Status of the Italian Freshwater Gastrotricha Biodiversity, with the Creation of an Interactive GIS-Based Web Map
Abstract
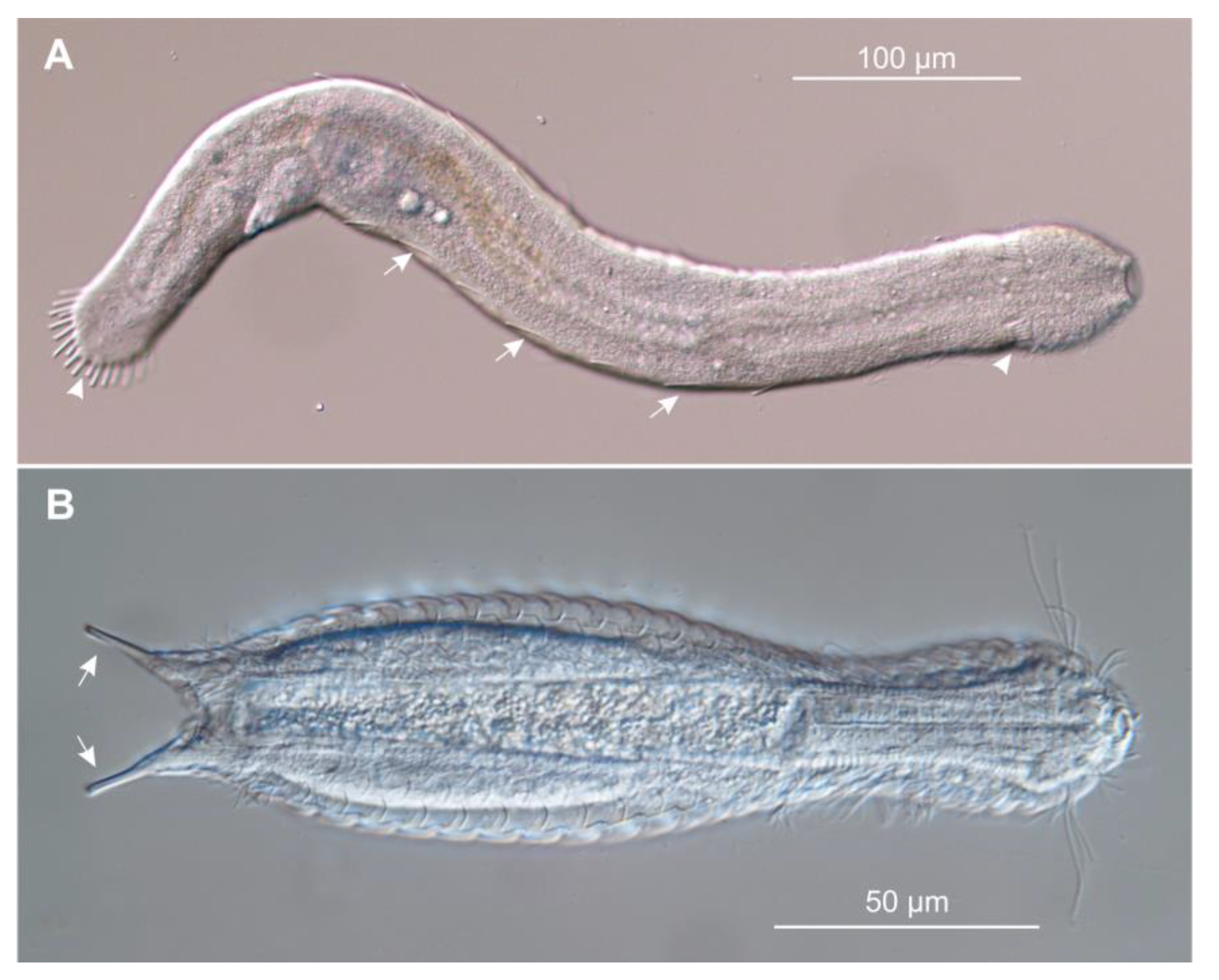
:1. Introduction
| Family CEPHALODASYIDAE Hummon & Todaro, 2010 [34 M spp.] |
| Genus Cephalodasys Remane, 1926 [14 M spp.] |
| Genus Dolichodasys Gagne, 1977 [3 M spp.] |
| Genus Mesodasys Remane, 1951 [8 M spp.] |
| Genus Paradasys Remane, 1934 [7 M spp.] |
| Genus Pleurodasys Remane, 1927 [2 M spp.] |
| Family DACTYLOPODOLIDAE Strand, 1929 [17 M spp.] |
| Genus Dactylopodola Strand, 1929 [11 M spp.] |
| Genus Dendrodasys Wilke, 1954 [6 M spp.] |
| Genus Dendropodola Hummon, Todaro & Tongiorgi, 1993 [1 M sp.] |
| Family HUMMONDASYIDAE Todaro, Leasi & Hochberg, 2014 [1 M sp.] |
| Genus Hummondasys Todaro, Leasi & Hochberg, 2014 [1 M sp.] |
| Family LEPIDODASYIDAE Remane, 1927 [9 M spp.] |
| Genus Lepidodasys Remane, 1926 [9 M spp.] |
| Family MACRODASYIDAE Remane, 1926 [56 M spp.] |
| Genus Kryptodasys Todaro, Dal Zotto, Kånneby, Hochberg, 2019 [7 M spp.] |
| Genus Macrodasys Remane, 1924 [32 M spp.] |
| Genus Thaidasys Todaro, Dal Zotto & Leasi, 2015 [1 M sp.] |
| Genus Urodasys Remane, 1926 [16 M spp.] |
| Family PLANODASYIDAE Rao & Clausen, 1970 [14 M spp.] |
| Genus Crasiella Clausen, 1968 [9 M spp.] |
| Genus Megadasys Schmidt, 1974 [3 M spp.] |
| Genus Planodasys Rao & Clausen, 1970 [2 M spp.] |
| Family REDUDASYIDAE Todaro et al. 2012 [1 M and 3 F spp.] |
| Genus Anandrodasys Todaro et al., 2012 [1 M sp.] |
| Genus Redudasys Kisielewski, 1987 [3 M spp.] |
| Family THAUMASTODERMATIDAE Remane, 1927 [176 M spp.] |
| Subfamily Diplodasinae Ruppert, 1978 [23 M spp.] |
| Genus Acanthodasys Remane, 1927 [13 M spp.] |
| Genus Diplodasys Remane, 1927 [10 M spp.] |
| Subfamily Thaumastodermatinae Remane, 1927 [154 M spp.] |
| Genus Chimaeradasys Kieneke & Todaro, 2020 [2 M spp.] |
| Genus Hemidasys Claparède, 1867 [1 M sp. *] |
| Genus Oregodasys Hummon, 2008 [16 M spp.] |
| Genus Pseudostomella Swedmark, 1956 [17 M spp.] |
| Genus Ptychostomella Remane, 1926 [13 M spp.] |
| Genus Tetranchyroderma Remane, 1926 [86 M spp.] |
| Genus Thaumastoderma Remane, 1926 [18 M spp.] |
| Family TURBANELLIDAE Remane, 1927 [64 M spp.] |
| Genus Desmodasys Clausen, 1965 [3 M spp.] |
| Genus Dinodasys Remane, 1927 [2 M spp.] |
| Genus Paraturbanella Remane, 1927 [24 M spp.] |
| Genus Prostobuccantia Evans & Hummon, 1991 [1 M sp.] |
| Genus Pseudoturbanella d’Hondt, 1968 [1 M sp.] |
| Genus Turbanella Schultze, 1853 [33 M spp.] |
| Family XENODASYIDAE Todaro, Guidi, Leasi & Tongiorgi, 2006 [4 M spp.] |
| Genus Chordodasiopsis Todaro, Guidi, Leasi & Tongiorgi, 2006 [1 M sp.] |
| Genus Xenodasys Swedmark, 1967 [3 M spp.] |
| INCERTAE SEDIS |
| Genus Marinellina Ruttner-Kolisko, 1955 [1 F sp.] |
| Suborder MULTITUBULATINA d’Hondt, 1971 [3 M spp.] |
| Family NEODASYIDAE Remane, 1929 [3 M spp.] |
| Genus Neodasys Remane, 1927 [3 M spp.] |
| Suborder PAUCITUBULATINA d’Hondt, 1971 [145 M * and 372 F spp.] |
| Family CHAETONOTIDAE Gosse, 1864 [112 M * and 320 F spp.] |
| Subfamily Chaetonotinae Kisielewski, 1991 [112 M and 319 F spp.] |
| Genus Arenotus Kisielewski, 1987 [1 F sp.] |
| Genus Aspidiophorus Voigt, 1903 [11 M and 24 F spp.] |
| Genus Bifidochaetus Kolicka & Kisielewski [2 F spp.] |
| Genus Caudichthydium Schwank, 1990 [3 M spp.] |
