There and Back Again—The Igatu Hotspot Siliciclastic Caves: Expanding the Data for Subterranean Fauna in Brazil, Chapada Diamantina Region
Abstract
:1. Introduction
2. Material and Methods
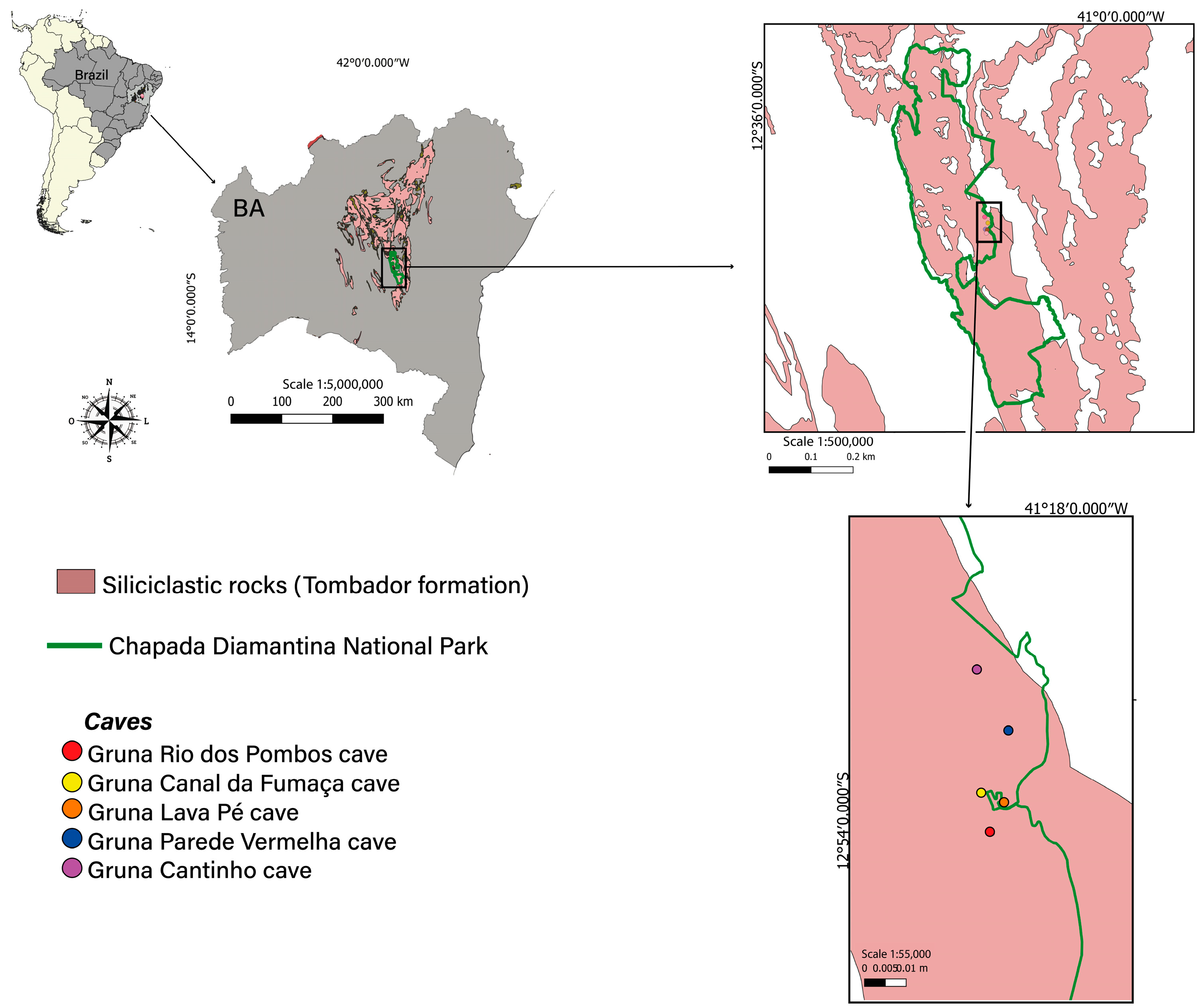
2.1. Igatu Region and Their Caves
2.2. Samplings, Determinations, Classification
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trajano, E. Evolution of Tropical Troglobites: Applicability of the Model of Quaternary Climatic Fluctuations. Mémoirs Biospéologie 1995, 22, 203–209. [Google Scholar]
- Trajano, E. Mapping Subterranean Biodiversity in Brazilian Karst Areas. In Mapping Subterranean Biodiversity; Culver, D.C., Deharveng, L., Gibert, J., Sasowsky, I., Eds.; Karst Waters Institute: Pennsylvania, PA, USA, 2001; pp. 67–70. [Google Scholar]
- Deharveng, L. Diversity Patterns in the Tropics. In Encyclopedia of Caves; Culver, D.C., White, W.B., Eds.; Academic Elsevier Press: Amsterdan, The Netherlands, 2005; pp. 166–170. [Google Scholar]
- Silva, M.S.; Ferreira, R.L. The First Two Hotspots of Subterranean Biodiversity in South America. Subterr. Biol. 2016, 19, 1–12. [Google Scholar] [CrossRef]
- Gallão, J.E.; Bichuette, M.E. Taxonomic Distinctness and Conservation of a New High Biodiversity Subterranean Area in Brazil. An. Acad. Bras. Cienc. 2015, 87, 209–217. [Google Scholar] [CrossRef]
- Trajano, E.; Gallão, J.E.; Bichuette, M.E. Spots of High Diversity of Troglobites in Brazil: The Challenge of Measuring Subterranean Diversity. Biodivers. Conserv. 2016, 25, 1805–1828. [Google Scholar] [CrossRef]
- Lourenço, W.R.; Baptista, R.L.C.; de Leão Giupponi, A.P. Troglobitic Scorpions: A New Genus and Species from Brazil. C. R. Biol. 2004, 327, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Bichuette, M.E.; De Pinna, M.C.C.; Trajano, E. A New Species of Glaphyropoma: The First Subterranean Copionodontine Catfish and the First Occurrence of Opercular Odontodes in the Subfamily (Siluriformes: Trichomycteridae). Neotrop. Ichthyol. 2008, 6, 301–306. [Google Scholar] [CrossRef]
- Bichuette, M.E.; Gallão, J.E. Under the Surface: What We Know about the Threats to Subterranean Fishes in Brazil. Neotrop. Ichthyol. 2021, 19, e210089. [Google Scholar] [CrossRef]
- CPRM. Projeto Chapada Diamantina: Parque Nacional da Chapada Diamantina—BA: Informações Básicas Para a Gestão Territorial: Diagnóstico do Meio Físico e da Vegetação; CPRM: Brasília, Brazil, 1994. [Google Scholar]
- Pedreira, A.J.; Serra Do Sincorá, B. Sítios Geológicos e Paleontológicos do Brasil; Schobbenhaus, C., Campos, D., Queiroz, E., Wing, M., Berbert-Born, M., Eds.; CPRM: Brasília, Brazil, 2001. [Google Scholar]
