Fish Diversity in Relation to Littoral Habitats in Three Basins of Lake Kivu (East Africa)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of Lake Kivu
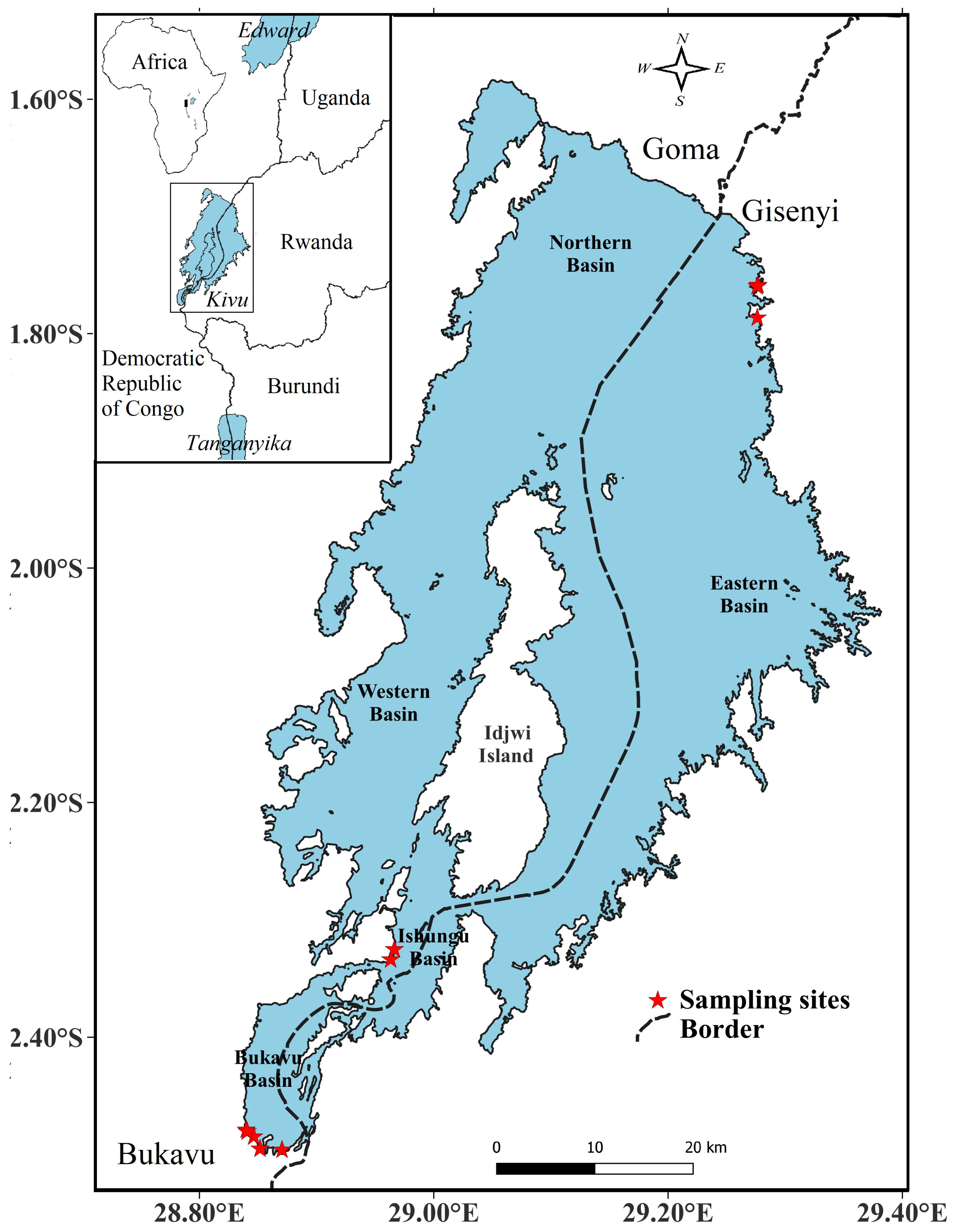
2.2. Site Description
2.3. Field Data Collection
2.4. Statistical Analyses
3. Results
3.1. Fish Distribution within the Littoral Zone of Lake Kivu
3.2. Fish Abundance at the Sites
3.2.1. Quantitative Approach
3.2.2. Diversity Indices: Hill N1 and Jaccard Similarity Index
3.3. Patterns and Factors Influencing Fish Assemblage in the Littoral Zone of Lake Kivu
3.3.1. Characterisation of Key Habitat Parameters at Each Site
3.3.2. Fish Taxa and Habitat Type Correlated Weakly for Indigenous Species but Strongly for Invasive Species
3.3.3. Habitat Variables Influencing the Fish Community in Lake Kivu Using the RDA Model
4. Discussion
4.1. Fish Diversity in Lake Kivu
4.1.1. Species Occurrence
4.1.2. Qualitative and Quantitative Fish Composition in the North and South at Specific Sites
4.2. Patterns of and Factors Influencing Fish Assemblage in Lake Kivu
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muvundja, F.A.; Masilya, P.M.; Kisekelwa, T.; Muhigirwa, B.C.; Natacha, P.; Lwikitcha, H.B.; Eric, M.R.; Désiré, A.B.; Déo, N.; Migeni, A.Z.; et al. Current status and strategic way forward for long-term management of Lake Kivu (East Africa). J. Great. Lakes Res. 2022. [Google Scholar] [CrossRef]
- Snoeks, J. The Haplochromines (Teleostei, Cichlidae) of Lake Kivu (East Africa): Taxonomic Revision with Notes on Their Ecology; MRAC: Tervuren, Belgium, 1994; p. 197. [Google Scholar]
- Snoeks, J.; De Vos, L.; Thys van den Audenaerde, D.F.E. The ichthyogeography of Lake Kivu. S. Afr. J. Sci. 1997, 93, 579–584. [Google Scholar]
- Snoeks, J.; Kaningini, B.; Masilya, P.; Nyina-Wamwiza, L.; Guillard, J.J. Fishes in Lake Kivu: Diversity and Fisheries. In Lake Kivu: Limnology and Biogeochemistry of a Tropical Great Lake; Descy, J.-P., Darchambeau, F., Schmid, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 107–126. [Google Scholar]
- Verbeke, J. Exploration Hydrobiologique des Lacs Kivu, Edouard et Albert (1952–1954); IRSNB: Bruxelles, Belgique, 1957; Volume III, p. 177. [Google Scholar]
- Kaningini, M.; Micha, J.-C.; Vandenhaute, J.; Platteau, J.-P.; Watongoka, H.; Melard, C.; Wilondja, M.K.; Isumbisho, M. Pêche du Sambaza au Filet Maillant Dans le Lac Kivu; Presses Universitaires de Namur: Namur, Belgium, 1999; p. 152. [Google Scholar]
- Guillard, J.; Darchambeau, F.; Masilya, P.; Descy, J.-P. Is the fishery of the introduced Tanganyika sardine (Limnothrissa miodon) in Lake Kivu (East Africa) sustainable? J. Great. Lakes Res. 2012, 38, 524–533. [Google Scholar] [CrossRef]
- Beadle, L.C. The Inland Waters of Tropical Africa: An Introduction to Tropical Limnology, 2nd ed.; Longman: Harlow, UK, 1981; p. 475. [Google Scholar]