| Genus Cephalionotus Garraffoni et al., 2017 [1 F sp.] |
| Genus Chaetonotus Ehrenberg, 1830 [47 M ** and 191 F spp.] |
| Genus Fluxiderma d’Hont, 1974 [3 F spp.] |
| Genus Halichaetoderma Rataj Križanová & Vdacný, 2023 [4 F spp.] |
| Genus Halichaetonotus Remane, 1936 [31 M spp.] |
| Genus Heterolepidoderma Remane, 1927 [15 M and 25 F spp.] |
| Genus Ichthydium Ehrenberg, 1830 [3 M and 29 F spp.] |
| Genus Lepidochaetus Kisielewski, 1991 [6 F spp.] |
| Genus Lepidodermella Blake, 1933 [2 M *** and 15 F spp.] |
| Genus Polymerurus Remane, 1926 [16 F spp.] |
| Genus Rhomballichthys Schwank, 1990 [1 F spp.] |
| Subfamily Undulinae Kisielewski, 1991 [1 F sp.] |
| Genus Undula Kisielewski, 1991 [1 F sp.] |
| Family DASYDYTIDAE Daday, 1905 [35 F spp.] |
| Genus Anacanthoderma Marcolongo, 1910 [2 F spp.] |
| Genus Chitonodytes Remane, 1936 [3 F spp.] |
| Genus Dasydytes Gosse, 1851 [9 F spp.] |
| Genus Haltidytes Remane, 1936 [6 F spp.] |
| Genus Ornamentula Kisielewski, 1991 [2 F spp.] |
| Genus Setopus Grünspan, 1908 [9 F spp.] |
| Genus Stylochaeta Hlava, 1904 [4 F spp.] |
| Family DICHAETURIDAE Remane, 1927 [4 F spp.] |
| Genus Dichaetura Lauterborn, 1913 [4 F spp.] |
| Family MUSELLIFERIDAE Leasi & Todaro, 2008 [7 M spp.] |
| Genus Diuronotus Todaro, Kristensen & Balsamo, 2005 [2 M spp.] |
| Genus Musellifer Hummon, 1969 [5 F spp.] |
| Family NEOGOSSEIDAE Remane, 1927 [11 F spp.] |
| Genus Kijanebalola Beauchamp, 1932 [4 F spp.] |
| Genus Neogossea Remane, 1927 [7 F spp.] |
| Family PROICHTHYDIDAE Remane, 1927 [2 F spp.] |
| Genus Proichthydium Cordero, 1918 [1 F spp.] |
| Genus Proichthydioides Sudzuki, 1971 [1 F spp.] |
| Family XENOTRICHULIDAE Remane, 1927 [26 M spp.] |
| Subfamily Draculiciterinae Ruppert, 1979 [1 M sp.] |
| Genus Draculiciteria Hummon, 1974 [1 M sp.] |
| Subfamily Xenotrichulinae Remane, 1927 [25 M spp.] |
| Genus Heteroxenotrichula Wilke, 1954 [9 M spp.] |
| Genus Xenotrichula Remane, 1927 [16 M spp.] |
2. Materials and Methods
2.1. Gastrotrichs
2.2. Data Matrix
2.3. Statistical Analysis
2.4. QGIS
2.4.1. Version and Plugins Used and Software Preparation
2.4.2. Creation of the Data Shapefile Layers
2.4.3. Web Map Preparation and Exportation
3. Results
3.1. Gastrotrichs


3.2. QGIS and Web Map




4. Conclusions
4.1. Gastrotrichs
| Nation | N. Species | N. Genera | N. Families | Reference |
|---|---|---|---|---|
| Denmark | 27 | 6 | 2 | [59] |
| France | 36 | 10 | 3 | [60] |
| Germany | 91 | 13 | 3 | [61] |
| Italy | 91 | 17 | 3 | Present study |
| Poland | 107 | 11 | 3 | [62] |
| Romania | 90 | 15 | 3 | [63] |
| Sweden | 54 | 9 | 2 | [64] |
| South Korea | 16 | 6 | 3 | [65] |
| Canada | 39 | 10 | 2 | [66] |
| U.S.A. | 60 | 13 | 4 | [19,67,68] |
| Brazil | 98 | 18 | 4 | [16,17,69,70,71,72,73,74,75,76,77] |
4.2. QGIS and Web Map
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balsamo, M.; Grilli, P.; Guidi, L.; d’Hondt, J.L. Gastrotricha—Biology, ecology and systematics. Families Dasydytidae, Dichaeturidae, Neogosseidae, Proichthydiidae. In Identification Guides to the Plankton and Benthos of Inland Waters; Backhuys Publishers: Leiden, The Netherlands, 2014; Volume 24. [Google Scholar]
- Todaro, M.A.; Telford, M.J.; Lockyer, A.E.; Littlewood, D.T.J. Interrelationships of the Gastrotricha and Their Place among the Metazoa Inferred from 18S rRNA Genes. Zool. Scr. 2006, 35, 251–259. [Google Scholar] [CrossRef]
