Abstract
The updated checklist of the marine crabs of Guinea-Bissau presented in this work is the result of consolidating decades of research, ongoing systematic revisions of the regional carcinofauna, and the inclusion of new records. DNA markers and morphological analyses for accurate identifications are integrated. Sixty-one species are mentioned in this list after reviewing specimens from scientific collections and literature. Of these 61 species listed, the presence of 51 species in Guinea-Bissau is confirmed, and 21 are reported for the first time in the area. A total of 98 sequences were obtained from 41 species (51 of 16S for 38 species and 47 of COI for 35 species), and 48 sequences (24 of 16S and 24 of COI) were the first ones obtained for 29 species. The maximum or minimum bathymetric range is expanded by 16 species, and for other six species, a color description is provided for the first time. Merocryptus obsoletus is synonymized as Merocryptus boletifer. The present study will be a useful baseline for gathering further ecological information about globally important marine taxa, not only in Guinea-Bissau but about African brachyurans in general.
1. Introduction
Marine ecosystems, when compared with many terrestrial ecosystems, have received less attention, and there is a clear need to expand our knowledge of them. This is especially true when it comes to deep-sea fauna. The challenges associated with collecting samples in the deep ocean have left many marine areas on our planet either unknown or poorly understood.
Decapod crustaceans constitute one of the dominant groups among megabenthic marine invertebrates. It is well established that they play a key role in linking lower and higher trophic levels [1,2], and they are essential for maintaining the healthy ecology of various ecosystems [3]. Within Decapoda, brachyuran crabs are among the most extensively studied groups of crustaceans [4] and represent one of the most diverse infraorders within the Crustacea [5], with over 7771 known species worldwide to date [6].
Although studies on deep-sea crabs from various regions have seen recent increases [4,7,8,9,10,11], there has been no published research focusing on deep-sea crabs in Guinea-Bissau waters.
A series of classic taxonomic studies included records of specimens collected off Guinea-Bissau or nearby regions [12,13,14,15,16]. However, none of them provided a minimal revision of deep-sea crabs in our study area. Consequently, no Brachyura checklists or integrative taxonomy studies in Guinea-Bissau have been published, leaving the diversity of crabs in this region still poorly understood.
Throughout the 20th century, the West African coast received extensive study, and the tropical brachyuran fauna of West Africa was thus considered one of the best-known in the world [12]. Several important works on decapods were written previously: Rathbun [17] published a list of all marine and freshwater brachyurans known from Senegal to Portuguese West Africa, now known as Angola; Capart [13] elaborated a guide of the brachyurans collected during the “Mercator” expedition spanning from Morocco and Guinea and from the Gulf of Guinea to Cape Town (South Africa); Monod [14] published, according to Manning and Holthuis [12], “a monumental compilation of information on West African Brachyura.”
There are also other small reports on decapods in general and in limited areas of West Africa [15,18,19,20,21,22,23]. Some recent studies have also focused on brachyuran crabs, such as that by Matos-Pita et al. [8].
Although the Brachyura is one of the most studied taxa within benthic communities, new checklists are recently being published even for areas that are considered well-known, such as the Iberian Peninsula (SW Europe) [24], Korea [4], the Persian-Arabian Gulf [25], São Paulo [26], and Mozambique [11].
Since the 1980s, numerous oceanographic cruises have been conducted to study marine ecosystems along the Atlantic coast of Africa (see Materials and Methods), with specimens collected during these cruises being deposited in various biological collections.
Species catalogs, particularly faunal checklists for specific taxa in defined areas, offer crucial taxonomic knowledge across various scientific disciplines. They provide information about biogeography, habitat, biology, species re-descriptions, taxonomic keys, illustrations, and more [4,7,9,11,18]. Furthermore, this knowledge is basic for environmental management [4].
Currently, the improvements in molecular genetic techniques (DNA barcoding) allow us to confirm identifications and explore genetic relationships between taxa. Therefore, studies combining morphological and molecular techniques are becoming more frequent and useful [11,26,27].
This work provides the first integrative study and updated checklist on the deep-sea crabs of Guinea-Bissau (Eastern Central-East Atlantic) through the taxonomic review and catalog of the specimens housed in two Spanish marine research institutions. This is the result of compiling all previous information for this area and updating the systematics according to the last changes proposed. Also, new data, including distribution, color, morphology, and DNA barcodes, are provided for most crab species examined in this work.
2. Materials and Methods
A comprehensive review was conducted on all specimens, involving taxonomic verification by comparing them with conspecifics collected in the waters of other regions. Literature and specific works of species, genera, or families were used, including the original descriptions.
This checklist was based on several previous works [12,13,14,28], and it was complemented by an extensive review of existing literature. The species and higher taxa were listed following the classification in Davie et al. [29], updated in DecaNet [30], and following recent changes provided by Davie [3]. Genera and species were listed alphabetically within their respective families, along with updated depth range information.
All examined specimens were sexed and measured in millimeters (mm), except for those specimens that were severely damaged or were not preserved after the cruisers. Two standard measurements were taken for each specimen: the maximum carapace width, measured at the base of the lateral spines or plates (cw), and the maximum carapace length (cl), measured as the length of the dorsal midline from the middle of the frontal region to the posterior margin of the carapace, excluding the pseudorostral or rostral spine. These measurements were provided in millimeters, following the format cw × cl. In a few cases, specimens were collected but not deposited in the previously mentioned collections; in such cases, “Material examined” was replaced with “Material collected”.
The collaboration of some participants in the fisheries research and oceanographic surveys where the specimens were collected has allowed access to color photographs of many species.
Marine fauna collections visited to review all the specimens collected in the waters of Guinea-Bissau and housed in Spanish institutions are: CRUST-IEOCD (Marine Fauna Collection, IEO-CSIC [31] https://www.gbif.org/dataset/317fd0a9-1bcf-4e94-bc3c-a685c4693c10 (accessed on 17 January 2024)), CBMR (Biological Reference Collections [32] https://www.gbif.org/dataset/1d743188-1e65-4d99-a814-fa3fd51f1490 (accessed on 17 January 2024)), and the Zariquiey Marine Crustacean Legacy Collection, CBMR-Zariquiey [33] https://www.gbif.org/dataset/791a7459-7b58-444c-af46-84db6f8576ef (accessed on 17 January 2024)), these last two belonging to the ICM-CSIC.
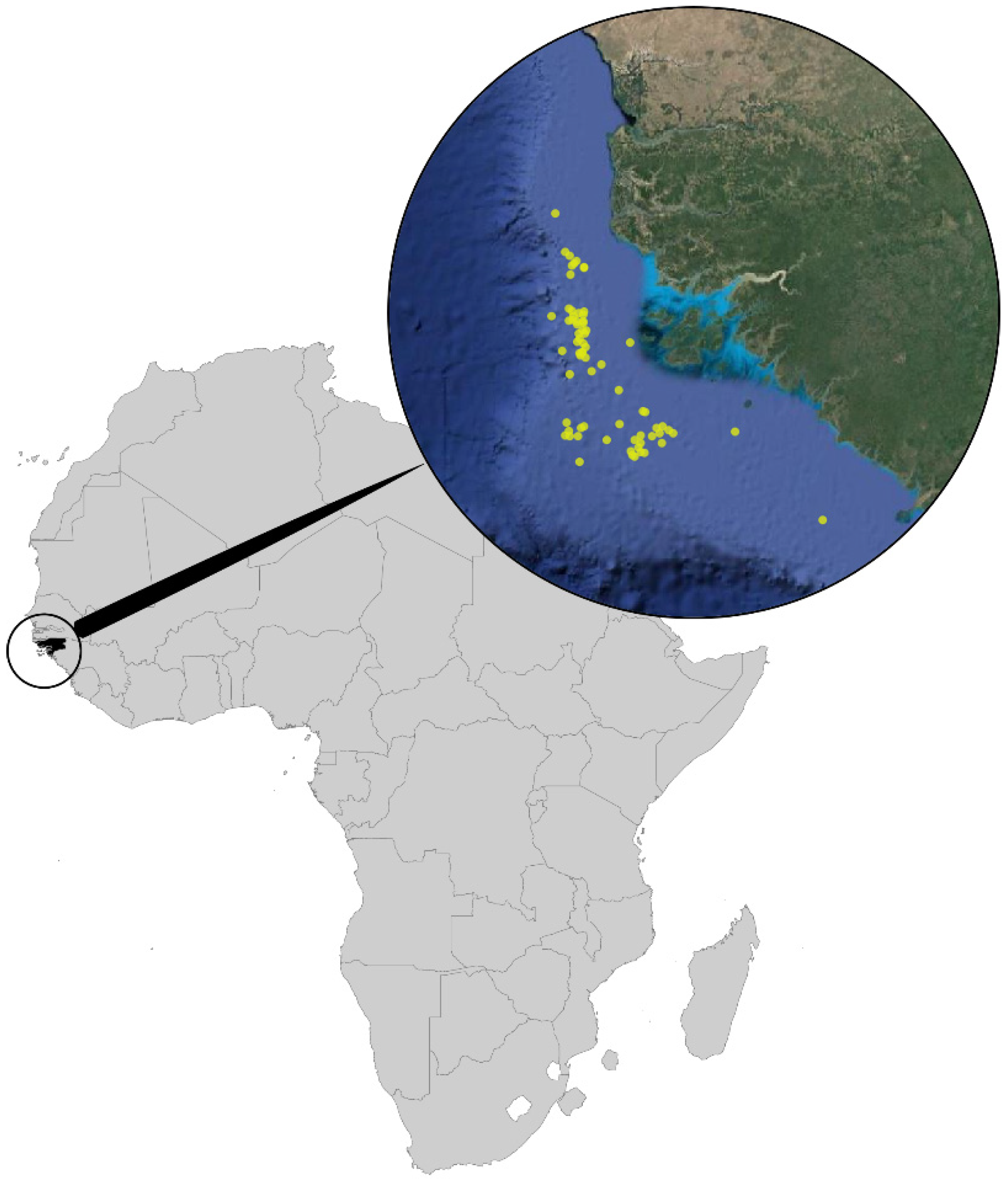
Most of the specimens examined were collected by epibenthic/demersal trawling fisheries research expeditions performed in the waters off West Africa in Guinea-Bissau waters (Figure 1). The cruises studied are:
- BISSAU0811: carried out by the Spanish Institute of Oceanography (IEO-CSIC) in November 2008, on board R/V Vizconde de Eza.
- CCLME2011: carried out under the EAF-Nansen project (Food and Agriculture Organization of the United Nations (FAO) and the Institute of Marine Research, Bergen, Norway), in October 2011 on board R/V Vizconde de Eza.
- CCLME2012: the same as above but performed in May 2012.
- LANGABISS 0111, 0211, and 0311: scientific observation survey in shrimp trawlers, permormed in Guinea-Bissau waters in 2011.
- GB1219: carried out by Spanish Institute of Oceanography (IEO-CSIC) between November and December 2019 on board R/V Vizconde de Eza.
- ICM84-85: scientific observer aboard a commercial fishing vessel: performed in Guinea-Bissau by Francesc Pagès (ICM-CSIC), 1984 and 1985 (ICM84-85 is a name given to identify that survey in this work; its real name is not known.).
Position (latitude and longitude) and depth of the sampled stations are summarized in Appendix A. The exact positions of the samples from the ICM84-85 cruises are not available.
Other abbreviations used (in alphabetical order) are: f.: female; G1 and G2 = male first and second gonopod, respectively; GB: Guinea-Bissau; m.: male; ov.: ovigerous; P2–P5 = first to last walking legs, respectively; Stn.: Station.
Total genomic DNA of a sample of the specimens studied herein was extracted from muscle tissue from one pereiopod, eye, or female pleopod, following a modified Chelex 10% protocol by Estoup et al. [34]. Target mitochondrial DNA from the 16S rRNA and COI genes was amplified with polymerase chain reaction (PCR) using the following cycling conditions: 2 min at 95 °C, and 35 cycles of 30 s at 95 °C, 30 s at 44–54 °C (depending on primer combination), and 30 s (16S) or 45 s (COI) at 72 °C, and a final 5 min at 72 °C. Primers 1472 (5′-AGA TAG AAA CCA ACC TGG-3′) [35], 16L2 (5′-TGC CTG TTT ATC AAA AAC AT-3′) [36] and 16L12 (5′-TGA CCG TGC AAA GGT AGG ATA A-3′) [37] were used to amplify a minimum of 450 bp and a maximum of 540 bp of 16S, while primers COH6 (5′-TAD ACT TCD GGR TGD CCA AAR AAY CA-3′) and COL6b (5′-ACA AAT CAT AAA GAT ATY GG-3′) [38], LCO1490 (5′-GGT CAA CAA ATC ATA AAG ATA TTG-3′) and HCO2198 (5′-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′) [39] and jgLCO1490 (5′-TNT CNA CNA AYC AYA ARG AYA TTG G-3′) and jgHCO2198 (5′-TAN ACY TCN GGR TGN CCR AAR AAY CA-3′) [40] allowed amplification of a maximum of 670 bp of COI. PCR products were sent to Stab Vida company to be purified and then bidirectionally sequenced. Sequences were edited using the software Chromas, version 2.6.4.
A BLAST (Basic Local Alignment Search Tool) on NCBI (National Center for Biotechnology Information) web facility on GenBank sequences database (http://www.ncbi.nlm.nih.gov/genbank/, accessed on 17 January 2024) was performed with the obtained final DNA sequences to obtain the best matches for species identification. The COI sequences were also searched in the official Barcode of Life database (BOLD) (http://v3.boldsystems.org/index.php/IDS_OpenIdEngine, accessed on 17 January 2024). Identifications were considered to belong to the same species when comparative sequences showed similarity values greater than 99%, with differences in 1–4 mutations in 16S and >97% (1–15 mutations) in COI, in this last case according to Meyer and Paulay [41]. Lower similarity values are highlighted as questionable species relationship that needs further study. All sequences obtained for both genes were deposited in Genbank. In the case of species included in the checklist but for which sequences could not be obtained in the present work, Genbank and BOLD databases were searched, and selected sequences for 16S and COI (when available) are provided. Some COI sequences deposited in BOLD and marked as “early-release” or “private” can be shown as results of a search and provide similarity data, but they are not available for downloading or further pair-base comparisons. For this reason, these sequences are mentioned as results in the “DNA barcodes” section but are considered in the present work as unpublished.
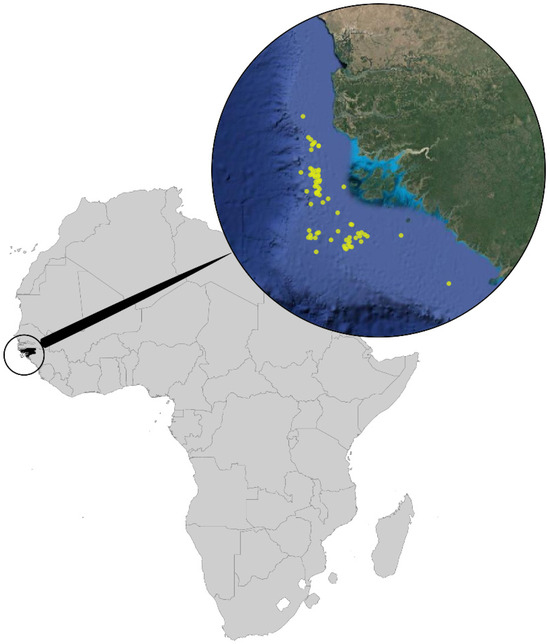
Figure 1.
Study area with collect sites in the continental shelf of Guinea-Bissau marked as yellow points.
Figure 1.
Study area with collect sites in the continental shelf of Guinea-Bissau marked as yellow points.
3. Results
3.1. Systematics of Species and Molecular Data
- Section PODOTREMATA Guinot, 1977
- Superfamily DROMIOIDEA De Haan, 1833 [in De Haan, 1833–1850]
- Family DROMIIDAE De Haan, 1833 [in De Haan, 1833–1850]
- Subfamily DROMIINAE De Haan, 1833 [in De Haan, 1833–1850]
- Dromia nodosa A. Milne-Edwards & Bouvier, 1898 (Figure 2A)
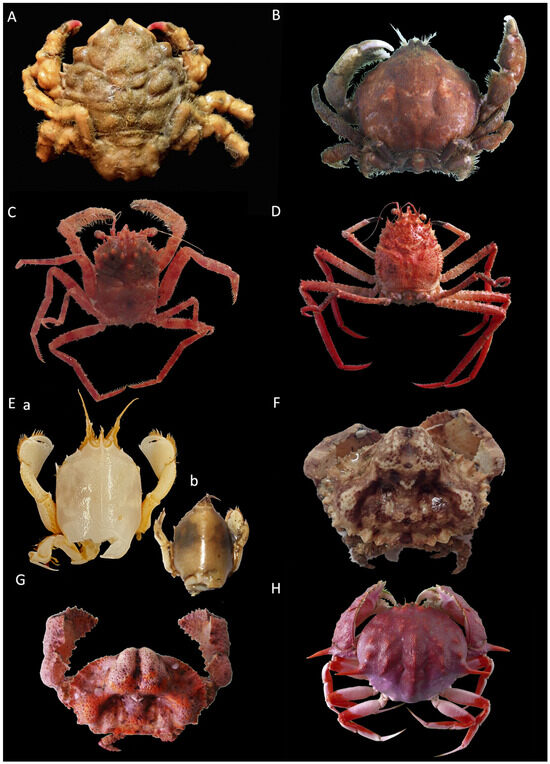
Figure 2.
(A) Dromia nodosa f. ov. 20.0 × 17.0 (cw × cl) IEOCD-GB19/2947; (B) Sternodromia spinirostris m. 40.6 × 36.0, IEOCD-GB08/178; (C) Homola barbata m. 20.1 × 23.0 IEOCD-GB08/191; (D) Paromola cuvieri f. 72.1 × 89.2; (E) Ranilia constricta 14.6 mm cw, (a) damaged specimen preserved in ethanol, (b) photo of fresh specimen, IEOCD-GB08/157; (F) Sakaila africana m. 28.7 × 20.4 IEOCD-GB19/2948; (G) S. africana f. 22.7 × 17.2, IEOCD-GB08/183; (H) Acanthocarpus brevispinis f. 53.1 × 49.8 IEOCD-GB19/2961.
Figure 2.
(A) Dromia nodosa f. ov. 20.0 × 17.0 (cw × cl) IEOCD-GB19/2947; (B) Sternodromia spinirostris m. 40.6 × 36.0, IEOCD-GB08/178; (C) Homola barbata m. 20.1 × 23.0 IEOCD-GB08/191; (D) Paromola cuvieri f. 72.1 × 89.2; (E) Ranilia constricta 14.6 mm cw, (a) damaged specimen preserved in ethanol, (b) photo of fresh specimen, IEOCD-GB08/157; (F) Sakaila africana m. 28.7 × 20.4 IEOCD-GB19/2948; (G) S. africana f. 22.7 × 17.2, IEOCD-GB08/183; (H) Acanthocarpus brevispinis f. 53.1 × 49.8 IEOCD-GB19/2961.
Material examined. Guinea-Bissau, GB1219, Stn. 18, November 2019, 65–70 m, f. ov. 21.9 × 20 (cw × cl) IEOCD-GB19/2967; GB1219, Stn. 82, 56 m, December 2019, f. ov. 20.0 × 17.0 IEOCD-GB19/2947.
Identification. The key provided by Forest [42] was used to determinate at the species level.
Distribution. The Morocco and Cape Verde Islands between 75 and 90 m [12,42].
Remarks. The figure of a D. nodosa specimen from Monod [14] actually belongs to Sternodromia monodi (Forest & Guinot, 1966) [15]. Forest and Guinot [15] described S. monodi as a new species from specimens collected in São Tomé, and upon reviewing the Monod’s D. nodosa specimens [14], they concluded that they belonged to S. monodi. Subsequently, Forest [42] affirmed this conclusion. Using the key provided by the last author [42], the specimens examined in this study correspond with the characteristic of the first rounded anterolateral tooth (unique in this genus). Carapace regions may be more or less marked, as observed in Forest [42]. This work provides the first records of D. nodosa in the waters of Guinea-Bissau and extends the worldwide minimum depth from 75 to 56 m.
DNA barcodes. Only the 16S sequence was obtained for the specimen IEOCD-GB19/2947. There are not any sequences of this genetic marker available for this species in any public database; therefore, this is the first one. The closer sequence for 16S is MF490178 from Dromia erythropus (97% similarity) from Brazil obtained by Mantelatto et al. [43], which falls in the expected intrageneric level.
- Sternodromia spinirostris (Miers, 1881) (Figure 2B)
Material examined. Guinea-Bissau, GB1219, Stn. 18, 65−70 m, November 2019, m. 19 × 17.6, m. 19.3 × 17.9, m. 17.3 × 15.9 (CW × CL, mm), IEOCD-GB19/2980; Bissau0811, Stn. 20, 52 m, October 2008, f. 23.1 × 21.8, m. 40.6 × 36.0, IEOCD-GB08/178; LANGABISS0211, Stn. 63, 48 m, May 2011, f. 30.3 × 27.9, IEOCD-LB0211/436-2; CCLME12, Stn. 43, 53 m, May 2012, f. ov. 28.5 × 26, f. 39.0 × 34.7, f. 38.2 × 32.5, m. 45.3 × 39.5, m. 40.1 × 35.7, IEOCD-CCLME12/1540; GB1219, Stn. 82, 56 m, July 2019, f. 35.3 × 32.2, ICMD002679; GB1219, Stn. 83, 71−75 m, m. 18.2 × 17, ICMD002681; CCLME12, Stn. 7, 47 m, May 2012, f. ov. 33.0 × 30.0, m. 24.3 × 22.3, f. 13 × 13.8, IEOCD-CCLME12/1588; LANGABISS0211, Stn. 63, 48 m, May 2011, f. 26.3 × 24.3, IEOCD-LB0211/436-1.
Identification. The work provided by Forest [42], where the genus Sternodromia was described, was used for species identification.
Distribution. West Africa, from Western Sahara to Angola, with other records in Western Sahara, Mauritania, The Cape Verde Islands, Senegal, Gambia, Guinea-Bissau, Guinea, Sierra Leone, Ivory Coast, Ghana, Nigeria, Cameroon, Gabon, Congo, and Zaire [12,13,14,15,42,44]. This species has also been reported in the Mediterranean Sea [45] at depths between 12−100 m [42] and 15−122 m [12]. In Guinea-Bissau, the species has been recorded at 60–73 m [15,42].
