The Mexican Balsam, Impatiens mexicana Rydb: A Redescription Based on Morphological and Phylogenetic Studies, with an Update of the Current Geographical Range of the Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material Sampling
2.2. Morphological Study
2.3. Phylogenetic and Genetic Distance Analysis
| Primer Name | Region-Primer Sequence | Source |
|---|---|---|
| ITS | ||
| ITS5 | 5′-GGAA GTA GAA GTC GTA ACA AGG-3′ | [63] |
| ITS4 | 5′-TCC TCC GCT TAT TGA TAT GC-3′ | |
| trnL-F | ||
| B49317 | 5′-CGAAATCGGTAGACGCTACG-3′ | [64] |
| A50272 | 5′-ATTTGAACTGGTGACACGAG-3′ | |
| atpB-rbcL | ||
| RBCL1 | 5′-GAATCCAACACTTGCTTTAGTCTCT-3′ | [65] |
| S2R | 5′ AGAAGTAGTAGGATTGATTCTCATA-3′ | |
3. Results
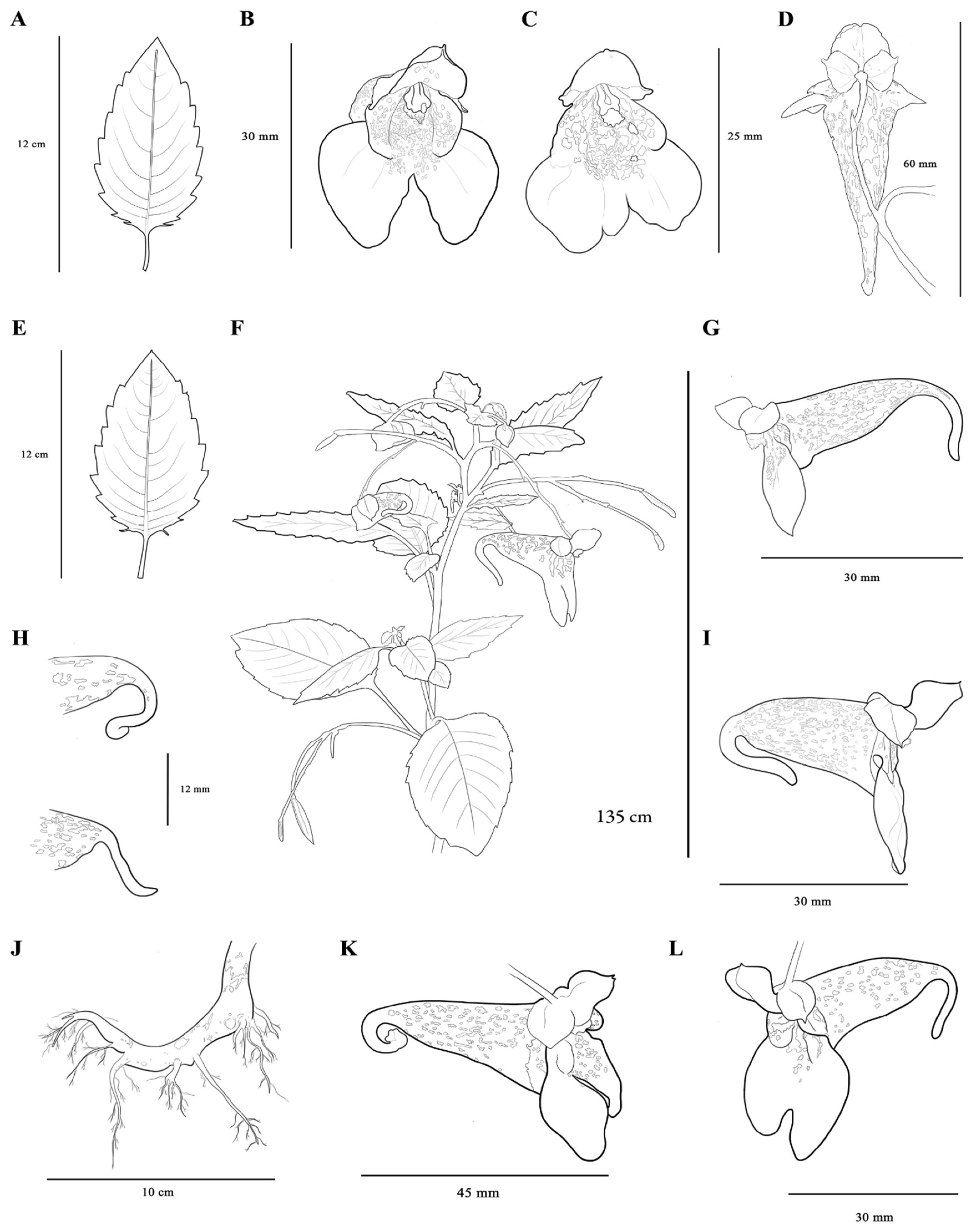
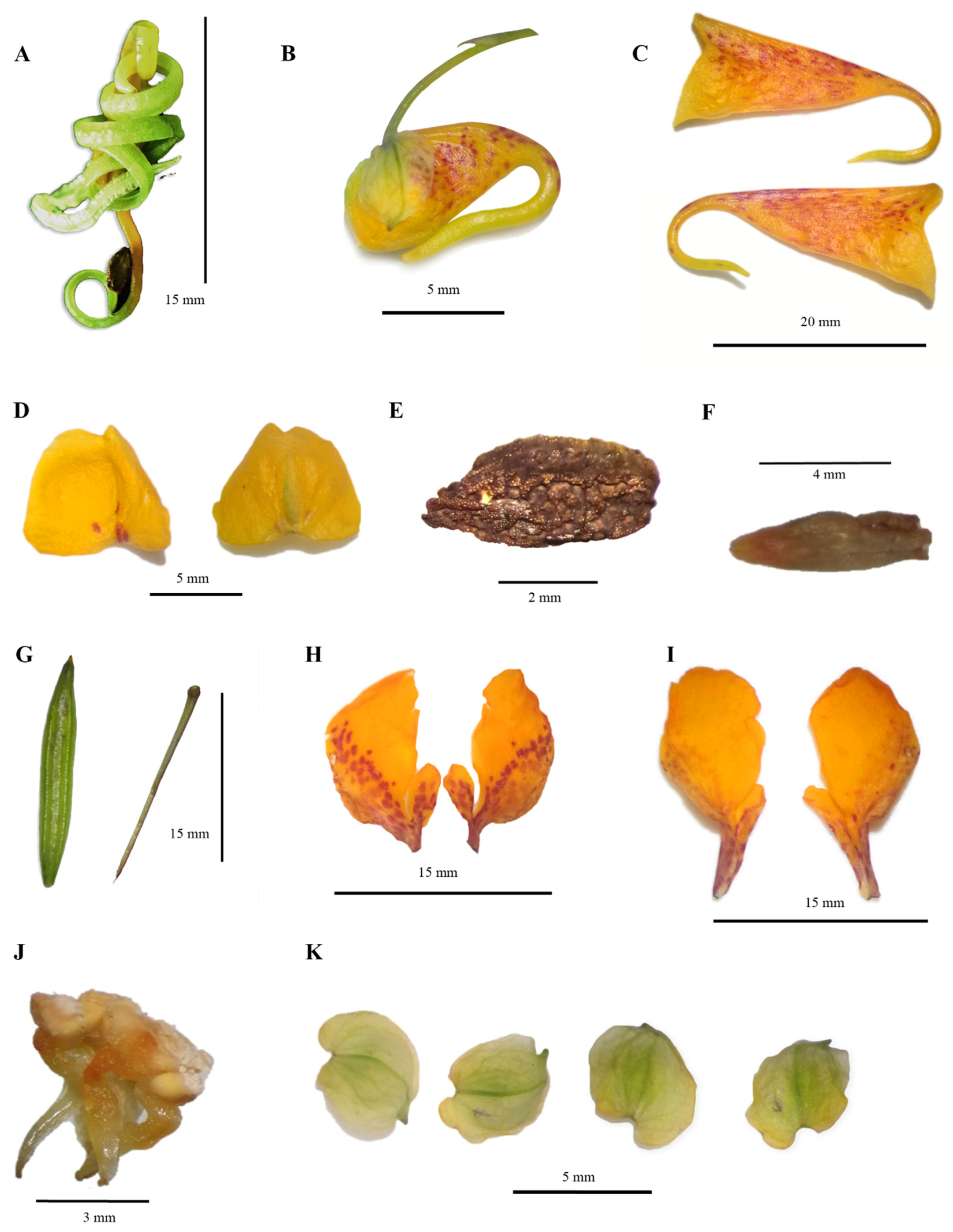
3.1. Redescription
3.1.1. Distribution, Habitat, and Ecology
3.1.2. Phenology
3.1.3. Floral Visitors
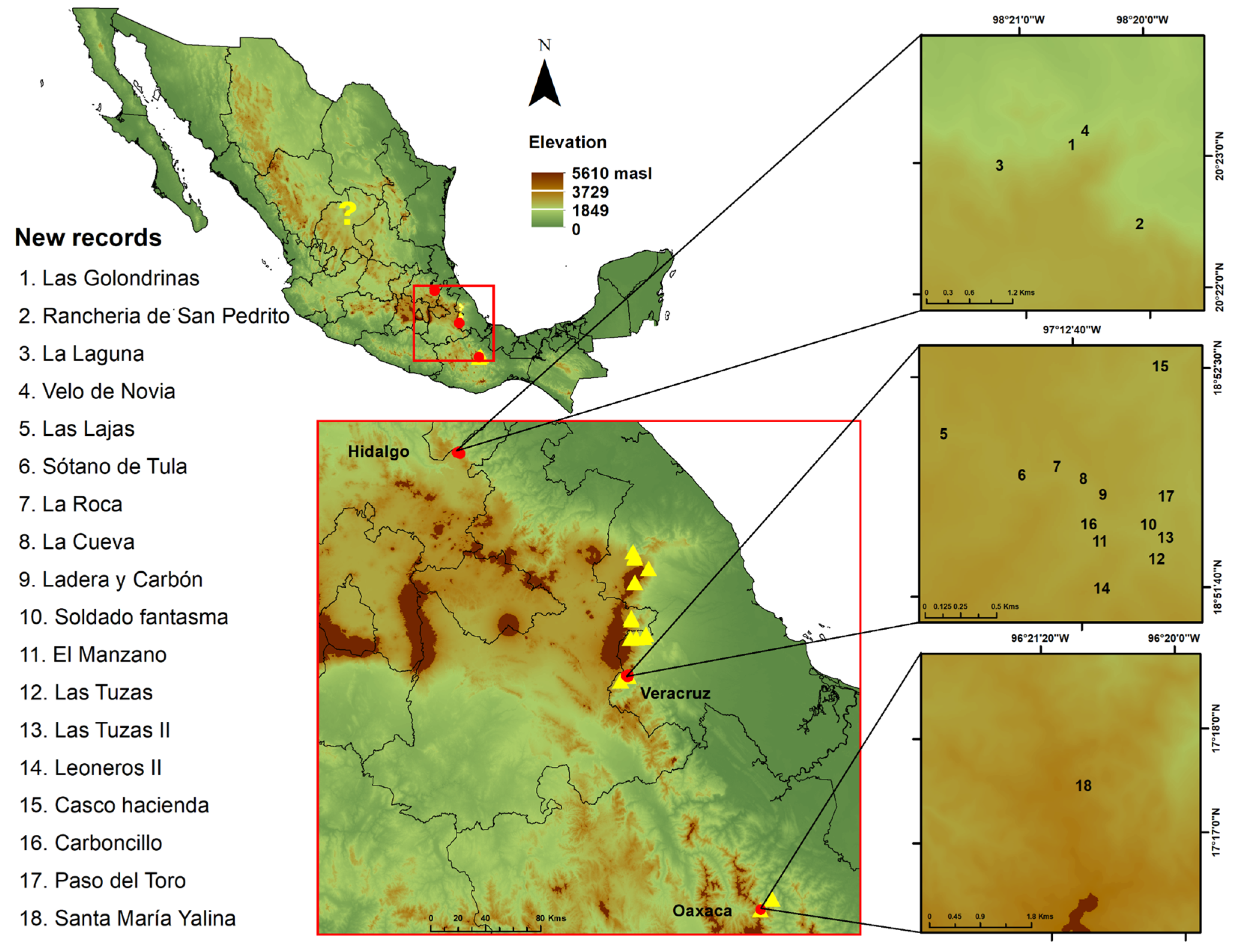
3.1.4. Studied Localities
3.1.5. Specimens Examined and Deposited
3.1.6. Remarks and Differential Diagnosis
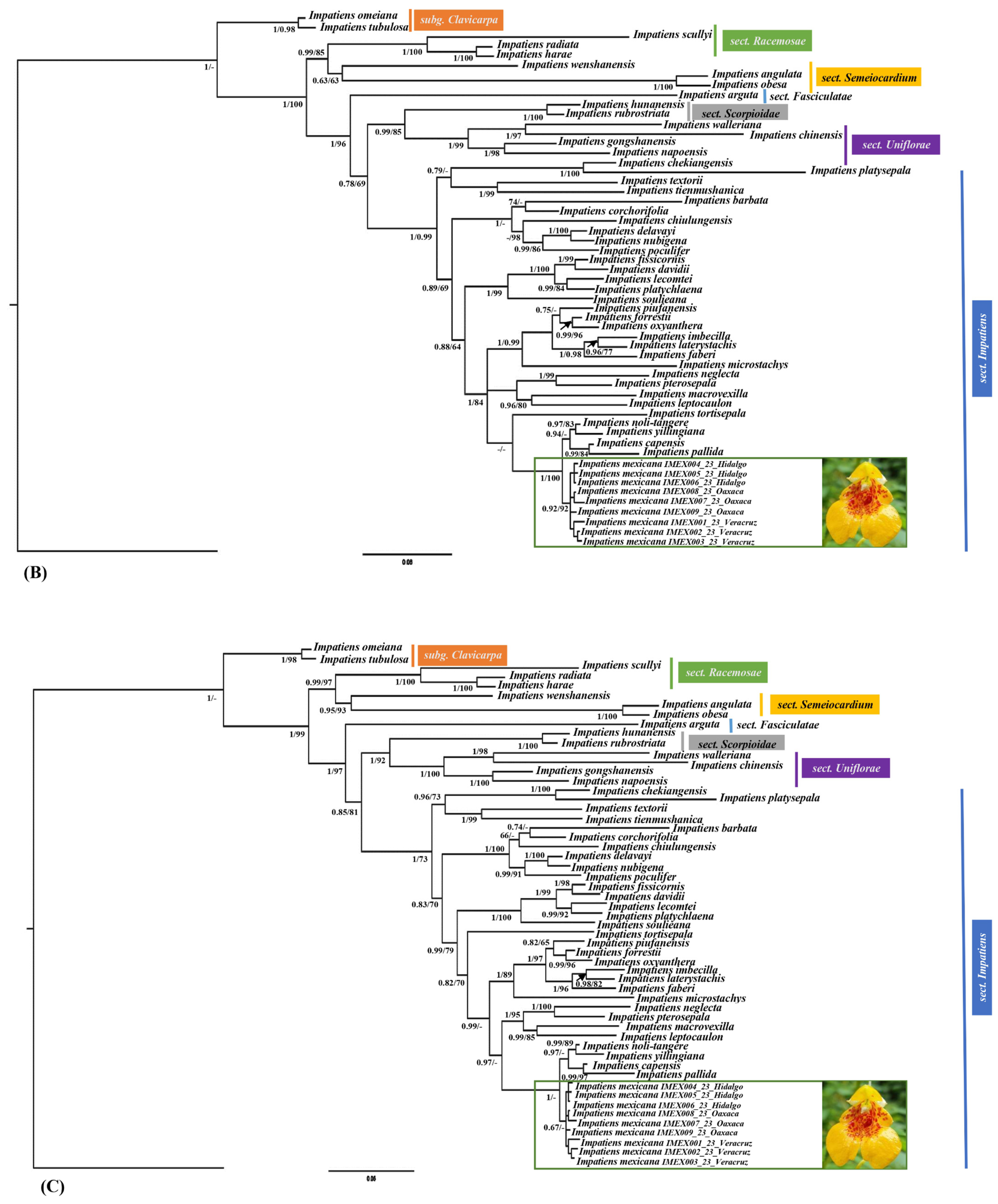
3.2. Phylogenetic and Distance Genetic Analyses
4. Discussion
4.1. A Morphological Discrimination of I. mexicana with American Balsam Congeners
4.2. Impatiens mexicana: Current Known Status
4.3. Molecular Studies: Phylogenetic Position and Genetic Distances of Impatiens mexicana
4.4. Limitations of the Study and Recommended Additional Research
4.5. Final Comments
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| State | Distribution. Locality-Town/Municipality/State | Coordinates/Longitude-latitude/% Altitude (masl) (If Available) | Collection-Institution Deposited/ | Source |
|---|---|---|---|---|
| Hidalgo | * Las Golondrinas Waterfalls, San Pedrito, Agua Blanca de Iturbide Population 1. | 98°20.201′, 20°22.501′ (−98.33, 20.37) %1925 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). AB0002UAEH-HGOM. | Present study |
| * Rancheria, San Pedrito, Agua Blanca de Iturbide. Population 2. | 98°20.699′, 20°22.864′ (−98.34, 20.38) %1906 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). AB0013UAEH-HGOM. | Present study | |
| * La Laguna, Agua Blanca de Iturbide. Population 3. | 98°20.546′, 20°23.130′ (98.35, 20.38) %2070 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). AB0014UAEH-HGOM. | Present study | |
| * Velo de Novia, Agua Blanca de Iturbide. Population 4. | 98°21.242′, 20°22.880′ (−98.34, 20.38) %1932 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). AB0025UAEH-HGOM. AB0026UAEH-HGOM. | Present study | |
| Oaxaca | 10 km South of Talea, Distrito de Villa Alta, Villa Talea de Castro. | 96°16. 7999′, 17°21′30″ (−96.26, 17.35) %2370 | Xalapa (INECOL) Herbarium. 14229. | [48] |
| Oaxaca | 10 km South of Talea, San Andres Solaga. | # 98°21.242′, 20°22.880′ (% −96.26, 17.35) %2370 | Mexico City (IBUNAM) 422421. | [48] |
| Oaxaca | * Santa María Yalina Population 18. | 96°21′4.74″, 17°17′23.04″ (−96.35, 17.28) %2657 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). OA0026UAEH-HGOM. OA0027UAEH-HGOM. OA0029UAEH-HGOM. | Present study |
| Oaxaca | Santa Maria Yalina. | 96°21′11.9″, 17°17′23.5″ (−96.35, 17.28) %2649 | Mexico City (IBUNAM). | [67] |
| Puebla | Chilchotla. | 97°10′28.829″, 19°14′20″ (−97.17, 19.23) %2160 | No deposited material in collection or institutions (Observation). | [33] |
| Puebla | Chilchotla waterfalls, Quimixtlan. | 97°10′16.99″, 19°15′2.65″ (−97.17, 19.23) %2100 | Xalapa (INECOL) Herbarium. XAL0145838. | [48] |
| Puebla | Chilchotla. | 97°10′23.887″, 19°15′6.635″ (−97.17, 19.25) %2110 | No deposited material in collection or institutions (Observation). | [33] |
| Veracruz | Near San Juan, Cofre del Perote, Xico. | 97°8′27.695″, 19°29′7.121″ (−97.14,19.48) %1150 | Xalapa (INECOL) Herbarium. | [43,47,48] |
| Veracruz | Southeast of Rinconada, near Cerro La Tolva, Las Minas. | 97°7′59.99″, 19°39′30″ (−97.13, 19.65) %1900 | IEB (INECOL) Herbarium. 14228. | [47,48] |
| Veracruz | Near Cofre de Perote Region, Las Minas. | 97°8′48.009″, 19°41′26.98″ (−97.14, 19.69) %1971 | No deposited material in collection or institutions (Observation). | [44,47,48] |
| Veracruz | 500 m Southwest of Maquistla, Road to Jacal, Calcahualco. | 97°10′59.999″ 19°7′ (−97.18, 19.11) %1960 | Xalapa (INECOL) Herbarium. V094128. 973417. | [47,48] |
| Veracruz | Cerro la Tolva, Las Minas. | 97°8′1.999″, 19°38′55″ (−97.13, 19.64) %2408 | Xalapa (INECOL) Herbarium. | [47,48] |
| Veracruz | Near San Juan, Cofre de Perote, Xico. | 97°8′27.695″, 19°29′7.121″ (% −97.14, 19.48) %2300 | Mexico City (IBUNAM). | [43,48] |
| Veracruz | Xico. | # 97°0′38.836″, 19°25′27.307″ (−97.01, 19.42) %2130 | Xalapa (INECOL) Herbarium. | [48] |
| Veracruz | Aserradero de Santa Cruz Muyuapan, Orizaba. | 97°15′44.432″ 18°50′16.528″ (−97.26, 18.83) %1830 | The New York Botanical Garden. | [24,48]. |
