Validated Inventories of Non-Indigenous Species (NIS) for the Mediterranean Sea as Tools for Regional Policy and Patterns of NIS Spread
Abstract
:1. Introduction
2. Methods
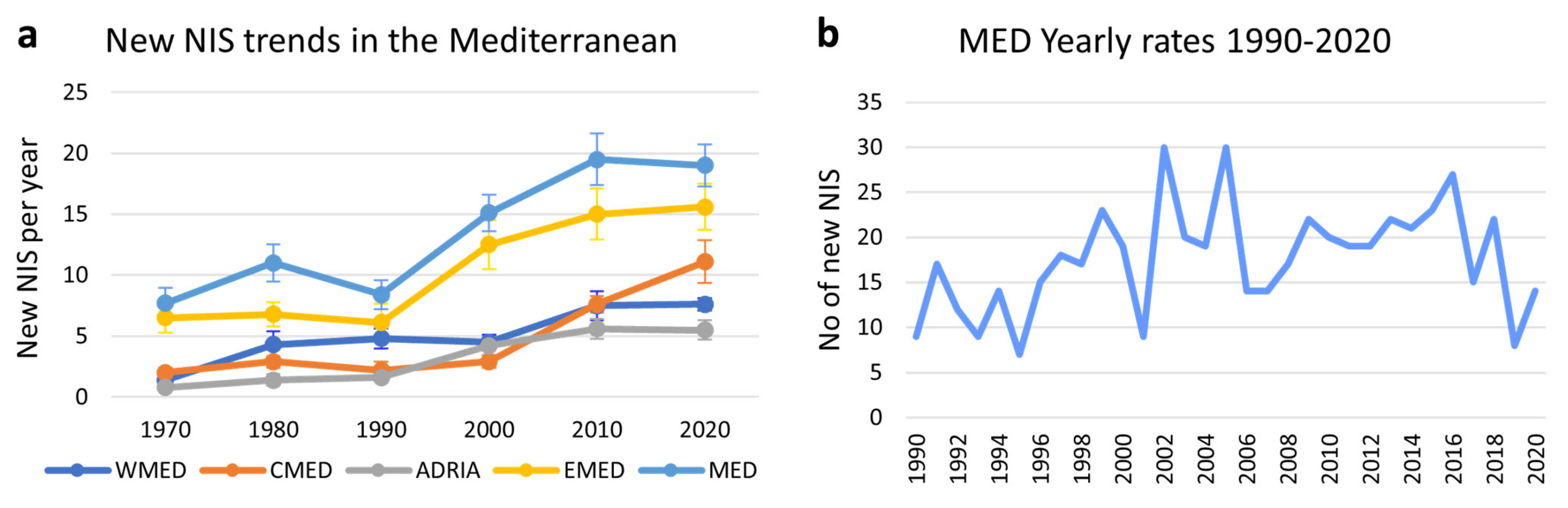
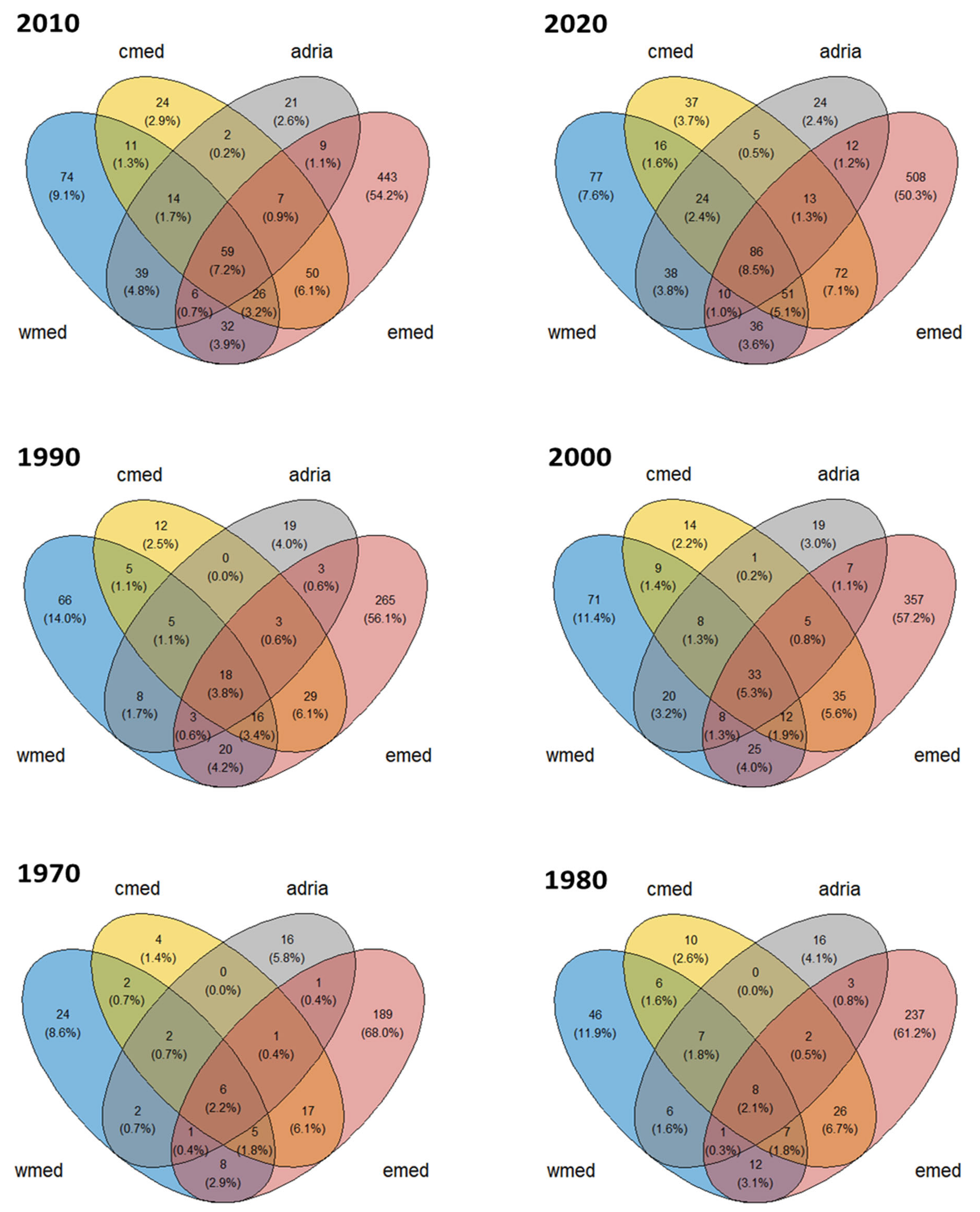
3. Results
4. Discussion
4.1. Species Validation and Uncertainties
4.2. Trends
4.3. Spatial Patterns of the Mediterranean Xenodiversity and Invasion Debt
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPBES. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Díaz, S., Settele, J., Brondízio, E.S., Ngo, H.T., Guèze, M., Agard, J., Arneth, A., Balvanera, P., Brauman, K.A., Butchart, S.H.M., et al., Eds.; IPBES Secretariat: Bonn, Germany, 2019; p. 56. [Google Scholar]
- Costello, M.J.; Dekeyzer, D.; Galil, B.S.; Hutchings, P.; Katsanevakis, S.; Pagad, S.; Robinson, T.B.; Turon, X.; Vandepitte, L.; Vanhoorne, B.; et al. Introducing the World Register of Introduced Marine Species (WRiMS). Manag. Biol. Invasions 2021, 12, 792–811. [Google Scholar] [CrossRef]
- Galil, B.S. A sea under siege—Alien species in the Mediterranean. Biol. Invasions 2000, 2, 177–186. [Google Scholar] [CrossRef]
- Galil, B.S. Taking stock: Inventory of alien species in the Mediterranean Sea. Biol. Invasions 2009, 11, 359–372. [Google Scholar] [CrossRef]
- Zenetos, A.; Galanidi, M. Mediterranean non indigenous species at the start of the 2020s: Recent changes. Mar. Biodivers. Rec. 2020, 13, 10. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; Garcia, E.L.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Meditter. Mar. Sci. 2022, 23, 196–212. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; Garcia, E.L.; Stern, N.; Tsiamis, K.; Galanidi, M. Corrigendum to the Review Article. Meditter. Mar. Sci. 2022, 23, 196–212, Erratum in 2022, 23, 876–878. [Google Scholar] [CrossRef]
- Jaureguiberry, P.; Titeux, N.; Wiemers, M.; Bowler, D.E.; Coscieme, L.; Golden, A.S.; Guerra, C.A.; Jacob, U.; Takahashi, Y.; Settele, J. The Direct Drivers of Recent Global Anthropogenic Biodiversity Loss. Sci. Adv. 2022, 8, eabm9982. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of marine invasive alien species on ecosystem services and biodiversity: A pan-European review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Galil, B.S. Poisonous and Venomous: Marine Alien Species in the Mediterranean Sea and Human Health. In CAB International 2018. Invasive Species and Human Health; Mazza, G., Tricarico, E., Eds.; CAB: Wallingford, UK, 2018; pp. 1–15. [Google Scholar]
- Tsirintanis, K.; Azzurro, E.; Crocetta, F.; Dimiza, M.; Froglia, C.; Gerovasileiou, V.; Langeneck, J.; Mancinelli, G.; Rosso, A.; Stern, N. Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat Invasions 2022, 3, 308–352. [Google Scholar] [CrossRef]
- UNEP/MAP. Decision IG 17/6: Implementation of the ecosystem approach to the management of human activities that may affect the Mediterranean marine and coastal environment. In Proceedings of the 15th Ordinary Meeting of the Contracting Parties to the Convention for the Protection of the Marine Environment and the Coastal Region of the Mediterranean and its Protocols, Almeria, Spain, 15–18 January 2008. UNEP(DEPI)/MED IG.17/10. [Google Scholar]
- UNEP/MAP. Decision IG.20/4: Implementing MAP ecosystem approach roadmap: Mediterranean Ecological and Operational Objectives, Indicators and Timetable for implementing. In Proceedings of the 16th Ordinary Meeting of the Contracting Parties to the Convention for the Protection of the Marine Environment and the Coastal Region of the Mediterranean and its Protocols, Marrakesh, Morocco, 3–5 November 2009. UNEP(DEPI)/MED IG.19/8. [Google Scholar]
- UNEP/MAP. Integrated Monitoring and Assessment Programme of the Mediterranean Sea and Coast and Related Assessment Criteria; UNEP/MAP: Athens, Greece, 2016; p. 26. [Google Scholar]
- European Comimission. European Commission Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008, establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Union 2008, L164, 19–40. [Google Scholar]
- European Commission. European Commission Decision (EC2017/848 of 17 May 2017 laying down criteria and methodological standards on good environmental status of marine waters and specifications and standardized methods for monitoring and assessment, and repealing Decision 2010/477/EU. Off. J. Eur. Union 2017, L125, 43–74. [Google Scholar]
- Marchini, A.; Galil, B.S.; Occhipinti-Ambrogi, A. Recommendations on standardizing lists of marine alien species: Lessons from the Mediterranean Sea. Mar. Pollut. Bull. 2015, 101, 267–273. [Google Scholar] [CrossRef]
