Seasonal Variations in Plant Species Diversity and Phylogenetic Diversity in Abandoned Farmland of China’s Huang–Huai Plain
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Surveys
2.2. Calculation of Plant Species Diversity Indices
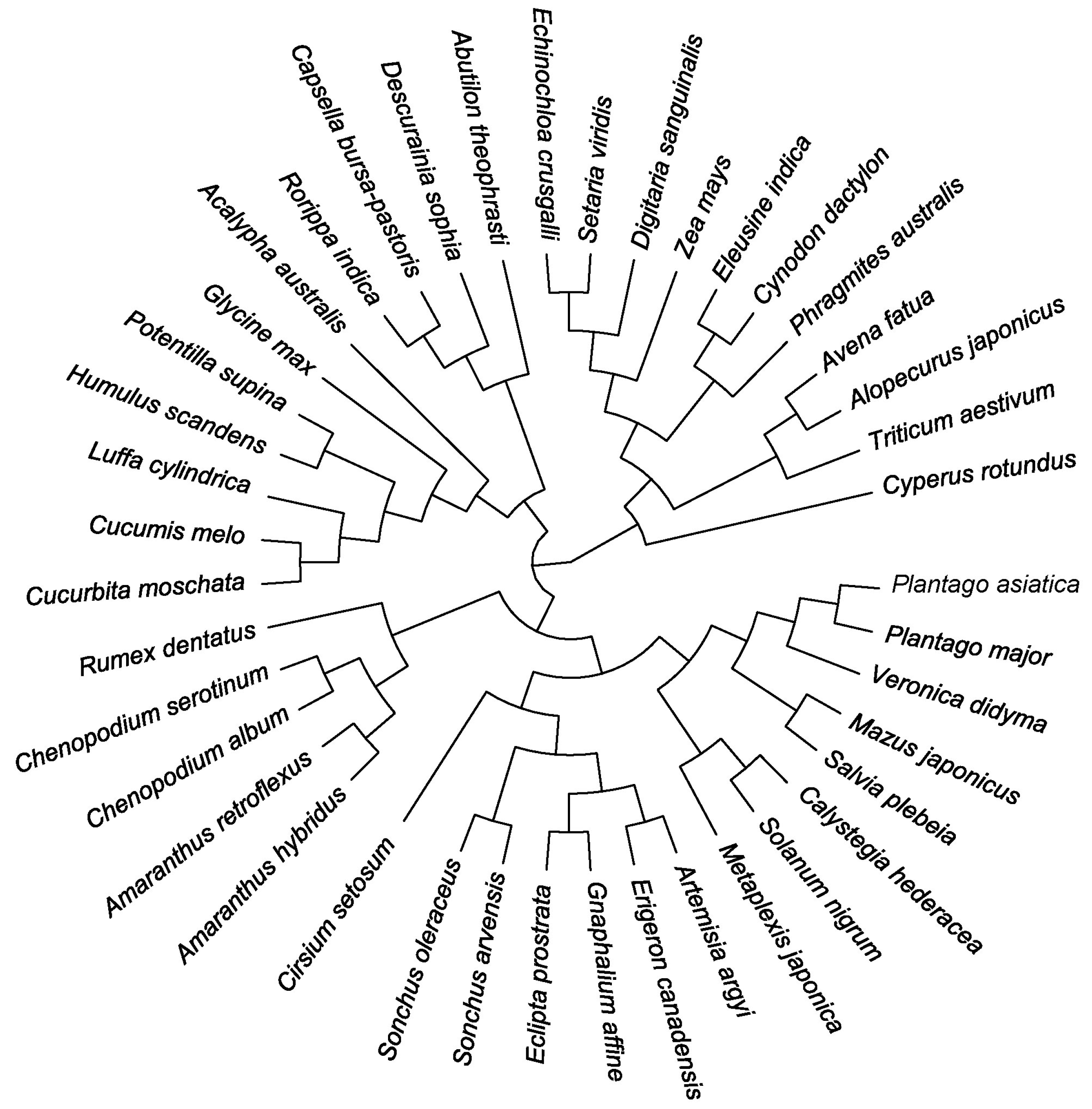
2.3. Construction of Phylogenetic Tree and Calculation of Phylogenetic Indices
2.4. Determination of Soil Physical and Chemical Properties
2.5. Data Analysis
3. Results
3.1. Seasonal Variations in Plant Community Structure
3.2. Seasonal Variations in Plant Species Diversity and Phylogenetic Diversity
3.2.1. Seasonal Variation in Plant Species Diversity
3.2.2. Seasonal Variations in Plant Phylogenetic Diversity and Phylogenetic Structure
3.3. Correlations among Various Plant Diversity Indices
3.4. Relationship between Plant Species Composition, Plant Diversity, and Soil Environmental Factors
4. Discussion
4.1. Seasonal Variation in Plant Species Diversity and Phylogenetic Diversity in Abandoned Farmland
4.2. Relationship between Plant Community, Plant Diversity, and Soil Environmental Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef]
- Lu, X.; Liang, G.; Tang, Q.; Ding, S.; Li, Q.; Zhang, X. Plant species of the non-agricultural habitats in the lower reaches of the Yellow River plain agro-landscape. Acta Ecol. Sin. 2014, 34, 789–797. [Google Scholar] [CrossRef]
- Pilling, D.; Belanger, J.; Hoffmann, I. Declining biodiversity for food and agriculture needs urgent global action. Nat Food 2020, 1, 144–147. [Google Scholar] [CrossRef]
- Tsioumani, E. The State of the World’s Biodiversity for Food and Agriculture: A Call to Action? Environ. Policy Law 2019, 49, 110–112. [Google Scholar] [CrossRef]
- Brooker, R.W.; George, T.S.; Homulle, Z.; Karley, A.J.; Newton, A.C.; Pakeman, R.J.; Schob, C. Facilitation and biodiversity-ecosystem function relationships in crop production systems and their role in sustainable farming. J. Ecol. 2021, 109, 2054–2067. [Google Scholar] [CrossRef]
- Hossain, M.M.; Begum, M.; Hashem, A.; Rahman, M.M.; Haque, M.E.; Bell, R.W. Continuous Practice of Conservation Agriculture for 3–5 Years in Intensive Rice-Based Cropping Patterns Reduces Soil Weed Seedbank. Agriculture 2021, 11, 895. [Google Scholar] [CrossRef]
- Canning, A.D. Rediscovering wild food to diversify production across Australia’s agricultural landscapes. Front. Sustain. Food Syst. 2022, 6, 865580. [Google Scholar] [CrossRef]
- Wretenberg, J.; Part, T.; Berg, A. Changes in local species richness of farmland birds in relation to land-use changes and landscape structure. Biol. Conserv. 2010, 143, 375–381. [Google Scholar] [CrossRef]
- Edlund, M.; Smith, H.G. Organic farming at local and landscape scales benefits plant diversity. Ecography 2010, 33, 514–522. [Google Scholar] [CrossRef]
- Hille, S.; Andersen, D.K.; Kronvang, B.; Baattrup-Pedersen, A. Structural and functional characteristics of buffer strip vegetation in an agricultural landscape—High potential for nutrient removal but low potential for plant biodiversity. Sci. Total Environ. 2018, 628–629, 805–814. [Google Scholar] [CrossRef]
