Abstract
Freshwater pearl mussels (FPMs, Margaritifera margaritifera, Linnaeus, 1758) are endangered and particularly vulnerable to climate change. To create effective conservation strategies, we studied their thermal tolerance and the impact of elevated water temperatures on growth and survival. Our experiments included field mesocosm studies in five FPM-streams in the Vogtland region (Germany) (2016 to 2020), as well as laboratory experiments at temperatures ranging from 1 to 26 °C. Growth of juvenile FPMs increased significantly within a temperature gradient from 12 to 21 °C. In the streams, maximum growth was 8.9 µm/d in surface water and 6.5 µm/d in the interstitial. The upper thermal tolerance for the mussels ranged from 22.1 to 22.9 °C, resulting in low survival during hot summer periods in 2018 and 2019. Warming during winter (+5 °C) did not significantly affect growth and survival, but survival during winter increased with the pre-overwintering shell length. Exceeding a shell length of about 1100 µm in December indicating gill development corelated to 50% survival. Shell length in December is primarily controlled by growth depending on water temperatures during summer. These findings define the thermal niche of juvenile FPMs (average summer temperatures of 14.5–21 °C) and have implications for water management, conservation strategies, and site selection for releasing captive-breeding mussels.
Keywords:
freshwater pearl mussel; thermal threshold; growth; survival; water temperature; summer; winter 1. Introduction
Global surface temperatures were 1.59 °C (over land) higher in 2011–2020 than from 1850–1900 [1] resulting in extreme heat events that caused widespread adverse impacts of terrestrial, freshwater, and ocean ecosystems worldwide. Global warming over the 21st century could range from 1.5 to 4 °C according to global modeled emissions pathways [1]. Climate warming in the northern temperate region is particularly pronounced in changes in thermal regimes and seasonality, with hot and longer summer and warmer winter and spring periods [1,2]. Besides temperature, climate warming affects precipitation and runoff patterns, thus causing episodes of flooding and droughts [3,4,5]. Water management often amplifies the negative impacts of climate warming on the thermal regimes of freshwater ecosystems by riparian tree removal, damming, hydroelectric schemes, thermal pollution, and changes in land use; thus, deviations from natural thermal conditions are expected to increase [6]. Increasing temperatures can exacerbate eutrophication symptoms in freshwater systems [7] that may be detrimental to freshwater organisms [5,8,9]. These changes have profound effects on the distribution of freshwater species [6] as well as phenology, species fitness, and survival, especially for ectotherms [10]. The thermal niche is generally those at which physiological functions are optimal and at which growth and fitness is maximized, if food availability is not limiting [11,12]. Therefore, water temperature is an important niche dimension for ectotherms [13]. Climate has long been considered among the strongest determinants of species distribution, often imposing physiological limits on where a species can occur.
The freshwater pearl mussel (FPM, Margaritifera margaritifera (Linnaeus, 1758)) is a Holarctic ectotherm that was widespread from the Holocene to the beginning of the 20th century [14]. During the last century, FPM populations have collapsed across much of their geographic range and are now among the most critically threatened freshwater organisms worldwide [14,15,16]. Scotland is considered to be a global stronghold for endangered M. margaritifera, supporting a significant proportion of the world’s extant natural populations [15]. However, it is apparent that >50% of Scottish populations may not be recruiting at sustainable levels. The declines in European FPM populations have been attributed to a wide array of human activities resulting in habitat destruction/alteration, siltation, water pollution, population fragmentation as well as declining of host fish populations, e.g., [5,15,17,18,19,20,21]. Considerable attention has been directed to monitor streams and to assess the effects of anthropogenic activities on the FPM population during the last decades [15]. Some threshold values of water-quality parameters (i.e., dissolved oxygen, acid-base chemistry, toleration of calcium ions in the water, and nutrient levels), habitat characteristics, and host fish abundances were identified and quantified by several studies that are summarized for European FPM populations in the European CEN standard [21]. Nevertheless, the identification of key stressors and drivers at particular localities was only partially successful with regard to the many changes that freshwater systems have undergone within the past century and a variety of factors potentially limiting species distribution. The fact that less experimental data are available causes uncertainty in identifying the causal mechanisms responsible for the observed decline in FPMs and targeting conservation strategies. Therefore, conservation measures were rarely successful (with less exceptions, e.g., River Lutter, [22]) and the collapse of FPM populations in Europe continued regardless of considerable restauration efforts in the streams during the past decades. Hence, it is very important to improve conservation strategies as freshwater mussel are important players in their aquatic ecosystem (Umbrella species) that provide important ecosystem services [14].
Hastie et al. [3] postulated that climate change must be considered a real threat to extant FPM populations. Effective conservation strategies require research to identify the effects of water temperature on reproduction, growth, and survival of FPMs, e.g., [3,23]. While it is crucial to understand the responses of FPMs for predicting the potential impacts of global warming, there has been relatively limited focus on assessing the effects of climatic warming on European FPMs. FPMs may be particularly sensitive to rapid climate change because: (i) they are adapted to cold-stenothermic environments [9]; (ii) adults have patchy distributions and limited mobility [24,25]; (iii) their complex life history [26] with the larva being highly depended on thermal sensitive host fishes (Salmo salar, Linnaeus, 1758 and Salmo trutta, Linnaeus, 1758) [14,18]; and (iv) they have long generation times [27] that reduce the likelihood of quick adaptation. Hastie et. al. [3] postulated a considerable degree of thermal tolerance for FPMs as natural mussel populations were alive in small streams in Scotland when water temperatures varied from 0 to 25 °C. Environmentally relevant estimates of upper thermal tolerances in FPM during summer and winter are urgently needed to assess the extent of changes in FPM populations that can be expected in response to global warming. Pandolfo et al. [28] postulated that (i) freshwater mussels in North America already might be living close to their upper thermal tolerances in some systems and (ii) acclimation temperature did not affect thermal tolerance for either life stage. Due to climate warming, we expect increases in extreme thermal events in headwater streams with low thermic buffer ability, which in turn might push FPMs toward their upper thermal tolerance limits [3]. Elevated water temperatures may amplify the negative effects of anthropogenic changes occurring throughout the watershed upstream of the FPMs and the surrounding area [3,5,9]. Although the temperature threshold is clearly a crucial indicator to define the thermal niche of FPMs, the critical upper and lower thermal limits for survival and normal functioning in this species are unknown. This knowledge might be, however, a precondition for selecting release sites of captive-breed FPMs and to adapt prospective conservation strategies to climate warming.
FPMs have a complex life cycle with a parasitic larval stage (glochidium) on gills of a specific host fish, a long juvenile period (12–20 years), and a 60 to 270 years lasting adult stage with continuous reproduction [26]. Mostly during the summer, the glochidia are released from the female mussel [3,29]. Then, the parasitic larvae attach and encyst on the gills of their host fish (only brown trout in Germany) and metamorphose into juveniles. Following 9–11 months, juveniles drop off from the host fish and settle onto the riverbed, where they spend at least the following 5 years. Optimal microhabitats for juvenile mussels are boulder-stabilized refugia containing sand or gravel for burrowing [30]. The first year after excystment constitutes the most sensitive life stage of FPMs to environmental stress [14,31,32,33] making this stage a preferable indicator to evaluate temperature effects [3,34]. The high sensitivity of FPMs during this stage may result from complex changes in the morphogenesis of the gills that correlate with changes in their feeding strategies [35]. Juvenile mussels may have different biological and environmental requirements depending upon their mode of feeding, and mortality may increase when developmental changes occur [36] due to an inability to meet energetic demands during intense morphogenesis [37]. Survival is the most common endpoint used for ecological and ecotoxicological studies of mussels [24]. Growth has been proven to be a sensitive parameter for assessing mussel responses to habitat quality [33]. The upper lethal temperature (LT50) is a metric frequently used to assess an organism’s thermal tolerance by estimating the temperature that is lethal to 50% of the population [28].
Given all these traits, FPMs provide a good opportunity to study consequences of climate change but our ability to accurately assess the effects of thermal disturbance on European FPMs is limited. Previous experimental and observational studies have examined growth and survival of freshwater mussels in captivity and in the wild in North America (see review [38]). But there are a few studies that examined the effects of temperature on survival or growth of juvenile FPMs [39,40,41,42,43]. While the majority of climate change research is focused on the growing season, less is known about the effects of temperature on mussel fitness during autumn or winter. Only Buddensiek [39] studied the potential influence of mussel size on survival after exposure of juvenile FPMs to headwater streams in Czechia during the winter. The aim of the present study is to assess the vulnerability to climate change of age-0 FPMs by (i) describing the direct temperature effects on growth and survival during summer and winter; (ii) analyzing time-delayed effects on survival during the first winter, and (iii) identifying the lower and upper temperature threshold to understand the effect of water temperature on survival and growth of FPMs during the first year after excystment, hereafter referred as age-0 mussels.
Buddensiek [39] stated that temperature effects are difficult to determine in field studies because the accelerated growth might be caused by temperature effects on metabolism or changes in food quality or uptake as observed later by Brauns et al. [44] or Grunicke et al. [45]. Therefore, our study combines field mesocosm studies in five streams and across four years with laboratory experiments at summer and winter conditions in semi-natural but controlled and replicated conditions. This experimental setup combining the research advantages from laboratory and field mesocosm experiments provides repeatability in a controlled setting, such as strict application of temperatures with the environmental complexity of field conditions. To address the thermal effects on age-0 FPMs, we determined growth and survival at different temperature regimes. We hypothesize that (i) the cold-stenothermic FPMs live close to their upper temperature limits during years with long-lasting hot and drought periods as observed during the summer 2018 and 2019; (ii) warming during winter periods will reduce survival; and (iii) survival rates during the first winter increase with shell length as a time-delayed effect of growth during summer. The successful re-establishment of viable FPM populations requires a holistic understanding of their ecological requirements, thermal niche, life history, and population dynamics.
2. Materials and Methods
2.1. Study Area
The study was conducted as a survey from June 2016 to April 2020, in five second-order mountain streams located in the Vogtland region (Saxony, Germany, altitudes between 500–600 m above sea level). All streams had historically large, viable populations of FPMs [46] that have been nearly extinct since 2010 [47]. To retain the genetic-potential of FPMs [48], a breeding station was established in the Vogtland region (Raun, Germany) in 2002 [49]. The rearing program has yielded promising results in releasing and the re-establishment of juvenile FPMs in the streams with historic FPM populations [49]. All studied mountain streams are tributaries of the Weiße Elster, a 257 km long stream with a catchment area of 5154 km2. The studied streams are not oligotrophic, but most stretches correspond to good ecological status concerning the Water Framework Directive (2000/60/EC). The headwater streams meander through meadows with riparian trees, agricultures, forests (mainly spruce plantations), and few small villages [44]. The five investigated streams (referred to as S1, S2, S3, S4, S5) are situated in close proximity to each other, and show similar abiotic and hydrological conditions such as width (1–3 m) or flow velocity (0.05–1.3, mean 0.44 ± 0.26 m/s). The riverbeds are covered with sand, gravel, or smaller stones and with fine sediment deposition in areas with reduced flow [25]. During our study period (2016–2020), there were high inter-annual variations in temperature, precipitation, and hydrology including both extended drought periods and exceptionally large floods (100-year return flood) partly in close succession. There were, however, no changes in general habitat quality due to construction sites or large-scale improvements that might explain any present patterns.
2.2. Experimental Specimens
As FPMs are protected under national legislation and within the European Union (IUCN, 2022), our experimental studies were performed with FPMs from a captive-breeding program in Saxony [49], applying standard propagation and culture methods following Hruška [50], modified after [45,47]. Host fish (Salmo trutta) from a local hatchery were infested with glochidia that were collected yearly in July or August from gravid females of a regional free-living, natural FPM population and were kept for 8-months at the fish farming station (Rösch, Bärnau, Germany). After glochidia grow and metamorphose on the gills into a tiny mussel, the fish were transferred to the mussel rearing station (Raun, Saxony, Germany) and excystment occurred when mussels reached a length of ~350 µm usually form April to May. The post-parasitic mussels were kept for the first 1–2 months in small plastic boxes (500 mL) at water temperatures between 15–16 °C. They were fed with a detritus–algae suspension [45]. The food mixture was refilled twice a week and dead mussels were removed. Mussels exceeding a shell length of about 600 µm were transferred randomly either to field mesocosms installed in each of the five streams or to the laboratory beakers at the Institute of Hydrobiology (Technical University of Dresden, Germany). Recent advances in mussel culturing produced large numbers of juveniles of similar size, age, and origin, which were necessary for fully replicated laboratory and mesocosm studies. Mussels from the rearing station placed in the experimental setups differed by no more than 2 weeks in age and <100 µm in shell length.
2.3. Temperature Measurement and Meteorological Data
Air temperatures (daily mean values) and daily precipitation for the study area were obtained from the SKlima database (http://sklima.de, accessed on 25 June 2021). Values were extracted from a meteorological station located in Elster, Bad-Sohl (E12.2736, N50.2706, altitude: 560 m). We used a historical base period (1961–1990) as the reference period for assessing climate change. Automatic temperature loggers (HOBO TidbiT MX Temperature 400′ Data Logger, Onset Computer Corporation, Bourne, Massachusetts, USA) were used to obtain water temperatures. Data loggers recorded temperatures every hour with a precision of ±0.1 degrees C. For each study site, a temperature logger was attached close to the mussel cages about 5 cm above the riverbed. A second logger was buried into the interstitial at a sediment depth of 3–5 cm.