| Veracruz | Nogales. | 97°12′19.378″ 18°51′54.414″ (−97.20, 18.86) %2205 | No deposited material in collection or institutions (Observation). | [33] |
| Veracruz | Northeast of Pico de Orizaba, Alpatlahuac and Calcahualco. | 97°5′15.681″, 19°7′13.549″ (−97.08, 19.12) %1750 | No deposited material in collection or institutions (Observation). | [47,48] |
| Veracruz | 500 m Southwest of Maxistla Road to Jacal, Calcahualco. | 97°8′, 31.999″, 19°7′10″ (−97.14, 19.11) %2650 | Xalapa (INECOL) Herbarium. | [47,48] |
| Veracruz | 500 m Southwest of Maxistla Road to Jacal, Calcahualco. | 97°10′58.8″, 19°7′1.2″ (−97.18, 19.11) %2510 | Mexico City (IBUNAM). | [47,48] |
| Veracruz | 500 m Southwest of Maxistla road to Jacal, Calcahualco. | 97°4′30″, 19°8′30″ (−97.07, 19.14) %1890 | IEB (INECOL) Herbarium. | [47,48] |
| Veracruz | Acajete, Xalapa. | 97°2′29″,19°34′36″ (−97.04, 19.57) %2389 | No deposited material in collection or institutions (Observation). | [33] |
| Veracruz | * Las Lajas Population 5. | 97°13.222′, 18°52.237′ (−97.22, 18.87) %2334 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). OR0009UAEH-HGOM. | Present study |
| Veracruz | * Sótano de Tula Population 6. | 97°12.916′, 18°52.072′ (−97.2152667, 18.8678667) %2308 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). OR0007UAEH-HGOM. | Present study |
| Veracruz | * La roca Population 7. | 97°12.774′18°52.100′ (−97.2129000, 18.8683333) %2325 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). OR0021UAEH-HGOM. | Present study |
| Veracruz | * La cueva Population 8. | 97°12.672′18°52.050′ (−97.2112, 18.8675) %2275 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). OR0028UAEH-HGOM. | Present study |
| Veracruz | * Ladera y Carbón Population 9. | 97°12.595′, 18°51.987′ (−97.2099167, 18.8664500) %2348 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). OR0011UAEH-HGOM. | Present study |
| Veracruz | * Soldado fantasma Population 10. | 97°12.427′, 18°51.868′ (−97.2071167, 18.8644667) %2233 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). OR0019UAEH-HGOM. | Present study |
| Veracruz | * El Manzano Population 11. | 97°12.627′18°51.846′ (−97.2104500, 18.8641000) %2269 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). OR0003UAEH-HGOM. | Present study |
| Veracruz | * Las Tuzas Population 12. | 97°12.408′, 18°51.736′ (−97.2068, 18.8622667) %2290 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). OR0017UAEH-HGOM. | Present study |
| Veracruz | * Las Tuzas II Population 13. | 97°12.370′, 18°51.816′ (−97.2061667, 18.8636000) %2245 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). OR0018UAEH-HGOM. | Present study |
| Veracruz | * Leoneros II Population 14. | 97°12.630′, 18°51.632′ (−97.2105000, 18.8605333) %2398 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). OR0001UAEH-HGOM. OR0002UAEH-HGOM. | Present study |
| Veracruz | * Casco de hacienda Population 15. | 97°12.367′, 18°52.468′ (−97.206531,18.874339) %2199 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). OR0005UAEH-HGOM. | Present study |
| Veracruz | * Carboncillo Population 16. | 97°12.674′, 18°51.877′ (−97.21123333, 18.8646167) %2208 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). OR0012UAEH-HGOM. | Present study |
| Veracruz | * Paso del Toro Population 17. | 97°12.361′, 18°51.974′ (−97.20601667, 18.8662333) %2204 | Herbario de la Universidad Autónoma del Estado de Hidalgo (UAEH-HGOM). OR0004UAEH-HGOM. | Present study |
| Zacatecas | ? Villa de Cos. | 102°31′58.389″, 23°57′1.67″ (−102.5328861, 23.9504638) %2204 | BM Herbarium NHM Natural History Museum, London. | [48] |
| Species | Specimen Voucher Deposited | Origin | atpB-rbcL | ITS | trnL-F |
|---|---|---|---|---|---|
| Subgen. Clavicarpa | |||||
| Impatiens omeiana Hook.f | * Ruchisansakun 236 (QBG) ** S.X. Yu 4093 | * China origin, cult. Chiang Mai, Thailand ** China: Sichuan | * KC905619 | * KC905505 | ** KP776152 |
| I. tubulosa Hemsl. ex F. B. Forbes & Hemsl | * S.X. Yu 3762 (PE) | * China: Guangxi | * KP776056 | * KP776108 | * KP776172 |
| Subgen. Impatiens, sect. Fasciculatae | |||||
| I. arguta Hook. f. & Thomson | * Yuan CN2k-74 (NEU), ** S.X. Yu 5406 (PE) | * China: Yunnan, ** Xizang | * DQ147812 | * AY348746 | ** KP776116 |
| Subgen. Impatiens, sect. Impatiens | |||||
| I. aurea Hook. f. Thomson (I. pallida) | * Janssens SJ008 (LV) ** L0388636 (L) | North American origin, cult. Holden arboretum | * DQ147813 | ** MH377175 | / |
| I. barbata H.F. Comber | * CN2k2-178 (NEU) **/ | * China: Yunnan **/ | * DQ147818 | * AY348750 | ** AB043658 |
| I. capensis Meerb. | * Janssens SJ009 (LV) ** W. Fritsch 1772(CAS) | * North American origin: Holden Arboretum (Cult.) ** Strybing Arboretum, San Francisco, U.S.A., bed 40B;P. | * DQ147823 | * AY348759 | **AF396206 |
| I. chekiangensis Y.L. Chen | * Qin et al. 20141 | * China: Zhejiang | * KP776014 | * KP776064 | * KP776121 |