- Egli, L.; Meyer, C.; Scherber, C.; Kreft, H.; Tscharntke, T. Winners and losers of national and global efforts to reconcile agricultural intensification and biodiversity conservation. Glob. Chang. Biol. 2018, 24, 2212–2228. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X. Progress and prospect on farmland abandonment. Acta Geogr. Sin. 2016, 71, 370–389. [Google Scholar] [CrossRef]
- Isbell, F.; Adler, P.R.; Eisenhauer, N.; Fornara, D.; Kimmel, K.; Kremen, C.; Letourneau, D.K.; Liebman, M.; Polley, H.W.; Quijas, S.; et al. Benefits of increasing plant diversity in sustainable agroecosystems. J. Ecol. 2017, 105, 871–879. [Google Scholar] [CrossRef]
- Robleño, I.; Storkey, J.; Solé-Senan, X.O.; Recasens, J. Using the response–effect trait framework to quantify the value of fallow patches in agricultural landscapes to pollinators. Appl. Veg. Sci. 2018, 21, 267–277. [Google Scholar] [CrossRef]
- Li, L.; Pan, Y.Z.; Zheng, R.B.; Liu, X.P. Understanding the spatiotemporal patterns of seasonal, annual, and consecutive farmland abandonment in China with time-series MODIS images during the period 2005–2019. Land Degrad. Dev. 2022, 33, 1608–1625. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, C.; Dong, J.D.; Ge, Q. Spatio-temporal pattern of cropland abandonment in China from 1992 to 2017: A Meta-analysis. Acta Geogr. Sin. 2019, 74, 411–420. [Google Scholar] [CrossRef]
- Swenson, N.G. The role of evolutionary processes in producing biodiversity patterns, and the interrelationships between taxonomic, functional and phylogenetic biodiversity. Am. J. Bot. 2011, 98, 472–480. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Jonathan Davies, T.; Regetz, J.; Kembel, S.W.; Cleland, E.; Oakley, T.H. Phylogenetic diversity metrics for ecological communities: Integrating species richness, abundance and evolutionary history. Ecol. Lett. 2010, 13, 96–105. [Google Scholar] [CrossRef]
- Jones, H.P.; Barber, N.A.; Gibson, D.J. Is phylogenetic and functional trait diversity a driver or a consequence of grassland community assembly? J. Ecol. 2019, 107, 2027–2032. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Kozak, K.H.; Fine, P.V.; Kembel, S.W. The merging of community ecology and phylogenetic biology. Ecol. Lett. 2009, 12, 693–715. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Ackerly, D.D. Functional trait and phylogenetic tests of community assembly across spatial scales in an Amazonian forest. Ecol. Monogr. 2010, 80, 401–422. [Google Scholar] [CrossRef]
- Emerson, B.C.; Gillespie, R.G. Phylogenetic analysis of community assembly and structure over space and time. Trends Ecol. Evol. 2008, 23, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, J.; Ma, Z.; Chang, F.; Liu, B.; You, S. Responses of plant diversity to seasonal changes in the lakeside wetland of Bosten Lake. Plant Sci. 2022, 40, 462–471. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, D.-D.; Huang, K.-C.; Liu, S.; Reyila, M.; Sun, Y.-F.; Li, J.-N.; Cui, B.-K. Seasonal variation in the soil fungal community structure of Larix gmelinii forests in Northeast China. Front. Microbiol. 2023, 14, 1106888. [Google Scholar] [CrossRef]
