Assessment of Common Ragweed (Ambrosia Artemisiifolia L.) Seed Predation in Crop Fields and Their Adjacent Semi-Natural Habitats in Hungary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Experimental Design
2.2. Assessment of Seed Predation
2.3. Weather Data Collection
2.4. Data Preparation and Statistical Analysis
3. Results
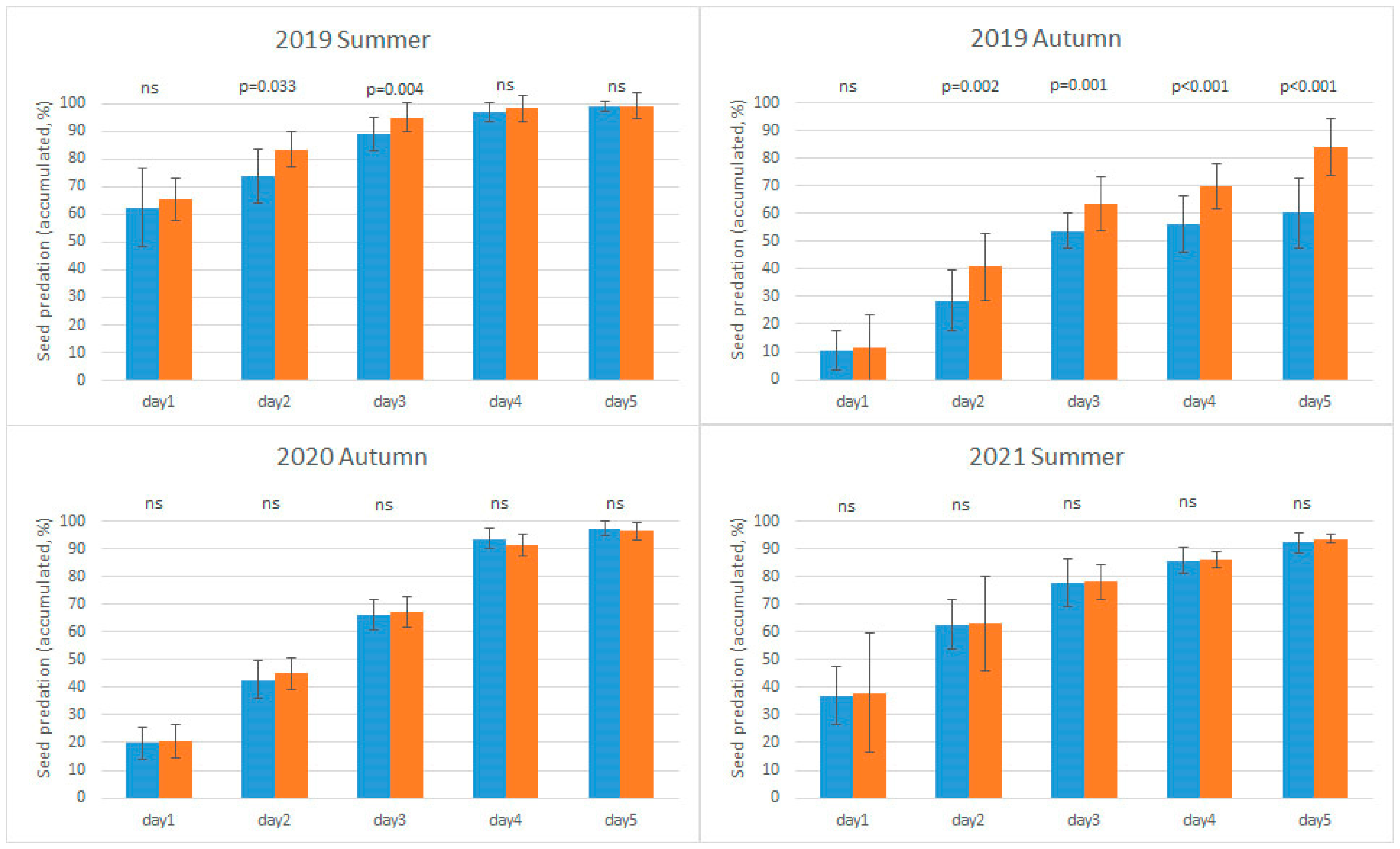
3.1. The Effects of Habitat and Survey Period on Seed Predation
3.2. The Effect of Weather Conditions on Seed Predation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Essl, F.; Biró, K.; Brandes, D.; Broennimann, O.; Bullock, J.M.; Chapman, D.S.; Chauvel, B.; Dullinger, S.; Fumanal, B.; Guisan, A.; et al. Biological Flora of the British Isles: Ambrosia artemisiifolia. J. Ecol. 2015, 103, 1069–1098. [Google Scholar]
- Müller-Schärer, H.; Chauvel, B.; Karrer, G.; Kazinczi, G.; Kudsk, P.; AGJM, O.L.; Schaffner, U.; Skjoth, C.; Smith, M.; Sun, Y.; et al. Cross-fertilizing weed science and plant invasion science to improve efficient management: An European challenge. Basic Appl. Ecol. 2018, 33, 1–13. [Google Scholar]
- Csontos, P.; Vitalos, M.; Barina, Z.; Kiss, L. Early distribution and spread of Ambrosia artemisiifolia in Central and Eastern Europe. Bot. Helv. 2010, 120, 75–78. [Google Scholar]
- Novák, R.; Magyar, M.; Simon, G.; Kadaravek, B.; Kadaravekné Guttyán, A.; Blazsek, K.; Erdélyi, K.; Farkas, G.; Gyulai, B.; Hornyák, A.; et al. Change in the spread of common ragweed in Hungary in the light of the national arable weed surveys (1947–2019). In Proceedings of the Conference of the International Ragweed Society, Budapest, Hungary, 8–9 February 2022. [Google Scholar]
- Fumanal, B.; Gaudot, I.; Bretagnolle, F. Seed-bank dynamics in the invasive plant, Ambrosia artemisiifolia L. Seed Sci. Res. 2008, 18, 101–114. [Google Scholar]
- Kazinczi, G.; Béres, I.; Pathy, Z.; Novák, R. Common ragweed (Ambrosia artemisiifolia L.): A review with special regards to the results in Hungary: II. Importance and harmful effect, allergy, habitat, allelopathy and beneficial characteristics. Herbologia 2008, 9, 93–118. [Google Scholar]
