Exploring the Diversity and Ancestry of Fine-Aroma Cacao from Tumaco, Colombia
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and DNA Extraction
2.2. Reduced Representation Library (RRL) Preparation and Sequencing
2.3. Data Processing and Sequencing Analysis
2.4. Population Structure, Genetic Diversity, and Maximum Likelihood Phylogenetic Reconstruction Analyses
3. Results
3.1. SNP Data
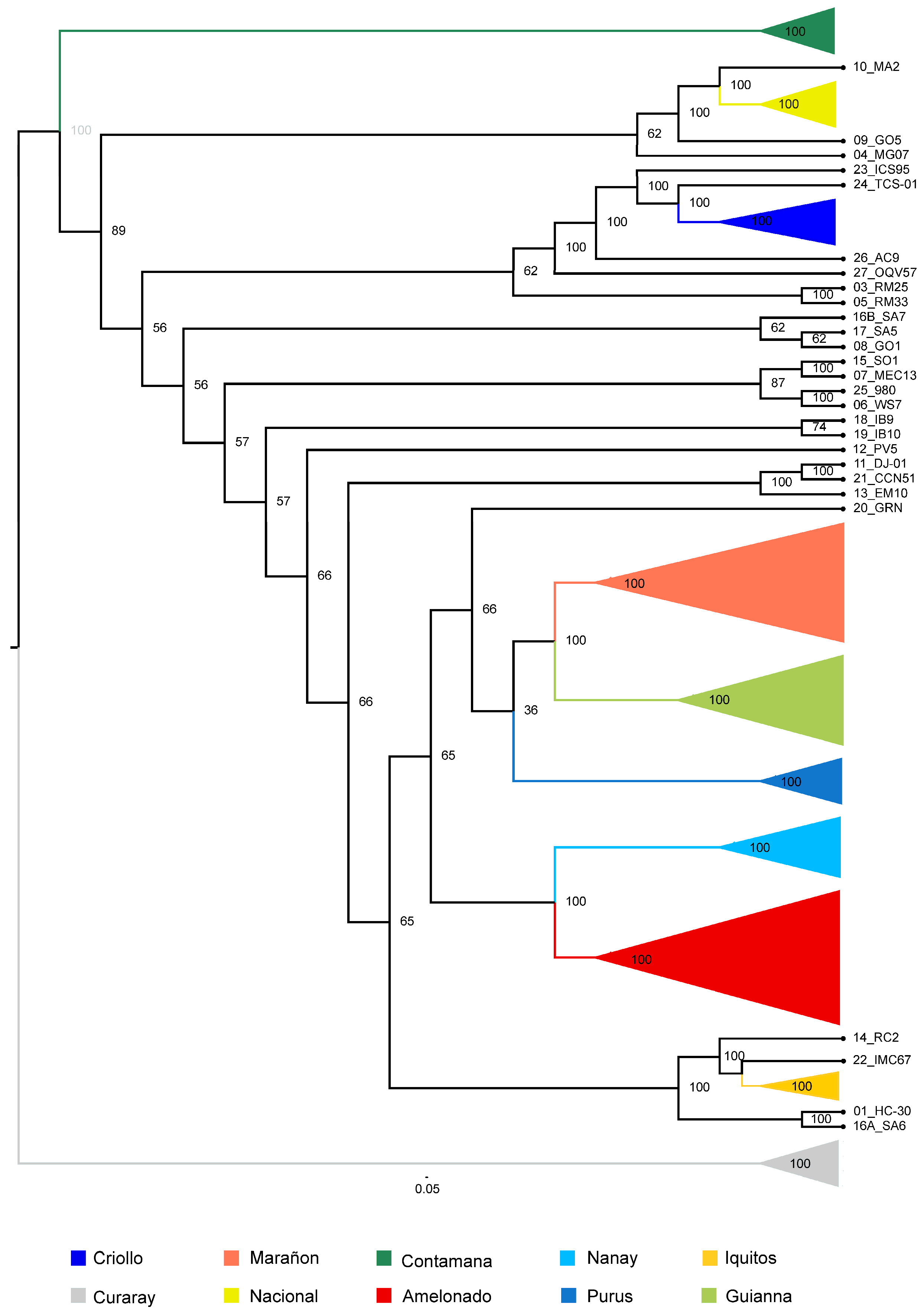
3.2. Analyses Using the Germplasm Data and Reference Populations (Dataset A)
3.3. Analysis Using Pacific Accessions from the Colombian Germplasm Bank (Dataset B)
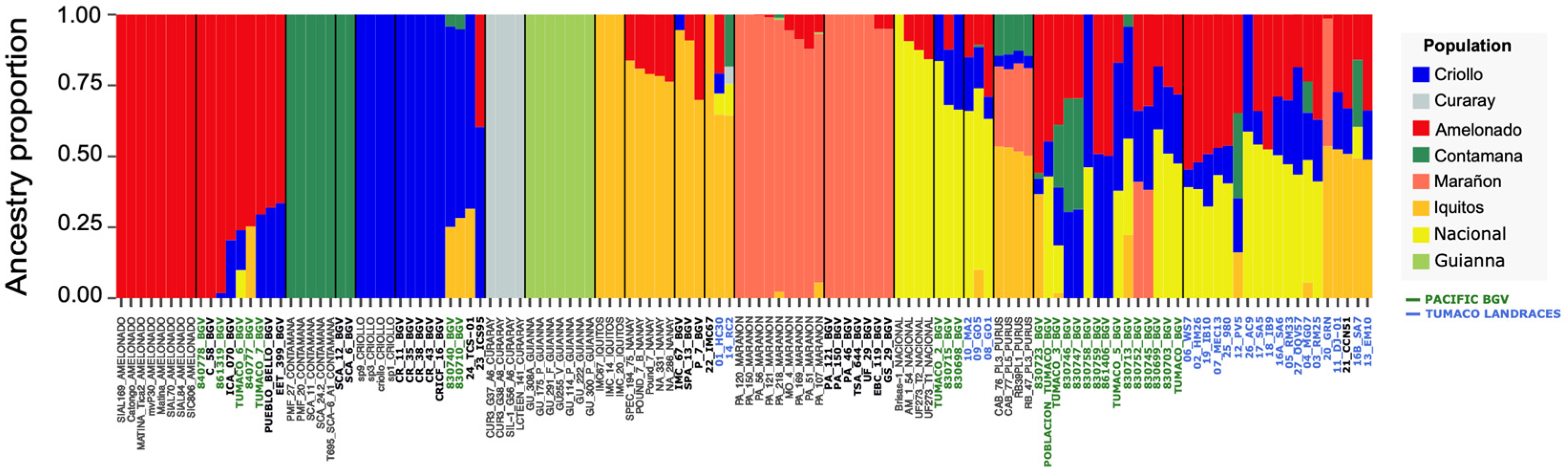
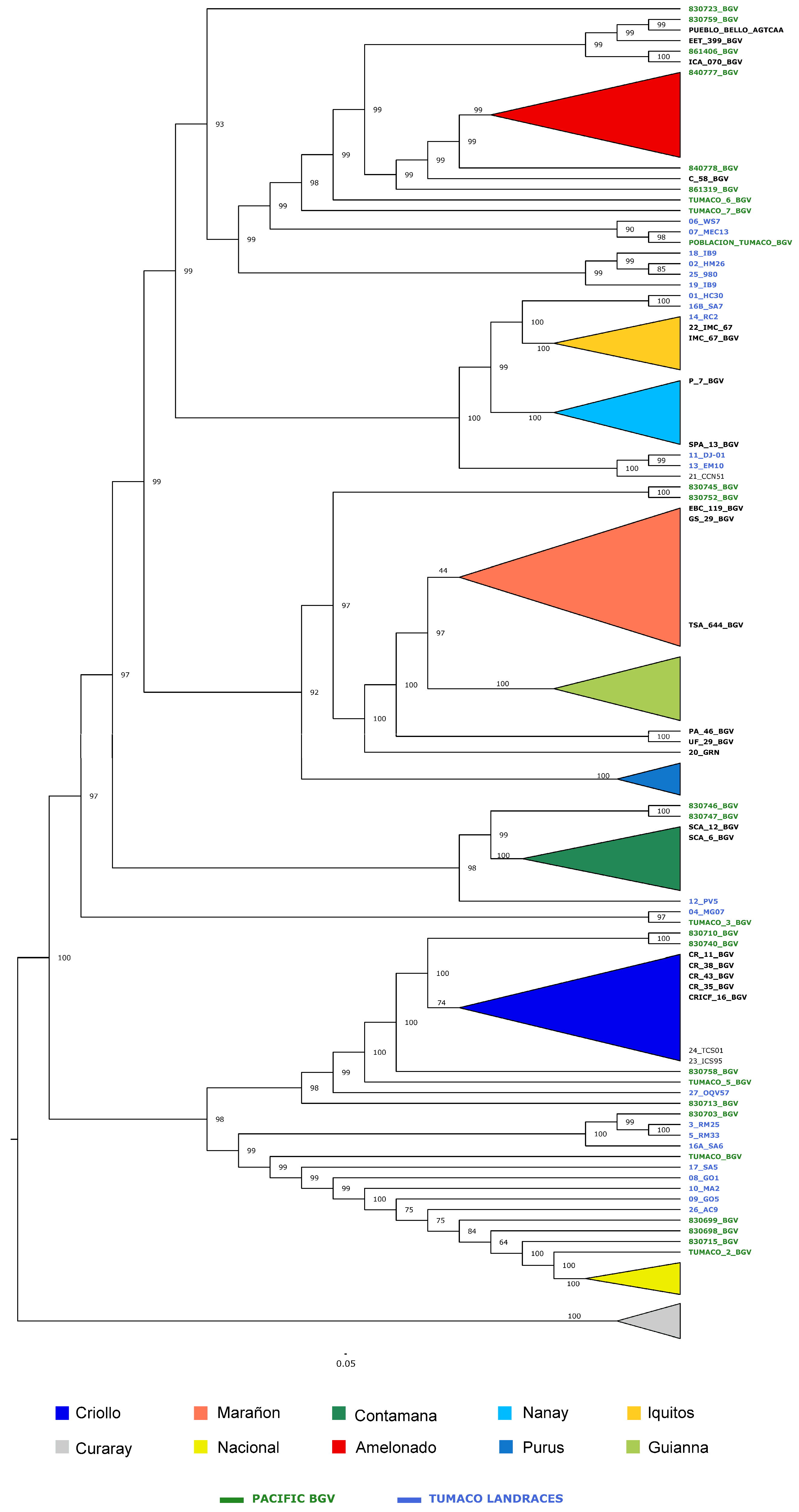
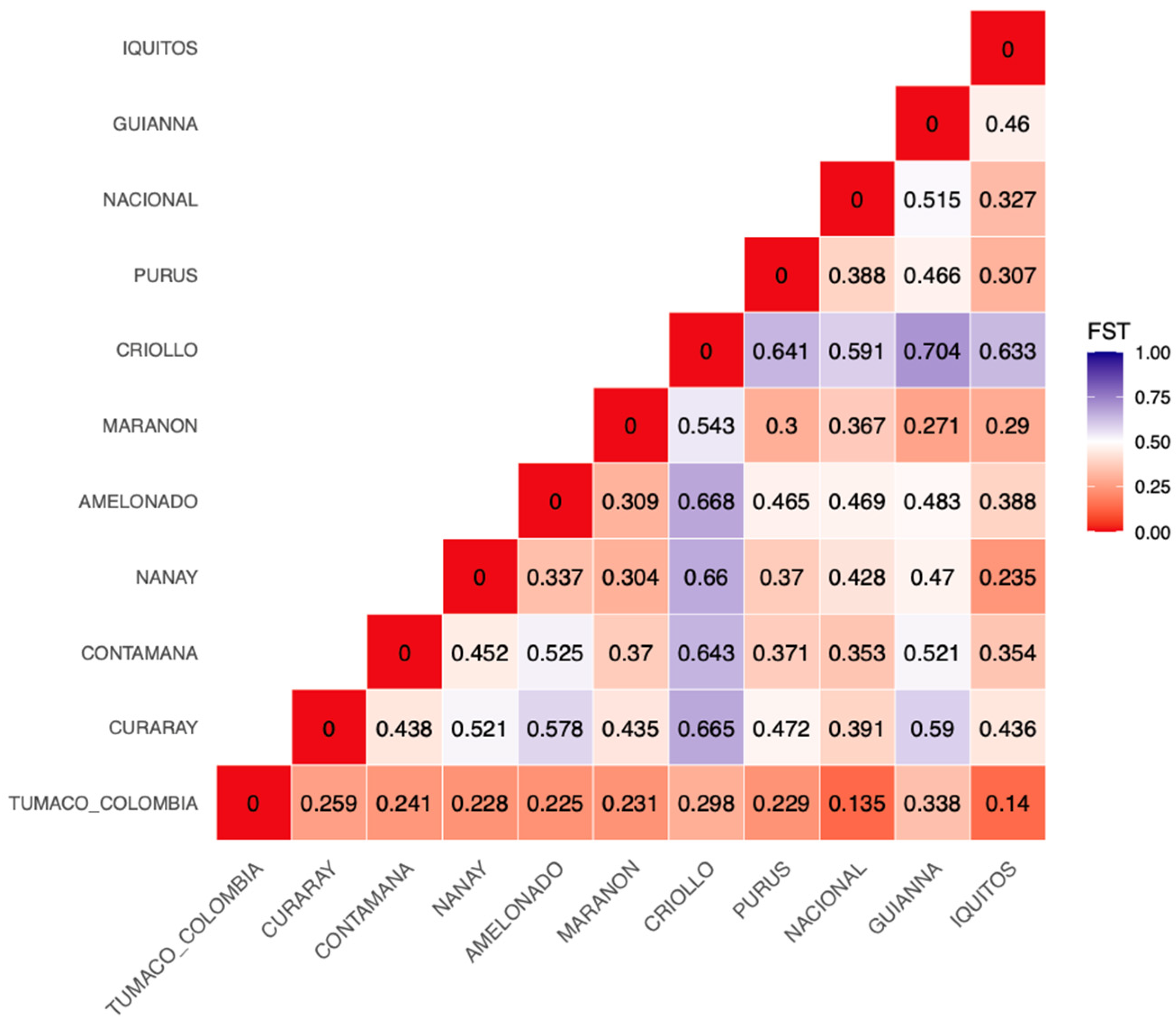
3.4. Analysis with the Reference Cacao Genetic Groups (Dataset C)
3.4.1. Genetic Structure and PCA with Reference Groups