- Struck, T.H.; Wey-Fabrizius, A.R.; Golombek, A.; Hering, L.; Weigert, A.; Bleidorn, C.; Klebow, S.; Iakovenko, N.; Hausdorf, B.; Petersen, M.; et al. Platyzoan Paraphyly Based on Phylogenomic Data Supports a Noncoelomate Ancestry of Spiralia. Mol. Biol. Evol. 2014, 31, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Egger, B.; Lapraz, F.; Tomiczek, B.; Müller, S.; Dessimoz, C.; Girstmair, J.; Škunca, N.; Rawlison, K.A.; Cameron, B.C.; Beli, E.; et al. A Transcriptomic-Phylogenomic Analysis of the Evolutionary Relationships of Flatworms. Curr. Biol. 2015, 25, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Laumer, C.E.; Bekkouche, N.; Kerbl, A.; Goetz, F.; Neves, R.C.; Sørensen, M.V.; Kristensen, R.M.; Hejnol, A.; Dunn, C.W.; Giribet, G.; et al. Spiralian Phylogeny Informs the Evolution of Microscopic Lineages. Curr. Biol. 2015, 25, 2000–2006. [Google Scholar] [CrossRef]
- Fromm, B.; Tosar, J.P.; Aguilera, F.; Friedländer, M.R.; Bachmann, L.; Hejnol, A. Evolutionary Implications of the microRNA- and piRNA Complement of Lepidodermella Squamata (Gastrotricha). ncRNA 2019, 5, 19. [Google Scholar] [CrossRef]
- Todaro, M.A.; Dal Zotto, M.; Kånneby, T.; Hochberg, R. Integrated Data Analysis Allows the Establishment of a New, Cosmopolitan Genus of Marine Macrodasyida (Gastrotricha). Sci. Rep. 2019, 9, 7989. [Google Scholar] [CrossRef]
- Kieneke, A.; Schmidt-Rhaesa, A. Gastrotricha. In Handbook of Zoology; De Gruyter: Berlin, Germany; München, Germany; Boston, MA, USA, 2014; Volume 3, pp. 1–134. [Google Scholar]
- Kieneke, A.; Todaro, M.A. Discovery of Two ‘Chimeric’ Gastrotricha and Their Systematic Placement Based on an Integrative Approach. Zool. J. Linn. Soc. 2021, 192, 710–735. [Google Scholar] [CrossRef]
- Hummon, W.D.; Todaro, M.A. Analytic Taxonomy and Notes on Marine, Brackish-Water and Estuarine Gastrotricha. Zootaxa 2010, 2392, 1. [Google Scholar] [CrossRef]
- Araujo, T.Q.; King-Trudeau, S.; VanDyke, J.; Hochberg, R. First Ultrastructural Description of an Apomictic Opsiblastic Egg in Freshwater Gastrotricha. J. Morphol. 2023, 285, e21659. [Google Scholar] [CrossRef]
- Giere, O. Patterns of Meiofauna Distribution. In Meiobenthology: The Microscopic Motile Fauna of Aquatic Sediments; Springer: Berlin/Heidelberg, Germany, 2009; pp. 243–265. [Google Scholar]
- Terhivuo, J.; Saura, A. Dispersal and Clonal Diversity of North-European Parthenogenetic Earthworms. Biol. Invasions 2006, 8, 1205–1218. [Google Scholar] [CrossRef]
- Ruttner-Kolisko, A. Rheomorpha neiswestnovae und Marinellina flagellata, Zwei Phylogenetisch Interessante Wurmtypen Aus Dem Susswasserpsammon. Osterr. Zool. Z. 1955, 6, 33–69. [Google Scholar]
- Kisielewski, J. Two New Interesting Genera of Gastrotricha (Macrodasyida and Chaetonotida) from the Brazilian Freshwater Psammon. Hydrobiologia 1987, 153, 23–30. [Google Scholar] [CrossRef]
- Todaro, M.A.; Dal Zotto, M.; Jondelius, U.; Hochberg, R.; Hummon, W.D.; Kånneby, T.; Rocha, C.E.F. Gastrotricha: A Marine Sister for a Freshwater Puzzle. PLoS ONE 2012, 7, e31740. [Google Scholar] [CrossRef] [PubMed]
- Garraffoni, A.R.S.; Araújo, T.Q.; Lourenço, A.P.; Guidi, L.; Balsamo, M. Integrative Taxonomy of a New Redudasys Species (Gastrotricha: Macrodasyida) Sheds Light on the Invasion of Fresh Water Habitats by Macrodasyids. Sci. Rep. 2019, 9, 2067. [Google Scholar] [CrossRef] [PubMed]