- Fumanal, B.; Plenchette, C.; Chauvel, B.; Bretagnolle, F. Which role can arbuscular mycorrhizal fungi play in the facilitation of Ambrosia artemisiifolia L. invasion in France? Mycorrhiza 2006, 17, 25–35. [Google Scholar]
- MacKay, J.; Kotanen, P.M. Local escape of an invasive plant, common ragweed (Ambrosia artemisiifolia L.), from above-ground and below-ground enemies in its native area. J. Ecol. 2008, 96, 1152–1161. [Google Scholar]
- Genton, B.J.; Shykoff, J.A.; Giraud, T. High genetic diversity in French invasive populations of common ragweed, Ambrosia artemisiifolia, as a result of multiple sources of introduction. Mol. Ecol. 2005, 14, 4275–4285. [Google Scholar]
- Friedman, J.; Barrett, S.C.H. High outcrossing in the annual colonizing species Ambrosia artemisiifolia (Asteraceae). Ann. Bot. 2008, 101, 1303–1309. [Google Scholar]
- Bohren, C.; Mermillod, G.; Delabays, N. Common ragweed (Ambrosia artemisiifolia L.) in Switzerland: Development of a nationwide concerted action. J. Plant Dis. Prot. 2006, 20, 497–503. [Google Scholar]
- Lavoie, C.; Jodoin, Y.; Merlis, A.G. How did common ragweed (Ambrosia artemisiifolia L.) spread in Quebec? A historical analysis using herbarium records. J. Biogeogr. 2007, 34, 1751–1761. [Google Scholar]
- Hunyadi, K.; Béres, I.; Kazinczi, G. Gyomnövények, Gyomirtás, Gyombiológia, 1st ed.; Mezőgazda Kiadó: Budapest, Hungary, 2000; pp. 477–585. [Google Scholar]
- Shergill, L.S.; Bejleri, K.; Davis, A.; Mirsky, S.B. Fate of weed seeds after impact mill processing in midwestern and mid-Atlantic United States. Weed Sci. 2020, 68, 92–97. [Google Scholar]
- Buttenschøn, R.M.; Waldispühl, S.; Bohren, C. Guidelines for Management of Common Ragweed, Ambrosia artemisiifolia; Technical Report; EUPHRESCO, University of Copenhagen: København, Denmark, 2010; p. 47. Available online: https://internationalragweedsociety.org/smarter/wp-content/uploads/Ambrosia-management-guidelines-2009_AMBROSIA-EUPHRESCO_eng.pdf (accessed on 25 July 2024).
