The Influence of Macroclimatic Drivers on the Macrophyte Phylogenetic Diversity in South African Estuaries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area, Data Acquisition, and Species and Biogeographical Classification
2.2. Macroclimatic Analyses
2.3. Phylogenetic Diversity Analyses
2.4. Phylogenetic Diversity Patterns in Relation to Bioclimatic Variables
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Species | Family | Clade | Climatic Zone | Habitat |
|---|---|---|---|---|
| Acrostichum aureum | Polypodiaceae | Polypodiales | Tropical | Mangrove |
| Avicennia marina | Acanthaceae | Asterids | Tropical.Subtropical | Mangrove |
| Barringtonia racemosa | Lecythidaceae | Asterids | Tropical | Swamp Forest |
| Bassia diffusa | Amaranthaceae | Caryophyllales | Temperate | Supratidal Salt Marsh |
| Bolboschoenus maritimus | Cyperaceae | Poales | All | Reeds and Sedges |
| Bruguiera gymnorrhiza | Rhizophoraceae | Rosids | Tropical.Subtropical | Mangrove |
| Centella asiatica | Asteraceae | Asterids | Temperate | Intertidal Salt Marsh |
| Chara vulgaris | Charophyceae | Charales | Temperate | Submerged Macrophytes |
| Nidorella ivifolia | Asteraceae | Asterids | Temperate | Supratidal Salt Marsh |
| Cotula coronopifolia | Asteraceae | Asterids | Temperate | Intertidal Salt Marsh |
| Cynodon dactylon | Poaceae | Poales | All | Supratidal Salt Marsh |
| Cyperus laevigatus | Cyperaceae | Poales | All | Reeds and Sedges |
| Disphyma crassifolium | Aizoaceae | Caryophyllales | Temperate | Supratidal Salt Marsh |
| Frankenia pulverulenta | Frankeniaceae | Caryophyllales | Temperate | Supratidal Salt Marsh |
| Halophila ovalis | Hydrocharitaceae | Alismatales | Temperate | Submerged Macrophytes |
| Hibiscus tiliaceus | Malvaceae | Malvids | Tropical | Swamp Forest |
| Isolepis cernua | Cyperaceae | Poales | All | Reeds and Sedges |
| Juncus acutus | Juncaceae | Poales | All | Reeds and Sedges |
| Limonium scabrum | Plumbaginaceae | Caryophyllales | Temperate | Supratidal Salt Marsh |
| Lumnitzera racemosa | Combretaceae | Rosids | Tropical | Mangrove |
| Phragmites australis | Poaceae | Poales | All | Reeds and Sedges |
| Plantago carnosa | Plantaginaceae | Asterids | Temperate | Intertidal Salt Marsh |
| Poecilolepis ficoidea | Asteraceae | Asterids | Temperate | Supratidal Salt Marsh |
| Rhizophora mucronata | Rhizophoraceae | Rosids | Tropical.Subtropical | Mangrove |
| Ruppia cirrhosa | Ruppiaceae | Alismatales | Temperate | Submerged Macrophytes |
| Salicornia meyeriana | Amaranthaceae | Caryophyllales | Temperate | Intertidal Salt Marsh |
| Salicornia uniflora | Amaranthaceae | Caryophyllales | Temperate | Intertidal Salt Marsh |
| Salicornia pachystachya | Amaranthaceae | Caryophyllales | Subtropical | Intertidal Salt Marsh |
| Samolus porosus | Theophrastaceae | Asterids | Temperate | Intertidal Salt Marsh |
| Salicornia capensis | Amaranthaceae | Caryophyllales | Temperate | Supratidal Salt Marsh |
| Salicornia decumbens | Amaranthaceae | Caryophyllales | Temperate | Intertidal Salt Marsh |
