Seagrass Blue Carbon Stock and Air–Sea CO2 Fluxes in the Karimunjawa Islands, Indonesia during Southeast Monsoon Season
Abstract
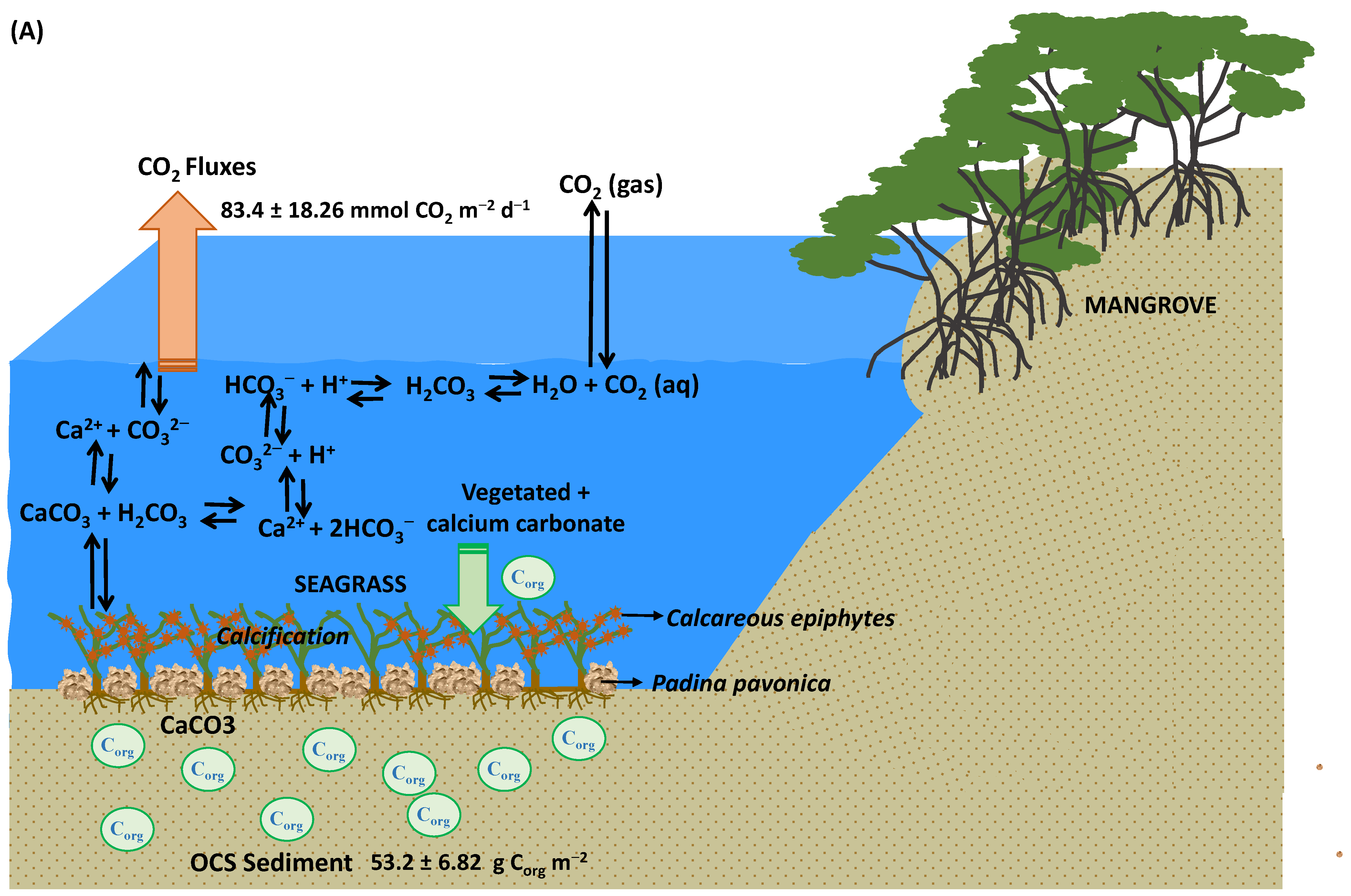
:1. Introduction
2. Materials and Methods
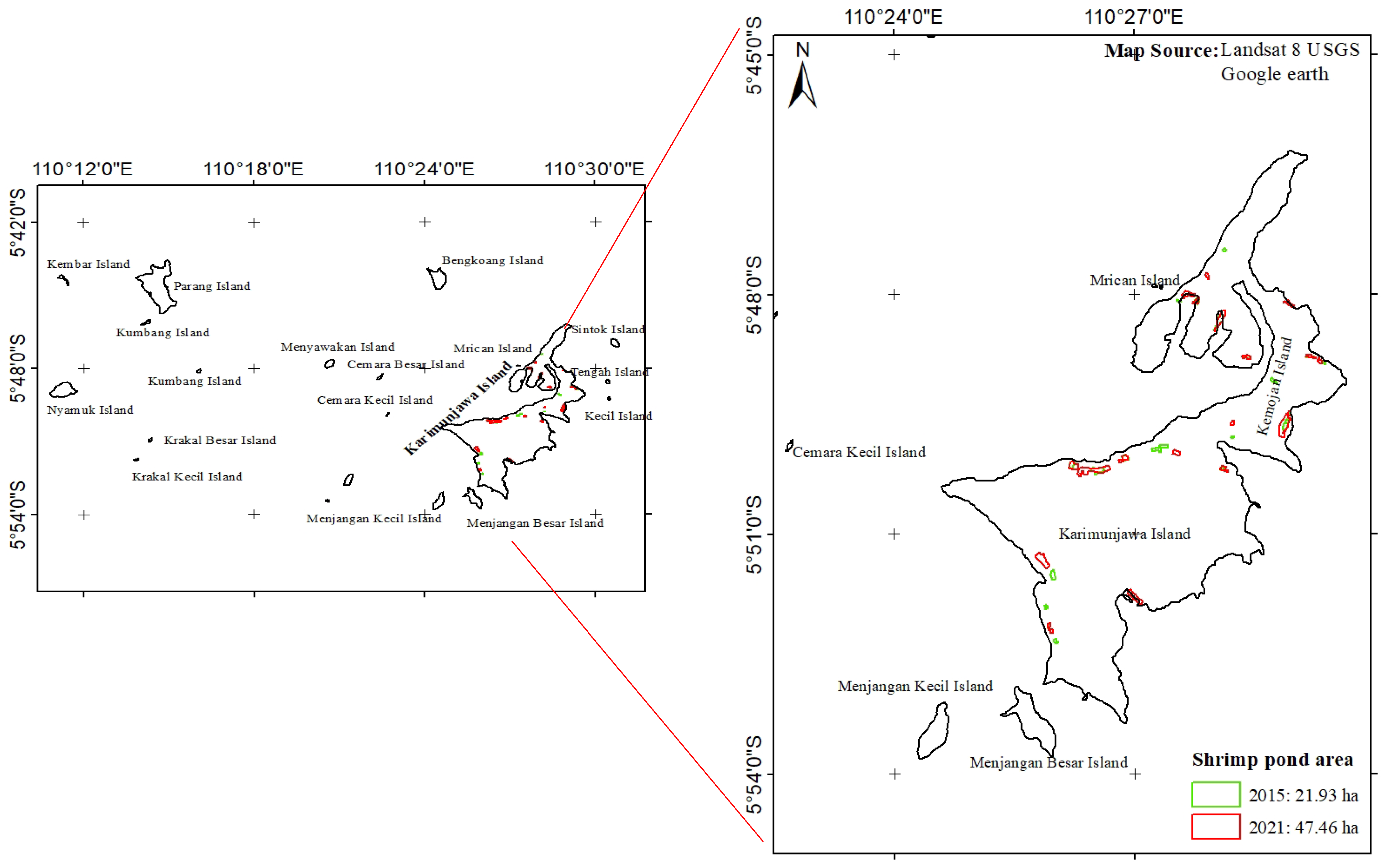
2.1. Study Sites
2.2. Data Collection
2.2.1. Primary Data
2.2.2. Secondary Data
2.3. Estimation of Carbon Stock and Storage
2.4. Estimation of Sea–Air CO2 Fluxes
2.5. Statistical Analyses
3. Results
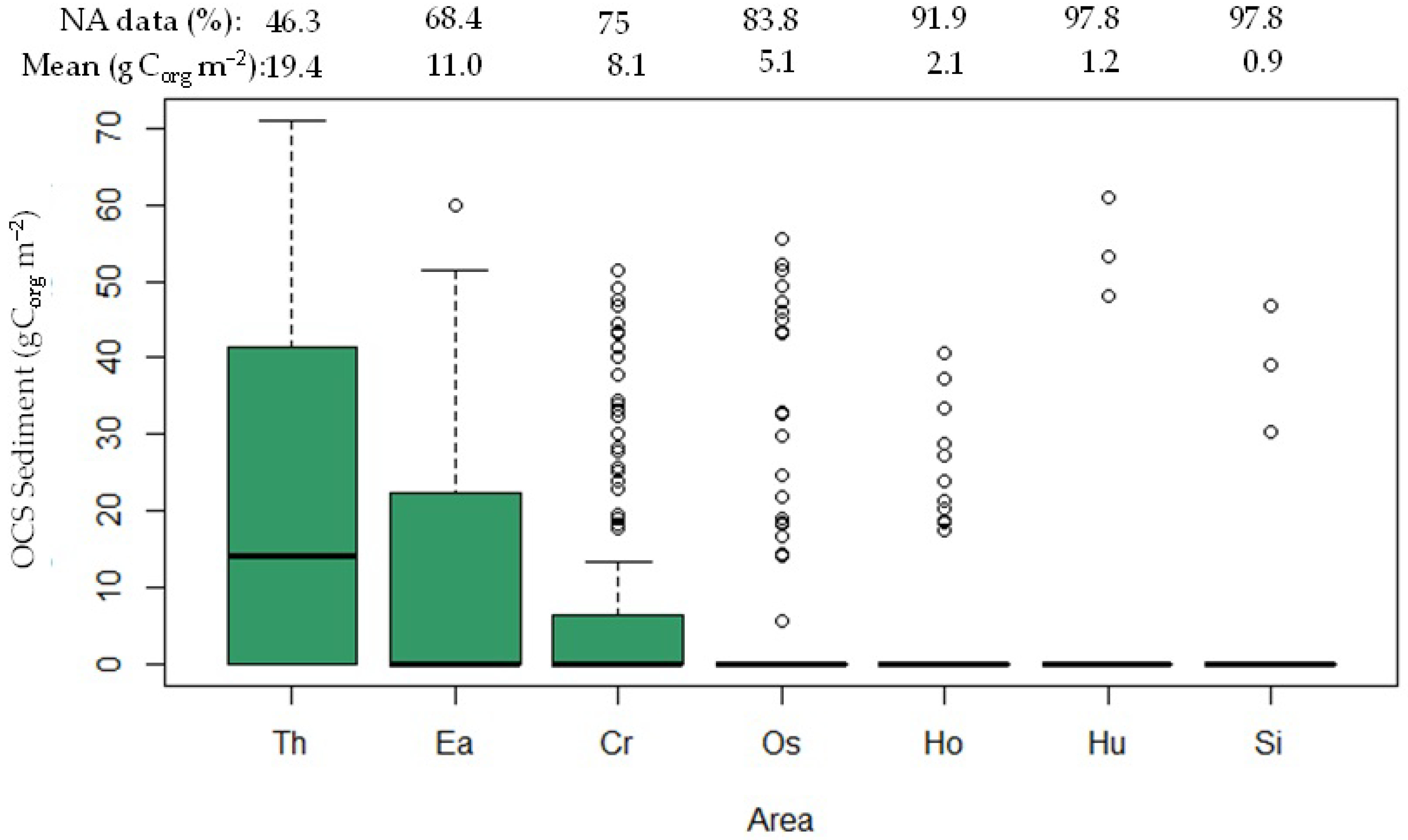
3.1. Organic Carbon Stock in the Seagrass Ecosystem
3.2. Air–Sea CO2 Fluxes
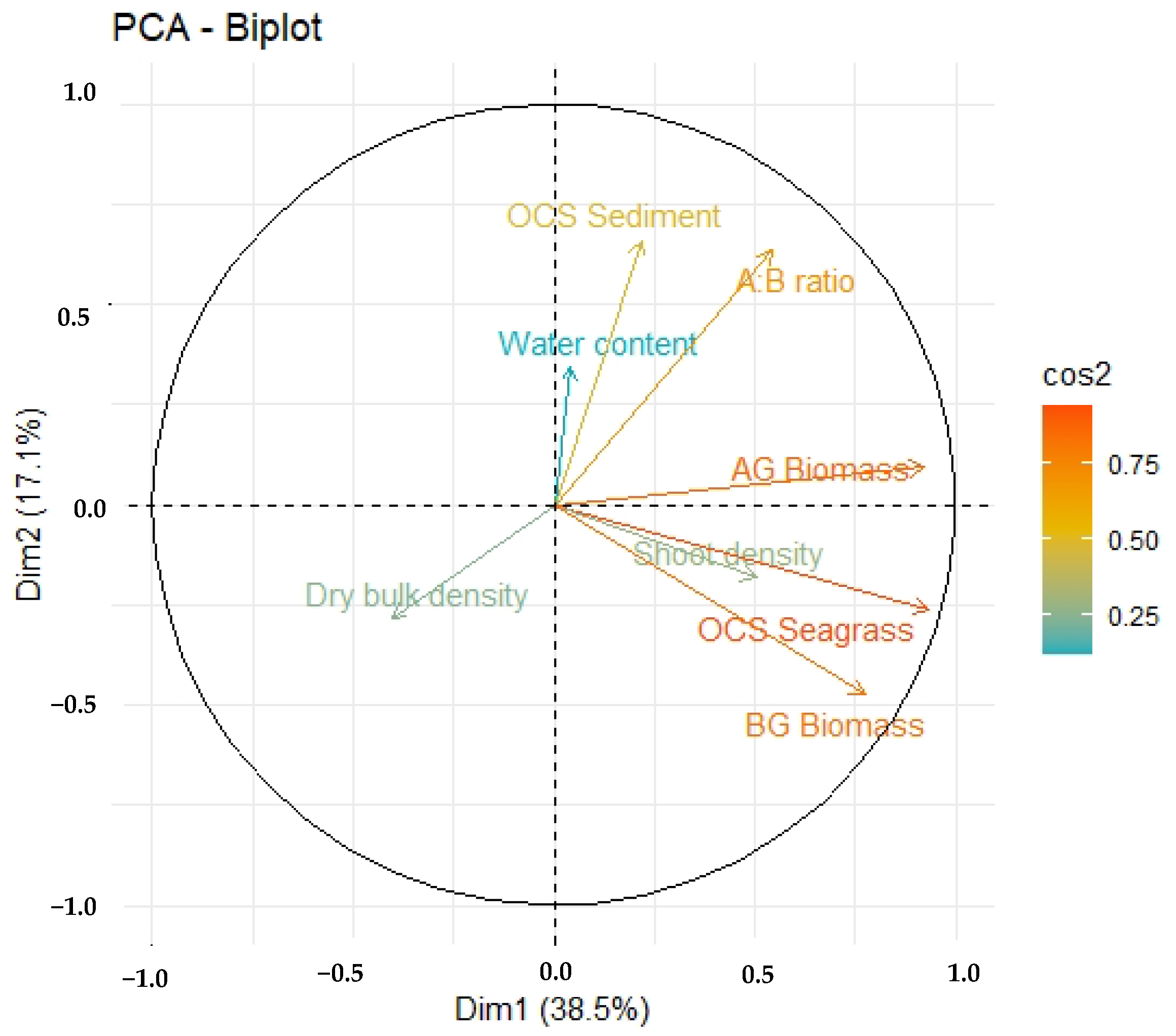
3.3. Organic Carbon Stock and Air–Sea CO2 Flux
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, T.; Olafsson, J.; Goddard, J.G.; Chipman, D.W.; Sutherland, S.C. Seasonal Variation of CO2 and Nutrients in the High-Latitude Oceans: A Comparative Study. Glob. Biogeochem. Cycles 1993, 7, 843–878. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Danie, J.; Michael, T.; Marco, B.; Sally, B.; Ines, C.; Arona, D.; Riyanti, D. Chapter 3: Impacts of 1.5 °C Global Warming on Natural and Human Systems. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Preindustrial Levels and Related Global Greenhouse Gas Emission Pathways; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; pp. 175–312. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. A Special Report of the Intergovernmental Panel on Climate Change; Pörtner, H.O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Nicolai, M., Okem, A., Petzold, J., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019. [Google Scholar]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.; Andrew, R.; Hauck, J.; Olsen, A.; Peters, G.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget 2020. Earth Syst. Sci. Data Discuss. 2020, 12, 3269–3340. [Google Scholar] [CrossRef]
- Gulev, S.K.; Thorne, P.W.; Ahn, J.; Dentener, F.J.; Domingues, C.M.; Gerland, S.; Gong, D.; Kaufman, D.S.; Nnamchi, H.C.; Quaas, J.; et al. Changing State of the Climate System. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 287–422. [Google Scholar] [CrossRef]
- Borges, A.V.; Morana, C.; Bouillon, S.; Servais, P.; Descy, J.P.; Darchambeau, F. Carbon Cycling of Lake Kivu (East Africa): Net Autotrophy in the Epilimnion and Emission of CO2 to the Atmosphere Sustained by Geogenic Inputs. PLoS ONE 2014, 9, e109500. [Google Scholar] [CrossRef]
- Tye, A.M.; Williamson, J.L.; Jarvie, H.P.; Dise, N.B.; Lapworth, D.J.; Monteith, D.; Sanders, R.; Mayor, D.J.; Bowes, M.J.; Bowes, M.; et al. Dissolved Inorganic Carbon Export from Rivers of Great Britain: Spatial Distribution and Potential Catchment-Scale Controls. J. Hydrol. 2022, 615, 128677. [Google Scholar] [CrossRef]
- Arora, V.K.; Jones, C.D.; Williams, R.; Katavouta, A.; Friedlingstein, P.; Brovkin, V.; Cadule, P.; Hajima, T.; Seferian, R.; Joetzjer, E.; et al. Carbon-Concentration and Carbon-Climate Feedbacks in CMIP6 Models. J. Clim. 2019. preprint. [Google Scholar]
- Christianson, A.B.; Cabré, A.; Bernal, B.; Baez, S.K.; Leung, S.; Pérez-Porro, A.; Poloczanska, E. The Promise of Blue Carbon Climate Solutions: Where the Science Supports Ocean-Climate Policy. Front. Mar. Sci. 2022, 9, 1–16. [Google Scholar] [CrossRef]
- Otero, P.; Padin, X.A.; Ruiz-Villarreal, M.; García-García, L.M.; Ríos, A.F.; Pérez, F.F. Net Sea-Air CO2 Flux Uncertainties in the Bay of Biscay Based on the Choice of Wind Speed Products and Gas Transfer Parameterizations. Biogeosciences 2013, 10, 2993–3005. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, Y.; Bao, Y.; Zhao, C.; Wang, G.; Zhang, Y.; Song, Z.; Wu, Z.; Qiao, F. Seasonal to Decadal Spatiotemporal Variations of the Global Ocean Carbon Sink. Glob. Chang. Biol. 2022, 28, 1786–1797. [Google Scholar] [CrossRef]
