Fish and Bivalve Therapeutants in Freshwater Mussel Captive Breeding—A First Summary of Practical Experiences in European Facilities
Abstract
1. Introduction
2. Materials and Methods
2.1. Practical Experiences of Fish Therapeutant Used in Mussel Captive Breeding Programs
- Mussel species;
- Host fish species;
- Kind of pathogen;
- Time of pathogen occurrence (glochidial encystment, parasitic phase, excystment phase, juvenile mussel rearing);
- Prevalence of pathogen at time of treatment (low, medium, high);
- Treatment (remedy, dosage, duration of application per treatment, frequency);
- Treatment success (full recovery, low, medium, or high losses, full loss);
- Effect on mussels (harmful, no visible effect);
- Additional remarks.
2.2. Application of Virkon S in Fish and Juvenile Mussel Rearing Tanks
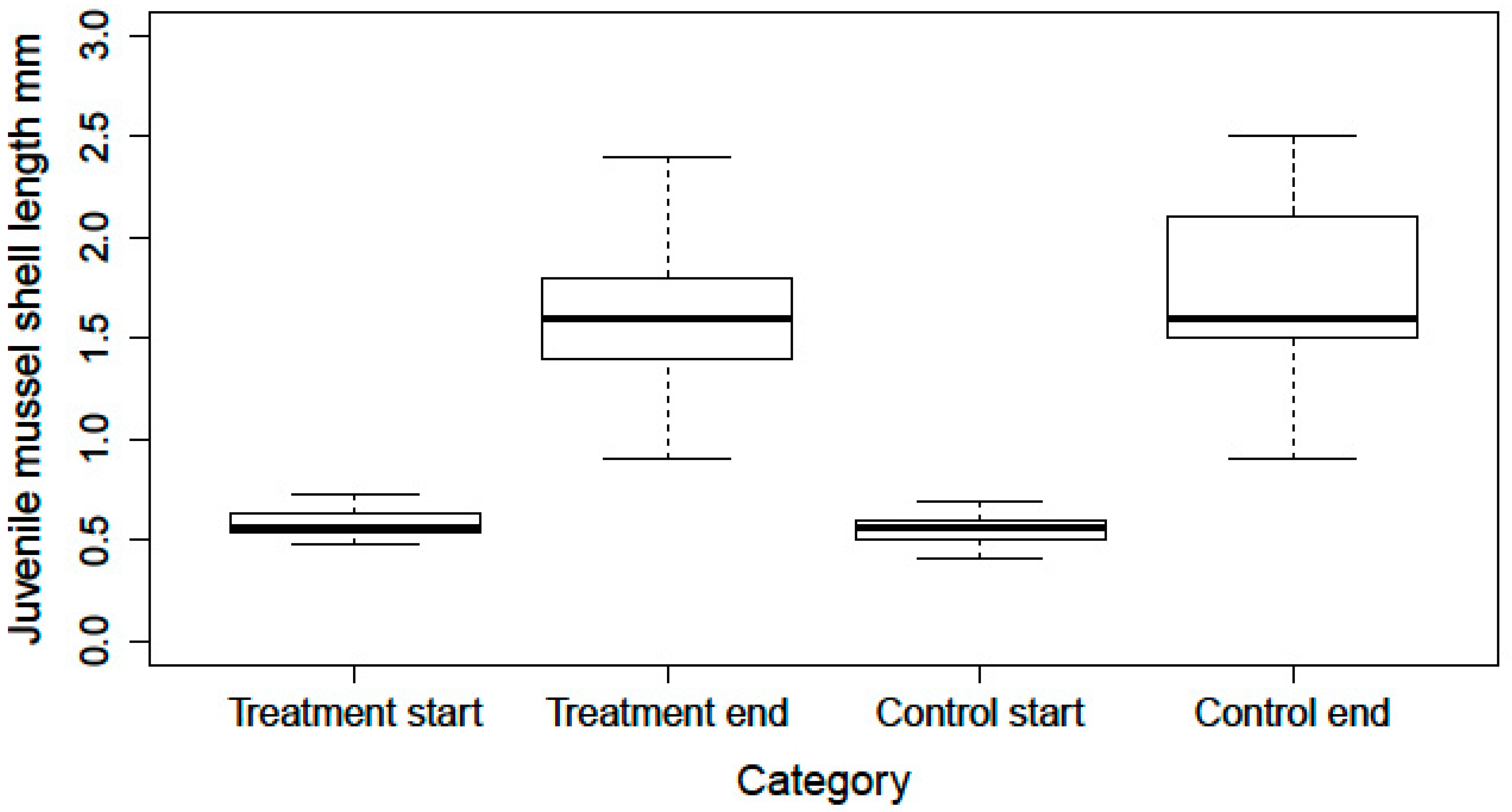
- Reactive treatment: Eight brown trout (Salmo trutta) during juvenile mussel excystment were reared in four standard 60 L glass aquaria. Each aquarium was separated into two compartments by a filter mat and stocked with two brown trout. Water was supplied from a pearl mussel stream and filtered over a 100 µm mesh before use. Water was recirculated with an exchange rate of 20% 1–2 times weekly. The water temperature was kept at 18 °C. Four fish in two aquaria exhibited symptoms of fungal skin infections, which was an opportunity for a preliminary test on the effect of Virkon S use for disinfection during juvenile mussel harvest. In these tanks, Virkon S was added to the water for 15 min on 3 consecutive days and on day 5. After each application, 80% of the water was exchanged and discarded into the sewage system. The two other aquaria with 4 healthy fish served as a control. During the complete excystment period, juvenile mussels were harvested daily and samples were checked for dead specimens. Furthermore, juvenile mussel survival and growth rates were monitored for a three-month period. Fish survival was followed up for one month after the treatment.
- Pro-active treatment: Six replicate rearing containers (Regalux clear box mini, Bahag AG, Mannheim, Germany) were set up, each with 10 juvenile, pedal-feeding freshwater pearl mussels. Juveniles had been reared for about four weeks after excystment to ensure only vital individuals were included. Three containers were treated twice with Virkon S on the first and third day of the experiment with an exposure lasting 15 min. The water was then completely changed but before use it was filtered through a 100 µm mesh. The other three replicates served as a control. In all containers, juvenile pearl mussels were fed with detritus, Nanno 3600® and Shellfish diet 1800® (both Reed Mariculture, Campbell, CA, USA), which were added at a concentration of 0.2 ppt each. Both groups were followed up for three months. Food and water were renewed every five days. Survival and growth rates between the beginning and the end of the experiment were calculated. The temperature was kept between 18–19 °C.