- Kånneby, T.; Wicksten, M.K. First Record of the Enigmatic Genus Redudasys Kisielewski, 1987 (Gastrotricha: Macrodasyida) from the Northern Hemisphere. Zoosystema 2014, 36, 723–733. [Google Scholar] [CrossRef]
- Kånneby, T.; Kirk, J.J. A New Species of Redudasys (Gastrotricha: Macrodasyida: Redudasyidae) from the United States. Proc. Biol. Soc. 2017, 130, 128–139. [Google Scholar] [CrossRef]
- Balsamo, M.; d’Hondt, J.-L.; Kisielewski, J.; Pierboni, L. Global diversity of gastrotrichs (gastrotricha) in fresh waters. In Freshwater Animal Diversity Assessment; Balian, E.V., Lévêque, C., Segers, H., Martens, K., Eds.; Developments in Hydrobiology; Springer: Dordrecht, The Netherlands, 2008; Volume 198, pp. 85–91. [Google Scholar]
- Kisielewski, J. Inland-Water Gastrotricha from Brazil. Annal. Zool. 1991, 43, 1–168. [Google Scholar]
- Curini-Galletti, M.; Artois, T.; Delogu, V.; De Smet, W.H.; Fontaneto, D.; Jondelius, U.; Leasi, F.; Martínez, A.; Meyer-Wachsmuth, I.; Nilsson, K.S.; et al. Patterns of Diversity in Soft-Bodied Meiofauna: Dispersal Ability and Body Size Matter. PLoS ONE 2012, 7, e33801. [Google Scholar] [CrossRef]
- Corti, B. Osservazioni Microscopiche Sulla Tremella e Sulla Circolazione Del Fluido in Una Pianta Acquajuola; Rocchi: Lucca, Italy, 1774. [Google Scholar]
- Maggi, L. Primo Elenco Dei Rotiferi o Sistolidi Della Valcuvia. Atti Soc. Ital. Sci. Nat. Pavia 1878, 21, 320. [Google Scholar]
- Issel, R. Saggio Sulla Fauna Termale Italiana. Atti Accad. Sci. Torino 1901, 36, 3–15. [Google Scholar]
- Zacharias, O. Osservazioni Limnologiche Su Alcuni Laghi Italiani. Acquacolt. Lomb. 1908, 8, 5. [Google Scholar]
- Marcolongo, I. Primo Contributo Allo Studio Dei Gastrotrichi Nel Lago-Stagno Craterico Di Astroni. Monit. Zool. Ital. 1910, 21, 315–318. [Google Scholar]
- Marcolongo, I. I Gastrotrichi Del Lago-Stagno Craterico Di Astroni. Atti R. Accad. Sci. Fis. Mat. Napoli Ser. A 1914, 2, 15. [Google Scholar]
- Mola, P. Gastrotricha Delle Acque Dolci Italiane. Internat. Rev. Ges. Hydrobiol. Hydrograph. 1932, 26, 394–423. [Google Scholar] [CrossRef]
- Balsamo, M. Prime Ricerche Sui Gastrotrichi Dulciacquicoli Italiani. Atti Soc. Tosc. Sc. Nat. Mem. 1977, 84, 87–150. [Google Scholar]
- Balsamo, M. Gastrotrichi Della Toscana: Il Lago Di Sibolla. Boll. Mus. Civ. St. Nat. Verona 1980, 7, 547–571. [Google Scholar]
- Balsamo, M. Secondo Contributo Alla Conoscenza Dei Gastrotrichi Delle Acque Dolci Italiane. Atti Soc. Tosc. Sc. Nat. Mem. 1980, 87, 97–126. [Google Scholar]
- Balsamo, M. Three New Gastrotrichs from a Tuscan-Emilian Apennine Lake. Boll. Zool. 1982, 49, 287–295. [Google Scholar] [CrossRef]
- Balsamo, M. Gastrotrichs from Lakes Bolsena, Chiusi and Montepulciano (Central Italy), with the Description of Four New Species. Boll. Zool. 1990, 57, 165–178. [Google Scholar] [CrossRef]
- Fregni, E.; Balsamo, M.; Tongiorgi, P. Interstitial Gastrotrichs from Lotic Italian Fresh Waters. Hydrobiologia 1998, 368, 175–187. [Google Scholar] [CrossRef]
- Kånneby, T.; Todaro, M.A.; Jondelius, U. A Phylogenetic Approach to Species Delimitation in Freshwater Gastrotricha from Sweden. Hydrobiologia 2012, 683, 185–202. [Google Scholar] [CrossRef]