| Salicornia natalensis | Amaranthaceae | Caryophyllales | Temperate | Intertidal Salt Marsh |
| Salicornia pillansii | Amaranthaceae | Caryophyllales | Temperate | Supratidal Salt Marsh |
| Salicornia tegetaria | Amaranthaceae | Caryophyllales | Temperate | Intertidal Salt Marsh |
| Schoenoplectus scirpoides | Cyperaceae | Poales | All | Reeds and Sedges |
| Schoenoplectus triqueter | Cyperaceae | Poales | All | Reeds and Sedges |
| Spartina maritima | Poaceae | Poales | All | Intertidal Salt Marsh |
| Spergularia media | Caryophyllaceae | Caryophyllales | Temperate | Supratidal Salt Marsh |
| Spergularia rubra | Caryophyllaceae | Caryophyllales | Temperate | Supratidal Salt Marsh |
| Sporobolus virginicus | Poaceae | Poales | All | Supratidal Salt Marsh |
| Stenotaphrum secundatum | Poaceae | Poales | All | Supratidal Salt Marsh |
| Stuckenia pectinata | Potamogetonaceae | Alismatales | Temperate | Submerged Macrophytes |
| Suaeda inflata | Amaranthaceae | Caryophyllales | Temperate | Supratidal Salt Marsh |
| Suaeda fruticosa | Amaranthaceae | Caryophyllales | Temperate | Supratidal Salt Marsh |
| Triglochin bulbosa | Juncaginaceae | Alismatales | Temperate | Intertidal Salt Marsh |
| Triglochin striata | Juncaginaceae | Alismatales | All | Intertidal Salt Marsh |
| Zostera capensis | Zosteraceae | Alismatales | All | Submerged Macrophytes |
References
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- CBOL Plant Working Group. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef]
- Ackerly, D. Conservatism and diversification of plant functional traits: Evolutionary rates versus phylogenetic signal. Proc. Natl. Acad. Sci. USA 2009, 106 (Suppl. S2), 19699–19706. [Google Scholar] [CrossRef] [PubMed]
- Kress, W.J.; García-Robledo, C.; Uriarte, M.; Erickson, D.L. DNA barcodes for ecology, evolution, and conservation. Trends Ecol. Evol. 2015, 30, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Veldkornet, D.A.; Adams, J.B.; Boatwright, J.S.; Rajkaran, A. Barcoding of estuarine macrophytes and phylogenetic diversity of estuaries along the South African coastline. Genome 2019, 62, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Yessoufou, K.; Bezeng, B.S.; Gaoue, O.G.; Bengu, T.; van der Bank, M. Phylogenetically diverse native systems are more resistant to invasive plant species on Robben Island, South Africa. Genome 2019, 62, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Gostel, M.R.; Kress, W.J. The expanding role of DNA barcodes: Indispensable tools for ecology, evolution, and conservation. Diversity 2022, 14, 213. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Cavender–Bares, J.; Kozak, K.H.; Fine, P.V.; Kembel, S.W. The merging of community ecology and phylogenetic biology. Ecol. Lett. 2009, 12, 693–715. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Chiu, C.H.; Jost, L. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through Hill numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]