| I. chiulungensis Y.L. Chen | * S.X. Yu et al. 3989 | * China: Sichuan | * KP776016 | * KP776066 | * KP776124 |
| I. corchorifolia Franch. | * Chassot & Yuan, 99-173 (NEU) ** S.X. Yu et al. 4596 (PE) | * China: Yunnan, ** Sichuan | * DQ147831 | * AY348767 | ** KP776127 |
| I. davidii Franch. | * H.N. Qin et al. 19960 (PE) | * China: Fujian, Anhui | * KP776020 | * KP776070 | * KP776129 |
| I. delavayi Franch. | * Chassot and Yuan 99–154 (NEU) ** S.X. Yu et al. 4783 (PE) | * China: Yunnan, ** Sichuan | * DQ147836 | * AY348773 | ** KP776130 |
| I. faberi Hook. f. | * Song S 007 (NEU) ** S.X. Yu 4091 | China: Sichuan | * DQ147841 | * AY348778 | ** KP776132 |
| I. fissicornis Maxim. | * Wang SH-003 (NEU) | * China: Shaanxi | * DQ147844 | * AY348782 | / |
| I. forrestii Hook. f. ex W.W. Smith | * Yuan, CN2k-79 (NEU) | * China: Yunnan | * DQ147847 | * AY348784 | / |
| I. imbecilla Hook. f. | * Hao 426 (NEU) | * China: Sichuan | * DQ147851 | * AY348796 | / |
| I. macrovexilla Y.L. Chen | * S.X. Yu s. n. (PE) | *China: Guangxi | * KP776034 | * KP776082 | * KP776142 |
| I. mexicana Rydb. | * AB0025UAEH-HGOM AB0026UAEH-HGOM AB0002UAEH-HGOM ** OR0007UAEH-HGOM,OR0001UAEH-HGOMOR0002UAEH-HGOM *** OA0026UAEH-HGOM,OA0027UAEH-HGOM,OA0029UAEH-HGOM | Mexico: * Hidalgo, ** Veracruz, *** Oaxaca | * OR525134,OR525135,OR525136, ** OR525137, OR525138, OR525139 *** OR525133, OR525132, OR525131 | * OR52572, OR525071, OR525070 ** OR525075, OR525074, OR525073 *** OR525069, OR525068, OR525067 | * OR525080, OR525079 ** OR525083, OR525082, OR52581 *** OR525078, OR525077, OR525076 |
| I. microstachys Hook. f. | * S.X. Yu et al. 5043 (PE) | * China: Sichuan | / | * KP776085 | * KP776144 |
| I. neglecta Y.L. Xu & Y.L. Chen | * H.N. Qin et al. 19942 (PE) | * China: Anhui | * KP776038 | * KP776087 | * KP776147 |
| I. noli-tangere L. | * KUMA:K08 | * Japan: Kumamoto, Gokanosho | * LC465215 | * LC465177 | * LC465233 |
| I. nubigena W.W. Smith | * S.X. Yu et al. 4800 | * S.X. Yu et al. 4800 | * KP776040 | * KP776089 | * KP776149 |
| I. lateristachys Y.L. Chen & Y.Q. Lu | * S.X. Yu et al. 4097 | * China: Sichuan | * KP776030 | * KP776078 | / |
| I. lecomtei Hook. f. | * Yuan CN2k2-202 (NEU) | * China: Yunnan | * DQ147855 | *AY348802 | / |
| I. leptocaulon Hook. f. | * S.X. Yu et al. 4019 (PE) | * China: Guangxi | * KP776032 | * KP776080 | * KP776140 |
| I. oxyanthera Hook. f. | * Song S008 (NEU) | * China: Sichuan | * DQ147865 | * AY348814 | / |
| I. platychlaena Hook. f. | * Song, S009 (NEU) ** S.X. Yu 4081 (PE) | China: Sichuan | * DQ147867 | * AY348818 | ** KP776154 |
| I. platysepala Y.L. Chen | * H.N. Qin et al. 19983A | * China: Anhui | * KP776044 | * KP776095 | * KP776155 |
| I. pterosepala Hook. f | * S.X. Yu s.n (PE) | * China: Hubei | * KP776046 | * KP776097 | * KP776158 |
| I. poculifer Hook. f | ** Yuan CN2K2-209 (NEU) | ** China: Yunnan | ** DQ147870 | ** AY348820 | / |
| I. soulieana Hook. f | * Yuan CN2k2-163 (NEU) ** S.X. Yu et al. 5053 | * China: Sichuan **/ | * DQ147880 | * AY348833 | ** KP776164 |
| I. tayemonii Hayata | * Zhengyu Jiang T2 (NEU) | * China: Taiwan | / | * AY348839 | / |
| I. textorii Miquel | * KUMA:K29 | * Japan: Kumamoto, Takamori-machi, Nojiri | * LC465213 | * LC465175 | * LC465231 |
| I. tienmushanica Y.L. Chen | * S.X. Yu et al. 20089 (PE) | * China: Zhejiang | * KP776053 | * KP776105 | * KP776169 |
| I. tortisepala Hook. f | *S.X. Yu et al. 4925 | *China: Sichuan | *KP776054 | *KP776106 | *KP776170 |
| I. yillingniana X.F.Jin, S.Z.Yang & L.Qian | * X. F. Jin 1901 (HTC). | * China: Zhejiang, Lin’an, Mt. Tianmu, Dashuwang. | * MN974549 | * MN974566 | * MN974583 |
| Subgen. Impatiens, sect. Racemosae | |||||
| I. scullyi Hook. F. | * Y.S. Chen et al. 652 (PE) | * China: Xizang | * KP776048 | * KP776100 | * KP776163 |
| I. radiata Hook. f. | * S.X. Yu et al. 4760 (PE)2 | * China: Sichuan | * KC905573 | * KC905523 | * KP776160 |
| I. harae H. Ohba & S. Akiyama. | * Y.S. Chen et al. 392 (PE) | * China: Xizang | * KP776025 | * KP776075 | * KP776136 |
| Subgen. Impatiens, sect. Scorpioidae | |||||
| I. hunanensis Y.L. Chen | * S.X. Yu 3759 (PE) | * China: Guangxi | * KP776028 | * KP776077 | * KP776137 |
| I. rubrostriata Hook. f. | * Yuan CN2k1-4 (NEU) ** S.X. Yu 3753 (PE) | China: Yunnan | * DQ147876 | * AY348828 | ** KP776161 |
| Subgen. Impatiens, sect. Semeiocardium | |||||
| I. angulata S.X. Yu, Y.L. Chen & H.N. Qin | * S.X. Yu 3777 (PE) | * China: Guangxi | * KP776010 | * KP776060 | * KP776113 |
| I. obesa Hook. f. | * S.X. Yu 3775 (PE) | * China: Guangxi | * KP776042 | * KP776091 | * KP776151 |
| I. wenshanensis S.H. Huang | * S.X. Yu 4044 (PE) | * China: Guangxi | * KP776057 | * KP776110 | * KP776175 |
| Subgen. Impatiens, sect. Uniflorae | |||||
| I. walleriana Hook.f. | * O. Phaehler & M. Schnell I08 (NEU) ** S3926 (BR) *** H. Fujihashi 3 (TI) | * Kenya, ** African origin: Nat. Bot. Gard. Meise (Cult.), *** Tokyo (Cult.). | *AY348849 | **DQ147892 | ***AB043641 |
