The Role of Retention Trees in Providing a Habitat for Bryophytes and Lichens in Young Forest Stands: A Mid-Term Perspective
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Convention on Biological Diversity. Strategic Plan for Biodiversity 2021-Microclimate in Forest Ecosystem and Landscape Ecology 2030. Available online: https://www.cbd.int/doc/strategic-plan/Post2020/Bubb.pdf (accessed on 21 December 2022).
- Pykälä, J. Habitat loss and deterioration explain the disappearance of populations of threatened vascular plants, bryophytes and lichens in a hemiboreal landscape. Glob. Ecol. Conserv. 2019, 18, e00610. [Google Scholar] [CrossRef]
- Gustafsson, L.; Bauhus, J.; Asbeck, T.; Augustynczik, A.L.D.; Basile, M.; Frey, J.; Gutzat, F.; Hanewinkel, M.; Helbach, J.; Jonker, M.; et al. Retention as an integrated biodiversity conservation approach for continuous-cover forestry in Europe. AMBIO 2019, 49, 85–97. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, L.; Kouki, J.; Sverdrup-Thygeson, A. Tree retention as a conservation measure in clear-cut forests of northern Europe: A review of ecological consequences. Scand. J. For. Res. 2010, 25, 295–308. [Google Scholar] [CrossRef]
- Gustafsson, L.; Hannerz, M.; Koivula, M.; Shorohova, E.; Vanha-Majamaa, I.; Weslien, J. Research on retention forestry in Northern Europe. Ecol. Process. 2020, 9, 3. [Google Scholar] [CrossRef]
- Kubiak, D.; Osyczka, P. Non-forested vs forest environments: The effect of habitat conditions on host tree parameters and the occurrence of associated epiphytic lichens. Fungal Ecol. 2020, 47, 100957. [Google Scholar] [CrossRef]
- Franklin, J.F.; Berg, D.R.; Thornburgh, D.A.; Tappeiner, J.C. Alternative silvicultural approaches to timber harvesting: Variable retention harvest systems. In Creating a Forestry for the 21st Century; Kohm, K.A., Franklin, J.F., Eds.; Island Press: Washington, DC, USA, 1997; pp. 111–140. [Google Scholar]
- Gustafsson, L.; Baker, S.C.; Bauhus, J.; Beese, W.J.; Brodie, A.; Kouki, J.; Lindenmayer, D.B.; Lõhmus, A.; Pastur, G.M.; Messier, C.; et al. Retention Forestry to Maintain Multifunctional Forests: A World Perspective. Bioscience 2012, 62, 633–645. [Google Scholar] [CrossRef] [Green Version]
- Rosenvald, R.; Lõhmus, P.; Rannap, R.; Remm, L.; Rosenvald, K.; Runnel, K.; Lõhmus, A. Assessing long-term effectiveness of green-tree retention. For. Ecol. Manag. 2019, 448, 543–548. [Google Scholar] [CrossRef]
- Lõhmus, A.; Lõhmus, P. First-Generation Forests Are Not Necessarily Worse than Long-Term Managed Forests for Lichens and Bryophytes. Restor. Ecol. 2007, 16, 231–239. [Google Scholar] [CrossRef]
- Lõhmus, A.; Lõhmus, P.; Vellak, K. Substratum diversity explains landscape-scale co-variation in the species-richness of bryophytes and lichens. Biol. Conserv. 2007, 135, 405–414. [Google Scholar] [CrossRef]
- Madžule, L.; Brūmelis, G.; Tjarve, D. Structures determining bryophyte species richness in a managed forest landscape in boreo-nemoral Europe. Biodivers. Conserv. 2012, 21, 437–450. [Google Scholar] [CrossRef]
- Moisejevs, R.; Motiejūnaitė, J.; Lõhmus, P. Lichen assemblages on Scots pine stumps and fine woody debris in hemiboreal post-harvest sites: The impact of site age and green tree retention. Nova Hedwig. 2019, 109, 247–266. [Google Scholar] [CrossRef]
- Storch, I.; Penner, J.; Asbeck, T.; Basile, M.; Bauhus, J.; Braunisch, V.; Dormann, C.F.; Frey, J.; Gärtner, S.; Hanewinkel, M.; et al. Evaluating the effectiveness of retention forestry to enhance biodiversity in production forests of Central Europe using an interdisciplinary, multi-scale approach. Ecol. Evol. 2020, 10, 1489–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perhans, K.; Appelgren, L.; Jonsson, F.; Nordin, U.; Söderström, B.; Gustafsson, L. Retention patches as potential refugia for bryophytes and lichens in managed forest landscapes. Biol. Conserv. 2009, 142, 1125–1133. [Google Scholar] [CrossRef]
- Rosenvald, R.; Lõhmus, A. For what, when, and where is green-tree retention better than clear-cutting? A review of the biodiversity aspects. For. Ecol. Manag. 2008, 255, 1–15. [Google Scholar] [CrossRef]