- Le Quéré, C.; Andres, R.J.; Boden, T.; Conway, T.; Houghton, R.A.; House, J.I.; Marland, G.; Peters, G.P.; Van Der Werf, G.R.; Ahlström, A.; et al. The Global Carbon Budget 1959–2011. Earth Syst. Sci. Data 2013, 5, 165–185. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass Ecosystems as a Globally Significant Carbon Stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Mazarrasa, I.; Marbà, N.; Lovelock, C.E.; Serrano, O.; Lavery, P.S.; Fourqurean, J.W.; Kennedy, H.; Mateo, M.A.; Krause-Jensen, D.; Steven, A.D.L.; et al. Seagrass Meadows as a Globally Significant Carbonate Reservoir. Biogeosciences 2015, 12, 4993–5003. [Google Scholar] [CrossRef]
- Bayley, D.; Marengo, I.; Pelembe, T. Giant Kelp ‘Blue Carbon ’ Storage and Sequestration Value in the Falkland Islands; Joint Nature Conservation Committee: Peterborough, UK, 2017; p. 31. [Google Scholar] [CrossRef]
- Egea, L.G.; Jiménez-Ramos, R.; Hernández, I.; Bouma, T.J.; Brun, F.G. Effects of Ocean Acidification and Hydrodynamic Conditions on Carbon Metabolism and Dissolved Organic Carbon (DOC) Fluxes in Seagrass Populations. PLoS ONE 2018, 13, e0192402. [Google Scholar] [CrossRef]
- Watanabe, K.; Kuwae, T. How Organic Carbon Derived from Multiple Sources Contributes to Carbon Sequestration Processes in a Shallow Coastal System? Glob. Chang. Biol. 2015, 21, 2612–2623. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.M.; Walton, M. Manual for the Creation of Blue Carbon Projects in Europe and the Mediterranean; IUCN, International Union for Conservation of Nature Center for Mediterranean Cooperation: Malaga, Spain, 2021. [Google Scholar]
- Samper-Villarreal, J.; Lovelock, C.E.; Saunders, M.I.; Roelfsema, C.; Mumby, P.J. Organic Carbon in Seagrass Sediments Is Influenced by Seagrass Canopy Complexity, Turbidity, Wave Height, and Water Depth. Limnol. Oceanogr. 2016, 61, 938–952. [Google Scholar] [CrossRef]
- Salinas, C.; Duarte, C.M.; Lavery, P.S.; Masque, P.; Arias-Ortiz, A.; Leon, J.X.; Callaghan, D.; Kendrick, G.A.; Serrano, O. Seagrass Losses since Mid-20th Century Fuelled CO2 Emissions from Soil Carbon Stocks. Glob. Chang. Biol. 2020, 26, 4772–4784. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, C.E.; Fourqurean, J.W.; Morris, J.T. Modeled CO2 Emissions from Coastal Wetland Transitions to Other Land Uses: Tidal Marshes, Mangrove Forests, and Seagrass Beds. Front. Mar. Sci. 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Akhand, A.; Watanabe, K.; Chanda, A.; Tokoro, T.; Chakraborty, K.; Moki, H.; Tanaya, T.; Ghosh, J.; Kuwae, T. Lateral Carbon Fluxes and CO2 Evasion from a Subtropical Mangrove-Seagrass-Coral Continuum. Sci. Total Environ. 2021, 752, 142190. [Google Scholar] [CrossRef]
- Macklin, P.A.; Suryaputra, I.G.N.A.; Maher, D.T.; Murdiyarso, D.; Santos, I.R. Drivers of CO2 along a Mangrove-Seagrass Transect in a Tropical Bay: Delayed Groundwater Seepage and Seagrass Uptake. Cont. Shelf Res. 2019, 172, 57–67. [Google Scholar] [CrossRef]
- Thorhaug, A.; Gallagher, J.B.; Kiswara, W.; Prathep, A.; Huang, X.; Yap, T.K.; Dorward, S.; Berlyn, G. Coastal and Estuarine Blue Carbon Stocks in the Greater Southeast Asia Region: Seagrasses and Mangroves per Nation and Sum of Total. Mar. Pollut. Bull. 2020, 160, 111168. [Google Scholar] [CrossRef]