2.4. Mesocosm Field Study: Cage Experiments
We placed age-0 mussels (first year after excystment) in two types of field mesocosm systems: (i) cages slightly modified after Buddensiek [39]; and (ii) substrate tubes (modified after [51]). Previous works confirmed that Buddensiek’s [39] cages are an appropriate system to keep juvenile mussels and to assess water-quality effects on juvenile growth and survival [33,47]. The modified Buddensiek cage system consisted of three perforated plates (polyacrylic, 125 × 85 mm with 50 holes of 10 mm diameter) and plastic gauzes (250 µm-mesh) placed between the plates and the two outer plates to create small compartments for the juvenile mussels (Figure 1A). Each compartment contained one or two mussels (in total 30–60 individuals per cage). To prevent food limitation, cages were exposed to the respective stream one week before the experiment to enable biofilm growth. The cages were fixed in the surface water (hereafter referred as surface water cage, SWC) facing moderate flow velocity (0.24 ± 0.14 m/s) at 0.2–0.4 m water depth on sites with stable gravel substrate about 3–5 cm above the sediment using iron rods (Figure 1A). The SWCs were cleaned at 7- to 14-day intervals to ensure that the gauzes remained permeable to the water current. In situ exposure of juvenile mussels was performed in five streams with three replicates and started annually in June (2016–2019) with in total 15 SWCs. The age (number of months passed after excystment), the mean length, and initial total number of the mussels exposed to the SWCs (Table 1) varied between the years (2016: 2 months, 733 ± 49 µm, n_ind = 225; 2017: 1.8 months, 813 ± 61 µm, n_ind = 450; 2018: 1.2 months, 660 ± 69 µm, n_ind = 900; 2019: 1.5 months, 650 ± 99 µm, n_ind = 750).

Figure 1.
Our study combines field mesocosm studies (A,B) with laboratory experiments (100 mL-beaker) (C) at summer and winter conditions in semi-natural but controlled and replicated conditions. Age-0 mussels (first year after excystment) were exposed to two types of field mesocosm systems: (A) cages slightly modified after Buddensiek [39] which are fixed in the surface water (hereafter referred as surface water cage, SWC) and (B) substrate tubes (modified after [51]) buried at 3–5 cm deep into the interstitial (hereafter referred as interstitial tubes, IT).

Table 1.
Experimental design to analyze growth and survival of FPMs during the first year after excystment (referred as age-0 mussels) with field mesocosms and laboratory studies.
To simulate in situ conditions resembling natural habitats of wild juvenile mussels (hyporheic zone), we used a second type of field mesocosm that was modified after Dury et al. [51]. Mussels were placed into cylindrical substrate tubes (protective cover for aquarium filters made of stainless steel, length 6.5 cm, diameter 2 cm, mesh size 400 µm) (Figure 1B). Tubes contain stream-specific gravel of grain size from 2 to 6.3 mm and 50 or 25 mussels, respectively. The top was sealed using plastic mesh (mesh size 250 µm) and zip ties. Six to nine tubes were buried in each of the five streams clustered in groups of three at 3–5 cm deep into the interstitial (hereafter referred as interstitial tubes, IT) in 2018 and 2019. We selected sites for IT exposure where the riverbed consisted mainly of gravel and small stones to ensure a good flow through the hyporheic zone (i.e., no obvious mud or silt layer on the sediment). The surrounding permeable gauze ensured the continuous inflow and outflow of interstitial water though the ITs and thus a supply of natural food particles as well as the removal of metabolic waste products. Before the experiments started, we determined the age, mean length, and total number of the mussels (n_ind) for all ITs (n_IT) (2018: 2 month, 797 ± 77 µm, n_ind = 2250, n_IT = 45; 2019: 2 month, 785 ± 57 µm, n_ind = 750, n_IT = 30) (Table 1).
ITs and SWCs were placed within a 5 to 10 m2 area in the study sites. After a 2- or 3-month exposure of mussels in the field mesocosms (Table 1), cages were carefully removed and opened at the end of August each year. Mussels were separated from gravel and detritus. Each mussel was photographed at the start and at the end of the experiment to measure shell length to the nearest 10 μm using the image analysis software ImageJ [52]. After counting living mussels using a binocular microscope and removing dead mussels, SWCs and ITs were placed in their respective streams until the next monitoring in November (before winter) and in April (after the first winter). In 2017, mussels in SWCs were measured at the end of November to determine growth during autumn (from September to November). According to the number of mussels surviving the summer period, replicates of SWCs or ITs were reduced in 2018 and 2019 and mussels of similar age and stream-history were pooled in one or two cages. Therefore, the total number of cages (n_SWC or n_IT) available for the field mesocosm studies of the age-0 FPMs during the winter period was as follows: 2017/18 (n_SWC = 15), 2018/19 (n_SWC = 12, n_IT = 33), and 2019/20 (n_SWC = 13).
2.5. Laboratory Experiments
In 2017, we established a standardized method (modified after [40]) to analyze survival and growth of juvenile mussels kept in cylindrical plastic beakers with natural food suspensions (total volume of 100 mL, Figure 1C) under laboratory conditions [45]. The beakers were stored under controlled (constant) temperature conditions in climate chambers (Binder KB 53, Binder GmbH, Tuttlingen, Germany) without light. To monitor the temperature, data loggers (HOBO TidbiT MX Temperature 400′ Data Logger) were placed in control beakers submerged in water to simulate the buffer function. Stream detritus collected from our study sites (S1, S2 or S3) was used as food suspension that consisted of sedimented fine particles characterized by an organic proportion of 4.8–7.8% of dry weight. Stream detritus was collected directly from the upper surface of the riverbed in flow calmed areas (for details see [45] on a biweekly basis during summer and monthly during the winter experiment). The collected stream detritus was immediately sieved to a fraction of <500 µm to eliminate macrozoobenthos and larger organic particles. The solution was stored under low light conditions (10–30 µE m−2s−1) (Li-Cor spherical quantum sensor, LI-189, Bad Homburg, Germany) with a 12 h of day and 12 h of night cycle, at 15 °C (summer) or 4 °C (winter), and it was constantly aerated using aquarium pumps.
The size fraction suitable for juvenile FPM consumption is between 28 and 31 µm according to the interfilamentary distance determined by quantitative histology [35,36]. To estimate the food quantity for FPMs in the laboratory experiments, the concentration of edible particulate organic carbon (POC < 30 µm) of the food suspensions was measured weekly using a carbon analyzer (C844, LECO, St. Joseph, Missouri, USA). To prepare the food suspension for the laboratory experiments, detritus was diluted with stream water to create a POC ranging from 75 to 210 mg C/L (at summer conditions) corresponding to a medium, non-limiting food level during the laboratory experiments [45]. Mussels were fed on a weekly basis, by removing them from the old food suspension and refilling each beaker with the new prepared solution. To check water quality for optimal FPM conditions (based on threshold values from [21]), we recorded oxygen (saturation and concentration), conductivity, and pH values throughout the experiment using a multiparameter probe (Multi 3630 IDS, WTW, Weilheim, Germany) twice: (i) before removing the mussels in the old food suspension, and (ii) in the new food solution before adding the mussels. Each mussel was photographed at the start and at the end of the experiment to estimate growth. During the weekly check, the mussels were visually inspected with a binocular. All alive individuals were counted to assess survival since the start of the experiment and the dead mussels were removed.
For the first summer laboratory experiment (Table 1), we used four temperature treatments, covering the full thermal range mussels experience actually in the streams during summer, classified as cold (12 °C), average (15 °C), slightly increased (18 °C), and high (21 °C). Temperatures of the treatments were constant during the exposure and mean values did not vary greatly from target temperatures (means 12.15 ± 0.45, 15.08 ± 0.45, 18.14 ± 0.21, 20.98 ± 0.19 °C). Detritus collected from S2 and S3 was used to prepare food suspensions for each temperature treatment (4 temp × 2 food sources), and each treatment was replicated 3 times in a completely randomized design. Each beaker contained 25 mussels at the beginning of the experiment (age: 1.5 month, mean length 671 ± 42 µm). The experiment lasted 88 days from June 7th to September 3rd in 2019. Food suspensions from S2 and S3 had a mean concentration of dissolved oxygen of 7.59 or 7.98 mg/L (far above limiting concentrations), a pH value of 7.20 or 7.60, and a conductivity of 211 or 349 μS/cm during the one-week exposure, respectively. The POC concentration (<30 µm) of the food suspensions after the one-week exposure were similar across all temperature treatments (S2: 99.6 ± 5.8 mg C/L, S3: 78.8 ± 3.4 mg C/L).
The second part of the summer laboratory experiment (Table 1, follow-up to the 88-day experiment in 2019) was performed to estimate thermal stress and mortality. Mussels were exposed to three water temperature treatments (18 (control), 22, and 26 °C) with food suspensions from stream S2 and S3 (3 temp x 2 food sources). Temperatures from 22 to 26 °C represent field conditions from current-day temperature that might be experienced in hot summer due to solar heating in the watercourse to projected future temperatures. For this experiment, all fit mussels from the first summer experiment were separated into two size classes that were randomly allocated to two replicates with larger-sized mussels (1002 ± 101 µm) and two with smaller-sized mussels (804 ± 61 µm). A total of 15 mussels (age: 4.5 month) were kept in each 100 mL-beaker. According to Pandolfo et al. [28], the experiment started after an acclimation period of 7 days. The laboratory experiment was run at nearly constant temperatures (mean ± SD 18.03 ± 0.11, 21.7 ± 0.09, 26.02 ± 0.05 °C) for two months. The food quantity (POC < 30 µm) in the experimental beakers amounted to 207 ± 45 mg C/L (S2) and 158 ± 22 mg C/L (S3). The food suspensions (from S2 and S3) in the beakers had an average oxygen concentration of 7.63 or 7.79 mg/L, pH values of 7.28 or 7.48, and a conductivity of 224 or 368 μS/cm, respectively. Thermal tolerances for the mussels were defined by the temperature that is lethal to 50% of the tested mussels (Lethal_upperT50) [28]). The Lethal_upperT50 was derived from logistic regression of survival and temperature. To validate the Lethal_upperT50 from the laboratory experiment, we estimate the realized upper thermal limit of FPMs from the summer field mesocosm studies (SWCs, 2016–2019).
In a winter laboratory experiment (Table 1), we determined the effects of current-day and projected future temperatures during winter on growth and overwintering survival of age-0 FPMs. Three water temperatures (1, 3, and 5 °C) were tested during the 98-day experiment (21 December 2017 to 29 March 2018). Besides the effect of temperature, we analyzed the impact of shell length and food source on growth and survival of juvenile mussels during their first winter period. Ca. 8-month old mussels from laboratory cultures were separated into two distinct groups based on size: (i) Large group (L): from 1150 to 1950 µm, mean length 1612 ± 77 µm; n = 65; and (ii) Small group (S): from 790 to 1350 µm, mean length 1131 ± 59 µm; n = 50. Shell lengths differed significantly between the size groups at the beginning of the experiment (paired t-test, t = 23.7, p < 0.001) due to different food sources during the summer (for details see [43]). During the winter experiment, we used stream detritus, sampled monthly from S1 and S2 (for preparation, see above). The mean POC (<30 µm) during the winter experiment amounted to 51 ± 16 mg C/L (stream detritus from S2) and 37 ± 18 mg C/L (S1). At the beginning of the winter experiment, five to seven mussels were added into 100 mL-beakers for the two size groups, Large and Small, respectively. Mussels were acclimatized to winter conditions for two weeks; they were gradually cooled (−0.5 °C/day) from 10 °C to the respective temperature conditions until the experiment started. The 36 beakers (3 temp × 2 shell length × 2 food sources each with 3 replicates) were divided randomly within the three climate chambers. After the 3.1-months exposure, the number of living mussels and the length of each mussel was recorded. We describe the correlative relationship between mean length of FPMs at the beginning of the experiment and the survival during the laboratory experiment. To validate the function derived from the laboratory winter experiment, we used data from the respective winter field mesocosms (2017/18 (SWC), 2018/19 (SWC, IT), and 2019/20 (SWC)) to fit the model at the field scale (length at the end of growing period and the survival during winter). From the correlation, a shell length at which 50% of the population of FPM survive their first winter period (Lethal_size50) was estimated for the laboratory and the field study.
2.6. Data Analyses
The statistical analysis was based on the response (survival or growth) of mussels present in one beaker or one field cage, respectively. To study the temperature effects on growth or survival in the field mesocosms (SWC and IT), the average daily mean of water temperature during summer (1 June to 31 August) from the probes exposed to the surface water and the interstitial, respectively, were used (average water temperature during summer, Tw_su). Peak water temperatures (Tw_peak) during the field study were determined for each site and year, respectively, from the daily maximum of water temperature of at least two consecutive days recorded using the probes. To estimate the temperature effects in laboratory experiments, we calculated the mean water temperature during the exposure of FPMs for each treatment.
Survival (%) was calculated by dividing the number of vital mussels within one SWC, IT, or beaker at the end of the experiment by the number at the first day of the experiment. The mean daily growth (µm/d) was calculated for each replicate from the difference of the mean shell length of all mussels in one beaker or cage at the end of exposure and the mean initial length (µm) divided by the experiment duration (days). To assess the maximum values observed for daily growth of FPMs at a given average water temperature in the field mesocosms (SWC), we used the highest values of the daily growth within each 0.5 °C-intervals of average water temperature across all streams and years.