- Severo Giudice, D.; Melo e Souza, R. Geologia E Geoturismo Na Chapada Diamantina. Gestión Turística 2010, 2020, 69–81. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T. Ecological and Evolutionary Classifications of Subterranean Organisms. In Encyclopedia of Caves; White, W.B., Culver, D.C., Pipan, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 376–379. [Google Scholar]
- Trajano, E.; Golovatch, S.I.; Geoffroy, J.-J.; Pinto-da-Rocha, R.; Fontanetti, C.S. Synopsis of Brazilian Cave-Dwelling Millipedes (Diplopoda). Pap. Avulsos de Zool. (São Paulo) 2000, 41, 259–287. [Google Scholar]
- Sierwald, P.; Spelda, J. MilliBase. Available online: https://www.millibase.org (accessed on 22 May 2023). [CrossRef]
- Bouzan, R.S.; Means, J.C.; Ivanov, K.; Ferreira, R.L.; Brescovit, A.D.; Moretti Iniesta, L.F. Worldwide Distribution of Cave-Dwelling Chelodesmidae (Diplopoda, Polydesmida). Int. J. Speleol. 2022, 51, 235–248. [Google Scholar] [CrossRef]
- Chagas, A.; Bichuette, M.E. A Synopsis of Centipedes in Brazilian Caves: Hidden Species Diversity That Needs Conservation (Myriapoda, Chilopoda). Zookeys 2018, 2018, 13–56. [Google Scholar] [CrossRef]
- Chagas, A.; Bichuette, M.E. A New Species of Scolopocryptops Newport: A Troglobitic Scolopocryptopine Centipede from a Remarkable Siliciclastic Area of Eastern Brazil (Scolopendromorpha, Scolopocryptopidae, Scolopocryptopinae). Zookeys 2015, 487, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Von Schimonsky, D.M.; Bichuette, M.E. Distribution of Cave-Dwelling Pseudoscorpions (Arachnida) in Brazil. J. Arachnol. 2019, 47, 110–123. [Google Scholar] [CrossRef]
- Moreno-González, J.A.; Pinto-da-Rocha, R.; Gallão, J.E. Bringing Order to a Complex System: Phenotypic and Genotypic Evidence Contribute to the Taxonomy of Tityus (Scorpiones, Buthidae) and Support the Description of a New Species. Zookeys 2021, 1075, 33–75. [Google Scholar] [CrossRef] [PubMed]
- Pinto-da-Rocha, R. Sinopse Da Fauna Cavernícola Do Brasil (1907–1994). Pap. Avulsos Zool. 1994, 39, 61–173. [Google Scholar] [CrossRef]
- Gallão, J.E.; Bichuette, M.E. Brazilian Obligatory Subterranean Fauna and Threats to the Hypogean Environment. Zookeys 2018, 746, 1–23. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Berbert-Born, M.; Souza-Silva, M. The Água Clara Cave System in Northeastern Brazil: The Richest Hotspot of Subterranean Biodiversity in South America. Diversity 2023, 15, 761. [Google Scholar] [CrossRef]
- Francke, O.F.; Monjaraz-Ruedas, R.; Cruz-López, J.A. Biodiversity of the Huautla Cave System, Oaxaca, Mexico. Diversity 2021, 13, 429. [Google Scholar] [CrossRef]
- Niemiller, M.L.; Slay, M.E.; Inebnit, T.; Miller, B.; Tobin, B.; Cramphorn, B.; Hinkle, A.; Jones, B.D.; Mann, N.; Niemiller, K.D.K.; et al. Fern Cave: A Hotspot of Subterranean Biodiversity in the Interior Low Plateau Karst Region of Alabama in the Southeastern United States. Diversity 2023, 15, 633. [Google Scholar] [CrossRef]