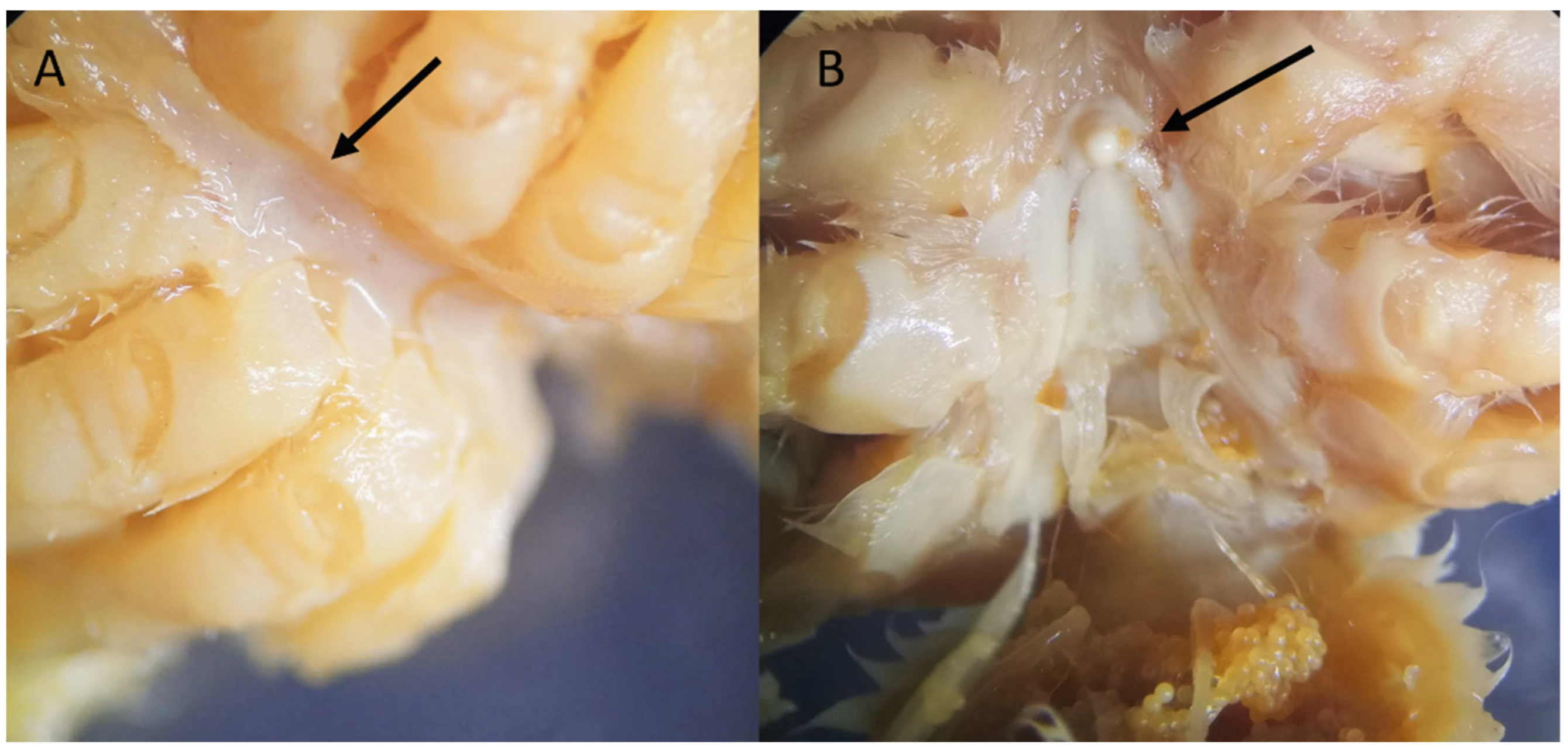
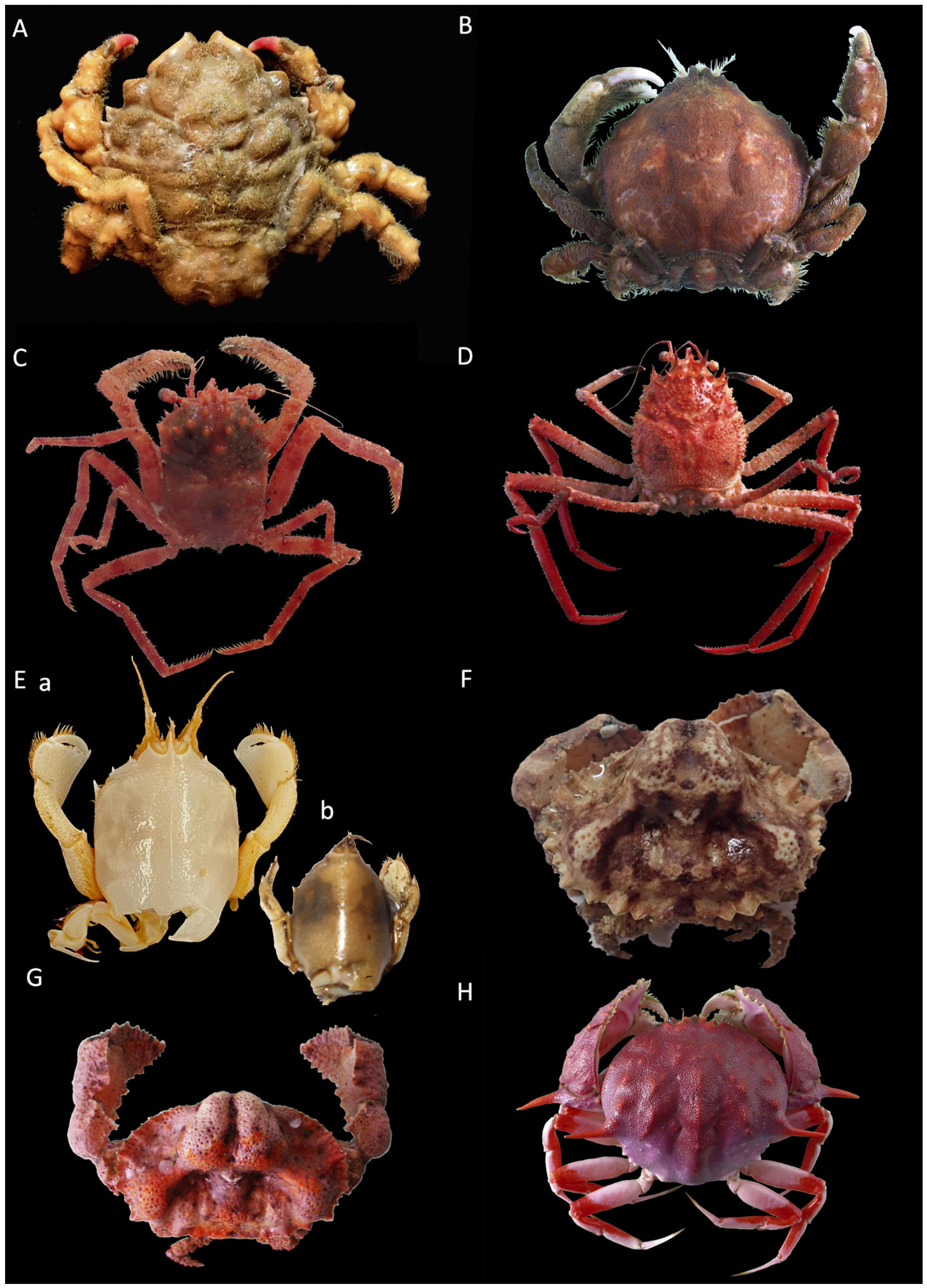
Remarks. While all specimens closely match the original and subsequent descriptions of S. spinirostris, it is worth remarking a distinguishing feature in one of the females examined, namely, the smallest one with code IEOCD-CCLME12/1588. Forest [42] elaborated a key for the Dromiidae species, and the first species that is separated from the rest is S. spinirostris, and one of the defining characteristics is “sillons sternaux de la femelle s’ouvrant à la base d’un fort tubercule conique median.” S. spinirostris is the only species within Dromiidae with a median round tubercle in the female sternum. This key could lead us to confusion because our specimen, IEOCD-CCLME12/1588 (a small female), lacks this medium tubercle (Figure 3A), but not the large ovigerous female (IEOCD-CCLME12/1588), which bears it (Figure 3B). Manning and Holthuis [12] clarified this topic and remarked that the young females did not show this tubercle. For this reason, young specimens of this species could be misidentified as Dromia monodi (Forest and Guinot, 1966) (presently, Sternodromia monodi). DNA data (see below) allows us to confirm this identification as S. spinirostris. Concerning bathymetric range, the present records reduce the minimum depth in Guinea-Bissau waters from 60 to 47 m.
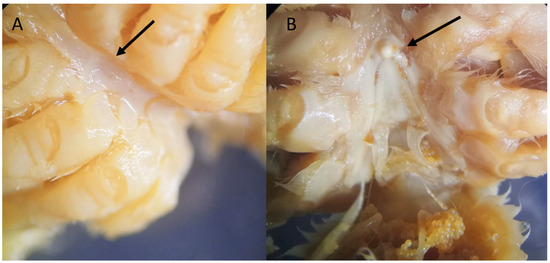
Figure 3.
Sternum of Sternodromia spinirostris (A) f. 13 mm cw, (B) f. ov. 30 mm cw (IEOCD-CCLME12/1588). Black arrows point out the location where the tubercle is present or absent.
Coloration observed. As previously described by Capart [13] and Forest [42], the carapaces of the studied specimens are brown to purplish-brown with a velvety appearance due to short, dense hairs. Additionally, as pointed out by Manning and Holthuis [12], the fingers of our specimens are white and hairless.
DNA barcodes. 16S and COI sequences were obtained for the specimens IEOCD-LB0211/436-2, IEOCD-GB19/2980, and IEOCD-CCLME12/1588. There are no sequences of these two genetic markers available for this species in any public database; therefore, the sequences obtained in the present study constitute the first for the species. The closest sequence for 16S is MF490178 from Dromia erythropus (97% similarity), obtained by Mantelatto et al. [43], and it could be considered an intrageneric level similarity. In the case of COI, the closest sequence belongs to Dromia personata (JQ306068), obtained by Matzen et al. [46] with a 90% similarity. For this gene, the distances obtained could be considered around the limit between intra- and intergeneric levels. The three specimens showed two haplotypes for 16S and COI, each one differentiated by just one mutation. IEOCD-GB19/2980 and IEOCD-CCLME12/1588 share the same haplotypes, while IEOCD-LB0211/436-2 presents the second haplotype for both genes.
- Superfamily HOMOLOIDEA De Haan, 1839 [in De Haan, 1833–1850]
- Family HOMOLIDAE De Haan, 1839 [in De Haan, 1833–1850]
- Homola barbata (Fabricius, 1793) (Figure 2C)
Material examined. Guinea-Bissau, Bissau0811, Stn. 52, 223 m, November 2008, m. 20.1 × 23.0 (cw × cl) IEOCD-GB08/191; LANGABISS0211, Stn. 77, 207 m, May 2011, m. 13.8 × 20.1, f. 23 × 26.8, IEOCD-LB0211/422.
Identification. Manning and Holthuis [12] was used to identify the species.
Distribution. Homola barbata is a species with a wide distribution that inhabits waters of the Eastern (from Portugal and Spain to South Africa), Western Atlantic, and Mediterranean Sea between 10 and 679 m [12,14,47,48,49]. The first record in the waters of Guinea-Bissau was made by Muñoz et al. [10] at depths of 223 m.
Remarks. This specimen constitutes the second record of H. barbata off Guinea-Bissau and reduces its minimum depth from 223 to 207 m.
Coloration observed. Orange-reddish in general, with lighter orange chelae, meri of the chelae, and all the articles of the walking legs with weaker, darker bands, almost maroon.
DNA barcodes. No DNA sequences were obtained for these specimens. One 16S sequence (MF490181) and five COI sequences (JQ306136, JQ306137, JQ348863, MF490091, MW264436) of specimens from Portugal, Morocco, Brazil, and the Northeast Atlantic are deposited and available in Genbank, and one COI sequence (BIM677-19) of a specimen from Israel is deposited and available in BOLD. When comparing the COI sequence of the specimen from Brazil with the rest, the percent of similarity is in a range of 95.97–96.48%, suggesting a congeneric difference. In this case, this sequence (MF490091) could belong to Homola minima Guinot & Richer de Forges, 1995, instead of H. barbata, since there is also a sequence of a specimen collected in Florida (US), deposited in BOLD (marked as private), that matches 100% with this sequence.
- Paromola cuvieri (Risso, 1816) (Figure 2D)
Material collected. Guinea-Bissau, Bissau0811, Stn. 28, 422–431 m, November 2008, f. 72.1 × 89.2 (cw × cl).
Identification. Capart [13] was used for the determination at the species level.
Distribution. Atlantic waters from Scotland to Angola, with records in the Azores and the Cape Verde Islands, Mauritania, and Mediterranean Sea, from 10 to 1000 m [8,12,13,24,50,51]. Recorded for the first time in the waters of Guinea-Bissau between 29 and 431 m by Muñoz et al. [10].
Remarks. No specimens of P. cuvieri were housed in a collection. This female was photographed, measured, and identified on board using Capart’s work [13].
Coloration observed. Our specimen shows the color described for this species by Capart [13]: “Couleur generale jaune orange, plus rouge a 1′avant de la carapace et sur les epines anterieures; les pattes un peu plus foncees”. We added to that description the presence of black fingers.
DNA barcodes. No DNA sequences could be obtained from this specimen because it was not deposited in a collection. Unfortunately, there are no sequences for any genetic marker for this species in public databases.
- Superfamily RANINOIDEA De Haan, 1839 [in De Haan, 1833–1850]
- Family RANINIDAE De Haan, 1839 [in De Haan, 1833–1850]
- Subfamily NOTOPODINAE Serène & Umali, 1972
- Ranilia constricta (A. Milne-Edwards, 1880) (Figure 2E)
Material examined. Guinea-Bissau, Bissau0811, Stn. 65, 29 m, November 2008, 14.6 mm cw (damaged specimen) IEOCD-GB08/157.
Identification. The specimen was strongly damaged. The original description and illustration made by Studer [52] as Notopus atlanticus and posterior remarks added by other authors as Ranilia atlantica [12,14] were used for the identification of the specimen.
Distribution. Ranilia constricta has a wide distribution, inhabiting waters on both sides of the Atlantic Ocean. Off-West Africa records are reported from: the Cape Verde Islands, Mauritania, Senegal, Guinea-Bissau, Congo, Sierra Leone, Equatorial Guinea (Annobon Island), and Ascension Island [10,12,16,53,54]. In West African waters, it has been reported at depths between 40 and 69 m, off Ascension Island at 110 m; in the western Atlantic, it has been recorded from the littoral zone to 481 m [12].
Remarks. The specimen examined herein was reported by Muñoz et al. [10] as the first record of the species off Guinea-Bissau.
Coloration observed. The carapace is orange-brown with numerous scattered white dots. The chelipeds and legs are beige, and the movable finger is orangish. No references to the live coloration have been found.
DNA barcodes. 16S and COI sequences were obtained for this specimen (IEOCD-GB08/157). There is only one sequence of 16S in Genbank, but the COI sequence is the first one obtained for this species. The 16S sequence matched 100% with the 16S sequence MF490212 from R. constricta (as Ranilia muricata in Genbank) obtained by Mantelatto et al. [43] and assigned to R. constricta by Mantelatto et al. [26]. However, the COI sequence presents 87.39% and 87.86% similarity with R. muricata (Florida, USA) and R. fornicata (Costa Rica), respectively. These sequences are deposited in BOLD but marked as private. There are also two COI sequences, MW124935 and MW124892, that present closer similarities (98.48 and 98.33%, respectively) at the intraspecific level and belong to two larvae from plankton of the Gulf Stream off Fort Pierce (Florida, USA) (USNM:IZ:1450125 and USNM:IZ:144967). More research, including more DNA markers, will be necessary to dilucidate the relationship and distribution between R. constricta, inhabiting the west and east parts of the Atlantic, and R. muricata, which is distributed only in the west Atlantic. The present molecular data supports the previous comments by Manning and Holthuis [12]. They did not find significant morphological differences between R. constricta from West Africa and R. constricta (as R. atlantica) from Ascension Island, Cuba, Barbados, and Florida; therefore, this would be considered an amphiatlantic species.
- Section EUBRACHYURA Saint Laurent, 1980
- Subsection HETEROTREMATA Guinot, 1977
- Superfamily AETHROIDEA Dana, 1851
- Family AETHRIDAE Dana, 1851
- Sakaila africana Manning & Holthuis, 1981 (Figure 2F,G)
Material examined. Guinea-Bissau, GB1219, Stn. 83, 71−75 m, December 2019, m. 28.7 × 20.4 (cw × cl) IEOCD-GB19/2948; Bissau 0811, Stn. 5, 105 m, October 2008, f. 22.7 × 17.2, IEOCD-GB08/183; Bissau0811, Stn. 19, 71 m, October 2008, m. 35 × 25.2, IEO-CD-GB08/184.
Identification. The original description made by Manning and Holthuis [12] and previous works where this species was misidentified with Osachila stimpsonii Studer, 1883 [14,15,55] were used for the determination.
Distribution. Off tropical West Africa, with records in Senegal, Gabon, and Equatorial Guinea (Annobon) [12,14,15], Cape Verde Island [56], and Guinea-Bissau [10], between 65 and 132 m.
Remarks. The two specimens from 2008 reported here were the first records of S. africana in Guinea-Bissau [10], and the male from 2019 is the second record in these waters at depths between 71 and 105 m.
Coloration observed. Coloration in real life is described for the first time. These specimens exhibit two different color patterns: the male IEOCD-GB19/2948 (Figure 3F) has brown carapace with darker brown and beige patches. Chela and walking legs show the same pattern; the female IEOCD-GB08/183 (Figure 3G) is brown, with some areas displaying an orange hue. The chela and walking legs exhibit a similar pattern. Notably, even after preservation in ethanol, this female specimen retains some orange areas.
DNA barcodes. 16S sequences were obtained for the three specimens: IEOCD-GB19/2948, IEOCD-GB08/183, and IEOCD-GB08/184. There are no sequences of 16S available for this species in any public database; therefore, these constitute the first ones. Each specimen presents a different haplotype, separated from each other by one or two mutations. COI sequences were obtained for two specimens, IEOCD-GB08/183 and IEOCD-GB08/184. Each specimen showed a different haplotype, separated apart by six mutations. These two sequencies fit 99.24 and 99.54% similarity with a sequence of Sakaila africana from Senegal deposited in BOLD (marked as early-release); therefore, these are the first COI sequences published and available for this species.
- Superfamily CALAPPOIDEA De Haan, 1833 [in De Haan, 1833–1850]
- Family CALAPPIDAE De Haan, 1833
- Acanthocarpus brevispinis Monod, 1946 (Figure 2H)
Material examined. Guinea-Bissau, GB1219, Stn. 10, 173−175 m, November 2019, f. 53.1 × 49.8, m. 62.0 × 56.1 (cw × cl) IEOCD-GB19/2961; Bissau0811, Stn. 28, 431 m, October 2008, m. 67.3 × 62.5, IEOCD-GB08/158; Bissau0811, Stn. 4, 385 m, October 2008, m. 57.5 × 55.0, IEOCD-GB08/159; LANGABISS0211, Stn. 47, 245 m, May 2011, m. 55 × 51.4, IEOCD-GB0211/424; ICM84-85, 247–267 m, January 1985, m. 49.2 × 29.4, f. 57.14 × 36.25, ICMD322/1998; 200–234 m, January 1985, m. 68.43 × 56.68, f. ov. 97.2 × 85.9, ICMD318/1998; 220–400 m, January 1985, m. 69.2 × 54.2 m. 74.6 × 55.9, ICMD319/1998; ICM84-85, 227–359 m, f. 78.8 × 53.69, ICMD321/1998; ICM84-85, 214 m, January 1985, m. 76.74 × 61.25 m. 71.61 × 56.23 f. 72.38 × 52.75, ICMD325/1998; ICM84-85, 247–267 m, January 1985, m. 69.32 × 55.28 m. 73.86 × 56.37 f. 78.9 × 52.65, ICMD323/1998; ICM84-85, 270–351, January 1985, f. 82.51 × 54.51 f. 70.19 × 52.19, ICMD320/1998; ICM84-85, 247–267, January 1985, f. 81.3 × 52.1 m. 72.8 × 57.1 m. 80.7 × 58.3 m. 74 × 56.3, ICMD324/1998.
Identification. The original description and illustrations provided by Monod [57] were used for the determination.
Distribution. West Africa, between Morocco and Angola, with records in Western Sahara, Mauritania, Senegal, Guinea, Guinea-Bissau, Gambia, Ivory Coast, Ghana, Nigeria, Sierra Leone, and Togo, between 100 and 500 m [8,10,12,14,28,57]. The first record in Guinea-Bissau was published by Muñoz et al. [10], between 213 and 517 m.
Remarks. Neither Manning and Holthuis [12], Monod [14], nor Sasaki [28] reported A. brevispinis in the waters of Guinea-Bissau, although Capart [13] described this species (as Acanthocarpus africanus), establishing its distribution range between 10° N and 11° S, being 10° N the limit between Guinea-Bissau and Guinea. The material examined here provides the second record of A. brevispinis in Guinea-Bissau, and although the first record was provided in 2012 by Muñoz et al. [10], the specimens from 1985 represent the first confirmed presence of the species in this area up to now. Also, the minimum depth is reduced from 213 to 200 m.
Coloration observed. Capart [13] described its live color as “carapace de blanc a rose, passant au mauve sur la partie anterieure des pinces; les epines orange; les pattes en partie orange, en partie blanches.” All of the specimens reviewed herein have bright red, almost maroon carapace, tubercles tip reddish, and posterolateral regions of carapace in pink-purple. Chelae with the outside red-maroon and the big spine of the merus in red, and the inner part between white and pink. Ambulatory legs with the ischium, first half of the merus, and the dactyls in pinkish white; second half of the merus; carpus; and top of the propodus in red. After two years of preservation in ethanol, specimens retain the pink color of the carapace and the orange color of the legs.
DNA barcodes. 16S sequences were obtained for two specimens, IEOCD-GB19/2961 and IEOCD-GB08/159. Both sequences represent a unique haplotype that matches 100% with the sequence KU206580 of the specimen ZMK2569 of A. brevispinis from Angola obtained by Ewers-Saucedo et al. [58]. A COI sequence was obtained for the specimen IEOCD-GB08/159. This sequence matched 100% and 99.69% with two sequences of A. brevispinis from the “Atlantic Ocean” deposited in BOLD (marked as early-release); therefore, this is the first COI sequence published and available for this species.
- Calappa galloides Stimpson, 1859 (Figure 4A)

Figure 4.
(A) Calappa galloides m. 56.7 × 44.3 (cw × cl) IEOCD-GB19/442; (B) Calappa pelii m. 32.3 × 27.9, IEOCD-GB08/196; (C) Calappa rubroguttata m. 5.7 × 42.3 IEOCD-GB19/2985; (D) Medorippe lanata f. 29.9 × 23.6 IEOCD-GB08/164; (E) Phyllodorippe armata f. 15.6 × 11 IEOCD-GB19/2953; (F) Ethusa rosacea m. 9.3 × 9.2 IEOCD-CCLME12/1511; (G) Ethusa rugulosa m. 15.8 × 15.7 IEOCD-GB08/163; (H) Ethusa vossi f. 8.8 × 9.9 IEOCD-GB19/2956.
Figure 4.
(A) Calappa galloides m. 56.7 × 44.3 (cw × cl) IEOCD-GB19/442; (B) Calappa pelii m. 32.3 × 27.9, IEOCD-GB08/196; (C) Calappa rubroguttata m. 5.7 × 42.3 IEOCD-GB19/2985; (D) Medorippe lanata f. 29.9 × 23.6 IEOCD-GB08/164; (E) Phyllodorippe armata f. 15.6 × 11 IEOCD-GB19/2953; (F) Ethusa rosacea m. 9.3 × 9.2 IEOCD-CCLME12/1511; (G) Ethusa rugulosa m. 15.8 × 15.7 IEOCD-GB08/163; (H) Ethusa vossi f. 8.8 × 9.9 IEOCD-GB19/2956.

Material examined. Guinea-Bissau, GB1219, Stn. 41, 28−29 m, November 2019, m. 56.7 × 44.3 (cw × cl) IEOCD-GB19/442.
Identification. To determine and clarify the taxonomical status of the specimen, several documents were used [12,14,59].
Distribution. Tropical Atlantic. In the eastern Atlantic, the species has a distribution from the Canary Islands to Angola, and in the central Atlantic, on Ascension Island, shore to 200 m [12,14,28,60,61].
Remarks. There is certain confusion about the identity of C. galloides. Manning and Holthuis [12] considered it a synonym of Calappa gallus (Herbst, 1803), and then C. galloides would have a wider range of distribution. Galil [59] clarified the status of these two species, maintaining the names C. gallus for the species that inhabits Indo-Pacific waters and C. galloides for the Tropical Atlantic species. This explains why Manning and Holthuis [12] and Monod [14] recorded C. gallus in their respective revisions of western African crabs instead of C. galloides. Even nowadays, there is some confusion in some biodiversity platforms about the distribution of these two species, such as WORMS [62] or GBIF [63], or in works such as Sasaki [28]. The present work provides the first record of C. galloides in Guinea-Bissau at a depth of 28–29 m.
Coloration observed. Reddish-brown with rose and beige patches scattered throughout the carapace and legs.
DNA barcodes. 16S and COI sequences were obtained for the specimen IEOCD-GB19/442. There are 16S and COI sequences (MK971242, and MN183812, respectively) in Genbank for a specimen of C. galloides (ULLZ13575) from Bocas del Toro (Panama) obtained by Venera-Ponton et al. [64]. The 16S sequences of both specimens differ in five mutations (including one gap) (98.99% similarity), and COI sequences show larger differences: 43 mutations in 658 bp (93.47% similarity). In BOLD, there are also two COI sequences (marked as private) from specimens from Louisiana (USA), with similar distances (93.22 and 93.39%). These data question the fact that both specimens belong to the same species; therefore, new studies including more specimens from both sides of the Atlantic will be necessary to clarify this question.
- Calappa pelii Herklots, 1851 (Figure 4B)
Material examined. Guinea-Bissau, GB1219, Stn. 6, 58−61 m, November 2019, m. 23.3 × 20.2 (cw × cl) IEOCD-GB19/2981; GB1219, Stn. 18, 65−70 m, November 2019, f. 22.6 × 20, m. 23.9 × 20.8, IEOCD-GB19/2982; GB1219, Stn. 83, 71−75 m, December 2019, f. 31.7 × 26.9, IEOCD-GB19/2929; GB1219, Stn. 29, 72−75 m, December 2019, m. 34.3 × 29, IEOCD-GB19/2986; Bissau0811, Stn. 96, 37 m, November 2008, f. ov. 72.2 × 60.7, IEOCD-GB08/161; Bissau0811, Stn. 20, 52 m, October 2008, m. 22.4 × 19.3, m. 20.2 × 18.3, m. 32.3 × 27.9, IEOCD-GB08/196; LANGABISS0111, Stn. 136, 17 m, May 2011, f. 72.5 × 59.2, IEOCD-LB0111/402; CCLME12, Stn. 47, 175 m, May 2012, m. 34.5 × 30.7, IEO-CD-CCLME12/1495; 270–351 m, January 1985, f. 65.7 × 54.5, f. 76.54 × 55.75, ICMD312/1998; ICM84-85, 240–334 m, January 1985, m. 87.56 × 70.86, m. 81.66 × 66.09, ICMD316/1998; ICM84-85, 55–58 m, February 1985, m. 86.13 × 68.41, f. ov. 83.19 × 68.9, m. 82.69 × 65.77, m. 68.37 × 58.6, m. 54.31 × 43.2, m. 31.9 × 28.71, ICMD314/1998; ICM84-85, 200–234 m, January 1985, f. 80.71 × 63.37, f. 75.78 × 60.94, f. 66.12 × 54.09, ICMD315/1998; ICM84-85, 180–200 m, January 1985, m. 65 × 54.57, f. 50.53 × 43.88, ICMD313/1998; ICM84-85, 47–52 m, February 1985, m. 89.1 × 67.7, m. 79.6 × 64.2, f. 74.6 × 61.9, ICMD317/1998.
Identification. Although Capart [13] provided a key for differentiating C. pelii from the other species of the genus in West Africa, such as Calappa rubroguttata Herklots, 1851, it was necessary to use other works for small specimens [12,14]. Young specimens of these two species are difficult to distinguish because their carapace colors are similar, and small C. rubroguttata do not show well-developed, marked red round spots on their carapace and chela. Manning and Holthuis [12] provided linear drawings of the carapace of young specimens of both species.
Distribution. Off West and Central Africa, between Western Sahara and Angola, in depths between 8–20 and 400 m, with records in Mauritania, Senegal, Guinea-Bissau, Sierra Leone, Ivory Coast, Ghana, Cameroon, Principe, Gabon, and Congo [12,13,14,15]. Also in the Mediterranean Sea [65].
Remarks. It is a species with a wide distribution throughout West Africa that also exhibits high genetic variability.
Coloration observed. Our specimens fit well with the color description made by Capart [13].
DNA barcodes. Sequences of 16S and COI were obtained for the specimen IEOCD-GB08/161. The 16S sequence fit 99.26 to 99.75% (one to three mutations) with three sequences (KU206597-KU206599) obtained by Ewers-Saucedo et al. [58] of the specimens of C. pellii (ZMK2565, ZMK2567, and ZMK2568) from Angola. The COI sequence shows a 98.02% similarity with the sequence KU853979, also obtained by Ewers-Saucedo et al. [58], but from an uncatalogued specimen of the Gulf of Guinea. However, there are five sequences in BOLD (marked as early-release), all from different locations in the East Atlantic (from the Gulf of Guinea to Portugal), and with similarity between 97.7 and 99.85%.
- Calappa rubroguttata Herklots, 1851 (Figure 4C)
Material examined. Guinea-Bissau, GB1219, Stn. 36, 45−46 m, November 2019, m. 57 × 42.3 (cw × cl) CRUST_GB19/2985; Bissau0811, Stn. 59, 22 m, November 2018, f. ov. 80.1 × 61.4, IEOCD-GB08/160; Bissau0811, Stn. 58, 41 m, November 2008, m. 20.7 × 17.1, IEOCD-GB08/162; CCLME12, Stn. 65, 30 m, May 2012, f. 56.7 × 42.8, IEO-CD-CCLME12/1534; 28 m, December 1984, m. 97.7 × 71.1, m. 106.9 × 75.1, ICMD308/1998; ICM84-85, 27 m, December 1984, m. 99.45 × 74.83, m. 107.11 × 81.72, ICMD309/1998.