| I_napoensis Y.L. Chen | * Yuan CN2k1-61 (NEU), ** S.X. Yu 3049 (PE) | China: * Yunnan, ** Guangxi | * DQ147861 | ** AY348811 | ** KP776146 |
| I. gongshanensis Y.L. Chen | * PT-ET 975 (PE) | * Myanmar: Putao | * KP776024 | * KP776074 | * KP776135 |
| I. chinensis L. | * Yuan CN2k1-49 (NEU), ** S.X. Yu 3656 (PE). | China: * Yunnan, ** Guangxi | * DQ147825 | * AY348761 | ** KP776122 |
| Outgroup | |||||
| Hydrocera triflora (L.) Wight & Arn. | ** Robyns 7260, 0249369 (L) | ** Sri Lanka | ** DQ147895 | ** AY348853 | / |

References
- Linnaeus, C. Species Plantarum, Exhibentes Plantas Rite Cognitas, Ad Genera Relatas, Cum Differentiis Specificis, Nominibus Trivialibus, Synonymis Selectis, Locis Natalibus, Secundum Systema Sexuale Digestas; Impensis Laurentius Salvius: Stockholm, Sweden, 1753; Volume 2, pp. 561–1199. [Google Scholar]
- Grey-Wilson, C. Impatiens of Africa; CRC Press: Rotterdam, The Netherlands, 1980; pp. 1–235. [Google Scholar]
- Douglas, G.W.; Straley, G.B.; Meidinger, D.; Pojar, J. Illustrated Flora of British Columbia, Volume 2, Dicotyledons (Balsaminaceae through Cuscutaceae); British Columbia Ministry of Environment, Lands and Parks, Ministry of Forests: Victoria, BC, Canada, 1998; pp. 1–401. [Google Scholar]
- Fischer, E. Balsaminaceae. In Flowering Plants Dicotyledons. The Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 6, pp. 20–25. [Google Scholar] [CrossRef]
- San, D.P.; Ruchisansakun, S. Impatiens latiflora, a new record of Impatiens sect. Uniflorae for Sagaing region, Myanmar. Thai For. Bull. (Bot.) 2022, 50, 191–194. [Google Scholar] [CrossRef]
- Gogoi, R.; Borah, S.; Dash, S.S.; Singh, P. Balsams of Eastern Himalaya: A Regional Revision; Botanical Survey: Kolkata, India, 2018; pp. 1–209. [Google Scholar]
- Utami, N.; Tihurua, E.F. Impatiens (Balsaminaceae) diversity and conservation status in Mount Singgalang, West Sumatra. Bul. Keb. Ray. 2021, 24, 136–140. [Google Scholar] [CrossRef]
- Ramasubbu, R.; Anjana, S.; Chandra-Prabha, A. A new species Impatiens L. (Balsaminaceae) from Kodaikkanal Wildlife sanctuary, India. Taiwania 2020, 65, 426–430. [Google Scholar] [CrossRef]
- Utami, N. Impatiens marroninus, a new species of Impatiens (Balsaminaceae) from Sumatra, Indonesia. Blumea 2020, 65, 10–11. [Google Scholar] [CrossRef]
- Lu, Z.C.; Pan, B.; Huang, F.Z.; Liu, Y. Impatiens gongchengensis (Balsaminaceae), a new species from Guangxi, Southern China. Taiwania 2020, 65, 1–4. [Google Scholar] [CrossRef]
- Mohan, V.; Francis, D.; Nampy, S.; Venugopal, D.K. Impatiens raktakesara (Balsaminaceae), a new species from the southern Western Ghats, India. Brittonia 2021, 73, 316–322. [Google Scholar] [CrossRef]
- Song, Y.X.; Peng, S.; Cong, Y.Y.; Zheng, Y.M. Impatiens rapiformis, a new species of Impatiens with root tuber from Yunnan, China. Nord. J. Bot. 2021, 39, e03151. [Google Scholar] [CrossRef]
- Song, Y.X.; Xiao, Y.; Peng, S.; Cong, Y.Y.; Hu, G.W. Two new species of Impatiens from China, and taxonomic insights into the Longifilamenta group, which is endemic to China. Plants 2021, 10, 1697. [Google Scholar] [CrossRef]
- Tanaka, N.; Aung, M.M.; Vermeulen, J.J. Impatiens katjae, a new species of Impatiens (Balsaminaceae) from Central Myanmar. Novon 2022, 30, 56–60. [Google Scholar] [CrossRef]
- Yu, S.X.; Janssens, S.B.; Zhu, X.Y.; Lidén, M.; Gao, T.G.; Wang, W. Phylogeny of Impatiens (Balsaminaceae): Integrating molecular and morphological evidence into a new classification. Cladistics 2015, 32, 179–197. [Google Scholar] [CrossRef]
- Song, Y.X.; Peng, S.; Mutie, F.M.; Jiang, H.; Ren, J.; Cong, Y.Y.; Hu, G.W. Evolution and taxonomic significance of seed micromorphology in Impatiens (Balsaminaceae). Front. Plant. Sci. 2022, 13, 835943. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.B.; Knox, E.B.; Huysmans, S.; Smets, E.F.; Merckx, V.S.F.T. Rapid radiation of Impatiens (Balsaminaceae) during Pliocene and Pleistocene: Result of a global climate change. Mol. Phylogenet. Evol. 2009, 52, 806–824. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.M.; Song, Y.; Geuten, K.; Rahelivololona, E.; Wohlhauser, S.; Fischer, E.; Smets, E.; Küpfer, P. Phylogeny and biogeography of Balsaminaceae inferred from ITS sequences. Taxon 2004, 53, 391–404. [Google Scholar] [CrossRef]
- Song, Y. Evolution and Biogeography of Balsaminaceae: Insights from Molecular Phylogeny. Ph.D. Thesis, Université de Neuchâtel, Neuchâtel, Switzerland, 2006. [Google Scholar]
- Ruchisansakun, S.; van der Niet, T.; Janssens, S.B.; Triboun, P.; Techaprasan, J.; Jenjittikul, T.; Suksathan, P. Phylogenetic analyses of molecular data and reconstruction of morphological character evolution in Asian Impatiens section Semeiocardium (Balsaminaceae). Syst. Bot. 2016, 40, 1063–1074. [Google Scholar] [CrossRef]