- Dickerson, C.T. Studies on the Germination, Growth, Development and Control of Common Ragweed (Ambrosia artemisiifolia L.). Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 1968. [Google Scholar]
- Kiss, J.; Penksza, K.; Tóth, F.; Kádár, F. Evaluation of fields and field margins in nature production capacity with special regard to plant protection. Agric. Ecosys. Environ. 1997, 63, 227–232. [Google Scholar]
- Kádár, F.; Hatvani, A.; Kiss, J.; Tóth, F. Futóbogarak előfordulása őszibúza-táblában és a táblaszegélyben (Coleoptera: Carabidae). Növényvédelem 2004, 40, 53–59. [Google Scholar]
- Janzen, D.H. Seed predation by animals. Ann. Rev. Ecol. Syst. 1971, 2, 465–492. [Google Scholar]
- Hulme, P.E.; Benkman, C.W. Granivory. In Plant-Animal Interactions: An Evolutionary Approach, 1st ed.; Herrera, C.M., Pellmyr, O., Eds.; Wiley-Blackwell: Oxford, UK, 2002; Volume 26, pp. 132–154. [Google Scholar]
- Tooley, J.; Brust, J. Weed Seed Predation by Carabid beetles. In The Agroecology of Carabid Beetles, 1st ed.; Holland, J.M., Ed.; Intercept: Andover, UK, 2002; pp. 215–230. [Google Scholar]
- Begon, M.; Townsend, C.R.; Harper, J.L. Ecology. From Individuals to Ecosystems; Wiley-Blackwell: Oxford, UK, 2006; p. 752. [Google Scholar]
- Karban, R.; Agrawal, A.A.; Thaler, J.S.; Adler, L.S. Induced plant responses and information content about risk of herbivory. Trends Ecol. Evol. 1999, 14, 443–447. [Google Scholar] [PubMed]
- Fuller, P.J.; Hay, M.E. Is glue production by seeds of Salvia columbariae deterrent to desert granivores? Ecology 1983, 64, 960–963. [Google Scholar]
- Gallandt, E.R.; Molloy, T.; Lynch, R.P.; Drummond, F.A. Effect of cover-cropping systems on invertebrate seed predation. Weed Sci. 2005, 53, 69–76. [Google Scholar]
- Cromar, H.E.; Murphy, S.D.; Swanton, C.J. Influence of tillage and crop residue on postdispersal predation of weed seeds. Weed Sci. 1999, 47, 184–194. [Google Scholar] [CrossRef]
- Westerman, P.R.; Wes, J.S.; Kropff, M.J.; van der Werf, W. Annual losses of weed seeds due to predation in organic cereal fields. J. Appl. Ecol. 2003, 40, 824–836. [Google Scholar]
- Labruyere, S.; Bohan, A.D.; Biju-Duval, L.; Ricci, B.; Petit, S. Local, neighbor and landscape effects on the abundance of weed seed-eating carabids in arable fields: A nationwide analysis. Basic Appl. Ecol. 2016, 17, 230–239. [Google Scholar]
- Griffiths, E.; Wratten, S.D.; Vickerman, G.P. Foraging by the carabid Agonum dorsale in the field. Ecol. Entom. 1985, 10, 181–189. [Google Scholar]
- Cardina, J.; Noroquay, H.M.; Stinner, B.R.; McCartney, D.A. Post-dispersal predation of velvetleaf (Abutilon threophrasti) seeds. Weed Sci. 1996, 44, 534–539. [Google Scholar]
- Petit, S.; Boursault, A.; Bohan, D.A. Weed seed choice by carabid beetles (Coleoptera: Carabidae): Linking field measurements with laboratory diet assessments. Eur. J. Entomol. 2014, 111, 615–620. [Google Scholar]
- Kulkarni, S.S.; Dosdall, L.M.; Willenborg, C.J. The role of ground beetles (Coleoptera: Carabidae) in weed seed consumption: A review. Weed Sci. 2015, 63, 335–376. [Google Scholar]