- May, R.M. Taxonomy as Destiny. Nature 1990, 347, 129–130. [Google Scholar] [CrossRef]
- Williams, P.H.; Humphries, C.J.; Vane-Wright, R.I. Measuring Biodiversity: Taxonomic Relatedness for Conservation Priorities. Aust. Syst. Bot. 1991, 4, 665–679. [Google Scholar] [CrossRef]
- Vane-Wright, R.I.; Humphries, C.J.; Williams, P.H. What to Protect?—Systematics and the Agony of Choice. Biol. Conserv. 1991, 55, 235–254. [Google Scholar] [CrossRef]
- Trajano, E.; Moreira, J.R.d.A. Estudo Da Fauna de Cavernas Da Província Espeleológica Arenítica Altamira—Itaituba, Pará. Rev. Bras. Biol. 1991, 51, 13–29. [Google Scholar]
- Zeppelini Filho, D.; Ribeiro, A.C.; Ribeiro, G.C.; Fracasso, M.P.A.; Pavani, M.M.; Oliveira, O.M.P.; de Oliveira, S.A.; Marques, A.C. Faunistic Survey of Sandstone Caves from Altinópolis Region, São Paulo State, Brazil. Pap. Avulsos Zool. 2003, 43, 93–99. [Google Scholar] [CrossRef]
- Reis, R.L.; Júnior, C.F.E.; Figueiredo, G.P.S.; Muriel-Cunha, J. Levantamento Preliminar da Biodiversidade da Caverna do Prudente, Província Espeleológica Arenitica Altamira-Itaituba, Ruropólis, Pará. In ANAIS do 32o Congresso Brasileiro de Espeleologia; Sociedade Brasileira de Espeleologia: Barreiras, BA, Brazil, 2013; pp. 115–119. [Google Scholar]
- Sorbo, C.F.; Bichuette, M.E. Preliminary survey of invertebrates in three sandstone caves from rio do sul, Brazil. Espeleo-Tema 2013, 24, 41–47. [Google Scholar]
- Gallo, J.S. Diversidade de Invertebrados Terrestres Em Cavernas Areníticas Do Estado de São Paulo, Com Ênfase Em Pseudonannolenidae (Diplopoda: Spirostreptida). Master’s Dissertation, Universidade Federal de São Carlos, São Carlos, CA, USA, 2017. [Google Scholar]
- Souza Silva, M.; Iniesta, L.F.M.; Lopes Ferreira, R. Invertebrates Diversity in Mountain Neotropical Quartzite Caves: Which Factors Can Influence the Composition, Richness, and Distribution of the Cave Communities? Subterr. Biol. 2020, 33, 23–43. [Google Scholar] [CrossRef]
- Culver, D.C.; Sket, B. Hotspots of Subterranean Biodiversity in Caves and Wells. J. Cave Karst Stud. 2000, 62, 11–17. [Google Scholar]
- Culver, D.C.; Deharveng, L.; Pipan, T.; Bedos, A. An Overview of Subterranean Biodiversity Hotspots. Diversity 2021, 13, 487. [Google Scholar] [CrossRef]





| Taxonomic Group | Taxon | Cave |
|---|---|---|
| Diplopoda: Polydesmida: cf. Chelodesmidae | Gen. sp. | Gruna Cantinho |
| Diplopoda: Polydesmida: Oniscodesmidae | Crypturodesmus sp. | Gruna Cantinho, Gruna da Parede Vermelha |
| Chilopoda: Scutigeromorpha: Pselliodidae | Sphendononema sp. | Gruna da Parede Vermelha, Gruna Canal da Fumaça |
| Chilopoda: Scolopendromorpha: Scolopocryptopidae | Scolopocryptops troglocaudatus Chagas-Jr and Bichuette, 2015 | Gruna Cantinho, Gruna Lava Pé |
| Chilopoda: Scolopendromorpha: Cryptopidae | Cryptops sp. | Gruna Lava Pé |