- Hatcher, P.E. Impatiens noli-tangere L. J. Ecol. 2003, 91, 147–167. [Google Scholar] [CrossRef]
- Smith, J.D. Undescribed plants from Guatemala and other Central American republics. XVIII. Bot. Gaz. 1897, 23, 235–251. [Google Scholar] [CrossRef]
- Elias, T.S. Part VI. Family 110: Balsaminaceae. In Flora of Panama; Woodson, R.E., Jr., Schery, R.W., Eds.; Annals of the Missouri Botanical Garden; Missouri Botanical Garden Press: St. Louis, MO, USA, 1967; Volume 54, pp. 21–24. [Google Scholar] [CrossRef]
- Rydberg, P.A. Family 6. Balsaminaceae. N. Am. Flora 1910, 25, 93–96. [Google Scholar]
- Rydberg, P.A. Studies on the Rocky Mountains flora. IV. Bull. Torrey 1901, 28, 20–38. [Google Scholar] [CrossRef]
- Quin, F.; Xue, T.; Zhang, X.; Yang, X.; Yu, J.; Gadagkar, S.R.; Yu, S. Past climate cooling and orogenesis of the Hengduan Mountains have influenced the evolution of Impatiens sect. Impatiens (Balsaminaceae) in the Northern hemisphere. BMC Plant Biol. 2023, 23, 600. [Google Scholar] [CrossRef]
- Meerburgh, N. Afbeeldingen van Zeldzaame Gewassen; Johannes le Mair: Te Leyden, The Netherlands, 1775; pp. 1–67. [Google Scholar] [CrossRef][Green Version]
- Moore, G.; Zika, P.F.; Rushworth, C.A. Impatiens ecornuta, a new name for Impatiens ecalcarata (Balsaminaceae), a jewelweed from the United States and Canada. Novon 2012, 22, 60–61. [Google Scholar] [CrossRef]
- Blankinship, J.W. Supplement to the flora of Montana. Sci. Stud. Montana Coll. Agric. Bot. 1905, 1, 33–109. [Google Scholar]
- Nuttal, T. The Genera of North American Plants and a Catalogue of the Species to the Year 1817; D. Heartt: Philadelphia, PA, USA, 1818; pp. 1–312. [Google Scholar] [CrossRef][Green Version]
- Zika, P.F. Impatiens × pacifica (Balsaminaceae), a new hybrid jewelweed from the Pacific Northwest Coast of North America. Novon 2006, 16, 443–448. [Google Scholar] [CrossRef]
- Ornduff, R. Hybridization and regional variation in Pacific Northwestern Impatiens (Balsaminaceae). Brittonia 1967, 19, 122–128. [Google Scholar] [CrossRef]
- iNaturalist. Available online: https://www.inaturalist.org (accessed on 30 June 2023).
- Luo, C.; Huang, W.; Sun, H.; Yer, H.; Li, X.; Li, Y.; Yan, B.; Wang, Q.; Wen, Y.; Huang, M.; et al. Comparative chloroplast genome analysis of Impatiens species (Balsaminaceae) in the karst area of China: Insights into genome evolution and phylogenomic implications. BMC Genom. 2021, 22, 571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, W.Q.; Ding, B.; Deng, H.P. Characterization of the complete chloroplast genome sequence of Impatiens pritzelii (Balsaminaceae): An endemic species from China. Mitochondrial DNA B Resour. 2019, 4, 4073–4074. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Zhang, Z.H.; Wang, M.Z.; Jin, X.J.; Lin, J.D.; Comes, H.P.; Chen, J.X.; Cui, R.N.; Duan, R.Q.; Li, P. Plastome evolution and phylogenomics of Impatiens (Balsaminaceae). Planta 2023, 257, 45. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Y.; Li, Z.; Xiang, M.; Dong, X.; Tang, X. The complete chloroplast genome of Impatiens mengtszeana (Balsaminaceae), an endemic species in China. Mitochondrial DNA B Resour. 2022, 7, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Cafa, G.; Baroncelli, R.; Ellison, C.A.; Kurose, D. Impatiens glandulifera (Himalayan balsam) chloroplast genome sequence as a promising target for populations studies. PeerJ 2020, 8, e8739. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Chen, X.; Li, T.; Zhou, A. Characterizing the complete chloroplast genome of the Impatiens davidii (Balsaminaceae). Mitochondrial DNA B Resour. 2022, 7, 466–467. [Google Scholar] [CrossRef]
- Godínez, J.L. Colectores de algas de México (1787–1954). Acta Bot. Mex. 2008, 75–97. [Google Scholar] [CrossRef]
- Hemsley, W.B. Bosquejo de la Historia de la Exploración Botánica de México; 1a Ser. La Naturaleza: Puebla, Mexico, 1891; pp. 1–15. [Google Scholar]
- Barnhart, J.H. Biographical Notes upon Botanists; G.K. Hall: Boston, MA, USA, 1965; Volume 2, pp. 1–549. [Google Scholar]
- Barringer, K. Flora de Veracruz. Balsaminaceae; Fascículo 64; Instituto de Ecología, A.C. Xalapa, Ver., University of California: Riverside, CA, USA, 1991; pp. 1–8. [Google Scholar]
- Sosa, V.; Vovides, A.P.; Castillo-Campos, G. Monitoring endemic plant extinction in Veracruz, México. Biodivers. Conserv. 1998, 7, 1521–1527. [Google Scholar] [CrossRef]
- Castillo-Campos, G.; Medina-Abreo, M.; Dávila-Aranda, P.D.; Zavala-Hurtado, J.A. Contribución al conocimiento del endemismo de la flora vascular en Veracruz, México. Acta Bot. Mex. 2005, 73, 19–57. [Google Scholar] [CrossRef]
- Villaseñor, J.L. Checklist of the native vascular plants of México. Rev. Mex. Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- Enciclovida. Available online: https://enciclovida.mx/ (accessed on 15 July 2022).