Identification. The same bibliography as for C. pelii was used for the identification of the specimens.
Distribution. Off West Africa, between Senegal and Angola, with records in Guinea-Bissau [10], Guinea, Sierra Leone, Liberia, Ivory Coast, Ghana, Cameroon, Gabon, and Congo, usually at depths shallower than 50 m [12,13,14,15,23,53,66,67,68,69].
Remarks. Specimens IEOCD-GB08/162 and IEOCD-GB08/160 were reported by Muñoz et al. [10] as first records of C. rugroguttata in Guinea-Bissau waters. The other specimens examined in this work provide the second record for this species, occurring at depths ranging from 22 to 46 m. The two males collected in 1985 were the first specimens found in these waters. Furthermore, the review and comparison of the specimens from Guinea-Bissau with those from other areas have provided the first record of C. rubroguttata off Mauritania. It is a single specimen collected in 2011 at a depth of 24 m, hosted at the CRUST-IEOCD collection, not published (https://www.gbif.org/occurrence/2564781517 (accessed on 17 January 2024)), and represents the northernmost record for this species.
Coloration observed. Our specimens fit well with the color description made by Capart [13].
DNA barcodes. Sequences of 16S and COI were obtained for the specimens IEOCD-GB08/160 and CRUST_GB19/2985. The 16S sequences of both specimens differ in three mutations, and both present a similarity of 95.54–95.43% (23 mutations) with respect to the sequence KU206602 of the specimen ZMK2571 of C. rubroguttata from Angola, obtained by Ewers-Salcedo et al. [58]. However, both sequences from Guinea-Bissau differ only in one (IEOCD-GB08/160) or four mutations (IEOCD-GB19/2985) from the specimen of C. rubroguttata IEOCD-GECU17/2157 from Equatorial Guinea (Genbank accession code PP118359). These data question the identity of the specimen from Angola. The COI sequences of the specimens from Guinea-Bissau represent two haplotypes that differ in two mutations; however, both sequences show similarities of 90.54 and 90.63% with respect to the single COI sequence of C. rubroguttata deposited in BOLD (marked as early-release). According to the map shown in BOLD, the specimen could have been collected in Senegal. The COI sequence of the specimen IEOCD-GECU17/2157 from Equatorial Guinea (Genbank accession code PP133847) presents a third haplotype that only differs in one and two mutations with respect to IEOCD-GB08/160 and IEOCD-GB19/2985, respectively. Data from 16S and COI suggest that, probably, in the Gulf of Guinea and close areas, two different species may be present, currently identified as C. rubroguttata.
- Supferfamily CANCROIDEA Latreille, 1802
- Family ATELECYCLIDAE Ortmann, 1893
- Atelecyclus rotundatus (Olivi, 1792)
Material collected. Guinea-Bissau, BISSAU0811, St 50, 1 November 2008, 256–257 m, four specimens, sex and size not available.
Identification. The key provided by Monod [14] was used.
Distribution. Eastern Atlantic, from Scotland and Scandinavia southward to South Africa, including the Mediterranean. Along the African Coast, the species has been recorded in Morocco, the Cape Verde Islands, Mauritania, Senegal, Guinea-Bissau, Sierra Leone, and Gabon, from the littoral to about 300 m [8,10,12,14,15,18,44,53,70].
Remarks. The specimens of A. rotundatus mentioned in this work were reported by Muñoz et al. [10], as the first record of this species in Guinea-Bissau waters. They were not preserved, housed in any collection, or photographed.
DNA barcodes. No DNA sequences could be obtained from the Bissauguinean specimens because they were not deposited in any collection. There are one 16S (FM207652) and 13 COI sequences available in Genbank: JQ305993, JQ305994, JQ306190, JQ306130, KT208453, KT208520, KT208692, KT208762, KT209131, KT209153, KT209240, MG935217, and MG935359. Furthermore, in BOLD there are seven COI sequences published (DECNB004-13, DECNB336-17, DECNB241-15, NLGIM073-16, NLGIM074-16, ILGEA104-20, NLMAR428-20), and three as early-release. All these sequences belong to specimens from European waters.
- Supferfamily DORIPPOIDEA MacLeay, 183
- Family DORIPPIDAE MacLeay, 1838
- Medorippe lanata (Linnaeus, 1767) (Figure 4D)
Material examined. Guinea-Bissau, GB1219, Stn. 32, 34−36 m, November 2019, f. ov. (moulting) 27.9 × 22.4 (cw × cl) IEOCD-GB19/2988; Bissau0811, Stn. 20, 52 m, October 2008, f. 29.9 × 23.6, IEOCD-GB08/164; Bissau0811, Stn. 59, 22 m, November 2008, f. ov. 16.1 × 11.8, IEOCD-GB08/165; LANGABISS0211, Stn. 63, 48 m, May 2011, m. 33.1 × 25.9, IEOCD-LB0211/434.
Identification. Manning and Holthuis [12] and Monod [14] were used.
Distribution. Eastern Atlantic, from Portugal and Spain to South Africa, the Mediterranean Sea, and off Mozambique. Off West Africa with records in Morocco, Western Sahara, Mauritania, Senegal, Guinea-Bissau, Sierra Leone, Ivory Coast Ghana, Cameroon, Congo, and Angola, between 10−100 m [10,12,14,47,49].
Remarks. This work provides the second record of M. lanata off Guinea-Bissau, with the registers IEOCD-GB19/2988 and IEOCD-LB0211/434. The specimens IEOCD-GB08/164 and IEOCD-GB08/165 were the first records included in Muñoz et al. [10]. Manning and Holthuis [12] described M. lanata as a species with a carapace as wide as long, although all of our individuals are wider than long, especially the IEOCD-LB0211/434. Concerning this feature, our specimens have a more similar proportion to Phyllodorippe armata (Miers, 1881). Some authors reported the presence of ovigerous females from March to June, August, and October [12,13,14,19]. We can add December to the period when the females of M. lanata are ovigerous. There is a record of M. lanata in American waters concerning a specimen housed in the National Museum of Natural History, Smithsonian Institution (https://www.gbif.org/occurrence/2397803968 (accessed on 17 January 2024)) from 1975, which we considered an identification error since in the record file of the museum (http://n2t.net/ark:/65665/3de63ed17-bc8a-4e41-92eb-9b5f7289ce09 (accessed on 17 January 2024)) there is a note showing: “Other identifications: Ethusina abyssicola Smith, 1884”, a species that inhabits those waters.
Coloration observed. Capart [13] and Manning and Holthuis [12] described the fresh color, and our specimens fit well with these descriptions.
DNA barcodes. Sequences of 16S and COI were obtained for the specimen IEOCD-GB19/2988. The sequence of 16S only differs in one mutation with respect to the only sequence (EU636950) of M. lanata deposited in Genbank that corresponds to one specimen from Israel (uncatalogued) obtained by Sin et al. [71]. The COI sequence represents a haplotype that differs from three to five mutations (99.54 to 99.15% similarity) with respect to the five sequences (EU636981, JQ305916–JQ305918, ON716031) desposited in Genbank corresponding to specimens from Israel and Italy. Other nine COI sequences (marked as early-release) deposited in BOLD show similarities in the same range, 99.21 to 99.85%, in specimens collected in the “Atlantic Ocean” and Mauritania. It is remarkable that there is such low genetic variability between specimens from localities so far away.
- Phyllodorippe armata (Miers, 1881) (Figure 4E)
Material examined. Guinea-Bissau, GB1219, Stn. 5, 43−44 m, November 2019, f. 15.6 × 11 (cw × cl) IEOCD-GB19/2953; GB1219, Stn. 6, 58−61 m, November 2019, m. 18 × 12.3, IEOCD-GB19/2954.
Identification. The specimens analyzed fit perfectly with the description and illustrations of P. armata (as Dorippe armata) made by Monod [14]. The genus Phyllodorippe was established by Manning and Holthuis [12], and it is the unique species of the genus.
Distribution. Off West Africa, from Western Sahara to Angola, with records in Mauritania, Senegal, Sierra Leone, Liberia, Ivory Coast, Ghana, Nigeria, Cameroon, São Tomé and Príncipe, and Congo at a depth of 2 to 100 m [12,14,15,19,23,44,66,68].
Remarks. Sin et al. [71] considered Phyllodorippe as a genus with ambiguous morphology, which shares certain characteristics with Dorippe and Medorippe and other features with Neodorippe, Heikeopsis, and Nobilum. In that study, they were unable to obtain tissue for genetic analysis. In this work, and thanks to the sequences obtained (see below), we can confirm that the genus Phyllodorippe is well-established. This work provides the first records of P. armata in the waters of Guinea-Bissau at depths ranging from 43 to 61 m.
DNA barcode. 16S and COI sequences were obtained for the specimen IEOCD-GB19/2953. There are no 16S sequences in Genbank of this species, and all closer sequences from species of the genera Philippidorippe, Medorippe, Dorippe, Neodorippe, Heikeopsis, or Paradorippe present < 90% of similarity. This is the first 16S sequence for this genus and species. The COI sequence matches 100% with five sequences of P. armata deposited in BOLD (marked as early-release) from specimens distributed from Mauritania to Angola (according to the map in BOLD). Therefore, this is the first COI sequence published and available for this genus and species. The other closer COI sequences belong to species of the genera Neodorippe, Heikeopsis, Dorippe, Medorippe, or Paradorippe, all of them <85% similar. Given the similarity data for 16S and COI sequences, the genus Phyllodorippe seems well supported and clearly distinguished from the other closer Dorippidae genera.
- Family ETHUSIDAE Guinot, 1977
- Ethusa rosacea A. Milne-Edwards & Bouvier, 1897 (Figure 4F)
Material examined. Guinea-Bissau, CCLME12, Stn. 53, 738 m, May 2012, m. 9.3 × 9.2, m. 7.5 × 6.7 (cw × cl), IEOCD-CCLME12/1511.
Identification. Although six specimens were collected in 2012, only two males were conserved. Illustrations from Capart [13] and the key provided by Monod [14] were used to identify our specimen at the species level.
Distribution. Eastern Atlantic, from the Canary Islands to Angola, with scattered records in Cape Verde, Mauritania, Gabon, Congo, Ivory Coast, and Liberia [8,12,13,14,72,73,74], from 100 to 1113 m.
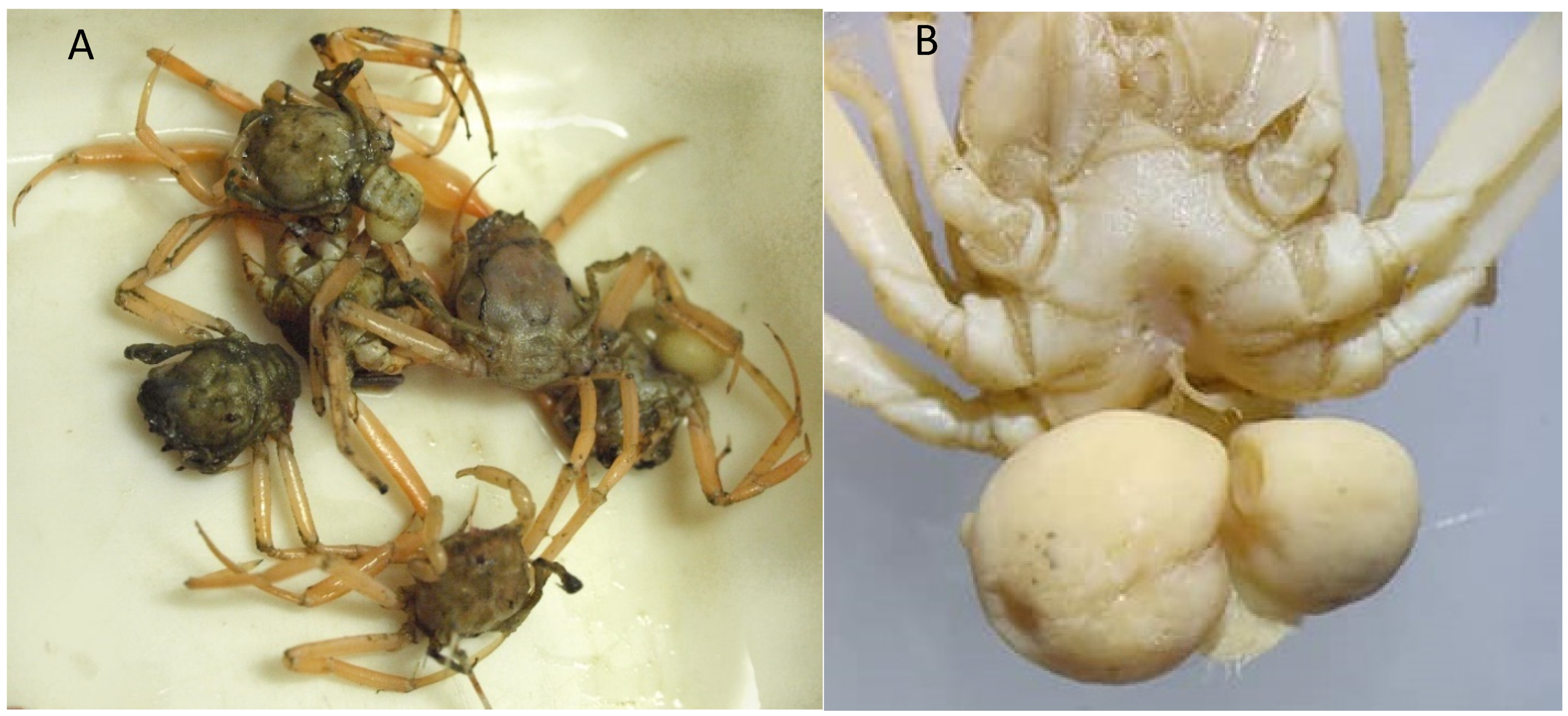
Remarks. These specimens represent the first record of E. rosacea in the waters of Guinea-Bissau, at a depth of 738 m. It is worth stating that three males were parasitized by Sacculina sp. (Figure 5).
Figure 5.
(A) Specimens of Ethusa rosacea collected in CCLME12; (B) E. rosacea male parasitized by two specimens of Sacculina sp.
Coloration observed. Our specimens agree well with the Milne-Edwards and Bouvier [72] description and illustration of E. rosacea color: “Les trois paires de pattes antérieures, les flanes, et la partie frontale du test sont d’un rouge pàle; le reste du corps est gris avec une légère Leinte rose sur le vivant.” Capart [13] also described the E. rosacea color: “Coleur brun clair, sauf la fase ventrale et les pinces, qui sont blanc-rose.” Our specimens partially agree with this description; their carapace is light brown, some of them with a slight reddish tone. One of the specimens shows a darker coloration. possibly due to the hairiness, which darkens the carapace. Legs differed from Capart’s description, with our specimens having light orange legs with some pink tone and darker dactyli.
DNA barcodes. No 16S or COI sequences were obtained for this species. There are no sequences for any genetic marker for this species in public databases.
- Ethusa rugulosa A. Milne-Edwards & Bouvier, 1897 (Figure 4G)
Material examined. Guinea-Bissau, Bissau0811, Stn. 43, 278 m, October 2008, m. 15.8 × 15.7 (cw × cl) IEOCD-GB08/163.
Identification. Illustrations from [7,8] and the key provided by Manning and Holthuis [12] were used for the identification.
Distribution. Eastern Atlantic, with records in the Cape Verde Islands, Mauritania, Senegal, Ivory Coast, Liberia, Sierra Leone, and Angola, between 20−275 m [8,12,28]. Only one register of E. rugulosa is available in GBIF (https://www.gbif.org/occurrence/2570175694 (accessed on 17 January 2024)), recorded in Netherlands. We consider this record as a mistake due to misidentification.
Remarks. This work provides the first specific record of E. rugulosa off Guinea-Bissau. This specimen was previously reported as Ethusa sp. by Muñoz et al. [10].
Coloration observed. Carapace is reddish brown, hairy, the branchial region is clearer, and the area between the branchial and cardiac regions has white tubercles. Chelipeds and walking legs are light pink but look brown because of the hairyness. The right chela is larger than the left, and the propodus is almost white. Dactyli of the walking legs are red.
DNA barcodes. Only the 16S sequence of the specimen IEOCD-GB08/163 was obtained. There is no 16S sequence for this species in Genbank; this is the first one. The closer 16S sequences belong to Ethusa sexdentata, with similarities of 85.15–85.40%.
- Ethusa vossi Manning & Holthuis, 1981 (Figure 4H)
Material examined. Guinea-Bissau, GB1219, Stn. 30, 49 m, November 2019, f. 8.8 × 9.9 (cw × cl) IEOCD-GB19/2956.
Identification. The original description made by Manning and Holthuis [12] was used for the determination.
Distribution. Off tropical West Africa, recorded from localities of Senegal, Angola, Guinea, Ivory Coast, Sao Tome and Principe, Sierra Leone, and Ghana, between 6−96 m [12,14,15,19,53,54,75].
Remarks. Ethusa vossi closely resembles Ethusa mascarpone (Herbts, 1758), which has a northern and Mediterranean distribution. Despite Manning and Holthuis [12] listing several differences between these two species, only one feature could be used for our female: “In both sexes the posterior margin of the orbit is smoother in E. mascarpone than in E. vossi, and females of the two species can be distinguished by this feature.” This work provides the first record for E. vossi in the waters of Guinea-Bissau.
DNA barcodes. The 16S and COI sequences were obtained for the specimen IEOCD-GB19/2956. The 16S sequence is the first known for this species. The closer 16S sequences belong to Ethusa sexdentata (Stimpson, 1858), with similarities of 87.37 and 87.58%. The COI sequence is also the first published for this species, although in BOLD there is data on one COI sequence of E. vossi from Senegal (according to the map) but it is not available. The closer COI sequence is one of E. mascarpone (ON16006), obtained by Mugnai et al. (unpublished) from a specimen from Italy (86.26% similarity). Comparing data from 16S and COI sequences of E. rugulosa, E. vossi (from this work), and Ethusa spp. (deposited in Genbank and BOLD), higher distances than expected at the intrageneric level were observed (<88% for 16S and <87% for COI), thus suggesting that further studies on intrageneric relationships within the genus Ethusa are needed.
- Superfamily GONEPLACOIDEA MacLeay, 1838
- Family EURYPLACIDAE Stimpson, 1871
- Machaerus oxyacanthus (Monod, 1956) (Figure 6A)
Material examined. Guinea-Bissau, LANGABISS0211, Stn. 63, 48m, December 2011 m. 19.1 × 12.0, m. 26.6 × 16..2 (cw × cl) IEOCD-LB0211/430; GB1219, Stn. 1, 83−84m, November 2019 f. 13.3 × 8.7, f. 11.7 × 8.4, m. 12.8 × 9, IEOCD-GB19/2972-1, m. 13.5 × 9.5, IEOCD-GB19/2972-2; ICM84-85, 43–47 m, January 1985, m. 30.6 × 19.4, m. 27.4 × 18.6, m. 31 × 19.1, m. 31.2 × 19.1, m. 28 × 18.6, m. 32.4 × 21.5, m. 35 × 22.6, m. 36.1 × 24.3, m. 28.8 × 18.5, m. 27 × 17.7, m. 31.9 × 21, m. 28.3 × 19.3, m. 32.6 × 20.6, ICMD244/1998; ICM84-85, 47–50 m, February 1985, f. 16.8 × 11.5, m. 23.5 × 14.6, m. 21.8 × 14.1, ICMD245/1998.
Identification. This species was described by Monod [14], as Pilumnoplax oxyacantha, within the Pseudorhombilinae Alcock, 1900. The present name, a new combination, and its current placement within the Euyplacidae was made by Manning and Holthuis [12]. The key provided by Monod [14] and the recent revision of Euriplacidae [76] were used.
Distribution. West Africa, from Mauritania to Angola, with records in Senegal, Guinea, Sierra Leone, Liberia, Ivory Coast, Ghana, Nigeria, Cameroon, Principe, and Congo, from 7 m to 73 m [12,14,15,19,23,28,77,78].
Remarks. This work provides the first record of M. oxyacanthus in the waters of Guinea-Bissau and expands its maximum depth from 73 to 84 m.
Coloration observed. Capart [13] described its coloration as “coleur beige claire avec taches brunâtres.” The specimens examined here were brown, with many small white, marble-like spots. In the middle of the gastric area, it shows a large, very bright white spot. It also has white areas on the capillary posterolateral edges. The chelipeds and legs are also brown, with the same pattern of white spots, white fingers on the chelipeds, and a white proximal region of the meri of the ambulatory legs.

Figure 6.
(A) Machaerus oxyacanthus m. 13.5 × 9.5 (cw × cl) IEOCD-GB19/2972; (B) Goneplax barnardi m. 25.5 × 14.6 IEOCD-GB08/190; (C) Atlantophila cristata m. 11.4 × 12.9 IEOCD-GB08/170; (D) Ilia spinosa f. 15.9 × 15.9 IEOCD-GB08/187; (E) Merocryptus boletifer f. ov. 13.5 × 11.9 IEOCD-GB08/188; (F) Pseudomyra mbizi m. 11.5 × 13.1 IEOCD-GB19/2939; (G) Scyramathia hertwigi m. 55.2 × 61.1 IEOCD-GB08/181; (H) Afropisa carinimana m. 7.2 × 10.3 IEOCD-GB08/182.
Figure 6.
(A) Machaerus oxyacanthus m. 13.5 × 9.5 (cw × cl) IEOCD-GB19/2972; (B) Goneplax barnardi m. 25.5 × 14.6 IEOCD-GB08/190; (C) Atlantophila cristata m. 11.4 × 12.9 IEOCD-GB08/170; (D) Ilia spinosa f. 15.9 × 15.9 IEOCD-GB08/187; (E) Merocryptus boletifer f. ov. 13.5 × 11.9 IEOCD-GB08/188; (F) Pseudomyra mbizi m. 11.5 × 13.1 IEOCD-GB19/2939; (G) Scyramathia hertwigi m. 55.2 × 61.1 IEOCD-GB08/181; (H) Afropisa carinimana m. 7.2 × 10.3 IEOCD-GB08/182.

DNA barcodes. The 16S sequence was obtained for the specimen IEOCD-LB0211/430, and COI sequences were obtained for IEOCD-LB0211/430 and IEOCD-GB19/2972-2. The 16S sequence is the first reported for this genus and species. The closer sequences (91.16–91.29% similarity) belong to Eucrate crenata (ON150678, NC_066715). The COI sequences represent two haplotypes, differentiated by one mutation. Each haplotype matched 100% with COI sequences of M. oxyacanthus deposited in BOLD (marked as early-release), and there is a third haplotype also deposited in BOLD (marked as private). Therefore, these two COI sequences are the first reported for this genus and species, published, and available. These two haplotypes also showed 90.63 and 90.78% similarity with the COI sequence of Machaerus atlanticus deposited in BOLD (as early-release). This is the second species of this genus, and, according to these results, the COI distances between the two species could be considered closer to the limit between intra- and intergeneric levels.
- Family GONEPLACIDAE MacLeay, 1838
- Goneplax barnardi (Capart, 1951) (Figure 6B)
Material examined. Guinea-Bissau, Bissau0811, Stn. 24, 435 m, October 2008, m. 34.9 × 19.9, m. 25.5 × 14.6, f. ov. 27.7 × 16.6 (cw × cl) IEOCD-GB08/190; LANGABISS0311, Stn. 148, 549 m, October 2011, m. 28.2 × 15.0, IEOCD-LB0311/443; ICM84-85, 227–359 m, January 1985, f. 26.4 × 15.1, ICMD243/1998.
Identification. The original description made by Capart [13], as Carcinoplax barnardi, was used for determination.
Distribution. Goneplax barnardi inhabits the waters of West Africa, from the Canary Islands to Angola, with records in Cape Verde, West Sahara, Mauritania, Guinea-Bissau, Ivory Coast, Ghana, Nigeria, and Gabon, at depths of 200 to 580 m [8,10,12,13,44,51]. In Guinea-Bissau, it was recorded at 435 m [10].
Remarks. This work provides the second record of G. barnardi in the waters of Guinea-Bissau and extends its maximum depth from 435 to 549 m.
Coloration observed. Our specimens fit well with the color description made by Capart [13] “coloration rose bistre, des laches noires a 1′extremité des doigts des pinces.”
DNA barcodes. Only the COI sequence was obtained from the specimen IEOCD-LB0311/443 and matched 100% with three sequences and 99.85% with one sequence of G. barnardi deposited in BOLD (marked as early-release). Therefore, this is the first COI sequence published and available for this species.
- Superfamily LEUCOSIOIDEA Samouelle, 1819
- Family LEUCOSIIDAE Samouelle, 1819
- Subfamily EBALIINAE Stimpson, 1871
- Atlantophila cristata (Miers, 1881) (Figure 6C)
Material examined. Guinea-Bissau, Bissau0811, Stn. 96, 37 m, November 2008 m. 11.4 × 12.9 (cw × cl), IEOCD-GB08/170.
Identification. The key provided by Monod [14] (where A. cristata is mentioned as Philyra cristata) was used for the determination at the species level. The genus Atlantophila was established by Galil [79].
Distribution. West Africa, from Senegal to the Congo, at depths between 4 and 25 m. Records in Senegal, Guinea-Bissau, Guinea, São Tomé and Principe, Sierra Leone, and Congo [14,15,23,53].
Remarks. The specimen studied here, collected at 37 m depth, was reported by Muñoz et al. [10] as a first record for Guinea-Bissau.
Coloration observed. Monod [14] described: “Moins gris-plombé [than in A. laevidorsalis], parfois plus ou moins orangé, les pinces rose saumon.” Our single specimen also has a certain gray tone on its carapace, mixed with light brown, forming a sinuous drawing. Meri of the chelipeds has a gray background, with the upper regions and tubercles orange. Propodus and fingers are orange with some gray lines; walking legs are orange with brown and white patches.
DNA barcodes. Only the COI sequence was obtained from the specimen IEOCD-GB08/170. There are no sequences of this species in any database; therefore, this is the first sequence for this genus and species. In BOLD, our specimen showed a 96.64% similarity with Atlantolocia laevidorsalis.