3. Results
3.1. Practical Experiences of Fish Therapeutant Used in Mussel Captive Breeding Programs
| Breeding Facility | Mussel Species | Host Fish Species | Pathogen | Occurrence Time | Prevalence at Time of Treatment | Treatment | Treatment Success | Effect on Mussels | Additional Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 9 | Margaritifera margaritifera | Salmo trutta/Salmo salar | Fungal infection | Glochidial encystment | medium | clay 1 kg/m3 | high | no | |
| 13 | Parasitic phase | low | Formaldehyde 35–38%, 250 ppt for 30 min, 2 days, 2 days rest, 1 day | high | no | ||||
| 2 | Parasitic and Excystment phase | low–medium | Salt, 2% for 20–30 min, 5 days | high | no | early application increases success | |||
| 10 | low | salt, different concentrations | high | no | |||||
| 2 | 4 g/m3 Virkon S, 3 days, 1 day pause, 1 day treatment | full recovery | no | ||||||
| 9 | Margaritifera margaritifera | Salmo trutta/Salmo salar | Ichthyobodo necator, Trichodina | Glochidial encystment | medium | Formalin 1:5000 | high | no | Do not use near to excystment |
| 5, 8 | Ichthyobodo necator | Parasitic and Excystment phase | low–medium | Salt, 2%, 60 min, up to 3 times per week | high–full recovery | no | |||
| 8 | Pyceze, 20 ppm | full recovery | no | ||||||
| 5 | Trichodina sp. | Parasitic and Excystment phase | medium | Salt, 2%, 60 min, up to 3 times per week | high | no | |||
| 13 | Ichthyobodo necator | low | Formaldehyde 35–38%, 250 ppt for 30 min, 2 days, 2 days rest, 1 day | medium | no | Treatment eliminates pathogen, but lesions in fins remain | |||
| 5 | Margaritifera margaritifera | Salmo trutta/Salmo salar | Dactylogyrus sp. | Parasitic and Excystment phase | low | Salt, 2%, 60 min, up to 3 times per week | high | no | |
| 5 | Margaritifera margaritifera | Salmo trutta/Salmo salar | Aeromonas salmonicida | Parasitic phase | high | florfénicol 20 mg active substance/kg fish/day, for 10 days; -> Nuflor® 300 mg florfénicol, oral, 1 mL/15 kg fish/day for 10 days, mixed with food | high | no | |
| 13 | low | Formaldehyde 35–38%, 250 ppt for 30 min, 2 days, 2 days rest, 1 day | medium | ||||||
| 5 | Margaritifera margaritifera | Salmo trutta/Salmo salar | Scyphidia sp. | Parasitic and Excystment phase | medium | Salt, 2%, 60 min, up to 3 times per week | high | no | |
| 5 | Margaritifera margaritifera | Salmo trutta/Salmo salar | Flavobacterium sp. | Parasitic phase | high | chloramin- T; bath, dosage dependend on pH: low pH, low dosage. pH +/− 7: 6 g/m3 for 60 min 1–2 times /day for 3 days, after that every 2 days for 3 treatments, after that every 3 days for another 3 treatments. Dosage can be lifted to a maximum of 15 g/m3 | full recovery | no | |