- GBIF. Global Biodiversity Information Facility. Available online: https://www.gbif.org/ (accessed on 15 July 2022).
- eFloraMEX. Las Plantas Vasculares de México. Available online: https://efloramex.ib.unam.mx/ (accessed on 10 November 2023).
- Villaseñor, J.L. El Bosque Húmedo de Montaña en México y sus Plantas Vasculares: Catálogo Florístico-Taxonómico, 1st ed.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO)-Universidad Nacional Autónoma de México (UNAM): Mexico City, Mexico, 2010; pp. 1–40. [Google Scholar]
- Rzedowski, J. Análisis preliminar de la flora vascular de los bosques mesófilos de montaña de México. Acta Bot. Mex. 1996, 35, 25–44. [Google Scholar] [CrossRef]
- Challenger, A.; Caballero, J. Utilización y Conservación de Los Ecosistemas Terrestres de México: Pasado, Presente y Futuro, 1st ed.; Universidad Nacional Autónoma de México/Agrupación Sierra Madre: Mexico City, Mexico, 1998; pp. 1–847. [Google Scholar]
- González-Espinosa, M.; Meave, J.A.; Ramírez-Marcial, N.; Toledo-Aceves, T.; Lorea-Hernández, F.G.; Ibarra-Manríquez, G. Los bosques de niebla de México: Conservación y restauración de su componente arbóreo. Ecosistemas 2012, 21, 36–54. [Google Scholar]
- Munsell, Color (Firm). Munsell Color Charts for Plant Tissues, 2nd ed.; Munsell Color, Macbeth Division of Kollmorgen Corporation: Baltimore, MD, USA, 1977. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Rozen, S.; Skaletsky, H.J. Primer3; Whitehead Institute for Biomedical Research and MIT Center for Genome Research: Cambridge, MA, USA, 1996; pp. 501–503. [Google Scholar]
- BOLDSYSTEMS. Barcode of Life Data Systems. Available online: https://www.boldsystems.org/ (accessed on 20 June 2022).
- GenBank-NCBI. Available online: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 15 June 2022).
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinformatics 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocol: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–321. [Google Scholar] [CrossRef]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant. Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef]
- Hoot, S.B.; Culham, A.; Crane, P.R. The utility of atpB gene sequences in resolving phylogenetic relationships: Comparison with rbcL and 18S ribosomal DNA sequences in the Lardizabalaceae. Ann. Missouri Bot. Gard. 1995, 82, 194–207. [Google Scholar] [CrossRef]
- Monzalvo, R. Personal Observation; School of Biological Sciences, Southern Illinois University: Carbondale, IL, USA, 2022. [Google Scholar]
- Velasco Gutierrez, K. Personal Communication; Sociedad para el Estudio de los Recursos Bióticos de Oaxaca A. C.: Oaxaca, Mexico, 2022. [Google Scholar]
- Weatherby, C.A. Color forms of Impatiens biflora. Rhodora 1917, 19, 115–118. [Google Scholar]
- Ornduff, R. Impatiens capensis in Oregon, native or naturalized? Leafl. W. Bot. 1966, 10, 317–319. [Google Scholar]
- Rust, R.W. Pollination in Impatiens capensis and Impatiens pallida (Balsaminaceae). J. Torrey Bot. 1977, 104, 361–367. [Google Scholar] [CrossRef]
- Schemske, D.W. Evolution of reproductive characteristics in Impatiens (Balsaminaceae): The significance of cleistogamy and chasmogamy. Ecology 1978, 59, 596–613. [Google Scholar] [CrossRef]
- Schmitt, J.; Ehrhardt, D.; Swartz, D. Differential dispersal of self-fertilized and outcrossed progeny in jewelweed (Impatiens capensis). Am. Nat. 1985, 126, 570–575. [Google Scholar] [CrossRef]
- Zimmerman, M.; Cook, S. Pollinator foraging, experimental nectar-robbing and plant fitness in Impatiens capensis. Am. Midl. Nat. 1985, 113, 84–91. [Google Scholar] [CrossRef]
- Wilson, P. Selection for pollination success and the mechanical fit of Impatiens flowers around bumblebee bodies. Biol. J. Linn. Soc. 1995, 55, 355–383. [Google Scholar] [CrossRef]
- Tabak, N.M.; von Wettberg, E. Native and introduced jewelweeds of the Northeast. Northeast. Nat. 2008, 15, 159–176. [Google Scholar] [CrossRef]
- Young, H.J. Selection on spur shape in Impatiens capensis. Oecologia 2008, 156, 535–543. [Google Scholar] [CrossRef]
- Jewelweeds and Touch-Me-Nots (Impatiens, Balsaminaceae) of the Pacific Northwest of North America. Available online: https://www.ou.edu/cas/botany-micro/ben/ben408.html (accessed on 14 April 2022).
- McDonald, S.; Caruso, C.M. Simulated nectar robbing does not affect pollinator-mediated selection on floral traits of Impatiens capensis. Int. J. Plant. Sci. 2019, 180, 922–927. [Google Scholar] [CrossRef]
- Panique, H.; Caruso, C.M. Simulated pollinator declines intensify selection on floral traits that facilitate selfing and outcrossing in Impatiens capensis. Am. J. Bot. 2020, 107, 148–154. [Google Scholar] [CrossRef]
- Lady Bird Johnson. Wildflower Center the University of Texas at Austin. Available online: https://www.wildflower.org/ (accessed on 25 July 2021).
- Monzalvo, R.; Escorcia-Guerrero, D.L. Personal Observation; School of Biological Sciences, Southern Illinois University: Carbondale, IL, USA; Laboratorio de Ecología de Poblaciones, Universidad Autónoma del Estado de Hidalgo: Mineral de la Reforma, Mexico, 2022. [Google Scholar]
- Fotos de Balsamina Naranja (Impatiens capensis). Available online: https://www.naturalista.mx/taxa/47888-Impatiens-capensis/browse_photos (accessed on 28 August 2023).
- Nguyen, T.; Monzalvo, R. Personal Observation; Department of Environmental Conservation, University of Massachusetts: Amherst, MA, USA; School of Biological Sciences, Southern Illinois University: Carbondale, IL, USA, 2023. [Google Scholar]
- USDA. United States Department of Agriculture. Available online: https://www.usda.gov/ (accessed on 15 October 2023).
- IPNI. International Plant Names Index and World Checklist of Vascular Plants. Available online: https://www.ipni.org/ (accessed on 15 October 2023).
- Waller, D.M. The relative costs of self-and cross-fertilized seeds in Impatiens capensis (Balsaminaceae). Am. J. Bot. 1979, 66, 313–320. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 5 June 2022).
- Whitfield, H.L.; Toczydlowski, R.H. Distinguishing Impatiens capensis from Impatiens pallida (Balsaminaceae) using leaf traits. Botany 2020, 98, 361–369. [Google Scholar] [CrossRef]
- Lu, Y.Q.; Chen, Y.L. Seed Morphology of Impatiens L. (Balsaminaceae) and its taxonomic significance. J. Syst. Evol. 1991, 29, 252–257. [Google Scholar]
- Utami, N.; Shimizu, T. Seed morphology and classification of Impatiens (Balsaminaceae). Blumea 2005, 50, 447–456. [Google Scholar] [CrossRef]
- Wang, J.S.; Lu, Y.F.; Xu, Y.L.; Jin, S.H.; Jin, X.F. Impatiens wuyiensis (Balsaminaceae), a new species from Fujian of Southeast China, based on morphological and molecular evidences. Bot. Stud. 2020, 61, 29. [Google Scholar] [CrossRef] [PubMed]
- Rewicz, A.; Myśliwy, M.; Adamowski, W.; Podlasiński, M.; Bomanowska, A. Seed morphology and sculpture of invasive Impatiens capensis Meerb. from different habitats. PeerJ 2020, 8, e10156. [Google Scholar] [CrossRef] [PubMed]
- Rewicz, A.; Adamowski, W.; Borah, S.; Gogoi, R. New data on seed coat micromorphology of several Impatiens spp. from Northeast India. Acta Soc. Bot. Pol. 2020, 89, 89312. [Google Scholar] [CrossRef]
- Melgarejo, J.L. Breve Historia de Veracruz; Biblioteca de la Facultad de Filosofía y Letras Universidad Veracruzana: Xalapa, Mexico, 1960; pp. 1–268. [Google Scholar]
- Gómez-Pompa, A.; Krömer, T.; Castro-Cortés, R. Atlas de la Flora de Veracruz: Un Patrimonio Natural en Peligro, 1st ed.; Comisión del Estado de Veracruz para la Conmemoración de la Independencia Nacional y la Revolución Mexicana: Gobierno del Estado de Veracruz; Universidad Veracruzana: Veracruz, Mexico, 2010; pp. 1–528. [Google Scholar]
- Ellis, E.A.; Martínez-Bello, M.; Monroy-Ibarra, R. Focos rojos para la conservación de la biodiversidad. In La Biodiversidad en Veracruz: Estudio de Estado; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO), Ed.; Gobierno del Estado de Veracruz, Universidad Veracruzana, Instituto de Ecología, A.C.: Mexico City, Mexico, 2011; pp. 351–368. [Google Scholar]
- Luna-Vega, I.; Alcántara, A.O. Florística del bosque mesófilo de montaña de Hidalgo. In Biodiversidad de la Sierra Madre Oriental; Luna, I., Morrone, J.J., Espinosa, D., Eds.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad-Universidad Nacional Autónoma de México: Mexico City, Mexico, 2004; pp. 169–192. [Google Scholar]
- Monzalvo, R.; García-Montes, M.A. Personal Observation; School of Biological Sciences, Southern Illinois University: Carbondale, IL, USA; Laboratorio de Genética, Universidad Autónoma del Estado de Hidalgo: Mineral de la Reforma, Mexico, 2022. [Google Scholar]
- Santa Maria Yalina, Villa Alta, Oaxaca. Plan Municipal 2010. Available online: https://www.economia.gob.mx/datamexico/es/profile/geo/santa-maria-yalina (accessed on 15 November 2023).