- Ilia spinosa Miers, 1881 (Figure 6D)
Material examined. Guinea-Bissau, Bissau0811, Stn. 56, Nov 2011, 46 m, f. 15.9 × 15.9 (cw × cl), IEOCD-GB08/187; GB1219, Stn. 31, 42 m, f. ov. 14.1 × 14.7, IEOCD-GB19/2955.
Identification. These specimens agree well with previous illustrations and morphological descriptions [12,13,14].
Distribution. Atlantic coasts of Mauritania to Angola, including the Canary Islands, the Cape Verde Islands, Mauritania, Senegal, Guinea-Bissau, Guinea, Sierra Leone, Liberia, Ivory Coast, Ghana, Nigeria, Cameroon, Principe, Equatorial Guinea (Annobon Islands), Gabon, Congo, and Angola, between 4 and 150 m [12,13,14,15,19,23,28,53,70,77,80].
Remarks. The first record was provided by Forest and Guinot [15] at 18 m. Consequently, this study extends the bathymetrical distribution of I. spinosa from 18 to 46 m in the waters of Guinea-Bissau.
Coloration observed. The specimens examined in this work differ from the brief color description provided by Capart [13]: “Le carapace est orange clair, les épines et appendices presque blancs.” Females in this study (Figure 6D) have a white carapace with dark orange tubercles, except in the gastric area, which is purple and adorned with dark orange and purple tubercles. The ventral side is white with a light orange antero-lateral edge featuring small dark orange tubercles. Pereiopods are white with some orange patches on the meri and carpi, lacking tubercles, and dactyli are white. The meri of the chelipeds are white with a purple cross line in the middle, large purple tubercles in the first half of the meri, and the second half with small light orange grains.
DNA barcodes. The 16S and COI sequences were obtained for the specimen IEOCD-GB19/2955. The 16S is the first sequence of this gene for this species. This 16S sequence presents a similarity of 91.85% with the sequence ON716024 of an Italian specimen of the other species of this genus, Ilia nucleus (Linnaeus, 1758), obtained by Mugnai et al. (unpublished). The COI sequence fit 99.03–99.19% and 98.87% with three and three sequences of I. spinosa, respectively, deposited in BOLD (marked as early-release). Also, present a similarity of 97.58% and 97.91% with the other two sequences of I. spinosa deposited in BOLD (marked as early-release). The closest sequences belong to specimens identified as from «Mauritania» and «Atlantic Ocean», and the two most distant are from specimens from «Senegal» and «Atlantic Ocean». This is the first COI sequence published and available for this species.
Synonym: Merocryptus obsoletus A. Milne-Edwards & Bouvier, 1898
Material examined. Guinea-Bissau, Bissau0811, Stn. 69, November 2008, 65 m, f. ov. 13.5 × 11.9 (cw × cl) IEOCD-GB08/188.
Identification. In order to identify at the species level and for further arguments given in Remarks, it was necessary to examine several documents [12,14,15,19,75,81,82].
Distribution. Following the proposed synonymy below (see Remarks), the distribution of Merocryptus boletifer has been expanded towards Central African waters, with a wide distribution from the Azores in Europa to Angola, as well as in the Mediterranean Sea, with records in the Eastern Atlantic in the Azores, Canary Islands, Morocco, Cape Verde Islands, Senegal, Guinea-Bissau, and Angola, at depths between 75 and 629 m [14,19,49,81,82,83]. The first record in Guinea-Bissau was published by Muñoz et al. [10] as Merocryptus obsoletus A. Milne-Edwards & Bouvier, 1898, concerning this same specimen.
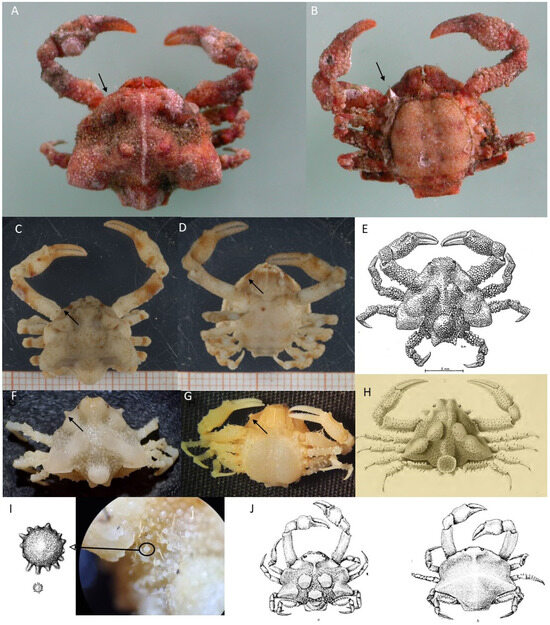
Figure 7.
(A,B) M. boletifer f. ov. IEOCD-GB08/188 from Guinea-Bissau; (C,D) M. boletifer f. (determined as Atlantotlos rhombifer), housed in Natural History Collection of the Bergen University Id. BETRA_Workshop_72; (E) Illustration of M. boletifer cited as M. obsoletus m. from Monod (1956); (F,G) M. boletifer f. CRUST-INFUECO/3454 from Canary Islands (Spain); (H) Illustration of M. boletifer m. from Milne-Edwars (1894); (I) Mushroom-shape projections that adorn the more depressed areas of the carapace of M. boletifer IEOCD-GB08/188; (J) Illustration of A. rhombifer from Capart (1951). Black arrows in figures (A–D,F,G) point out the pterygostomic spine or the place where it should be.
Figure 7.
(A,B) M. boletifer f. ov. IEOCD-GB08/188 from Guinea-Bissau; (C,D) M. boletifer f. (determined as Atlantotlos rhombifer), housed in Natural History Collection of the Bergen University Id. BETRA_Workshop_72; (E) Illustration of M. boletifer cited as M. obsoletus m. from Monod (1956); (F,G) M. boletifer f. CRUST-INFUECO/3454 from Canary Islands (Spain); (H) Illustration of M. boletifer m. from Milne-Edwars (1894); (I) Mushroom-shape projections that adorn the more depressed areas of the carapace of M. boletifer IEOCD-GB08/188; (J) Illustration of A. rhombifer from Capart (1951). Black arrows in figures (A–D,F,G) point out the pterygostomic spine or the place where it should be.

Remarks. Milne-Edwards and Bouvier [82] described M. boletifer, based on a male from the Azores captured at 75 m depth, and, in 1898, the same authors [81] described the species M. obsoletus, based on a male from the Cabo Verde Islands collected at 454 m depth. These authors mentioned that both species were very similar, but M. boletifer“a des champignons moins nombreux, des épines très développées sur toutes les pattes et des lobes branchiaux tranchants sur leur pourtour le plus externe.” Also, according to the descriptions, the pterygostomian region of M. boletifer shows a strong, granular, and pointed projection, very reduced in M. obsoletus, in which it cannot be seen dorsally. Also, Milne-Edwards and Bouvier [81] referred to the fact that M. obsoletus was especially close to M. lambriformis (Milne Edwards 1873) from the Pacific (Upolu, Samoa). Monod [14] captured three males from Gorée (Senegal) and one male of M. obsoletus from Morocco of M. obsoletus, providing a good illustration. He also mentioned that Forest questioned the taxonomic validity of M. obsoletus and M. boletifer, and after reviewing the holotypes of M. obsoletus (male), a female specimen of M. boletifer, and a male with an intermediate form, he was not able to answer this question, raising the possibility of a sexual dimorphism. This left the door open to considering that M. obsoletus and M. boletifer would really be the same species (M. obsoletus being the female of M. boletifer). Our specimen, a female (Figure 7A,B), was compared with a male of M. boletifer (Figure 7F,G) from the Canary Islands, housed in the CBMR, and with a female identified as Atlantotlos rhombifer Doflein, 1904, deposited in Bergen (Figure 7C,D) (BOLDSYSTEMS https://v3.boldsystems.org/index.php/Taxbrowser_Taxonpage?taxid=321786 (accessed on 17 January 2024)). Our female and the female deposited in Bergen fit perfectly with the illustration provided by Monod for M. obsoletus [14] (Figure 7E), while the male from the Canary Islands fitted with the description made by Milne-Edwards [82] for M. boletifer (Figure 7H). As Milne-Edwards [82] already pointed out, concerning the peculiarity of projections in certain areas of M. boletifer, the female from Guinea-Bissau also shows similar tubercles (Figure 7I). The most differentiating feature between both species is the presence of a large pterygostomic spine in the male of M. boletifer (see Figure 6, black arrow), which is not observed in the females of M. obsoletus: neither in the female collected in Guinea-Bissau, nor in the figure of Monod, nor in the images of a female deposited in Bergen, identified as A. rhombifer but belonging to M. boletifer (see DNA barcode paragraph).
Coloration observed. Orange-brown, with a lighter stripe in the middle of the shell, from the frontal to the rear edge.
DNA barcodes. The 16S and COI sequences were obtained from the ovigerous female IEOCD-GB08/188. This is not the first sequence obtained for this genus, but it is the first 16S sequence for this species. The COI sequence matched 100% with the sequence of Atlantotlos rhombifer deposited in BOLD as early-release. Considering the controversy with the other species of the genus, 16S and COI sequences were obtained for the M. boletifer specimen CRUST-INFUECO/3454 from the Canary Islands (Genbank accession codes PP118360 and PP133848, respectively). COI sequences of M. obsoletus and M. boletifer matched 100%, and 16S sequences only differed in one mutation. After sequencing both species (the present document), M. obsoletus and M. boletifer, the validity of M. obsoletus cannot be supported, with results suggesting strongly that both species could be considered synonymous, as other authors, such as Monod [14], questioned. The valid species should accordingly be M. boletifer, described four years before. Additional studies, including specimens from different points of the species distribution, will be necessary to clarify this conflict. We have compared the image in BOLD of the DNA voucher specimen of Atlantotlos rhombifer UMBergen_MBOWA_brach23 collected in Cape Verde Islands (according to the map in BOLD) (Figure 7C,D), with the original description by Doflein [75] that included an illustration of a female A. rhombifer (Figure 7I), and with the illustrations of a male and a female provided by Capart [13] (Figure 7J). We suggest that this individual was not correctly identified and that it is a specimen of M. boletifer, a female very similar to that examined in the present study. Although Forest and Guinot [15] synonymized the two genera (Atlantotlos Doflein, 1904, and Merocryptus A. Milne Edwards, 1873), Monod [14] stated that this subject was not yet clear: “Je ne suis pas certain du tout que le genre Atlantotlos soit distinct de Merocryptus.” However, Manning and Holthuis [12] found differences in the male gonopods and decided to keep the two species as valid species.
- Pseudomyra mbizi Capart, 1951 (Figure 6F)
Material examined. Guinea-Bissau. GB1219, Stn. 18, 65−70 m, November 2019, m. 11.5 × 13.1 (cw × cl), IEOCD-GB19/2939; GB1219, Stn. 85, 105−106 m, December 2019, f. ov. 11.7 × 12.9, f. 10.8 × 12.8, IEOCD-GB19/2957; CCLME12, Stn. 56, 74 m, May 2012, m. 20.5 × 22.2, f. 18.6 × 20.9, f. 9.8 × 11.1, f. 10 × 6.5 × 7.7, 11.5, f. 10.6 × 12.1, m. 21.1 × 23.1, m. 17.7 × 19.2, IEOCD-CCLME12/1541; ICM84-85, 47–50 m, February 1985, m. 17.31 × 19.46, m. 19.45 ×20.98, m. 19.49 × 21.52, m. 21.54 × 23.37, m. 19.79 × 21.3, m. 13.51 × 15.45, ICMD211/1998; ICM84-85, 53–55 m, February 1985, m. 16.8 × 18.9, m. 13.3 × 15.1, m. 15.9 × 17.8, ICMD212/1998; ICM84-85, 43–50 m, December 1984, m. 21.3 × 24.3, m. 20.4 × 22.9, m. 15.8 × 18, m. 20.2 × 22.2, m. 15.2 × 18.1, m. 19.9 × 22.1, m. 14.7 × 17.9, m. 19.7 × 22.3, m. 18.2 × 21.2, m. 20.3 × 22.9, m. 24.2 × 26.1, m. 20.4 × 22.8, m. 17.1 × 19.3, m. 20.2 × 22.1, m. 18.1 × 19.4, m. 22 × 24.5, m. 19.2 × 20.5, m. 20 × 21.9, m. 22 × 23.8, m. 19.9 × 22.3, m. 19.9 × 22, m. 20 × 21.4, m. 16.3 × 19, m. 19.5 × 21.2, m. 20.6 × 21.9, m. 17.6 × 20.7, m. 20.4 × 22.6, ICMD210/1998.
Identification. Our specimens fit well with the original description made by Capart [13].
Distribution. West African coast, from scattered localities between Mauritania and Angola, with records in Senegal, Liberia, Ivory Coast, Ghana, Nigeria, Cameroon, Gabon, and Congo, at depths between 12 and 300 m [8,12,13,15,23,68,77,84].
Remarks. This work provides the first record for P. mbizi in the waters of Guinea-Bissau at depths ranging from 43 to 106 m.
Coloration observed. Capart [13] described the color of P. mbizi as “rose orange avec extremités des pinces et pattes plus claires.” Our specimens fit well with this description, and we add in that the tips of the posterior spines end in bone color, similar to that of the legs.
DNA barcodes. 16S and COI sequences were obtained for the specimen IEOCD-GB19/2939. There were no 16S sequences for this monotypic genus; the sequences obtained in the present work constitute the first for the species and the genus. The COI sequence matched in a range from 98.87 to 99.51% similarity with 13 sequences of P. mbizi deposited in BOLD as early-release. These DNA voucher specimens are from the North Atlantic Ocean (one) (the closer haplotype to IEOCD-GB19/2939), Guinea-Bissau (three), Guinea (one), and the Atlantic Ocean (eight), according to the Tree-Based Identification of BOLD. This is the first COI sequence published and available for this species and genus.
- Superfamily MAJOIDEA Samouelle, 1819
- Family EPIALTIDAE MacLeay, 1838
- Scyramathia hertwigi (Doflein in Chun, 1900) (Figure 6G)
Material examined. Guinea-Bissau, Bissau0811, Stn. 30, 550 m, November 2008, m. 55.2 × 61.1 (cw × cl) (deformed rostrum) IEOCD-GB08/181; LANGABISS0111, Stn. 203, 551 m, f. (damaged specimen) IEOCD-LB0111/404.
Identification. The key provided by Lee et al. [85] was used for the identification, as well as the remarks made by Macpherson [22].
Distribution. Scyramathia hertwigi is known from its type locality, off Cape Point and Agulhas Bank, South Africa, and Namibia, between 250 and 622 m [22,85], as well as from areas of the south Atlantic along the African coast. The first record in the waters of Guinea-Bissau was made by Muñoz et al. [86] at a depth of 500 m. It is also known along the southwesternmost edge of the Indian Ocean.
Remarks. The present individuals provide the second record of S. hertwigi off Guinea-Bissau, as well as for the Eastern Central Atlantic, and extend the maximum depth for this species from 500 to 551 m. One of our specimens, the male collected in 2008, showed a deformed rostrum, with the left rostral spine curved inward. The specimen IEOCD-GB08/181 was reported by Muñoz et al. [10] as Rochinia carpentieri (Wyville Thomson, 1873).
Coloration observed. The only known description of the S. hertwigi coloration was made by Macpherson [22]: “color marrón claro con tonalidades rosáceas.” Our specimens show a brown carapace with some darker areas and light brown tubercle edges. Chelipeds are orange-brown, with lighter fingers; ambulatory legs are light brown with an apink tone; dactyls are dark brown. A color picture of S. hertwigi is herein published for the first time (Figure 6G).
DNA barcode. Only the 16S sequence was obtained for the specimens IEOCD-GB08/181 and IEOCD-LB0111/404, as well as for a specimen from Namibia, IEOCD-NB07/2445 (Genbank accession code PP118361), also deposited in CRUST_IEOCD. The three sequences showed 100% similarity and matched 99.54% (two mutations in 431 bp) with the sequence MK309600 of Scyramathia umbonata, obtained by Colavite et al. [87] from the specimen ULLZ13797 from the Gulf of Mexico. This similarity value is clearly higher than expected at the interspecific level; additional genetic markers, such as COI, and further morphological studies will be necessary to clarify their taxonomic relationships.
- Subfamily PISINAE Dana, 1851
- Afropisa carinimana (Miers, 1879) (Figure 6H)
Material examined. Guinea-Bissau, Bissau0811, Stn. 87, 38 m, November 2008, m. 7.2 × 10.3 (cw × cl) IEOCD-GB08/182.
Identification. For the determination, the keys provided by Monod [14] (where the species is mentioned as Pisa carinimana) and by Muñoz et al. [86], where the new genus Afropisa was established, were used.
Distribution. From the south-west of Spain [16,74,88,89] to Angola, with records in Western Sahara, Mauritania, Senegal, Guinea, Guinea-Bissau, Equatorial Guinea, Sierra Leone, Ivory Coast, Ghana, Nigeria, Principe, Gabon, and Congo, depth ranges from 2 to 110 m [12,13,14,19,86,90].
Remarks. Manning and Holthuis [12] mentioned that Forest and Guinot [15] had recorded A. caninimana in the waters of Guinea-Bissau. However, upon reviewing Forest and Guinot [15] for the present document, it was found that the reference was based on an illustration of a right pleopod from a male collected in Guinea, not Guinea-Bissau. This specimen of A. carinimana was included in Muñoz et al. [10], but as Pisa calva Forest and Guinot, 1966.
Coloration observed. Brown carapace, with some darker longitudinal patches, and small beige tubercles scattered all over the carapace. Rostral spines are also beige, with some small brown patches. Walking legs with brown and beige transversal bands; chelipeds beige with brown tubercles.
DNA barcodes. The 16S sequence of this specimen was recently published by Muñoz et al. [86](OP326678). The COI sequence was not obtained by these authors or in the present work. In Muñoz et al. [86] reported 16S and COI sequences for specimens from Mauritania, the Canary Islands, Madeira, and localities from the South of Spain with similarity values within the intraspecific level.
- Apiomithrax bocagei (Ozorio, 1887) (Figure 8A)
Figure 8.
(A) Apiomithrax bocagei m. 26 × 24.8 (cw × cl) IEOCD-GB19/2968; (B) Pisa armata m. 29.0 × 31.3, IEOCD-GB08/180; (C) Inachus aguarii m. 16.5 × 19.9 IEOCD-GB08/192; (D) Inachus angolensis f. ov. 14.2 × 15.4 IEOCD-GB19/2952; (E) Macropodia gilsoni m. 12.4 × 17.8 IEOCD-GB19/2941; (F) Macropodia hesperiae m. 5.9 × 10.5 IEOCD-GB08/189; (G) Macropodia rostrata m. 11.1 × 17.4 IEOCD-GB08/186; (H) Stenorhynchus lanceolatus m. 9.1 × 12.6 × 21.0, IEOCD-GB08/195.
Figure 8.
(A) Apiomithrax bocagei m. 26 × 24.8 (cw × cl) IEOCD-GB19/2968; (B) Pisa armata m. 29.0 × 31.3, IEOCD-GB08/180; (C) Inachus aguarii m. 16.5 × 19.9 IEOCD-GB08/192; (D) Inachus angolensis f. ov. 14.2 × 15.4 IEOCD-GB19/2952; (E) Macropodia gilsoni m. 12.4 × 17.8 IEOCD-GB19/2941; (F) Macropodia hesperiae m. 5.9 × 10.5 IEOCD-GB08/189; (G) Macropodia rostrata m. 11.1 × 17.4 IEOCD-GB08/186; (H) Stenorhynchus lanceolatus m. 9.1 × 12.6 × 21.0, IEOCD-GB08/195.
Material examined. Guinea-Bissau, LANGABISS0211, Stn. 179, 26 m, May 2011, m. 30.0 × 27.5, m. 39.3 × 34.3, m. 40.4 × 36.3, m. 30.3 × 27.8 (cw × cl) IEOCD-LB0211/431; LANGABISS0211, Stn. 63, 48 m, f. 33.4 × 29.8, f. ov. 27.7 × 26.4, IEOCD-LB0211/432; GB1219, Stn. 5, 43−44 m m. 17.5 × 17.3, m. 26 × 24.8, IEOCD-GB19/2968.
Identification. The document where the genus Apiomithrax was established [91] and the re-description of the two species belonging to this new genus made by Monod [14] were used for determination at the species level. Previously, A. bocagei was placed within Micropisa.
Distribution. West Africa, where it has been recorded from numerous localities between Western Sahara and southern Angola, with records in Guinea-Bisssau, Sierra Leone, Ivory Coast, Ghana, Principe, Congo, and Angola [12,14,15,19,53,66,68,86,92], from the shore to 140 m.
Remarks. Our specimens confirm the presence of A. bocagei in the waters of Guinea-Bissau, being the second record of this species in these waters.
DNA barcodes. 16S and COI sequences were obtained for the specimen IEOCD-GB19/2968. This is the first known 16S sequence for this species and shows a large distance with respect to the other species of the genus, A. violaceus, with 84.99% similarity (68 mutations in 453 bp) with respect to the sequence MF490139 obtained by Mantelatto et al. [43]. The COI sequence, the first for this species, also showed large distances with respect to the sequence MF49070 of the specimen of A. violaceus obtained by Mantelatto et al. [43], with a similarity of 83.41%, which would put under question that the two species belong to the same genus.
- Pisa armata (Latreille, 1803) (Figure 8B)
Material examined. Guinea-Bissau, GB1219, Stn. 30, 49 m, November 2019, m. 35.6 × 34.9 × 52.1 (cw × cl×cl with rostral spines), IEOCD-GB19/2987; GB1219, Stn. 29, 72−75 m, November 2019, f. 30.4 × 30 (cw × cl), IEOCD-GB19/2940; Bissau0811, Stn. 5, 105 m, October 2008, f. 27.7 × 27.8, m. 24.6 × 25.3, IEOCD-GB08/179; Bissau0811, Stn. 69, 65 m, October 2008, m. 29.0 × 31.3, IEOCD-GB08/180.
Identification. Our specimens agree well with the descriptions and figures made by Monod [14] and the revision of the genus made by Muñoz et al. [86].
Distribution. Eastern Atlantic, from the southern North Sea and England to Angola, and Mediterranean Sea, with records off Africa in Morocco, Western Sahara, Mauritania, Cape Verde, Guinea-Bissau, Equatorial Guinea, Sierra Leone, and Liberia [10,12,13,14,16,90,93,94].
Remarks. Specimens IEOCD-GB08/179 and IEOCD-GB08/180 were reported by Muñoz et al. [10] as first recorded in the waters of Guinea-Bissau. Therefore, the present work provides the second record for P. armata in the study area, between 39 and 105 m depth.
DNA barcodes. 16S sequences were obtained only for the specimen IEOCD-GB08/180 and for a specimen of Pisa armata (IEOCD-CCLME12/1594, Genbank accession code PP118362) from the geographically close Guinea Conakry. These sequences fitted 99.24 to 99.81% similarity with respect to seven sequences (OP326649 to OP326656) of P. armata recently published by Muñoz et al. [86] from Equatorial Guinea, Mauritania, the Canary Islands, the Gulf of Cadiz in the Atlantic, and the Balearic Islands in the Mediterranean. COI sequences were not obtained from the Guinea-Bissau specimens, but Muñoz et al. [86] obtained six COI sequences (OP362242 to OP362247) of specimens from Mauritania, the Canary Islands, the Gulf of Cadiz in the Atlantic, and the Balearic Islands in the Mediterranean. Two additional COI sequences, from the North Sea (KT208450, KT208481), are available in Genbank, and another one in BOLD, from the Gulf of Cadiz (IPID078-10) (as Pisa nodipes). All these COI sequences from different and distant localities showed similarity values within the intraspecific level.
- Family INACHIDAE MacLeay, 1838
- Capartiella longipes (Capart, 1951)
Material examined. Guinea-Bissau, GB1219, Stn. 30, 49 m, November 2019, m. 6.7 × 7.8 (cw × cl), IEOCD-GB19/2969.
Identification. The original description by Capart [13] (as Achaeus longipes) was used for the determination at the species level. Manning and Holthuis [12] described Capartiella as a new genus and established the new combination for C. longipes.
Distribution. Tropical West Africa, from Senegal to Angola, with some records in Ivory Coast, Ghana, and Benin [13,15,23]; sublittoral, at depths between 35 and 82 m.
Remarks. This work provides the first record of C. longipes off Guinea-Bissau. at 49 m depth. The revision of the specimens housed at the CRUST-IEOCD for this work allowed to identify the first records of this species in Western Sahara waters: (https://www.gbif.org/occurrence/2564780725, (accessed on 17 January 2024) https://www.gbif.org/occurrence/2564781160, (accessed on 17 January 2024) https://www.gbif.org/occurrence/2564780586 (accessed on 17 January 2024)). These three Sahara specimens represent the northernmost record for the species. The maximum depth for this species is also extended by the specimens reported herein, from 82 to 113 m, according to data from the specimen collected in 2012 in the waters of Western Sahara (https://www.gbif.org/occurrence/2564781160 (accessed on 17 January 2024)). The maximum depth for this species also extends from 82 to 113 m, according to a specimen collected in 2012 in the waters of Western Sahara (https://www.gbif.org/occurrence/2564781160 (accessed on 17 January 2024)).
DNA barcodes. 16S and COI sequences were obtained from the specimen IEOCD-GB19/2969. The 16S sequence is the first known for the species of this monotypic genus. The closest sequences available are those of Inachus spp. (87.84–89.22% similarity), followed by Macropodia and Achaeus spp. The COI sequence is also the first COI sequence for this species, although there are two unpublished sequences (early-release) in BOLD that fit 99.37% similarity with the specimens MBOWA_brach 143 and 199 deposited at the Natural History Collections of the University of Bergen (Norway). The closest sequences are also those of Inachus spp. (86.07–88.02% similarity), followed by Macropodia spp. and Achaeus spp. These 16S and COI data support and confirm the placement of Capartiella as a separate genus within the Inachidae.
- Subfamily INACHINAE MacLeay, 1838
- Inachus aguiarii de Brito Capello, 1876 (Figure 8C)
Material examined. Guinea-Bissau, Bissau0811, Stn. 69, November 2008, 65 m, m. 16.5 × 19.9 (cw × cl) IEOCD-GB08/192.
Identification. For the determination at the species level, the key and illustrations provided by Manning and Holthuis [12] and the illustrations by Monod [14] did not provide enough information to properly identify all the species collected. The key provided by García Raso et al. [95] points out that “males of I. thoracicus and I. aguiarii are morphologically identical,” and for this reason, a molecular identification was needed.