- Petit, S.; Trichard, A.; Biju-Duval, L.; Mclaughlin, B.; Bohan, D.A. Interactions between conservation agricultural practice and landscape composition promote weed seed predation by invertebrates. Agricult. Ecosys. Environ. 2017, 240, 45–53. [Google Scholar] [CrossRef]
- Saska, P.; Honěk, A.; Martinková, Z. Preferences of carabid beetles (Coleoptera: Carabidae) for herbaceous seeds. Acta Zool. Acad. Sci. Hung. 2019, 65, 57–76. [Google Scholar]
- Kádár, F.; Lövei, G. Bogarak—Coleoptera. In Biológiai Védekezés Természetes Ellenségekkel, 1st ed.; Balázs, K., Mészáros, Z., Eds.; Mezőgazdasági Kiadó: Budapest, Hungary, 1989; pp. 117–125. [Google Scholar]
- Brown, P.R.; Singleton, G.R.; Tann, C.R.; Mock, I. Increasing sowing depth to reduce mouse damage to winter crops. Crop Prot. 2003, 22, 653–660. [Google Scholar]
- Harper, J.L. Population Biology of Plants; Academic Press: New York, NY, USA, 1977; p. 892. [Google Scholar]
- Shuler, R.E.; DiTommaso, A.; Losey, J.E.; Mohler, C.L. Postdispersal Weed Seed Predation Is Affected by Experimental Substrate. Weed Sci. 2008, 56, 889–895. [Google Scholar]
- Hulme, P.E. Post-dispersal seed predation in grassland: Its magnitude and sources of variation. J. Ecol. 1994, 82, 645–652. [Google Scholar]
- O’Rourke, M.E.; Heggenstaller, A.H.; Liebman, M.; Rice, M.E. Post-dispersal weed seed predation by invertebrates in conventional and low-external-input crop rotation systems. Agric. Ecosyst. Environ. 2006, 116, 280–288. [Google Scholar] [CrossRef]
- Baraivar, B. Seed Predation and Weed Seed Predation for Weed Control in Winter Cereals. Ph.D. Thesis, University of Lleida, Lleida, Spain, 2011. [Google Scholar]
- Daedlow, D.; Westerman, P.R.; Baraibar, B.; Rouphael, S.; Gerowitt, B. Weed seed predation rate in cereals as a function of seed density and patch size, under high predation pressure by rodents. Weed Res. 2014, 54, 186–195. [Google Scholar] [CrossRef]
- Kiss, J.; Kádár, F.; Kozma, E.; Tóth, I. Importance of various habitats in agricultural landscape related to integrated pest management: A preliminary study. Landsc. Urban Plan. 1993, 27, 191–198. [Google Scholar] [CrossRef]
- Kiss, J.; Kádár, F.; Tóth, I.; Kozma, E.; Tóth, F. Occurrence of predatory arthropods in winter wheat and in the field edge. Ecologie 1994, 25, 127–132. [Google Scholar]
- Booman, G.C.; Laterra, P.; Comparatore, V.; Murillo, N. Post-dispersal predation of weed seeds by small vertebrates: Interactive influences of neighbor land use and local environment. Agric. Ecosyst. Environ. 2009, 129, 277–285. [Google Scholar] [CrossRef]
- Deroulers, P.; Bretagnolle, V. The consumption pattern of 28 species of carabid beetles (Carabidae) to a weed seed, Viola arvensis. Bull. Entomol. Res. 2019, 109, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, M.; Maruyama, K.; Yamashita, M.; Sawada, H.; Inagaki, H.; Asai, M. Quantifying the ecosystem service of non-native weed seed predation in traditional terraced paddy fields. Weed Biol. Manag. 2021, 21, 192–201. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Trichard, A.; Ricci, B.; Ducourtieux, C.; Petit, S. The spatiotemporal distribution of weed seed predation differs between conservation agriculture and conventional tillage. Agricult. Ecosys. Environ. 2014, 188, 40–47. [Google Scholar] [CrossRef]