| 5 | Parasitic phase | high | florfénicol 20 mg active substance/kg fish/day, for 10 days; -> Nuflor® 300 mg florfénicol, oral, 1 mL/15 kg fish/day for 10 days, mixed with food | high | no obvious effect | ||||
| 2 | Excystment phase | low-medium | 4 g/m3 Virkon S, 3 days, 1 day pause, 1 day treatment | high | no | ||||
| 2 | Margaritifera margaritifera | Salmo trutta/Salmo salar | Ichthyophtirius multifiliis | Excystment phase | medium–high | Salt, 2% for 20–30 min, 5 days | low–medium | no | |
| 2 | Excystment phase | medium–high | 4 g/m3 Virkon S, 3 days, 1 day pause, 1 day treatment | high | no | ||||
| 1 | Glochidial encystment | high | Acetic acid, ppm in flow-through tanks once a day for 3 days | full loss | pathogen pressure probably already too high in combination with high water temperature | ||||
| 1 | Margaritifera margaritifera | Salmo trutta | different ectodermal pathogens | Glochidial encystment, excystment | low | Removal of infected fish/fish with unusual behavior, disinfection of tanks during glochidial encystment | high | no | |
| 12 | Pseudunio auricularius | Salaria fluviatilis | Ichthyophtirius multifiliis | Glochidial encystment, Parasitic phase | high | Salt baths (1–3% salinity), 30 min | medium | no | |
| 12 | Pseudunio auricularius | Acipenser baeri | no pathogens detected yet | ||||||
| 12 | Potomida littoralis | Barbus graellsi | Fungal infection | Before glochidial encystment | high | Acriflavine + sulfate and copper chloride | low-medium | not assessed | |
| 3, 4, 6, 7, 11 | Margaritifera margaritifera | Salmo trutta/Salmo salar | No treatment in parasitic and excystment phase | ||||||
| 5 | Unio crassus | Phoxinus phoxinus | No treatment in parasitic and excystment phase |
3.2. Application of Virkon S in Fish and Juvenile Mussel Rearing Tanks
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zieritz, A.; Sousa, R.; Aldridge, D.C.; Douda, K.; Esteves, E.; Ferreira-Rodríguez, N.; Mageroy, J.H.; Nizzoli, D.; Osterling, M.; Reis, J.; et al. A global synthesis of ecosystem services provided and disrupted by freshwater bivalve molluscs. Biol. Rev. 2022, 97, 1967–1998. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, C.C. Ecosystem services provided by freshwater mussels. Hydrobiologia 2018, 810, 15–27. [Google Scholar] [CrossRef]
- Atkinson, C.L.; Vaughn, C.C.; Forshay, K.J.; Cooper, J.T. Aggregated filter-feeding consumers alter nutrient limitation: Consequences for ecosystem and community dynamics. Ecology 2013, 94, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Spooner, D.E.; Vaughn, C.C. Context-dependent effects of freshwater mussels on stream benthic communities. Freshw. Biol. 2006, 51, 1016–1024. [Google Scholar] [CrossRef]