| Arachnida: Acari: Mesostigmata: Pachylaepidae | Gen. sp. | Gruna Cantinho |
| Arachnida: Acari: Mesostigmata: Dithinozerconidae | Gen. sp. | Gruna Rio dos Pombos |
| Arachnida: Acari: Sarcoptiformes: Oehserchestidae | Gen. sp. | Gruna da Parede Vermelha |
| Arachnida: Scorpiones: Buthidae | Troglorhopalurus translucidus Lourenço, Baptista and Giupponi, 2004 | Gruna da Parede Vermelha, Gruna Canal da Fumaça, Gruna Cantinho, Gruna Lava Pé, Gruna Rio dos Pombos |
| Arachnida: Araneae: Theraphosidae | Tmesiphantes hypogeus Bertani, Bichuette and Pedroso, 2013 | Gruna da Parede Vermelha |
| Arachnida: Araneae: Ctenidae | Ctenus igatu Polotow, Cizauskas and Brescovit, 2022 | Gruna Canal da Fumaça |
| Arachnida: Araneae: Gnaphosidae: Prodidominae | Gen. sp. | Gruna Rio dos Pombos |
| Arachnida: Araneae: Ochyroceratidae | Ochyrocera sp. | Gruna Cantinho |
| Arachnida: Araneae: Pholcidae | Metagonia sp. | Gruna Rio dos Pombos, Gruna Cantinho |
| Arachnida: Araneae: Telemidae | Gen. sp. | Gruna da Parede Vermelha |
| Arachnida: Opiliones: Gonyleptidae | Discocyrtus pedrosoi Kury, 2008 | Gruna da Parede Vermelha, Gruna Canal da Fumaça, Gruna Cantinho, Gruna Lava Pé, Gruna Rio dos Pombos |
| Arachnida: Opiliones: Tricommatidae | Gen. sp. | Gruna Cantinho |
| Arachnida: Pseudoscorpiones: Chernetidae | Spelaeochernes sp. | Gruna da Parede Vermelha |
| Arachnida: Pseudoscorpiones: Chthoniidae | Pseudochthonius sp. | Gruna da Parede Vermelha |
| Arachnida: Pseudoscorpiones: Syarinidae | Gen. sp. | Gruna da Parede Vermelha |
| Arachnida: Palpigradi: Eukoeneniidae | Eukoenenia sp. | Gruna Lava Pé, Gruna Cantinho |
| Malacostraca: Isopoda: Philosciidae | Metaprosekia igatuensis Campos-Filho, Fernandes and Bichuette, 2020 | Gruna Rio dos Pombos |
| Malacostraca: Isopoda: Philosciidae | Benthana xiquinhoi Campo-Filho, Bichuette and Taiti, 2019 | Gruna Lava Pé, Gruna da Parede Vermelha |
| Malacostraca: Isopoda: Philosciidae | Gen. sp. | Gruna da Parede Vermelha |
| Malacostraca: Isopoda: Plathyartridae | Trichorhina sp. | Gruna Rio dos Pombos, Gruna Lava Pé |
| Malacostraca: Isopoda: Platyarthridae | Gen. sp. | Gruna da Parede Vermelha, Gruna Rio dos Pombos |
| Collembola: Entomobryomorpha: Entomobryidae | Verhoeffiella sp. | Gruna da Parede Vermelha |
| Collembola: Entomobryomorpha: Entomobryidae: Heteromurinae: Heteromurini | Gen. sp. | Gruna Cantinho, Gruna Rio dos Pombos |
| Collembola: Entomobryomorpha: Paronellidae | Troglopedetes sp. | Gruna da Parede Vermelha, Gruna Cantinho |
| Diplura: Projapygidae | Gen. sp. | Gruna Rio dos Pombos |
| Insecta: Zygentoma: Nicoletiidae | Gen. sp. | Gruna Canal da Fumaça |
| Insecta: Blattaria: Blattellidae | Gen. sp. | Gruna da Parede Vermelha |
| Insecta: Coleoptera: Scydmaenidae | Gen. sp. | Gruna Cantinho |
| Insecta: Coleoptera: Staphylinidae: Pselaphinae | Gen. sp. | Gruna da Parede Vermelha |