- Kånneby, T.; Todaro, M.A.; Jondelius, U. Phylogeny of Chaetonotidae and Other Paucitubulatina (Gastrotricha: Chaetonotida) and the Colonization of Aquatic Ecosystems. Zool. Scr. 2013, 42, 88–105. [Google Scholar] [CrossRef]
- Balsamo, M.; Tongiorgi, P. Gastrotricha. In Checklist delle Specie della Fauna d’Italia; Calderini: Bologna, Italy, 1995; pp. 1–11. [Google Scholar]
- Todaro, M.A.; Balsamo, M. Gastrotricha. In Checklist of the Italian Fauna; Società Italian di Biologia Marina: Genova, Italy, 2021. [Google Scholar]
- Campos, A.; Garraffoni, A.R.S. A Synopsis of Knowledge, Zoogeography and an Online Interactive Map of Brazilian Marine Gastrotrichs. PeerJ 2019, 7, e7898. [Google Scholar] [CrossRef] [PubMed]
- Grünspan, T. Beiträge Zur Systematik der Gastrotrichen. Zool. Jahrb. Abt. Syst. 1908, 26, 214–256. [Google Scholar]
- Manfredi, P. Brevi Note Intorno Alla Fauna Della Gora Di Bertonico. Ann. Biol. Lacustre 1927, 15, 110–117. [Google Scholar]
- Balsamo, M.; Kisielewski, J. Chaetonotus Fluviatilis, a New Freshwater Gastrotrich. Boll. Zool. 1986, 53, 111–114. [Google Scholar] [CrossRef]
- Balsamo, M.; Todaro, M.A. Life History Traits of Two Chaetonotids (Gastrotricha) Under Different Experimental Conditions. Invertebr. Reprod. Dev. 1988, 14, 161–176. [Google Scholar] [CrossRef]
- Bertolani, R.; Balsamo, M. Tardigradi e Gastrotrichi Del Trentino: Il Lago Di Tovel. Studi Trentini Sci. Nat. 1989, 65, 83–93. [Google Scholar]
- Balsamo, M.; Fregni, E.; Tongiorgi, P. Marine and Freshwater Gastrotricha from the Island of Montecristo (Tuscan Archipelago, Italy), with the Description of New Species. Boll. Zool. 1994, 61, 217–227. [Google Scholar] [CrossRef]
- Balsamo, M.; Fregni, E. Gastrotrichs from Interstitial Fresh Water, with a Description of Four New Species. Hydrobiologia 1995, 302, 163–175. [Google Scholar] [CrossRef]
- Balsamo, M.; Todaro, M.A. Gastrotrichi Del Trentino: Viotte Del Monte Bondone. Studi Trentini Sci. Nat. 1995, 70, 9–22. [Google Scholar]
- Leasi, F.; Rothe, B.H.; Schmidt-Rhaesa, A.; Todaro, M.A. The Musculature of Three Species of Gastrotrichs Surveyed with Confocal Laser Scanning Microscopy (CLSM). Acta Zool. 2006, 87, 171–180. [Google Scholar] [CrossRef]
- Leasi, F.; Todaro, M.A. The Muscular System of Musellifer delamarei (Renaud-Mornant, 1968) and Other Chaetonotidans with Implications for the Phylogeny and Systematization of the Paucitubulatina (Gastrotricha). Biol. J. Linn. Soc. 2008, 94, 379–398. [Google Scholar] [CrossRef]
- Schwank, P. Gastrotricha. In Susswasserfauna von Mitteleuropa; Schwoerbel, J., Zwick, P., Eds.; Gustav Fisher Verlag: Stuttgart, Germany, 1990; p. 252. [Google Scholar]
- WoRMS—World Register of Marine Species. Available online: https://www.marinespecies.org/ (accessed on 14 January 2023).
- Kolicka, M.; Dabert, M.; Olszanowski, Z.; Dabert, J. Sweet or Salty? The Origin of Freshwater Gastrotrichs (Gastrotricha, Chaetonotida) Revealed by Molecular Phylogenetic Analysis. Cladistics 2020, 36, 458–480. [Google Scholar] [CrossRef] [PubMed]
- QGIS.org. QGIS Geographic Information System. QGIS Association. 2023. Available online: http://www.qgis.org (accessed on 24 September 2023).
- ISTAT—Confini delle Unità Amministrative a Fini Statistici al 1° Gennaio. 2023. Available online: https://www.istat.it/it/archivio/222527 (accessed on 14 January 2023).