- Wahyudi, A.J.; Rahmawati, S.; Irawan, A.; Hadiyanto, H.; Prayudha, B.; Hafizt, M.; Afdal, A.; Adi, N.S.; Rustam, A.; Hernawan, U.E.; et al. Assessing Carbon Stock and Sequestration of the Tropical Seagrass Meadows in Indonesia. Ocean Sci. J. 2020, 55, 85–97. [Google Scholar] [CrossRef]
- Wahyudi, A.J.; Febriani, F. Country-Specific Emission Factor for Developing a Tier 3 System of Indonesia’s Seagrass Carbon Inventory. IOP Conf. Ser. Earth Environ. Sci. 2021, 944, 012058. [Google Scholar] [CrossRef]
- Ganefiani, A.; Suryanti, S.; Latifah, N. Potensi Padang Lamun sebagai Penyerap Karbon di Perairan Pulau Karimunjawa, Taman Nasional Karimunjawa. SAINTEK Perikan. Indones. J. Fish. Sci. Technol. 2019, 14, 115. [Google Scholar] [CrossRef]
- Ramadona, D.N.; Ain, C.; Febrianto, S.; Suryanti, S.; Latifah, N. Carbon Storage Potential of Seagrass Meadows in Pokemon Beach, Karimunjawa. J. Ilmu Teknologi. Kelautan Tropis. 2021, 13, 319–332. [Google Scholar] [CrossRef]
- Latifah, N.; Febrianto, S.; Wirasatriya, A.; Endrawati, H.; Zainuri, M.; Suryanti, S.; Hidayat, A.N. Air-Sea Flux of CO2 in the Waters of Karimunjawa Island, Indonesia. Saintek Perikan. Indones. J. Fish. Sci. Technol. 2020, 16, 171–178. [Google Scholar] [CrossRef]
- Latifah, N.; Endrawati, H.; Febrianto, S. Distribusi Spasial Fluks Karbon Dioksida Di Perairan Karimunjawa, Indonesia. J. Ilmu Teknol. Kelaut. Trop. 2019, 11, 357–368. [Google Scholar] [CrossRef]
- Kartadikaria, A.R.; Watanabe, A.; Nadaoka, K.; Adi, N.S.; Prayitno, H.B.; Soemorumekso, S.; Muchtar, M.; Triyulianti, I.; Setiawan, A.; Suratno, S.; et al. CO2 Sink/Source Characteristics in the Tropical Indonesian Seas. J. Geophys. Res. Ocean. 2015, 120, 7842–7856. [Google Scholar] [CrossRef]
- Wirasatriya, A.; Sugianto, D.N.; Maslukah, L.; Ahkam, M.F.; Wulandari, S.Y.; Helmi, M. Carbon Dioxide Flux in the Java Sea Estimated from Satellite Measurements. Remote Sens. Appl. Soc. Environ. 2020, 20, 100376. [Google Scholar] [CrossRef]
- Aryetti, F.; Pratomo, D.G.; Pribadi, C.B. Utilization of Bathymetry and Sea Surface Current Data for Coral Reef Habitats in The Karimunjawa Islands. IOP Conf. Ser. Earth Environ. Sci. 2023, 1127, 012041. [Google Scholar] [CrossRef]
- Wungo, G.L.; Mussadun, M.; Ma’rif, S. Edukasi Penerapan Konsep Ecotourism Di Kepulauan Karimunjawa. J. Pasopati 2020, 2, 142–149. [Google Scholar]
- Hach Company. Scope and Application: For Water, Wastewater and Seawater. Nitrate; Hach Company: Loveland, CO, USA, 2014; Volume 584. [Google Scholar]
- Hach Company. DR/2010 Spectrophotometer Procedures Manual; Hach Company: Loveland, CO, USA, 2010. [Google Scholar]
- Hach Company. Scope and Application: For Water, Wastewater and Seawater. Phosporus, Reactive (Orthophospahate); Hach Company: Loveland, CO, USA, 2014; Volume 584. [Google Scholar]
- Rahmawati, S.; Hernawan, U.E.; Rustam, A. The Seagrass Carbon Content of 0.336 of Dry Weight Can Be Applied in Indonesian Seagrasses. AIP Conf. Proc. 2019, 2120, 030012. [Google Scholar] [CrossRef]
- Wanninkhof, R.; McGill, W. A Cubic Relationship between Air-Sea CO2 Exchage and Wind Speed. Geophys. Res. Lett. 1999, 26, 1889–1892. [Google Scholar] [CrossRef]
- Takahashi, T.; Sutherland, S.C.; Sweeney, C.; Poisson, A.; Metzl, N.; Tilbrook, B.; Bates, N.; Wanninkhof, R.; Feely, R.A.; Sabine, C.; et al. Global Sea-Air CO2 Flux Based on Climatological Surface Ocean PCO2, and Seasonal Biological and Temperature Effects. Deep. Res. Part II Top. Stud. Oceanogr. 2002, 49, 1601–1622. [Google Scholar] [CrossRef]