- Latombe, G.; Canavan, S.; Hirsch, H.; Hui, C.; Kumschick, S.; Nsikani, M.M.; Potgieter, L.J.; Robinson, T.B.; Saul, W.-C.; Turner, S.C.; et al. A four-component classification of uncertainties in biological invasions: Implications for management. Ecosphere 2019, 10, e02669. [Google Scholar] [CrossRef]
- Golani, D.; Orsi-Relini, L.; Massutí, E.; Quignard, J.P. CIESM Atlas of Exotic Species in the Mediterranean. Vol. 1. Fishes; Briand, F., Ed.; CIESM Publishers: Monaco, 2002; p. 254. [Google Scholar]
- Golani, D.; Azzurro, E.; Dulčić, J.; Massutí, E.; Orsi-Relini, L. Atlas of Exotic Fishes in the Mediterranean Sea; Briand, F., Ed.; CIESM Publishers: Paris, France, 2021; p. 365. [Google Scholar]
- Galil, B.S.; Froglia, C.; Noel, P.Y. Crustacean Decapods and Stomatopods. In CIESM Atlas of Exotic Species in the Mediterranean; Briand, F., Ed.; CIESM Publishers: Monaco, 2002; Volume 2, p. 192. [Google Scholar]
- Galil, B.S.; Froglia, C.; Noel, P. Looking back, looking ahead: The CIESM Atlas, Crustaceans. Manag. Biol. Invasions 2015, 6, 171–175. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Russo, G.; Templado, J. CIESM Atlas of Exotic Species in the Mediterranean: Vol. 3. Molluscs; Briand, F., Ed.; CIESM Publishers: Monaco, 2004; p. 376. [Google Scholar]
- Verlaque, M.; Ruitton, S.; Mineur, F.; Boudouresque, C.-F. CIESM Atlas of Exotic Species in the Mediterranean: 4. Macrophytes; Briand, F., Ed.; CIESM: Monaco, 2015; p. 364. ISBN 978-92-990003-4-2. [Google Scholar]
- Galil, B.S. Alien species in the Mediterranean Sea—Which, when, where, why? Hydrobiologia 2008, 606, 105–116. [Google Scholar] [CrossRef]
- Galil, B.S.; Marchini, A.; Occhipinti-Ambrogi, A. East is east and West is west? Management of marine bioinvasions in the Mediterranean Sea. Estuar. Coast. Shelf Sci. 2018, 201, 7–16. [Google Scholar] [CrossRef]
- Zenetos, A.; Cinar, M.E.; Pancucci-Papadopoulou, M.A.; Harmelin, J.G.; Furnari, G.; Andaloro, F.; Bellou, N.; Streftaris, N.; Zibrowius, H. Annotated list of marine alien species in the mediterranean with records of the worst invasive species. Mediterr. Mar. Sci. 2005, 6, 63–118. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Verlaque, M.; Cinar, M.E.; Raso, J.E.G.; Bianchi, C.N.; Morri, C.; Azzurro, E.; Bilecenoglu, M.; Froglia, C.; et al. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part, I. Spatial distribution. Mediterr. Mar. Sci. 2010, 11, 381–493. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Morri, C.; Rosso, A.; Violanti, D.; Garcia Raso, J.E.; Cinar, M.E.; Almogi-Labin, A.; Ates, A.S.; Azzurro, E.; et al. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr. Mar. Sci. 2012, 13, 328–352. [Google Scholar] [CrossRef]
- Zenetos, A.; Cinar, M.E.; Crocetta, F.; Golani, D.; Rosso, A.; Servello, G.; Shenkar, N.; Turon, X.; Verlaque, M. Uncertainties and validation of alien species catalogues: The Mediterranean as an example. Estuar. Coast. Shelf Sci. 2017, 191, 171–187. [Google Scholar] [CrossRef]
- Grimes, S.; Benabdi, M.; Babali, N.; Refes, W.; Boudjellal-Kaidi, N.; Seridi, H. Biodiversity changes along the Algerian coast (Southwest Mediterranean basin): From 1834 to 2017: A first assessment of introduced species. Mediterr. Mar. Sci. 2018, 19, 156–179. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tsiamis, K.; Ioannou, G.; Michailidis, N.; Zenetos, A. Inventory of alien marine species of Cyprus. Mediterr. Mar. Sci. 2009, 10, 109–134. [Google Scholar] [CrossRef]
- Pečarević, M.; Mikuš, J.; Cetinić-Bratoš, J.; Dulčić, J.; Čalić, M. Introduced marine species in Croatian waters (Eastern Adriatic Sea). Mediterr. Mar. Sci. 2013, 14, 224–237. [Google Scholar] [CrossRef]
- Galil, B.S. Seeing red: Alien species along the Mediterranean coast of Israel. Aquat. Invasions 2007, 2, 281–312. [Google Scholar] [CrossRef]
- Galil, B.S.; Mienis, H.K.; Hoffman, R.; Goren, M. Non-indigenous species along the Israeli Mediterranean coast: Tally, policy, outlook. Hydrobiologia 2021, 848, 2011–2029. [Google Scholar] [CrossRef]
- Occhipinti-Ambrogi, A.; Marchini, A.; Cantone, G.; Castelli, A.; Chimenz, C.; Cormaci, M.; Froglia, C.; Furnari, G.; Gambi, M.C.; Giaccone, G.; et al. Alien species along the Italian coasts: An overview. Biol. Invasions 2011, 13, 215–237. [Google Scholar] [CrossRef]
- Servello, G.; Andaloro, F.; Azzurro, E.; Castriota, L.; Catra, M.; Chiarore, A.; Crocetta, F.; D’Alessandro, M.; Denitto, F.; Froglia, C.; et al. Marine alien species in Italy: A contribution to the implementation of descriptor D2 of the marine strategy framework directive. Mediterr. Mar. Sci. 2019, 20, 1–48. [Google Scholar] [CrossRef]
- Di Muri, C.; Lazic, T.; Rosati, I.; Pierri, C.; Boggero, A.; Corriero, G.; Basset, A. Alien and native species in Italian marine and transitional waters. Biodivers. Data J. 2023, 11, e101464. [Google Scholar] [CrossRef]