We calculated Spearman correlation coefficients between growth, survival, and water temperature. Regression models were used to determine growth using water temperatures as the input parameter for field and laboratory experiments. The upper thermal threshold (Lethal_upperT50) values were derived from logistic regression of survival rates during summer and the mean water temperature (laboratory summer experiments) or the peak water temperature (field studies). This measure estimates the temperature that is lethal to 50% of the mussel population. Differences in growth or survival of mussels between treatments during the summer laboratory experiments were tested with a two-way Analysis of Variance (ANOVA) followed by Tukey’s HSD pairwise comparison of means. Analysis of Variance (ANOVA) of growth and survival was preceded by tests for normality (Shapiro–Wilk test) and homogeneity of variances (Equal Variance Test after Brown–Forsythe). The effects of temperature, shell length, and food source on survival during the winter laboratory experiment were analyzed using a three-way ANOVA. Analyses were conducted using Sigma Plot (version 14.0, Systat Software GmbH 2022, Frankfurt, Germany).
Our final goal was to obtain estimates of winter survival of age-0 FPMs as a function of average water temperature during summer, assuming that survival over winter is a function of mussel shell length. Consequently, survival during winter (surv_wi) is a function of both water temperature (Tw_su) and growth (gro_su) corresponding to the mussel shell length in the beginning of winter (L_wi). We developed a predictive model to estimate the lower average water temperature during summer (Lethal_lowerT50), which facilitate sufficient growth to ensure that 50% of the age-0 mussels survive during the first winter period. The model was calibrated from the results of (1) the mesocosm field experiments (SWC and ITs) across all streams and years and (2) the winter laboratory experiment (Table 1). Calculations were separated for summer and winter periods for (1). The assumptions behind our approach are the following: (i) mussels detach from the host fish in the beginning of May at a size of ~350 µm (L_exsys), followed (ii) by a 4-month period of high growth rates during summer (May to August). (iii) Growth during summer is controlled by water temperature, and therefore daily growth for FPMs exposed to the surface water (SWC) and the interstitial (ITs) was determined from the average water temperature data during summer (Tw_su) of the two habitats. (iv) Total growth during autumn (from September to November, gro_aut) was integrated by adding 110 µm to mussel lengths at the end of August according to the mean growth determined from the mesocosm field studies (SWC) in 2017. (v) Winter survival (surv_wi) depends on the total length of age-0 FPMs at the beginning of the winter period (L_wi). Based on this approach and the main results from the field mesocosm and the winter laboratory experiments, the following equations was derived (Table 2).

Table 2.
Input variables and equations for the predictive model to estimate winter survival of age-0 FPMs as a function of average water temperature during summer.
After applying the model, we obtained two sigmoidal functions that predict the winter survival of FPMs kept in surface and interstitial waters, respectively, as a function of average summer temperatures (Table 2). These functions were used to calculate the Lethal_lowerT50 values (temperature that is lethal to 50% of the population during winter) for both habitats.
3. Results
3.1. Environmental Data
Interannual variability of temperatures was high during the study period. In 2016 and 2017, the average of daily air temperatures during summer exceeded values from the historical base period (1961–1990, 14.7 °C) by ~2 °C. The summer periods in 2018 and 2019 were the warmest on record, at 3.1 and 3.8 °C above the average air temperature, respectively. The summer periods were very dry in 2018 and 2019 (with only 49 and 36 mm/month), but normal in 2016 and 2017 (with 72 and 99 mm/month) compared to the historical base period (1961–1990: 77 mm/month). In May 2018, an extreme flood in the studied streams was caused by excessive rainfall in a short period of time (151 mm/day). An extended dry period (lasting about 1.5 months) followed the flood. The mean air temperature during winter (December to March) in 2017/18 (−0.5 °C) was quite similar to the reference period (1961–1990: −0.9 °C), whereas an increase by 2.2 and 3.1 °C was observed during winter 2018/19 and 2019/20 (compared to the historical base period).
Daily water temperatures in the investigated streams during the summer ranged from 7.5 to 23.9 °C in surface waters and from 9.4 to 22.7 °C in interstitial waters across the four years. Average summer temperatures of surface water in the four years varied from 14.1 to 16.3 °C across streams (Figure 2A) and from 14.7 to 16.7 °C for interstitial loggers (Figure 2B). In 2018, warming of water temperature was delayed by ca. 2 weeks due to the extreme flooding event at the end of May. Monthly averages of water temperature across the years and streams ranged from 10.3 to 13.1 °C during September, followed by a period with 8.3–10.6 °C during October and 5.0 to 6.9 °C during November. Average water temperatures during winter (December to March) varied between 2.9 and 4.3 °C across streams and years.
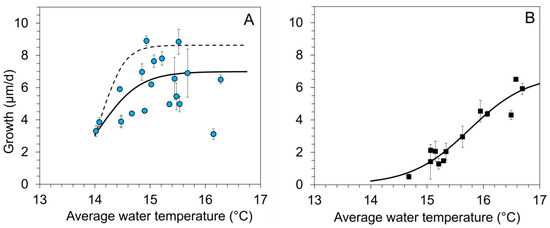
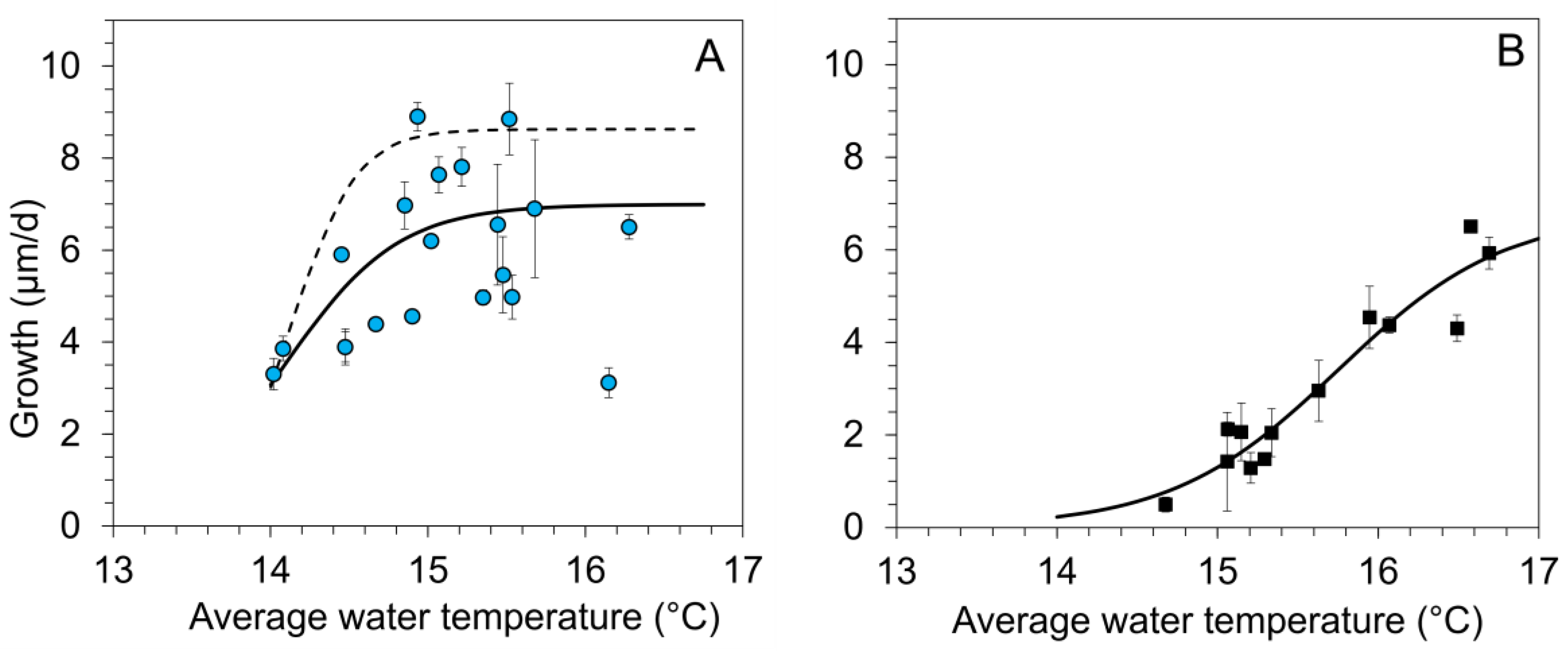
Figure 2.
Regression analysis of daily growth rates (means ± 1 SE of the replicates per stream) during summer and the corresponding average water temperature (Tw_su) (from June to August) for age-0 FPMs exposed to five headwater streams (A) to the surface water (surface water cage, SWC, 2016–2019) and (B) to the hyporheic zone in interstitial tubes (IT, 2018 and 2019). A nonlinear regression (sigmoid, 3 parameters) between average water temperature during summer (Tw_su) and growth of age-0 FPMs was calculated for (A) maximum daily growth (dashed line): gro_max_SWC = 8.628/(1 + exp(−(Tw_su − 14.38)/0.207)); r2 = 0.74, p < 0.001); mean daily growth (solid line): gro_mean_SWC = 6.991/(1 + exp(−(Tw_su − 14.09)/0.361)); (r2 = 0.37, p < 0.001); (B) mean daily growth in the interstitial tubes (solid line): gro_mean_IT = 7.318/(1 + exp(−(Tw_su − 15.88)/0.572)); (r2 = 0.93, p < 0.001).
3.2. Mussel Survival and Growth in Mesocosm Experiments
Survival of mussels in surface water cages (SWC) during the summer period averaged 64.9 ± 24.1% across streams and years (2016–2019) and varied between 10 and 100%. Survival in the interstitial tubes (IT) ranged from 0 to 97% with an average of 46.2 ± 34.1%. Average summer temperatures did not correlate significantly to mussel survival in ITs or SWCs (Spearman Rank Order Correlation: p = 0.86 and p = 0.18), respectively.
Summer average of water temperatures exerted a highly significant influence on mussel growth both in SWC and ITs (Spearman Rank Order Correlation: SWC: p = 0.003, n = 60, correlation coefficient = 0.44; IT: p < 0.001, n = 65, correlation coefficient = 0.65). For SWCs (Figure 2A), the mean daily growth during the summer period ranged from 3.0 to 8.9 µm/d (mean across all years and streams 5.37 ± 1.73 µm/d). Highest growth values (corresponding to the upper envelope curve in Figure 2A) were observed in 2017 and 2016. In 2017, the mean initial mussel length increased by up to 220% during the 92-day lasting exposure to the streams, resulting in a maximum total length of 1480 µm at the end of August. For mussels kept in SWCs, we determined a logistic regression between average water temperature and mussel growth during summer. Daily growth was low at water temperatures below 14 °C. Average summer water temperatures rising from 14 to 15.5 °C increased daily growth of age-0 FPMs by about 3.4 µm/d per degree Celsius warming. A further increase in average water temperatures was not related to higher growth; in some cases, it resulted in decreased growth rates (Figure 2A).
For mussels kept in ITs buried in the riverbed, the mean daily growth during the summer ranged from 0.5 to 6.5 µm (Figure 2B) (mean ± SD across all streams and the two years: 3.13 ± 1.94 µm/d). Daily growth of mussels in ITs increased linearly with summer averages of interstitial temperatures increasing from 15 to 16.5 °C by about 2.7 µm/d per degree Celsius warming (Figure 2B).
During the autumn exposure of juveniles in SWCs (from September to November), the mean growth amounted to 108 ± 44 µm, corresponding to a daily growth of 1.16 ± 0.58 µm/d (n_SWC = 10) at average water temperatures of 9.1 ± 1.8 °C.
3.3. Summer Laboratory Experiments
Survival rates during the first summer laboratory experiment varied between 90 and 100% for all temperatures and food sources, indicating favorable experimental conditions for the age-0 FPMs (Figure 3A). Survival was not significantly affected by temperature (two-way ANOVA; F3,23 = 2.21, p = 0.126). The food source had a significant effect on survival rates (F1,23 = 8.319, p = 0.011), with a higher survival for detritus from stream S2 (Figure 3A).
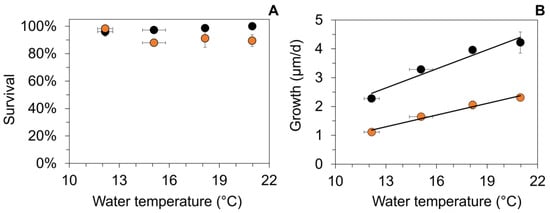
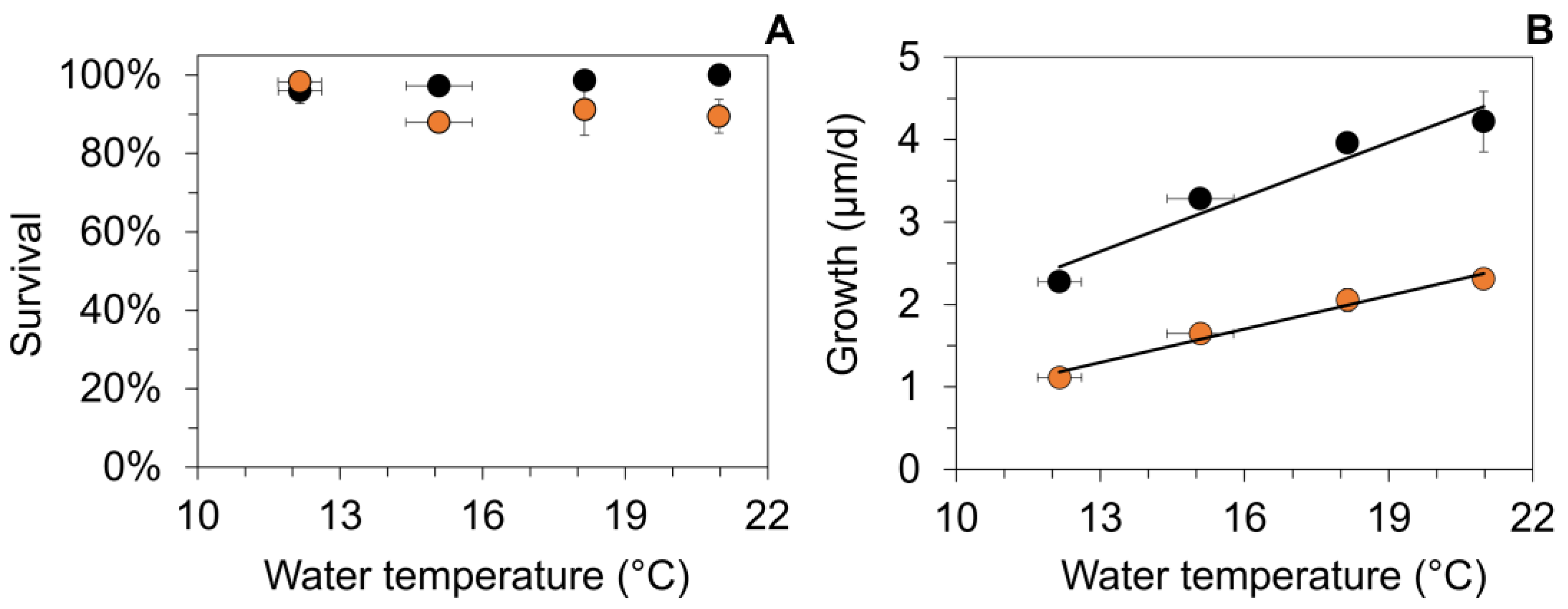
Figure 3.