- Ehrenberg, C.G. Die infusionsthierchen als vollkommene organismen. In Ein Blick in das Tiefere Organische Leben der Natur; L. Voss: Leipzig, Germany, 1838. [Google Scholar]
- Kolicka, M. New Chaetonotus (Wolterecka semovitus sp. nov. (Gastrotricha: Chaetonotida: Chaetonotidae) from a Palm House in Vienna (Austria). Annal. Zool. 2019, 69, 447. [Google Scholar] [CrossRef]
- Rataj Križanová, F.; Vďačný, P. A Huge Undescribed Diversity of the Subgenus Hystricochaetonotus (Gastrotricha, Chaetonotidae, Chaetonotus) in Central Europe. Eur. J. Taxon. 2022, 840, 1–93. [Google Scholar] [CrossRef]
- Grilli, P.; Kristensen, R.M.; Balsamo, M. Contribution to the Knowledge of Freshwater Gastrotricha from Denmark. Steenstrupia 2010, 32, 79–92. [Google Scholar]
- Grilli, P.; d’Hondt, J.L.; Balsamo, M. Contribution à La Connaissance Des Gastrotriches (Gastrotricha) Dulcicoles Des Eaux Françaises. Bull. Soc. Zool. Fr. 2010, 135, 93–107. [Google Scholar]
- Schmidt-Rhaesa, A. German Freshwater Gastrotricha. Gastrotricha World Portal. 2006. Available online: http://www.gastrotricha.unimore.it/ (accessed on 5 October 2023).
- Kolicka, M. Polish Freshwater Gastrotricha. Gastrotricha World Portal. 2015. Available online: http://www.gastrotricha.unimore.it/ (accessed on 5 October 2023).
- Rudescu, L. Gastrotricha; Academiei Republicii Socialiste România: Bucharest, Romania, 1967. [Google Scholar]
- Kånneby, T. Swedish Freshwater Gastrotricha. Gastrotricha World Portal. 2014. Available online: http://www.gastrotricha.unimore.it/ (accessed on 5 October 2023).
- Lee, J.M. Biodiversity of Gastrotricha from Korea. In Proceedings of the Joint Academic Conference of the Korea Association of Marine Science and Technology, Jeju-si, Republic of Korea, 21–23 May 2015. [Google Scholar]
- Kånneby, T. Canadian Freshwater Gastrotricha. Gastrotricha World Portal. 2014. Available online: http://www.gastrotricha.unimore.it/ (accessed on 5 October 2023).
- Kånneby, T.; Weiss, M.J. U.S. Freshwater Gastrotricha. Gastrotricha World Portal. 2016. Available online: http://www.gastrotricha.unimore.it/ (accessed on 5 October 2023).
- Kirk, J.J. Lepidodermella weissi, New Species (Gastrotricha: Chaetonotida: Chaetonotidae) from Northwestern Oregon, U.S.A. Proc. Biol. Soc. Wash. 2021, 134, 116–126. [Google Scholar] [CrossRef]
- Bizzarri, N.; Todaro, M.A. Brazilian Freshwater Gastrotricha. Gastrotricha World Portal. 2010. Available online: http://www.gastrotricha.unimore.it/ (accessed on 5 October 2023).
- Garraffoni, A.R.S.; Araújo, T.Q. Chave de Identificação de Gastrotricha de Águas Continentais e Marinhas Do Brasil. Pap. Avulsos Zool. 2010, 50, 535–552. [Google Scholar] [CrossRef]
- Garraffoni, A.R.S.; Melchior, M.P. New Species and New Records of Freshwater Heterolepidoderma (Gastrotricha: Chaetonotidae) from Brazil with an Identification Key to the Genus. Zootaxa 2015, 4057, 551. [Google Scholar] [CrossRef] [PubMed]
- Minowa, A.K.; Garraffoni, A.R.S. A New Species of Haltidytes Remane, 1936 (Gastrotricha: Chaetonotida: Dasydytidae) from an Urban Lagoon in Brazil with a Phylogenetic Reconstruction of the Genus Based on Morphological Data. Zool. Anz. 2017, 269, 100–109. [Google Scholar] [CrossRef]
- Garraffoni, A.R.S.; Araújo, T.Q.; Lourenço, A.P.; Guidi, L.; Balsamo, M. A New Genus and New Species of Freshwater Chaetonotidae (Gastrotricha: Chaetonotida) from Brazil with Phylogenetic Position Inferred from Nuclear and Mitochondrial DNA Sequences. Syst. Biodivers. 2017, 15, 49–62. [Google Scholar] [CrossRef]
- Minowa, A.K.; Garraffoni, A.R.S. Assessing Biodiversity Shortfalls of Freshwater Meiofauna from the Atlantic Forest: New Species, Distribution Patterns and the First Total-Evidence Phylogeny of Semiplanktonic Gastrotricha. Mol. Phylogenet. Evol. 2020, 152, 106926. [Google Scholar] [CrossRef]
- Guidetti, E.B.; Campos, A.; Batistão, A.R.; Silva, A.T.D.; Bilatto, C.G.; Salgado, K.A.; Araújo, T.Q.; Garraffoni, A.R.S. Gastrotrichs and Tardigrades in a Remnant of Atlantic Forest (Serra Do Japi, SP, Brazil). Biota Neotrop. 2021, 21, e20201165. [Google Scholar] [CrossRef]