- Zhu, Y.; Shang, S.; Zhai, W.; Dai, M. Satellite-Derived Surface Water PCO2 and Air-Sea CO2 Fluxes in the Northern South China Sea in Summer. Prog. Nat. Sci. 2009, 19, 775–779. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Y.; Feng, M.; Wang, T.; Zhang, N.; Wijffels, S. Decadal Trends of the Upper Ocean Salinity in the Tropical Indo-Pacific since Mid-1990s. Sci. Rep. 2015, 5, 16050. [Google Scholar] [CrossRef] [PubMed]
- Sisma-Ventura, G.; Bialik, O.M.; Yam, R.; Herut, B.; Silverman, J. PCO2 Variability in the Surface Waters of the Ultra-Oligotrophic Levantine Sea: Exploring the Air–Sea CO2 Fluxes in a Fast Warming Region. Mar. Chem. 2017, 196, 13–23. [Google Scholar] [CrossRef]
- Yan, H.; Yu, K.; Shi, Q.; Lin, Z.; Zhao, M.; Tao, S.; Liu, G.; Zhang, H. Air-Sea CO2 Fluxes and Spatial Distribution of Seawater PCO2 in Yongle Atoll, Northern-Central South China Sea. Cont. Shelf Res. 2018, 165, 71–77. [Google Scholar] [CrossRef]
- Weiss, R.F. Carbon Dioxide in Water and Seawater: The Solubility of a Non-Ideal Gas. Mar. Chem. 1974, 2, 203–215. [Google Scholar] [CrossRef]
- Wanninkhof, R. Relationship between Wind Speed and Gas Exchange over the Ocean. J. Geophys. Res. 1992, 97, 7373–7382. [Google Scholar] [CrossRef]
- Raymond, P.A.; Cole, J.J. Technical Notes and Comments: Gas Exchange in Rivers and Estuaries: Choosing a Gas Transfer Velocity. Estuaries 2001, 24, 312–317. [Google Scholar] [CrossRef]
- Borges, A.V.; Vanderborght, J.P.; Schiettecatte, L.S.; Gazeau, F.; Ferrón-Smith, S.; Delille, B.; Frankignoulle, M. Variability of the Gas Transfer Velocity of CO2 in a Macrotidal Estuary (the Scheldt). Estuaries 2004, 27, 593–603. [Google Scholar] [CrossRef]
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. Guide to Best Practices for Ocean CO2 Measurements; North Pacific Marine Science Organization: Sidney, Australia, 2007; Volume 3, ISBN 1-897176-07-4. [Google Scholar]
- Williams, L.J. Newman-Keuls Test and Tukey Test 1 Pairwise Comparisons. Encylopedia Res. Des. 2010, 1, 897–902. [Google Scholar]
- Park, S.R.; Moon, K.; Kim, S.H.; Lee, K.S. Growth and Photoacclimation Strategies of Three Zostera Species Along a Vertical Gradient: Implications for Seagrass Zonation Patterns. Front. Mar. Sci. 2021, 8, 594779. [Google Scholar] [CrossRef]
- Kim, S.H.; Suonan, Z.; Qin, L.Z.; Kim, H.; Park, J.I.; Kim, Y.K.; Lee, S.; Kim, S.G.; Kang, C.K.; Lee, K.S. Variability in Blue Carbon Storage Related to Biogeochemical Factors in Seagrass Meadows off the Coast of the Korean Peninsula. Sci. Total Environ. 2022, 813, 152680. [Google Scholar] [CrossRef] [PubMed]
- Gullstrom, M.; Lyimo, L.D.; Dahl, M.; Eggertsen, M.; Anderberg, E.; Rasmusson, L.M.; Mtwana, L.; Linderholm, H.W.; Knudby, A.; Bjo, M. Blue Carbon Storage in Tropical Seagrass Meadows Relates to Carbonate Stock Dynamics, Plant—Sediment Processes, and Landscape Context: Insights from the Western Indian Ocean. Ecosystems 2018, 21, 551–566. [Google Scholar] [CrossRef]
- Ricart, A.M.; Pérez, M.; Romero, J. Landscape Configuration Modulates Carbon Storage in Seagrass Sediments. Estuar. Coast. Shelf Sci. 2017, 185, 69–76. [Google Scholar] [CrossRef]
- Duarte, C.M.; Marbà, N.; Gacia, E.; Fourqurean, J.W.; Beggins, J.; Barrón, C.; Apostolaki, E.T. Seagrass Community Metabolism: Assessing the Carbon Sink Capacity of Seagrass Meadows. Glob. Biogeochem. Cycles 2010, 24, 1–8. [Google Scholar] [CrossRef]