Distribution. Eastern Atlantic, from Portugal to Guinea (with records in Morocco, Western Sahara, and Mauritania), and Mediterranean Sea. Sublittoral, at depths between 20 and 200 m [8,12,14].
Remarks. Manning and Holthuis [12] provide a key to differentiate I. aguiarii from Inachus thoracius Roux, 1830, but when the specimen is a male, it is not possible to separate them because males of both species have a sternal callosity, and the criteria of the relation between the length of the walking legs and the carapace is not valid (see García Raso et al. [95]). In the case of males, only the use of DNA barcoding allowed them to confirm their identity with certainty. This specimen of I. aguarii was reported by Muñoz et al. [10] as Inachus sp. Consequently, this is now the first record of the species off Guinea-Bissau.
Coloration observed. Brown-reddish, with red chelae. Merus and carpus of the chelipeds are brown-reddish, and propodus and dactyli are almost beige.
DNA barcodes. 16S and COI sequences were obtained for this specimen. The 16S sequence matched 100% with the sequences KU163290 and KU163291 of two I. aguiarii specimens (MHNUSC250044-1 and -2) obtained by Cuesta et al. [96]. The COI sequence only differs in four mutations (99.38% similarity) from the sequence KU163294 of the specimen MNHUSC250044-1 and showed a similarity of 99.39% with respect to two early-release sequences in BOLD of I. aguiarii from Portugal.
- Inachus angolensis Capart, 1951 (Figure 8D)
Material examined. Guinea-Bissau, CCLME11, Stn. 44, 104 m, October 2011, f. ov. 18.3 × 19.7 (cw × cl), IEOCD-CCLME11/720; GB1219, Stn. 85, Dic. 2019, 105−106 m, f. 13.9 × 14.6, IEOCD-GB19/2942; GB1219, Stn. 17, 71−76 m, November 2019, f. ov. 14.2 × 15.4, IEOCD-GB19/2952.
Identification. Three specimens fit perfectly with the original description made by Capart [13]. Different works [12,14,15] were used to differentiate them from the closest Inachus (especially from Inachus communissimus Rizza, 1839).
Distribution. West Africa from Western Sahara to South Africa, with records in Mauritania, Senegal, Ivory Coast, Ghana, Cameroon, Gabon, Congo, and Namibia, in depths between 46 and at least 400 m, of mud, sandy mud, and sandy bottoms [8,12,13,14,22,28].
Remarks. This work provides the first record of I. angolensis off Guinea-Bissau at depths of 71 to 106 m.
Coloration observed. I. angolensis from Guinea-Bissau showed a dark purple carapace, with chelipeds and walking legs beige, darker meri than other parts, and dactyli almost white.
DNA barcodes. 16S and COI sequences were obtained for the specimen IEOCD-GB19/2942. These are the first sequences known and published for this species. The 16S sequence shows a 99.02% similarity with the sequence MK309594 of Inachus dorsettensis (specimen ULLZ11658 from the Mediterranean Sea) obtained by Colavite et al. [87]. The COI sequence fit between 99.95 and 100% with nine sequences of I. angolensis deposited in BOLD (as private and early-release). The following higher similarities are with I. communissimus and I. dorsettensis: 96.06% (KC866321) and 95.38–95.74% (JQ306010, JQ306203, JQ306204, JQ306207, JQ306209, and JQ306211), respectively.
- Macropodia gilsoni (Capart, 1951) (Figure 8E)
Material examined. Guinea-Bissau. GB1219, Stn. 83, 71−75 m, f. 11.5 × 17.2, m. 12.4 × 17.8 (cw × cl) IEOCD-GB19/2941-1 and 2; GB1219, Stn. 7, 110 m, m. 11.1 × 17, IEOCD-GB19/2943; GB1219, Stn. 20, 83−84 m, m. 12.9 × 20.8, IEOCD-GB19/2944.
Identification. The original description by Capart [13] (as Achaeopsis gilsoni) was used to determine the species. Different works [12,14,97] were useful to clarify the differences with other species, such as Macropodia intermedia Bouvier, 1940, or Macropodia tenuirostris (Leach, 1814).
Distribution. West Africa, from Mauritania to Angola, with records in Senegal, Guinea-Bissau, Gabon, Sierra Leone, Ivory Coast, Cameroon, and Congo, in depths between 37 and about 200 m. [8,15,77,98].
Remarks. Manning and Holthuis [12] summarized the differences between the close species M. gilsoni and M. intermedia. Three features were pointed out to differentiate them, but any of them is optimal. The rostrum length of our specimens, shorter than the antennal peduncle, is the main character identifying our specimens as M. gilsoni (Figure 9).
Our work provides the second record of M. gilsoni in the waters of Guinea-Bissau and expands the maximum depth in this area from 73 to 110 m.
Coloration observed. Carapace is light brown brown with the deeper areas in the dark; walking legs are reddish-pink; chelipeds have yellow ischium; and merus, carpus, and propodius are brown.
DNA barcodes. 16S and COI sequences were obtained for the specimens IEOCD-GB19/2941-1 and IEOCD-GB19/2944. Both specimens showed the same haplotypes for 16S and COI, and both constitute the first published sequences for this species. The 16S sequence shows the highest similarity (98.57 and 98.81%) with two sequences of Macropodia longipes (A. Milne-Edwards & Bouvier, 1899) (JN412692 and KC866333, respectively) of two specimens, FCDOPB086-09 (from the Northeast Atlantic, Portugal) and UB2011_ML (from the Western Mediterranean), respectively. The COI haplotype matched 100% and 99.85% of similarity with six (three and three) sequences (early-release) of M. gilsoni deposited in BOLD. However, it matched 100% with 14 sequences of Macropodia tenuirostris (Leach, 1814) and with four sequences of M. tenuirostris (as M. longipes), published in Genbank, and with five and two sequences (early-release) of M. rostrata (as M. parva) and M. longirostris in BOLD, as well as with other 20 sequences of M. tenuirostris at >99%. All this is a clear example of the strong difficulties in identifying specimens of Macropodia. These molecular data could indicate that M. gilsoni is a spinier African form of M. tenuirostris (and support the idea that M. longipes is synonymous with M. tenuirostris).
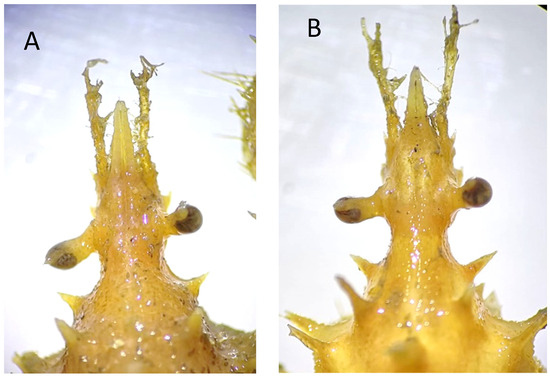
Figure 9.
Rostra of (A) Macropodia gilsoni f.; and of (B) M. gilsoni m. IEOCD-GB19/2941-1 and 2.
Figure 9.
Rostra of (A) Macropodia gilsoni f.; and of (B) M. gilsoni m. IEOCD-GB19/2941-1 and 2.
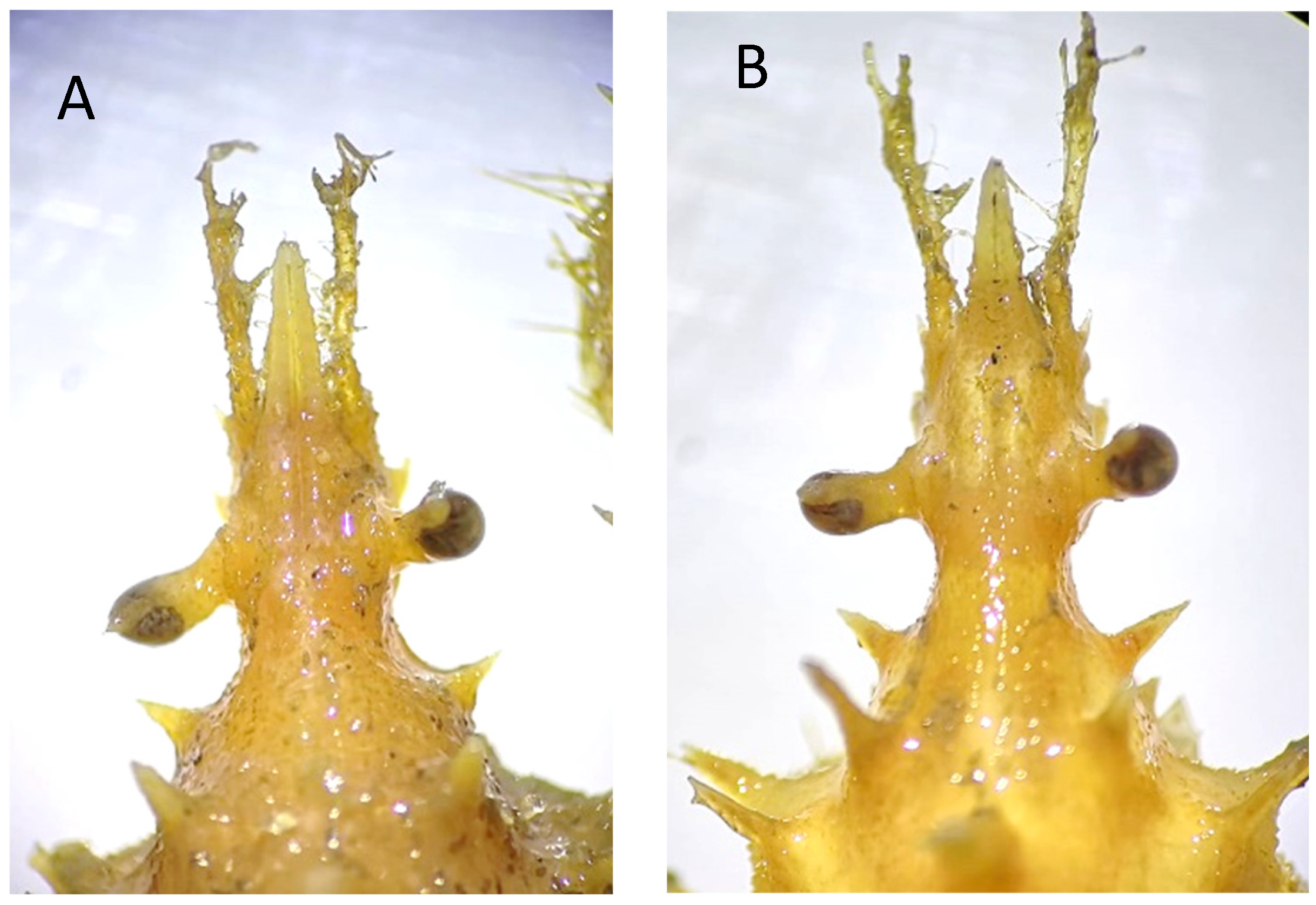
- Macropodia hesperiae Manning & Holthuis, 1981 (Figure 8F)
Material examined. Guinea-Bissau. Bissau0811, Stn. 1, 102 m, October 2008, m. 6.7 × 11.5, m. 5.9 × 10.5 (cw × cl) IEOCD-GB08/189-1, IEOCD-GB08/189-2; GB1219, Stn. 30, 49 m, m. 3.4 × 5.8, m. 5.8 × 9.0, m. 5.9 × 7.8 IEOCD-GB19/2958.
Identification. Our specimens agree well with the original description made by Manning and Holthuis [12].
Distribution. Off Mauritania between 80−102 m on sand [8], and Gulf of Guinea, from off Senegal, the Ivory Coast, Ghana, and Nigeria, at depths between 46−49 and 82−97 m, on mud, sand, coral, and rocky bottom [12,15].
Remarks. There is a record depth of Macropodia aff hesperiae [74], but Matos-Pita et al. [8] questioned this record, and it was not considered in a world list of decapods [28]. d’Udekem d’Acoz [99] also reported M. aff hesperiae in European waters, but based on the Canary Islands record, and remarked that the true M. hesperiae inhabits the waters of the tropical East Atlantic. We have decided not to consider this record from the Canary Islands, following previous doubts [8,99]. Our findings constitute the first record of M. hesperiae in the waters of Guinea-Bissau. Reviewing the crab sizes reported in the literature, IEOCD-GB08/189-2 is the largest male recorded (9.4 mm was the maximum CL recorded for a male [12]). Live color is described, and G1 of the male is photographed for the first time (Figure 10). The record with code IEOCD-GB08/189 (1 and 2) was cited by Muñoz et al. [10] as Macropodia doracis Manning and Holthuis, 1981, but after an exhaustive review of the specimens, we can confirm that the individual belongs to M. hesperiae.
Coloration observed. In live, the whole specimen has bone color with a darker carapace. Some light orange patches in carpi, chelipeds, and dorsal carapace.
DNA barcodes. 16S and COI sequences were obtained for the specimens IEOCD-GB08/189-2 and IEOCD-GB19/2958. These are the first sequences known and published for this species. The 16S sequences differ from each other in four mutations and show a large distance from other congeneric species with 16S sequences in Genbank, with 92.32% of similarity with M. longirostris (OQ701326) and 92.62% of similarity with M. rostrata (KC866334) as the closest species. The COI sequences presented two haplotypes that differed in one mutation and matched 99.18 and 99.51% of similarity with two sequences (early-release) of Achaeus monodi deposited in BOLD, but the photographs of these specimens do not correspond to A. monodi and should be attributed to M. hesperiae. The problem with the identification was probably due to the typical short rostrum of this species, which is more similar to that of Achaeus than to the majority of Macropodia species.
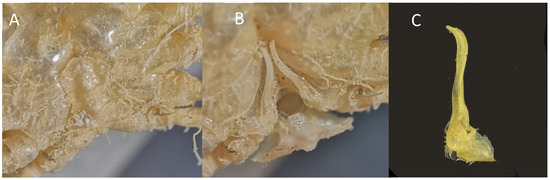
Figure 10.
Macropodia hesperieae, male IEOCD-GB08/189-1. (A) Pleon; (B) First gonopods; (C) G1.
Figure 10.
Macropodia hesperieae, male IEOCD-GB08/189-1. (A) Pleon; (B) First gonopods; (C) G1.
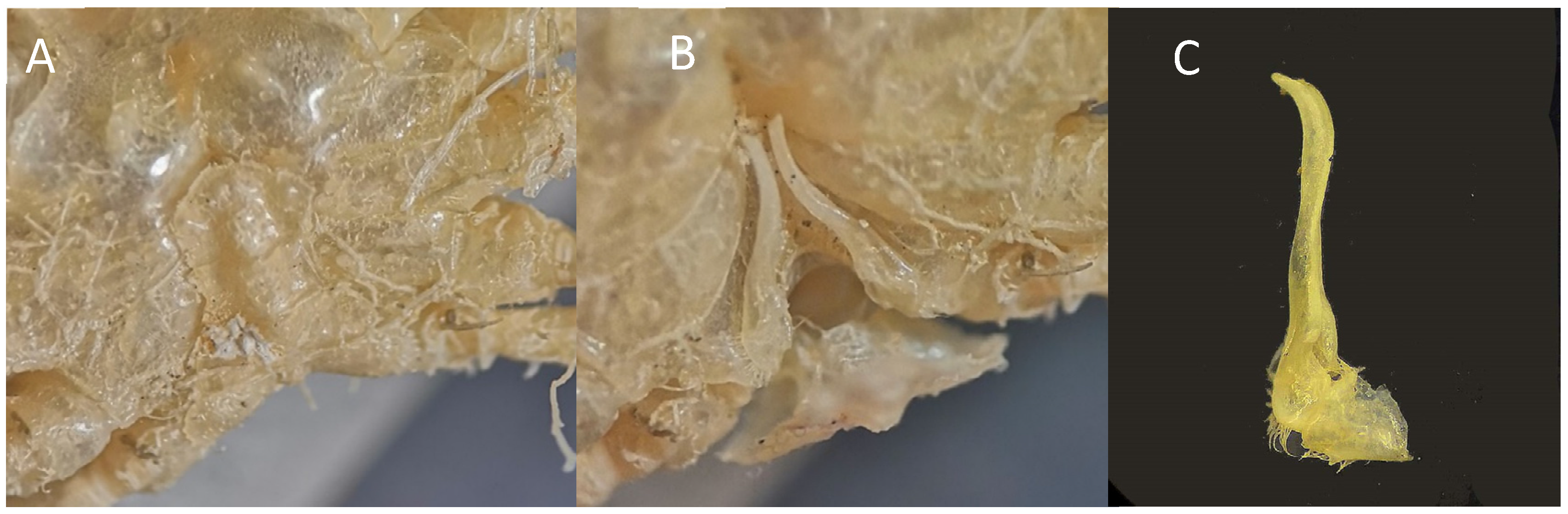
- Macropodia rostrata (Linnaeus, 1761) (Figure 8G)
Material examined. Guinea-Bissau, Bissau0811, Stn. 61, November 2008, 25 m, m. 11.1 × 17.4 (cw × cl), IEOCD-GB08/186-1; CCLME12, Stn. 49, May. 2012, 42 m, f. ov. 10.3 × 14.2, IEOCD-CCLME12/1488.
Identification. Keys provided by Manning and Holthuis [12] (as Macropodia spinulosa) and Forest [97] were used.
Distribution. The species inhabits the Eastern Atlantic, from Norway to Angola, with records in West Africa in Cape Verde, Senegal, Guinea, Guinea-Bissau, Sierra Leone, Ivory Coast, Ghana, Nigeria, Principe, Equatorial Guinea, Gabon, Congo, and Angola [10,13,14,15,52,68] and the Mediterranean Sea [99], as European and Mediterranean references compilation), sublittoral, at depths between one and 126 m.
Remarks. This work provides the second record of M. rostrata in the waters off Guinea-Bissau. The specimen, IEOCD-GB08/186-1, was covered with hydrozoans.
DNA barcodes. 16S and COI sequences were obtained for the specimen IEOCD-GB08/186-1. The 16S sequence matched 99.31 and 99.51% (three or two mutations) with M. rostrata (as M. parva) (KF452979) from the Spanish East Atlantic, and M. rostrata (OQ701327) from the Gulf of Cadiz, respectively. The best match of the COI sequence was with the sequence KF452901 of M. rostrata (as M. parva) (99.54% similarity), but there are another 18 sequences of M. rostrata (as M. parva) and M. rostrata matching between 99.03 and 99.53% of similarity in Genbank. Therefore, all the close 16S and COI sequences belong to M. rostrata, thus supporting the synonymization of M. spinulosa (as it was previously known in this region) with M. rostrata.
- Family INACHOIDIDAE Dana, 1851
- Subfamily STENORHYNCHINAE Dana, 1851
- Stenorhynchus lanceolatus (Brullé, 1837) (Figure 8H)
Material examined. Guinea-Bissau, GB1219, Stn. 29, 72−75 m, November 2019, f. ov. 13.8 × 20 (c × cl without rostrum) m. 19.4 × 28.8 (broken rostrum), m. 18 × 27.7, CRUST_GB19/2990; Bissau0811, Stn. 21, 46 m, October 2008, m. 10.7 × 13.9 × 30.7, IEOCD-GB08/193; Bissau0811, Stn. 19, 71 m, October 2008, m. 16.3 × 21.1 × 5.8, IEOCD-GB08/194; Bissau0811, Stn. 58, 41 m, November 2008, f. 13.2 × 19.0 × 34.5, m. 9.1 × 12.6 × 21.0, IEOCD-GB08/195; LANGABISS0211, Stn. 65, 51 m, May. 2011, f. ov. 16.2 × 24.3 (broken rostrum), IEOCD-LB0211/419; ICM84-85, 47–50 m, February 1985, m. 14.89 × 21.5, m. 14.09 × 18.5, m. 10.79 × 16.3, m. 15.16 × 20.14, m. 12.96 × 17.56, ICMD239/1998; ICM84-85, 53–55 m, January 1985, m. 17.1 × 22, ICMD241/1998; ICM84-85, 220 m, February 1985, m. 17.4 × 22.1, ICMD240/1998.
Identification. Capart [13] and Yang [100] were used for the determination of our specimens.
Distribution. Stenorhynchus lanceolatus is an eastern Atlantic species that inhabits waters from Madeira and the Canary Islands [83] to Angola, with records in several localities of different regions [6,7,8,39,69], at depths between 6 and 96 m, including off Guinea-Bissau from 60 to 73 m [10,15].
Remarks. Up until 1967, all the authors considered that the African species of Stenorhynchus were S. seticornis (Herbst, 1788) [13,14]. Yan [100] clarified that S. lanceolatus is the correct name for the West African species. This work extends the depth range of this species from 60–73 m to 45–220 m.
Coloration observed. Specimens reviewed in this works fit with the color description made by Milne-Edwards and Bouvier [83].
DNA barcodes. The 16S sequence was obtained for the specimen IEOCD-LB0211/419, and the COI sequence was obtained for this specimen and IEOCD-GB19/2990. The 16S sequence is the first known and published for this species. This sequence showed similarities of 89.57 to 90.89% with four sequences of S. seticornis (KM188468, MK971240, MK971602, and MF490218) in Genbank. The COI sequences provided herein are the first known and published for this species. Each specimen in Genbank shows a different haplotype that differs in five mutations. The closest published COI sequences correspond to three of S. seticornis, two (MK971602, MF490122) (82.72 to 83.1%, similarity) in Genbank, and one (AMINV025-08) (82.87–83.1%) in BOLD. There are other close sequences (private) belonging to S. yangi (eight sequences, 90.52 to 91.28% similarity), S. debilis (one sequence, 83.18–83.26% similarity), and S. seticornis (16 sequences, 82.72–83.17% similarity) in BOLD.
- Family MAJIDAE Samouelle, 1819
- Subfamily MAJINAE Samouelle, 1819
- Eurynome aspera (Pennant, 1777) (Figure 11A)
Material examined. Guinea-Bissau, CCLME12, Stn. 38, 142–148 m, m. 9.2 × 9.9 (cw × cl), IEO-CD-CCLME12/1635.
Identification. Monod [14,15,101] were used for determination at level species.
Distribution. Widely distributed in the eastern Atlantic, from Norway to South Africa, including the Mediterranean, at depths between 10 and 550 m. Records in western Africa: Morocco, Mauritania, Sierra Leone, Equatorial Guinea, Angola, and South Africa [14,15,53,93,102,103].
Remarks. The present work provides the first record for E. aspera in the waters of Guinea-Bissau.
DNA barcodes. No sequences could be obtained from this specimen. There are no 16S sequences available for this species in Genbank, but there are six COI sequences: two from the North Sea (KT208884, KT208554), two from Sweden (MG935322, MG935352) in Genbank, and two from Norway (DECNB195-15, DECNB236-15) in BOLD.

Figure 11.
(A) Eurynome aspera m. 9.2 × 9.9 (cw × cl) IEO-CD-CCLME12/1635; (B) Parthenopoides massena f. ov. 14.6 × 12.4 IEOCD-GB08/167; (C) Solenolambrus noordendei m. 11.9 × 11.6 IEOCD-CCLME12/1638; (D) Spinolambrus notialis m. 20.7 × 18.5 IEOCD-GB19/2989 (a) in ethanol; (b) details of the color; (E) Pilumnus stebbingi f. 9.4 × 6.9, IEOCD-GB08/175; (F) Chaceon maritae f. ov. 97.2 × 85.9, ICMD002054; (G) Bathynectes piperitus m. 82.7 × 57.3, IEOCD-GB08/172; (H) Macropipus rugosus m. 40.9 × 29.2, IEOCD-GB08/169.
Figure 11.
(A) Eurynome aspera m. 9.2 × 9.9 (cw × cl) IEO-CD-CCLME12/1635; (B) Parthenopoides massena f. ov. 14.6 × 12.4 IEOCD-GB08/167; (C) Solenolambrus noordendei m. 11.9 × 11.6 IEOCD-CCLME12/1638; (D) Spinolambrus notialis m. 20.7 × 18.5 IEOCD-GB19/2989 (a) in ethanol; (b) details of the color; (E) Pilumnus stebbingi f. 9.4 × 6.9, IEOCD-GB08/175; (F) Chaceon maritae f. ov. 97.2 × 85.9, ICMD002054; (G) Bathynectes piperitus m. 82.7 × 57.3, IEOCD-GB08/172; (H) Macropipus rugosus m. 40.9 × 29.2, IEOCD-GB08/169.

- Superfamily PARTHENOPOIDEA MacLeay, 1838
- Family PARTHENOPIDAE MacLeay, 1838
- Subfamily PARTHENOPINAE MacLeay, 1838
- Parthenopoides massena (P. Roux, 1830 [in P. Roux, 1828–1830]) (Figure 11B)
Material examined. Guinea-Bissau, Bissau0811, Stn. 59, 22 m, November 2008, f. ov. 16.4 × 14.5, f. ov. 14.6 × 12.4 (cw × cl), IEOCD-GB08/167.
Identification. Illustrations and key provided by Monod [14] and Tan and Ng [104] were used for the determination at the species level.
Distribution. Eastern Atlantic, from Brittany, Atlantic coast of France, southward to the Congo, including the Mediterranean, with records in Western Africa in Morocco, Cape Verde, Senegal, Guinea-Bissau, Guinea, Sierra Leone, Liberia, Ghana, Nigeria, Cameroon, Sao Tomé and Principe, Equatorial Guinea, and Gabon, between 91 and 240 m [14,15,23,49,53,77,105,106]. Muñoz et al. [10] reported P. massena for first time in Guinea-Bissau, at a depth of 22 m.
Remarks. This specimen was previously reported by Muñoz et al. [10]. Due to the need to compare the specimen with others (P. massena), we can also confirm the presence of this species in the waters of Western Sahara, concerning some specimens housed in the CRUST-IEOCD collections: (https://www.gbif.org/occurrence/2564780409 (accessed on 17 January 2024), https://www.gbif.org/occurrence/3946721957 (accessed on 17 January 2024), https://www.gbif.org/occurrence/3946721960 (accessed on 17 January 2024)) which represent the first record of this species in Western Sahara. As previously published [14,15], we can confirm the presence of ovigerous females in November. Zariquiey [49] reported ovigerous females only between April and September.
Coloration observed. As Miers [107] and other authors [49,108] had already remarked concerning the morphological variability of P. massena, we have found a large variability in coloration, too.
DNA barcodes. No 16S or COI sequences were obtained for this specimen. In Genbank, there are three sequences of 16S (KP057808, KP057811, and KP057812) and three sequences of COI (KP057815, KP057818, and KP057819) obtained by Marco-Herrero et al. [109] for adults and larvae from the Mediterranean Sea.