- Chen, Y.; Han, M.; Yuan, X.; Cao, G.; Zhu, B. Seasonal changes in soil properties, microbial biomass and enzyme activities across the soil profile in two alpine ecosystems. Soil Ecol. Lett. 2021, 3, 383–394. [Google Scholar] [CrossRef]
- Sorce, C.; Nannipieri, F.; Ciccarelli, D. Ecophysiological and morpho-functional responses of mediterranean plants on coastal dunes: How they vary across seasons. Estuar. Coast. Shelf Sci. 2021, 259, 107480. [Google Scholar] [CrossRef]
- Ren, H.; Han, G.; Li, M.H.; Gao, C.; Jiang, L. Ethylene-regulated leaf lifespan explains divergent responses of plant productivity to warming among three hydrologically different growing seasons. Glob. Chang. Biol. 2021, 27, 4169–4180. [Google Scholar] [CrossRef]
- Martello, F.; dos Santos, J.S.; Silva-Neto, C.M.; Cassia-Silva, C.; Siqueira, K.N.; de Ataide, M.V.R.; Ribeiro, M.C.; Collevatti, R.G. Landscape structure shapes the diversity of plant reproductive traits in agricultural landscapes in the Brazilian Cerrado. Agr. Ecosyst. Environ. 2023, 341, 108246. [Google Scholar] [CrossRef]
- Swierszcz, S.; Nobis, M.; Maslak, M.; Smieja, A.; Kojs, P.; Nowak, S.; Nowak, A. Varied response of underground and aboveground plant matter: Functional diversity of three different vegetational types after translocation to reclaimed postindustrial land. Land Degrad. Dev. 2019, 30, 2287–2297. [Google Scholar] [CrossRef]
- Dembicz, I.; Velev, N.; Boch, S.; Janisova, M.; Palpurina, S.; Pedashenko, H.; Vassilev, K.; Dengler, J. Drivers of plant diversity in Bulgarian dry grasslands vary across spatial scales and functional-taxonomic groups. J. Veg. Sci. 2021, 32, e12935. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Chen, J.; Xiao, Y.H.; Ni, Y.L.; Zhang, R.Y.; Ye, W.H.; Lian, J.Y. Effects of soil resource availability on patterns of plant functional traits across spatial scales. Ecol. Evol. 2022, 12, e8587. [Google Scholar] [CrossRef]
- Walther, G.; Jandt, U.; Kattge, J.; Romermann, C. Functional rarity of plants in German hay meadows—Patterns on the species level and mismatches with community species richness. Ecol. Evol. 2022, 12, e9375. [Google Scholar] [CrossRef] [PubMed]
- Mugnai, M.; Ferretti, G.; Gesuelli, E.; Nuti, L.; Di Natale, S.; Corti, E.; Viciani, D.; Lazzaro, L. Site dependence of local variations in taxonomic and functional diversity of plant communities in semi-natural dry grasslands. Plant Ecol. 2023, 224, 95–111. [Google Scholar] [CrossRef]
- Liu, M.X.; Xu, L.; Mu, R.L.; Zhang, G.J.; Yu, R.X.; Li, L. Plant community assembly of alpine meadow at different altitudes in Northeast Qinghai-Tibet Plateau. Ecosphere 2023, 14, e4354. [Google Scholar] [CrossRef]