- Scherson, R.A.; Thornhill, A.H.; Urbina-Casanova, R.; Freyman, W.A.; Pliscoff, P.A.; Mishler, B.D. Spatial phylogenetics of the vascular flora of Chile. Mol. Phylogenetics Evol. 2017, 112, 88–95. [Google Scholar] [CrossRef]
- Cadotte, M.W. Experimental evidence that evolutionarily diverse assemblages result in higher productivity. Proc. Natl. Acad. Sci. USA 2013, 110, 8996–9000. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.R.; Halliday, F.W.; Lopez, B.E.; Palmquist, K.A.; Wilfahrt, P.A.; Hurlbert, A.H. Using trait and phylogenetic diversity to evaluate the generality of the stress–dominance hypothesis in eastern North American tree communities. Ecography 2014, 37, 814–826. [Google Scholar] [CrossRef]
- Goberna, M.; Navarro–Cano, J.A.; Verdú, M. Opposing phylogenetic diversity gradients of plant and soil bacterial communities. Proc. R. Soc. B Biol. Sci. 2016, 283, 20153003. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]
- Barber, N.A.; Jones, H.P.; Duvall, M.R.; Wysocki, W.P.; Hansen, M.J.; Gibson, D.J. Phylogenetic diversity is maintained despite richness losses over time in restored tallgrass prairie plant communities. J. Appl. Ecol. 2017, 54, 137–144. [Google Scholar] [CrossRef]
- Fine, P.V.; Kembel, S.W. Phylogenetic community structure and phylogenetic turnover across space and edaphic gradients in western Amazonian tree communities. Ecography 2011, 34, 552–565. [Google Scholar] [CrossRef]
- Daru, B.H.; Le Roux, P.C. Marine protected areas are insufficient to conserve global marine plant diversity. Glob. Ecol. Biogeogr. 2016, 25, 324–334. [Google Scholar] [CrossRef]
- Navarro-Cano, J.A.; Ferrer-Gallego, P.P.; Laguna, E.; Ferrando, I.; Goberna, M.; Valiente-Banuet, A.; Verdú, M. Restoring phylogenetic diversity through facilitation. Restor. Ecol. 2016, 24, 449–455. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Shang, K.; Zhao, M.; Kong, J.; Wang, X.; Wang, Y.; Song, H.; Zhang, O.; Lv, X.; et al. A taxonomic and phylogenetic perspective on plant community assembly along an elevational gradient in subtropical forests. J. Plant Ecol. 2021, 14, 702–716. [Google Scholar] [CrossRef]
- Staab, M.; Bruelheide, H.; Durka, W.; Michalski, S.; Purschke, O.; Zhu, C.D.; Klein, A.M. Tree phylogenetic diversity promotes host–parasitoid interactions. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160275. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.R. The Geographical Distribution of Animals, with a Study of the Relations of Living and Extinct Faunas as Elucidating the Past Changes of the Earth’s Surface; Harper & Brothers: New York, NY, USA, 1876; Volume 1. [Google Scholar]
- Collart, F.; Wang, J.; Patiño, J.; Hagborg, A.; Söderström, L.; Goffinet, B.; Magain, N.; Hardy, O.J.; Vanderpoorten, A. Macroclimatic structuring of spatial phylogenetic turnover in liverworts. Ecography 2021, 44, 1474–1485. [Google Scholar] [CrossRef]
- Willig, M.R.; Kaufman, D.M.; Stevens, R.D. Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 273–309. [Google Scholar] [CrossRef]