- Sciberras, M.; Schembri, P.J. A critical review of records of alien marine species from the Maltese Islands and surrounding waters (Central Mediterranean). Mediterr. Mar. Sci. 2007, 8, 41–66. [Google Scholar] [CrossRef]
- Evans, J.; Barbara, J.; Schembri, P.J. Updated review of marine alien species and other ‘newcomers’ recorded from the Maltese Islands (Central Mediterranean). Mediterr. Mar. Sci. 2015, 16, 225–244. [Google Scholar] [CrossRef]
- Zibrowius, H.; Bitar, G. Invertébrés marins exotiques sur la côte du Liban. Leban. Sci. J. 2003, 4, 67–74. [Google Scholar]
- Bitar, G.; Ramos-Esplá, A.; Ocaña, O.; Sghaier, Y.; Forcada, A.; Valle, C.; El Shaer, H.; Verlaque, M. Introduced marine macroflora of Lebanon and its distribution on the Levantine coast. Mediterr. Mar. Sci. 2017, 18, 138–155. [Google Scholar] [CrossRef]
- Bariche, M.; Fricke, R. The marine ichthyofauna of Lebanon: An annotated checklist, history, biogeography, and conservation status. Zootaxa 2020, 4775, 1–157. [Google Scholar] [CrossRef] [PubMed]
- Bazairi, H.; Sghaier, Y.R.; Benamer, I.; Langar, H.; Pergent, G.; Bourass, E.M.; Verlaque, M.; Souissi, J.B.; Zenetos, A. Alien marine species of Libya: First inventory and new records in El-Kouf National Park (Cyrenaica) and the neighbouring areas. Mediterr. Mar. Sci. 2013, 14, 451–462. [Google Scholar] [CrossRef]
- Shakman, E.; Eteayb, K.; Taboni, I.; Ben Abdalla, A. Status of marine alien species along the Libyan coast. J. Black Sea Mediterr. Environ. 2019, 25, 188–209. [Google Scholar]
- Farrag, M.M.S.; El-Naggar, H.A.; Abou-Mahmoud, M.A.; Alabssawy, A.N.; Ahmed, H.O.; Abo-Taleb, H.A.; Kapiris, K. Marine Biodiversity Patterns off Alexandria Area, Southeastern Mediterranean Sea, Egypt. Environ. Monit. Ass. 2019, 191, 367–394. [Google Scholar] [CrossRef]
- Petović, S.; Marković, O.; Đurović, M. Inventory of non-indigenous and cryptogenic marine benthic species of the south-east Adriatic Sea, Montenegro. Acta Zool. Bulg. 2019, 71, 47–52. [Google Scholar]
- Massé, C.; Viard, F.; Humbert, S.; Antajan, E.; Auby, I.; Bachelet, G.; Bernard, G.; Bouchet, V.M.P.; Burel, T.; Dauvin, J.-C.; et al. An Overview of Marine Non-Indigenous Species Found in Three Contrasting Biogeographic Metropolitan French Regions: Insights on Distribution, Origins and Pathways of Introduction. Diversity 2023, 15, 161. [Google Scholar] [CrossRef]
- Pancucci-Papadopoulou, M.A.; Zenetos, A.; Corsini-Foka, M.; Politou, C. Update of marine aliens in Hellenic waters. Mediterr. Mar. Sci. 2005, 6, 147–158. [Google Scholar] [CrossRef]
- Zenetos, A.; Pancucci-Papadopoulou, M.A.; Zogaris, S.; Papastergiadou, E.; Vardakas, L.; Aligizaki, K.; Economou, A.N. Aquatic alien species in Greece: Tracking sources, patterns and effects on the ecosystem. J. Biol. Res. 2009, 12, 135–172. [Google Scholar]
- Zenetos, A.; Katsanevakis, S.; Poursanidis, D.; Crocetta, F.; Damalas, D.; Apostolopoulos, G.; Gravili, C.; Vardala-Theodorou, E.; Malaquias, M. Marine alien species in Greek Seas: Additions and amendments by 2010. Mediterr. Mar. Sci. 2011, 12, 95–120. [Google Scholar] [CrossRef]
- Zenetos, A.; Arianoutsou, M.; Bazos, I.; Balopoulou, S.; Corsini-Foka, M.; Dimiza, M.; Drakopoulou, P.; Katsanevakis, S.; Kondylatos, G.; Koutsikos, N.; et al. ELNAIS: A collaborative network on aquatic alien species in Hellas (Greece). Manag. Biol. Invasions 2015, 6, 185–196. [Google Scholar] [CrossRef]
- Zenetos, A.; Corsini-Foka, M.; Crocetta, F.; Gerovasileiou, V.; Karachle, P.K.; Simboura, N.; Tsiamis, K.; Pancucci-Papadopoulou, M.-A. Deep cleaning of alien and cryptogenic species records in the Greek Seas (2018 update). Manag. Biol. Invasions 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Zenetos, A.; Karachle, P.K.; Corsini-Foka, M.; Gerovasileiou, V.; Simboura, N.; Xentidis, N.J.; Tsiamis, K. Is the trend in new introductions of marine non-indigenous species a reliable criterion for assessing good environmental status? The case study of Greece. Mediterr. Mar. Sci. 2020, 21, 775–793. [Google Scholar] [CrossRef]
- Lipej, L.; Mavrič, B.; Orlando-Bonaca, M.; Malej, A. State of the art of the marine non-indigenous flora and fauna in Slovenia. Mediterr. Mar. Sci. 2012, 13, 243–249. [Google Scholar] [CrossRef]
- Png-Gonzalez, L.; Comas-González, R.; Calvo-Manazza, M.; Follana-Berná, G.; Ballesteros, E.; Díaz-Tapia, P.; Falcón, J.M.; García Raso, J.E.; Gofas, S.; González-Porto, M.; et al. Updating the National Baseline of Non-Indigenous Species in Spanish Marine Waters. Diversity 2023, 15, 630. [Google Scholar] [CrossRef]