- Viana, J.L.; Dalling, J.W. Soil fertility and water availability effects on trait dispersion and phylogenetic relatedness of tropical terrestrial ferns. Oecologia 2022, 198, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, S.; Hejda, M.; Ejtehadi, H.; Farzam, M.; Memariani, F.; Pysek, P. Effects of livestock grazing on soil, plant functional diversity, and ecological traits vary between regions with different climates in northeastern Iran. Ecol. Evol. 2019, 9, 8225–8237. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.T.; Zhang, J.; Wang, Z. Responses and Indicators of Composition, Diversity, and Productivity of Plant Communities at Different Levels of Disturbance in a Wetland Ecosystem. Diversity 2021, 13, 252. [Google Scholar] [CrossRef]
- Hauchhum, R.; Singson, M.Z. Tree species composition and diversity in abandoned Jhum lands of Mizoram, North East India. Trop. Ecol. 2020, 61, 187–195. [Google Scholar] [CrossRef]
- Cao, G.; Tsuchiya, K.; Zhu, W.; Okuro, T. Vegetation dynamics of abandoned paddy fields and surrounding wetlands in the lower Tumen River Basin, Northeast China. Peerj 2019, 7, e6704. [Google Scholar] [CrossRef]
- Liu, K.; Liang, T.; Qiang, W.Y.; Du, G.Z.; Baskin, J.M.; Baskin, C.C.; Bu, H.Y.; Yang, H.; Xiao, S. Changes in seed germination strategy along the successional gradient from abandoned cropland to climax grassland in a subalpine meadow and some implications for rangeland restoration. Agr. Ecosyst. Environ. 2020, 289, 106746. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Tang, Q.; Liang, G.; Ding, S. Characteristics of non-agricultural habitat plant functional groups in agricultural landscape of Huanghuaihai Plain. Acta Ecol. Sin. 2019, 39, 6721–6730. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 668. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Margalef, R. Diversidad de especies en las comunidades naturales. P. Inst. Biol. Apl. 1951, 9, 15–27. [Google Scholar]
- Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; Stevens, P.F. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Jin, Y.; Qian, H.V. PhyloMaker2: An updated and enlarged R package that can generate very large phylogenies for vascular plants. Plant Divers. 2022, 44, 335–339. [Google Scholar] [CrossRef]
- Yu, G. Data Integration, Manipulation and Visualization of Phylogenetic Trees; Chapman and Hall/CRC: New York, NY, USA, 2022. [Google Scholar]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and Community Ecology. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; Kembel, S.W. Phylocom: Software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 2008, 24, 2098–2100. [Google Scholar] [CrossRef]
- Page, A.L. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1983. [Google Scholar]
- Rooij, G.H.d. Methods of Soil Analysis. Part 4. Physical Methods. Vadose Zone J. 2004, 3, 722–723. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.Y.; Tan, Y.; Fan, H.; Ruan, H. Fertilizer regime impacts on abundance and diversity of soil fauna across a poplar plantation chronosequence in coastal Eastern China. Sci. Rep. 2016, 6, 20816. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Simko, T.W.a.V. R Package ‘Corrplot’: Visualization of a Correlation Matrix (Version 0.92). Available online: https://github.com/taiyun/corrplot (accessed on 14 November 2022).