- Munyandamutsa, P.; Lazaro, W.J.; Kassam, D.; Mtethiwa, A. Species specificity and sexual dimorphism in tooth shape among the three sympatric haplochromine species in Lake Kivu cichlids. Ecol. Evol. 2020, 10, 5694–5711. [Google Scholar] [CrossRef]
- Kaningini, M.B. Etude de la Croissance, de la Reproduction et de L’exploitation de Limnothrissa miodon au Lac Kivu, Bassin de Bukavu (Zaïre). Ph.D. Thesis, Université de Namur, Namur, Belgium, 1995. [Google Scholar]
- Masilya, M.P.; Darchambeau, F.; Isumbisho, M.; Descy, J.-P. Diet overlap between the newly introduced Lamprichthys tanganicanus and the Tanganyika sardine in Lake Kivu, Eastern Africa. Hydrobiologia 2011, 675, 75–86. [Google Scholar] [CrossRef]
- Schmid, M.; Halbwachs, M.; Wehrli, B.; Wüest, A. Weak mixing in Lake Kivu: New insights indicate increasing risk of uncontrolled gas eruption. Geochem. Geophys. Geosyst. 2005, 6, Q07009. [Google Scholar] [CrossRef]
- Muvundja, F.A.; Pasche, N.; Bugenyi, F.W.B.; Isumbisho, M.; Müller, B.; Namugize, J.-N.; Rinta, P.; Schmid, M.; Stierli, R.; Wüest, A. Balancing nutrient inputs to Lake Kivu. J. Great. Lakes Res. 2009, 35, 406–418. [Google Scholar] [CrossRef]
- Darchambeau, F.; Sarmento, H.; Descy, J.-P. Primary production in a tropical large lake: The role of phytoplankton composition. Sci. Total Environ. 2014, 473–474, 178–188. [Google Scholar] [CrossRef]
- Tietze, K.; Geyh, M.; Müller, H.; Schröder, L.; Stahl, W.; Wehner, H. The genesis of the methane in Lake Kivu (Central Africa). Geol. Rundsch. 1980, 69, 452–472. [Google Scholar] [CrossRef]
- Schmid, M.; Wüest, A. Stratification, Mixing and Transport Processes in Lake Kivu. In Lake Kivu: Limnology and Biogeochemistry of a Tropical Great Lake; Descy, J.-P., Darchambeau, F., Schmid, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 13–29. [Google Scholar]
- Kling, G.; MacIntyre, S.; Steenfelt, J.S.; Hirslund, F. Lake Kivu Gas Extraction. Report on Lake Stability; Report 2006, n° 62721-0001.
- Pasche, N.; Schmid, M.; Vazquez, F.; Schubert, C.J.; Wüest, A.; Kessler, J.D.; Pack, M.A.; Reeburgh, W.S.; Bürgmann, H. Methane sources and sinks in Lake Kivu. J. Geophys. Res. Biogeosci. 2011, 116, G03006. [Google Scholar] [CrossRef]
- Blees, J.; Niemann, H.; Wenk, C.B.; Zopfi, J.; Schubert, C.J.; Kirf, M.K.; Veronesi, M.; Hitz, C.; Lehmann, M.F. Micro-aerobic bacterial methane oxidation in the chemocline and anoxic water column of deep south-Alpine Lake Lugano (Switzerland). Limnol. Oceanogr. 2014, 59, 311–324. [Google Scholar] [CrossRef]
- Obolewski, K. Epiphytic macrofauna on water soldiers (Stratiotes aloides L.) in Slupia River oxbows. Oceanol. Hydrobiol. Stud. 2005, 34, 37–54. [Google Scholar]
- Glińska-Lewczuk, K.; Burandt, P.; Kujawa, R.; Kobus, S.; Obolewski, K.; Dunalska, J.; Grabowska, M.; Lew, S.; Chormański, J. Environmental factors structuring fish communities in floodplain lakes of the undisturbed system of the Biebrza River. Water 2016, 8, 146. [Google Scholar] [CrossRef]