3.4.2. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, D.; Motilal, L. Origin, Dispersal, and Current Global Distribution of Cacao Genetic Diversity. In Cacao Diseases: A History of Old Enemies and New Encounters; Bailey, B.A., Meinhardt, L.W., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–31. ISBN 978-3-319-24789-2. [Google Scholar]
- Valles, R.R. Comissão Executiva do Plano da Lavoura Cacaueira (CEPLAC); Centro de Pesquisas do Cacau (CEPEC). In Ciência, Tecnologia e Manejo Do Cacaueiro; CEPLAC/CEPEC/SEFIS: Ilhéus, Brazil, 2012. [Google Scholar]
- Federación Nacional de Cacaoteros (FEDECACAO). Presentación: El Sector Cacaotero En Colombia En Reunión de Acercamiento FEDECACAO—Incentivo al Seguro Agropecuario ISA 2020; FEDECACAO-MADR-FASECOLDA-FINAGRO: Bogotá, Colombia, 2020. [Google Scholar]
- Montoya-Restrepo, I.A.; Montoya-Restrepo, L.A.; Lowy-Ceron, P.D. Oportunidades Para La Actividad Cacaotera En El Municipio de Tumaco, Nariño, Colombia. Entramado 2015, 11, 48–59. [Google Scholar] [CrossRef]