- Spearman, C. The proof and measurement of association between two things. Am. J. Psychol. 1961, 15, 72–101. [Google Scholar] [CrossRef]
- Fishman, G. Monte Carlo: Concepts, Algorithms, and Applications; Springer Science & Business Media: Berlin, Germany, 2013; pp. 1–698. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; pp. 1–3871. [Google Scholar]
- Kompała-Bąba, A.; Sierka, E.; Dyderski, M.K.; Bierza, W.; Magurno, F.; Besenyei, L.; Błońska, A.; Ryś, K.; Jagodziński, A.M.; Woźniak, G. Do the dominant plant species impact the substrate and vegetation composition of post-coal mining spoil heaps? Ecol. Eng. 2020, 143, 105685. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.; Ren, Z.; Zhou, X.; Du, G. N-Induced Species Loss Dampened by Clipping Mainly Through Suppressing Dominant Species in an Alpine Meadow. Front. Plant Sci. 2022, 13, 815011. [Google Scholar] [CrossRef]
- Doležal, J.; Lanta, V.; Mudrák, O.; Lepš, J.; Wilson, S. Seasonality promotes grassland diversity: Interactions with mowing, fertilization and removal of dominant species. J. Ecol. 2018, 107, 203–215. [Google Scholar] [CrossRef]
- Mao, J.; Shi, X.; Thornton, P.E.; Piao, S.; Wang, X. Causes of spring vegetation growth trends in the northern mid–high latitudes from 1982 to 2004. Environ. Res. Lett. 2012, 7, 014010. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, D.; Wang, R.; Liu, J.; Du, N. Altitudinal Patterns of Species Diversity and Phylogenetic Diversity across Temperate Mountain Forests of Northern China. PLoS ONE 2016, 11, e0159995. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S. Plant community diversity will decline more than increase under climatic warming. Philos. Trans. R. Soc. 2020, 375, 20190106. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ren, H.; Wang, Z.; Li, Z.; Liu, Y.; Wang, Z.; Li, Y.; Zhang, R.; Zhao, M.; Chang, S.X. Additive negative effects of decadal warming and nitrogen addition on grassland community stability. J. Ecol. 2020, 108, 1442–1452. [Google Scholar] [CrossRef]
- Natalia, R.; Sergey, K.; Michał, S.; Xianyong, C.; Snezhana, Z. Postglacial history of the Steppe Altai: Climate, fire and plant diversity. Quat. Sci. Rev. 2020, 249, 106616. [Google Scholar] [CrossRef]
- Harrison, S.; Spasojevic, M.J.; Li, D. Climate and plant community diversity in space and time. Proc. Natl. Acad. Sci. USA 2020, 117, 4464–4470. [Google Scholar] [CrossRef]
- Zani, D.; Crowther, T.W.; Mo, L.; Renner, S.S.; Zohner, C.M. Increased growing-season productivity drives earlier autumn leaf senescence in temperate trees. Science 2020, 370, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.M. Assembly of Species Communities; Harvard University Press: Cambridge, MA, USA, 1975; pp. 342–444. [Google Scholar]
- Chai, Y.; Xu, J.; Liu, H.; Liu, Q.; Zhang, C.; Kang, M.; Liang, C.; Wang, R.; Gao, X.; Zhang, F.; et al. Species composition and phylogenetic structure of major shrublands in North China. China J. Plant Ecol. 2019, 43, 793–805. [Google Scholar] [CrossRef]
- Weiher, E.; Keddy, P.A. Assembly rules, null models, and trait dispersion: New questions from old patterns. Oikos 1995, 74, 159–164. [Google Scholar] [CrossRef]
- Barfknecht, D.F.; Gibson, D.J. Are metapopulation species drivers of metacommunity structure in sandstone outcrop communities? J. Veg. Sci. 2023, 34, e13167. [Google Scholar] [CrossRef]