- Parthenopoides sp.
Material examined. Guinea-Bissau, GB1219, Stn. 31, 42 m, November 2019, f. ov. 11.7 × 11.1 (cw × cl) IEOCD-GB19/2959.
Remarks. It is a specimen that morphologically falls between three Parthenopinae: Parthenopoides, Ochtholambrus Tan & Ng, 2007, and Neikolambrus Tan & Ng, 2003. The first two have a West African distribution. Monod [14] pointed out the intraspecific variability of P. massena. In fact, this author considered the existence of five morphological varieties (f. typica, f. atlantica, f. pulchella (=goreensis), f. rugosa, and f. bicarinata); the variety pulchella is Ochtholambrus pulchellus (A. Milne-Edwards, 1868) nowadays. Molecularly, this specimen cannot be assigned to a valid species with sequences deposited in public databases (see DNA barcodes).
DNA barcodes. 16S and COI sequences were obtained for the specimen IEOCD-GB19/2959. The 16S sequences closer to this 16S sequence are those three sequences of P. massena mentioned above (KP057808, KP057811, and KP057812) with 97.90–98.54% similarity (8 mutations) that could be considered intrageneric distances. The COI sequence matches 99.39% with a sequence of Daldorfia bouvieri deposited in BOLD as early-release, but the morphology of this specimen (according to the photo in BOLD) does not correspond with D. bouvieri. The other closer sequences belong to P. massena; the three sequences mentioned above (KP057815, KP057818, and KP057819) have 97.13–97.52% similarity (differing in 15–19 mutations) that could be considered intrageneric distances. This specimen, as well as the specimen incorrectly identified as D. bouvieri, could belong to a new undescribed species of the monotypic Parthenopoides.
- Solenolambrus noordendei (Capart, 1951) (Figure 11C)
Material examined. Guinea-Bissau, GB1219, Stn. 84, 102−103 m, December 2019 m. 12.6 × 11.3, m. 12.3 × 11.5, IEOCD-GB19/2962, m. 11.8 × 10.9, IEOCD-GB19/2962-1 (cw × cl); CCLME12, Stn. 48, 63 m, May 2012 m. 11.9 × 11.6, IEOCD-CCLME12/1638.
Identification. The original description and illustration made by Capart [13] as Heterocrypta noordendei and the key provided by Tan and Ng [104] were used for the determination at the genus level.
Distribution. Tropical West Africa, from localities between Western Sahara and Angola, has records in Mauritania, Liberia, Nigeria, Sierra Leone, Ivory Coast, Ghana, and the Congo [8,13,53,108,110], between 55 and 220 m [13,108].
Remarks. This work provides the first record of S. noordendei in the waters of Guinea-Bissau between 63 and 103 m deep. The record from Western Sahara is yet unpublished and corresponds to a male housed in the CRUST-IEOCD Collection (https://www.gbif.org/occurrence/2564781035 (accessed on 17 January 2024)), being the first record of S. noordendei in the waters of Western Sahara and the northernmost record of this species.
Coloration observed. Capart did not describe the live color of S. noordendei. Our specimens have a carapace with brownish-pink deeper regions, ridges, elevated regions, and tubercles in beige. Walking legs and chelipeds are beige, with the proximal regions of merus and propodus in pink. Cheliped tubercles are light pink or beige.
DNA barcode. 16S and COI sequences were obtained for the specimen IEOCD-GB19/2962-1. The 16S sequence is the first known and published for this genus and species. The COI sequence matched 99.52 to 100% similarity with 11 sequences of S. noordendei deposited in BOLD and marked as early-release. These DNA voucher specimens are from the North Atlantic Ocean (one, the closer haplotype to IEOCD-GB19/2962), Senegal (five), and the Atlantic Ocean (five), according to the Tree-Based Identification of BOLD. Therefore, this is the first COI sequence published and available for this genus and species.
Material examined. Guinea-Bissau, GB1219, Stn. 85, 105−106 m, December 2019, m. 20 × 18.5, m. 20.7 × 18.5 (cw × cl) IEOCD-GB19/2989; GB1219, Stn. 20, 83−84 m, November 2019, m. 15.9 × 14.1, IEOCD-GB19/2991; GB1219, Stn. 17, 71−76 m, November 2019, m. 21.5 × 17.7, IEOCD-GB19/2938; GB1219, Stn. 84, 102−103 m, December 2019, f. ov. 15.8 × 13.1, IEOCD-GB19/2946; Bissau0811, Stn. 21, 46 m, October 2008, m. 24.2 × 17.8, IEOCD-GB08/166; GB1219, Stn. 83, 71−75 m, December 2019, f. 17.4 × 14.2, IEOCD-GB19/2951; ICM84-85, 33 m, December 1984, m. 45 × 36.1, ICMD238/1998; ICM84-85, 43–47 m, Jav. 1985, m. 30.9 × 25.3, ICMD237/1998; ICM84-85, 33 m, December 1984, f. ov. 20.5 × 15.6, f. 23.2 × 19.5, m. 26.1 × 21.1, m. 23.7 × 19.4, m. 30 × 23.4, m. 27.4 × 24.5, ICMD238/1998.
Identification. Our specimens fit perfectly with the original description made by Manning and Holthuis [12], except for the female IEOCD-GB19/2951 (see comments in Remarks).
Distribution. Spinolambrus notialis inhabits waters from the Western Sahara to Angola, with records in Mauritania, Senegal, Guinea-Bissau, Sierra Leone, Ivory Coast, Ghana, Cameroon, and Gabon, 18 to 162 m [8,13,15,23,44,53,54,77,93]. Forest and Guinot [15] recorded S. notialis for the first time (as Lambrus macrocheles) in the waters of Guinea-Bissau, at 60–70 m.
Remarks. The present document diminishes the minimum depth recorded for the species from 60 to 33 m and increases the maximum from 70 to 106 m in Guinea-Bissau waters. We also report the presence of ovigerous females in December, thus expanding the previously known time span of ovigerous female occurrences observed [12,14,15]. The female IEOCD-GB19/2951, with 17.4 of cw, shows a smooth dorsal carapace surface without high tubercles; tubercles are present along the longitudinal carapace midline; these are low, without a shaped tip, in comparison with other specimens of the species; the branchial tubercles, except the last one, are very diminished; the rostrum does not bear any tooth at either side, as occurs in S. notialis. This female has the appearance of being age-worn, but her size is smaller than the other specimens examined (Figure 12A,B). However, there are no remarkable differences in its COI sequence with respect to another specimen (IEOCD-GB19/2989), with just two mutations (intraspecific variability). Additionally, it is close to other specimens from the region (see DNA barcode).
DNA barcode. The 16S sequence was obtained for the specimen IEOCD-GB08/166, which is the first 16S sequence for this species. The COI sequence was obtained for two specimens, IEOCD-GB19/2951 and IEOCD-GB19/2989, which showed two different haplotypes that differ in two mutations. These sequences match 99.85 and 99.69% of similarity with four sequences of S. notialis from Senegal and the Atlantic Ocean deposited in BOLD (as early-release). Therefore, these are the first COI sequences published and available for S. notialis.
Figure 12.
(A,B) Spinolambrus notialis, f. IEOCD-GB19/2951; (C) Illustration of S. notialis (as Lambrus machochelos from Monod, 1956).
Figure 12.
(A,B) Spinolambrus notialis, f. IEOCD-GB19/2951; (C) Illustration of S. notialis (as Lambrus machochelos from Monod, 1956).
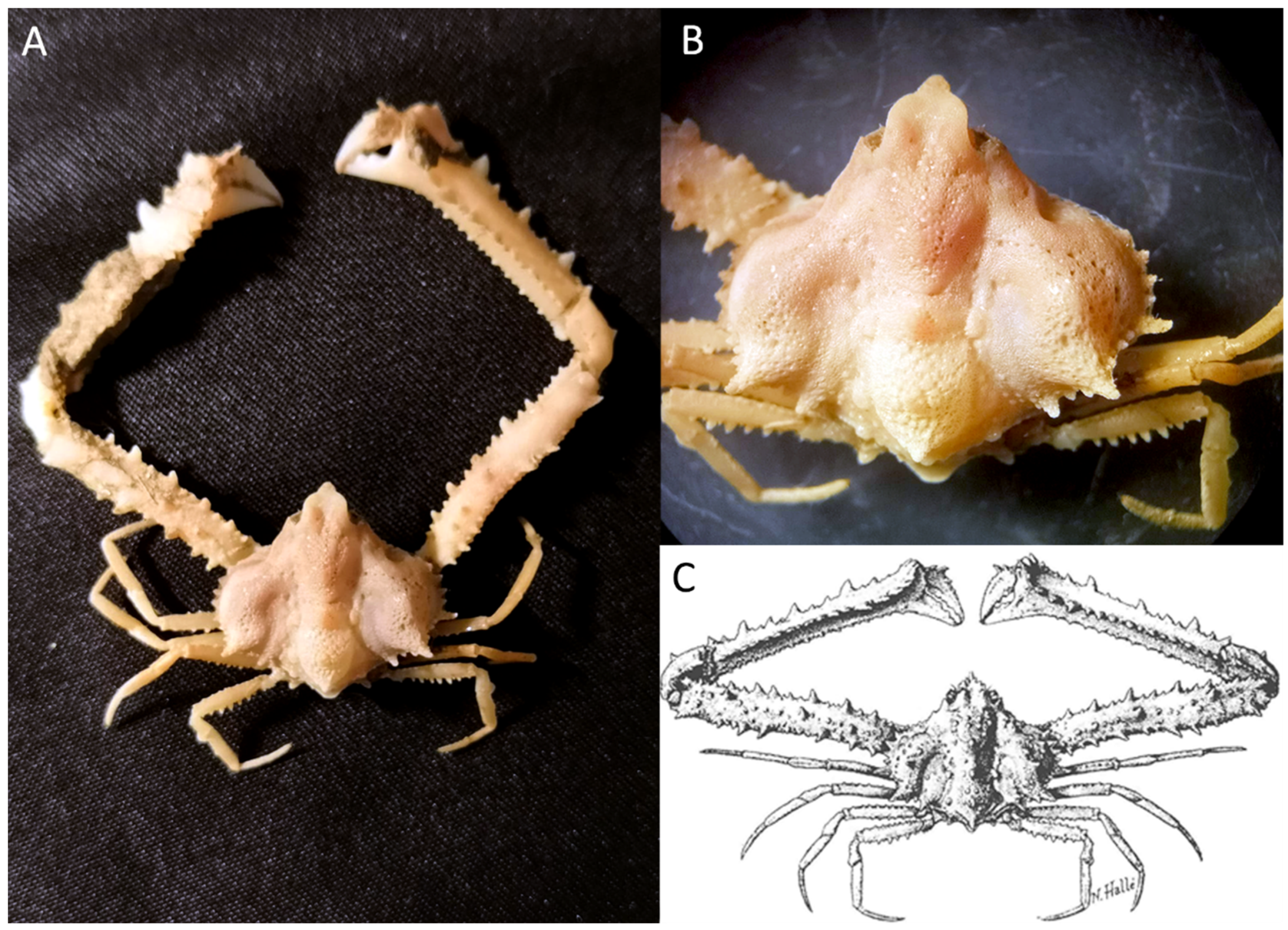
- Superfamily PILUMNOIDEA Samouelle, 1819
- Family PILUMNIDAE Samouelle, 1819
- Subfamily PILUMNINAE Samouelle, 1819
- Pilumnus stebbingi Capart, 1951 (Figure 11E)
Material examined. Guinea-Bissau, Bissau0811, Stn. 58, 41 m, November 2008, m. 9.4 × 7, f. 8 × 5.8, f. 6.7 × 5.2 (cw × cl) IEOCD-GB08/175; Bissau0811, Stn. 20, 52 m, October 2008, m. 7.8 × 5.6, f. 9.4 × 6.9, IEOCD-GB08/176; GB1219, Stn. 44, 49 m, November 2019, f. 7.5 × 5, f. ov. 10.4 × 7.1, f. ov. 10.7 × 7.7, m. 11.3 × 8, IEOCD-GB19/2970-1, m. 12.2 × 8.7, IEOCD-GB19/2970-2; GB1219, Stn. 33, 43 m, November 2019, f. 8.4 ×5.5, m. 11.3 × 8.4, ICMD002682; GB1219, Stn. 30, 41 m, November 2019, f. ov. 6.6 × 5.4, f. ov. 7 × 5.1, f. ov. 6.2 × 4.9, f. 7.5 × 5.8, IEOCD-GB19/2971.
Identification. Original description and key made by Capart [13] were used for the determination at the species level.
Distribution. Pilumnus stebbingi is known from scattered localities from Western Sahara to Angola, including records in Senegal, Guinea-Bissau, Guinea, Sierra Leone, Equatorial Guinea, and Principe [12,13,14,15] at depths between 10 and 73 m. In Guinea-Bissau, it has been recorded at depths ranging from 60 to 73 m [15].
Remarks. This work provides the second record of P. stebbingi in the waters of Guinea-Bissau and reduces the mininum depth from 60 to 41 m. Monod [14] and Maurin [44] reported ovigerous females in May, June, and September. Five of the seven females we presently examined, captured in November, were ovigerous.
Coloration observed. Two remarks on P. stebbingi coloration are known, one made by Monod [14] concerning specimens from Guinea: “épines rouges sur les pattes, qui sont annelées de rouge,” and the other made by Rossignoli [23]: “Caracteristique par son tomentum épais, feutre, de couleur grise a gris-noir avec, sur les pinces, des tubercules epineux rouges.” Our specimens were red-brown, with a dense red integument and red eyes, with no signs of gray or black.
DNA barcodes. Only the 16S sequence was obtained for specimen IEOCD-GB19/2970-1. This constitutes the first 16S sequence for this species. There is only one COI sequence in Genbank for this species (MF504146), from a specimen (USNM 1277872) of Equatorial Guinea deposited by Magalhães et al. (unpublished).
- Superfamily PORTUNOIDEA Rafinesque, 1815
- Family GERYONIDAE Colosi, 1923
- Subfamily GERYONINAE Colosi, 1923
- Chaceon maritae (Manning & Holthuis, 1981) (Figure 11F)
Material examined. Guinea-Bissau, Bissau0811, Stn. 24, 435 m (damaged specimen), IEOCD-GB08/241; ICM84-85, 162–210 m, 1985, f. ov. 115.4 × 97.6, f. ov. 97.2 × 85.9, ICMD002054.
Identification. Descriptions and remarks provided by different authors were used [12,22,111,112]. Manning and Holthuis [112] contains useful lists with the mean differences between C. affinis and C. maritae and between C. maritae and C. quinquedens. Other consulted bibliography used is [12,22,111,112].
Distribution. West African species, known from localities between Western Sahara and Angola, with records in Mauritania, Senegal, Guinea-Bissau, Liberia, Ivory Coast, and Ghana, between 300 and 800 m [8,11,14,44,113,114].
Remarks. The present specimen is the second record for this species in the waters of Guinea-Bissau, between 162 and 210 m; this is the minimum depth recorded for this species. For a long time, there was notorious taxonomic confusion within the genus Chaceon. Many authors, including [14,47,115], misidentified C. affinis, C. maritae, and Chaceon quinquedens (Smith, 1879) (as Geryon), for instance, considering Geryon affinis and Geryon quinquedens as synonymous.
DNA barcodes. 16S and COI sequences were obtained for the specimen ICMD002054. The 16S sequence matched 100% with one sequence of C. maritae (LN809920) and 99.73% with another (LN809929) of the specimens from Western Sahara and Namibia obtained by Hernández et al. [111]. The COI sequence matched 100% with one sequence of C. maritae (LN809898) and 99.79% with two others (LN809916, LN809915) of the specimens from Western Sahara and Namibia obtained by Hernández et al. [111].
- Family POLYBIIDAE Ortmann, 1893
- Bathynectes piperitus Manning & Holthuis, 1981 (Figure 11G)
Material examined. Guinea-Bissau, Bissau0811, Stn. 49, 420 m, November 2009, m. 82.7 × 57.3 (cw × cl) IEOCD-GB08/172; LANGABISS0111, Stn. 82, 194 m, March 2011, m. 62.0 × 40.0, IEOCD-LB0111/400; CCLME11, Stn. 23, 450 m, October 2011, f. ov. 88.7 × 60, IEO-CD-CCLME11/503; ICM84-85, 220–400 m, January 1985, m. 49.18 × 30.75, f. 47.76 × 29.82, ICMD247/1998; ICM84-85, 227–259 m, January 2015, m. 72.2 × 54.2, ICMD248/1998; ICM84-85, 240–387 m, January 1985, f. 80.1 × 55.1, m. 83.9 × 57.8, m. 76.7 × 50.2, m. 61.9 × 40.8, ICMD246/1998; ICM84-85, 162–210 m, January 1985, f. ov. 60.9 × 42, m. 83.35 × 59.91, m. 81.4 × 51.4, f. 76.8 × 55.54, ICMD299/2000; ICM84-85, 247–267 m, f. 64.19 × 42.81, f. 61.86 × 37.2, ICMD249/1998; ICM84-85, 214–237 m, January 1985, m. 33.8 × 21, ICMD250/1998.
Identification. The original description made by Manning and Holthuis [12] was used for the identification of this specimen. Monod [14] and Zariquiey Álvarez [49] were also necessary to check with the other species of the genus known to occur in the study area or in nearby areas, namely B. maravigna (Prestandrea, 1839).
Distribution. African species occur from Cape Verde to Angola, with records in Western Sahara, Mauritania, Senegal, Guinea-Bissau, Guinea, Liberia, Ivory Coast, Ghana, Gabon, and Namibia, from 200 to 628 m [6,7,8,15,26,27,41,42,49,50]. In Guinea-Bissau, it was previously recorded by Capart [13] between 320–360 m.
Remarks. This record provides the second record for B. piperitus in the waters of Guinea-Bissau, reduces the minimum depth of occurrence of the species to 162 m, and extends the maximum depth up to 450 m off Guinea-Bissau. The specimens from 1985 represent the first confirmed presence of the species in this area. The male with code IEOCD-GB08/172 was included as B. maravigna in Muñoz et al. [10]. Due to the revision of B. piperitus from Guinea-Bissau and the comparison with specimens from other areas, we herein provide the first record of B. piperitus in Equatorial Guinea, which belongs to a specimen with the collection code IEO-CD-G.ECU17/2079 housed in the CRUST-IEOCD https://www.gbif.org/occurrence/2605328439 (accessed on 17 January 2024).
Coloration observed. Manning and Holthuis [12] listed three different color descriptions, one new color pattern described by them, and the other two based on previous studies made by Capart [13] and Monod [14]. Our specimens fit well with Manning and Holthuis [12] description: orange carapace with a white spot in the frontal, cardiac, and metabranchial regions; orange chelipeds with white spine tips; walking legs with broad orange bands over the distal part of the merus, carpus, and the proximal half of the propodus. The specimens under this study differ from Manning and Holthuis description [12] in the apex of the lateral spine, which is clearer and not dark, almost black.
DNA barcodes. 16S and COI sequences were obtained for the specimen IEO-CD-CCLME11/503. There are no sequences of B. piperitus in any public database; therefore, these are the first sequences known and published. The 16S sequence fits 98.87% of similarity with the sequence KT365526 of one specimen of Bathynectes longispina from Florida, obtained by Evans [116], and 98.18% of similarity with the sequence FM207770 of one specimen of B. maravigna from the Alboran Sea (Western Mediterranean), obtained by Schubart and Reuschel [117]. The closest published COI sequences to this of B. piperitus belong to the other two species of this genus. One deposited in Genbank (KT365693) of B. longispina (96.95% similarity), and other 10 sequences of B. maravigna (96.93–96.44% similarity) deposited in Genbank and BOLD. Currently, there is no molecular data on the other two species of the genus, B. brevispina and B. longipes, in any public database.
- Liocarcinus corrugatus (Pennant, 1777)
Material collected. Guinea-Bissau, BISSAU0811, st 100, 22 m, November 2008, 1 specimen.
Identification. The specimen was neither preserved nor housed in any collection. Zariquiey Álvarez [49] was used for on-board identification.
Distribution. Eastern Atlantic, from England to Angola, Mediterranean. In West Africa, with records in the Cape Verde Islands, Western Sahara, Mauritania, Senegal, Guinea-Bissau, and Angola, from littoral to 60 m [8,10,13,14,18].
Remarks. Prior to Plagge et al. [118], L. corrugatus had an Indo-West Pacific distribution. These authors later assigned the records from the Indo-West Pacific to Liocarcinus strigilis (Stimpson, 1858). A specimen was reported by Muñoz et al. [10] as the first record of L. corrugatus in Guinea-Bissau waters. This specimen was determined and photographed on board, but it was neither conserved nor housed in any collection.
DNA barcodes. No DNA sequences could be obtained from this specimen. However, there are in Genbank several available sequences of 16S, COI, and H3 for this species. For identification purposes, the best sequences are probably those of COI (KP795935–KP795937) gathered by Plagge et al. [118], which clarify the differences between populations of L. corrugatus and the consideration of L. strigilis as the species with Asian distribution previously reported as L. corrugatus.
- Macropipus rugosus (Doflein, 1904) (Figure 11H)
Material examined. Guinea-Bissau, GB1219, Stn. 46, 164−165 m, November 2019, m. 37.8 × 26.2, m. 37.1 × 25.7, m. 28 × 20.2, m. 39.9 × 27.2 (cw × cl) IEOCD-GB19/2945; GB1219, Stn. 82, 56 m, December 2019, m. 17.6 × 12.5, IEOCD-GB19/2949; GB1219, Stn. 6, 58−61 m, November 2019, m. 36.3 × 25.8, IEOCD-GB19/2950; GB1219, Stn. 30, 49 m, November 2019, f. 16 × 11.7, IEOCD- GB19/2960; Bissau0811, Stn. 1, 102 m, m. 39.7 × 27.1, m. 38.1 × 27.2, IEOCD-GB08/168; Bissau0811, Stn. 77, 218 m, November 2008, f. 32.4 × 23.3, m. 40.9 × 29.2, IEOCD-GB08/169; LANGABISS0211, Stn. 101, 209 m, May 2011, f. 35.1 × 24.9, f. 30.6 × 21.7, f. 34 × 24.4, IEOCD-LB0211/423; LANGABISS0311, Stn. 160, 329 m, f. 40.1 × 27.6, IEOCD-LB0311/449; CCLME11, Stn. 29, 130 m, October 2011, m. 31.2 × 21.7, m. 33.1 × 24.0, m. 30.9 × 21.3, IEOCD-CCLME11/500.
Identification. For identification, [13,14] (both as Portunus tuberculatus) and [15,119] (both as M. rugosus) were used.
Distribution. Off West Africa, from Mauritania to Angola, in several locations (Morocco, West Sahara, Mauritania, Senegal, Guinea-Bissau, Sierra Leone, Liberia, Ivory Coast, Cameroon, São Tomé and Príncipe, Gabon, Congo, and Angola [12], at moderate depths, from 5 to 400 m. In the waters of Guinea-Bissau, it has been reported by several authors [10,15,19] between 60 and 174 m.
Remarks. This document extends the depth range of M. rugosus in the waters of Guinea-Bissau from 60−174 m [15] to 49−319 m. Both [13,14] included Portunus tuberculatus P. Roux, 1830, in their respective catalogues of species of West Africa, but after a revision made by Manning and Holthuis [12], these authors concluded that the correct species inhabiting those waters was Macropipus rugosus.
Coloration observed. Capart [13] described the coloration of M. rugosus (as M. tuberculatus) as follows: “gris-jaune avec laches roses, prenant un aspect nacre après fixation.” Manning and Holthuis [12] considered that Capart [13] had made a mistake and described Macropipus australis Guinot, 1961. Our specimens fit well with the description made by Monod [14]: brown carapace, with some pinkish patches, with iridescent posterolateral edges. Chelae and ambulatory legs are light brown and orange, with the back in bone color and some iridescent patches. After two years in ethanol, the iridescence remains in carapace and chelae.
DNA barcodes. 16S and COI sequences were obtained for the specimen IEOCD-GB19/2945. These are the first 16S and COI sequences published for this species. The 16S sequence fits at 91.91% similarity with the sequence FM208769 of M. tuberculatus. The COI sequence matched 100%, 99.84%, and 99.68% with nine, one, and three COI sequences, respectively, of M. rugosus deposited in BOLD (as early-release and private) and from specimens from Guinea-Bissau, Mauritania, and the Atlantic Ocean.
- Family PORTUNIDAE Rafinesque, 1815
- Subfamily PORTUNINAE Rafinesque, 1815
- Callinectes amnicola (de Rochebrune, 1883) (Figure 13A)

Figure 13.
(A) Callinectes amnicola f. 78.4 × 61.3 IEOCD-LB0211/421; (B) Callinectes marginatus f. ov. 92.9 × 45.7 CRUST-GB19/3728; (C) Callinectes pallidus m. 26.6 × 10.6 CRUST-GB19/3727; (D) Sanquerus validus m. 230.1 × 112 from Bissau0811; (E) Charybdis helleri m. 71.5 × 44.8 IEOCD-LB0211/426; (F) Cronius ruber (a) f. 67.6 × 40.9 IEOCD-GB08/171, (b) m. 58.6 × 35.9, IEOCD-LB0211/428; (G) Paraxanthias eriphioides m. 31.3 × 18.3 IEOCD-GB08/177.
Figure 13.
(A) Callinectes amnicola f. 78.4 × 61.3 IEOCD-LB0211/421; (B) Callinectes marginatus f. ov. 92.9 × 45.7 CRUST-GB19/3728; (C) Callinectes pallidus m. 26.6 × 10.6 CRUST-GB19/3727; (D) Sanquerus validus m. 230.1 × 112 from Bissau0811; (E) Charybdis helleri m. 71.5 × 44.8 IEOCD-LB0211/426; (F) Cronius ruber (a) f. 67.6 × 40.9 IEOCD-GB08/171, (b) m. 58.6 × 35.9, IEOCD-LB0211/428; (G) Paraxanthias eriphioides m. 31.3 × 18.3 IEOCD-GB08/177.

Material examined. Guinea-Bissau, Bissau0811, Stn. 99, 25 m, f. (damaged specimen) IEOCD-GB08/173; LANGABISS0211, Stn. 51, 256–262 m, May 2011, f. 78.4 × 61.3, IEOCD-LB0211/421.