- Lafaux Castillo, M.P. Evaluación de Dos Sistemas de Producción Del Cultivo de Cacao (Theobroma cacao L.), En La Vereda San Luis Robles Tumaco y Sus Impactos Socioeconómicos y Ambientales. Master’s Thesis, Universidad de Manizales, Manizales, Colombia, 2022. [Google Scholar]
- Bacca, P.P.; Alarcon, K.A.; González, J.C.; Guzmán, F.A.; Coronado, R.A.; Romero Barrera, Y. Evaluación de Cuatro Genotipos de Cacao En Nariño, Colombia. Rev. Mex. Cienc. Agric. 2023, 14, e3331. [Google Scholar] [CrossRef]
- Unidad de Planificación Rural Agropecuaria (UPRA), Evaluaciones Agropecuarias Municipales. Agronet, 2021. Available online: https://www.agronet.gov.co/estadistica/paginas/home.aspx?cod=1 (accessed on 4 November 2024).
- Ballesteros, W. Caracterización Morfológica de Árboles Elite de Cacao (Theobroma cacao L.) en el municipio de Tumaco, Nariño, Colombia. Master’s Thesis, Universidad de Nariño, Pasto, Colombia, 2011. [Google Scholar]
- Agencia UNAL. Cacaoteros y Transformadores de Cacao de Tumaco Reciben Asistencia Técnica Especializada de La UNAL. Agencia UNAL, 2024. Available online: https://agenciadenoticias.unal.edu.co/detalle/cacaoteros-y-transformadores-de-cacao-de-tumaco-reciben-asistencia-tecnica-especializada-de-la-unal (accessed on 5 November 2024).
- Rodriguez-Medina, C.; Arana, A.C.; Sounigo, O.; Argout, X.; Alvarado, G.A.; Yockteng, R. Cacao Breeding in Colombia, Past, Present and Future. Breed. Sci. 2019, 69, 373–382. [Google Scholar] [CrossRef]
- Hyman, S. CCN-51: Are We Barking up the Wrong (Fruit) Tree? 2024. Available online: https://cocoarunners.com/blog/ccn-51-are-we-barking-up-the-wrong-fruit-tree (accessed on 5 November 2024).