- Zamora-Marín, J.M.; Herrero-Reyes, A.A.; Ruiz-Navarro, A.; Oliva-Paterna, F.J. Non-indigenous aquatic fauna in transitional waters from the Spanish Mediterranean coast: A comprehensive assessment. Mar. Pollut. Bull. 2023, 191, 114893. [Google Scholar] [CrossRef]
- Ali, M. An updated Checklist of Marine fishes from Syria with an emphasis on alien species. Meditter. Mar. Sci. 2018, 19, 388–393. [Google Scholar] [CrossRef]
- Ammar, I. Updated list of alien macrozoobenthic species along the Syrian coast. Int. J. Aquat. Biol. 2019, 7, 180–194. [Google Scholar]
- Amor, K.O.-B.; Rifi, M.; Ghanem, R.; Draeif, I.; Zaouali, J.; Ben Souissi, J. Update of alien fauna and new records from Tunisian marine waters. Mediterr. Mar. Sci. 2016, 17, 124–143. [Google Scholar] [CrossRef]
- Sghaier, Y.R.; Zakhama-Sraieb, R.; Mouelhi, S.; Vazquez, M.; Valle, C.; Ramos-Espla, A.A.; Astier, J.M.; Verlaque, M.; Charfi Cheikhrouha, F. Review of alien marine macrophytes in Tunisia. Mediterr. Mar. Sci. 2016, 17, 109–123. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bilecenoglu, M.; Ozturk, B.; Katagan, T.; Aysel, V. Alien species on the coasts of Turkey. Mediterr. Mar. Sci. 2005, 6, 119–146. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bilecenoglu, M.; Ozturk, B.; Katagan, T.; Yokes, M.B.; Aysel, V.; Dagli, E.; Acik, S.; Ozcan, T.; Erdogan, H. An updated review of alien species on the coasts of Turkey. Mediterr. Mar. Sci. 2011, 12, 257–315. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bilecenoglu, M.; Yokes, M.B.; Ozturk, B.; Taskin, E.; Bakir, K.; Dogan, A.; Acik, S. Current status (as of end of 2020) of marine alien species in Turkey. PLoS ONE 2021, 16, e0251086. [Google Scholar] [CrossRef] [PubMed]
- UNEP/MAP. Integrated Monitoring and Assessment Guidance. In Report of the 19th Ordinary Meeting of the Contracting Parties to the Convention for the Protection of the Marine Environment and the Coastal Region of the Mediterranean and Its Protocols; UNEP/MAP: Athens, Greece, 2016; UNEP(DEPI)/MED IG.22/Inf.7. [Google Scholar]
- Tsiamis, K.; Palialexis, A.; Stefanova, K.; Gladan, Ž.N.; Skejić, S.; Despalatović, M.; Cvitković, I.; Dragičević, B.; Dulčić, J.; Vidjak, O.; et al. Non-indigenous species refined national baseline inventories: A synthesis in the context of the European Union’s Marine Strategy Framework Directive. Mar. Pollut. Bull. 2019, 145, 429–435. [Google Scholar] [CrossRef]
- Tsiamis, K.; Palialexis, A.; Connor, D.; Antoniadis, S.; Bartilotti, C.; Bartolo, G.A.; Berggreen, U.C.; Boschetti, S.; Buschbaum, C.; Canning-Clode, J.; et al. Marine Strategy Framework Directive-Descriptor 2, Non-Indigenous Species, Delivering Solid Recommendations for Setting Threshold Values for Non-Indigenous Species Pressure on European Seas; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar] [CrossRef]
- Zenetos, A.; Tsiamis, K.; Galanidi, M.; Carvalho, N.; Bartilotti, C.; Canning-Clode, J.; Castriota, L.; Chainho, P.; Comas-González, R.; Costa, A.C.; et al. Status and Trends in the Rate of Introduction of Marine Non-Indigenous Species in European Seas. Diversity 2022, 14, 1077. [Google Scholar] [CrossRef]
- UNEP/MAP—SPA/RAC. Monitoring and Assessment Scales, Assessment Criteria and Thresholds Values for the IMAP Common Indicator Related to Non-Indigenous Species. In Proceedings of the Meeting of the Ecosystem Approach Correspondence Group on Monitoring (CORMON) Biodiversity and Fisheries, Online, 10–11 June 2021. UNEP/MED WG. 500/7. [Google Scholar]
- UNEP/MAP. Baseline for the IMAP Common Indicator 6 related to Non-Indigenous Species. In Proceedings of the 9th Meeting of the Ecosystem Approach Coordination Group, Online, 5 July 2022. UNEP/MED WG.521/Inf.8. [Google Scholar]
- UNEP/MAP. Report of the 20th Ordinary Meeting of the Contracting Parties to the Convention for the Protection of the Marine Environment and the Coastal Region of the Mediterranean and Its Protocols; UNEP/MAP: Tirana, Albania, 2017. [Google Scholar]
- UNEP/MED WG.520/3. 2023 Med QSR structure and outline content template, and status of Common Indicators and Ecological Objectives. In Proceedings of the Meeting of the Ecosystem Approach Correspondence Group on Monitoring (CORMON) Biodiversity and Fisheries, Online, 28–29 March 2022. [Google Scholar]
- WoRMS Editorial Board. World Register of Marine Species. 2022. Available online: http://www.marinespecies.org (accessed on 25 May 2022).
- CBD. Pathways of Introduction of Invasive Species, Their Prioritization and Management. UNEP/CBD/SBSTTA/18/9/Add.1. Secretariat of the Convention on Biological Diversity, Montréal. 2014, p. 18. Available online: https://www.cbd.int/doc/meetings/sbstta/sbstta-18/official/sbstta-18-09-add1-en.pdf (accessed on 10 June 2023).