Mean (±1 SE) survival (A) and daily growth (B) of age-0 FPMs after an 88-day exposure to four temperature treatments (Tw) at two food sources during the first laboratory experiment (LEx) in summer. Symbol color indicates food source used for the respective treatment of the laboratory experiments (detritus from streams S2: (black circles) and S3 (orange circles)). (A) Water temperatures did not correlate significantly to mussel survival during the exposure (S2: p = 0.054; S3, p = 0.89). (B) Linear regression between the water temperature during the summer laboratory experiment and growth of age-0 FPMs for the two different food treatments: gro_LEx_S2 (µm/d) = 0.221 × Tw − 0.229, r2 = 0.94, p < 0.001; gro_LEx_S3 (µm/d) = 0.136 × Tw − 0.470; r2 = 0.97, p < 0.001.
Daily growth was significantly affected (two-way ANOVA) by the temperature (F1,23 = 154.8, p < 0.001) and food source (F3,23 = 27.3, p < 0.001). Growth rates were significantly higher when mussels fed on stream detritus from S2 in comparison to detritus from S3 (Figure 3B). The mean daily growth ranged from 1.1 (at 12 °C) to 4.2 µm (at 21 °C, Figure 3B). Linear regression analysis revealed a significant increase in daily growth of juvenile mussels at elevated water temperatures both for S2 and S3 (p < 0.001, Figure 3B), but with relatively low rates of increase.
To determine the upper temperature limit of age-0 FPMs, survival from a follow-up laboratory experiment with three temperatures (18, 22, 26 °C) was used. After a 2-month exposure during the second summer experiment, survival of juveniles in the control treatment (18 °C) amounted to 72.9 ± 6.2% for S2 and 86.6 ± 9.2% for S3. At 22 °C, mean survival rates dropped to 26.2 ± 7.1% (S2) or 48.7 ± 20.5% (S3), and at 26 °C no surviving mussels were found (Figure 4A). We combined the results from the two parts of the summer laboratory experiment in Figure 4A for the two food sources, respectively, and estimated Lethal_upperT50-values of 22.1 °C (S2) and 22.2 °C (S3) from the logistic regression.
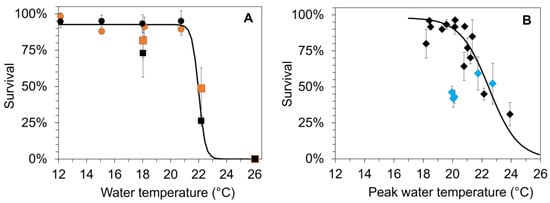
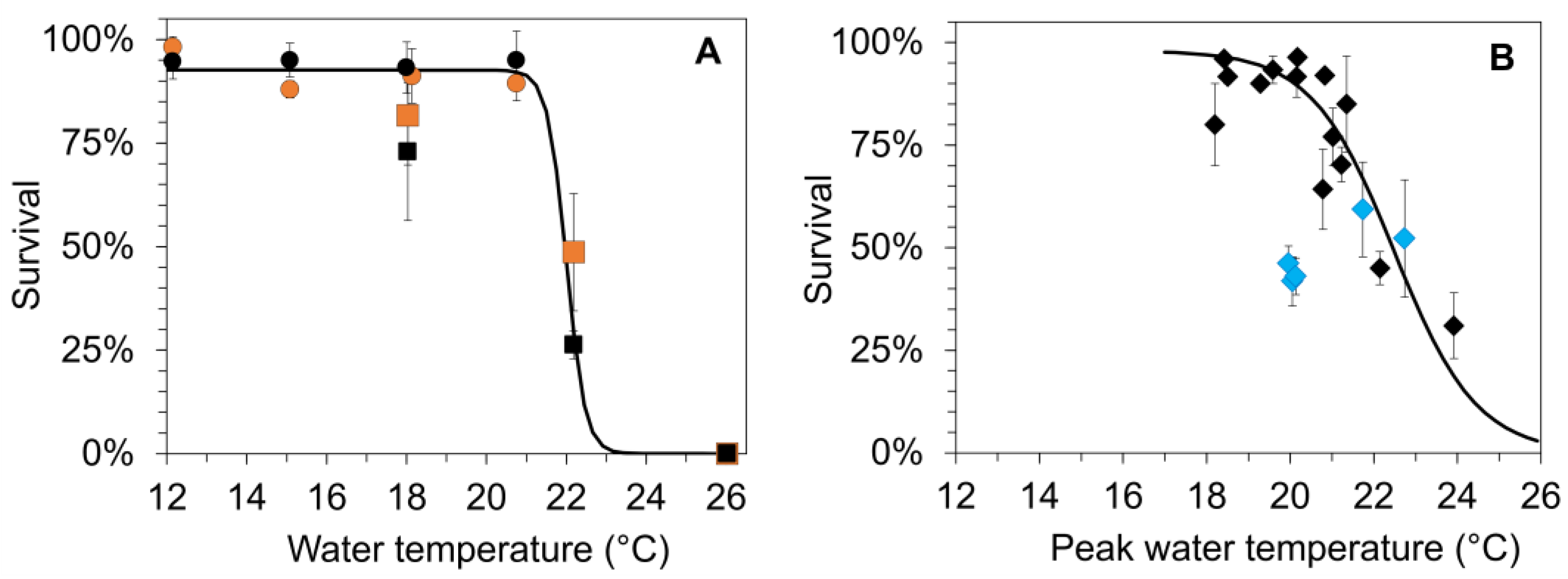
Figure 4.
(A) Mean survival (±SD) of age-0 FPMs during laboratory exposure (LEx) in climate chambers at six temperature treatments (Tw) and two food sources (stream detritus from S2 (black) and S3 (orange)). The figure combines the results from the first (circles, 12, 15, 18, 21 °C) and the second (squares symbols, 18, 22 and 26 °C) part of the laboratory experiments in summer. (B) Mean survival (±SD) of age-0 FPMs in the surface water cages (SWC) after the exposure to five streams during summer (2016–2019); water temperatures based on peak temperatures (Tw_peak) determined in the surface waters. The blue diamond symbols correspond to values that were affected by the extreme flooding event at the end of May 2018. The Lethal_upperT50 values (temperature resulting in 50% mortality of FPMs) were calculated by a nonlinear regression (sigmoid, 3 parameters) between water temperature and survival from (A) surv_LEx = 93.99/(1 + exp(−(Tw − 22.1)/−0.33)), r2 = 0.98, p < 0.001, Lethal_upperT50_Lex = 22.1 °C; (B) surv_SWC = 91.65/(1 + exp(−(Tw_peak − 22.9)/−1.77)), r2 = 0.37, p = 0.011, Lethal_upperT50_SWC = 22.9 °C.
For the mesocosm field study, peak temperature of surface water ranged from 18.4 to 23.9 °C throughout the whole experiment (Figure 4B), with significant differences between streams and years (two-way Analysis of Variance, year: F3,17 = 11.29, p = 0.001; stream: F4,17 = 8.60, p = 0.003). Peak temperatures did not exceed 21 °C in 2016 and 2017. In 2018 and 2019, peak temperatures exceeded 21 °C in two streams for a period of 6 or 11 consecutive days, whereas peak temperatures >22 °C were observed only at 3 or 5 days. Mean survival of juvenile mussels in SWCs did not fall below 75% during summer when peak temperatures did not exceed 21 °C. The further increase in peak water temperatures in 2018 and 2019 resulted in a rapid decrease in survival to 30% (Figure 4B). The peak temperature related to 50% survival (Lethal_upperT50) across the five streams, and four years amounted to 22.9 °C. Survival rates measured in 2018 after the extreme flood event were lower than expected from the water temperatures (Figure 4B blue diamond symbols).
3.4. Winter Experiments
During the 3-month winter laboratory experiment (wi_LEx) the mean temperatures in the three climate cabinets were 1.09 ± 0.30 °C, 3.27 ± 0.17 °C, and 5.3 ± 0.27 °C. Mean (± SD) daily growth was estimated at 0.43 ± 0.06 µm/d across all treatments (Figure 5A). Results of a three-way ANOVA for daily growth indicated no significant effects of temperature (F2,35 = 0.58, p = 0.57), shell length at the start (F1,35 = 0.06, p = 0.81), or food source used (F1,35 = 3.85, p = 0.07).
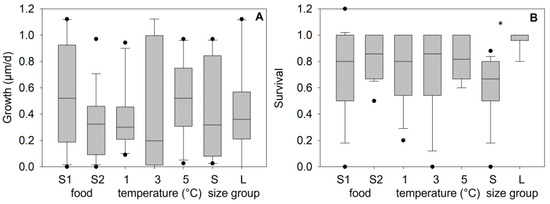
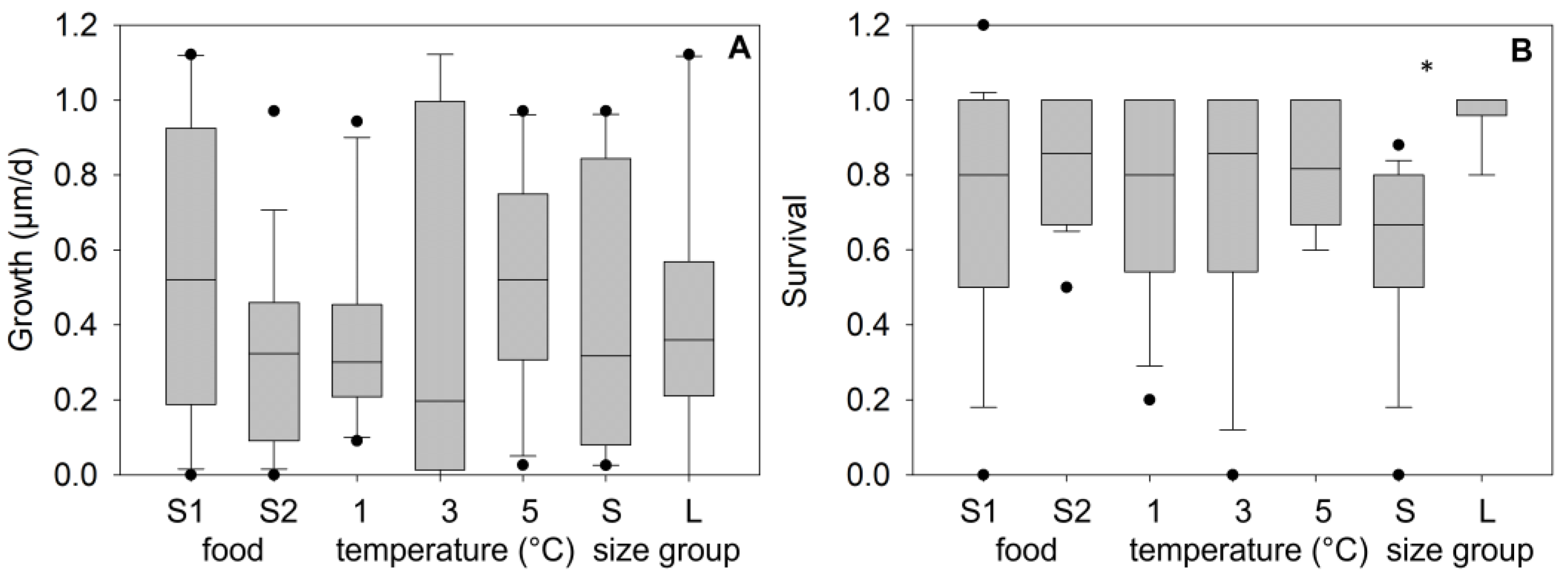
Figure 5.
Growth (A) and survival (B) of age-0 FPMs after 3-months exposure to different treatments during the laboratory winter experiment (wi_LEx). Treatments consider food sources from two streams (S1, S2) combined with three temperature treatments (low-, intermediate-, and high-water temperature) and two size classes of mussels at the start of the experiment (Large mussels “L” from 1150 to 1950 µm, n = 65, Small mussels “S” from 790 to 1350 µm, n = 50). The symbol * indicates significant differences between the treatments at <1% risk level.