- Fenton, N.J.; Frego, K.A.; Sims, M.R. Changes in forest floor bryophyte (moss and liverwort) communities 4 years after forest harvest. Can. J. Bot. 2003, 81, 714–731. [Google Scholar] [CrossRef]
- Lundström, J.; Jonsson, F.; Perhans, K.; Gustafsson, L. Lichen species richness on retained aspens increases with time since clear-cutting. For. Ecol. Manag. 2013, 293, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Caners, R.T.; Macdonald, S.E.; Belland, R.J. Responses of boreal epiphytic bryophytes to different levels of partial canopy harvest. Botany 2010, 88, 315–328. [Google Scholar] [CrossRef]
- Rudolphi, J.; Gustafsson, L. Forests regenerating after clear-cutting function as habitat for bryophyte and lichen species of conservation concern. PLoS ONE 2011, 6, e18639. [Google Scholar] [CrossRef] [Green Version]
- Jönsson, M.; Perhans, K.; Appelgren, L.; Gustafsson, L. Bryophytes of conservation concern decline and traits change in retention patches during two decades following forest harvest. Biol. Conserv. 2022, 273, 109647. [Google Scholar] [CrossRef]
- Nilsson, S.G. Selecting biodiversity indicators to set conservation targets: Species, structures, or processes? In Setting Conservation Targets for Managed Forest Landscape; Villard, M.-A., Jonsson, B.G., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 79–108. [Google Scholar]
- Czerepko, J.; Gawryś, R.; Szymczyk, R.; Pisarek, W.; Janek, M.; Haidt, A.; Kowalewska, A.; Piegdoń, A.; Stebel, A.; Kukwa, M.; et al. How sensitive are epiphytic and epixylic cryptogams as indicators of forest naturalness? Testing bryophyte and lichen predictive power in stands under different management regimes in the Białowieża forest. Ecol. Indic. 2021, 125, 107532. [Google Scholar] [CrossRef]
- Lõhmus, A.; Lõhmus, P.; Runnel, K. A simple survey protocol for assessing terrestrial biodiversity in a broad range of ecosystems. PLoS ONE 2018, 13, e0208535. [Google Scholar] [CrossRef]
- Klein, J.; Low, M.; Thor, G.; Sjögren, J.; Lindberg, E.; Eggers, S. Tree species identity and composition shape the epiphytic lichen community of structurally simple boreal forests over vast areas. PLoS ONE 2021, 16, e0257564. [Google Scholar] [CrossRef] [PubMed]
- Sjörs, H. Amphi-Atlantic zonation, nemoral to arctic. In North Atlantic Biota and Their History; Löve, A., Löve, D., Eds.; The Macmillan Company: New York, NY, USA, 1963; pp. 109–125. [Google Scholar]
- Latvian Environment, Geology and Meteorology Centre (LEGMC). Available online: https://videscentrs.lvgmc.lv/ (accessed on 21 December 2022).
- Meža Resursi. Meža Resursu Statistikas Dati. Available online: https://www.zm.gov.lv/lv/meza-resursi (accessed on 21 December 2022).
- Bušs, K. Meža Ekoloģija un Tipoloģija; Zinātne: Rīga, Latvija, 1981; p. 64. [Google Scholar]
- Gerra-Inohosa, L.; Pušpure, I.; Bambe, B. Epifītisko sūnu un ķērpju sugu daudzveidība uz izcirtumos atstātajiem kokiem jaunaudzēs. Mežzinātne 2015, 29, 35–57. [Google Scholar]
- Smith, C.; Aptroot, A.; Coppins, B.; Fletcher, A.; Gilbert, O.; James, P.; Wolseley, P. The Lichens of Great Britain and Ireland; MPG Books Group: London, UK, 2009; p. 1046. [Google Scholar]
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An annotated checklist of bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Āboliņa, A.; Piterāns, A.; Bambe, B. Latvijas Kērpji un Sūnas. Taksonu Saraksts; Saule: Daugavpils, Latvija, 2015; p. 213. [Google Scholar]
- Auniņš, A. Eiropas Savienības Aizsargājamie Biotopi Latvijā. Noteikšanas Rokasgrāmata. 2. Precizētais Izdevums; Latvijas Dabas fonds, Vides Aizsardzības un Reģionālās Attīstības Ministrija: Rīga, Latvija, 2013; p. 320. [Google Scholar]
- Arnqvist, G. Mixed models offer no freedom from degrees of freedom. Trends Ecol. Evol. 2019, 35, 329–335. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R. A Language and Environment for Statistical Computing. 2020. Available online: http://www.R-project.org (accessed on 21 December 2022).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2016, 67, 48. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package_. R Package Version 2.6-4. 2022. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 21 December 2022).