- Pergl, J.; Brundu, G.; Harrower, C.A.; Cardoso, A.C.; Genovesi, P.; Katsanevakis, S.; Lozano, V.; Perglová, I.; Rabitsch, W.; Richards, G.; et al. Applying the Convention on Biological Diversity Pathway Classification to alien species in Europe. Neobiota 2020, 62, 333–363. [Google Scholar] [CrossRef]
- Langeneck, J.; Lezzi, M.; Del Pasqua, M.; Musco, L.; Gambi, M.C.; Castelli, A.; Giangrande, A. Non-indigenous polychaetes along the coasts of Italy: A critical review. Mediterr. Mar. Sci. 2020, 21, 238–275. [Google Scholar] [CrossRef]
- Gómez, F. Comments on the non-indigenous microalgae in the European seas. Mar. Pollut. Bull. 2019, 148, 1–2. [Google Scholar] [CrossRef]
- Zenetos, A.; Meriç, E.; Verlaque, M.; Galli, P.; Boudouresque, C.F.; Giangrande, A.; Çinar, M.E.; Bilecenoglu, M. Additions to the annotated list of marine alien biota in the Mediterranean with special emphasis on Foraminifera and Parasites. Mediterr. Mar. Sci. 2008, 9, 119–166. [Google Scholar] [CrossRef]
- Yan, L. ggvenn: Draw Venn Diagram by ‘ggplot2’, R Package Version 0.1.9; 2021. Available online: https://CRAN.R-project.org/package=ggvenn (accessed on 5 May 2023).
- Bitar, G. Occurrence of Abudefduf spp. (Pisces: Pomacentridae) in the Lebanese coastal waters (eastern Mediterranean)—Morphological traits and visual evidence. Medit. Mar. Sci. 2021, 22, 715–723. [Google Scholar] [CrossRef]
- Dragičević, B.; Fricke, R.; Ben Soussi, J.; Ugarković, P.; Dulčić, J.; Azzurro, E. On the occurrence of Abudefduf spp. (Pisces: Pomacentridae) in the Mediterranean Sea: A critical review with new records. BioInvasions Rec. 2021, 10, 188–199. [Google Scholar] [CrossRef]
- Galanidi, M.; Zenetos, A. Data-Driven Recommendations for Establishing Threshold Values for the NIS Trend Indicator in the Mediterranean Sea. Diversity 2022, 14, 57. [Google Scholar] [CrossRef]
- Mavrič, B.; Orlando-Bonaca, M.; Fortič, A.; Francé, J.; Mozetič, P.; Slavinec, P.; Pitacco, V.; Trkov, D.; Vascotto, I.; Zamuda, L.L.; et al. Monitoring Species Diversity and Abundance of Non-Indigenous Species in the Slovenian Sea (In Slovenian). Final Project Report. Marine Biology Station Piran, National Institute of Biology, 2021, Report 195. p. 83. Available online: http://www.ribiski-sklad.si/f/docs/Dokumenti_1/koncno_porocilo_NIS_2018-2021_s_CIP.pdf (accessed on 2 June 2023).
- Stulpinaite, R.; Hyams-Kaphzan, O.; Langer, M.R. Alien and cryptogenic Foraminifera in the Mediterranean Sea: A revision of taxa as part of the EU 2020 Marine Strategy Framework Directive. Mediterr. Mar. Sci. 2020, 21, 719–758. [Google Scholar] [CrossRef]
- Meric, E.; Avşar, N.; Yokes, M.; Dincer, F. Atlas of Recent Benthic foraminifera from Turkey. Micropaleontology 2014, 60, 211–398. [Google Scholar] [CrossRef]
- Albano, P.G.; Sabbatini, A.; Lattanzio, J.; Päßler, J.F.; Steger, J.; Hua, Q.; Kaufman, D.K.; Szidat, S.; Zuschin, M.; Negri, A. Alleged Lessepsian foraminifera prove native and suggest Pleistocene range expansions into the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2022, 700, 65–78. [Google Scholar] [CrossRef]
- Erdoğan-Dereli, D.; Cinar, M.E. Levinsenia species (Annelida: Polychaeta: Paraonidae) from the Sea of Marmara with descriptions of two new species. Zootaxa 2021, 4908, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Giangrande, A.; Putignano, M.; Licciano, M.; Gambi, M.C. The Pandora’s box: Morphological diversity within the genus Amphiglena Claparède, 1864 (Sabellidae, Annelida) in the Mediterranean Sea, with description of nine new species. Zootaxa 2021, 4949, 201–239. [Google Scholar] [CrossRef]
- Çinar, M.E.; Dağli, E.; Erdoğan-Dereli, D. The diversity of polychaetes (Annelida: Polychaeta) in a long-term pollution monitoring study from the Levantine coast of Turkey (Eastern Mediterranean), with the descriptions of four species new to science and two species new to the Mediterranean fauna. J. Nat. Hist. 2022, 56, 1383–1426. [Google Scholar] [CrossRef]
- Çinar, M.E. Syllis ergeni: A new species of Syllidae (Annelida: Polychaeta) from Izmir Bay (Aegean Sea, eastern Mediterranean Sea). Zootaxa 2005, 1036, 43–54. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bakir, K.; Öztürk, B.; Katağan, T.; Doğan, A.; Açik, Ş.; Kurt-Sahin, G.; Özcan, T.; Dağli, E.; Bitlis-Bakir, B.; et al. Macrobenthic fauna associated with the invasive alien species Brachidontes pharaonis (Mollusca: Bivalvia) in the Levantine Sea (Turkey). J. Mar. Biol. Assoc. 2017, 97, 613–628. [Google Scholar] [CrossRef]
- Ben-Eliahu, M.N. Errant polychaete cryptofauna (excluding Syllidae and Nereidae) from rims of similar intertidal vermetid reefs on the Mediterranean coast of Israel and in the Gulf of Elat. Isr. J. Ecol. Evol. 1976, 25, 156–177. [Google Scholar]
- Cinar, M.E. Description of a new fireworm, Eurythoe turcica sp. nov. (Polychaeta: Amphinomidae), from the Levantine coast of Turkey (eastern Mediterranean), with re-descriptions of Eurythoe parvecarunculata Horst and Amphinome djiboutiensis Gravier based on type material. J. Nat. Hist. 2008, 42, 1975–1990. [Google Scholar]
- Furnari, G.; Cormaci, M.; Serio, D. The Laurencia complex (Rhodophyta, Rhodomelaceae) in the Mediterranean Sea: An overview. Cryptogam. Algol. 2001, 22, 331–373. [Google Scholar] [CrossRef]
- Machín-Sánchez, M.; Gil-Rodríguez, M.C.; Haroun, R. Phylogeography of the red algal Laurencia complex in the Macaronesia region and nearby coastal areas: Recent advances and future perspectives. Diversity 2018, 10, 10. [Google Scholar] [CrossRef]
- Tsiamis, K.; Panayotidis, P.; Zenetos, A. Alien marine macrophytes in Greece: A review. Bot. Mar. 2008, 51, 237–246. [Google Scholar] [CrossRef]
- Gerakaris, V.; Lardi, P.L.; Issaris, Y. First Record of the Tropical Seagrass Species Halophila decipiens Ostenfeld in the Mediterranean Sea. Aquat. Bot. 2020, 160, 103151. [Google Scholar] [CrossRef]
- García-Escudero, C.A.; Tsigenopoulos, C.S.; Gerakaris, V.; Tsakogiannis, A.; Apostolaki, E.T. Its DNA Barcoding Reveals That Halophila stipulacea Still Remains the Only Non-Indigenous Seagrass of the Mediterranean Sea. Diversity 2022, 14, 76. [Google Scholar] [CrossRef]
- Orlando-Bonaca, M.; Lipej, L.L.; Bonanno, G. Non-indigenous macrophytes in Adriatic ports and transitional waters: Trends, taxonomy, introduction vectors, pathways and management. Mar. Pollut. Bull. 2019, 145, 656–672. [Google Scholar] [CrossRef]
- Zenetos, A. Trend in alien species in the Mediterranean. An answer to Galil, 2009 “Taking stock: Inventory of alien species in the Mediterranean Sea”. Biol. Invasions 2010, 12, 3379–3381. [Google Scholar] [CrossRef]
- Marchini, A.; Cardeccia, A. Alien amphipods in a sea of troubles: Cryptogenic species, unresolved taxonomy and overlooked introductions. Mar. Biol. 2017, 164, 69. [Google Scholar] [CrossRef]
- Zenetos, A.; Gratsia, E.; Cardoso, A.; Tsiamis, K. Time lags in reporting of biological invasions: The case of Mediterranean Sea. Meditter. Mar. Sci. 2019, 20, 469–475. [Google Scholar] [CrossRef]
- Stæhr, P.A.U.; Carbonell, A.; Guerin, L.; Kabuta, S.H.; Tidbury, H.; Viard, F. Trends in New Records of Non-Indigenous Species (NIS) Introduced by Human Activities. In OSPAR 2023: The 2023 Quality Status Report for the Northeast Atlantic; OSPAR Commission: London, UK, 2022; Available online: https://oap.ospar.org/en/ospar-assessments/quality-status-reports/qsr-2023/indicatorassessments/trends-new-records-nis (accessed on 10 June 2023).
