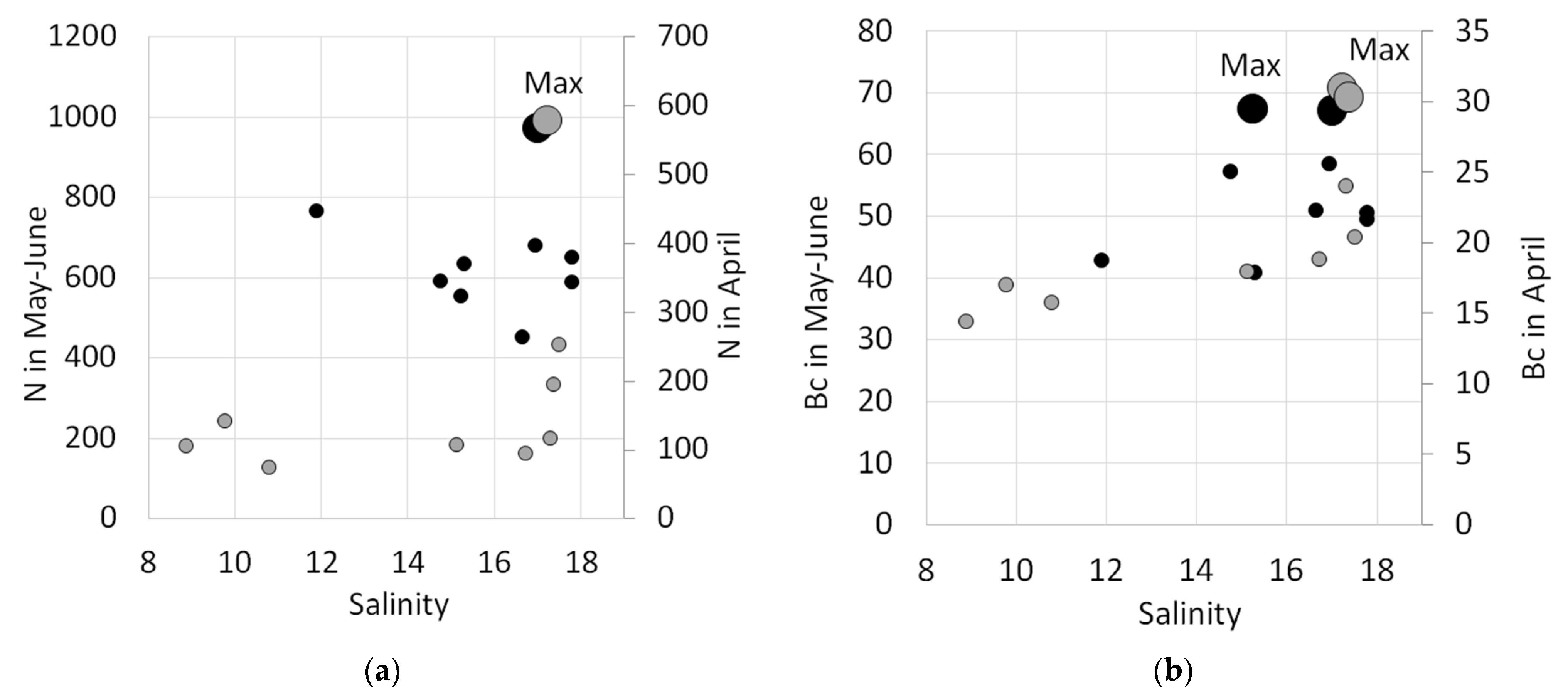

| Impacted by | Date | Number of Stations | Surface T Range, °C | Surface S Range | Depth at Station Range, m | Distance from Mouth, km | Sampling from River |
|---|---|---|---|---|---|---|---|
| Tuapse | 31.05.2018 | 6 (6) | 22.2–22.6 | 16.2–18.1 | 4–14 | 2.4 | + |
| Ashe | 31.05.2018 | 2 (2) | 22.7–22.9 | 16.4–17.3 | 3–4 | 0.5 | |
| Psezuapse | 29.05.2018 | 2 (2) | 22.5–23.1 | 15.2–17.7 | 3–8 | 0.6 | + |
| Shahe | 02.06.2018 | 5 (9) | 20.9–22.5 | 11.9–17.7 | 2–9 | 1 | + |
| Kodor | 02.04.2019 | 12 | 9.5–10.2 | 8.9–17.8 | 3–55 | 4.5 | + |
| River | Data | T,°C | S | chl a, μg L−1 | % Phaeo | PP, μgC L−1 d−1 | Fv/Fm | P-PO4, μM | Si, μM | N-NO3, μM | Trophic Level 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tuapse | 31.05.2018 | 24 | 0.1 | 3.64 | 16% | 1809.5 | 0.564 | 4.4 | 98.9 | 13.2 | Meso |
| Psezuapse | 29.05.2018 | 22 | 0.1 | 0.2 | 61% | 1.6 | 0.277 | 0.6 | 107.5 | 18.6 | Oligo |
| Shahe | 02.06.2018 | 22 | 0.1 | 0.3 | 56% | 4.7 | 0.510 | 0.6 | 121.7 | 14.8 | Oligo |
| Kodor | 02.04.2019 | 9 | 0.01 | 0.1 | 82% | 0.8 | 0.284 | 1.4 1 | 128.5 1 | 16.3 1 | Oligo |
| NMR | DP | TR | DCP | M | |||
|---|---|---|---|---|---|---|---|
| April (Kodor Influence) | Stations | 3a, 6a, 7a | 4a | 2a | 5a, 9a, 11a | 13a | |
| Salinity | <12 | 14.8–15.1 | 15.5–17 | 17–17.5 | >17.7 | ||
| Temperature, °C | 9.5–9.6 | 10 | 10.2 | 9.9–10.2 | 10 | ||
| Bc, µgC L−1 (Bw, µg L1) | Diat | 0.9–1.8 (14.4–30.8) | 2 (24.1) | 1 (14.6) | 1.2–1.6 (22.5–25.5) | 0.9 (12.1) | |
| Dino | 10.8–12.3 (70.3–106.1) | 12.7 (86.4) | 16.8 (139) | 18.1–25.5 (144.1–424) | 16.1 (144.2) | ||
| Cocc | 0.9–2.4 (2–15.4) | 0.5 (3.2) | 0.8 (5.5) | 1.4–9.5 (9.1–62) | 2.5 (15.7) | ||
| Nutrients, µM | N-NO3 | 9.4–9.9 | 3.6 | 2.7 | 1.2–2.2 | 2.1 | |
| N-NH4 | 0.2–0.4 | 0.2 | 0.3 | 0.1–0.3 | 0.3 | ||
| P-PO4 | 0–0.1 | 0.1 | 0 | 0–0.1 | 1 | ||
| Function | chl a, µg L−1 | 1.4–2 | 1.8 | 2.1 | 1.8–2.4 | 2 | |
| PP, µgC L−1 day−1 | 12–17.9 | 16.1 | 17.9 | 23.9–28.5 | 21.9 | ||
| Fv/Fm | 0.55–0.59 | 0.53 | 0.59 | 0.62–0.65 | 0.58 | ||