- Kisekelwa, T.; Snoeks, J.; Ibala Zamba, A.; Amzati, G.S.; Isumbisho, M.; Masilya, P.M.; Lemmens, P.; Vreven, E. Association between Labeobarbus spp. (Teleostei: Cyprinidae) and environmental variables in the Luhoho basin (Eastern Congo River basin; DRC). J. Fish Biol. 2021, 99, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Rice, W.R. Analyzing tables of statistical tests. Evolution 1989, 43, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Greenstreet, S.P.; Rogers, S.I. Indicators of the health of the North Sea fish community: Identifying reference levels for an ecosystem approach to management. ICES J. Mar. Sci. 2006, 63, 573–593. [Google Scholar] [CrossRef]
- Marcon, E. Mesure de la Biodiversité; UMR: Guyane, France, 2010; p. 58. [Google Scholar]
- Daly, A.J.; Baetens, J.M.; De Baets, B. Ecological Diversity: Measuring the Unmeasurable. Mathematics 2018, 6, 199. [Google Scholar] [CrossRef]
- Kisekelwa, T.; Snoeks, J.; Vreven, E. An annotated checklist of the fish diversity of the fish fauna of the river systems draining the Kahuzi-Biega National Park (Upper Congo: Eastern DR Congo). J. Fish Biol. 2020, 96, 700–721. [Google Scholar] [CrossRef]
- Dube, T.; Pinceel, T.; De Necker, L.; Wepener, V.; Lemmens, P.; Brendonck, L. Lateral hydrological connectivity differentially affects the community characteristics of multiple groups of aquatic invertebrates in tropical wetland pans in South Africa. Fresh. Biol. 2019, 64, 2189–2203. [Google Scholar] [CrossRef]
- Mwaijengo, G.N.; Vanschoenwinkel, B.; Trevor, D.; Njau, K.N.; Brendonck, L. Seasonal variation in benthic macroinvertebrate assemblages and water quality in an Afrotropical river catchment, northeastern Tanzania. Limnologica 2020, 82, 25780. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Phil. Trans. R. Soc. 2016, A374, 20150202. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, H.; Vilmi, A.; Tolonen, K.T.; Tang, X.; Qin, B.; Gong, Z.; Heino, J. Relative roles of spatial processes, natural factors and anthropogenic stressors in structuring a lake macroinvertebrate metacommunity. Sci. Total Environ. 2017, 601–602, 1702–1711. [Google Scholar] [CrossRef]
- Mwaijengo, G.N.; Msigwa, A.; Njau, K.N.; Brendonck, L.; Vanschoenwinkel, B. Where does land use matter most? Contrasting land use effects on river quality at different spatial scales. Sci. Total Environ. 2020, 715, 134825. [Google Scholar] [CrossRef]
- Blanchet, G.F.; Legendre, P.; Borcard, D. Forward selection of explanatory variables. Ecology 2008, 89, 2623–2632. [Google Scholar] [CrossRef]
- Legendre, E.; Gallagher, D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: New York, NY, USA, 1977; p. 579. [Google Scholar]