- Magpali, L.; Machado, D.R.P.; Araújo, T.Q.; Garraffoni, A.R.S. Long Distance Dispersal and Pseudo-Cryptic Species in Gastrotricha: First Description of a New Species (Chaetonotida, Chaetonotidae, Polymerurus) from an Oceanic Island with Volcanic Rocks. Eur. J. Taxon. 2021, 746, 62–93. [Google Scholar] [CrossRef]
- Minowa, A.; Senna Garraffoni, A.R. Seek and You Shall Find: New Species of the Rare Genus Ornamentula (Gastrotricha: Chaetonotida) and First Record Outside of Type-Locality. Zoologia 2021, 38, 1–9. [Google Scholar] [CrossRef]



| Order CHAETONOTIDA Remane, 1925 Rao & Clausen, 1970 |
| Suborder PAUCITUBULATINA d’Hondt, 1971 |
| Family Chaetonotidae Zelinka, 1889 |
| Genus Aspidiophorus Voigt, 1904 |
| Aspidiophorus heterodermus Saito, 1937 |
| Aspidiophorus oculifer Kisielewski, 1981 |
| Aspidiophorus ophiodermus Balsamo, 1982 |
| Aspidiophorus paradoxus (Voigt, 1902) |
| Aspidiophorus squamulosus Roszczak, 1936 |
| Genus Chaetonotus Ehrenberg, 1830 |
| Chaetonotus acanthocephalus Valkanov, 1937 |
| Chaetonotus acanthodes Stokes, 1887 |
| Chaetonotus acanthophorus Stokes, 1887 |
| Chaetonotus aemilianus Balsamo, 1978 |
| Chaetonotus arethusae Balsamo & Todaro, 1995 |
| Chaetonotus balsamoe Kisielewski, 1997 |
| Chaetonotus benacensis Balsamo & Fregni, 1995 |
| Chaetonotus bisacer Greuter, 1917 |
| Chaetonotus brachyurus Balsamo, 1981 |
| Chaetonotus brevispinosus Zelinka, 1889 |
| Chaetonotus cestacanthus Balsamo, 1990 |
| Chaetonotus chuni Voigt, 1901 |
| Chaetonotus daphnes Balsamo & Todaro, 1995 |
| Chaetonotus decemsetosus Marcolongo, 1910 |
| Chaetonotus disjunctus Greuter, 1917 |
| Chaetonotus dracunculus Balsamo, 1990 |
| Chaetonotus enormis Stokes, 1887 |
| Chaetonotus fluviatilis Balsamo & Kisielewski, 1986 |
| Chaetonotus heideri Brehm, 1917 |
| Chaetonotus heterospinosus Balsamo, 1978 |
| Chaetonotus hirsutus Marcolongo, 1910 |
| Chaetonotus hystrix Metschnikoff, 1865 |
| Chaetonotus italicus Balsamo & Todaro, 1995 |
| Chaetonotus laroides Marcolongo, 1910 |
| Chaetonotus larus (Müller, 1773) |
| Chaetonotus longispinosus Stokes, 1887 |
| Chaetonotus lunatospinosus Balsamo, 1981 |
| Chaetonotus macrochaetus Zelinka, 1889 |
| Chaetonotus maximus Ehrenberg, 1830 |
| Chaetonotus microchaetus Preobrajenskaia, 1926 |
| Chaetonotus minimus Marcolongo, 1910 |
| Chaetonotus multispinosus Grünspan, 1908 |
| Chaetonotus mutinensis Balsamo, 1978 |
| Chaetonotus naiadis Balsamo & Todaro, 1995 |
| Chaetonotus octonarius Stokes, 1887 |
| Chaetonotus oculifer Kisielewski, 1981 |
| Chaetonotus oplites Balsamo & Fregni, 1995 |
| Chaetonotus paucisetosus Marcolongo, 1910 |
| Chaetonotus pentacanthus Balsamo, 1981 |
| Chaetonotus persetosus Zelinka, 1889 |
| Chaetonotus polyspinosus Greuter, 1917 |
| Chaetonotus poznaniensis Kisielewski, 1981 |
| Chaetonotus pungens Balsamo, 1990 |
| Chaetonotus robustus Davison, 1938 |
| Chaetonotus schultzei Metchnikoff, 1865 |
| Chaetonotus similis Zelinka, 1889 |
| Chaetonotus simrothi Voigt, 1909 |
| Chaetonotus sphagnophilus Kisielewki, 1981 |
| Chaetonotus spinulosus Stokes, 1887 |
| Chaetonotus succinctus Voigt, 1901 |
| Chaetonotus trispinosus Balsamo, 1990 |
| Chaetonotus uncinus Voigt, 1902 |
| Genus Fluxiderma d’Hondt, 1974 |
| Fluxiderma concinnum (Stokes, 1887) |
| Genus Heterolepidoderma Remane, 1927 |
| Heterolepidoderma brevitubulatum Kisielewski, 1981 |
| Heterolepidoderma gracile Remane, 1927 |
| Heterolepidoderma lamellatum Balsamo & Fregni, 1995 |
| Heterolepidoderma macrops Kisielewski, 1981 |
| Heterolepidoderma majus Remane, 1927 |
| Heterolepidoderma multiseriatum Balsamo, 1978 |
| Heterolepidoderma ocellatum (Metschnikoff, 1865) |
| Heterolepidoderma pineisquamatum Balsamo, 1981 |
| Genus Ichthydium Ehrenberg, 1830 |
| Ichthydium diacanthum Balsamo & Todaro, 1995 |
| Ichthydium forcipatum Voigt, 1901 |
| Ichthydium forficula Remane, 1927 |
| Ichthydium fossae d’Hondt, 1971 |
| Ichthydium plicatum Balsamo & Fregni, 1995 |
| Ichthydium podura (Müller, 1773) |
| Ichthydium squamigerum Balsamo & Fregni, 1995 |