- Brown, J.H. Why are there so many species in the tropics? J. Biogeogr. 2014, 41, 8–22. [Google Scholar] [CrossRef]
- Das, A.A.; Ratnam, J. The thermal niche and phylogenetic assembly of evergreen tree metacommunities in a mid-to-upper tropical montane zone. Proc. R. Soc. B 2022, 289, 20220038. [Google Scholar] [CrossRef]
- Zanne, A.E.; Tank, D.C.; Cornwell, W.K.; Eastman, J.M.; Smith, S.A.; FitzJohn, R.G.; McGlinn, D.J.; O’meara, B.C.; Moles, A.T.; Reich, P.B.; et al. Three keys to the radiation of angiosperms into freezing environments. Nature 2014, 506, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M. The impact of climate change on mangrove forests. Curr. Clim. Chang. Rep. 2015, 1, 30–39. [Google Scholar] [CrossRef]
- Osland, M.J.; Enwright, N.M.; Day, R.H.; Gabler, C.A.; Stagg, C.L.; Grace, J.B. Beyond just sea–level rise: Considering macroclimatic drivers within coastal wetland vulnerability assessments to climate change. Glob. Chang. Biol. 2016, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gabler, C.A.; Osland, M.J.; Grace, J.B.; Stagg, C.L.; Day, R.H.; Hartley, S.B.; Enwright, N.M.; From, A.S.; McCoy, M.L.; McLeod, J.L. Macroclimatic change expected to transform coastal wetland ecosystems this century. Nat. Clim. Chang. 2017, 7, 142–147. [Google Scholar] [CrossRef]
- Molina–Venegas, R.; Ottaviani, G.; Campetella, G.; Canullo, R.; Chelli, S. Biogeographic deconstruction of phylogenetic and functional diversity provides insights into the formation of regional assemblages. Ecography 2022, 2022, e06140. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Diniz-Filho, J.A.F.; Jaramillo, C.A.; Soeller, S.A. Climate, niche conservatism, and the global bird diversity gradient. Am. Nat. 2007, 170, S16–S27. [Google Scholar] [CrossRef]
- Carter, B.E.; Misiewicz, T.M.; Mishler, B.D. Spatial phylogenetic patterns in the North American moss flora are shaped by history and climate. J. Biogeogr. 2022, 49, 1327–1338. [Google Scholar] [CrossRef]
- Janzen, D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Colwell, R.K.; Rangel, T.F. A stochastic, evolutionary model for range shifts and richness on tropical elevational gradients under Quaternary glacial cycles. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3695–3707. [Google Scholar] [CrossRef]
- Cubino, J.P.; Cavender-Bares, J.; Hobbie, S.E.; Pataki, D.E.; Avolio, M.L.; Darling, L.E.; Larson, K.L.; Hall, S.J.; Groffman, P.M.; Trammell, T.L.E.; et al. Drivers of plant species richness and phylogenetic composition in urban yards at the continental scale. Landsc. Ecol. 2019, 34, 63–77. [Google Scholar] [CrossRef]
- Daru, B.H.; Elliott, T.L.; Park, D.S.; Davies, T.J. Understanding the processes underpinning patterns of phylogenetic regionalization. Trends Ecol. Evol. 2017, 32, 845–860. [Google Scholar] [CrossRef]