Identification. Keys and illustrations provided by different works [12,13,14,120] as Callinectes latimanus were used for the determination of the specimens at the species level.
Distribution. Off west Africa, from Mauritania to Angola, with records in Cape Verde, Senegal, Gambia, Sierra Leone, Liberia, Ivory Coast, Ghana, Nigeria, and Cameroon, from inshore and estuaries, from 0 to 30 m [66,68,77,105,112,120,121,122,123].
Remarks. This study establishes a record for this species at a depth of 256–262 m, extending the maximum depth beyond shallow waters and the platform. This depth has been verified to eliminate the possibility of contamination errors, and the specimen originates from previous fishing stations. However, all fishing stations conducted prior to Stn. 51 from the LANGABISS0211 survey were at depths exceeding 200 m. Therefore, we can confidently consider the depth of 256–262 m as a reliable data point. This work provides the first record of C. amnicola in the waters of Guinea-Bissau. The female with code IEOCD-GB08/173 was misidentified and reported by Muñoz et al. [10] under the name Callinectes marginatus (A. Milne-Edwards, 1861). After thorough revision, we can confirm that this specimen is a C. amnicola since the granules from the anterior part of the carapace to the epibranchial ridges are larger and more apart than those located on the posterior half of the carapace [12].
Coloration observed. Several remarks on coloration can be found in the literature: Williams [120]: “a mottled olive coloration persists at least as long as 20 years in some preserved specimens”; Capart [13]: “uniforme bleuatre (exemplaires fixes, orange clair)”; Gauld [66]: “olive-brown with pale legs”. This pattern, however, does not fit the females we examined. They have brown-reddish carapace, green walking legs, a fifth pereiopod with some reddish patches in merus, carpus, and propodus, and important red hairiness; chelipeds with merus and carpus; propodus orange with a pale ventral area; and green brown-reddish fingers.
DNA barcodes. The 16S sequence was obtained for the specimen IEOCD-GB08/173. This is the first 16S sequence known and published for an adult specimen of this species, and it matched 100% with the 16S sequence (MW264140) of a megalopa of this species collected from plankton samples (MEGMF24) from the Atlantic Ocean by Marco-Herrero et al. [124]. The COI sequence was obtained for the specimens IEOCD-GB08/173 and IEOCD-LB0211/421, which showed the same haplotype and matched 100% with HVDBC074-11 and HVDBC075-11 (from Namibia) and 99.85% and 99.70% with the specimens ULLZ4650 from Ghana (MG462523) and MEGMF24 (MW264441) from the Atlantic Ocean, respectively.
- Callinectes marginatus (A. Milne-Edwards, 1861) (Figure 13B)
Material examined. Guinea-Bissau, GB1219, Stn. 39, 43–44 m, November 2019, f. ov. 92.9 × 45.7 (cw × cl) CRUST-GB19/3728.
Identification. Keys and illustrations provided by different authors [12,13,14,120] were used for the determination of the specimen at the species level.
Distribution. Off southern Florida, through the Caribbean Sea to south central Brazil and Bermuda, and off Africa, from the Cape Verde Islands to Angola, with records in Gambia, Guinea, Sierra Leone, Ghana, Togo, Equatorial Guinea, Congo, and Nigeria [15,19,66,120,125,126]. The first record for C. marginatus in the waters of Guinea-Bissau was published by Muñoz et al. [10]. But, after a revision made for this work, we can confirm that it was a mistake, with the correct determination being C. amnicola.
Remarks. This work provides the first records for C. marginatus in the waters of Guinea-Bissau, after having confirmed the error in the determination of the specimen by Muñoz et al. [10].
Coloration observed. Uniform gray-green carapace, as Rossignoli [23] described, or mottled white and black [127]. Our specimen shows this color pattern also in the legs, although lighter. Rossignoli [23] pointed out some blue areas. In our specimen, a shadow of a blue spot on the palm and proximal internal part of the chela fingers can be appreciated, as Rossignoli [23] described.
DNA barcodes. 16S and COI sequences were obtained for the specimen CRUST-GB19/3728. The 16S sequence fits 99.27%, 99.23%, 99.04%, and 97.88% with five 16S sequences of C. larvatus (specimens ULLZ5171, ULLZ13476, ULLZ13405, ULLZ13372, and ULLZ13406) from Venezuela (DQ407678) and Panama (MK971243, MK9711586, MK971577, and MK971444), obtained by Robles et al. [128] and Venera-Ponton et al. [64]. It also fits 98.87% and 97.47% with two sequences (KT365527, MW081862) of C. marginatus from Florida (UF11403) and Brazil (USP:CCDB:6104) by Evans [116] and Mantelatto et al. [26], respectively. The COI sequence showed larger distances with respect to five sequences of C. larvatus (94.83–94.98% similarity) and two of C. marginatus (94.79–94.82%). This is strange considering that the COI sequences belong to the same specimens with 16S sequences matching >99% (i.e., ULLZ13405, ULLZ13406) of C. larvatus and the same specimen of C. marginatus (UF11403) with 98.87% for 16S present a 94.82% similarity in COI. The taxonomic relationships between C. larvatus (Western Atlantic) and C. marginatus (East Atlantic) are not clear, and they are considered sister species. However, they show very short genetic distances. In this case, C. larvatus showed higher similarity with C. marginatus from Guinea-Bissau than this last with C. marginatus from Florida and Brazil (which could perhaps be considered as C. larvatus, too). More studies, including more specimens and new genetic markers, are really needed to clarify the relationships between American and African Callinectes species.
- Callinectes pallidus (de Rochebrune, 1883) (Figure 13C)
Material examined. Guinea-Bissau, Bissau0811, Stn. 100, 22 m, November 2008, m. 31.2 × 12.2 (cw × cl), IEOCD-GB08/174; GB1219, Stn. 87, December 2019, 29−31 m, m. 26.6 × 10.6, CRUST-GB19/3727.
Identification. Keys and illustrations provided by several authors [13,14,120] (as Callinectes gladiator) were used for the determination of the specimens at the species level.
Distribution. West Africa from France to Angola, with other records in Mauritania, Senegal, Guinea, Sierra Leone, Liberia, Ghana, Ivory Coast and Nigeria, Cameroon, Equatorial Guinea, Gabon, Congo, Benin, Sao Tomé and Principe, from shore to depths of 35 m on sand, sandy mud, or gravel [14,15,19,53,66,68,69,77,120,129,130]. Two specimens were recorded (as Callinectes exasperatus) in the Gulf of Cadiz (Spain) [131].
Remarks. It is necessary to clarify that C. pallidus is a synonym for C. gladiator. Manning and Holthuis [12] synonymized both species under the name Callinectes pallidus (De Rochebrune, 1883), a new combination, clarifying that one was really the juvenile stage of the other. Currently, both species are still considered valid on the checklist of crabs of the world [28,84] and in some platforms (https://www.marinespecies.org/aphia.php?p=taxdetails&id=442768 (accessed on 17 January 2024), https://eol.org/pages/46508456 (accessed on 17 January 2024)). However, for other platforms, such as https://www.sealifebase.se/nomenclature/SpeciesList.php?genus=Callinectes (accessed on 17 January 2024), both species are synonyms. This work provides the first record for C. pallidus in the waters of Guinea-Bissau.
Coloration observed. Manning and Holthuis [12] summarize several color descriptions for C. pallidus from different authors, but since our specimen CRUST-GB19/3727 is a juvenile, it does not fit well with any description. Our specimen has carapace, chelipeds, and walking legs mottled grey-green, without any dark pinces or spines, nor blue walking legs, as Irvine [127] described.
DNA barcodes. 16S and COI sequences were obtained from the specimens IEOCD-GB08/174 and CRUST-GB19/3727. Both specimens present the same haplotypes for the two genes. The 16S sequences are the first known and published 16S sequences for this species and fit 98.90% (six mutations) and 98.48% (seven mutations) of similarity with two sequences of Callinectes exasperatus (Gerstaecker, 1856), DQ407682 (ULLZ4366 from Venezuela), and KX060432 (CCDB:802 from Brazil) obtained by Robles et al. [128] and Negri et al. [132], respectively. The COI sequences match 100% with a COI sequence of C. pallidus (MG462536) of the specimen ULLZ4649 from Ghana obtained by Windsor et al. [133].
- Sanquerus validus (Herklots, 1851) (Figure 13D)
Material collected. Guinea-Bissau, Bissau0811, Stn. 100, 22 m, November 2008, m. 230.1 × 112; BISSAU0811, st 77, 216–217 m, November 2008, two specimens (size and sex not available).
Identification. Three specimens were collected in November 2008. Capart [13], Monod [14] (as Neptunus validus), and Manning and Holthuis [12] (as Portunus validus) were used for determination. The specimens were not preserved or housed in any collection.
Distribution. This is an insular species in the Eastern Atlantic, with records in Madeira, the Cape Verde Islands, Guinea-Bissau, Gambia, Ghana, Liberia, Guinea, Ivory Coast, Equatorial Guinea, Sao Tome Island, Benin, Togo, Cameroon, Sierra Leone, Congo, and Ascension Island, in moderate depths of 7 to about 309 m [23,53,66,68,77,134,135,136,137].
Remarks. These specimens were cited by Muñoz et al. [10] as the first record of S. validus in the waters of Guinea-Bissau.
DNA barcodes. No DNA sequences could be obtained for this species since no specimen was deposited in a collection. In Genbank, there is only one 16S sequence (MG515587) of a specimen from the Lagos Lagoon (Nigeria) obtained by Mantelatto et al. [138].
- Subfamily THALAMITINAE Paulson, 1875
- Charybdis (Charybdis) hellerii (A. Milne-Edwards, 1867) (Figure 13E)
Material examined. Guinea-Bissau, LANGABISS0211, Stn. 229, 31 m, m. 71.5 × 44.8 (cw × cl), IEOCD-LB0211/426.
Distribution. The native distribution of C. helleri is in the Indo-Pacific Ocean [139,140], and it is considered an invasive crab, colonizing the Mediterranean Sea [141,142], the Western Atlantic [132], with an isolated record in northwest Spain [96], and West Africa [139]. Dessouassi et al. [139] have cited this species in Benin in 2019. In that work, the authors pointed out an unpublished record in Guinea-Bissau, referring to our specimen reviewed here: https://www.gbif.org/occurrence/2564780164 (accessed 17 January 2024).
Remarks. Our specimen is the first record for C. helleri in the waters of Guinea-Bissau. Some authors have reported variation in pubescence in this species, and it is possible to find both pubescent and glabrous specimens in the same population [132,140]. The male reported here is a glabrous type.
Coloration observed. Milne-Edwards [143] reported the coloration for C. helleri as “greenish, the legs with shades of violet, this color turning red on the chelipeds, the tips of fingers being black.” Our specimen has orange carapace, although some green tone, two white spots in the posterolateral regions, and the lateral spine tips are in black; orange chelipes; palms with the ventral side near the finger, in white, and black fingers; walking legs are bluish, with orange dactyls.
DNA barcodes. 16S and COI sequences were obtained for the specimen IEOCD-LB0211/426. The 16S sequence matches 100% with nine sequences deposited in Genbank (KX060443, KX060500, KX060502, KX060503, KX060532, KX060544, KT365540, MF693855, NC_060062) of specimens from Hawai’i, Australia, Hong Kong, Indonesia, Brazil, and the Philippines. But there are more than 50 other sequences with similarities between 98.68 and 99.82%. The COI sequence presents the same haplotype as the sequence KX060311 of a specimen from Hong Kong obtained by Negri et al. [132]. There are more than 150 COI sequences in Genbank; the 100 closest have similarities between 97.13 and 99.85%.
- Cronius ruber (Lamarck, 1818) (Figure 13(Fa,b))
Material examined. Guinea-Bissau, Bissau0811, Stn. 96, 37 m, November 2008, f. 67.6 × 40.9 (cw × cl) IEOCD-GB08/171; LANGABISS0211, Stn. 63, 48 m, May 2011, m. 65.6 × 41.0, m. 58.6 × 35.9, IEOCD-LB0211/428; ICM84-85, 33 m, Dec 1984, f. 48 × 29.4, f. ov. 47.9 × 30.2, f. ov. 55 × 33.5, f. 60.2 × 36.6, m. 71.4 × 44.2, m. 62.7 × 38.2, ICMD213/1998; ICM84-85, 43–47 m, January 1985, f. 64.0 × 40.4, ICMD214/1998.
Identification. The description made by Capart [13] and the figure of Monod [14] were used for the determination at the species level.
Distribution. Species with a wide distribution in the eastern Pacific as well as in the western and eastern Atlantic, with records off West Africa in Mauritania, Canary Islands, Cape Verde, Guinea-Bissau, Guinea, Sierra Leone, Ivory Coast, Ghana, São Tomé and Principe, Equatorial Guinea, and Angola, in shallow water to a depth of 69 m [10,13,15,19,23,51,53,68,69,144].
Remarks. The first record in the waters of Guinea-Bissau was made by Muñoz et al. [10] at 37 m, based on the specimen IEOCD-GB08/171. This work reports the second record of C. ruber in Guinea-Bissau and extends the maximum depth range from 33–48 m. Specimens collected in 1984 were the first specimens collected for C. ruber in these waters.
Coloration observed. Between our specimens, we can find two color patterns: the greenish one similar to those described by González et al. [145] (Figure 13(Fb)) and the reddish pattern described by Capart [13] (Figure 13(Fa)).
DNA barcodes. Only the 16S sequence was obtained for the specimen IEOCD-LB0211/428. This sequence matches 100% with the sequence FJ152143 of a C. ruber specimen (SMF31986) from Ghana and fits at 99.03 to 99.81 with the other six sequences of C. ruber (FJ152144-152146, KT365546, KY111025, KY111026) from Guinea, the Canary Islands, Brazil, and Mexico. In Genbank, there are also five COI sequences for specimens of C. ruber from Canary Island, Guinea, Brazil, and Florida (KY111027, KY111028, KT365725, MT623339, and MW124931).
- Superfamily XANTHOIDEA Guinot, 1967
- Family XANTHIDAE MacLeay, 1838
- Subfamily EUXANTHINAE Alcock, 1898
- Monodaeus sp.
Material examined. Guinea-Bissau, GB1219, Stn. 20, 83−84 m, m. 12.3 × 7.6 (cw × cl), IEOCD-GB19/2973.
Remarks. The specimen was very damaged and does not allow for a clear morphological comparison.
DNA barcodes. Only the COI sequence was obtained for the specimen IEOCD-GB19/2973. This sequence does not match >97% with any sequence in any public database. The closest COI sequences are more than 20 sequences of Monadeus couchii (Couch, 1851) (94.53–93.74% similarity), HM751010 (95.30% similarity) of M. rectifrons (Crosnier, 1967), MZ43482 (94.32% similarity), and HM751031 (94.30%) of M. tuberculidens (Rathbun, 1911), two sequences early-release in BOLD (94.32% similarity) of M. rouxi (Capart, 1951), and HM751029 (93.79% similarity) of M. cristulatus. Considering these data, this specimen probably belongs to a new undescribed species of Monodaeus or close genus, or to other Monodaeus species not previously recorded in the East Atlantic and without molecular data published as M. arnaudi Guinot & Macpherson, 1988, and M. pettersoni Garth, 1985.
- Subfamily XANTHINAE MacLeay, 1838
- Paraxanthias eriphioides (A. Milne-Edwards, 1867) (Figure 13G)
Material examined. Guinea-Bissau, Bissau0811, Stn. 69, 65 m, m. 31.3 × 18.3 (cw × cl) IEOCD-GB08/177.
Identification. The key and illustrations provided by Capart [13] and Monod [14] were used for the identification.
Distribution. Eastern Atlantic, with few records in the Azores, Cape Verde, and Guinea-Bissau, sublittoral, 10–85 m [10,14,108].
Remarks. Our specimen here reviewed was already cited by Muñoz et al. [10] as the first record of P. eriphioides in Guinea-Bissau. Besides reviewing specimens of this species housed in the CRUST-IEOCD, we have found two males from Equatorial Guinea (IEO-CD-G.ECU17/2107, https://www.gbif.org/occurrence/2605328447 (accessed 17 January 2024)) collected in August 2017 at 69 m that are the first record of this species in the waters of Equatorial Guinea. Also, additional records of P. eriphioides in the waters of Angola and Congo can be found on online platforms (WORMS, GBIF). However, reviewing the bibliography related to this species, we question the veracity of these records. Capart [13] cited three observations of P. eriphioides in Angola (https://www.gbif.org/occurrence/3024589687 (accessed 17 January 2024) https://www.gbif.org/occurrence/3025546624 (accessed 17 January 2024), https://www.gbif.org/occurrence/3024611123 (accessed 17 January 2024), https://www.gbif.org/occurrence/3025573075 (accessed 17 January 2024)) and two in Congo (https://www.gbif.org/occurrence/3025573075 (accessed 17 January 2024), https://www.gbif.org/occurrence/3026000168 (accessed 17 January 2024)), but at the same time, himseelf put under question their identifications as P. eriphioides. Effectively, years later, Monod [14] confirmed the incorrect identifications by Capart [13] and confirmed that the records and illustrations made by him belong to Pseudomedaeus africanus (Monod, 1956). Back to the records in Angola and Congo, we can track the year of collection and who determined these specimens, and we found that they were collected in 1948 and 1949, and Monod determined them as species level. Therefore, it is clear that these records are the same that Capart [13] included in his work as P. eriphioides with doubts, and they are really P. africanus. We recommended the correction of these records in GBIF.
Coloration observed. Our specimen is lively red, with fingers in deep red and white tips. Walking legs are red, with some irregular lines in beige. Dactyls seem to be brown because of their hairiness.
DNA barcodes. No DNA sequences could be obtained from this specimen. However, there are 16S (MZ413004) and COI (MZ400991) sequences in Genbank of the specimen ULLZ11965 from an undetermined locality obtained by Mendoza et al. [146].
3.2. Annotated Checklist of Deep-Sea Brachyuran Crabs from Guinea-Bissau
The checklist (Table 1) has been compiled considering classical literature from the region as well as the most recent studies and publications, in addition to the data obtained from this work (324 specimens belonging to 16 superfamilies studied). The systematics followed for the creation of this checklist have been carried out considering the most recent changes.

Table 1.
Checklist of the deep-sea crabs from Guinea-Bissau. *: species recorded in this study; R: references of the first record in Guinea-Bissau; pw: present work, first record for Guinea-Bissau; 16S and COI: GenBank accession codes of selected 16S rRNA and COI sequences known for each species, in bold those obtained in this study, and underlined those new contributions; NAD: no available data.
The present annotated checklist comprises 16 superfamilies, 24 families, 15 subfamilies, and 61 species. Of the 61 species compiled in this checklist, 59 were identified at the species level and two at the genus level, namely, Monodaeus sp. and Parthenopoides sp., which could probably be new yet undescribed species. Nineteen of the listed species are reported for the first time in the area: the Dromiidae Dromia nodosa, the Calappidae Calappa galloides, the Dorippoidea Phyllodorippe armata, Ethusa rosacea, E. rugulosa, and E. vossi, the Euryplacidae Machaerus oxyacanthus, the Leucosiidae Pseudomyra mbizi, the Epialtidae Afropisa carinimana, the Inachidae Capartiella longipes, Inachus aguiarii, I. angolensis, and Macropodia hesperiae, the Inachoididae Eurynome aspera, the Parthenopidae Solenolambrus noordendei, and the Portunidae Callinectes amnicola, C. marginatus, C. pallidus, and Charybdis helleri. Twenty-two additional species are reported for the second time in the area: Sternodromia spinirostris, Homola barbata, Paromola cuvieri, Ranilia constricta, Sakaila africana, Acanthocarpus brevispinis, Calappa rubroguttata, Medorippe lanata, Goneplax barnardi, Ilia spinosa, Scyramathia hertwigi, Pisa armata, Apiomithrax bocagei, Macropodia gilsoni, Macropodia rostrata, Parthenopoides massena, Spinolambrus notialis, Pilumnus stebbingi, Chaceon maritae, Bathynectes piperitus, Cronius ruber, and Paraxanthias eriphioides.
Afropisa calva (Forest & Guinot, 1966), Macropodia doracis Manning & Holthuis, 1981 and Scyramathia carpenteri (Thomson, 1873) were included by Sasaki [28] as species recorded in the waters of Guinea-Bissau. These three records come from Muñoz et al. [10], being incorrect identifications, which have been discussed in the Results section. Therefore, these species have not been included in the present checklist.
Callinectes marginatus is also included by Sasaki [28] off Guinea-Bissau, but with reference to Muñoz et al. [10], where the specimen was also misidentified (see Results section).
Liocarcinus marmoreus (Leach, 1814) is also cited in Guinea-Bissau by Sasaki [28]. Although this record was published by Muñoz et al. [10], it is based on a single specimen. This record must be taken with caution because the specimen was not preserved, there are no on-board photographs, and there is no certainty that it was that species, another Liocarcinus, or a closely related genus. Therefore, L. marmoreus has not been included in the checklist.
Bathynectes maravigna (Prestandrea, 1839) has not been included in the current checklist either, although it was cited in Guinea-Bissau by Muñoz et al. [10]. After the review conducted in this work, the specimens included in that study (see Results section) were identified as Bathynectes piperitus.
4. Discussion and Conclusions
We report herein the updated checklist of the so-far-known deep-sea brachyuran crabs from Guinea-Bissau, based on the revision of 324 specimens housed in three Spanish marine collections and the related literature comprised by 150 references. To perform this checklist, many publications have been used. Among others, Forest and Guinot [15] and Muñoz et al. [10] are the authors that most contributed to the first records in Guinea-Bissau waters, followed by Monod [14], Capart [13], Guinot and Ribeiro [19], and Crosnier [110].
It is worth pointing out that some of the species herein reported for the first time in Guinea-Bissau are previously misidentified specimens published in Muñoz et al. [10], as indicated in the text. Due to the need to check and compare our specimens from Guinea-Bissau with other individuals of the same species from different areas, two new records have been reported in the Western Sahara (Capartiella longipes [13,15,23] and Parthenopoides massena [14,15,23,49,53,77,105,106]), one in the waters of Mauritania (Calappa rubroguttata [12,13,14,15,23,53,66,67,68,69]), and two more in Equatorial Guinea (Bathynectes piperitus [6,7,8,15,26,27,41,42,49,50] and Paraxanthias eriphioides [10,14,108]), these being the first records of these species in their respective regions. For three species, Calappa rubroguttata, Capartiella longipes [13,15,23], and Solenolambrus noordendei [8,13,53,108,110], their observations represent the northernmost record in their distribution.
The worldwide bathymetric range has been expanded for Dromia nodosa [12,42], Machaerus oxyacanthus [12,14,15,19,23,28,77,78], Capartiella longipes [13,15,23], Stenorhynchus lanceolatus [6,7,8,39,69], Chaceon maritae [8,11,14,44,113,114], Bathynectes piperitus [6,7,8,15,26,27,41,42,49,50], and Macropipus rugosus [10,12,15,19]. Additionally, in the waters of Guinea-Bissau, the bathymetric range has increased for Sternodromia spinirostris [12,13,14,15,42,44], Homola barbata [10,12,14,47,48,49], Acanthocarpus brevispinis [8,10,12,14,28,57], Goneplax barnardi [8,10,12,13,44,51], Ilia spinosa [12,13,14,15,19,23,28,53,70,77,80], Macropodia gilsoni [8,15,77,98], Spinolambrus notialis [8,13,15,23,44,53,54,77,93], Pilumnus stebbingi [12,13,14,15], Callinectes amnicola [66,68,77,105,112,120,121,122,123], and Cronius ruber [10,13,15,19,23,51,53,68,69,144]. For details, see the Remarks sections for each species.
There is new information on the fresh coloration of some species, and for the first time, pictures of seven species in live conditions are presented (Ranilia constricta, Sakaila africana, Scyramathia hertwigi, Phyllodorippe armata, Macropodia hesperiae, Pilumnus stebbingi, and Callinectes pallidus).
After reviewing the specimens included in this work and considering the literature studied, we have concluded that certain species, such as Medorippe lanata at the National Museum of Natural History, Smithsonian Institution, as well as Atlantotlos rhombifer, Achaeus monodi, and Daldorfia bouvieri at the Museum of Bergen, need to be reevaluated due to the possibility of misidentification.
Concerning genetic information, a total of 98 sequences (51 of 16S rRNA and 47 of COI) were successfully obtained for 51 specimens, representing 41 species. Unfortunately, DNA sequences could not be obtained for specimens of Homola barbata, Ethusa rosacea, Eurynome aspera, Parthenopoides massena, and Paraxanthias eriphioides. However, sequences for 16S and/or COI are available for all of them in GenBank, except for Ethusa rosacea. In the cases of Paromola cuvieri, Atelecyclus rotundatus, and Sanquerus validus, the specimens were not deposited in any museums or collections. Consequently, it was not possible to obtain DNA sequences from them. Molecular data for Paromola cuvieri are also absent from public databases.
Seventeen of the species for which the COI sequence was obtained matched >98% with sequences deposited, early-release or private, in BOLD, but that means that we could obtain the results of the search in the BOLD system in terms of species identification and percent similarity, but the sequences are not available (unpublished) and further comparisons are not possible.
From the molecular data obtained, we can highlight certain results.
The 16S and COI data of Calappa rubroguttata suggest the presence of two distinct species, likely coexisting in the Gulf of Guinea and surrounding areas, which are currently identified under the same scientific name.
When comparing data from 16S and COI sequences of Ethusa rugulosa, Ethusa vossi (from this work), and other Ethusa spp. deposited in Genbank and BOLD, higher than expected distances at the intrageneric level were observed, thus suggesting that further studies on intrageneric relationships in Ethusa and related genera, such as Ethusina, are needed.
The molecular data (16S and COI sequences) support the establishment and confirm the placement of the genera Phyllodorippe and Capartiella in Dorippidae and Inachidae, respectively.
New studies, incorporating more specimens from both sides of the Atlantic and employing additional genetic markers, would be necessary to clarify the status and taxonomic relationships among certain species, such as Calappa galloides, Ranilia constricta/R. muricata, Apiomitrax bocagei/A. violaceus, Scyramathia hertwigi/S. umbonata, and Callinectes marginatus/C. larvatus.
A revision of the genus Macropodia is also needed. The clarification of the taxonomic status of Macropodia gilsoni, Macropodia tenuirostris, and Macropodia intermedia requires a morphological study involving more specimens as well as obtaining new DNA sequences from additional genetic markers.
The specimens identified as Parthenopoides sp. and Monodaeus sp. in this study require further investigation to elucidate whether they represent new, yet undescribed, species.