- Craney, T.A.; Surles, J.G. Model-dependent variance inflation factor cutoff values. Qual. Eng. 2002, 14, 391–403. [Google Scholar] [CrossRef]
- Thompson, C.G.; Kim, R.S.; Aloe, A.M.; Betsy, J.B. Extracting the Variance Inflation Factor and Other Multicollinearity Diagnostics from Typical Regression Results. Basic Appl. Soc. Psychol. 2017, 39, 81–90. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; Hara, R.B.O.; Simpson, G.L.; Solymos, P.; et al. Vegan Community Ecology Package Version 2.4-1. 2016. Available online: http://CRAN.R-project.org/package=vegan (accessed on 8 May 2020).
- Ter Braak, C.J.F. Canoco—An extension of decorana to analyze species-environment relationships. Vegetatio 1988, 75, 159–160. [Google Scholar] [CrossRef]
- Joyeuse, M. Contribution à L’étude du Peuplement de la Faune Ichtyologique Dans le Bassin Nord du Lac Kivu. Licence Thesis, ISP of Bukavu, Bukavu, Congo, 2020. [Google Scholar]
- Mazambi, L.J. Diversité Ichtyologique de la Rivière Ruzizi en Amont et en Aval Des Centrales Hydroélectriques Ruzizi I et II. Master’s Thesis, University of Abomey Calavi, Cotonou, Benin, 2016. [Google Scholar]
- Walsh, S.J.; Chapman, L.J.; Rosenberger, A.E.; Chapman, C.A. Redescription of Amphilius jacksonii (Siluriformes: Amphiliidae) with habitat and life-history notes. Ichthyol. Explor. Freshw. 2000, 11, 163–174. [Google Scholar]
- Akonkwa, D.B. Effets des Changements Climatiques et Des Pratiques de Pêche sur Les Ressources Halieutiques du Lac Kivu (Afrique de l’Est). Ph.D. Thesis, Université d’Abomey-Calavi, Cotonou, Benin, 2017. [Google Scholar]
- Fryer, G. The trophic interrelationships and ecology of some littoral communities of Lake Nyasa with especial reference to the fishes, and a discussion of the evolution of a group of rock-frequenting Cichlidae. Proc. Zool. Soc. Lond. 1959, 132, 153–281. [Google Scholar] [CrossRef]
- Beadle, L.C. The Evolution of species in the Lakes of East Africa. Uganda J. 1962, 26, 44–54. [Google Scholar]
- Kocher, T.D. Adaptive evolution and explosive speciation: The cichlid fish model. Nat. Rev. Genet. 2004, 5, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Salzburger, W. Understanding explosive diversification through cichlid fish genomics. Nat. Rev. Genet. 2018, 19, 705–717. [Google Scholar] [CrossRef]
- Seehausen, O.; Magalhaes, I.S. Geographical mode and evolutionary mechanism of ecological speciation in Cichlid Fish. In Research of the Causes of Evolution: From Field Observations to Mechanisms; Grant, P.R., Grant, B.R., Eds.; Princeton: Singapore, 2010; pp. 282–308. [Google Scholar]
- Seehausen, O.; Samwel-Terry, A.; Bouton, N. Habitat and ecology of rock-dwelling cichlids. In Lake Victoria Rock Cichlids: Cichlids: Taxonomy, Ecology and Distribution; Seehausen, O., Ed.; Verduijn Cichlids: Zevenhuizen, Germany, 1996; pp. 36–43. [Google Scholar]