- Ye, J.; Lu, L.; Liu, B.; Yang, T.; Zhang, J.; Hu, H.; Li, R.; Lu, A.; Liu, H.; Mao, L.; et al. Phylogenetic delineation of regional biota: A case study of the Chinese flora. Mol. Phylogenetics Evol. 2019, 135, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.M.; Zheng, Y.S. Exploring the species and phylogenetic diversity, phylogenetic structure of mixed communities along the coastal gradient. A case study in a subtropical island, China. Appl. Ecol. Environ. Res. 2022, 20, 3129–3141. [Google Scholar] [CrossRef]
- Flood, M.C.; Burgess, K.S.; Kruse, L.M.; Ballenger, J.; Worthy, S.J. Comparison of phylogenetic and taxonomic diversity of pitcher plant bogs in Georgia’s Coastal Plain. Plant Ecol. 2023, 224, 523–537. [Google Scholar] [CrossRef]
- Daru, B.H.; Holt, B.G.; Lessard, J.P.; Yessoufou, K.; Davies, T.J. Phylogenetic regionalization of marine plants reveals close evolutionary affinities among disjunct temperate assemblages. Biol. Conserv. 2017, 213, 351–356. [Google Scholar] [CrossRef]
- Coelho, J.F.R.; Lima, S.M.Q.; Petean, F.D.F. Phylogenetic conservatism of abiotic niche in sympatric Southwestern Atlantic skates. Mar. Biol. Res. 2020, 16, 458–473. [Google Scholar] [CrossRef]
- Veldkornet, D.A.; Rajkaran, A. Predicting shifts in the geographical distribution of two estuarine plant species from the subtropical and temperate regions of South Africa. Wetlands 2019, 39, 1179–1188. [Google Scholar] [CrossRef]
- Veldkornet, D.A.; Adams, J.B.; Potts, A.J. Where do you draw the line? Determining the transition thresholds between estuarine salt marshes and terrestrial vegetation. S. Afr. J. Bot. 2015, 101, 153–159. [Google Scholar] [CrossRef]
- Adams, J.B.; Veldkornet, D.; Tabot, P. Distribution of macrophyte species and habitats in South African estuaries. S. Afr. J. Bot. 2016, 107, 5–11. [Google Scholar] [CrossRef]
- Emanuel, B.P.; Bustamante, R.H.; Branch, G.M.; Eekhout, S.; Odendaal, F.J. A zoogeographic and functional approach to the selection of marine reserves on the west coast of South Africa. S. Afr. J. Mar. Sci. 1992, 12, 341–354. [Google Scholar] [CrossRef]
- van Niekerk, L.; Adams, J.; James, N.; Lamberth, S.; MacKay, C.; Turpie, J.; Rajkaran, A.; Weerts, S.; Whitfield, A. An Estuary Ecosystem Classification that encompasses biogeography and a high diversity of types in support of protection and management. Afr. J. Aquat. Sci. 2020, 45, 199–216. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef]
- Bridgewater, P.B.; Cresswell, I.D. Biogeography of mangrove and saltmarsh vegetation: Implications for conservation and management in Australia. Mangroves Salt Marshes 1999, 3, 117–125. [Google Scholar] [CrossRef]
- Saintilan, N. Biogeography of Australian saltmarsh plants. Austral Ecol. 2009, 34, 929–937. [Google Scholar] [CrossRef]
- Osland, M.J.; Hartmann, A.M.; Day, R.H.; Ross, M.S.; Hall, C.T.; Feher, L.C.; Vervaeke, W.C. Microclimate influences mangrove freeze damage: Implications for range expansion in response to changing macroclimate. Estuaries Coasts 2019, 42, 1084–1096. [Google Scholar] [CrossRef]