- Valenti, T.W.; Cherry, D.S.; Currie, R.J.; Neves, R.J.; Jones, J.W.; Mair, R.; Kane, C.M. Chlorine toxicity to early life stages of freshwater mussels (Bivalvia: Unionidae). Environ. Toxicol. Chem. 2006, 25, 2512–2518. [Google Scholar] [CrossRef]
- Ferreira-Rodríguez, N.; Beggel, S.; Geist, J.P.; Modesto, V.; Österling, M.; Riccardi, N.; Sousa, R.; Urbańska, M. Freshwater Mussels as Sentinels for Safe Drinking Water Supply in Europe. ACS ES&T Water 2023, 3, 3730–3735. [Google Scholar] [CrossRef]
- Augspurger, T.; Keller, A.E.; Black, M.C.; Cope, W.G.; Dwyer, F.J. Water quality guidance for protection of freshwater mussels (Unionidae) from ammonia exposure. Environ. Toxicol. Chem. 2006, 22, 2569–2575. [Google Scholar] [CrossRef]
- Allen, D.C.; Vaughn, C.C. Complex hydraulic and substrate variables limit freshwater mussel species richness and abundance. J. N. Am. Benthol. Soc. 2010, 29, 383–394. [Google Scholar] [CrossRef]
- Stoeckl, K.; Denic, M.; Geist, J. Conservation status of two endangered freshwater mussel species in Bavaria, Germany: Habitat quality, threats, and implications for conservation management. Aquat. Conserv. 2020, 30, 647–661. [Google Scholar] [CrossRef]
- Taeubert, J.E.; Martinez, A.M.P.; Gum, B.; Geist, J. The relationship between endangered thick-shelled river mussel (Unio crassus) and its host fishes. Biol. Conserv. 2012, 155, 94–103. [Google Scholar] [CrossRef]
- Strayer, D.L.; Downing, J.A.; Haag, W.R.; King, T.L.; Layzer, J.B.; Newton, T.J.; Nichols, J.S. Changing perspectives on pearly mussels, North America’s most imperiled animals. BioScience 2004, 54, 429–439. [Google Scholar] [CrossRef]
- Nakamura, K.; Sousa, R.; Mesquita-Joanes, F. Collapse of native freshwater mussel populations: Prospects of a long-term study. Biol. Conserv. 2023, 279, 109931. [Google Scholar] [CrossRef]
- da Silva, J.P.; Sousa, R.; Gonçalves, D.V.; Miranda, R.; Reis, J.; Teixeira, A.; Varandas, S.; Lopes-Lima, M.; Filipe, A.F. Streams in the Mediterranean Region are not for mussels: Predicting extinctions and range contractions under future climate change. Sci. Total Environ. 2023, 883, 163689. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, J.; Bespalaya, Y.; Bódis, E.; Burlakova, L.; Van Damme, D.; et al. Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biol. Rev. 2016, 92, 572–607. [Google Scholar] [CrossRef] [PubMed]
- Geist, J.; Thielen, F.; Lavictoire, L.; Hoess, R.; Altmueller, R.; Baudrimont, M.; Blaize, C.; Campos, M.; Carroll, P.; Daill, D.; et al. Captive breeding of European freshwater mussels as a conservation tool: A review. Aquat. Conserv. 2023, 33, 1321–1359. [Google Scholar] [CrossRef]