- Lucy, F.E.; Roy, H.; Simpson, A.; Carlton, J.T.; Hanson, J.M.; Magellan, K.; Campbell, M.L.; Costello, M.J.; Pagad, S.; Hewitt, C.L.; et al. INVASIVESNET towards an international association for open knowledge on invasive alien species. Manag. Biol. Invasions 2016, 7, 131–139. [Google Scholar] [CrossRef]
- Roy, H.; Groom, Q.; Adriaens, T.; Agnello, G.; Antic, M.; Archambeau, A.; Bacher, S.; Bonn, A.; Brown, P.; Brundu, G. Increasing understanding of alien species through citizen science (Alien-CSI). Res. Ideas Outcomes 2018, 4, e31412. [Google Scholar] [CrossRef]
- UNEP/MAP-PAP/RAC-SPA/RAC, MET and NAPA. Integrated Monitoring Programme—Albania; PAP/RAC, GEF Adriatic Project: Split, Croatia, 2021; p. 140+Annexes. [Google Scholar]
- UNEP/MAP-PAP/RAC-SPA/RAC and MESPU. Integrated Monitoring Programme—Montenegro; PAP/RAC, GEF Adriatic Project: Split, Croatia, 2021; p. 130+Annexes. [Google Scholar]
- Tempesti, J.; Mangano, M.C.; Langeneck, J.; Maltagliati, F.; Lardicci, C.; Castelli, A. Non-indigenous species in Mediterranean ports: A knowledge baseline. Mar. Environ. Res. 2020, 161, 105056. [Google Scholar] [CrossRef]
- Samaha, C.; Zu Dohna, H.; Bariche, M. Analysis of Red Sea fish species’ introductions into the Mediterranean reveals shifts in introduction patterns. J. Biogeogr. 2016, 43, 1797–1807. [Google Scholar] [CrossRef]
- Solow, A.R.; Costello, C.J. Estimating the rate of species introductions from the discovery record. Ecology 2004, 85, 1822–1825. [Google Scholar] [CrossRef]
- UNEP/MAP; Plan Bleu. United Nations Environment Programme/Mediterranean Action Plan and Plan Bleu. In State of the Environment and Development in the Mediterranean; UNEP/MAP: Nairobi, Kenya, 2020; p. 341. [Google Scholar]
- Ferrario, J.; Caronni, S.; Occhipinti-Ambrogi, A.; Marchini, A. Role of commercial harbours and recreational marinas in the spread of non-indigenous fouling species. Biofouling 2017, 33, 651–660. [Google Scholar] [CrossRef]
- Ulman, A.; Ferrario, J.; Occhipinti-Ambrogi, A.; Arvanitidis, C.; Bandi, A.; Bertolino, M.; Bogi, C.; Chatzigeorgiou, G.; Çiçek, B.A.; Deidun, A.; et al. A massive update of non-indigenous species records in Mediterranean marinas. PeerJ 2017, 5, e3954. [Google Scholar] [CrossRef]
- Ulman, A.; Ferrario, J.; Forcada, A.; Seebens, H.; Arvanitidis, C.; Occhipinti-Ambrogi, A.; Marchini, A. Alien Species Spreading via Biofouling on Recreational Vessels in the Mediterranean Sea. J. Appl. Ecol. 2019, 56, 2620–2629. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Florido, M.; Ros, M.; González-Romero, P.; Guerra-García, J.M. Impoverished mobile epifaunal assemblages associated with the invasive macroalga Asparagopsis taxiformis in the Mediterranean Sea. Mar. Environ. Res. 2018, 141, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Morri, C.; Montefalcone, M.; Gatti, G.; Vassallo, P.; Paoli, C.; Bianchi, C.N. An Alien Invader is the Cause of Homogenization in the Recipient Ecosystem: A Simulation-Like Approach. Diversity 2019, 11, 146. [Google Scholar] [CrossRef]
- Daniel, B.; Piro, S.; Francour, P. Lessepsian Rabbitfish Siganus luridus reached the French Mediterranean Coasts. Cybium Rev. Int. Ichtyol. 2009, 33, 163–164. [Google Scholar]
- ICES. Working Group on Introductions and Transfers of Marine Organisms (WGITMO). In ICES Scientific Reports; ICES: Copenhagen, Denmark, 2011; Volume 4. [Google Scholar]
- Schickele, A.; Guidetti, P.; Giakoumi, S.; Zenetos, A.; Francour, P.; Raybaud, V. Improving predictions of invasive fish ranges combining functional and ecological traits with environmental suitability under climate change scenarios. Glob. Chang. Biol. 2021, 27, 6086–6102. [Google Scholar] [CrossRef] [PubMed]
- Nykjaer, L. Mediterranean Sea surface warming 1985–2006. Clim. Res. 2009, 39, 11–17. [Google Scholar] [CrossRef]
- Raitsos, D.E.; Beaugrand, G.; Georgopoulos, D.; Zenetos, A.; Pancucci-Papadopoulou, A.M.; Theocharis, A.; Papathanassiou, E. Global climate change amplifies the entry of tropical species into the Eastern Mediterranean Sea. Limnol. Oceanogr. 2010, 55, 1478–1484. [Google Scholar] [CrossRef]
- MedECC. Climate and Environmental Change in the Mediterranean Basin—Current Situation and Risks for the Future. In First Mediterranean Assessment Report; Cramer, W., Guiot, J., Marini, K., Eds.; Union for the Mediterranean, Plan Bleu, UNEP/MAP: Marseille, France, 2020; p. 632. [Google Scholar] [CrossRef]
- Di Martino, V.; Stancanelli, B. The alien lionfish, Pterois miles (Bennett, 1828), enters the Adriatic Sea, Central Mediterranean Sea. J. Black Sea Mediterr. Environ. 2021, 27, 104–108. [Google Scholar]
- Dragičević, B.; Ugarković, P.; Krželj, M.; Zurub, D.; Dulčić, J. New record of Pterois cf. miles (Actinopterygii: Scorpaeniformes: Scorpaenidae) from the eastern middle Adriatic Sea (Croatian waters): Northward expansion. Acta Ichthyol. Piscat. 2021, 51, 379–383. [Google Scholar] [CrossRef]
- Fortič, A.; Al-Sheikh, R.R.; Almajid, Z.; Badreddine, A.; Baez, J.C.; Belmonte-Gallegos, A.; Bettoso, N.; Borme, D.; Camisa, F.; Caracciolo, D.; et al. New records of introduced species in the Mediterranean Sea (April 2023). Mediterr. Mar. Sci. 2023, 24, 182–202. [Google Scholar] [CrossRef]
- Bianchi, C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 2007, 580, 7–21. [Google Scholar] [CrossRef]