- Yando, E.S.; Jones, S.F.; James, W.R.; Colombano, D.D.; Montemayor, D.I.; Nolte, S.; Raw, J.L.; Ziegler, S.L.; Chen, L.; Daffonchio, D.; et al. An integrative salt marsh conceptual framework for global comparisons. Limnol. Oceanogr. Lett. 2023, 1–20. [Google Scholar] [CrossRef]
- Ewanchuk, P.J.; Bertness, M.D. Structure and organization of a northern New England salt marsh plant community. J. Ecol. 2004, 92, 72–85. [Google Scholar] [CrossRef]
- Charrier, G.; Martin-StPaul, N.; Damesin, C.; Delpierre, N.; Hänninen, H.; Torres-Ruiz, J.M.; Davi, H. Interaction of drought and frost in tree ecophysiology: Rethinking the timing of risks. Ann. For. Sci. 2021, 78, 40. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological responses to drought, salinity, and heat stress in plants: A review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Zou, D.; Su, X.; Cai, H.; Luo, A.; Jiang, K.; Zhang, X.; Xu, X.; Shrestha, N.; et al. Phylogenetic niche conservatism and variations in species diversity–climate relationships. Ecography 2021, 44, 1856–1868. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. Plant responses to multifactorial stress combination. New Phytol. 2022, 234, 1161–1167. [Google Scholar] [CrossRef]
- Klanderud, K.; Vandvik, V.; Goldberg, D. The importance of biotic vs. abiotic drivers of local plant community composition along regional bioclimatic gradients. PLoS ONE 2015, 10, e0130205. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Cheng, X.L.; Cubino, J.P.; Balfour, K.; Zhu, Z.X.; Wang, H.F. Drivers of spontaneous and cultivated species diversity in the tropical city of Zhanjiang, China. Urban For. Urban Green. 2022, 67, 127428. [Google Scholar] [CrossRef]
- De Pauw, K.; Meeussen, C.; Govaert, S.; Sanczuk, P.; Vanneste, T.; Bernhardt-Römermann, M.; Bollmann, K.; Brunet, J.; Calders, K.; Cousins, S.A.; et al. Taxonomic, phylogenetic and functional diversity of understorey plants respond differently to environmental conditions in European forest edges. J. Ecol. 2021, 109, 2629–2648. [Google Scholar] [CrossRef]
- Zellweger, F.; De Frenne, P.; Lenoir, J.; Vangansbeke, P.; Verheyen, K.; Bernhardt–Römermann, M.; Baeten, L.; Hédl, R.; Berki, I.; Brunet, J.; et al. Forest microclimate dynamics drive plant responses to warming. Science 2020, 368, 772–775. [Google Scholar] [CrossRef]
- Ringelberg, J.J.; Koenen, E.J.; Sauter, B.; Aebli, A.; Rando, J.G.; Iganci, J.R.; de Queiroz, L.P.; Murphy, D.J.; Gaudeul, M.; Bruneau, A.; et al. Precipitation is the main axis of tropical plant phylogenetic turnover across space and time. Sci. Adv. 2023, 9, eade4954. [Google Scholar] [CrossRef]
- Harrison, T.D.; Whitfield, A.K. Temperature and salinity as primary determinants influencing the biogeography of fishes in South African estuaries. Estuar. Coast. Shelf Sci. 2006, 66, 335–345. [Google Scholar] [CrossRef]
- Wooldridge, T.H.; Adams, J.B.; Fernandes, M. Biotic responses to extreme hypersalinity in an arid zone estuary, South Africa. S. Afr. J. Bot. 2016, 107, 160–169. [Google Scholar] [CrossRef]
- Harvey, P.H.; Pagel, M.D. The Comparative Method in Evolutionary Biology; Oxford University Press: Oxford, UK, 1991; Volume 239. [Google Scholar]