- Snoeks, J. How well known is the ichthyodiversity of the large East African lakes? Adv. Ecol. Res. 2000, 31, 17–38. [Google Scholar] [CrossRef]
- Lévêque, C.; Agnèse, F. Diversity of African fish: Heritage of evolution. In The Inland Water Fishes of Africa: Diversity, Ecology and Human Use; Paugy, D., Lévêque, C., Otero, O., Eds.; IRD: Paris, France; MRAC: Tervuren, Belgium, 2017; pp. 127–145. [Google Scholar]
- Dunn, I.G. Ecological notes on the Haplochromis (Pisces: Cichlidae) species-flock of Lake George, Uganda (East Africa). J. Fish Biol. 1975, 7, 651–666. [Google Scholar] [CrossRef]
- Sturmbauer, C. Explosive speciation in cichlid fishes of the African Great Lakes: A dynamic model of adaptive radiation. J. Fish Biol. 1998, 53, 18–36. [Google Scholar] [CrossRef]
- Muderhwa, N.; Lushombo, M. The introduction of the endemic fish species, Lamprichthys tanganicanus (Poeciliidae), from Lake Tanganyika into Lake Kivu: Possible causes and effects. Aquat. Ecosyst. Health Manag. 2010, 13, 203–213. [Google Scholar] [CrossRef]
- Coulter, G.W. Lake Tanganyika and Its Life; Oxford University Press: Oxford, UK, 1991; p. 362. [Google Scholar]
- Heino, J.; Melo, A.S.; Siqueira, T.; Soininen, J.; Valanko, S.; Bini, L.M. Metacommunity organisation, spatial extent and dispersal in aquatic systems: Patterns, processes and prospects. Freshw. Biol. 2015, 60, 845–869. [Google Scholar] [CrossRef]




| Clay (%) | Sand (%) | Cobble/Pebble (%) | Large Coarse (%) | Cover (%) | Macrophytes (%) | |
|---|---|---|---|---|---|---|
| North basin | ||||||
| (1) Sandy patch | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| (2) Rock and macrophytes | 0.0 | 0.0 | 0.0 | 80.0 | 0.0 | 20.0 |
| (3) Rocky shore | 0.0 | 0.0 | 0.0 | 100.0 | 10.0 | 0.0 |
| (4) Rocks mixed with sand | 0.0 | 60.0 | 0.0 | 40.0 | 0.0 | 0.0 |
| (5) Rocky shore | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| (6) Rocky shore—shaded | 0.0 | 0.0 | 0.0 | 100.0 | 10.0 | 0.0 |
| (7) Sandy patch | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| South basin | ||||||
| (8) Ishungu with macrophytes | 25.0 | 25.0 | 50.0 | 0.0 | 0.0 | 100.0 |
| (9) Ishungu with a rocky shore | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| (10) Kalengera with a denuded shore | 50.0 | 25.0 | 25.0 | 0.0 | 0.0 | 0.0 |
| (11) Kalengera with macrophytes | 50.0 | 25.0 | 25.0 | 0.0 | 0.0 | 100.0 |
| (12) Alleluia Port with a denuded shore | 0.0 | 25.0 | 50.0 | 25.0 | 0.0 | 0.0 |
| (13) SNCC beach with macrophytes | 50.0 | 25.0 | 25.0 | 0.0 | 0.0 | 100.0 |
| (14) Governor’s park with macrophytes | 50.0 | 25.0 | 25.0 | 0.0 | 5.0 | 100.0 |
| North | South | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ishungu | Bukavu | ||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | C (%N) | 8 | 9 | 10 | 11 | 12 | 13 | 14 | C (%S) | C (%LK) | |
| Clupeidae | |||||||||||||||||