- Villaseñor, J.L.; Ortiz, E.; Sánchez-González, A. Riqueza y distribución de la flora vascular del estado de Hidalgo, México. Rev. Mex. Biodiv. 2022, 93, e933920. [Google Scholar] [CrossRef]
- Miguel-Vazquez, M.I.; Espejo-Serna, M.A.; Ceja-Romero, J.; Cerros-Tlatilpa, R. The angiosperms epiphytes of Puebla, Mexico: Richness and distribution. Bot. Sci. 2020, 98, 585–596. [Google Scholar] [CrossRef]
- Mendoza-García, A.; Lezama, P.T.; Santiago, J.R. El endemismo en la flora fanerogámica de la Mixteca Alta, Oaxaca-Puebla, México. Acta Bot. Mex. 1994, 27, 53–73. [Google Scholar] [CrossRef]
- Jin, X.F.; Yang, S.Z.; Chen, Z.H.; Qian, L. Impatiens yilingiana sp. nov. and I. huangyanensis subsp. attenuata subsp. nov. (Balsaminaceae) from Zhejiang, eastern China. Nord. J. Bot. 2008, 26, 207–213. [Google Scholar] [CrossRef]
- Ramírez-Barahona, S.; Eguiarte, L.E. The role of glacial cycles in promoting genetic diversity in the Neotropics: The case of cloud forests during the Last Glacial Maximum. Ecol. Evol. 2013, 3, 725–738. [Google Scholar] [CrossRef]
- Vargas-Rodriguez, Y.L.; Platt, W.J.; Vázquez-García, J.A.; Boquin, G. Selecting relict montane cloud forests for conservation priorities: The case of Western Mexico. Nat. Areas. J. 2010, 30, 156–173. [Google Scholar] [CrossRef]
- Quan, X.; Zhou, S.L. Molecular identification of species in Prunus sect. Persica (Rosaceae), with emphasis on evaluation of candidate barcodes for plants. J. Syst. Evol. 2011, 49, 138–145. [Google Scholar] [CrossRef]
- Susanto, A.H.; Nuryanto, A.; Daryono, B.S. High connectivity among Synedrella nodiflora populations in Java Island based on intergenic spacer atpB-rbcL. Biosaintifika: J. Biol. Biol. Educ. 2018, 10, 41–47. [Google Scholar] [CrossRef]
- Doi, K.; Kaga, A.; Tomooka, N.; Vaughan, D.A. Molecular phylogeny of Genus Vigna subgenus Ceratotropis Based on rDNA ITS and atpB-rbcL intergenic spacer of cpDNA sequences. Genetica 2002, 114, 129–145. [Google Scholar] [CrossRef]
- González, D.; Vovides, A.P. Low intralineage divergence in Ceratozamia (Zamiaceae) detected with nuclear ribosomal DNA ITS and chloroplast DNA trnL-F non-coding region. Syst. Bot. 2002, 27, 654–661. [Google Scholar]
- Eriksson, T.; Hibbs, M.S.; Yoder, A.D.; Delwiche, C.F.; Donoghue, M.J. The phylogeny of Rosoideae (Rosaceae) based on sequences of the internal transcribed spacers (ITS) of nuclear ribosomal DNA and the trnL/F region of chloroplast DNA. Int. J. Plant Sci. 2003, 164, 197–211. [Google Scholar] [CrossRef]
- Rannala, B.; Yang, Z. Species delimitation. In Phylogenetics in Genomic Era; Section 5.5; Scornavacca, C., Delsuc, F., Galtier, N., Eds.; No Commercial Publisher; 2020; pp. 5.5:1–5.5:18. Available online: https://discovery.ucl.ac.uk/id/eprint/10097360/ (accessed on 15 November 2023).
- Jiao, X.; Flouri, T.; Yang, Z. Multispecies coalescent and its applications to infer species phylogenies and cross-species gene flow. Natl. Sci. Rev. 2021, 8, nwab127. [Google Scholar] [CrossRef] [PubMed]
- Hillis, D.M.; Chambers, E.A.; Devitt, T.J. Contemporary methods and evidence for species delimitation. Ichthyol. Herpetol. 2021, 109, 895–903. [Google Scholar] [CrossRef]




| Morphological Attributes and Geographic Information | Rydberg 1910 | Barringer 1991 | Present Study |
|---|---|---|---|
| Total height | 40 cm or more. | 60 cm. | 24–235 cm. |
| Stem | 40 cm. Glabrous, striate, light green or tinged with purple, slender. | No information. | Glabrous; light or medium green; tinged, aqueous; oval bumps; occasionally purple pigmentation. |
| Leaves | Alternate, dentate with obliquely triangular, thin, and mucronate teeth; acute at both ends 2–5 L × 1–3 W cm; somewhat paler beneath. | Ovate, roughly dentate, apiculate teeth; the base is obtuse; rounded to 2.5–6 L × 1.5–3.2 W cm. | Alternate, ovate dentate with obliquely triangular mucronate teeth; apiculate or rarely truncate form at apex; dark green in the adaxial face and pale green in the abaxial face; reticulate venation. 6.07–69 L × 4–42 W. |
| Petiole | 1–3 cm. | 10–20 eglandular. | 1–69 glabrous. |
| Stipe | No information. | 1–2 cm. | 2–47. |
| * Root | No information. | No information. | Primary root 150 L × 10 W with several adventitious roots. |
| * Cleistogamous flowers morphology | No information. | No information. | Flower is bright to pale yellow. Munsell’s color codes: (5Y 9/12, 5Y 9/8, 5Y 9/10) 13–16 L x 7–8 W. Reflexed spur 9–10, occasionally green in the distal region. Munsell’s color codes: (5GY 9/12, 5GY 9/10). Mottling patterns can be diffused or concentrated. |
| Distal lobes of the united lateral petals | No information. | Different. | Doblariform 6–17 L × 5–15.5 W. |
| Basal lobe of the united lateral petals | Somewhat shorter; boot-shaped; acute. | Entire and different. | Orbicular, oblong, and subequal; 1.5–6 L × 1–4.5 W. |
| * Flower color | No information. | No information. | Vivid to vivid-pale and strong to pale yellow closest to Munsell’s colors code: (7.5Y 9/12, 7.5Y 9/10, 5Y 9/12, 7.5Y 9/12). |
| Color and disposition of spots in the chasmogamous flower | Large purplish spots. | No information. | Intense to pale red spots. Munsell’s color codes: (5R 4/14, 5R 5/14 and 7.5 R 5/16) covering the dorsal surface (approximately ¾ of of the region) and lateral regions of the lower sepal, while in the spur the dots fade as they reach at the distal zone, in part of the lobes, spots do not cover all of them (internal face, ¾ of basal lobe and ½ or less of distal lobe) red spots-mark lines, individuals vary, presenting diffuse patterns or condensed and intense. |
| Lateral sepals | Obliquely ovate; abruptly acuminate; 5–6 L. | Free sepals. | Lateral sepals ovate with dichotomous to longitudinal venation; pale green-white, greener in the middle zone, sometimes with gray spots; 2.5–7 L × 2.5–7 W. |
| Lateral united petals | Oblong; truncate; 15 L. | United, bilobated, 0.8–1.0 cm L. | Orbicular, reniform or obcorded lobes that mostly do not overlap each other, and occasionally overlap themselves 10–24 L × 4–13 W. |
| Upper petal | Broadly ovate. | Spurred without crest 2–4 (medium-hump-spur). | Orbicular; yellow; occasionally has some red spots in the mid-posterior region; 4.5–10 L × 3.5–11.5 W; has a white or green large horn in the middle that finishes in a concave tip, and a horn with different lengths and orientations. |
| Spur | Reflexed. | No information. | Recurved (slightly, medium, extremely and may end in the shape of a hook); perpendicular; reflexed. The spur may present at distal tip of spur may present a slightly or accentuated bifurcation and, occasionally, two bulges at the extremes 4–16 L. |
| Capsule | Clavate, acute; 15–18 L × 3 W. | Glabrous and glaucous; fusiform; 1–1.5 cm L. | Glabrous and glaucous; usually elongated fusiform; rarely short fusiform; 5.14–26.5 L × 1.13–6 W. |
| * Seeds | No information. | No information. | Ellipsoid green seeds with a brown external tegument, wart series, and ornamentation in a polar region; a rectangular-square form; 2–5.5 L × 0.75–4.0 W. |
| Phenology | No information. | August. | April–October (May vary between populations). |
| Habitat | Cloud forests. | No information. | Tropical mountain cloud forests; conserved; usually near water bodies (flood lands, rivers, waterfalls, and slopes); secondary forests in open fields. |
| Altitude (masl) | No information. | 2300. | 1906–2657. |
| Traits and Geographic Information | I. mexicana | I. aurella | I. capensis | I. ecornuta | I. noli-tangere | I. pallida | I. turrialbana |
|---|---|---|---|---|---|---|---|
| References | ([24,43], Present study) | [3,24,25,31,32] | [3,24,32,68,69,70,71,72,73,75,76,77,78,79,86] | [3,24,29,31,32] | [1,21,30,32,33] | [24,30,71] | [22,23,24] |