- Patterson, M.A.; Mair, R.A.; Eckert, N.L.; Gatenby, C.M.; Brady, T.; Jones, J.W.; Simmons, B.R.; Devers, J.L. Freshwater Mussel Propagation for Restoration, 1st ed.; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Douda, K. Host-dependent vitality of juvenile freshwater mussels: Implications for breeding programs and host evaluation. Aquaculture 2015, 445, 5–10. [Google Scholar] [CrossRef]
- Denic, M. Comparison of two different field cages for semi-natural rearing of juvenile freshwater pearl mussels, Margaritifera margaritifera (Linnaeus, 1758) (Bivalvia: Unionoidea: Margaritiferidae). Folia Malacol. 2018, 26, 189–195. [Google Scholar] [CrossRef]
- Geist, J.; Bayerl, H.; Stoeckle, B.C.; Kuehn, R. Securing genetic integrity in freshwater pearl mussel propagation and captive breeding. Sci. Rep. 2021, 11, 16019. [Google Scholar] [CrossRef]
- Waller, D.L.; Cope, W.G. The status of mussel health assessment and a path forward. Freshw. Mollusk Biol. Conserv. 2019, 22, 26–42. [Google Scholar] [CrossRef]
- Meyer, F.P. Communications: Solutions to the Shortage of Approved Fish Therapeutants. J. Aquat. Anim. Health 1989, 1, 78–80. [Google Scholar] [CrossRef]
- Lieke, T.; Meinelt, T.; Hoseinifar, S.H.; Pan, B.; Straus, D.L.; Steinberg, C.E. Sustainable aquaculture requires environmental-friendly treatment strategies for fish diseases. Rev. Aquacult. 2020, 12, 943–965. [Google Scholar] [CrossRef]
- Marwaha, J.; Jensen, K.H.; Jakobsen, P.J.; Geist, J. Duration of the parasitic phase determines subsequent performance in juvenile freshwater pearl mussels (Margaritifera margaritifera). Ecol. Evol. 2017, 7, 1375–1383. [Google Scholar] [CrossRef]
- Eybe, T.; Thielen, F.; Bohn, T.; Sures, B. The first millimetre–rearing juvenile freshwater pearl mussels (Margaritifera margaritifera L.) in plastic boxes. Aquat. Conserv. 2013, 23, 964–975. [Google Scholar] [CrossRef]
- Lavictoire, L.; Moorkens, E.; Ramsey, A.D.; Sinclair, W.; Sweeting, R.A. Effects of substrate size and cleaning regime on growth and survival of captive-bred juvenile freshwater pearl mussels, Margaritifera margaritifera (Linnaeus, 1758). Hydrobiologia 2016, 766, 89–102. [Google Scholar] [CrossRef]
- Tedesco, P.; Fioravanti, M.L.; Galuppi, R. In vitro activity of chemicals and commercial products against Saprolegnia parasitica and Saprolegnia delica strains. J. Fish Dis. 2019, 42, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.S.; Choi, T.J. The efficacy of Virkon S for the control of saprolegniasis in common carp, Cyprinus carpio L. PeerJ 2018, 6, e5706. [Google Scholar] [CrossRef] [PubMed]
- Koski, P.; Anttila, P.; Kuusela, J. Killing of Gyrodactylus salaris by heat and chemical disinfection. Acta Vet. Scand. 2015, 58, 1–6. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 28 November 2023).