- Lõhmus, A.; Lõhmus, P. Epiphyte communities on the trunks of retention trees stabilise in 5 years after timber harvesting, but remain threatened due to tree loss. Biol. Conserv. 2010, 143, 891–898. [Google Scholar] [CrossRef]
- Chen, J.; Saunders, S.C.; Crow, T.R.; Naiman, R.J.; Brosofske, K.D.; Mroz, G.D.; Brookshire, B.L.; Franklin, J.F. Microclimate in forest ecosystem and landscape ecology: Variations in local climate can be used to monitor and compare the effects of different management regimes. Bioscience 1999, 49, 288–297. [Google Scholar] [CrossRef] [Green Version]
- Ódor, P.; Király, I.; Tinya, F.; Bortignon, F.; Nascimbene, J. Patterns and drivers of species composition of epiphytic bryophytes and lichens in managed temperate forests. For. Ecol. Manag. 2013, 306, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Snäll, T.; Ribeiro, P.J., Jr.; Rydin, H. Spatial occurrence and colonisations in patch-tracking metapopulations: Local conditions versus dispersal. Oikos 2003, 103, 566–578. [Google Scholar] [CrossRef]
- Mežaka, A.; Brūmelis, G.; Piterāns, A. Tree and stand-scale factors affecting richness and composition of epiphytic bryophytes and lichens in deciduous woodland key habitats. Biodivers. Conserv. 2012, 21, 3221–3241. [Google Scholar] [CrossRef]
- Schei, F.H.; Blom, H.H.; Gjerde, I.; Grytnes, J.-A.; Heegaard, E.; Sætersdal, M. Conservation of epiphytes: Single large or several small host trees? Biol. Conserv. 2013, 168, 144–151. [Google Scholar] [CrossRef]
- Jüriado, I.; Liira, J.; Paal, J.; Suija, A. Tree and stand level variables influencing diversity of lichens on temperate broad-leaved trees in boreo-nemoral floodplain forests. Biodivers. Conserv. 2008, 18, 105–125. [Google Scholar] [CrossRef]
- Jüriado, I.; Paal, J. Epiphytic lichen synusiae and functional trait groups of species in boreo-nemoral deciduous forests are influenced by host tree properties and environmental factors. Nord. J. Bot. 2019, 37, e01939. [Google Scholar] [CrossRef]
- Király, I.; Ódor, P. The effect of stand structure and tree species composition on epiphytic bryophytes in mixed deciduous–coniferous forests of Western Hungary. Biol. Conserv. 2010, 143, 2063–2069. [Google Scholar] [CrossRef]