- Boza, E.J.; Motamayor, J.C.; Amores, F.M.; Cedeño-Amador, S.; Tondo, C.L.; Livingstone, D.S.; Schnell, R.J.; Gutiérrez, O.A. Genetic Characterization of the Cacao Cultivar CCN 51: Its Impact and Significance on Global Cacao Improvement and Production. J. Am. Soc. Hortic. Sci. 2014, 139, 219–229. [Google Scholar] [CrossRef]
- Motamayor, J.C.; Mockaitis, K.; Schmutz, J.; Haiminen, N.; Livingstone, D., III; Cornejo, O.; Findley, S.D.; Zheng, P.; Utro, F.; Royaert, S.; et al. The Genome Sequence of the Most Widely Cultivated Cacao Type and Its Use to Identify Candidate Genes Regulating Pod Color. Genome Biol. 2013, 14, r53. [Google Scholar] [CrossRef]
- Thomas, E.; van Zonneveld, M.; Loo, J.; Hodgkin, T.; Galluzzi, G.; van Etten, J. Present Spatial Diversity Patterns of Theobroma cacao L. in the Neotropics Reflect Genetic Differentiation in Pleistocene Refugia Followed by Human-Influenced Dispersal. PLoS ONE 2012, 7, e47676. [Google Scholar] [CrossRef]
- Osorio-Guarín, J.A.; Berdugo-Cely, J.; Coronado, R.A.; Zapata, Y.P.; Quintero, C.; Gallego-Sánchez, G.; Yockteng, R. Colombia a Source of Cacao Genetic Diversity As Revealed by the Population Structure Analysis of Germplasm Bank of Theobroma cacao L. Front. Plant Sci. 2017, 8, 1994. [Google Scholar] [CrossRef]
- Osorio-Guarín, J.A.; Berdugo-Cely, J.A.; Coronado-Silva, R.A.; Baez, E.; Jaimes, Y.; Yockteng, R. Genome-Wide Association Study Reveals Novel Candidate Genes Associated with Productivity and Disease Resistance to Moniliophthora spp. in Cacao (Theobroma cacao L.). G3 Genes Genomes Genet. 2020, 10, 1713–1725. [Google Scholar] [CrossRef]
- González-Orozco, C.E.; Osorio-Guarín, J.A.; Yockteng, R. Phylogenetic Diversity of Cacao (Theobroma cacao L.) Genotypes in Colombia. Plant Genet. Resour. 2022, 20, 203–214. [Google Scholar] [CrossRef]
- Ruiz Erazo, X.A. Diversidad Genética de Cacao Theobroma cacao L. Con Marcadores Moleculares Microsatelites; Universidad Nacional de Colombia-Sede Palmira: Palmira, Colombia, 2015. [Google Scholar]
- Morillo, Y.; Morillo, A.C.; Muñoz, J.E.; Ballesteros, W.; Gonzalez, A. Caracterización Molecular Con Microsatélites Amplificados al Azar (RAMs) de 93 Genotipos de Cacao (Theobroma cacao L.). Agron. Colomb. 2014, 32, 315–325. [Google Scholar] [CrossRef]
- Motamayor, J.C.; Lachenaud, P.; da Silva e Mota, J.W.; Loor, R.; Kuhn, D.N.; Brown, J.S.; Schnell, R.J. Geographic and Genetic Population Differentiation of the Amazonian Chocolate Tree (Theobroma cacao L). PLoS ONE 2008, 3, e3311. [Google Scholar] [CrossRef]
- Zhang, D.; Boccara, M.; Motilal, L.; Butler, D.R.; Umaharan, P.; Mischke, S.; Meinhardt, L. Microsatellite Variation and Population Structure in the “Refractario” Cacao of Ecuador. Conserv. Genet. 2008, 9, 327–337. [Google Scholar] [CrossRef]
- Bustamante, D.E.; Motilal, L.A.; Calderon, M.S.; Mahabir, A.; Oliva, M. Genetic Diversity and Population Structure of Fine Aroma Cacao (Theobroma cacao L.) from North Peru Revealed by Single Nucleotide Polymorphism (SNP) Markers. Front. Ecol. Evol. 2022, 10, 895056. [Google Scholar] [CrossRef]
- Arevalo-Gardini, E.; Meinhardt, L.W.; Zuñiga, L.C.; Arévalo-Gardni, J.; Motilal, L.; Zhang, D. Genetic Identity and Origin of “Piura Porcelana” a Fine-Flavored Traditional Variety of Cacao (Theobroma cacao) from the Peruvian Amazon. Tree Genet. Genomes 2019, 15, 11. [Google Scholar] [CrossRef]
- Kilian, B.; Graner, A. NGS Technologies for Analyzing Germplasm Diversity in Genebanks. Brief. Funct. Genom. 2012, 11, 38–50. [Google Scholar] [CrossRef]
- Peterson, B.K.; Weber, J.N.; Kay, E.H.; Fisher, H.S.; Hoekstra, H.E. Double Digest RADseq: An Inexpensive Method for De Novo SNP Discovery and Genotyping in Model and Non-Model Species. PLoS ONE 2012, 7, e37135. [Google Scholar] [CrossRef]
- Davey, J.W.; Hohenlohe, P.A.; Etter, P.D.; Boone, J.Q.; Catchen, J.M.; Blaxter, M.L. Genome-Wide Genetic Marker Discovery and Genotyping Using Next-Generation Sequencing. Nat. Rev. Genet. 2011, 12, 499–510. [Google Scholar] [CrossRef]
- Baird, N.A.; Etter, P.D.; Atwood, T.S.; Currey, M.C.; Shiver, A.L.; Lewis, Z.A.; Selker, E.U.; Cresko, W.A.; Johnson, E.A. Rapid SNP Discovery and Genetic Mapping Using Sequenced RAD Markers. PLoS ONE 2008, 3, e3376. [Google Scholar] [CrossRef]
- Osorio-Guarín, J.A.; Quackenbush, C.R.; Cornejo, O.E. Ancestry Informative Alleles Captured with Reduced Representation Library Sequencing in Theobroma cacao. PLoS ONE 2018, 13, e0203973. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 20 September 2023).