- Lavery, P.S.; Mateo, M.Á.; Serrano, O.; Rozaimi, M. Variability in the Carbon Storage of Seagrass Habitats and Its Implications for Global Estimates of Blue Carbon Ecosystem Service. PLoS ONE 2013, 8, e73748. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating Loss of Seagrasses across the Globe Threatens Coastal Ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef]
- Alongi, D.M.; Murdiyarso, D.; Fourqurean, J.W.; Kauffman, J.B.; Hutahaean, A.; Crooks, S.; Lovelock, C.E.; Howard, J.; Herr, D.; Fortes, M.; et al. Indonesia’s Blue Carbon: A Globally Significant and Vulnerable Sink for Seagrass and Mangrove Carbon. Wetl. Ecol. Manag. 2015, 23, 3–13. [Google Scholar] [CrossRef]
- McLeod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A Blueprint for Blue Carbon: Toward an Improved Understanding of the Role of Vegetated Coastal Habitats in Sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, S.; Zhang, J. Eutrophication Indirectly Reduced Carbon Sequestration in a Tropical Seagrass Bed Eutrophication Indirectly Reduced Carbon Sequestration in a Tropical Seagrass Bed. Plant Soil 2018, 426, 135–152. [Google Scholar] [CrossRef]
- Asplund, M.E.; Dahl, M.; Ismail, R.O.; Arias-Ortiz, A.; Deyanova, D.; Franco, J.N.; Hammar, L.; Hoamby, A.I.; Linderholm, H.W.; Lyimo, L.D.; et al. Dynamics and Fate of Blue Carbon in a Mangrove–Seagrass Seascape: Influence of Landscape Configuration and Land-Use Change. Landsc. Ecol. 2021, 36, 1489–1509. [Google Scholar] [CrossRef]
- Avnimelech, Y.; Ritvo, G.; Meijer, L.E. Water Content, Organic Carbon and Dry Bulk Density in Flooded Sediments Water Content, Organic Carbon and Dry Bulk Density in Flooded Sediments. Aquac. Eng. 2001, 25, 25–33. [Google Scholar] [CrossRef]
- Susana, T. Karbon Dioksida. Oseana 1988, XIII, 1–11. [Google Scholar]
- Curbelo-Hernández, D.; Santana-Casiano, J.M.; González, A.G.; González-Dávila, M. Air-Sea CO2 Exchange in the Strait of Gibraltar. Front. Mar. Sci. 2021, 8, 745304. [Google Scholar] [CrossRef]
- Fitranti, B.A.; Juliandri, D.; Herunadi, B. Potensi Pelepasan dan Penyerap CO2 Kaitannya Dengan Suhu Dan Salinitas Di Perairan Teluk Banten. J. Akuatika 2013, 2, 174–182. [Google Scholar]
- Basah, P.B.; Bontang, K.; Timur, K. Distribusi Karbon Anorganik Dan Fluks CO2. Lingkungan Tropis 2016, 6, 149–158. [Google Scholar]
- Afdal. Fluks CO2 Di Perairan Pesisir Pulau Lombok, Nusa Tenggara Barat CO2 Flux in the Coastal Waters of Lombok, West Nusa Tenggara. Oseanologi Limnol. 2016, 1, 91–103. [Google Scholar]
- Cai, W.J.; Dai, M.; Wang, Y. Air-Sea Exchange of Carbon Dioxide in Ocean Margins: A Province-Based Synthesis. Geophys. Res. Lett. 2006, 33, 2–5. [Google Scholar] [CrossRef]
- Ito, R.G.; Tavano, V.M.; Mendes, C.R.B.; Garcia, C.A.E. Sea-Air CO2 Fluxes and PCO2 Variability in the Northern Antarctic Peninsula during Three Summer Periods (2008–2010). Deep. Res. Part II Top. Stud. Oceanogr. 2018, 149, 84–98. [Google Scholar] [CrossRef]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A Comprehensive Quantification of Global Nitrous Oxide Sources and Sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Zheng, Z.; Luo, X.; Wei, H.; Zhao, W.; Qi, D. Analysis of the Seasonal and Interannual Variations of Air-Sea CO2 Flux in the Chukchi Sea Using A Coupled Ocean-Sea Ice-Biogeochemical Model. J. Geophys. Res. Ocean. 2021, 126, e2021JC017550. [Google Scholar] [CrossRef]
- Curbelo-Hernández, D.; González-Dávila, M.; González, A.G.; González-Santana, D.; Santana-Casiano, J.M. CO2 Fluxes in the Northeast Atlantic Ocean Based on Measurements from a Surface Ocean Observation Platform. Sci. Total Environ. 2021, 775, 145804. [Google Scholar] [CrossRef]