| May–June (Ashe, Psezuapse, Shahe Influence) | Stations | 24 | 3,25,26 | 16,27 | 28 | 15,30 | |
| Salinity | <12 | 14.8–15.1 | 15.5–17 | 17–17.5 | >17.7 | ||
| Temperature, °C | 21.8 | 22.6–23.1 | 23–23.2 | 22.8 | 22.5–22.8 | ||
| Bc, µgC L−1 (Bw, µg L−1) | Diat | 14.8 (186.3) | 32.1–45.9 (338.6–694.2) | 16.8–27.4 (250.1–442.5) | 1 (12) | 5.1–6.2 (76.1–96.4) | |
| Dino | 20.8 (128.7) | 8.0–30.6 (55.6–226.8) | 15.8–24.8 (168.8–201.9) | 50.2 (367.8) | 35–36.1 (209.1–243.9) | ||
| Cocc | 5.5 (34.9) | 3.2–4.7 (20–35.8) | 5.1–5.8 (32.7–36.3) | 15.4 (100.4) | 6.2–10.4 (38.1–65.4) | ||
| Nutrients, µM | N-NO3 | 8.2 | 2.3 | 0.2–1.8 | 0 | 1–1.6 | |
| N-NH4 | 6.4 | 5.2 | 4.5–6.5 | 4.3 | 5.2 | ||
| P-PO4 | 3.4 | 0.5–1 | 0.8 | 0.4–0.6 | |||
| Function | chl a, µg L−1 | 0.7 | 0.7 | 0.8–0.9 | 1.1 | 0.6 | |
| PP, µgC L−1 day−1 | 25.1 | 24.6 | 20.5–23.3 | 17.7 | 5.8–31.4 | ||
| Fv/Fm | 0.56 | 0.48–0.52 | 0.5–0.66 | 0.58 | 0.57 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergeeva, V.M.; Mosharov, S.A.; Shulga, N.A.; Kremenetskiy, V.V.; Khlebopashev, P.V.; Matorin, D.N. Response of the Coastal Phytoplankton Community to the Runoff from Small Rivers in the Northeastern Black Sea. Diversity 2023, 15, 857. https://doi.org/10.3390/d15070857
Sergeeva VM, Mosharov SA, Shulga NA, Kremenetskiy VV, Khlebopashev PV, Matorin DN. Response of the Coastal Phytoplankton Community to the Runoff from Small Rivers in the Northeastern Black Sea. Diversity. 2023; 15(7):857. https://doi.org/10.3390/d15070857
Chicago/Turabian StyleSergeeva, Valentina M., Sergey A. Mosharov, Natalia A. Shulga, Viacheslav V. Kremenetskiy, Pavel V. Khlebopashev, and Dmitry N. Matorin. 2023. "Response of the Coastal Phytoplankton Community to the Runoff from Small Rivers in the Northeastern Black Sea" Diversity 15, no. 7: 857. https://doi.org/10.3390/d15070857
APA StyleSergeeva, V. M., Mosharov, S. A., Shulga, N. A., Kremenetskiy, V. V., Khlebopashev, P. V., & Matorin, D. N. (2023). Response of the Coastal Phytoplankton Community to the Runoff from Small Rivers in the Northeastern Black Sea. Diversity, 15(7), 857. https://doi.org/10.3390/d15070857