- Krueger, F. Trim Galore. Babraham Bioinformatics 2018. Available online: https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 20 September 2023).
- Argout, X.; Martin, G.; Droc, G.; Fouet, O.; Labadie, K.; Rivals, E.; Aury, J.M.; Lanaud, C. The Cacao Criollo Genome v2.0: An Improved Version of the Genome for Genetic and Functional Genomic Studies. BMC Genom. 2017, 18, 730. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce Framework for Analyzing Next-Generation DNA Sequencing Data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Broad Institute. Picard Tool Toolkit. 2019. Available online: http://broadinstitute.github.io/picard/ (accessed on 31 October 2024).
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T. The Variant Call Format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Cornejo, O.E.; Yee, M.-C.; Dominguez, V.; Andrews, M.; Sockell, A.; Strandberg, E.; Livingstone, D.; Stack, C.; Romero, A.; Umaharan, P.; et al. Population Genomic Analyses of the Chocolate Tree, Theobroma cacao L., Provide Insights into Its Domestication Process. Commun. Biol. 2018, 1, 167. [Google Scholar] [CrossRef]
- Danecek, P.; McCarthy, S.A. BCFtools/Csq: Haplotype-Aware Variant Consequences. Bioinformatics 2017, 33, 2037–2039. [Google Scholar] [CrossRef]
- Francis, R.M. Pophelper: An R Package and Web App to Analyse and Visualize Population Structure. Mol. Ecol. Resour. 2017, 17, 27–32. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.W.; Daly, M.J. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2. Wiley Interdiscip Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Alexander, D.H.; Lange, K. Enhancements to the ADMIXTURE Algorithm for Individual Ancestry Estimation. BMC Bioinform. 2011, 12, 246. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R Package for Genetic Analysis of Populations with Clonal, Partially Clonal, and/or Sexual Reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef]
- Gruber, B.; Unmack, P.J.; Berry, O.F.; Georges, A. Dartr: An r Package to Facilitate Analysis of SNP Data Generated from Reduced Representation Genome Sequencing. Mol. Ecol. Resour. 2018, 18, 691–699. [Google Scholar] [CrossRef]
- Granato, I.S.C.; Galli, G.; de Oliveira Couto, E.G.; e Souza, M.B.; Mendonça, L.F.; Fritsche-Neto, R. SnpReady: A Tool to Assist Breeders in Genomic Analysis. Mol. Breed. 2018, 38, 102. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Fouet, O.; Loor Solorzano, R.G.; Rhoné, B.; Subía, C.; Calderón, D.; Fernández, F.; Sotomayor, I.; Rivallan, R.; Colonges, K.; Vignes, H.; et al. Collection of Native Theobroma cacao L. Accessions from the Ecuadorian Amazon Highlights a Hotspot of Cocoa Diversity. Plants People Planet 2022, 4, 605–617. [Google Scholar] [CrossRef]
- Argout, X.; Droc, G.; Fouet, O.; Rouard, M.; Labadie, K.; Rhoné, B.; Rey Loor, G.; Lanaud, C. Pangenomic Exploration of Theobroma cacao: New Insights into Gene Content Diversity and Selection During Domestication. BioRxiv 2023. [Google Scholar] [CrossRef]
- Viana, C. Colombian Cocoa and Its Influence on World Patisseries. Colombia One. 2024. Available online: https://colombiaone.com/2024/11/09/colombian-cocoa (accessed on 4 December 2024).