| Limnothrissa miodon (Boulenger, 1906) (i) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 14 | 141 | 15 | 0 | 0 | 0 | 6 | 7 | 57 | 36 |
| Cyprinidae | |||||||||||||||||
| Enteromius kerstenii (Peters, 1868) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Procatopodidae | |||||||||||||||||
| Lamprichthys tanganicanus (Boulenger, 1898) (i) | 2 | 145 | 374 | 183 | 274 | 357 | 15 | 100 | 58 | 385 | 7 | 7 | 27 | 3 | 200 | 100 | 100 |
| Cichlidae | |||||||||||||||||
| Coptodon rendalli (Boulenger, 1897) (i) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Haplochromis adolphifrederici (Boulenger, 1914) | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 57 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 36 |
| H. astatodon Regan, 1921 | 0 | 12 | 4 | 7 | 2 | 13 | 1 | 86 | 0 | 4 | 3 | 0 | 1 | 2 | 4 | 71 | 79 |
| H. crebridens Snoeks, de Vos, Coenen, and Thys van den Audenaerde, 1990 | 0 | 9 | 0 | 0 | 2 | 1 | 17 | 57 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 57 | 57 |
| H. gracilior Boulenger, 1914 | 5 | 8 | 0 | 14 | 7 | 2 | 0 | 71 | 0 | 0 | 2 | 3 | 4 | 0 | 0 | 43 | 57 |
| H. graueri Boulenger, 1914 | 6 | 3 | 0 | 26 | 0 | 0 | 0 | 43 | 0 | 1 | 6 | 17 | 0 | 12 | 8 | 57 | 57 |
| H. insidae Snoeks, 1994 | 0 | 7 | 3 | 0 | 0 | 0 | 4 | 43 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 21 |
| H. microchrysomelas Snoeks, 1994 | 8 | 15 | 4 | 2 | 2 | 0 | 0 | 71 | 2 | 3 | 5 | 4 | 0 | 9 | 2 | 86 | 79 |
| H. occultidens Snoeks, 1988 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 43 | 29 |
| H. olivaceus Snoeks, de Vos, Coenen, and Thys van den Audenaerde, 1990 | 1 | 8 | 6 | 1 | 1 | 2 | 0 | 86 | 14 | 5 | 9 | 0 | 4 | 3 | 0 | 71 | 79 |
| H. paucidens Regan, 1921 | 0 | 4 | 4 | 3 | 2 | 0 | 0 | 57 | 0 | 0 | 0 | 0 | 4 | 1 | 2 | 43 | 50 |
| H. rubescens Snoeks, 1994 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 14 | 0 | 1 | 3 | 0 | 6 | 0 | 0 | 43 | 29 |
| H. scheffersi Snoeks and Thys van den Audenaerde 1987 | 13 | 1 | 2 | 8 | 6 | 1 | 5 | 100 | 2 | 1 | 1 | 3 | 5 | 1 | 3 | 100 | 100 |
| H. vittatus (Boulenger, 1901) | 1 | 5 | 0 | 3 | 7 | 0 | 0 | 57 | 1 | 2 | 0 | 3 | 3 | 2 | 3 | 86 | 71 |
| Oreochromis niloticus (Linnaeus, 1758) | 0 | 0 | 0 | 1 | 1 | 0 | 3 | 43 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 29 |
| Total number of specimens | 36 | 219 | 398 | 251 | 304 | 377 | 46 | 222 | 417 | 37 | 38 | 57 | 40 | 231 | |||
| Species richness | 7 | 13 | 8 | 13 | 10 | 8 | 7 | 9 | 9 | 9 | 7 | 10 | 10 | 9 | |||
| Species richness per basin | 17 | 12 | 13 | ||||||||||||||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | (13) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) Sandy patch | |||||||||||||
| (2) Rock and macrophytes | 0.00 * | ||||||||||||
| (3) Rocky shore | 0.03 | 0.57 | |||||||||||
| (4) Rocks mixed with sand | 0.00 * | 0.93 | 0.57 | ||||||||||
| (5) Rocky shore | 0.01 * | 0.81 | 0.68 | 0.93 | |||||||||