- Ben Saadi, C.; Cayuela, L.; de Dios, G.B.; de Aledo, J.G.; Matas-Granados, L.; Salinas, N.; Cuadros, M.D.L.; Macia, M.J. Latitudinal patterns and environmental drivers of taxonomic, functional, and phylogenetic diversity of woody plants in western Amazonian terra firme forests. Front. Plant Sci. 2022, 13, 978299. [Google Scholar] [CrossRef]
- Ouyang, J.; Liu, G.; Zhou, W. Community assembly of riparian vegetation in the middle and lower reaches of the Yangtze River. Plant Sci. J. 2020, 38, 751–761. [Google Scholar] [CrossRef]
- Ankit, A.; Kamali, S.; Singh, A. Genomic & structural diversity and functional role of potassium (K(+)) transport proteins in plants. Int. J. Biol. Macromol. 2022, 208, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Anil Kumar, S.; Kaniganti, S.; Hima Kumari, P.; Sudhakar Reddy, P.; Suravajhala, P.; Suprasanna, P.; Kishor, P.B.K. Functional and biotechnological cues of potassium homeostasis for stress tolerance and plant development. Biotechnol. Genet. Eng. Rev. 2022, 5, 1–44. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Ge, Y.; Wang, J. Positive effects of plant diversity on soil microbial biomass and activity are associated with more root biomass production. J. Plant Interact. 2017, 12, 533–541. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vazquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, D.; Wang, H.; Lv, G. Discriminating ecological processes affecting different dimensions of alpha- and beta-diversity in desert plant communities. Ecol. Evol. 2022, 12, e8710. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.L.; Yuan, L.X.; Nizamani, M.M.; Zhu, Z.X.; Friedman, C.R.; Wang, H.F. Taxonomic and phylogenetic diversity of vascular plants at Ma’anling volcano urban park in tropical Haikou, China: Reponses to soil properties. PLoS ONE 2018, 13, e0198517. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.H.; Gou, Q.Q.; Zhang, Y.; Liu, J.; Gao, M. Succession of a natural desert vegetation community after long-term fencing at the edge of a desert oasis in northwest China. Front. Plant Sci. 2023, 14, 1091446. [Google Scholar] [CrossRef]
- An, H.B.; Zhang, B.; Thomas, B.; Beck, R.; Willms, W.D.; Li, Y.J.; Hao, X.Y. Short term recovery of vegetation and soil after abandoning cultivated mixedgrass prairies in Alberta, Canada. Catena 2019, 173, 321–329. [Google Scholar] [CrossRef]
- Ge, J.M.; Wang, S.; Fan, J.; Gongadze, K.; Wu, L.H. Soil nutrients of different land-use types and topographic positions in the water-wind erosion crisscross region of China’s Loess Plateau. Catena 2020, 184, 104243. [Google Scholar] [CrossRef]
- Song, X.; Fang, C.; Yuan, Z.Q.; Li, F.M. Long-Term Growth of Alfalfa Increased Soil Organic Matter Accumulation and Nutrient Mineralization in a Semi-Arid Environment. Front. Env. Sci. 2021, 9, 649346. [Google Scholar] [CrossRef]







| Species | Family | Importance Values | ||
|---|---|---|---|---|
| Spring | Summer | Autumn | ||
| Chenopodium serotinum | Amaranthaceae | 42.52% | 19.51% | 0.32% |
| Alopecurus japonicus | Poaceae | 9.85% | 0.00% | 0.00% |
| Cyperus rotundus | Cyperaceae | 4.29% | 1.95% | 0.00% |
| Descurainia sophia | Brassicaceae | 6.09% | 0.00% | 0.00% |
| Abutilon theophrasti | Malvaceae | 6.31% | 16.35% | 6.17% |
| Rumex dentatus | Polygonaceae | 2.54% | 0.61% | 0.00% |