- Pérez, E.; Guzmán, R.; Álvarez, C.; Lares, M.; Martínez, K.; Suniaga, G.; Pavani, A. Cacao, Cultura y Patrimonio: Un Hábitat de Aroma Fino En Venezuela. RIVAR 2021, 8, 146–162. [Google Scholar] [CrossRef]
- Sánchez Arizo, V.H.; Zambrano Mendoza, J.L.; Iglesias, C. La Cadena de Valor Del Cacao En América Latina y El Caribe; INIAP, Estación Experimental Santa Catalina: Quito, Ecuador, 2019. [Google Scholar]
- Pérez-Zuñiga, J.I.; Moreno, B.A.; Segura, J.; Mejia, R.J.; Ortiz, C.C.; Alarcon, K.A. Selección de Árboles Promisorios de Cacao (Theobroma cacao L.) Por Su Alta Producción En Dos Zonas Cacaoteras Del Departamento de Nariño. 2024; in preparation. [Google Scholar]
- Gopaulchan, D.; Motilal, L.A.; Kalloo, R.K.; Mahabir, A.; Moses, M.; Joseph, F.; Umaharan, P. Genetic Diversity and Ancestry of Cacao (Theobroma cacao L.) in Dominica Revealed by Single Nucleotide Polymorphism Markers. Genome 2020, 63, 583–595. [Google Scholar] [CrossRef]
- Lukman; Zhang, D.; Susilo, A.W.; Dinarti, D.; Bailey, B.; Mischke, S.; Meinhardt, L.W. Genetic Identity, Ancestry and Parentage in Farmer Selections of Cacao from Aceh, Indonesia Revealed by Single Nucleotide Polymorphism (SNP) Markers. Trop Plant Biol. 2014, 7, 133–143. [Google Scholar] [CrossRef]
- Céspedes-Del Pozo, W.H.; Blas-Sevillano, R.; Zhang, D. Assessing Genetic Diversity of Cacao (Theobroma cacao L.) Nativo Chuncho in La Convención, Cusco-Perú. In Proceedings of the International Symposium on Cocoa Research (ISCR), Lima, Peru, 13–17 November 2017. [Google Scholar]
- Wahlund, S. Zusammensetzung von Populationen Und Korrelationserscheinungen Vom Standpunkt Der Vererbungslehre Aus Betrachtet. Hereditas 1928, 11, 65–106. [Google Scholar] [CrossRef]
- Schnell, R.J.; Olano, C.T.; Brown, J.S.; Meerow, A.W.; Cervantes-Martinez, C.; Nagai, C.; Motamayor, J.C. Retrospective Determination of the Parental Population of Superior Cacao (Theobroma cacao L.) Seedlings and Association of Microsatellite Alleles with Productivity. J. Am. Soc. Hortic. Sci. 2005, 130, 181–190. [Google Scholar] [CrossRef]
- Motamayor, J.C.; Risterucci, A.-M.; Lopez, P.A.; Ortiz, C.F.; Moreno, A.; Lanaud, C. Cacao Domestication I: The Origin of the Cacao Cultivated by the Mayas. Heredity 2002, 89, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Martínez, W.J.; Johnson, E.S.; Somarriba, E.; Phillips-Mora, W.; Astorga, C.; Mischke, S.; Meinhardt, L.W. Genetic Diversity and Spatial Structure in a New Distinct Theobroma cacao L. Population in Bolivia. Genet. Resour. Crop Evol. 2012, 59, 239–252. [Google Scholar] [CrossRef]
- Thomas, E.; Imán Correa, S.A.; Atkinson, R.; Zavaleta, D.; Rodriguez, C.; Lastra, S.; Murrieta, E.; Farfán, A.; Castro, J.; Ramírez, J. Diversidad Genética de Cacao En El Perú. In Catalogue of Cocoas from Peru; Thomas, E., Lastra, S., Zavaleta, D., Eds.; Bioversity International: Rome, Italy, 2023. [Google Scholar]
- Bailey, B.A.; Meinhardt, L.W. Cacao Diseases; Bailey, B.A., Meinhardt, L.W., Eds.; Springer International Publishing: Cham, Switherland, 2016; ISBN 978-3-319-24787-8. [Google Scholar]
- Pound, F.J. Cacao and Witches’ Broom Disease (Marasmius Perniciosus) of South America with Notes on Other Species of Theobroma; Yuille’s Printery: Port of Spain, Trinidad and Tobago, 1938. [Google Scholar]
- Pound, F.J. A Note on the Cocoa Population of South America. In Report and Proceedings of the 1945 Cocoa Conference; The Colonial Office, His Majesty’s Stationary Office: London, UK, 1945; pp. 131–133. [Google Scholar]