| Leaves | Alternate; ovate; dentate with obliquely triangular mucronate teeth; apex reticulate and rarely truncate; apiculate at base. Reticulate green venation in adaxial and abaxial faces; 6.07–69 mm L × 4–42 mm W. | Alternate; ovate or oval; thin; bright green; a little paler beneath; 2–8 cm L × 1–4 cm W; coarsely dentate with obliquely triangular, finely mucronate teeth. | Alternate; glaucous or pale leaves that are ovate or elliptic and crenate; egg-shaped to elliptic; shallowly and remotely saw-toothed; 2–12 cm L, 1–6 cm W. | Alternate; ovate; elliptic; ovate-elliptic; elliptic-lanceolate; with obliquely triangular mucronate teeth; adaxial face light green and paler abaxial face; 2–10 cm L, 1–5 cm W. | Opposite becoming alternate; ovate-elliptic to ovate-lanceolate or oblong; margin serrate to subcordate; often glandular near base; 1.5–10 cm L × 1.5 cm W. | Alternate; thin; bright green; somewhat paler beneath; oval or ovate; 3–15 cm L; 2–9 cm W; crenate-dentate; with rounded or obliquely triangular mucronate teeth. | Leaves opposite; uppermost crowded to appear whorled; oblong-elliptic; 2–7.5 cm L, 1–3 cm W; remotely serrate above the middle, or often entirely; apiculate with a mucro. * Reticulate green venation in the adaxial face and green to reddish in the abaxial face. |
| Flower color (chasmogamous) | Vivid to vivid-pale and strong to pale yellow, the closest to Munsell’s color codes: 7.5Y 9/12, 7.5Y 9/10, 5Y 9/12, 7.5Y 9/12. | Orange color. | Wide color variation, including orange, orange-brown, lemon-yellow, pale and intense yellow and orange-red. Forma citrina: Perianth with a lemon-yellow color Forma albiflora: Flowers with a white or cream color. | Orange to pale yellow. | Intense yellow. | Light sulfur-yellow; citron yellow. | Scarlet color. * Yellow tones may be inside and outside the upper petal. |
| Lateral united petals | Orbicular; reniform or obcorded lobes that mostly do not overlap each other but occasionally overlap themselves; 10–24 mm L × 4–13 mm W. | Spatulate form. | Obovate; rounded-spatulate; 13.5 mm L × 7.4 mm W. | Orbicular or reniform; notched; oblanceolate or oblong; 12–14 mm L × 6 mm W. | Oblique and irregularly lobed; 22 mm L × 14.4 mm W. | Broadly obliquely spatulate; 23.1 mm L × 14.6 mm W. | Oblong-elliptic with two oblong or ovate lobes; 4 mm L × 3–4 mm W; emarginate at apex. |
| Geographical distribution | Central and southern Mexico (Hidalgo, Puebla, Veracruz, and Oaxaca). | Southwestern Canada: British Columbia; North-West United States; Washington, Idaho, and Montana [32]. | Widely distributed in the northeastern United States, its native range includes all eastern states, most Canadian provinces, many mid-western states, and the Pacific northwest [75]. | Southwestern Canada: British Columbia and North-West United States: Washington, Oregon, Idaho, and Montana [87]. | America; southwestern to central Canada; EUA; Alaska, Washington, Oregon, and California; Temperate Eurasia [87]. | Southeastern Canada; Mid-West, North-East, and South USA [87]. | Costa Rica: the Alajuela, San José and Cartago provinces; Panamá: the Chiriquí and Bocas del Toro provinces [48]. |
| Color and disposition of spots in the chasmogamous flower | Intense to pale red spots that can be constant or dispersed; dots can be alargated into lines, covering dorsal (approximately ¾ of the ventral region) and lateral regions of the lower sepal. In part of the lobes, spots do not cover everything (internal face, ¾ of basal lobe and ½ or more of distal lobe). Munsell’s color codes: 5R 4/14, 7.5R 5/16, and 5R 5/14. | Unspotted. | Posterior sepal orange and rarely pink; usually spotted (red or purple), but sometimes wholly unspotted. $ 1.-Yellow perianth with intense orange lateral petals. $ 2.-Yellow perianth with uniform light orange mottling completely covering the complete lateral petals and inner upper petal. $ 3.-Yellow perianth, with orange spots in the inner upper petal and spots that do not invade most of the 50% of lateral surface. $ 4.-Yellow perianth with dark orange uniform or dispersed spots that cover the lateral petal and inner upper petal. | Unspotted. | Unspotted or with small brownish/red spots. | Usually unspotted or with minute reddish or brownish dots. | Unspotted or * rarely dark spots in the middle region of the lower sepal and on the spur. |
| Spur | 4–16 mm L, recurved: slightly (110°), medium (180°), and extremely (270°); reflexed (45°), and perpendicular (90°) spur whose distal tip may present a slightly or accentuated bifurcation; Rarely spotted. | 8 mm L; recurved, abruptly recurved and S-curved; cylindric spur, curved or deflexed forward. | Recurved, reflexed and perpendicular (88° to 420°). | Spurless flowers. | Curved 6–12 mm L; rarely bent through 90°. | Recurved or bent at a right angle; very short (3–8 mm L), usually notched. | Incurved; inflated at the apex; 8 mm L; is * rarely slightly spotted. |
| Lateral sepals | Ovate-rounded; pale green-white; greener in the middle zone, sometimes with gray spots; 2.5–7 mm L × 2.5–7 mm W; Dichotomous to longitudinal venation; main vein in the middle is greener than the secondary venation. | Ovate; abruptly acuminate; 4–5 mm L; “Sepals pouched”. | Obovate; short acuminate; 5–6.3 mm L; “Sepals pouched”. | Oblique-elliptical; oval; abruptly acuminate at the apex; 6 mm L. | Flat and oblique; green. | Broadly ovate; acuminate; light green; 5–7 mm L. | Orbicular-ovate; apiculate; subcordate at the base; 8 mm L × 7 mm W; * Sepals yellow and yellow-red to red; Longitudinally ribbed venation; main vein in the middle is yellow and the secondary venation is red. |
| Habitat | Tropical mountain cloud forests (TMCF) with primary and secondary vegetation, which are near water bodies and, sometimes, valleys. | Moist streambanks and meadows in the steppe or lower montane zones; swamps and wet places. | Moist habitats; forests, in the lowland; steppe and lower montane zones; Lakes, pools and riverbanks; woodlands. | Moist forests in the mountain zone and wet soils. | Damp to wet; occasionally waterlogged soils, mainly in damp woods; on the edges of rivers, streams, and lakes. | Riverbanks and wet grounds; dry sites. | Valleys; near rivers. |
| Altitude (masl) | 1906–2657 | No information | 39–1334 | No information | 700–2000 | No information | 1300–2850 [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monzalvo, R.; Escorcia-Guerrero, D.L.; García-Montes, M.A.; Rewicz, A.; Rewicz, T.; Manríquez-Morán, N.L. The Mexican Balsam, Impatiens mexicana Rydb: A Redescription Based on Morphological and Phylogenetic Studies, with an Update of the Current Geographical Range of the Species. Diversity 2024, 16, 87. https://doi.org/10.3390/d16020087
Monzalvo R, Escorcia-Guerrero DL, García-Montes MA, Rewicz A, Rewicz T, Manríquez-Morán NL. The Mexican Balsam, Impatiens mexicana Rydb: A Redescription Based on Morphological and Phylogenetic Studies, with an Update of the Current Geographical Range of the Species. Diversity. 2024; 16(2):87. https://doi.org/10.3390/d16020087
Chicago/Turabian StyleMonzalvo, René, Diana Lizbeth Escorcia-Guerrero, Mario Adolfo García-Montes, Agnieszka Rewicz, Tomasz Rewicz, and Norma L. Manríquez-Morán. 2024. "The Mexican Balsam, Impatiens mexicana Rydb: A Redescription Based on Morphological and Phylogenetic Studies, with an Update of the Current Geographical Range of the Species" Diversity 16, no. 2: 87. https://doi.org/10.3390/d16020087
APA StyleMonzalvo, R., Escorcia-Guerrero, D. L., García-Montes, M. A., Rewicz, A., Rewicz, T., & Manríquez-Morán, N. L. (2024). The Mexican Balsam, Impatiens mexicana Rydb: A Redescription Based on Morphological and Phylogenetic Studies, with an Update of the Current Geographical Range of the Species. Diversity, 16(2), 87. https://doi.org/10.3390/d16020087