Survival rates after the winter exposure were controlled significantly by the initial shell length (F1,35 = 56.184, p < 0.001) and by the food source (F1,35 = 9.03, p = 0.006), but no effect of winter temperatures was determined (F2,35 = 0.74, p = 0.89) (three-way ANOVA). The survival values in the group “Large” (start length > 1150 µm) varied from 80 to 100% (mean ± SD: 97.8 ± 6.4%, n = 18), whereas survival values for mussels in the group “Small” (start length = 790–1350 µm) ranged between 20 and 88% (62.6 ± 17.5%, n = 18), indicating significant differences between both size groups (p < 0.001, Figure 5B). A sigmoidal regression between the shell length in December (start of the laboratory winter experiment, L_wi) and survival after the 3-month exposure described the correlation significantly (surv_wi_LEx = 0.99(1 + exp(−(L_wi − 1059.9)/108.5)), r2 = 0.98, p < 0.001, n = 12, Lethal_size50_wi_Lex = 1060 µm).
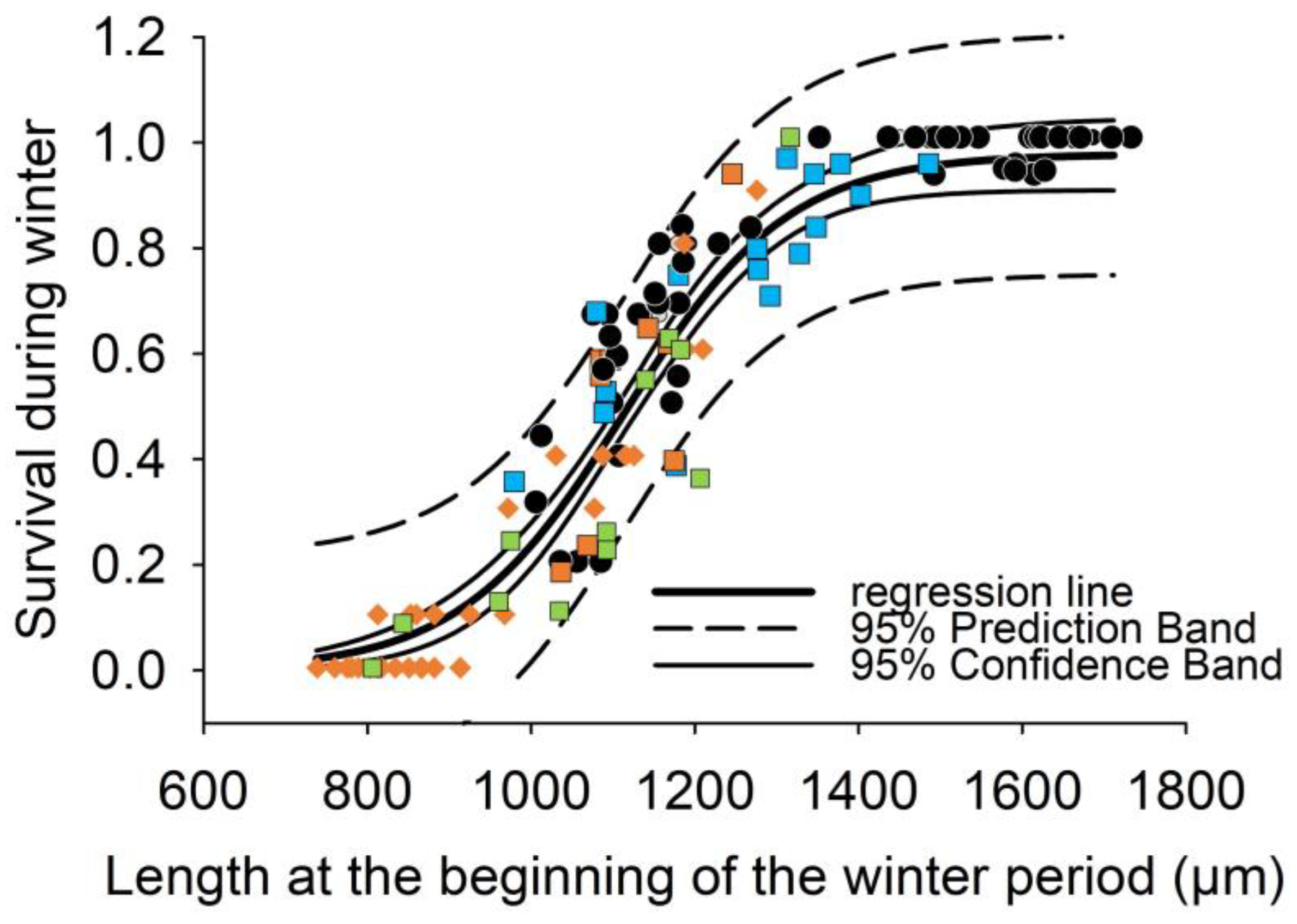
In the field mesocosm studies, mean survival during the first winter varied between 32 ± 27% and 73 ± 19% for mussels in SWCs, but only 19 ± 16% of the juvenile mussels exposed to the interstitial (IT) survived. We used a sigmoidal regression to model the relationship between the shell length at the end of growing period and survival during the winter period across three years and five streams (surv_wi_field = 0.98(1 + exp(−(L_wi − 1127)/99.6)), r2 = 0.89, p < 0.001, n = 74, Lethal_size50_field = 1127 µm). Combining length-survival-data from winter laboratory and winter field experiments, survival of FPMs during their first winter was described significantly by the sigmoidal function in Figure 6 (surv_wi = 0.97/(1 + exp(−(L_wi − 1107)/97.2)), r2 = 0.92, p < 0.001, Lethal_size50 = 1107 µm) indicating a threshold effect of shell length on survival. The prediction band in Figure 6 accounts for both the uncertainty of estimating a value and the random variation of individual values sampled, whereas the confidence interval describes how accurate the estimate is in accounting for sampling error. Accordingly, shell length at the end of growing period accounted for more than 90% of the variability in winter survival. A pre-overwintering shell length of 1107 µm corresponds to 50% survival during winter (Lethal_size50). Mussels with a length less than 900 µm at the end of growing period had a survival rate of under 10% and survival decreased to 0% for mussels below a shell length of 800 µm. Survival rates higher than 80% were observed only if mussels exceeded a pre-overwintering shell length of 1260 µm. If mussels are 100 µm bigger at the end of growing period (within a size range from 900 to 1300 µm), they are by 22% more likely to survive the winter period.
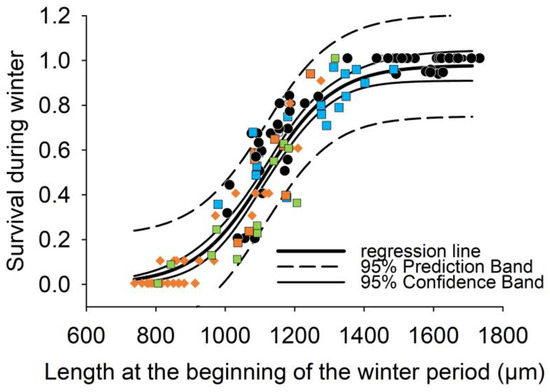
Figure 6.
Regression analysis of mussel length (L_wi) at the end of first growing period (for field studies in November, for lab experiments in December) and survival during the first winter period (surv_wi) from the laboratory winter experiment (wi_LEx) in 2017/18 (n = 18, black circles) and from the mesocosm field studies (wi_field) of age-0 mussels in surface water cages (SWC) started in 2017 (n = 15, blue squares), in 2018 (n = 12, orange squares) and in 2019 (n = 13, green squares) and in the interstitial tubes (IT) started in 2018 (n = 33, orange diamonds). Regression considering data both from laboratory and field mesocosm experiments are described by a sigmoid function with three parameters surv_wi (%) = 0.97/(1 + exp(−(L_wi-1107)/97.2)), r2 = 0.92, p < 0.001)., resulting in a Lethal_size50 of 1107 µm corresponding to a survival rate of 50% during the first winter.
Our results indicate that summer growth and winter survival correlate. Combing significant correlations from Figure 2 and Figure 6, a predictive model (for details see methods, Table 2) was developed to estimate the lowest average water temperature during summer (Lethal_lowerT50) that facilitates sufficient growth to reach a pre-overwintering shell length of 1107 µm. This size (Lethal_size50) at the end of the growing period results in 50% survival of the age-0 FPMs during the first winter period. The model was calibrated for conditions in the surface and interstitial water separately using the empirical results from the field mesocosm experiments for SWCs and ITs during summer (Figure 2A,B). According to the two sigmoidal functions in Figure 7, an 50% survival of FPMs during the first winter period can be predicted if average water temperatures during summer (indicating Lethal_lowerT50) exceed 14.5 °C in the surface water and 16.4 °C in the interstitial zone.
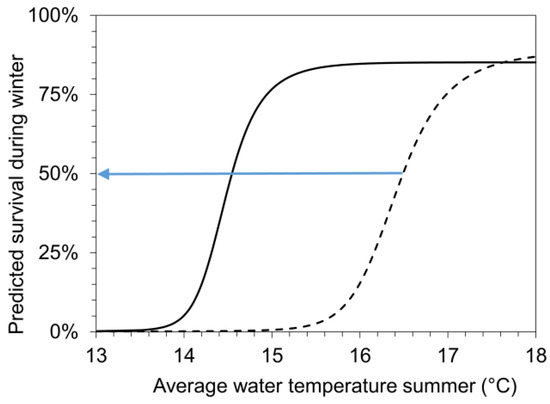
Figure 7.
A model (for details see methods, Table 2) was developed to predict the winter survival of FPMs as a function of average summer temperatures (Tw_su). We obtained two sigmoid functions that predict the winter survival of FPMs exposed to surface waters (solid line: surface water cage; SWC) and to hyporheic zone (dashed line: interstitial tubes; IT) of five headwater streams, respectively. These functions were used to estimate the lowest average water temperature during summer (Lethal_lowerT50), which facilitates sufficient growth to reach a shell length of 1107 µm (Lethal_size50) at the end of growing period, resulting in 50% survival of the age-0 FPMs during the first winter period (as marked by the blue arrow). Lethal_lowerT50-values amounted to 14.5 °C (for surface water) and 16.4 °C in the interstitial zone.
Figure 7.
A model (for details see methods, Table 2) was developed to predict the winter survival of FPMs as a function of average summer temperatures (Tw_su). We obtained two sigmoid functions that predict the winter survival of FPMs exposed to surface waters (solid line: surface water cage; SWC) and to hyporheic zone (dashed line: interstitial tubes; IT) of five headwater streams, respectively. These functions were used to estimate the lowest average water temperature during summer (Lethal_lowerT50), which facilitates sufficient growth to reach a shell length of 1107 µm (Lethal_size50) at the end of growing period, resulting in 50% survival of the age-0 FPMs during the first winter period (as marked by the blue arrow). Lethal_lowerT50-values amounted to 14.5 °C (for surface water) and 16.4 °C in the interstitial zone.
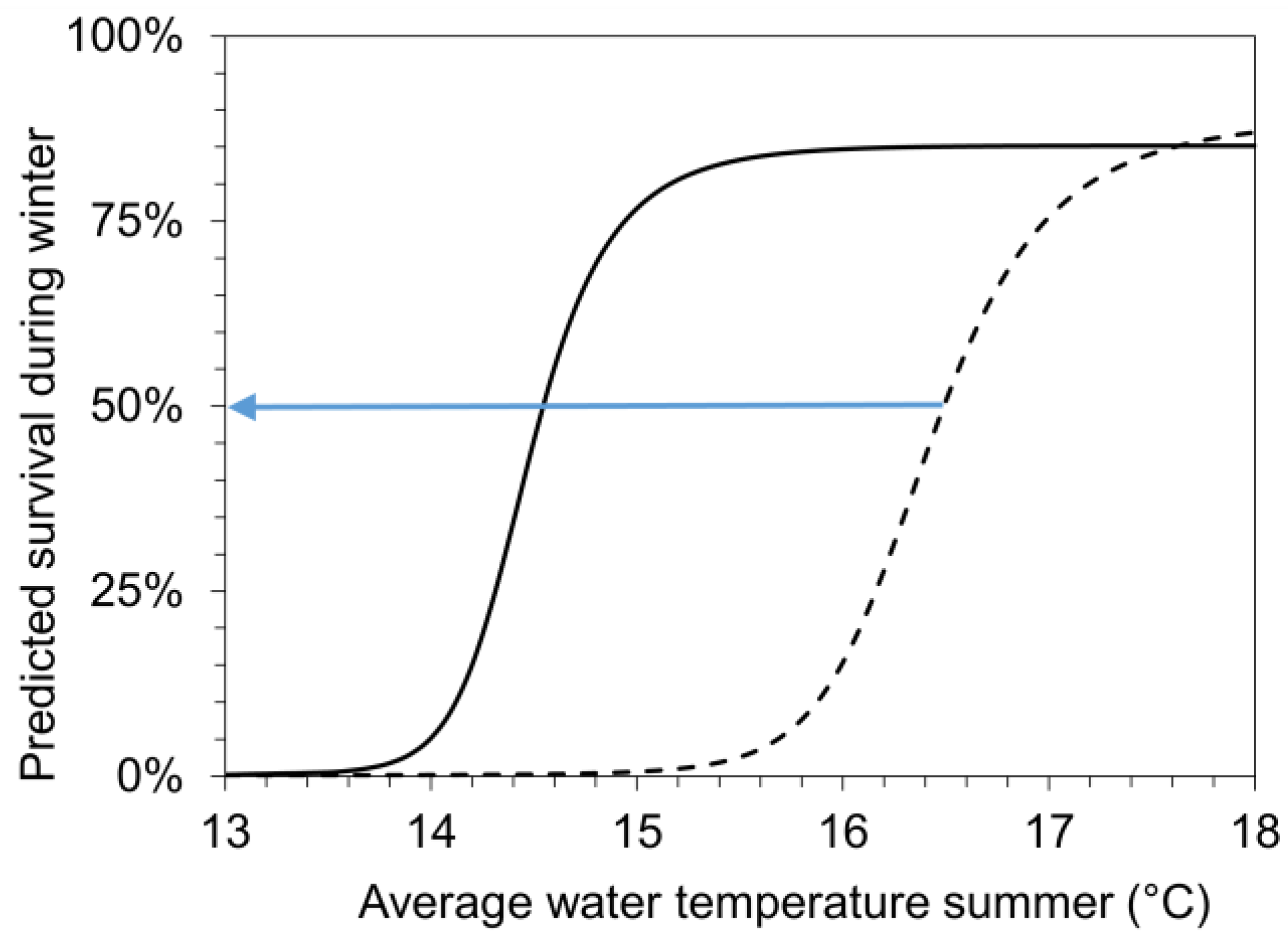
solid line: surv_wi_SWC = 0.846/(1 + exp(−(Tw_su − 14.49)/0.199)), p < 0.001
dashed line: surv_wi_IT = 0.859/(1 + exp(−(Tw_su − 16.41)/0.278)), p < 0.001.