- Carbonne, B.; Bohan, D.A.; Petit, S. Key carabid species drive spring weed seed predation of Viola arvensis. Biol. Contr. 2020, 141, 104148. [Google Scholar] [CrossRef]
- Osman, M.G.A.; Szalai, M.; Zalai, M.; Dorner, Z.; Kiss, J. Measurement of post-dispersal invertebrate seed predation of some relevant weed species in maize fields in Hungary: An ecosystem service provided in crop fields contributing to weed management. Plant Prot. Sci. 2022, 58, 351–359. [Google Scholar] [CrossRef]
- Osman, M.G.A.; Szalai, M.; Zalai, M.; Dorner, Z.; Kiss, J. Assessing the Importance of natural Regulating Mechanisms in Weed Management: The Case of Weed Seed Predation in a Winter Wheat Field and in Adjacent Semi-Natural Habitat in Northern Hungary. Agronomy 2022, 12, 2666. [Google Scholar] [CrossRef]
- Dennis, P.; Fry, G.L.A. Field margins: Can they enhance natural enemy population densities and general arthropod diversity on farmland? Agric. Ecosys. Environ. 1992, 40, 95–115. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Jacob, S.H.; Minkey, D.M.; Gallagher, R.S.; Borger, C.P. Variation in post-dispersal weed seed predation in a crop field. Weed Sci. 2006, 54, 148–155. [Google Scholar] [CrossRef]
- González, E.; Seidl, M.; Kadlec, T.; Ferrante, M.; Knapp, M. Distribution of ecosystem services within oilseed rape fields: Effects of field defects on pest and weed seed predation rates. Agric. Ecosys. Environ. 2020, 295, 106894. [Google Scholar] [CrossRef]
- Frank, K.; Petrie, B.; Fisher, J.; Leggett, W.C. Transient dynamics of an altered large marine ecosystem. Nature 2011, 477, 86–89. [Google Scholar] [CrossRef]
- Crawley, M.J. Seed predators and population dynamics. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CAB International: Wallingford, UK, 1992; pp. 157–191. [Google Scholar]
- Alignier, A.; Meiss, H.; Petit, S.; Reboud, X. Variation of post-dispersal weed seed predation according to weed species, space and time. J. Plant Dis. Prot. 2008, 21, 221–226. [Google Scholar]
- Noroozi, S.; Alizadeh, H.; Mashhadi, H.R. Temperature influences postdispersal predation of weed seeds. Weed Biol. Manag. 2016, 16, 24–33. [Google Scholar] [CrossRef]
- Westerman, P.R. Are many little hammers effective? Velvetleaf (Abutilon theophrasti) population dynamics in two- and four-year crop rotation systems. Weed Sci. 2005, 53, 382–392. [Google Scholar] [CrossRef]
- Brust, G.E.; House, G.J. Weed seed destruction by arthropods and rodents in low-input soybean agroecosystems. Am. J. Altern. Agric. 1988, 3, 19–25. [Google Scholar]
- Swanton, C.J.; Griffith, J.T.; Cromar, H.E.; Booth, B.D. Pre and post-dispersal weed seed predation and its implications to agriculture. In Proceedings of the Brighton Crop Protection Council Conference, Brighton, UK, 15–18 November 1999; pp. 829–834. [Google Scholar]
- Leskovsek, R.; Datta, A.; Simoncic, A.; Knezevic, S.Z. Influence of nitrogen and plant density on the growth and seed production of common ragweed (Ambrosia artemisiifolia L.). J. Pest. Sci. 2012, 85, 527–539. [Google Scholar] [CrossRef]