- Dimitriadis, C.; Galanidi, M.; Zenetos, A.; Corsini-Foka, M.; Giovos, I.; Karachle, P.K.; Konstantinidoy, I.F.; Kytinou, E.; Issaris, Y.; Azzurro, E.; et al. Updating the occurrences of Pterois miles in the Mediterranean Sea, with considerations on thermal boundaries and future range expansion. Mediterr. Mar. Sci. 2020, 21, 62–69. [Google Scholar] [CrossRef]
- Hewitt, C.L.; Campbell, M.L.; Coutts, A.D.M.; Dahlstrom, A.; Shields, D.; Valentine, J. Species biofouling risk assessment. In Report for the Department of Agriculture, Fisheries and Forestry; National Centre for Marine Conservation: Copenhagen, Denmark, 2011; p. 172. [Google Scholar]
- NOBANIS. Invasive Alien Species. Pathway Analysis and Horizon Scanning for Countries in Northern Europe; TemaNord 2015:517; NOBANIS: Copenhagen, Denmark, 2015. [Google Scholar] [CrossRef]
- Darling, J.A.; Galil, B.S.; Carvalho, G.R.; Rius, M.; Viard, F.; Piraino, S. Recommendations for developing and applying genetic tools to assess and manage biological invasions in marine ecosystems. Mar. Policy 2017, 85, 54–64. [Google Scholar] [CrossRef]
- Livi, S.; Varella, S.; Castriota, L.; Maggio, T.; Lomartire, M.; Vivona, P.; Corinaldesi, C.; Dell’Anno, A. The integration of environmental DNA metabarcoding in the monitoring programmes for Descriptor 2 of the MSFD. In Proceedings of the 2nd Mediterranean Symposium on the non-indigenous Species, Genova, Italy, 22–23 September 2022; Bouafif, C., Ouerghi, A., Eds.; SPA/RAC Publications: Tunis, Tunisia, 2022; p. 150. [Google Scholar]
- Fonseca, V.G.; Davison, P.I.; Creach, V.; Stone, D.; Bass, D.; Tidbury, H.J. The Application of eDNA for Monitoring Aquatic Non-Indigenous Species: Practical and Policy Considerations. Diversity 2023, 15, 631. [Google Scholar] [CrossRef]
- UNEP-MAP-RAC/SPA. Action Plan Concerning Species Introductions and Invasive Species in the Mediterranean Sea; RAC/SPA: Tunis, Tunisia, 2005; p. 30. [Google Scholar]
- UNEP-MAP. Action Plan Concerning Species Introductions and Invasive Species in the Mediterranean Sea; UN Environment/MAP: Athens, Greece, 2017; p. 14. [Google Scholar]
- UNEP/MAP SPA/RAC. Draft Updated Action Plan Concerning Species Introductions and Invasive Species in the Mediterranean Sea; UNEP/MAP: Malta, 2023; UNEP/MED WG.548/6. [Google Scholar]
- UNEP-MAP. Post-2020 Strategic Action Programme for the Conservation of Biodiversity and Sustainable Management of Natural Resources in the Mediterranean Region (Post-2020 SAP BIO). In Report of the Online Advisory Committee Meeting, 2 April 2020; SPA/RAC: Tunis, Tunisia, 2020; p. 49. [Google Scholar]
- UNEP First Draft of the Post-Biodiversity Framework. In Proceedings of the Open Ended Working Group on the Post-Global Biodiversity Framework, Online, 23 August–3 September 2021; Convention on Biological Diversity: Montreal, QC, Canada, 2021.




| Phylum | Species | Notes |
|---|---|---|
| Annelida | Armandia intermedia Fauvel, 1902 | QR in Zenetos et al. [6]/MEC expert judgement |
| Annelida | Eunice cf tubifex Crossland, 1904 | QR in Zenetos et al. [6] |
| Annelida | Eurythoe complanata (Pallas, 1766) | Likely alien polychaeta |
| Annelida | Eurythoe laevisetis Fauvel, 1914 | Likely alien polychaeta |
| Annelida | Lumbrinerides neogesae Miura, 1981 | Likely alien polychaeta |
| Annelida | Metasychis gotoi (Izuka, 1902) | Likely alien polychaeta |
| Annelida | Neopseudocapitella brasiliensis Rullier & Amoureux, 1979 | Likely alien polychaeta |
| Annelida | Novafabricia infratorquata (Fitzhugh, 1973) | Inconsistent status assignement |
| Annelida | Oenone cf. fulgida (Lamarck, 1818) | Likely alien polychaeta |
| Annelida | Pista unibranchia Day, 1963 | Likely alien polychaeta |
| Annelida | Polydora colonia Moore, 1907 | Inconsistent status assignement |
| Arthropoda | Calappa pelii Herklots, 1851 | Inconsistent status assignement |
| Arthropoda | Latigammaropsis togoensis (Schellenberg, 1925) | Inconsistent status assignement |
| Arthropoda | Percnon gibbesi (H. Milne Edwards, 1853) | Inconsistent status assignement |
| Arthropoda | Amphibalanus improvisus (Darwin, 1854) | Inconsistent status assignement |
| Bryozoa | Amathia verticillata (delle Chiaje 1822) | Inconsistent status assignement |
| Bryozoa | Microporella harmeri Hayward, 1988 | Inconsistent status assignement |
| Chlorophyta | Bryopsis pennata J.V.Lamouroux 1809 | Inconsistent status assignement—excluded in Verlaque et al. [24] |
| Chlorophyta | Caulerpa denticulata Decaisne, 1841 | QR in Zenetos et al. [6] |
| Chordata/Ascidiacea | Ascidiella aspersa (Müller, 1776) | Inconsistent status assignement |
| Chordata/Ascidiacea | Diplosoma listerianum (Milne Edwards, 1841) | Inconsistent status assignement |
| Chordata/Teleostei | Abudefduf spp. | WMED, ADRIA, EMED—Specimens of the genus Abudefduf visually classified may belong to different cryptic species of genus Abudefduf [80,81]. Molecularly identified specimens of the introduced species are retained as valid records |
| Chordata/Teleostei | Aphanius dispar (Rüppell, 1829) | QR in Zenetos et al. [6] |
| Chordata/Teleostei | Cephalopholis taeniops (Valenciennes, 1828) | Inconsistent status assignement |
| Chordata/Teleostei | Enchelycore anatina (Lowe, 1838) | Inconsistent status assignement |
| Chordata/Teleostei | Ophioblennius atlanticus (Valenciennes, 1836) | Inconsistent status assignement |
| Chordata/Teleostei | Pseudotolithus senegallus (Cuvier, 1830) | QR in Zenetos et al. [6]/MB expert judgement |