4. Discussion
Our study integrated field and laboratory experiments to estimate the effects of water temperature on growth and survival of juvenile FPMs during their first summer and winter period after excystment. The experimental setup combined repeatability in a controlled setting with the ecological complexity of the field conditions that enabled this study to estimate species’ temperature tolerances. Each of the three experimental setups used (surface water cages after Buddensiek (SWC), interstitial tubes (IT), 100 mL-beakers in the laboratory) proved to be suitable to assess the response of mussels as survival reached nearly 100% in all systems. Previous studies have shown a principal influence of temperature on growth or survival of FPMs, e.g., [39,40,41,42]. Our study describes such effects while addressing the FPMs’ full thermal gradient from current day to projected future temperatures during summer and winter and considering interstitial habitats beside the surface water. Experimental data were employed to assess the influence of thermal changes on the observed population decline in FPMs and to derive conservation strategies. Our field study involved two successive years (2018 and 2019) that were exceptionally warm and dry during the summer period. These years may serve as examples of near-future changes to be expected during climate change scenarios [1]. In contrast, temperatures and precipitation during the summer periods in 2016 and 2017 and in winter 2017/18 are in accordance with the local historical base period (1961–1990). Our empirical analysis shows that moderate change can have detrimental effects on growth and survival of juvenile FPMs if upper or lower temperature thresholds are exceeded or undercut.
4.1. Direct Temperature Effects on Mussel Growth
Growth rates of juvenile FPMs were positively correlated to water temperatures within a gradient from 12 to 21 °C for the laboratory experiments (Figure 3B). In situ experiments for juvenile Lampsilis cardium (Rafinesque, 1820) in streams in Kentucky, USA have shown a comparable correlation between water temperature (20–25 °C) and growth [34]. In our five streams, growth was generally low at water temperatures below 14 °C (Figure 2). Our findings corroborate with previous results, i.e., [33] from different headwater streams where the mean water temperature was around 13.5 °C during summer. The period in which water temperatures of 14 °C were exceeded permanently started in our investigated streams in May or latest in the beginning of June and was terminated at the end of August or in the first days of September, indicating that the period with good growth conditions (corresponding to the summer period) lasted between 3 and 4 months. Length increments of up to 220% observed for mussels kept in surface water cages (SWCs) during the summer period were similar to values published by Hruška [50] (increments of up to 250%), Eybe et al. [40] with values between 150–200%, and Lavictoire et al. [42] (170–220%). For FPMs deployed to SWCs, the relationship between water temperature and growth was described by a sigmoidal regression with a saturation at water temperatures exceeding 15.4 °C and with a maximum growth of about 9 µm/d (Figure 2A). We could not determine optimum temperature for juvenile FPM growth for this study due to the following factors: (i) the observed differences in the relationships between temperature and growth from SWC and IT (Figure 2A,B), (ii) the variability in growth at water temperatures >15.4 °C in SWC, and (iii) the absence of a growth saturation in the summer laboratory experiments. We believe that the function describing maximum growth of FPMs in SWCs for the respective average summer temperature across the years and streams (Figure 2A) provides the clearest evidence for the direct impact of temperature on growth. Other factors, apart from water temperature, have minimal influence on growth in this context. The growth performance corresponds to maximum values determined for juvenile FPMs by Scheder et al. [41], but it did not reach values (20 µm/d) observed temporarily for juvenile FPMs by Hruška [53]. Daily growth > 6 µm was observed in the surface water in two of our study sites (S3, S5) in years with wet, moderate warm summer periods (2016, 2017). Accordingly, growth rates in most of our streams fell below the maximum values expected for optimal conditions.
Water temperatures in the upper (3–5 cm) sediment layers were similar to water temperatures measured about 5 cm above the riverbed due to water exchange. Despite similar temperatures, growth was lower in ITs at water temperature below 16.2 °C than expected from the temperature–growth relationship for mussels from SWCs (Figure 2A,B). At higher water temperatures, however, mean daily growth of mussels in SWCs and ITs was comparable (Figure 2A,B). It is known that growth can be constrained by other factors besides temperature (e.g., food source, habitat quality, physico-chemical criteria, flow velocity, discharge [5,30,54]) which might indirectly shift the temperature window for high growth rates. Field experiments with juvenile mussels have proven that, besides temperature, food quantity [34,55] and food quality [40,45,47] are the most important predictors for mussel growth. Grunicke et al. [45] combined the analysis of the food quality of different samples from stream detritus sources with an analysis of food quantity of juvenile FPMs, indicating a saturation curve between food quantity and growth. According to these results [45], the growth rates of FPMs were not limited by food quantity during our summer laboratory studies. Higher growth rates at similar temperatures for mussels fed on stream detritus from S2 compared to S3 (Figure 3B) can be attributed to the higher food quality (corresponding to high concentrations of EPA (Eicosapentaenoic acid, 20: 5n−3)) that Grunicke at al. [45] detected in S2. Higher dietary intakes of EPA are associated with improving the growth of juvenile FPMs [45] and other freshwater invertebrates [56]. A high production of EPA was found when the algae (e.g., Pavlova lutheri, Butcher, 1952) was cultivated at temperature of 18 °C at low light intensity (40 μmol m−2·s−1) [57], corresponding well to physical conditions in the surface water at our study sites. We assume that EPA levels might be very low in the ITs and in our laboratory experiments as these experiments were carried out in the dark, which might explain the lower growth of mussels in ITs compared to the SWCs. Further investigation is needed to understand the interactions between variables such as water temperature, irradiation, food quality, or fatty acids on growth.
Growth of FPMs during autumn (at water temperatures of 8–13 °C) of 1.16 ± 0.58 µm/d falls below the lowest daily growth observed in SWCs during summer; therefore, about 80% of annual growth is realized during the summer period. Concerning winter conditions, we analyzed a wide range of temperatures, which goes far beyond the winter temperatures of the historical base period (by ±6 °C compared to 1961–1990) of our study sites. Contrary to assumptions concerning winter dormancy of wild M. margaritifera [58], we detected a slight growth during our laboratory experiments, even at water temperatures of 1 °C. Other previous studies [41,42] that kept FPMs in climate chambers at 6 °C, determined a mean daily growth of ~0.5 µm, confirming our results. Our laboratory experiment revealed no significant effect of winter temperatures on growth rates at water temperatures ranging from 1 to 5.3 °C. Thus, we postulate that individual variability might have a larger impact on population growth during winter than elevated water temperatures.
4.2. Direct Temperature Effects on Survival
Our results have shown that juvenile FPMs can tolerate a wide temperature range, indicating that they have some physiological capacity to cope with warming to about 21–22 °C. Whereas Hastie et al. [3] postulated a thermal tolerance for FPMs in small streams in Scotland up to a water temperature of 25 °C, in our study the decreasing survival during the mesocosm field studies and the laboratory summer experiments (Figure 4) highlights their vulnerability to water temperatures above 21 °C. An exposure to 26 °C for two months during the laboratory summer experiment, which simulated future heating events, resulted in a complete collapse of the FPM population. Lethal_upperT50s of juvenile FPMs, which were detected for food sources from two different streams (S2, S3) during the summer laboratory experiments, amounted to 22.1 to 22.2 °C, indicating a high similarity. As Ganser et al. [24] postulated that potential laboratory artifacts may limit applicability of these results to field conditions, the thermal tolerance of age-0 FPMs was estimated for the field mesocosm studies across five streams and the summer period of four years. Given that temperature fluctuates widely on a daily or weekly basis under natural conditions, peak temperatures rather than daily averages were used to determine Lethal_upperT50s (Figure 4B) for the field study. We found that Lethal_upperT50s determined from the peak temperatures in surface water and the mean temperatures in the climate cabinets were quite similar at 22.9 °C and 22.1 °C (Figure 4), despite the differences in the duration of the heating events and the possible impact of other environmental factors in the field ecosystems. The slightly lower Lethal_upperT50 from the laboratory experiments may result from the permanent exposures of FPMs to high temperatures in the climate chambers. The time periods that mussels in SWCs were exposed to temperatures close to the Lethal_upperT50 differ strongly among years and streams. Only in the two years with extreme hot and drought conditions (2018 and 2019) did water temperatures increased to >22 °C due to direct solar heating. In these years, peak temperatures resulting in thermal stress were experienced for 3 to 11 consecutive days in two of the studied streams (S3, S4) in stretches with less natural shading by riparian vegetation. Besides the future increases in peak temperatures, the frequency and duration of exceptionally warm periods in summer may be detrimental, particularly to mussels in small streams with low thermic buffer ability [3]. While there is no study describing upper thermal threshold values of European juvenile FPMs, they have been described for juveniles of other Unioniformes species in North America whose determined LT50-values range from 25.3 to 38.8 °C [24,28,59]. Consequently, LT50-values for Unioniformes in North America are considerably higher than the Lethal_upperT50 determined for FPMs in this study, indicating a lower thermal tolerance and a higher vulnerability of FPMs to climate warming. By combining data from the laboratory summer experiments with data from the mesocosm field study (Figure 4A,B), an upper thermal limit of 22.3 °C was determined for age-0 FPMs, which defines a threshold value for conservation of FPMs under conditions of ongoing climate warming.
Former studies have noted that mussels and other species are able to escape thermal stress when buried in the cooler sediment layers during times of heat stress compared to individuals at the surface [60]. In our streams and also in 23 streams in the USA [34], water temperatures in the upper sediment layers were nearly similar to water temperatures above the riverbed; nevertheless, sediment layers deeper than 5 cm may serve as thermal refuges. Newton et al. [61] suggest that the ability of sediments to act as thermal buffers for mussels in rivers may be site-specific depending on particle size, ground water influence, or water content. However, the burrowing behavior of freshwater mussels (Lampsilis spp.) was already significantly reduced at water temperatures above 22 °C [59].
Survival of juveniles exposed to the surface water (SWCs) in the streams that were disturbed by the extremely high discharge during the flood in 2018 was clearly below the values expected from water temperatures (Figure 4B). It is known that mussels use terrestrial organic matter directly or after further processing as an important food source [44,56]. We assume that FPM juveniles were food limited during the summer in 2018 due to the combined effect of (i) the flood that eliminated the organic material completely from the riverbed, the substrate, and the riparian zone; and (ii) the drought period (immediately following the flooding event) blocking surface inflow. Both events resulted in less terrestrial organic material entering the streams, consequently limiting food sources for FPM juveniles [44].
4.3. Indirect Temperature Effects on Survival during Winter
Our laboratory experiment simulated warming during winter (by ±6 °C compared to the reference period 1961–1990). Despite the large temperature gradient (1 to 5.3 °C), survival of juvenile FPMs did not respond significantly to elevated temperatures (Figure 5B). The key factor for survival during the first winter period derived both from the laboratory and field mesocosm studies was the shell length at the beginning of the winter period (Figure 6). In addition to previous studies [39], we considered age-0 FPMs with a wide gradient in length from 790 to 1900 µm at the end of first growing period. During our laboratory experiment, mussels of the group ”Large” (length from 1150 to 1950 µm) are more likely to survive than the smaller size group (from 790 to 1350 µm) (Figure 5B). As shown in Figure 6, which integrates all the results from the laboratory and mesocosm field studies, a sigmoid function described the relationship between mussel shell length at the end of the growing period and survival during the first winter period significantly: survival increases slowly up to a length of 900 µm, then rises sharply (900–1300 µm), and finally goes to saturation (>1300 µm). Overall, confirming Buddensiek’s [39] observation, one can expect little to no surviving mussels when FPM lengths are below 900 µm. Mussels with a size of about 1100 µm (Lethal_size50) correspond to a 50% survival during the first winter period. The Lethal_size50-values derived from the laboratory experiment and from the field studies (Figure 6) differed only by 67 µm, offering strong support for this value. Winter survival increased to 80% if pre-overwintering shell length exceeded 1250 µm (Figure 6), amplifying the importance of good growth during the first summer. Variations in the growth of individuals affected their chances of survival and are thought to be a pivotal determination for stability of FPM populations.