| Gastropoda: Stylommatophora: Systrophiidae | Happia sp. | Gruna da Parede Vermelha, Gruna Canal da Fumaça, Gruna Lava Pé, Gruna Rio dos Pombos |
| Actinopterygii: Siluriformes: Trichomycteridae | Copionodon sp. | Gruna da Parede Vermelha, Gruna Canal da Fumaça, Gruna Cantinho, Gruna Lava Pé, Gruna Rio dos Pombos |
| Actinopterygii: Siluriformes: Trichomycteridae | Glaphyropoma spinosum Bichuette, de Pinna and Trajano, 2008 | Gruna da Parede Vermelha, Gruna Canal da Fumaça, Gruna Cantinho, Gruna Lava Pé, Gruna Rio dos Pombos |
| Region | Geomorphological Information | Number of Caves | TR/STY | Total of Species | References |
|---|---|---|---|---|---|
| Altamira and Medicilândia—North Brazil | Altamira—Itaituba | 7 | 2 | 62 | [29] |
| Altinópolis—Southeastern Brazil | Serra Geral, Botucatu Formation | 9 | 0 | 83 | [30] |
| Rurópolis—North Brazil | Altamira—Itaituba | 1 | 0 | 16 | [31] |
| Manoel Viana and São Pedro do Sul—South Brazil | Serra Geral, Botucatu Formation | 3 | 0 | 30 | [32] |
| Chapada Diamantina—Northeastern Brazil | Serra do Espinhaço, Tombador Formation | 11 | 25 | 162 | [5] |
| Altinópolis—Southeastern Brazil | Serra Geral, Botucatu Formation | 8 | 0 | 131 | [33] |
| Lima Duarte—Southeastern Brazil | Andrelândia geological group | 20 | 6 | 469 | [34] |
| Itirapina—Southeastern Brazil | Serra Geral, Botucatu Formation | 1 | 3 | 67 | E.L.B. Carvalho, undergraduated monograph (unpubl. data) |
| Altamira—North Brazil | Altamira—Itaituba | 26 | 17 | 596 | M.E. Bichuette, unpubl. data |
| Chapada Diamandina—Northeastern Brazil | Serra do Espinhaço, Tombador Formation | 5 | 37 | 184 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallão, J.E.; Ribeiro, D.B.; Gallo, J.S.; Bichuette, M.E. There and Back Again—The Igatu Hotspot Siliciclastic Caves: Expanding the Data for Subterranean Fauna in Brazil, Chapada Diamantina Region. Diversity 2023, 15, 991. https://doi.org/10.3390/d15090991
Gallão JE, Ribeiro DB, Gallo JS, Bichuette ME. There and Back Again—The Igatu Hotspot Siliciclastic Caves: Expanding the Data for Subterranean Fauna in Brazil, Chapada Diamantina Region. Diversity. 2023; 15(9):991. https://doi.org/10.3390/d15090991
Chicago/Turabian StyleGallão, Jonas Eduardo, Deyvison Bonfim Ribeiro, Jéssica Scaglione Gallo, and Maria Elina Bichuette. 2023. "There and Back Again—The Igatu Hotspot Siliciclastic Caves: Expanding the Data for Subterranean Fauna in Brazil, Chapada Diamantina Region" Diversity 15, no. 9: 991. https://doi.org/10.3390/d15090991
APA StyleGallão, J. E., Ribeiro, D. B., Gallo, J. S., & Bichuette, M. E. (2023). There and Back Again—The Igatu Hotspot Siliciclastic Caves: Expanding the Data for Subterranean Fauna in Brazil, Chapada Diamantina Region. Diversity, 15(9), 991. https://doi.org/10.3390/d15090991