- Semesi, I.S.; Beer, S.; Björk, M. Seagrass Photosynthesis Controls Rates of Calcification and Photosynthesis of Calcareous Macroalgae in a Tropical Seagrass Meadow. Mar. Ecol. Prog. Ser. 2009, 382, 41–47. [Google Scholar] [CrossRef]
- Hofmann, L.C.; Bischof, K. Ocean Acidification Effects on Calcifying Macroalgae. Aquat. Biol. 2014, 22, 261–279. [Google Scholar] [CrossRef]
- Kalokora, O.J.; Buriyo, A.S.; Asplund, M.E.; Gullström, M.; Mtolera, M.S.P.; Björk, M. An Experimental Assessment of Algal Calcification as a Potential Source of Atmospheric CO2. PLoS ONE 2020, 15, e0231971. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.O.; Asplund, M.E.; Gullström, M.; George, R.; Dahl, M.; Buriyo, A.S.; Mtolera, M.S.P.; Björk, M. Effects of Calcification on Air-Water CO2 Fluxes in Tropical Seagrass Meadows: A Mesocosm Experiment. J. Exp. Mar. Biol. Ecol. 2023, 561, 151864. [Google Scholar] [CrossRef]
- Banerjee, K.; Paneerselvam, A.; Ramachandran, P.; Ganguly, D.; Singh, G.; Ramesh, R. Seagrass and Macrophyte Mediated CO2 and CH4 Dynamics in Shallow Coastal Waters. PLoS ONE 2018, 13, e0249253. [Google Scholar] [CrossRef]
- Cui, L.; Jiang, Z.; Huang, X.; Chen, Q.; Wu, Y.; Liu, S.; Li, J.; Macreadie, P.I. Eutrophication Reduces Seagrass Contribution to Coastal Food Webs. Ecosphere 2021, 12, e03626. [Google Scholar] [CrossRef]
- Duarte, C.M. Allometric Scaling of Seagrass Form and Productivity. Mar. Ecol. Prog. Ser. 1991, 77, 289–300. [Google Scholar] [CrossRef]
- Cebrián, J.; Duarte, C.M.; Marbà, N.; Enríquez, S. Magnitude and Fate of the Production of Four Co-Occurring Western Mediterranean Seagrass Species. Mar. Ecol. Prog. Ser. 1997, 155, 29–44. [Google Scholar] [CrossRef]
- Ware, J.R.; Smith, S.V.; Reaka-Kudla, M.L. Coral Reefs: Sources or Sinks of Atmospheric CO2? Coral Reefs 1991, 11, 127–130. [Google Scholar] [CrossRef]
- Frankignoulle, M.; Canon, C.; Gattuso, J.-P. Marine Calcification as a Source of Carbon Dioxide: Positive Feedback of Increasing Atmospheric CO2. Limnol. Oceanogr. 1994, 39, 458–462. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Serrano, O.; Maher, D.T.; Duarte, C.M.; Beardall, J. Addressing Calcium Carbonate Cycling in Blue Carbon Accounting. Limnol. Oceanogr. Lett. 2017, 2, 195–201. [Google Scholar] [CrossRef]
- Chou, W.-C.; Fan, L.-F.; Hung, C.-C.; Shih, Y.-Y.; Huang, W.-J.; Lui, H.-K.; Chen, T.-Y. Dynamics of O2 and PCO2 in a Southeast Asia Seagrass Meadow: Metabolic Rates and Carbon Sink Capacity. Front. Mar. Sci. 2023, 10, 1076991. [Google Scholar] [CrossRef]
- Chou, W.C.; Fan, L.F.; Yang, C.C.; Chen, Y.H.; Hung, C.C.; Huang, W.J.; Shih, Y.Y.; Soong, K.; Tseng, H.C.; Gong, G.C.; et al. A Unique Diel Pattern in Carbonate Chemistry in the Seagrass Meadows of Dongsha Island: The Enhancement of Metabolic Carbonate Dissolution in a Semienclosed Lagoon. Front. Mar. Sci. 2021, 8, 717685. [Google Scholar] [CrossRef]
- Van Dam, B.R.; Lopes, C.C.; Polsenaere, P.; Price, R.M.; Rutgersson, A.; Fourqurean, J.W. Water Temperature Control on CO2 Flux and Evaporation over a Subtropical Seagrass Meadow Revealed by Atmospheric Eddy Covariance. Limnol. Oceanogr. 2021, 66, 510–527. [Google Scholar] [CrossRef]
- Saderne, V.; Geraldi, N.R.; Macreadie, P.I.; Maher, D.T.; Middelburg, J.J.; Serrano, O.; Almahasheer, H.; Arias-Ortiz, A.; Cusack, M.; Eyre, B.D.; et al. Role of Carbonate Burial in Blue Carbon Budgets. Nat. Commun. 2019, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, A.; Ó Corcora, T.C.; Hukriede, W.; Schubert, P.R.; Reusch, T.B.H. Substantial Seagrass Blue Carbon Pools in the Southwestern Baltic Sea Include Relics of Terrestrial Peatlands. Front. Mar. Sci. 2022, 9, 1–17. [Google Scholar] [CrossRef]