The sigmoidal shape of the estimated survivorship curve (Figure 6) implied that winter survival corresponds to a threshold effect of shell length that might be related to complex morphogenesis correlating to changes in feeding ecology [35,36]. The timing in transition from pedal feeding (using few simple ciliated filaments on the mussel’s foot) to filter feeding (using the gills) has proven to be crucial for the survival of juvenile FPMs [3], especially when food sources are scarce. Schartum et al. [35] described the development of the filtering organ (ctenidium) in juveniles of FPMs (shell length 1–3 mm) by quantitative histology. The mussel size at which a functional transition from simple I-shaped filaments to folded structures of a gill basket started at a length of 1100 µm, corresponding to the Lethal_size50 of 1107 µm derived from our study (Figure 6). Studies by Lavictoire et al. [36] confirmed that the inner demibranch filaments began to develop when shell lengths of FPMs exceed about 1130 µm. This development is related to the fundamental transformation from pedal feeding to gradually becoming capable of filter feeding. Only the development of the gills allows bidirectional particle transport and the potential for selection and for volume regulation of ingested material on the gill [36]. Additionally, for every 123 μm increase in mussel length, FPMs develop an additional filament within their filter apparatus [35] improving efficiency of filter feeding. Accordingly, we postulate that the group of large mussels benefited from the development of a gill basket, which allowed a more efficient food uptake of suspended food particles and resulted in an additional energy input [37] and significant higher survival rates during winter (Figure 5B). Once juvenile FPMs grow from about 800 µm to 1800 µm (Figure 6) in length, small differences in size are crucial to survive during winter.
Combining results from summer and winter experiments makes it possible to estimate how winter survival is indirectly influenced by the temperature-mediated growth of age-0 mussels during summer (Figure 7). Our predictive model (Table 2) calculated the lower average water temperature during summer, which facilitates sufficient growth such that FPMs (kept in the surface or the interstitial water) reach the critical mussel length (Lethal_size50 = 1107 µm). Therefore, the daily growth during a 4-month lasting summer period (May to August) should be at least 5.2 µm/d. For a shorter summer period (only 3 months from June to August), a daily growth of at least 6.5 µm is required, which corresponds to the maximum growth values observed for FPMs in our mesocosm field studies (Figure 2). Due to the higher growth performance for juveniles kept in SWCs at water temperatures up to 16.2 °C, the Lethal_size50 was reached even at average summer temperatures of 14.5 °C compared to 16.4 °C for juveniles at interstitial conditions (ITs) (Figure 7). These values define the lower temperature threshold that must be reached in the different habitats for FPMs during summer. The difference between the Lethal_lowerT50-values estimated for SWCs and ITs could result from the lower food quality for mussels living in the hyporheic zone compared to the surface water (as discussed above) but also from differences in water-quality parameters [21]. Besides the factors already mentioned, a larger shell length at the beginning of winter can be supported by an extension of the growing period due to (i) an earlier start of excystment from gills of the host fish [18,29], (ii) a longer duration of summer conditions with water temperatures >14.5 °C, or (iii) a delayed start of winter corresponding to a prolonged fall growing season. Generally, longer growing seasons may allow FPMs to store additional energy [10], resulting in a higher length in December. While the effect of the pre-overwintering shell length on winter survival (Lethal_size50) can be generalized for age-0 FPMs, the lower temperature threshold that is required to exceed the Lethal_size50 might be more specific concerning the stream, habitat, and food quality. Additional research is required to validate the Lethal_lowerT50 for different field conditions.
4.4. Implications for Conservation Strategies
Important tasks to improve long-term survival in the context of conservation programs of European FPMs are (i) to increase survival and growth during captive breeding, (ii) to reduce the time that mussels spend in hatcheries, (iii) to select the best sites to release mussels from captive-breeding stations, and (iv) to restore the habitats and the catchment into which mussels are released, e.g., [14,48,49]. This study added two results that may improve future conservation strategies: (i) it is necessary for the FPMs to reach the critical pre-overwintering shell length (Lethal_size50 = 1100 mm) by the end of the first growing period, and (ii) optimal thermal conditions for juvenile survival and growth are given within a temperature window of average summer water temperatures between 14.5 and 21 °C (corresponding to the thermal niche if there is no drought), which is important for selecting the ideal release sites of captive-breed FPMs. FPM juveniles can grow faster under optimal thermal and food conditions during the first season, potentially reducing time spent in hatcheries and improving survival rates of individuals (Figure 6) when reintroduced to the wild [38]. The temperature window derived from our study corresponded well to average summer water temperatures (15–18 °C) observed in the Lutter River (Luneburg Heathlands, northern Germany), where a conservation program of FPMs was implemented successfully during the last decades, resulting in a functional pearl mussel stock [22]. In two study sites in Vogtland mountains (S3, S4), average summer temperatures >15 °C were observed yearly even in the epirhithral, but in the epirhithral of the three other streams (S1, S2, S5), the lower temperature threshold (14.5 °C) was exceeded only in years with above-average warming during the summer period. Our study supports the previous observations of Hruška [23] and Hastie et al. [3] that FPMs appeared to recruit well in headwater streams during wet years at warmer-than-average summer temperatures. Sometimes, water temperatures in the upstream regions can be reduced by groundwater inflow [9].
The strong relationship between water temperature and growth could indicate that summer temperatures in many epirhithral stretches, which often represent last FPM-refugia, fall below the lower temperature threshold to ensure sufficient growth to survive the first winter. This could result in a bottleneck for effective conservation of FPMs but also for other species, as the epirhithral of streams are often more favorable due to (i) low anthropogenic disturbance and water pollution [21] as well as (ii) better habitat quality compared to the meta- or hyporhitral. Therefore, stretches in the epirhithral represent better habitat quality for the mussels than regions downstream, which are often too degraded. Otherwise, epirhithral stretches are often characterized by water temperatures <14.5 °C, low edible POC, and less algae biomass. Furthermore, there is an increasing risk that these stretches will dry out in summer under conditions of climate warming as observed in 2018 and 2019. Large FPM beds in the historic distribution areas of the Vogtland mountains are documented mainly in the meta- or hyporhitral stretches [46]. If water temperatures increase with distance from the spring at 0.6 °C/km [61], optimal habitats for large mussel beds would be ~4–7 km downstream from the spring. On the other hand, FPMs in the metarhithral already may be living close to their upper thermal thresholds during hot and dry summer periods (as observed in 2018 and 2019), which could be the new normal. Water temperatures in studied streams increased to up to 24 °C due to direct solar heating of surface water in stretches without shading by riparian vegetation. The shading effect of deciduous trees lining the riverbank may reduce daily peak water temperature by 4.6 °C downstream when canopy cover in the 10 m buffer changes from fully unshaded to shaded [8]. One of the key strategies available to minimize the effects of climate warming on freshwater mussels could be developing woody vegetation (using native species like Alnus glutinosa, (L.) Gaertner) in a 10 m buffer directly adjacent to the riverbank. Today, most of the Scottish FPM populations are found in catchments denuded of native riparian and floodplain woodland [3]. Moreover, a high density of FPMs within a mussel bed could act as a thermal buffer for individuals due to the aggregate acting as a unit, thus increasing thermal inertia [62] and providing evidence to release higher numbers of mussels at one site instead along a river stretch.
Other studies indicate that the physical restoration of FPM habitats alone will not help to restore mussel populations if the suitable host species is lacking [18,21,43]. Brown trout (their obligate host fish) are adapted to cold-water temperatures with a temperature optimum between 12 and 18 °C [11] and with an incipient lethal temperature of 24.7 °C [12]. Studies by Hitt et al. [63] demonstrated that brown trout moved into thermal refugia or cold-water tributaries when water temperatures exceed 20 °C. Consequently, warming during summer can cause a spatial separation between host fish and FPMs at the period of glochidia release in July–August, which may prevent infestation by FPM larvae reducing reproduction success [18,43,64]. If water temperatures exceed 22 °C, additionally, survival of FPMs and their host fish decreases. As the upper thermal limit of the brown trout is lower than the L_upperL50 of FPMs (this study), water temperatures exceeding 20 °C should be avoided for an effective conservation of FPMs. Accounting for summer conditions at the Iberian Peninsula, FPMs are expected to contract their distribution if mean temperatures go above 20 °C [43] supporting the threshold temperature.
5. Conclusions
Water temperature is a robust environmental variable affecting growth and survival of juvenile mussels. A moderate warming to >14.5 °C during the summer period is currently beneficial for FPMs during their most sensitive life stage, especially when mussels live in the epirhithral stretches. Strong warming (>20 °C) of river sections with FPM stocks during the summer period harms the mussel population and their host fish and should thus be avoided. The present study illustrates that temperature-driven changes in mussel fitness are highly dependent on seasonal patterns of warming: Warming during winter had no direct effect on growth and survival, but water temperatures during summer may control juvenile survival during winter. Therefore, warming induces not only instantaneous but also time-delayed responses, which may explain site and stream-specific differences in the viability of freshwater mussel population, a fact that might be relevant to adapting conservation strategies.
Author Contributions
Conceptualization, A.W. and T.U.B.; methodology, A.W., D.L. and F.G.; software, A.W.; validation, A.W., D.L., and F.G.; formal analysis, A.W.; investigation, A.W., D.L. and F.G.; resources, A.W., F.G. and T.U.B.; data curation, A.W., D.L. and F.G.; writing—original draft preparation, A.W.; writing—review and editing, A.W., D.L., F.G. and T.U.B.; visualization, A.W.; supervision, T.U.B.; project administration, T.U.B.; funding acquisition, T.U.B. and A.W. All authors have read and agreed to the published version of the manuscript.
Funding
The study was embedded in the joint project ArKoNaVera and funded by a grant from the Federal Ministry of Education and Research Germany, grant no. 01LC1313B and 01LC1313A.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank Jana Schneider, Thomas Schiller, Michael Lange, Felix Eissenhauer, and Antje Kuhr for help with the field and laboratory work. We thank Jing Vir Leong for grammar checking and their valuable comments to improve the text.
Conflicts of Interest
The authors declare no conflicts of interest.
Nature Conservation
Mussels used in this study originated from a captive-breeding program and were used with the permission of the authority for nature conservation of the federal state of Saxony (Germany).