- Harvey, P.H.; Rambaut, A. Comparative analyses for adaptive radiations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1599–1605. [Google Scholar] [CrossRef]
- Losos, J.B. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 2008, 11, 995–1003. [Google Scholar] [CrossRef]
- Richardson, J.E.; Whitlock, B.A.; Meerow, A.W.; Madriñán, S. The age of chocolate: A diversification history of Theobroma and Malvaceae. Front. Ecol. Evol. 2015, 3, 120. [Google Scholar] [CrossRef]
- Qian, H.; Deng, T. Species invasion and phylogenetic relatedness of vascular plants on the Qinghai-Tibet Plateau, the roof of the world. Plant Divers. 2023, in press. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Rodriguez, M.A.; Weller, S.G. Global angiosperm family richness revisited: Linking ecology and evolution to climate. J. Biogeogr. 2011, 38, 1253–1266. [Google Scholar] [CrossRef]
- Wu, Y.; Ricklefs, R.E.; Huang, Z.; Zan, Q.; Yu, S. Winter temperature structures mangrove species distributions and assemblage composition in China. Glob. Ecol. Biogeogr. 2018, 27, 1492–1506. [Google Scholar] [CrossRef]
- Barreda, V.D.; Palazzesi, L.; Tellería, M.C.; Olivero, E.B.; Raine, J.I.; Forest, F. Early evolution of the angiosperm clade Asteraceae in the Cretaceous of Antarctica. Proc. Natl. Acad. Sci. USA 2015, 112, 10989–10994. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.Á.; Crespo, M.B.; Martínez-Azorín, M.; Mucina, L. Taxonomic identity and evolutionary relationships of South African taxa related to the Spergularia media group (Caryophyllaceae). Plant Syst. Evol. 2021, 307, 24. [Google Scholar] [CrossRef]
- Veldkornet, D.A.; Potts, A.J.; Adams, J.B. The distribution of salt marsh macrophyte species in relation to physicochemical variables. S. Afr. J. Bot. 2016, 107, 84–90. [Google Scholar] [CrossRef]
- Steffen, S.; Ball, P.; Mucina, L.; Kadereit, G. Phylogeny, biogeography and ecological diversification of Sarcocornia (Salicornioideae, Amaranthaceae). Ann. Bot. 2015, 115, 353–368. [Google Scholar] [CrossRef]
- Piirainen, M.; Liebisch, O.; Kadereit, G. Phylogeny, biogeography, systematics and taxonomy of Salicornioideae (Amaranthaceae/Chenopodiaceae)—A cosmopolitan, highly specialized hygrohalophyte lineage dating back to the Oligocene. Taxon 2017, 66, 109–132. [Google Scholar] [CrossRef]
- Linder, H.P.; Lehmann, C.E.; Archibald, S.; Osborne, C.P.; Richardson, D.M. Global grass (Poaceae) success underpinned by traits facilitating colonization, persistence and habitat transformation. Biol. Rev. 2018, 93, 1125–1144. [Google Scholar] [CrossRef]
- Yao, P.C.; Gao, H.Y.; Wei, Y.N.; Zhang, J.H.; Chen, X.Y.; Li, H.Q. Evaluating sampling strategy for DNA barcoding study of coastal and inland halo–tolerant Poaceae and Chenopodiaceae: A case study for increased sample size. PLoS ONE 2017, 12, e0185311. [Google Scholar] [CrossRef]
- Potts, A.J.; Veldkornet, D.A.; Adams, J.B. A phylogeographic break in a South African coastal saltmarsh macrophyte, Juncus kraussii. S. Afr. J. Bot. 2016, 107, 80–83. [Google Scholar] [CrossRef]