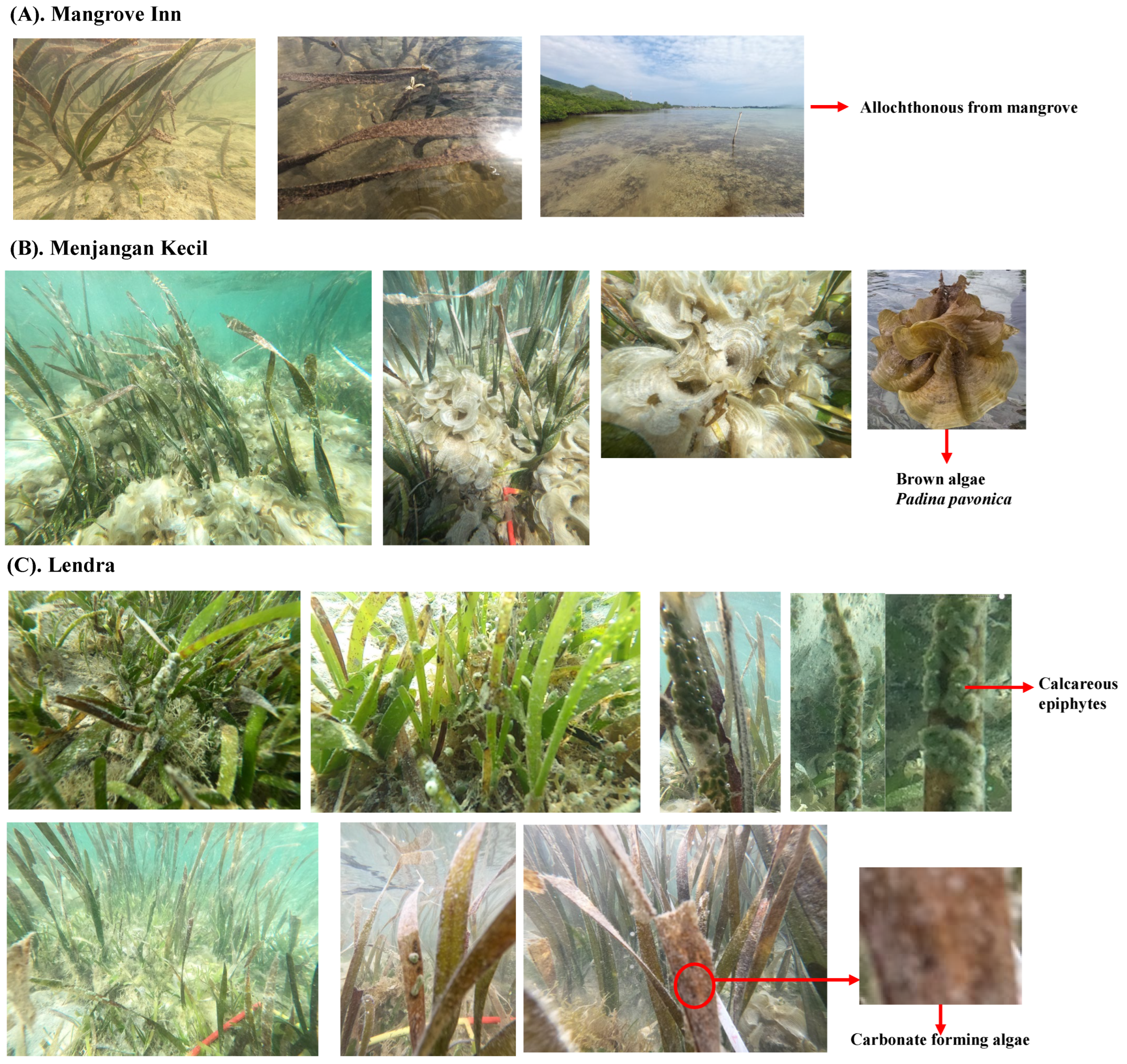

| Parameters | Unit | Procedure | Tools/Methods |
|---|---|---|---|
| Temperature | °C | In situ measurement | Thermometer and logger |
| pH | - | In situ measurement | pH meter |
| DO | mg L−1 | In situ measurement | DO meters |
| CO2atm | ppm | In situ measurement | CO2 meters |
| Salinity | ‰ | In situ measurement | Refractometer |
| Nitrate | ppm | Laboratory measurements (100 mL sample) | Spectrophotometer/Spectrophotometry with a wavelength of 500 nm, nitraver 5 nitrate reagent powder pillow [35] |
| Silicate | ppm | Laboratory measurements (100 mL sample) | Spectrophotometer/Spectrophotometry with a wavelength of 452 nm, molybdate reagent powder pillows [36] |
| Total phosphate | ppm | Laboratory measurements (100 mL sample) | Spectrophotometer/Spectrophotometry with a wavelength of 890 nm, phosver 3 phospate reagent powder pillow [37] |
| Chlorophyll-a | mg m−3 | Laboratory measurements (1500 mL sample) | Spectrophotometer/Spectrophotometry with a wavelength of 630, 647, 664, and 750 nm [36] |
| Study Sites | Seagrass Species | Shoot Density (Shoots m−2) | Tissue Carbon Content/OCC (%) | Biomass (gDW m−2) | A:B Ratio | OCS (gCorg m−2) | OCS-Sg (gCorg m−2) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| AG | BG | AG | BG | AG | BG | |||||
| East | ||||||||||
| Bobby | ||||||||||
| 2019 (n = 22) | Ea, Th, Cr, Ho, Hu | 446.5 ± 172.7 | 6.5 ± 0.8 | 7.6 ± 1.0 | 2.8 ± 0.3 | 4.5 ± 0.7 | 0.7 ± 0.0 | 15.6 ± 1.4 | 24.6 ± 3.9 | 40.17 ± 4.8 |
| 2022 (n = 32) | Ea, Th, Cr, Os, Ho | 212.7 ± 40.2 | 4.9 ± 1.2 | 8.3 ± 1.4 | 3.6 ± 0.5 | 2.2 ± 0.5 | 1.9 ± 0.3 | 16.0 ± 2.9 | 15.2 ± 3.3 | 28.5 ± 6.2 |
| Pokemon | ||||||||||
| 2019 (n = 18) | Ea, Th, Cr, Ho | 278.2 ± 85.3 | 4.9 ± 0.0 | 5.6 ± 0.0 | 1.3 ± 0.2 | 2.1 ± 0.4 | 0.5 ± 0.1 | 9.3 ± 0.5 | 14.8 ± 0.8 | 18.7 ± 3.6 |
| Barakuda | ||||||||||
| 2019 (n = 18) | Ea, Th, Cr, Hu | 158.2 ± 51.2 | 5.0 ± 0.0 | 5.6 ± 0.0 | 1.4 ± 0.0 | 2.6 ± 0.2 | 0.6 ± 0.0 | 7.8 ± 0.3 | 14.4 ± 1.3 | 22.2 ± 1.4 |
| West | ||||||||||
| Mangrove Inn | ||||||||||
| 2019 (n = 18) | Ea, Th | 39.6 ± 4.5 | 4.9 ± 0.0 | 5.6 ± 0.0 | 2.3 ± 0.2 | 3.8 ± 0.3 | 0.6 ± 0.1 | 13.2 ± 0.9 | 21.3 ± 1.8 | 34.5 ± 2.1 |
| 2022 (n = 34) | Ea, Th, Cr, Os, Ho | 74.8 ± 16.5 | 7.9 ± 1.5 | 9.7 ± 1.8 | 4.4 ± 0.4 | 3.5 ± 1.1 | 2.1 ± 0.5 | 21.1 ± 2.4 | 21.5 ± 6.6 | 43.3 ± 8.1 |
| Alang–Alang | ||||||||||
| 2019 (n = 22) | Ea, Th, Cr, Os | 156.4 ± 40.1 | 5.4 ± 1.0 | 6.2 ± 1.1 | 2.1 ± 0.2 | 2.6 ± 0.2 | 0.8 ± 0.1 | 12.0 ± 1.1 | 14.6 ± 1.2 | 26.6 ± 1.8 |
| 2022 (n = 40) | Ea, Th, Cr, Os, Ho | 188.8 ± 33.4 | 7.8 ± 1.1 | 11.5 ± 1.8 | 4.7 ± 0.8 | 3.5 ± 0.5 | 1.3 ± 0.1 | 22.3 ± 4.8 | 20.3 ± 3.3 | 42.6 ± 6.6 |
| Mrican | ||||||||||
| 2019 (n = 20) | Ea, Th, Cr, Os | 107.6 ± 12.7 | 5.4 ± 0.5 | 6.1 ± 0.6 | 1.8 ± 0.2 | 3.0 ± 0.4 | 0.6 ± 0.1 | 10.1 ± 1.1 | 16.1 ± 2.1 | 26.1 ± 2.7 |
| Cemara Kecil | ||||||||||
| 2022 (n = 18) | Th | 205.2 ± 29.0 | 4.5 ± 0.5 | 5.3 ± 0.6 | 1.6 ± 0.3 | 1.7 ± 0.2 | 0.9 ± 0.2 | 10.2 ± 1.4 | 10.8 ± 0.5 | 18.9 ± 2.4 |
| North | ||||||||||
| Lendra | ||||||||||
| 2022 (n = 36) | Ea, Th, Cr, Os, Ho | 250 ± 35.0 | 9.2 ± 1.0 | 10.4 ± 1.2 | 3.1 ± 0.5 | 3.3 ± 0.4 | 0.9 ± 0.1 | 18.4 ± 3.5 | 19.3 ± 2.3 | 37.7 ± 5.2 |
| South | ||||||||||
| Pelabuhan | ||||||||||
| 2022 (n = 46) | Ea, Th, Cr, Ho, Si | 339.7 ± 37.9 | 8.2 ± 0.9 | 9.2 ± 1.0 | 4.3 ± 0.6 | 2.7 ± 0.3 | 1.7 ± 0.2 | 26.7 ± 3.4 | 16.0 ± 1.8 | 42.7 ± 4.8 |