References
- Pörtner, H.O.; Roberts, D.C.; Tignor, M.; Poloczanska, E.S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; et al. (Eds.) Climate Change 2022: Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Wagner, A.; Hülsmann, S.; Paul, L.; Paul, R.J.; Petzoldt, T.; Sachse, R.; Schiller, T.; Zeis, B.; Benndorf, J.; Berendonk, T.U. A phenomenological approach shows a high coherence of warming patterns in dimictic aquatic systems across latitude. Mar. Biol. 2012, 159, 2543–2559. [Google Scholar] [CrossRef] [PubMed]
- Hastie, L.C.; Cosgrove, P.J.; Ellis, N.; Gaywood, M.J. The threat of climate change to freshwater pearl mussel populations. AMBIO J. Hum. Environ. 2003, 32, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.W.; Nobilis, F. Long-term changes in river temperature and the influence of climatic and hydrological factors. Hydrol. Sci. J. 2007, 52, 74–85. [Google Scholar] [CrossRef]
- Santos, R.M.B.; Fernandes, L.S.; Varandas, S.G.P.; Pereira, M.G.; Sousa, R.; Teixeira, A.; Lopes-Lima, M.; Cortes, R.; Pacheco, F.A.L. Impacts of climate change and land-use scenarios on Margaritifera margaritifera, an environmental indicator and endangered species. Sci. Total Environ. 2015, 511, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Caissie, D. The thermal regime of rivers: A review. Freshw. Biol. 2006, 51, 1389–1406. [Google Scholar] [CrossRef]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.W.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; De Meester, L.; et al. Allied attack: Climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef]
- Kail, J.; Palt, M.; Lorenz, A.; Hering, D. Woody buffer effects on water temperature: The role of spatial configuration and daily temperature fluctuations. Hydrol. Process. 2021, 35, e14008. [Google Scholar] [CrossRef]
- Hoess, R.; Generali, K.A.; Kuhn, J.; Geist, J. Impact of Fish Ponds on Stream Hydrology and Temperature Regime in the Context of Freshwater Pearl Mussel Conservation. Water 2022, 14, 2490. [Google Scholar] [CrossRef]
- Zani, P.A. Climate change trade-offs in the side-blotched lizard (Uta stansburiana): Effects of growing-season length and mild temperatures on winter survival. Physiol. Biochem. Zool. 2008, 81, 797–809. [Google Scholar] [CrossRef]
- Elliott, J.M. Quantitative Ecology and the Brown Trout; Oxford University Press: Oxford, UK, 1994; 286p. [Google Scholar]
- Elliott, J.M. Pools as refugia for brown trout during two summer droughts: Trout responses to thermal and oxygen stress. J. Fish Biol. 2000, 56, 938–948. [Google Scholar] [CrossRef]
- Magnuson, J.J.; Crowder, L.B.; Medvick, P.A. Temperature as an ecological resource. Am. Zool. 1979, 19, 331–343. [Google Scholar] [CrossRef]
- Geist, J. Strategies for the conservation of endangered freshwater pearl mussels (Margaritifera margaritifera L.): A synthesis of conservation genetics and ecology. Hydrobiologia 2010, 644, 69–88. [Google Scholar] [CrossRef]
- Young, M.R.; Cosgrove, P.J.; Hastie, L.C. The Extent of, and Causes for, the Decline of a Highly Threatened Naiad: Margaritifera margaritifera. In Ecology and Evolution of the Freshwater Mussels Unionoida; Bauer, G., Wächtler, K., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2001; Volume 145. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, J.; Bespalaya, Y.; Bódis, E.; Burlakova, L.; Van Damme, D.; et al. Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biol. Rev. 2017, 92, 572–607. [Google Scholar] [CrossRef] [PubMed]
- Strayer, D.L. Use of flow refuges by unionid mussels in rivers. J. N. Am. Benthol. Soc. 1999, 18, 468–476. [Google Scholar] [CrossRef]
- Hastie, L.C.; Young, M.R. Timing of spawning and glochidial release in Scottish freshwater pearl mussel (Margaritifera margaritifera) populations. Freshw. Biol. 2003, 48, 2107–2117. [Google Scholar] [CrossRef]
- Geist, J. Integrative freshwater ecology and biodiversity conservation. Ecol. Indic. 2011, 11, 1507–1516. [Google Scholar] [CrossRef]
- Österling, E.M. Timing, growth and proportion of spawners of the threatened unionoid mussel Margaritifera margaritifera: Influence of water temperature, turbidity and mussel density. Aquat. Sci. 2015, 77, 1–8. [Google Scholar] [CrossRef]
- Boon, P.J.; Cooksley, S.L.; Geist, J.; Killeen, I.J.; Moorkens, E.A.; Sime, I. Developing a standard approach for monitoring freshwater pearl mussel (Margaritifera margaritifera) populations in European rivers. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1365–1379. [Google Scholar] [CrossRef]
- Altmüller, R.; Dettmer, R. Successful species protection measures for the Freshwater Pearl Mussel (Margaritifera margaritifera) through the reduction of unnaturally high loading of silt and sand in running waters—Experiences within the scope of the Lutterproject. Inform. D. Naturschutz Niedersachs. 2006, 26, 192–204. [Google Scholar]
- Hruška, J. The freshwater pearl mussel in South Bohemia: Evaluation of the effect of temperature on reproduction, growth and age structure of the population. Arch. Für Hydrobiol. 1992, 126, 181–191. [Google Scholar] [CrossRef]
- Ganser, A.M.; Newton, T.J.; Haro, R.J. The effects of elevated water temperature on native juvenile mussels: Implications for climate change. Freshw. Sci. 2013, 32, 1168–1177. [Google Scholar] [CrossRef]
- Eissenhauer, F.; Grunicke, F.; Wagner, A.; Linke, D.; Kneis, D.; Weitere, M.; Berendonk, T.U. Active movement to coarse grained sediments by globally endangered freshwater pearl mussels (Margaritifera margaritifera). Hydrobiologia 2023, 850, 985–999. [Google Scholar] [CrossRef]
- Bauer, G. Threats to the freshwater pearl mussel Margaritifera margaritifera L. in central Europe. Biol. Conserv. 1988, 45, 239–253. [Google Scholar] [CrossRef]
- Rowe, C.L. The calamity of so long life: Life histories, contaminants, and potential emerging threats to long-lived vertebrates. Bioscience 2008, 58, 623–631. [Google Scholar] [CrossRef]
- Pandolfo, T.J.; Cope, W.G.; Arellano, C.; Bringolf, R.B.; Barnhart, M.C.; Hammer, E. Upper thermal tolerances of early life stages of freshwater mussels. J. N. Am. Benthol. Soc. 2010, 29, 959–969. [Google Scholar] [CrossRef]
- Taeubert, J.E.; Gum, B.; Geist, J. Variable development and excystment of freshwater pearl mussel (Margaritifera margaritifera L.) at constant temperature. Limnologica 2013, 43, 319–322. [Google Scholar] [CrossRef]
- Hastie, L.C.; Boon, P.J.; Young, M.R. Physical microhabitat requirements of freshwater pearl mussels, Margaritifera margaritifera (L.). Hydrobiologia 2000, 429, 59–71. [Google Scholar] [CrossRef]
- Geist, J.; Auerswald, K. Physicochemical stream bed characteristics and recruitment of the freshwater pearl mussel (Margaritifera margaritifera). Freshw. Biol. 2007, 52, 2299–2316. [Google Scholar] [CrossRef]
- Gum, B.; Lange, M.; Geist, J. A critical reflection on the success of rearing and culturing juvenile freshwater mussels with a focus on the endangered freshwater pearl mussel (Margaritifera margaritifera L.). Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 743–751. [Google Scholar] [CrossRef]
- Denic, M.; Taeubert, J.E.; Lange, M.; Thielen, F.; Scheder, C.; Gumpinger, C.; Geist, J. Influence of stock origin and environmental conditions on the survival and growth of juvenile freshwater pearl mussels (Margaritifera margaritifera) in a cross-exposure experiment. Limnologica 2015, 50, 67–74. [Google Scholar] [CrossRef]
- Haag, W.R.; Culp, J.J.; McGregor, M.A.; Bringolf, R.; Stoeckel, J.A. Growth and survival of juvenile freshwater mussels in streams: Implications for understanding enigmatic mussel declines. Freshw. Sci. 2019, 38, 753–770. [Google Scholar] [CrossRef]
- Schartum, E.; Mortensen, S.; Pittman, K.; Jakobsen, P.J. From pedal to filter feeding: Ctenidial organogenesis and implications for feeding in the postlarval freshwater pearl mussel Margaritifera margaritifera (Linnaeus, 1758). J. Molluscan Stud. 2017, 83, 36–42. [Google Scholar] [CrossRef]
- Lavictoire, L.; Ramsey, A.D.; Moorkens, E.A.; Souch, G.; Barnhart, M.C. Ontogeny of juvenile freshwater pearl mussels, Margaritifera margaritifera (Bivalvia: Margaritiferidae). PLoS ONE 2018, 13, e0193637. [Google Scholar] [CrossRef] [PubMed]
- Veniot, A.; Bricelj, V.; Beninger, P. Ontogenetic changes in gill morphology and potential significance for food acquisition in the scallop Placopecten magellanicus. Mar. Biol. 2003, 142, 123–131. [Google Scholar] [CrossRef]
- Carey, C.S.; Jones, J.W.; Hallerman, E.M.; Butler, R.S. Determining optimum temperature for growth and survival of laboratory-propagated juvenile freshwater mussels. N. Am. J. Aquac. 2013, 75, 532–542. [Google Scholar] [CrossRef]
- Buddensiek, V. The culture of juvenile freshwater pearl mussels Margaritifera margaritifera L. in cages: A contribution to conservation programmes and the knowledge of habitat requirements. Biol. Conserv. 1995, 74, 33–40. [Google Scholar] [CrossRef]
- Eybe, T.; Thielen, F.; Bohn, T.; Sures, B. The first millimetre–rearing juvenile freshwater pearl mussels (Margaritifera margaritifera L.) in plastic boxes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2013, 23, 964–975. [Google Scholar] [CrossRef]
- Scheder, C.; Lerchegger, B.; Jung, M.; Csar, D.; Gumpinger, C. Practical experience in the rearing of freshwater pearl mussels (Margaritifera margaritifera): Advantages of a work-saving infection approach, survival, and growth of early life stages. Hydrobiologia 2014, 735, 203–212. [Google Scholar] [CrossRef]
- Lavictoire, L.; Moorkens, E.; Ramsey, A.D.; Sinclair, W.; Sweeting, R.A. Effects of substrate size and cleaning regime on growth and survival of captive-bred juvenile freshwater pearl mussels, Margaritifera margaritifera (Linnaeus, 1758). Hydrobiologia 2016, 766, 89–102. [Google Scholar] [CrossRef]
- da Silva, J.P.; Gonçalves, D.V.; Lopes-Lima, M.; Anastácio, P.M.; Banha, F.; Frimpong, E.; Gama, M.; Miranda, R.; Reis, J.; Filipe, A.F.; et al. Predicting climatic threats to an endangered freshwater mussel in Europe: The need to account for fish hosts. Freshw. Biol. 2022, 67, 842–856. [Google Scholar] [CrossRef]
- Brauns, M.; Berendonk, T.; Berg, S.; Grunicke, F.; Kneis, D.; Krenek, S.; Schiller, T.; Schneider, J.; Wagner, A.; Weitere, M. Stable isotopes reveal the importance of terrestrially derived resources for the diet of the freshwater pearl mussel (Margaritifera margaritifera). Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 2496–2505. [Google Scholar] [CrossRef]
- Grunicke, F.; Wagner, A.; von Elert, E.; Weitere, M.; Berendonk, T. Riparian detritus vs. stream detritus: Food quality determines fitness of juveniles of the highly endangered freshwater pearl mussels (Margaritifera margaritifera). Hydrobiologia 2023, 850, 729–746. [Google Scholar] [CrossRef]
- Baer, O. Die Flussperlmuschel Margaritifera margaritifera (L.): Ökologie, umweltbedingte Reaktionen und Schutzproblematik einer vom Aussterben bedrohten Tierart. Die neue Brehm-Bücherei Bd. 619. Westarp Wissenschaften; Spektrum Akademischer Verlag: Magdeburg/Heidelberg, Germany, 1995; 118p. [Google Scholar]
- Lange, M.; Selheim, H. Growing factors of juvenile freshwater pearl mussels and their characteristics in selected pearl mussel habitats in Saxony (Germany). Ferrantia 2011, 64, 30–37. [Google Scholar]
- Geist, J.; Thielen, F.; Lavictoire, L.; Hoess, R.; Altmueller, R.; Baudrimont, M.; Blaize, C.; Campos, M.; Carroll, P.; Daill, D.; et al. Captive breeding of European freshwater mussels as a conservation tool: A review. Aquat. Conserv. Mar. Freshw. Ecosyst. 2023, 33, 1321–1359. [Google Scholar] [CrossRef]
- Jecke, F.; Denic, M.; Bayerl, H.; Findeis, T.; Geist, J.; Grunicke, F.; Schmidt, T.; Wagner, A.; Berendonk, T.U. Projekt ArKoNaVera: Sechs Jahre Artenschutz für die Flussperlmuschel (Margaritifera margaritifera). Nat. Und Landsch. 2022, 97, 373–380. [Google Scholar] [CrossRef]
- Hruška, J. Nahrungsansprüche der Flussperlmuschel und deren halbnatürliche Aufzucht in der Tschechischen Republik. Heldia 1999, 4, 69–79. [Google Scholar]
- Dury, P.; Pasco, P.Y.; Capoulade, M. Rearing and reinforcing Freshwater Pearl Mussel of the Armorican Massif. Programme LIFE+ NAT FR 2010–2013, 583. Poster, International Meeting “Improving the environment for the freshwater pearl mussel”, Kefermarkt, Österreich. 13–14 November 2013. Available online: https://www.life-moule-perliere.org/scripts/files/63f810b5317ed8.30261888/2013-11-13_poster_dopdf-br.pdf (accessed on 14 April 2017).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Hruška, J. Problematik der Rettung ausgewählter oligotropher Gewässersysteme und deren natürlicher Lebensgemeinschaften in der Tschechischen Republik. Lindberger Hefte 1995, 5, 98–123. [Google Scholar]
- Quinlan, E.; Gibbins, C.; Malcolm, I.; Batalla, R.; Vericat, D.; Hastie, L. A review of the physical habitat requirements and research priorities needed to underpin conservation of the endangered freshwater pearl mussel Margaritifera margaritifera. Aquat. Conserv. Mar. Freshw. Ecosyst. 2015, 25, 107–124. [Google Scholar] [CrossRef]
- Hyvärinen, H.S.; Chowdhury, M.M.R.; Taskinen, J. Pulsed flow-through cultivation of Margaritifera margaritifera: Effects of water source and food quantity on the survival and growth of juveniles. Hydrobiologia 2021, 848, 3219–3229. [Google Scholar] [CrossRef]
- Guo, F.; Kainz, M.J.; Sheldon, F.; Bunn, S.E. The importance of high-quality algal food sources in stream food webs–current status and future perspectives. Freshw. Biol. 2016, 61, 815–831. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Stengel, D.B. Interactive effects of light and temperature on pigments and n-3 LC-PUFA-enriched oil accumulation in batch-cultivated Pavlova lutheri using high-bicarbonate supply. Algal Res. 2017, 23, 113–125. [Google Scholar] [CrossRef]
- Dunca, E.; Mutvei, H. Comparison of microgrowth pattern in Margaritifera margaritifera shells from south and north Sweden. Am. Malacol. Bull. 2001, 16, 239–250. [Google Scholar]
- Archambault, J.M.; Cope, W.G.; Kwak, T.J. Survival and behaviour of juvenile unionid mussels exposed to thermal stress and dewatering in the presence of a sediment temperature gradient. Freshw. Biol. 2014, 59, 601–613. [Google Scholar] [CrossRef]
- Jost, J.; Helmuth, B. Morphological and ecological determinants of body temperature of Geukensia demissa, the Atlantic ribbed mussel, and their effects on mussel mortality. Biol. Bull. 2007, 213, 141–151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zwieniecki, M.A.; Newton, M. Influence of streamside cover and stream features on temperature trends in forested streams of western Oregon. West. J. Appl. For. 1999, 14, 106–113. [Google Scholar] [CrossRef]
- Newton, T.; Sauer, J.; Karns, B. Water and sediment temperatures at mussel beds in the upper Mississippi River basin. Freshw. Mollusk Biol. Conserv. 2013, 16, 53–62. [Google Scholar] [CrossRef]
- Hitt, N.P.; Snook, E.L.; Massie, D.L. Brook trout use of thermal refugia and foraging habitat influenced by brown trout. Can. J. Fish. Aquat. Sci. 2017, 74, 406–418. [Google Scholar] [CrossRef]
- Pandolfo, T.J.; Kwak, T.J.; Cope, W.G. Thermal tolerances of freshwater mussels and their host fishes: Species interactions in a changing climate. Freshw. Mollusk Biol. Conserv. 2012, 15, 69–82. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).