| Weather Factors [Unit] | 2019 Summer | 2019 Autumn | 2020 Autumn | 2021 Summer |
|---|---|---|---|---|
| Avg. (Min–Max) | ||||
| Minimum temperature [°C] | 18.7 (17.3–20.5) | 5.7 (0.9–11.1) | 7.2 (3.6–10.8) | 17.8 (16.1–22.3) |
| Maximum temperature [°C] | 29.1 (26.6–32.7) | 11.4 (9.8–14.6) | 14.9 (13.4–16.7) | 31.8 (29.7–37.0) |
| Average temperature [°C] | 23.9 (21.4–26.9) | 8.0 (5.4–12.2) | 10.5 (9.3–12.1) | 25.1 (23.3–28.7) |
| Relative humidity [%] | 77.0 (73.4–81.7) | 87.5 (80.3–92.2) | 90.2 (84.8–93.8) | 62.9 (59.7–64.8) |
| Air pressure [mbar] | 1019 (1015–1023) | 1009 (997–1025) | 1016 (1012–1020) | 1013 (1011–1015) |
| Precipitation [mm] | 2.4 (0.0–11.8) | 4.1 (0.0–14.0) | 1.7 (0.0–7.6) | 0.0 (0.0–0.0) |
| Solar radiation [Wh m−2] | 6199 (5014–7438) | 1120 (596–2402) | 1394 (580–2234) | 6882 (5800–7482) |
| Factor Variable | d.f. | ANOVA | Tukey HSD/T-Test | |||
|---|---|---|---|---|---|---|
| F | p-Value | Group | Avg. Value (Pred.%) | Sign. * | ||
| Habitat | 1 | 37.840 | <0.001 | Crop field | 87.85 | a |
| SNH | 93.64 | b | ||||
| Period | 3 | 153.334 | <0.001 | 2019 Summer | 99.13 | C |
| 2019 Autumn | 72.43 | A | ||||
| 2020 Autumn | 97.05 | C | ||||
| 2021 Summer | 93.00 | B | ||||
| Habitat × Period | 3 | 31.372 | <0.001 | |||
| Weather Factors | Summer Periods | Autumn Periods | ||||||
|---|---|---|---|---|---|---|---|---|
| Crop Field | SNH | Crop Field | SNH | |||||
| p | Corr. | p | Corr. | p | Corr. | p | Corr. | |
| Minimum temperature | ns | ns | <0.001 | 0.43 | <0.001 | 0.49 | ||
| Maximum temperature | ns | 0.001 | −0.28 | ns | ns | |||
| Average temperature | ns | 0.023 | −0.17 | <0.001 | 0.40 | <0.001 | 0.37 | |
| Humidity | 0.001 | 0.25 | <0.001 | 0.40 | <0.001 | 0.46 | <0.001 | 0.55 |
| Air pressure | <0.001 | 0.42 | <0.001 | 0.34 | ns | ns | ||
| Precipitation | 0.005 | 0.20 | 0.013 | 0.19 | ns | ns | ||
| Solar radiation | ns | 0.000 | −0.31 | 0.000 | −0.35 | <0.001 | −0.52 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorner, Z.; Osman, M.G.A.; Kukorellyné Szénási, Á.; Zalai, M. Assessment of Common Ragweed (Ambrosia Artemisiifolia L.) Seed Predation in Crop Fields and Their Adjacent Semi-Natural Habitats in Hungary. Diversity 2024, 16, 609. https://doi.org/10.3390/d16100609
Dorner Z, Osman MGA, Kukorellyné Szénási Á, Zalai M. Assessment of Common Ragweed (Ambrosia Artemisiifolia L.) Seed Predation in Crop Fields and Their Adjacent Semi-Natural Habitats in Hungary. Diversity. 2024; 16(10):609. https://doi.org/10.3390/d16100609
Chicago/Turabian StyleDorner, Zita, Mohammed Gaafer Abdelgfar Osman, Ágnes Kukorellyné Szénási, and Mihály Zalai. 2024. "Assessment of Common Ragweed (Ambrosia Artemisiifolia L.) Seed Predation in Crop Fields and Their Adjacent Semi-Natural Habitats in Hungary" Diversity 16, no. 10: 609. https://doi.org/10.3390/d16100609
APA StyleDorner, Z., Osman, M. G. A., Kukorellyné Szénási, Á., & Zalai, M. (2024). Assessment of Common Ragweed (Ambrosia Artemisiifolia L.) Seed Predation in Crop Fields and Their Adjacent Semi-Natural Habitats in Hungary. Diversity, 16(10), 609. https://doi.org/10.3390/d16100609