| Menjangan kecil | ||||||||||
| 2022 (n = 63) | Ea, Th, Cr, Os, Ho | 273.6 ± 28.9 | 9.2 ± 1.3 | 11.0 ± 1.5 | 3.9 ± 0.6 | 4.1 ± 0.5 | 0.9 ± 0.1 | 18.8 ± 3.5 | 22.0 ± 3.0 | 41.0 ± 6.1 |
| Study Sites | Water Content (%) | Dry Bulk Density (g cm−3) | %LOI | SOCC (%) | OCS-S (gCorg m−2) |
|---|---|---|---|---|---|
| East | |||||
| Bobby | |||||
| 2019 (vegetated; n = 9) | 18.7 ± 0.8 | 0.0051 ± 0.0005 | 23.0 ± 0.7 | 9.5 ± 0.3 | 39.9 ± 0.9 |
| 2022 (vegetated; n = 12) | 22.7 ± 1.7 | 0.0048 ± 0.0004 | 28.7 ± 1.7 | 12.0 ± 0.7 | 47.3 ± 1.7 |
| 2022 (unvegetated; n = 2) | 41.7 ± 8.4 | 0.0047 ± 0.0006 | 34.7 ± 1.9 | 14.6 ± 0.8 | 37.8 ± 3.6 |
| Pokemon | |||||
| 2019 (vegetated; n = 9) | 15.8 ± 0.6 | 0.0053 ± 0.0005 | 20.0 ± 0.7 | 8.3 ± 0.3 | 35.3 ± 1.0 |
| Barakuda | |||||
| 2019 (vegetated; n = 9) | 15.0 ± 0.7 | 0.0049 ± 0.0002 | 19.5 ± 0.7 | 8.0 ± 0.3 | 34.8 ± 1.0 |
| West | |||||
| Mangrove Inn | |||||
| 2019 (vegetated; n = 9) | 21.1 ± 0.8 | 0.0035 ± 0.0002 | 25.3 ± 0.8 | 10.6 ± 0.4 | 42.3 ± 1.0 |
| 2022 (vegetated; n = 10) | 19.0 ± 1.4 | 0.0036 ± 0.0002 | 36.0 ± 5.2 | 15.2 ± 2.2 | 63.6 ± 10.4 |
| 2022 (unvegetated; n = 2) | 16.3 ± 2.2 | 0.0070 ± 0.0002 | 27.4 ± 0.1 | 11.4 ± 0.0 | 48.8 ± 1.1 |
| Alang–Alang | |||||
| 2019 (vegetated; n = 9) | 17.8 ± 1.1 | 0.0043 ± 0.0004 | 22.0 ± 1.1 | 9.1 ± 0.5 | 38.1 ± 0.3 |
| 2022 (vegetated; n = 10) | 18.6 ± 1.2 | 0.0041 ± 0.0004 | 27.0 ± 1.6 | 11.3 ± 0.7 | 47.1 ± 3.3 |
| 2022 (unvegetated; n = 2) | 14.1 ± 0.4 | 0.0072 ± 0.0000 | 22.6 ± 0.3 | 9.4 ± 0.1 | 41.2 ± 0.3 |
| Mrican | |||||
| 2019 (vegetated; n = 9) | 11.1 ± 1.7 | 0.0046 ± 0.0004 | 13.7 ± 1.8 | 5.6 ± 0.8 | 24.7 ± 2.9 |
| Cemara Kecil | |||||
| 2022 (vegetated; n = 10) | 31.9 ± 3.5 | 0.0033 ± 0.0004 | 33.2 ± 3.3 | 13.9 ± 1.4 | 48.2 ± 4.8 |
| 2022 (unvegetated; n = 2) | 21.6 ± 0.6 | 0.0059 ± 0.0006 | 17.9 ± 2.2 | 7.4 ± 0.9 | 29.5 ± 3.9 |
| North | |||||
| Lendra | |||||
| 2022 (vegetated; n = 10) | 19.6 ± 1.3 | 0.0046 ± 0.0002 | 31.7 ± 1.4 | 13.3 ± 0.6 | 54.6 ± 3.0 |
| 2022 (unvegetated; n = 2) | 21.1 ± 1.9 | 0.0048 ± 0.0000 | 9.7 ± 3.4 | 3.8 ± 1.5 | 15.7 ± 6.3 |
| South | |||||
| Pelabuhan | |||||
| 2022 (vegetated; n = 15) | 16.5 ± 1.0 | 0.0045 ± 0.0003 | 22.1 ± 1.0 | 9.2 ± 0.4 | 38.9 ± 1.8 |
| 2022 (unvegetated; n = 2) | 25.0 ± 2.7 | 0.0063 ± 0.0003 | 20.2 ± 2.2 | 8.3 ± 0.9 | 31.8 ± 2.5 |
| Menjangan kecil | |||||
| 2022 (vegetated; n = 15) | 27.3 ± 2.6 | 0.0039 ± 0.0004 | 26.7 ± 1.2 | 11.1 ± 0.5 | 41.1 ± 2.4 |
| 2022 (unvegetated; n = 2) | 27.0 ± 0.7 | 0.0061 ± 0.0000 | 27.4 ± 0.2 | 6.9 ± 0.1 | 25.6 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latifah, N.; Ningsih, N.S.; Kartadikaria, A.R.; Wirasatriya, A.; Febrianto, S.; Adi, N.S.; Hamzah, F. Seagrass Blue Carbon Stock and Air–Sea CO2 Fluxes in the Karimunjawa Islands, Indonesia during Southeast Monsoon Season. Diversity 2023, 15, 978. https://doi.org/10.3390/d15090978
Latifah N, Ningsih NS, Kartadikaria AR, Wirasatriya A, Febrianto S, Adi NS, Hamzah F. Seagrass Blue Carbon Stock and Air–Sea CO2 Fluxes in the Karimunjawa Islands, Indonesia during Southeast Monsoon Season. Diversity. 2023; 15(9):978. https://doi.org/10.3390/d15090978
Chicago/Turabian StyleLatifah, Nurul, Nining Sari Ningsih, Aditya Rakhmat Kartadikaria, Anindya Wirasatriya, Sigit Febrianto, Novi Susetyo Adi, and Faisal Hamzah. 2023. "Seagrass Blue Carbon Stock and Air–Sea CO2 Fluxes in the Karimunjawa Islands, Indonesia during Southeast Monsoon Season" Diversity 15, no. 9: 978. https://doi.org/10.3390/d15090978