| Ichthydium tanytrichum Balsamo, 1983 |
| Genus Lepidochaetus Kisielewski, 1991 |
| Lepidochaetus zelinkai (Grünspan, 1908) |
| Genus Lepidodermella Blake, 1933 |
| Lepidodermella squamata (Dujardin, 1841) |
| Genus Polymerurus Remane, 1927 |
| Polymerurus nodicaudus (Voigt, 1901) |
| Polymerurus nodifurca (Marcolongo, 1910) |
| Polymerurus rhomboides (Stokes, 1887) |
| Polymerurus serraticaudus (Voigt, 1901) |
| Genus Romballichthys Schwank, 1990 |
| Romballichthys punctatus (Greuter, 1917) |
| Family Dasydytidae Daday, 1905 |
| Genus Anacanthoderma Marcolongo, 1910 |
| Anacanthoderma paucisetosum (Marcolongo, 1910) |
| Anacanthoderma punctatum Marcolongo, 1910 |
| Genus Chitonodytes Remane, 1967 |
| Chitonodytes longisetosus (Metschnikoff, 1865) |
| Genus Dasydytes Gosse, 1851 |
| Dasydytes ornatus Voigt, 1909 |
| Genus Haltidytes Remane, 1936 |
| Haltidytes crassus (Greuter, 1917) |
| Haltidytes saltitans (Stokes, 1887) |
| Genus Setopus Grünspan, 1908 |
| Setopus tongiorgii (Balsamo, 1983) |
| Genus Stylochaeta Hlava, 1904 |
| Stylochaeta fusiformis (Spencer, 1890) |
| Stylochaeta stylifera (Voigt, 1901) |
| Family Dichaeturidae Remane, 1927 |
| Genus Dichaetura Lauterborn, 1913 |
| Dichaetura capricornia (Metschnikoff, 1865) |
| Family Neogosseidae Remane, 1927 |
| Genus Neogossea Remane, 1927 |
| Neogossea antennigera (Gosse, 1851) |
| Region | N. Localities and (Sites) | N. Species | N. Genera | N. Families | N. Type Localities |
|---|---|---|---|---|---|
| Abruzzo | - | - | - | - | - |
| Basilicata | - | - | - | - | - |
| Calabria | - | - | - | - | - |
| Campania | 1 (1) | 18 | 4 | 2 | 1 |
| Emilia Romagna | 14 (18) | 28 | 8 | 1 | 3 |
| Friuli Venezia Giulia | - | - | - | - | - |
| Lazio | 5 (7) | 22 | 7 | 1 | 3 |
| Liguria | 1 (1) | 2 | 1 | 1 | 0 |
| Lombardia | 3 (3) | 12 | 5 | 2 | 0 |
| Marche | - | - | - | - | - |
| Molise | - | - | - | - | - |
| Piemonte | - | - | - | - | - |
| Puglia | - | - | - | - | - |
| Sardegna | 21 (24) | 34 | 13 | 3 | 0 |
| Sicilia | - | - | - | - | - |
| Toscana | 10 (12) | 41 | 11 | 2 | 5 |
| Trentino Alto Adige | 4 (14) | 27 | 9 | 2 | 4 |
| Umbria | - | - | - | - | - |
| Valle d’Aosta | - | - | - | - | - |
| Veneto | 2 (2) | 15 | 4 | 1 | 1 |
| Italy | 61 (81) | 92 | 17 | 3 | 17 |
| Region | N. Specie per Locality (mean ± s.d.) | Most Common Species (% Localities) | N. Endemites |
|---|---|---|---|
| Abruzzo | - | - | - |
| Basilicata | - | - | - |
| Calabria | - | - | - |
| Campania | 18 ± N.A. | N.A. | 4 |
| Emilia Romagna | 4.57 ± 3.08 | C. aemilianus (12.5%) | 3 |
| Friuli Venezia Giulia | - | - | - |
| Lazio | 9.40 ± 5.50 | L. squamata (14.9%) | 1 |
| Liguria | 2 ± N.A. | N.A. | 0 |
| Lombardia | 4.67 ± 5.51 | N.A. | 0 |
| Marche | - | - | - |
| Molise | - | - | - |
| Piemonte | - | - | - |
| Puglia | - | - | - |
| Sardegna | 1.81 ± 0.93 | N.A. | 0 |
| Sicilia | - | - | - |
| Toscana | 7.60 ± 6.24 | N.A. | 3 |
| Trentino Alto Adige | 12.75 ± 15.24 | C. hystrix (13.7%) | 0 |
| Umbria | - | - | - |
| Valle d’Aosta | - | - | - |
| Veneto | 8 ± 9.90 | H. ocellatum (12.5%) | 1 |
| Italy | 5.33 ± 6.13 | L. squamata (8.4%) | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saponi, F.; Todaro, M.A. Status of the Italian Freshwater Gastrotricha Biodiversity, with the Creation of an Interactive GIS-Based Web Map. Diversity 2024, 16, 17. https://doi.org/10.3390/d16010017
Saponi F, Todaro MA. Status of the Italian Freshwater Gastrotricha Biodiversity, with the Creation of an Interactive GIS-Based Web Map. Diversity. 2024; 16(1):17. https://doi.org/10.3390/d16010017
Chicago/Turabian StyleSaponi, Francesco, and M. Antonio Todaro. 2024. "Status of the Italian Freshwater Gastrotricha Biodiversity, with the Creation of an Interactive GIS-Based Web Map" Diversity 16, no. 1: 17. https://doi.org/10.3390/d16010017
APA StyleSaponi, F., & Todaro, M. A. (2024). Status of the Italian Freshwater Gastrotricha Biodiversity, with the Creation of an Interactive GIS-Based Web Map. Diversity, 16(1), 17. https://doi.org/10.3390/d16010017