- Allen, J.B. Geographical Variation and Population Biology in Wild Theobroma cacao. Ph.D. Thesis, The University of Edinburgh, Edinburgh, UK, 1988. [Google Scholar]
- Arévalo-Gardini, E.; Balbin-Coronado, V.; Zúñiga-Luna, H.; Chirinos, M. Genetic Diversity and Biochemical Characterization of Cacao (Theobroma cacao L.) Populations in the Peruvian Amazon. Genetic Resources and Crop Evolution. Genet. Resour. Crop Evol. 2019, 66, 1025–1037. [Google Scholar]
- Ceccarelli, V.; Lastra, S.; Loor Solórzano, R.G.; Chacón, W.W.; Nolasco, M.; Sotomayor Cantos, I.A.; Plaza Avellán, L.F.; López, D.A.; Fernández Anchundia, F.M.; Dessauw, D.; et al. Conservation and Use of Genetic Resources of Cacao (Theobroma cacao L.) by Gene Banks and Nurseries in Six Latin American Countries. Genet. Resour. Crop Evol. 2022, 69, 1283–1302. [Google Scholar] [CrossRef]
- Lanaud, C.; Vignes, H.; Utge, J.; Valette, G.; Rhoné, B.; Garcia Caputi, M.; Angarita Nieto, N.S.; Fouet, O.; Gaikwad, N.; Zarrillo, S.; et al. A Revisited History of Cacao Domestication in Pre-Columbian Times Revealed by Archaeogenomic Approaches. Sci. Rep. 2024, 14, 2972. [Google Scholar] [CrossRef]


| Dataset | Populations Included | Number of Samples | Number of SNPs |
|---|---|---|---|
| A | TP, RP, BGV | 295 | 359,950 |
| B | TP, RP, BGVss | 126 | 161,394 |
| C | TP, RP | 80 | 1,359,540 |
| Population | Number of Genotypes | Observed Heterozygosity (Ho) | Expected Heterozygosity (He) |
|---|---|---|---|
| AMELONADO | 10 | 0.15 | 0.12 |
| CONTAMANA | 4 | 0.18 | 0.16 |
| CRIOLLO | 4 | 0.02 | 0.02 |
| CURARAY | 4 | 0.11 | 0.12 |
| TUMACO | 23 | 0.15 | 0.23 |
| GUIANNA | 7 | 0.16 | 0.12 |
| IQUITOS | 3 | 0.31 | 0.19 |
| MARANON | 9 | 0.26 | 0.20 |
| NACIONAL | 4 | 0.27 | 0.19 |
| NANAY | 5 | 0.2 | 0.15 |
| PURUS | 4 | 0.17 | 0.15 |
| Genotype | Observed Heterozygosity (Ho) |
|---|---|
| 14_RC2 | 0.3 |
| 03_RM25 | 0.22 |
| 18_IB9 | 0.21 |
| 05_RM33 | 0.21 |
| 16A_SA6 | 0.16 |
| 06_WS7 | 0.16 |
| 09_GO5 | 0.16 |
| 12_PV5 | 0.15 |
| 15_SO1 | 0.15 |
| 17_SA5 | 0.15 |
| 11_DJ01 | 0.14 |
| 27_OQV57 | 0.14 |
| 07_MEC13 | 0.14 |
| 13_EM10 | 0.13 |
| 16B_SA7 | 0.13 |
| 20_GRN | 0.12 |
| 25_980 | 0.12 |
| 10_MA2 | 0.11 |
| 08_GO1 | 0.11 |
| 01_HC30 | 0.1 |
| 04_MG07 | 0.1 |
| 26_AC9 | 0.09 |
| 19_IB10 | 0.08 |
| Source | df | SS | MS | Var | % |
|---|---|---|---|---|---|
| Between Pops | 10 | 22,002,932 | 2,200,293.2 | 297,746.2 | 54.8 |
| Within Pops | 66 | 16,184,655 | 245,222.0 | 245,222.0 | 45.6 |
| Total | 76 | 38,187,587 | 502,468.3 | 542,968.2 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgadillo-Duran, P.; Berdugo-Cely, J.A.; Mejía-Salazar, J.; Pérez-Zúñiga, J.I.; Yockteng, R. Exploring the Diversity and Ancestry of Fine-Aroma Cacao from Tumaco, Colombia. Diversity 2024, 16, 754. https://doi.org/10.3390/d16120754
Delgadillo-Duran P, Berdugo-Cely JA, Mejía-Salazar J, Pérez-Zúñiga JI, Yockteng R. Exploring the Diversity and Ancestry of Fine-Aroma Cacao from Tumaco, Colombia. Diversity. 2024; 16(12):754. https://doi.org/10.3390/d16120754
Chicago/Turabian StyleDelgadillo-Duran, Paola, Jhon A. Berdugo-Cely, Julián Mejía-Salazar, José Ives Pérez-Zúñiga, and Roxana Yockteng. 2024. "Exploring the Diversity and Ancestry of Fine-Aroma Cacao from Tumaco, Colombia" Diversity 16, no. 12: 754. https://doi.org/10.3390/d16120754
APA StyleDelgadillo-Duran, P., Berdugo-Cely, J. A., Mejía-Salazar, J., Pérez-Zúñiga, J. I., & Yockteng, R. (2024). Exploring the Diversity and Ancestry of Fine-Aroma Cacao from Tumaco, Colombia. Diversity, 16(12), 754. https://doi.org/10.3390/d16120754