- Nakamura, K.; Elbaile, E.; Salinas, C.; Mesquita-Joanes, F.; Sousa, R.; Guerrero-Campo, J.; Ruiz-Zarzuela, I.; de Blas, I. Captive breeding of Margaritifera auricularia (Spengler, 1793) and its conservation importance. Aquat. Conserv. 2019, 29, 1771–1784. [Google Scholar] [CrossRef]
- Modesto, V.; Ilarri, M.; Souza, A.T.; Lopes-Lima, M.; Douda, K.; Clavero, M.; Sousa, R. Fish and mussels: Importance of fish for freshwater mussel conservation. Fish Fish. 2018, 19, 244–259. [Google Scholar] [CrossRef]
- Karvonen, A.; Rintamäki, P.; Jokela, J.; Tellervo Valtonen, E. Increasing water temperature and disease risks in aquatic systems: Climate change increases the risk of some, but not all, diseases. Int. J. Parasitol. 2010, 40, 1483–1488. [Google Scholar] [CrossRef]
- Isaksen, T.E.; Karlsbakk, E.; Sundnes, G.A.; Nylund, A. Patterns of Ichthyobodo necator sensu stricto infections on hatchery-reared Atlantic salmon Salmo salar in Norway. Dis. Aquat. Org. 2010, 88, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Gillis, P.L.; Salerno, J.; McKay, V.L.; Bennett, C.J.; Lemon, K.L.; Rochfort, Q.J.; Prosser, R.S. Salt-laden winter runoff and freshwater mussels; assessing the effect on early life stages in the laboratory and wild mussel populations in receiving waters. Arch. Environ. Contam. Toxicol. 2022, 82, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Beggel, S.; Geist, J. Acute effects of salinity exposure on glochidia viability and host infection of the freshwater mussel Anodonta anatina (Linnaeus, 1758). Sci. Total Environ. 2015, 502, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Karreman, G.A.; Gaunt, P.S.; Endris, R.G.; Saint-Erne, N. Therapeutants for fish. In Fish Diseases and Medicine; Smith, A.S., Ed.; e-book; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 321–348. [Google Scholar]
- Rach, J.J.; Brady, T.; Schreier, T.M.; Aloisi, D. Safety of fish therapeutants to glochidia of the plain pocketbook mussel during encystment on largemouth bass. N. Am. J. Aquacult. 2006, 68, 348–354. [Google Scholar] [CrossRef]

| Treatment | Control | |||
|---|---|---|---|---|
| Aquarium 1 | Aquarium 2 | Aquarium 1 | Aquarium 2 | |
| Mean number of juvenile mussels harvested per fish. | 295 | 331 | 395 | 335 |
| Total number of juvenile mussels after harvest. | 590 | 662 | 790 | 670 |
| Total number of juvenile mussels after 3 months. | 544 | 601 | 737 | 622 |
| Survival rate in % after 3 months. | 92 | 91 | 93 | 93 |
| Average shell length in mm after 3 months. | 1.56 | 1.48 | 1.52 | 1.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denic, M.; Nakamura, K.; Varela-Dopico, C.; Strachan, B.; Daill, D.; Gaehrken, J.; Taylor, J.; Grunicke, F. Fish and Bivalve Therapeutants in Freshwater Mussel Captive Breeding—A First Summary of Practical Experiences in European Facilities. Diversity 2024, 16, 78. https://doi.org/10.3390/d16020078
Denic M, Nakamura K, Varela-Dopico C, Strachan B, Daill D, Gaehrken J, Taylor J, Grunicke F. Fish and Bivalve Therapeutants in Freshwater Mussel Captive Breeding—A First Summary of Practical Experiences in European Facilities. Diversity. 2024; 16(2):78. https://doi.org/10.3390/d16020078
Chicago/Turabian StyleDenic, Marco, Keiko Nakamura, Catarina Varela-Dopico, Ben Strachan, Daniel Daill, Jakob Gaehrken, John Taylor, and Felix Grunicke. 2024. "Fish and Bivalve Therapeutants in Freshwater Mussel Captive Breeding—A First Summary of Practical Experiences in European Facilities" Diversity 16, no. 2: 78. https://doi.org/10.3390/d16020078
APA StyleDenic, M., Nakamura, K., Varela-Dopico, C., Strachan, B., Daill, D., Gaehrken, J., Taylor, J., & Grunicke, F. (2024). Fish and Bivalve Therapeutants in Freshwater Mussel Captive Breeding—A First Summary of Practical Experiences in European Facilities. Diversity, 16(2), 78. https://doi.org/10.3390/d16020078