- Király, I.; Nascimbene, J.; Tinya, F.; Ódor, P. Factors influencing epiphytic bryophyte and lichen species richness at different spatial scales in managed temperate forests. Biodivers. Conserv. 2012, 22, 209–223. [Google Scholar] [CrossRef] [Green Version]
- Barkman, J.J. Phytosociology and Ecology of Cryptogamic Epiphytes; Van Gorcum & Company. N. V.: Assen, The Netherlands, 1958; p. 628. [Google Scholar]
- Hedenås, H.; Hedström, P. Conservation of epiphytic lichens: Significance of remnant aspen (Populus tremula) trees in clear-cuts. Biol. Conserv. 2007, 135, 388–395. [Google Scholar] [CrossRef]
- Ellis, C.J. Lichen epiphyte diversity: A species, community and trait-based review. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 131–152. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Engels, P.; Sotiaux, A. Trends in diversity and abundance of obligate epiphytic bryophytes in a highly managed landscape. Ecography 2004, 27, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Nascimbene, J.; Dainese, M.; Sitzia, T. Contrasting responses of epiphytic and dead wood-dwelling lichen diversity to forest management abandonment in silver fir mature woodlands. For. Ecol. Manag. 2013, 289, 325–332. [Google Scholar] [CrossRef]
- Larrieu, L.; Paillet, Y.; Winter, S.; Bütler, R.; Kraus, D.; Krumm, F.; Lachat, T.; Michel, A.K.; Regnery, B.; Vandekerkhove, K. Tree related microhabitats in temperate and Mediterranean European forests: A hierarchical typology for inventory standardization. Ecol. Indic. 2018, 84, 194–207. [Google Scholar] [CrossRef]
- Timonen, J.; Gustafsson, L.; Kotiaho, J.S.; Mönkkönen, M. Hotspots in cold climate: Conservation value of woodland key habitats in boreal forests. Biol. Conserv. 2011, 144, 2061–2067. [Google Scholar] [CrossRef]
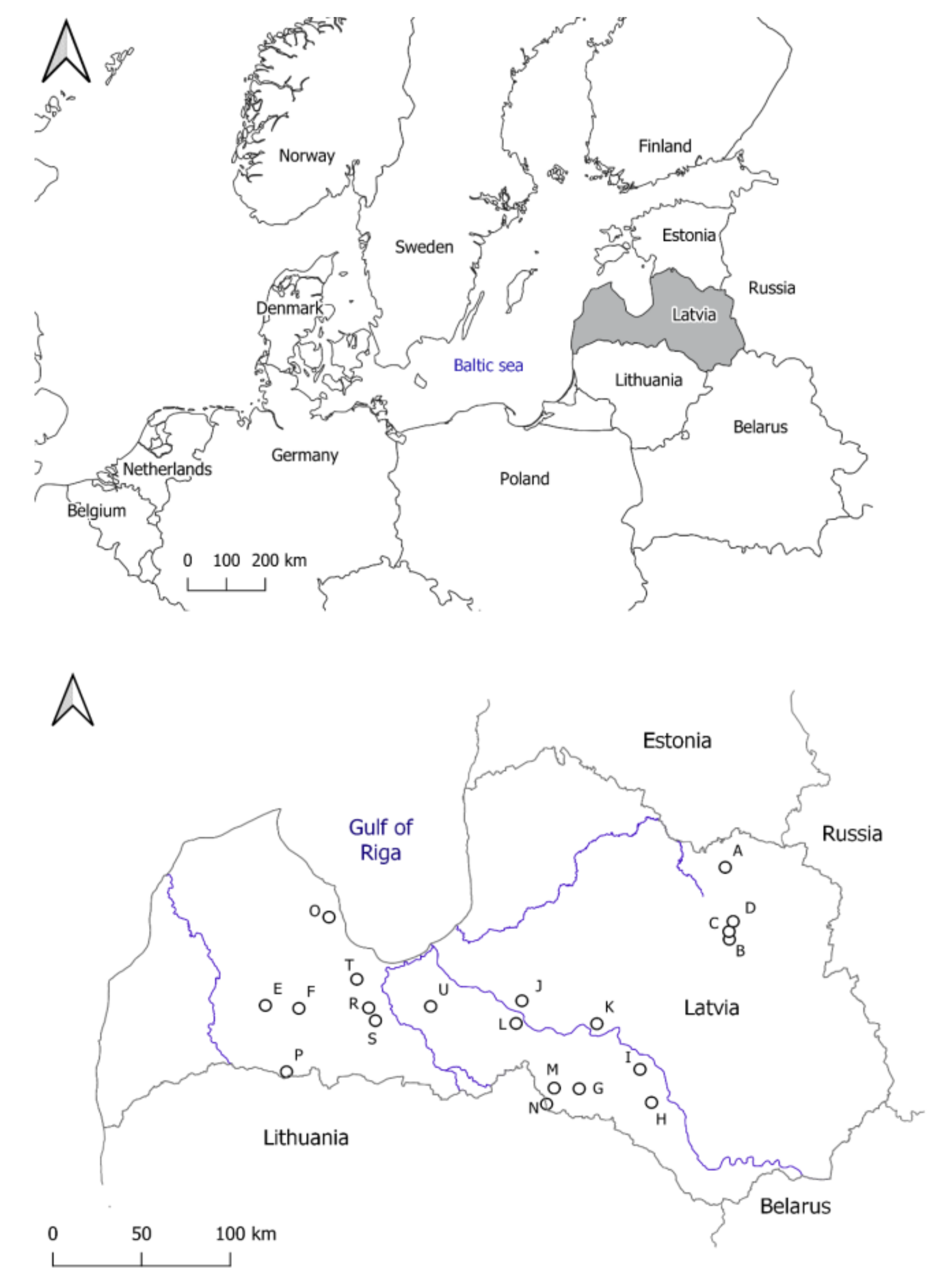


| Study Plot | The Number of Surveyed Trees by Species | Year of Clearfelling | Forest Type/Fertility | Soil | Coordinates | |
|---|---|---|---|---|---|---|
| E | N | |||||
| A | 1 Algl, 4 Bepe | 2003 | Myrtillosa turf.mel/mesotrophic | drained peat | 487,543 | 6,365,430 |
| B | 2 Frex, 3 Potr | 2003 | Oxalidosa/eutrophic | dry mineral | 488,020 | 6,323,578 |
| C | 5 Potr | 2003 | Oxalidosa/eutrophic | dry mineral | 488,040 | 6,328,124 |
| D | 3 Ulgl | 2003 | Aegopodiosa/eutrophic | dry mineral | 490,620 | 6,333,859 |
| E | 2 Potr, 3 Quro | 2002 | Myrtilloso-polytrichosa/mesotrophic | wet mineral | 591,961 | 6,288,735 |