| Chordata/Teleostei | Siganus javus (Linnaeus, 1766) | QR in Zenetos et al. [6] |
| Chordata/Teleostei | Trachurus indicus Nekrasov, 1966 | QR in Zenetos et al. [6] |
| Cnidaria | Filellum serratum (Clarke, 1879) | Inconsistent status assignement |
| Echinodermata | Ophiactis savignyi (Müller & Troschel, 1842) | Inconsistent status assignement |
| Foraminifera | Amphisorus hemprichii Ehrenberg, 1840 | Inconsistent status assignement |
| Foraminifera | Articulina alticostata Cushman, 1944 | QR this work |
| Foraminifera | Articulina mayori Cushman, 1922 | QR this work |
| Foraminifera | Astacolus insolitus (Schwager, 1866) | QR this work |
| Foraminifera | Bulimina biserialis Millett, 1900 | QR this work |
| Foraminifera | Cymbaloporetta plana (Cushman, 1915) | Inconsistent status assignement |
| Foraminifera | Cymbaloporetta squammosa (d’Orbigny, 1839) | Inconsistent status assignement |
| Foraminifera | Dentalina albatrossi (Cushman, 1923) | QR this work |
| Foraminifera | Euthymonacha polita (Chapman, 1904) | Inconsistent status assignement |
| Foraminifera | Heterocyclina tuberculata (Möbius, 1880) | QR this work |
| Foraminifera | Peneroplis pertusus (Forsskål in Niebuhr, 1775) | Inconsistent status assignement |
| Foraminifera | Polymorphina fistulosa Williamson, 1858 | Inconsistent status assignement |
| Foraminifera | Pseudonodosaria brevis (d’Orbigny, 1846) | QR this work |
| Foraminifera | Pyramidulina perversa (Schwager, 1866) | QR this work |
| Foraminifera | Quinqueloculina sp. C | QR this work |
| Foraminifera | Sigmoihauerina bradyi (Cushman, 1917) | QR this work |
| Foraminifera | Triloculina sp. A | QR this work |
| Foraminifera | Triloculinella asymmetrica (Said, 1949) | QR this work |
| Foraminifera | Vaginulinopsis sublegumen Parr, 1950 | QR this work |
| Mollusca | Spondylus nicobaricus Schreibers, 1793 | QR in Zenetos et al. [6] |
| Mollusca | Bursatella leachii Blainville, 1817 | Inconsistent status assignement |
| Mollusca | Thecacera pennigera (Montagu, 1813) | Inconsistent status assignement |
| Myzozoa | Alexandrium taylorii Balech | Inconsistent status assignement |
| Ochrophyta | Ectocarpus siliculosus var. hiemalis (P.L.Crouan & H.M.Crouan) Gallardo, 1992 | Inconsistent status assignement |
| Ochrophyta | Pylaiella littoralis (Linnaeus) Kjellman, 1872 | Inconsistent status assignement |
| Porifera | Niphates toxifera Vacelet, Bitar, Carteron, Zibrowius & Pérez, 2007 | Inconsistent status assignement |
| Rhodophyta | Pyropia koreana (M.S.Hwang & I.K.Lee) M.S.Hwang, H.G.Choi Y.S.Oh & I.K.Lee, 2011 | Inconsistent status assignement |
| Rhodophyta | Acanthophora nayadiformis (Delile) Papenfuss, 1968 | Inconsistent status assignement |
| Rhodophyta | Anotrichium okamurae Baldock, 1976 | Inconsistent status assignement (A. furcellatum in FR) |
| Rhodophyta | Ganonema farinosum (Lamouroux) Fan & Wang, 1974 | Inconsistent status assignement |
| Rhodophyta | Laurencia minuta Vandermeulen, Garbary & Guiry 1990 | Inconsistent status assignement |
| Rhodophyta | Palisada maris-rubri (K.W.Nam & Saito) K.W.Nam, 2007 | Inconsistent status assignement |
| Rhodophyta | Spyridia aculeata (Bory de Saint-Vincent) Papenfuss, 1968 | Inconsistent status assignement |
| Rhodophyta | Vertebrata fucoides (Hudson) Kuntze, 1891 | Inconsistent status assignement |
| MED | WMED | CMED | ADRIA | EMED | |
|---|---|---|---|---|---|
| Macrophytes | 143 | 90 | 52 | 55 | 72 |
| Annelida | 83 | 34 | 29 | 21 | 74 |
| Mollusca | 222 | 40 | 46 | 30 | 197 |
| Arthropoda | 188 | 65 | 46 | 38 | 149 |
| Fishes | 171 | 42 | 65 | 26 | 139 |
| Ascidiacea | 29 | 18 | 20 | 6 | 17 |
| Cnidaria | 42 | 21 | 10 | 13 | 31 |
| Bryozoa | 32 | 7 | 10 | 5 | 30 |
| Foraminifera | 47 | 6 | 8 | 6 | 44 |
| Miscellanea | 49 | 15 | 17 | 11 | 34 |
| Total | 1006 | 337 | 303 | 211 | 787 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galanidi, M.; Aissi, M.; Ali, M.; Bakalem, A.; Bariche, M.; Bartolo, A.G.; Bazairi, H.; Beqiraj, S.; Bilecenoglu, M.; Bitar, G.; et al. Validated Inventories of Non-Indigenous Species (NIS) for the Mediterranean Sea as Tools for Regional Policy and Patterns of NIS Spread. Diversity 2023, 15, 962. https://doi.org/10.3390/d15090962
Galanidi M, Aissi M, Ali M, Bakalem A, Bariche M, Bartolo AG, Bazairi H, Beqiraj S, Bilecenoglu M, Bitar G, et al. Validated Inventories of Non-Indigenous Species (NIS) for the Mediterranean Sea as Tools for Regional Policy and Patterns of NIS Spread. Diversity. 2023; 15(9):962. https://doi.org/10.3390/d15090962
Chicago/Turabian StyleGalanidi, Marika, Mehdi Aissi, Malek Ali, Ali Bakalem, Michel Bariche, Angela G. Bartolo, Hocein Bazairi, Sajmir Beqiraj, Murat Bilecenoglu, Ghazi Bitar, and et al. 2023. "Validated Inventories of Non-Indigenous Species (NIS) for the Mediterranean Sea as Tools for Regional Policy and Patterns of NIS Spread" Diversity 15, no. 9: 962. https://doi.org/10.3390/d15090962
APA StyleGalanidi, M., Aissi, M., Ali, M., Bakalem, A., Bariche, M., Bartolo, A. G., Bazairi, H., Beqiraj, S., Bilecenoglu, M., Bitar, G., Bugeja, M., Carbonell, A., Castriota, L., Chalabi, A., Çinar, M. E., Dragičević, B., Dulčić, J., El-Haweet, A. E. A., Farrag, M. M. S., ... Zenetos, A. (2023). Validated Inventories of Non-Indigenous Species (NIS) for the Mediterranean Sea as Tools for Regional Policy and Patterns of NIS Spread. Diversity, 15(9), 962. https://doi.org/10.3390/d15090962