| Humulus scandens | Moraceae | 5.99% | 12.87% | 20.24% |
| Sonchus arvensis | Asteraceae | 2.62% | 2.43% | 2.39% |
| Echinochloa crusgalli | Poaceae | 4.38% | 18.27% | 23.12% |
| Setaria viridis | Poaceae | 1.72% | 9.48% | 10.44% |
| Chenopodium album | Amaranthaceae | 2.88% | 4.49% | 2.69% |
| Cucumis acidus | Cucurbitaceae | 1.30% | 5.76% | 3.32% |
| Digitaria sanguinalis | Poaceae | 2.40% | 3.98% | 26.03% |
| Season | Shannon | Simpson | Pielou | Margalef | PD | NTI | NRI |
|---|---|---|---|---|---|---|---|
| Spring | 1.79 ± 0.24 | 0.74 ± 0.08 | 0.78 ± 0.07 | 1.87 ± 0.32 | 936 ± 121 | −0.2 ± 1.34 | −0.5 ± 0.89 |
| Summer | 1.72 ± 0.12 | 0.79 ± 0.03 | 0.91 ± 0.02 | 1.3 ± 0.19 | 682 ± 66.7 | 0.08 ± 0.77 | −0.2 ± 0.66 |
| Autumn | 1.62 ± 0.18 | 0.77 ± 0.03 | 0.91 ± 0.03 | 1.16 ± 0.27 | 566 ± 124 | 1.12 ± 0.59 | 1.55 ± 1.4 |
| Shannon | Simpson | Pielou | Margalef | PD | NTI | NRI | |
|---|---|---|---|---|---|---|---|
| Shannon | 0 *** | 0.023 * | 0 *** | 0 *** | 0.157 | 0.167 | |
| Simpson | 0 *** | 0 *** | 0.033* | 0.105 | 0.714 | 0.776 | |
| Pielou | 0.023 * | 0 *** | 0 *** | 0 *** | 0.129 | 0.017 * | |
| Margalef | 0 *** | 0.033 * | 0 *** | 0 *** | 0.025 * | 0.001 ** | |
| PD | 0 *** | 0.105 | 0 *** | 0 *** | 0 *** | 0 *** | |
| NTI | 0.157 | 0.714 | 0.129 | 0.025 * | 0 *** | 0 *** | |
| NRI | 0.167 | 0.776 | 0.017 * | 0.001 ** | 0 *** | 0 *** |
| Soil Factors | Unit | 0–10 cm | 10–20 cm | 0–20 cm | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M ± SD | R2 | p | M ± SD | R2 | p | M ± SD | R2 | p | ||
| pH | - | 8.13 ± 0.06 | 0.501 | 0.036 * | 8.17 ± 0.10 | 0.246 | 0.301 | 8.15 ± 0.08 | 0.352 | 0.132 |
| AK | mg/kg | 246.60 ± 16.86 | 0.648 | 0.009 ** | 234.70 ± 11.95 | 0.562 | 0.036 * | 240.65 ± 13.46 | 0.758 | 0.002 ** |
| AP | mg/kg | 54.34 ± 13.06 | 0.139 | 0.528 | 42.54 ± 6.42 | 0.307 | 0.207 | 48.44 ± 6.54 | 0.052 | 0.806 |
| AN | mg/kg | 28.33 ± 2.31 | 0.214 | 0.334 | 26.47 ± 3.05 | 0.577 | 0.023 * | 2740 ± 2.47 | 0.429 | 0.102 |
| SOM | g/kg | 10.92 ± 2.36 | 0.47 | 0.056 | 10.95 ± 2.44 | 0.549 | 0.026 * | 10.94 ± 2.35 | 0.493 | 0.038 * |
| SMC | % | 16.76 ± 1.39 | 0.288 | 0.222 | 18.07 ± 1.46 | 0.365 | 0.123 | 17.41 ± 1.33 | 0.368 | 0.127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, P.; Lu, X.; Li, W.; Zhang, J.; Li, P.; Li, Y.; Wang, K.; Ding, S. Seasonal Variations in Plant Species Diversity and Phylogenetic Diversity in Abandoned Farmland of China’s Huang–Huai Plain. Diversity 2023, 15, 922. https://doi.org/10.3390/d15080922
Yan P, Lu X, Li W, Zhang J, Li P, Li Y, Wang K, Ding S. Seasonal Variations in Plant Species Diversity and Phylogenetic Diversity in Abandoned Farmland of China’s Huang–Huai Plain. Diversity. 2023; 15(8):922. https://doi.org/10.3390/d15080922
Chicago/Turabian StyleYan, Peisen, Xunling Lu, Wanying Li, Jian Zhang, Peikun Li, Yan Li, Kaiyue Wang, and Shengyan Ding. 2023. "Seasonal Variations in Plant Species Diversity and Phylogenetic Diversity in Abandoned Farmland of China’s Huang–Huai Plain" Diversity 15, no. 8: 922. https://doi.org/10.3390/d15080922
APA StyleYan, P., Lu, X., Li, W., Zhang, J., Li, P., Li, Y., Wang, K., & Ding, S. (2023). Seasonal Variations in Plant Species Diversity and Phylogenetic Diversity in Abandoned Farmland of China’s Huang–Huai Plain. Diversity, 15(8), 922. https://doi.org/10.3390/d15080922