| F | 4 Potr, 1 Quro | 2002 | Oxalidosa/eutrophic | dry mineral | 610,873 | 6,287,850 |
| G | 1 Bepe, 1 Frex, 3 Potr | 2004 | Oxalidosa turf.mel./eutrophic | drained peat | 399,666 | 6,240,728 |
| H | 1 Algl, 1 Frex, 1 Potr, 2 Quro | 2004 | Myrtillosa turf.mel./mesotrophic | drained peat | 440,062 | 6,230,958 |
| I | 1 Pisy, 4 Tico | 2004 | Oxalidosa/eutrophic | dry mineral | 434,386 | 6,250,437 |
| J | 1 Frex, 3 Potr, 1 Tico | 2002 | Dryopteriosa/mesotrophic | wet mineral | 369,643 | 6,293,094 |
| K | 3 Bepe, 2 Pisy | 2004 | Oxalidosa/eutrophic | dry mineral | 411,258 | 6,277,966 |
| L | 3 Algl, 1 Bepe, 1 Potr | 2002 | Mercurialiosa mel./eutrophic | drained mineral | 365,764 | 6,280,126 |
| M | 3 Acpl, 1 Potr, 1 Quro | 2004 | Aegopodiosa/eutrophic | dry mineral | 385,608 | 6,241,691 |
| N | 1 Bepe, 3 Quro, 1 Ulgl | 2004 | Oxalidosa/eutrophic | dry mineral | 380,903 | 6,232,653 |
| O | 1 Algl, 1 Frex, 1 Potr, 2 Tico | 2003 | Myrtillosa mel./mesotrophic | drained mineral | 625,533 | 6,341,470 |
| P | 2 Bepe, 1 Potr, 2 Quro | 2002 | Oxalidosa/eutrophic | dry mineral | 605,520 | 6,250,790 |
| R | 1 Potr, 4 Quro | 2002 | Myrtillosa mel./mesotrophic | drained mineral | 650,214 | 6,289,832 |
| S | 4 Potr, 1 Quro | 2002 | Oxalidosa/eutrophic | dry mineral | 654,327 | 6,282,632 |
| T | 1 Potr, 4 Quro | 2003 | Myrtilloso-polytrichosa/mesotrophic | wet mineral | 642,772 | 6,306,155 |
| U | 3 Algl, 1 Quro, 1 Tico | 2002 | Mercurialiosa mel./eutrophic | drained mineral | 318,092 | 6,292,116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerra-Inohosa, L.; Matisons, R.; Jansone, D.; Jansons, Ā.; Lībiete, Z. The Role of Retention Trees in Providing a Habitat for Bryophytes and Lichens in Young Forest Stands: A Mid-Term Perspective. Diversity 2023, 15, 870. https://doi.org/10.3390/d15070870
Gerra-Inohosa L, Matisons R, Jansone D, Jansons Ā, Lībiete Z. The Role of Retention Trees in Providing a Habitat for Bryophytes and Lichens in Young Forest Stands: A Mid-Term Perspective. Diversity. 2023; 15(7):870. https://doi.org/10.3390/d15070870
Chicago/Turabian StyleGerra-Inohosa, Linda, Roberts Matisons, Diāna Jansone, Āris Jansons, and Zane Lībiete. 2023. "The Role of Retention Trees in Providing a Habitat for Bryophytes and Lichens in Young Forest Stands: A Mid-Term Perspective" Diversity 15, no. 7: 870. https://doi.org/10.3390/d15070870