| (6) Rocky shore—shaded | 0.05 | 0.81 | 0.68 | 0.93 | 0.93 | ||||||||
| (7) Sandy patch | 0.80 | 0.00 * | 0.07 | 0.01 * | 0.03 | 0.12 | |||||||
| (8) Ishungu with macrophytes | 0.11 | 0.91 | 0.91 | 0.91 | 0.91 | 0.91 | 0.16 | ||||||
| (9) Ishungu with a rocky shore | 0.03 | 0.59 | 0.74 | 0.59 | 0.59 | 0.59 | 0.04 | 0.47 | |||||
| (10) Kalengera with a denuded shore | 0.23 | 0.01 * | 0.10 | 0.01 * | 0.11 | 0.11 | 0.32 | 0.31 | 0.11 | ||||
| (11) Kalengera with macrophytes | 0.16 | 0.01 * | 0.11 | 0.01 * | 0.06 | 0.11 | 0.33 | 0.31 | 0.14 | 1.00 | |||
| (12) Alleluia Port with a denuded shore | 0.16 | 0.06 | 0.16 | 0.11 | 0.28 | 0.33 | 0.23 | 0.31 | 0.19 | 0.45 | 0.47 | ||
| (13) SNCC beach with macrophytes | 0.13 | 0.01 * | 0.10 | 0.03 | 0.11 | 0.11 | 0.23 | 0.31 | 0.11 | 0.65 | 0.88 | 0.66 | |
| (14) Governor’s park with macrophytes | 0.01 * | 0.45 | 1.00 | 0.59 | 0.59 | 0.59 | 0.01 * | 0.88 | 0.88 | 0.03 | 0.03 | 0.06 | 0.03 |
| Sites | HillN1 Diversity |
|---|---|
| North | |
| (1) Sandy patch | 5.10 |
| (2) Rock and macrophytes | 4.01 |
| (3) Rocky shore | 1.39 |
| (4) Rocks mixed with sand | 2.97 |
| (5) Rocky shore | 1.67 |
| (6) Rocky shore—shaded | 1.31 |
| (7) Sandy patch | 4.62 |
| South | |
| (8) Ishungu with macrophytes | 2.75 |
| (9) Ishungu with a rocky shore | 1.48 |
| (10) Kalengera with a denuded shore | 7.24 |
| (11) Kalengera with macrophytes | 4.95 |
| (12) Alleluia Port with a denuded shore | 5.87 |
| (13) SNCC beach with macrophytes | 6.96 |
| (14) Governor’s park with macrophytes | 1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisekelwa, T.; Alimasi, W.; Mazambi, L.; Mwaijengo, G.N.; Hyangya, L.; Muzungu, H.; Joyeuse, M.; Lubala, A.; Kubota, M.; Wüest, A.; et al. Fish Diversity in Relation to Littoral Habitats in Three Basins of Lake Kivu (East Africa). Diversity 2023, 15, 1014. https://doi.org/10.3390/d15091014
Kisekelwa T, Alimasi W, Mazambi L, Mwaijengo GN, Hyangya L, Muzungu H, Joyeuse M, Lubala A, Kubota M, Wüest A, et al. Fish Diversity in Relation to Littoral Habitats in Three Basins of Lake Kivu (East Africa). Diversity. 2023; 15(9):1014. https://doi.org/10.3390/d15091014
Chicago/Turabian StyleKisekelwa, Tchalondawa, Wilondja Alimasi, Lutete Mazambi, Grite N. Mwaijengo, Lwikitcha Hyangya, Heri Muzungu, Mudagi Joyeuse, Amani Lubala, Musombwa Kubota, Alfred Wüest, and et al. 2023. "Fish Diversity in Relation to Littoral Habitats in Three Basins of Lake Kivu (East Africa)" Diversity 15, no. 9: 1014. https://doi.org/10.3390/d15091014
APA StyleKisekelwa, T., Alimasi, W., Mazambi, L., Mwaijengo, G. N., Hyangya, L., Muzungu, H., Joyeuse, M., Lubala, A., Kubota, M., Wüest, A., Muvundja, A., & Masilya, M. (2023). Fish Diversity in Relation to Littoral Habitats in Three Basins of Lake Kivu (East Africa). Diversity, 15(9), 1014. https://doi.org/10.3390/d15091014