- Adams, J.B. Distribution and status of Zostera capensis in South African estuaries—A review. S. Afr. J. Bot. 2016, 107, 63–73. [Google Scholar] [CrossRef]
- Phair, N.L.; Toonen, R.J.; Knapp, I.S.S.; von der Heyden, S. Anthropogenic pressures negatively impact genomic diversity of the vulnerable seagrass Zostera capensis. J. Environ. Manag. 2020, 255, 109831. [Google Scholar] [CrossRef] [PubMed]
- Phair, N.L.; Nielsen, E.S.; von der Heyden, S. Applying genomic data to seagrass conservation. Biodivers. Conserv. 2021, 30, 2079–2096. [Google Scholar] [CrossRef]
- von Mering, S.; Kadereit, J.W. Phylogeny, biogeography and evolution of Triglochin L. (Juncaginaceae)—Morphological diversification is linked to habitat shifts rather than to genetic diversification. Mol. Phylogenetics Evol. 2015, 83, 200–212. [Google Scholar] [CrossRef] [PubMed]

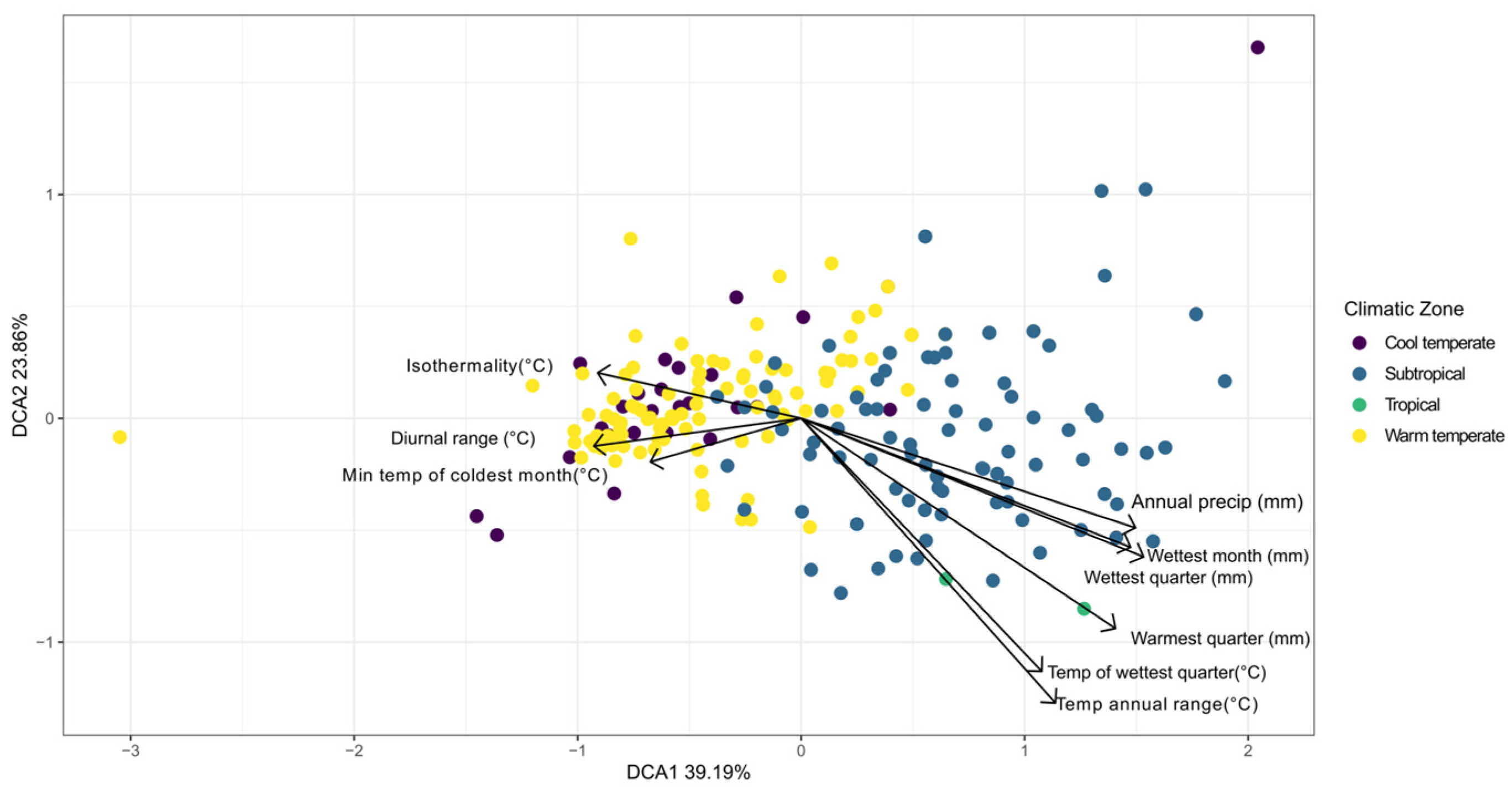
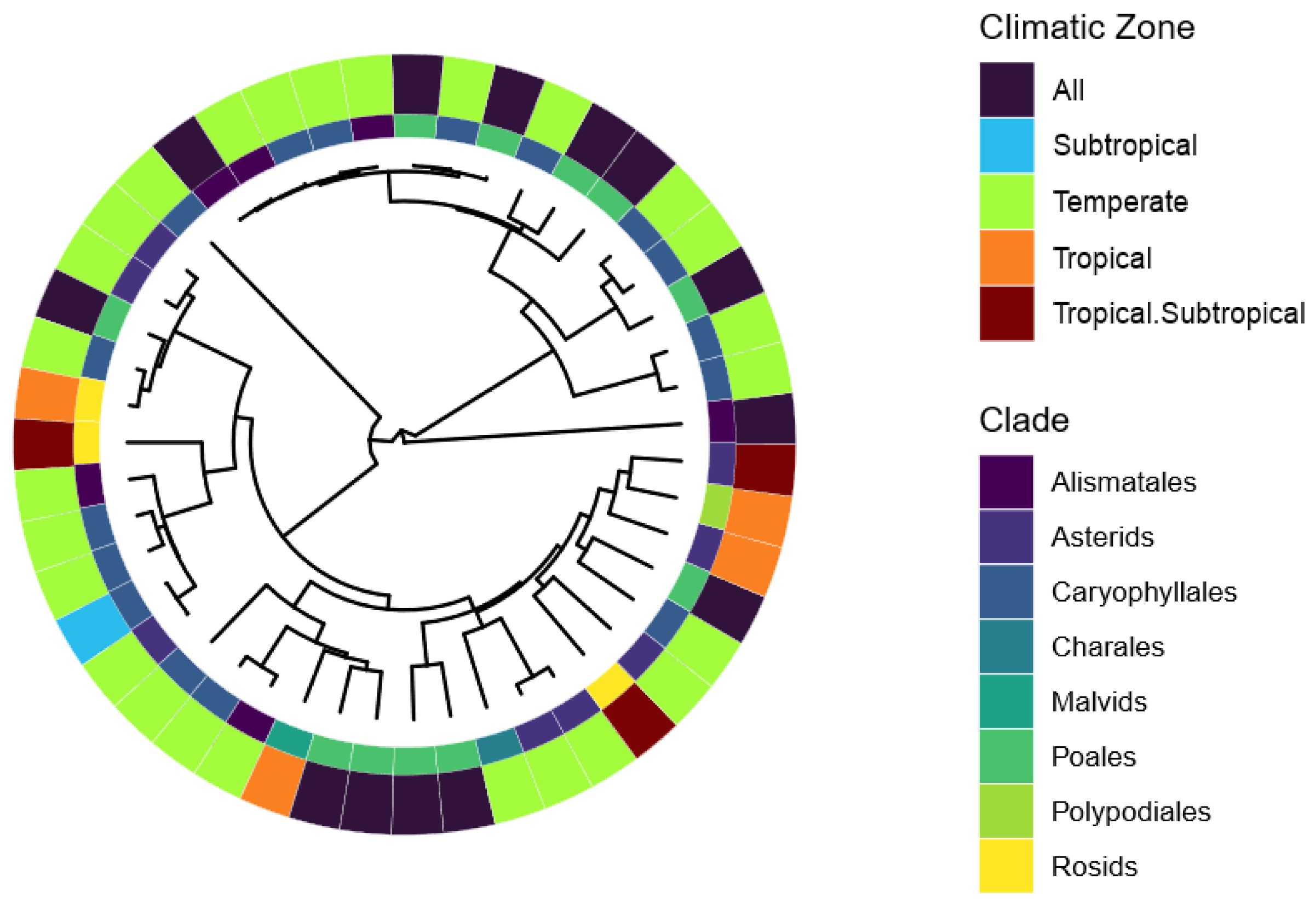
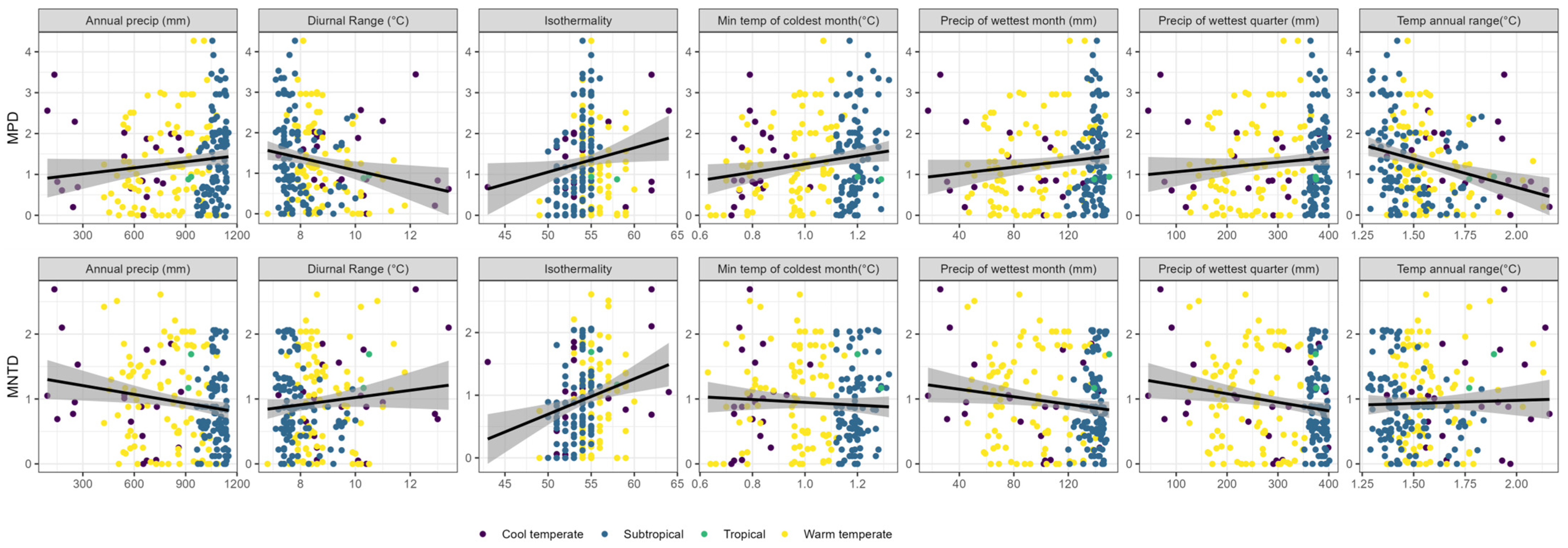
| Bioclimatic Variable | Cool Temperate | Warm Temperate | Subtropical | Tropical |
|---|---|---|---|---|
| Mean Diurnal Range (°C) | 9.91 (7.2; 13.4) | 7.86 (7.1; 10) | 10.4 (10.3; 10.5) | 8.8 (6.8; 11.8) |
| Isothermality (°C) | 54.48 (43; 64) | 55.40 (49; 60) | 53.23 (50; 56) | 56.5 (55; 58) |
| Temperature Seasonality (°C) | 28.44 (19.25; 41.03) | 22.96 (19.16; 31.62) | 22.59 (19.61; 28.12) | 24.98 (23.6; 26.36) |
| Minimum temperature of coldest month (°C) | 0.80 (0.72; 1.02) | 0.95 (0.63; 1.15) | 1.19 (1.12; 1.32) | 1.25 (1.2; 1.29) |
| Temperature annual range (°C) | 1.80 (1.37; 2.16) | 1.572 (1.37; 2.13) | 1.47 (1.28; 1.84) | 1.87(1.77; 1.89) |
| Mean temperature of the wettest quarter (°C) | 1.35 (1.2; 1.56) | 1.79 (1.36; 2.17) | 2.30 (2.15; 2.52) | 2.54 (2.52; 2.55) |
| Annual precipitation (mm) | 526 (93; 873) | 768 (424; 1087) | 1078 (966; 1150) | 924 (916; 932) |
| Precipitation of wettest month (mm) | 84 (17; 143) | 90 (46; 147) | 138 (119; 148) | 144 (139; 150) |
| Precipitation of wettest quarter (mm) | 237 (45; 399) | 241(126; 365) | 379 (352; 401) | 374 (374; 375) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veldkornet, D.A. The Influence of Macroclimatic Drivers on the Macrophyte Phylogenetic Diversity in South African Estuaries. Diversity 2023, 15, 986. https://doi.org/10.3390/d15090986
Veldkornet DA. The Influence of Macroclimatic Drivers on the Macrophyte Phylogenetic Diversity in South African Estuaries. Diversity. 2023; 15(9):986. https://doi.org/10.3390/d15090986
Chicago/Turabian StyleVeldkornet, Dimitri Allastair. 2023. "The Influence of Macroclimatic Drivers on the Macrophyte Phylogenetic Diversity in South African Estuaries" Diversity 15, no. 9: 986. https://doi.org/10.3390/d15090986
APA StyleVeldkornet, D. A. (2023). The Influence of Macroclimatic Drivers on the Macrophyte Phylogenetic Diversity in South African Estuaries. Diversity, 15(9), 986. https://doi.org/10.3390/d15090986