Merocryptus obsoletus is proposed to be synonymized with Merocryptus boletifer based on molecular and morphological evidence, supported by doubts expressed by some authors in the literature [14,81,82]. M. boletifer was described by Milne-Edwards and Bouvier in 1894 [82], and its name should prevail over M. obsoletus, described in 1898 [81].
Through this work, the importance and usefulness of natural history collections are demonstrated. Thanks to the taxonomic and molecular review of specimens housed in the three Spanish collections, gaps in the knowledge of deep-sea African crabs, specifically in the waters of Guinea-Bissau, have been filled.
The utilization of integrative taxonomy [26,27], involving the combination of molecular techniques and morphological analyses used in this study of brachyurans from Guinea-Bissau, has facilitated precise and reliable identification of certain species. Additionally, it has brought attention to taxonomic issues at the specific or generic level that need to be addressed in the near future.
All these data—DNA sequences, distribution, depth range, color, and habitat—provide a baseline for future studies not only in the fields of taxonomy and phylogeny but also in ecology, fisheries, and population dynamics. Additionally, considering potential changes in species distribution due to global warming, natural dispersal, or range extension, it could aid in detecting the presence of new or exotic species in this area and other adjacent zones in the Gulf of Guinea.
Author Contributions
Conceptualization, I.M. and J.A.C.; methodology, I.M. and J.A.C.; software, J.A.C.; validation, I.M., J.E.G.-R., P.A. and J.A.C.; formal analysis, J.A.C.; investigation, I.M., P.A., J.E.G.-R. and J.A.C.; resources, I.M. and J.A.C.; data curation, I.M.; writing—original draft preparation, I.M.; writing—review and editing, I.M., J.E.G.-R., P.A. and J.A.C.; supervision, J.E.G.-R. and J.A.C.; project administration, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Information of the specimens examined can be find in the CRUST-IEOCD Collection https://www.gbif.org/dataset/317fd0a9-1bcf-4e94-bc3c-a685c4693c10 and in the CBRM Collection https://www.gbif.org/dataset/1d743188-1e65-4d99-a814-fa3fd51f1490.
Acknowledgments
We would like to thank the curator, Elena Guerrero, for letting us access the specimens housed in the CBRM-General at ICM-CSIC. We also extend our gratitude to Ignacio Sobrino and Eva García-Isarch for leading the oceanographic campaigns conducted by IEO-CSIC in the waters of Guinea-Bissau and to all the Bissauguinean, Spanish, and Norwegian colleagues who have helped at some point in the collection of specimens on board oceanographic surveys. We want to thank five anonymous reviewers for their comments, corrections, suggestions, complete and detailed revisions, and contributions. Additionally, we are grateful to Enrique Macpherson for the initial identification of the specimens deposited at CBMR-General (ICM-CSIC) in 1985 and to Carlos Sánchez (ICMAN-CSIC) for the laboratory work.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Trawling stations list from the surveys considered in this work. Stn: station; Min_depth: minimum depth reached during trawling; Max_depth: maximum depth reached during trawling; Lat_ini and Long_ini: latitude and longitude at the initial position of trawling; Lat_fin and Long_fin: latitude and longitude at the final position of trawling.
Table A1.
Trawling stations list from the surveys considered in this work. Stn: station; Min_depth: minimum depth reached during trawling; Max_depth: maximum depth reached during trawling; Lat_ini and Long_ini: latitude and longitude at the initial position of trawling; Lat_fin and Long_fin: latitude and longitude at the final position of trawling.
| Survey | Stn | Date | Min_depht | Max_depth | Lat_ini | Long_ini | Lat_fin | Long_fin |
|---|---|---|---|---|---|---|---|---|
| BISSAU0811 | 1 | 23-10-2008 | 102 | 103 | 11.5685 | −17.1903 | 11.5537 | −17.1920 |
| BISSAU0811 | 4 | 23-10-2008 | 374 | 385 | 11.4715 | −17.2200 | 11.4864 | −17.2228 |
| BISSAU0811 | 5 | 23-10-2008 | 102 | 105 | 11.4770 | −17.1854 | 11.4615 | −17.1849 |
| BISSAU0811 | 19 | 26-10-2008 | 71 | 71 | 11.1129 | −17.1065 | 11.1274 | −17.1110 |
| BISSAU0811 | 20 | 26-10-2008 | 52 | 52 | 11.1410 | −17.0919 | 11.1278 | −17.0815 |
| BISSAU0811 | 21 | 26-10-2008 | 46 | 47 | 11.0858 | −17.0543 | 11.0709 | −17.0527 |
| BISSAU0811 | 24 | 27-10-2008 | 433 | 435 | 10.4201 | −17.2470 | 10.4048 | −17.2455 |
| BISSAU0811 | 28 | 28-10-2008 | 422 | 431 | 10.3326 | −17.2245 | 10.3479 | −17.2248 |
| BISSAU0811 | 30 | 28-10-2008 | 550 | 553 | 10.2864 | −17.2605 | 10.2717 | −17.2575 |
| BISSAU0811 | 43 | 31-10-2008 | 277 | 278 | 10.0811 | −16.5700 | 10.0714 | −16.5582 |
| BISSAU0811 | 49 | 1-11-2008 | 448 | 451 | 10.2801 | −17.2126 | 10.2949 | −17.2174 |
| BISSAU0811 | 50 | 11-1-2008 | 256 | 257 | 10.2773 | −17.1278 | 10.2650 | −17.1191 |
| BISSAU0811 | 52 | 1-11-2008 | 211 | 224 | 10.2371 | −16.5470 | 10.2229 | −16.5455 |
| BISSAU0811 | 56 | 2-11-2008 | 46 | 46 | 10.2056 | −16.2626 | 10.2196 | −16.2679 |
| BISSAU0811 | 58 | 2-11-2008 | 40 | 41 | 10.3112 | −16.2908 | 10.3268 | −16.2912 |
| BISSAU0811 | 59 | 3-11-2008 | 22 | 28 | 10.3256 | −15.5112 | 10.3117 | −15.5178 |
| BISSAU0811 | 61 | 3-11-2008 | 24 | 25 | 10.3070 | −16.1464 | 10.3222 | −16.1464 |
| BISSAU0811 | 65 | 3-11-2008 | 29 | 29 | 10.3788 | −16.2604 | 10.3894 | −16.2717 |
| BISSAU0811 | 69 | 4-11-2008 | 65 | 70 | 10.2802 | −16.3708 | 10.2915 | −16.3803 |
| BISSAU0811 | 77 | 6-11-2008 | 216 | 218 | 10.3777 | −17.0672 | 10.3922 | −17.0700 |
| BISSAU0811 | 87 | 8-11-2008 | 38 | 38 | 10.5251 | −16.4345 | 10.5384 | −16.4417 |
| BISSAU0811 | 96 | 9-11-2008 | 35 | 37 | 11.3696 | −17.0417 | 11.3652 | −17.0317 |
| BISSAU0811 | 99 | 10-11-2008 | 24 | 25 | 11.5635 | −17.0710 | 11.5787 | −17.0681 |
| BISSAU0811 | 100 | 10-11-2008 | 22 | 22 | 12.0127 | −17.0674 | 12.0278 | −17.0677 |
| CCLME2011 | 29 | 26-10-2011 | 130 | 141 | 10.2388 | −16.8323 | 10.2685 | −16.8585 |
| CCLME2011 | 44 | 28-10-2011 | 99 | 104 | 11.1583 | −17.2957 | 11.1923 | −17.3142 |
| LANGABISS0111 | 63 | 23-3-2011 | 197 | 197 | 10.1039 | −16.4427 | 10.2204 | −16.4954 |
| LANGABISS0111 | 82 | 27-3-2011 | 194 | 194 | 10.2103 | −16.4848 | 10.1200 | −16.4424 |
| LANGABISS0111 | 136 | 4-5-2011 | 17 | 17 | 11.2400 | −16.5905 | 11.1016 | −16.5747 |
| LANGABISS0111 | 203 | 19-4-2011 | 551 | 558 | 10.0100 | −17.1110 | 09.5173 | −17.0213 |
| LANGABISS0211 | 47 | 9-5-2011 | 243 | 245 | 10.1243 | −16.5853 | 10.0703 | −16.5321 |
| LANGABISS0211 | 51 | 10-5-2011 | 256 | 262 | 10.0657 | −16.5424 | 10.0143 | −16.4852 |
| LANGABISS0211 | 63 | 12-5-2011 | 48 | 48 | 11.3642 | −17.0852 | 11.3548 | −17.0955 |
| LANGABISS0211 | 65 | 12-5-2011 | 49 | 51 | 10.3603 | −17.0957 | 11.3548 | −17.0958 |
| LANGABISS0211 | 77 | 14-5-2011 | 203 | 207 | 10.1110 | −16.4848 | 10.1949 | −16.5106 |
| LANGABISS0211 | 101 | 18-5-2011 | 209 | 209 | 10.1621 | −16.5106 | 10.0744 | −16.4851 |
| LANGABISS0211 | 179 | 31-5-2011 | 26 | 37 | 11.4446 | −17.0742 | 11.3637 | −17.0427 |
| LANGABISS0211 | 229 | 8-6-2011 | 29 | 31 | 11.5325 | −17.0746 | 11.4601 | −17.0745 |
| LANGABISS0311 | 148 | 13-10-2011 | 549 | 686 | 12.1744 | −17.2637 | 12.0741 | −17.2425 |
| LANGABISS0311 | 160 | 15-10-2011 | 183 | 329 | 11.5920 | −17.2213 | 12.0700 | −17.2107 |
| CCLME2012 | 38 | 16-5-2012 | 144 | 148 | 10.4023 | −16.704 | 10.382 | −16.6877 |
| CCLME2012 | 43 | 18-5-2012 | 52 | 54 | 10.7518 | −16.7134 | 10.7722 | −16.7288 |
| CCLME2012 | 47 | 19-5-2012 | 173 | 176 | 10.9196 | −17.212 | 10.8972 | −17.1964 |
| CCLME2012 | 48 | 19-5-2012 | 62 | 65 | 10.9487 | −16.9875 | 10.9696 | −17.0035 |
| CCLME2012 | 49 | 19-5-2012 | 41 | 42 | 11.0166 | −16.8912 | 10.9959 | −16.8719 |
| CCLME2012 | 53 | 19-5-2012 | 730 | 738 | 11.5181 | −17.4045 | 11.5348 | −17.4036 |
| CCLME2012 | 56 | 20-5-2012 | 74 | 75 | 11.9418 | −17.2047 | 11.9315 | −17.203 |
| CCLME2012 | 65 | 21-5-2012 | 29 | 31 | 12.5706 | −17.361 | 12.5485 | −17.3472 |
| GB1219 | 1 | 22-11-2019 | 26 | 27 | 12.0241 | −17.0676 | 12.0092 | −17.0683 |
| GB1219 | 5 | 22-11-2019 | 43 | 44 | 11.3384 | −17.0617 | 11.3246 | −17.0684 |
| GB1219 | 6 | 22-11-2019 | 58 | 61 | 11.3375 | −17.1195 | 11.3529 | −17.1187 |
| GB1219 | 7 | 23-11-2019 | 110 | 110 | 12.0384 | −17.1949 | 12.0254 | −17.2015 |
| GB1219 | 10 | 23-11-2019 | 173 | 175 | 12.1391 | −17.2128 | 12.1249 | −17.2085 |
| GB1219 | 17 | 24-11-2019 | 71 | 76 | 12.0809 | −17.1429 | 12.0924 | −17.1528 |
| GB1219 | 18 | 24-11-2019 | 65 | 70 | 11.5462 | −17.1204 | 11.532 | −17.1163 |
| GB1219 | 20 | 25-11-2019 | 83 | 84 | 11.431 | −17.1412 | 11.4163 | −17.1438 |
| GB1219 | 29 | 26-11-2019 | 72 | 75 | 11.1366 | −17.1141 | 11.1214 | −17.1095 |
| GB1219 | 30 | 27-11-2019 | 49 | 49 | 11.2848 | −17.1108 | 11.2692 | −17.112 |
| GB1219 | 31 | 27-11-2019 | 42 | 42 | 11.2352 | −17.0751 | 11.2208 | −17.0809 |
| GB1219 | 32 | 27-11-2019 | 34 | 36 | 11.1939 | −17.0503 | 11.1792 | −17.0462 |
| GB1219 | 33 | 27-11-2019 | 49 | 49 | 11.1456 | −17.0852 | 11.1321 | −17.0795 |
| GB1219 | 36 | 27-11-2019 | 45 | 46 | 10.5349 | −16.4641 | 10.5232 | −16.4535 |
| GB1219 | 39 | 28-11-2019 | 43 | 44 | 10.3612 | −16.3174 | 10.3504 | −16.3063 |
| GB1219 | 41 | 28-11-2019 | 28 | 29 | 10.3439 | −16.1925 | 10.333 | −16.1822 |
| GB1219 | 46 | 29-11-2019 | 164 | 165 | 10.2853 | −16.4811 | 10.2738 | −16.4707 |
| GB1219 | 82 | 7-12-2019 | 56 | 56 | 11.2521 | −17.1381 | 11.2376 | −17.1327 |
| GB1219 | 83 | 7-12-2019 | 71 | 75 | 11.4632 | −17.1381 | 11.4754 | −17.1291 |
| GB1219 | 84 | 7-12-2019 | 102 | 103 | 11.5146 | −17.1792 | 11.5298 | −17.178 |
| GB1219 | 85 | 8-12-2019 | 105 | 106 | 12.0617 | −17.1721 | 12.0461 | −17.1762 |
| GB1219 | 87 | 8-12-2019 | 26 | 31 | 11.4645 | −17.0721 | 11.4492 | −17.0769 |
References
- Wenner, E.L.; Boesch, D.F. Distribution Patterns of Epibenthic Decapod Crustacea along the Shelf-Slope Coenocline, Middle Atlantic Bight, U.S.A. Bull. Biol. Soc. Wash. 1979, 3, 106–133. [Google Scholar]
- Cartes, J.E. Feeding Strategies and Partition of Food Resources in Deep-Water Decapod Crustaceans (400–2300 m). J. Mar. Biol. Assoc. UK 1998, 78, 509–524. [Google Scholar] [CrossRef]
- Davie, P.J.F. Crabs: A Global Natural History; Princeton University Press: Princeton, NJ, USA, 2021; p. 224. ISBN 978-0-691-23013-9. [Google Scholar]
- Lee, S.; Lee, C.; Noh, J.; Song, S.J.; Khim, J.S. First Comprehensive Ecological Checklist of Brachyura in Korea: 1879–2020. Mar. Pollut. Bull. 2021, 171, 112742. [Google Scholar] [CrossRef]
- Tsang, L.M.; Schubart, C.D.; Ahyong, S.T.; Lai, J.C.Y.; Au, E.Y.C.; Chan, T.-Y.; Ng, P.K.L.; Chu, K.H. Evolutionary History of True Crabs (Crustacea: Decapoda: Brachyura) and the Origin of Freshwater Crabs. Mol. Biol. Evol. 2014, 31, 1173–1187. [Google Scholar] [CrossRef]
- Bánki, O.; Roskov, Y.; Döring, M.; Ower, G.; Hernández Robles, D.R.; Plata Corredor, C.A.; Stjernegaard Jeppesen, T.; Örn, A.; Vandepitte, L.; Hobern, D.; et al. Catalogue of Life Checklist (Version 2024-01-24). Catalogue of Life. 2024. Available online: https://www.catalogueoflife.org/data/metadata (accessed on 17 January 2024).
- Takeda, M.; Ahyong, S.T.; Ohtsuchi, N.; Komatsu, H. Crabs (Crustacea, Decapoda) from the Seas of East and Southeast Asia Collected by the RV Hakuhō Maru (KH-72-1 Cruise), 4. South China Sea. Bull. Natl. Mus. Nat. Sci. 2022, 48, 147–191. [Google Scholar] [CrossRef]
- Matos-Pita, S.; Castillo Oñate, S.; Ramil, F. Contribution to the Knowledge of the Deep Brachyuran Fauna (Crustacea: Decapoda) in Waters off Mauritania (NW Africa). J. Mar. Biol. Assoc. UK 2016, 1, 1–33. [Google Scholar] [CrossRef]
- Padate, V.; Cubelio, S.; Takeda, M. Rare Deep-Water Crabs (Crustacea: Decapoda) from Indian Waters, with Description of One New Species. Nauplius 2021, 29, 1–21. [Google Scholar] [CrossRef]
- Muñoz, I.; García-Isarch, E.; Sobrino, I.; Burgos, C.; Funny, R.; González-Porto, M. Distribution, Abundance and Assemblages of Decapod Crustaceans in Waters off Guinea-Bissau (North-West Africa). J. Mar. Biol. Assoc. UK 2012, 92, 475–494. [Google Scholar] [CrossRef]
- Muñoz, I.; García-Isarch, E.; Cuesta, J. Annotated and Updated Checklist of Marine Crabs (Decapoda: Brachyura) of Mozambique Supported by Morphological and Molecular Data from Shelf and Slope Species of the “MOZAMBIQUE” Surveys. Zootaxa 2021, 5056, 1–67. [Google Scholar] [CrossRef]
- Manning, R.B.; Holthuis, L.B. West African Brachyuran Crabs. Smithson. Contrib. Zool. 1981, 306, 1–379. [Google Scholar] [CrossRef]
- Capart, A. Crustacés Décapodes Brachyures; Expédition Océan. Belge Dans Eaux Côtières Afr. L’Atlantique Sud 1948–1949 Résultats Sci; Institut Royal des Sciences Naturelles de Belgique: Bruxelles, Belgium, 1951; Volume 3, pp. 11–205. [Google Scholar]
- Monod, T. Hippidea et Brachyura Ouest-Africains; Memoires de l’Institut francais d’Afrique noire; IFAN: Dakar, Senegal, 1956; Volume 45, pp. 1–674. [Google Scholar]
- Forest, J.; Guinot, D. Crustacés Décapodes: Brachyoures. In Campagne de La Calypso Dans Le Golfe de Guinée et Aux Îles Principe, São Tomé, et Annobon (1956); Résultats Scientifiques Des Campagnes de La “Calypso”; Fascicule VII, 16; l’Institut océanographique de Monaco: Monaco-Ville, Monaco, 1966; Volume 44, pp. 23–124. [Google Scholar]
- Fransen, C.H.J.M. Preliminary Report on Crustacea Collected in the Eastern Part of the North Atlantic during the CANCAP and Mauritania Expeditions of the Former Rijksmuseum van Natuurlijke Historie, Leiden; Natural Historie Museum: Leiden, The Netherlands, 1991; 200p. [Google Scholar]
- Rathbun, M.J. The Decapod Crustaceans of West Africa. Proc. U. S. Natl. Mus. 1900, 22, 271–316. [Google Scholar] [CrossRef]
- González, J.A. Checklists of Crustacea Decapoda from the Canary and Cape Verde Islands, with an Assessment of Macaronesian and Cape Verde Biogeographic Marine Ecoregions. Zootaxa 2018, 4413, 401. [Google Scholar] [CrossRef]
- Guinot, D.; Ribeiro, A. Sur Une Collection de Crustacés Brachyoures Des Îles Du Cap-Vert de l’Angola; Memórias da Junta de Investigacões do Ultramar: Lisboa, Portugal, 1962; Volume 40, pp. 9–94. [Google Scholar]
- Macpherson, E. Biogeography and Community Structure of the Decapod Crustacean Fauna off Namibia (Southeast Atlantic). J. Crustac. Biol. 1991, 11, 401–415. [Google Scholar] [CrossRef]
- Macpherson, E. New Records of Decapods Crustaceans from the Coast off Namibia. South West Africa, with the Descriptions of Two New Species. Investig. Pesq. 1988, 52, 51–66. [Google Scholar]
- Macpherson, E. Crustáceos Decápodos capturados en las costas de Namibia. Result. Exped. Científicas Suppl. Investig. Pesq. 1983, 11, 3–80. [Google Scholar]
- Rossignoli, M. Catalogue de Crustaces Decapodes Brachyoures, Anomoures et Macroures Littoraux En Collection Au Centre d’Oceanographie de Pointe-Noire. Trauaux Cent. Oceanogr. Pointe-Noire 1962, 2, 111–138. [Google Scholar]
- Marco-Herrero, E.; Abello, P.; Drake, P.; García Raso, J.; González-Gordillo, J.; Guerao, G.; Palero, F.; Cuesta, J. Annotated Checklist of Brachyuran Crabs (Crustacea: Decapoda) of the Iberian Peninsula (SW Europe). Sci. Mar. 2015, 79, 243–256. [Google Scholar] [CrossRef]
- Yasser, A.; Naser, M. Checklist of the Marine Brachyura of the North Western Persian-Arabian Gulf, with the New Record of Phalangipus persicus Griffin, 1973 (Crustacea: Brachyura: Epialtidae MacLeay, 1838) from the Iraqi Coast. Acta Aquat. Turc. 2021, 17, 525–531. [Google Scholar]
- Mantelatto, F.; Tamburus, A.; Magalhães, T.; Buranelli, R.; Terossi, M.; Negri, M.; Castilho, A.; Costa, R.; Zara, F. Monograph Checklist of Decapod Crustaceans from the Coast of the São Paulo State (Brazil) Supported by Integrative Molecular and Morphological Data: III. Infraorder Brachyura Latreille, 1802. Zootaxa 2020, 4872, 1–108. [Google Scholar] [CrossRef]
- Mantelatto, F.; Tamburus, A.; Batista, A.; Rossi, N.; Buranelli, R.; Pantaleão, J.; Teles, J.; Zara, F.; Carvalho, F.; Bochini, G.; et al. Checklist of Decapod Crustaceans from the Coast of the So Paulo State (Brazil) Supported by Integrative Molecular and Morphological Data: V. Dendrobranchiata and Pleocyemata [Achelata, Astacidea, Axiidea, Caridea (Alpheoidea and Processoidea Excluded), Gebiidea, Stenopodidea]. Zootaxa 2022, 5121, 1–74. [Google Scholar] [CrossRef]
- Sasaki, J. The Species List of Decapoda, Euphausiacea, and Stomatopoda, All of the World. Version. 07-8.12-Worldwide Species List of Decapoda, Euphausiacea and Stomatopoda—2023; Local Independent Administrative Agency Hokkaido Research Organization, Resources Management and Enhancement Division, Abashiri Fisheries Research Institute, Fisheries Research Department: Hokkaido, Japan, 2023; 17923p. [Google Scholar] [CrossRef]
- Davie, P.J.F.; Guinot, D.; Ng, P.K.L. Systematics and Classification of Brachyura. In Treatise on Zoology—Anatomy, Taxonomy, Biology. The Crustacea, 9 C (I), Decapoda: Brachyura; Castro, P., Davie, P.J.F., Guinot, D., Schram, F., Von Vaupel Klein, J.C., Eds.; Part 2; Brill: Leiden, The Netherlands; Boston, MA, USA, 2015; pp. 1049–1130. [Google Scholar]
- DecaNet (Ed.) DecaNet. 2023. Available online: https://www.Decanet.Info (accessed on 30 October 2023). [CrossRef]
- Muñoz, I.; Serrano, M.; García, E. Colección de Crustáceos Marinos (CRUST-IEOCD). Version 1.30. Instituto Español de Oceanografía. Centro Oceanográfico de Cádiz (CSIC). Occurrence Dataset. 2023. Available online: https://www.gbif.org/dataset/317fd0a9-1bcf-4e94-bc3c-a685c4693c10 (accessed on 30 October 2023).
- Guerrero, E.; Abelló, P.; Lombarte, A.; Villanueva, R.; Ramón, M.; Sabatés, A.; Santos, R. Marine Biological Reference Collections: CBMR-General (ICM-CSIC). Version 1.31. Institut de Ciències Del Mar (CSIC). Occurrence Dataset. 2023. Available online: https://ipt.gbif.es/resource?r=cbr-icm (accessed on 30 October 2023).
- Santos-Bethencourt, R.; Guerrero, E.; Lombarte, A.; Abelló, P. Zariquiey Marine Crustacean Legacy Collection. CBMR-Zariquiey (ICM-CSIC). Version 1.14. Institut de Ciències Del Mar (CSIC). Occurrence Dataset. 2023. Available online: https://www.gbif.org/dataset/791a7459-7b58-444c-af46-84db6f8576ef (accessed on 30 October 2023).
- Estoup, A.C.R.L.; Largiader, C.R.; Perrot, E.; Chourrout, D. Rapid One-Tube DNA Extraction for Reliable PCR Detection of Fish Polymorphic Markers and Transgenes. Mol. Mar. Biol. Biotechnol. 1996, 5, 295–298. [Google Scholar]
- Crandall, K.A.; Fitzpatrick, J.F.J. Crayfish Molecular Systematics: Using a Combination of Procedures to Estimate Phylogeny. Syst. Biol. 1996, 45, 1–26. [Google Scholar] [CrossRef]
- Schubart, C.D.; Cuesta, J.A.; Felder, D.L. Glyptograpsidae, a New Brachyuran Family from Central America: Larval and Adult Morphology, and a Molecular Phylogeny of the Grapsoidea. J. Crust. Biol. 2002, 22, 28–44. [Google Scholar] [CrossRef]
- Schubart, C.D.; Diesel, R.; Hedges, S.B. Rapid Evolution to Terrestrial Life in Jamaican Crabs. Nature 1998, 393, 363–365. [Google Scholar] [CrossRef]
- Schubart, C.D.; Huber, M.G.J. Genetic Comparisons of German Populations of the Stone Crayfish, Austropotamobius torrentium (Crustacea: Astacidae). Bull. Fr. Pêche Piscic. 2006, 380–381, 1019–1028. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome C Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Geller, J.; Meyer, C.; Parker, M.; Hawk, H. Redesign of PCR Primers for Mitochondrial Cytochrome c Oxidase Subunit I for Marine Invertebrates and Application in All-Taxa Biotic Surveys. Mol. Ecol. Resour. 2013, 13, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.P.; Paulay, G. DNA Barcoding: Error Rates Based on Comprehensive Sampling. PLoS Biol. 2005, 3, e422. [Google Scholar] [CrossRef]
- Forest, J. Les Dromies de l’Atlantique Oriental. Description de Sternodromia gen. nov. et Deux Espèces Nouvelles Du Genre Dromia Weber (Crustacea Decapoda Dromiidae). Ann. Inst. Océan. 1974, 50, 71–123. [Google Scholar]
- Mantelatto, F.; Terossi, M.; Negri, M.; Buranelli, R.; Robles, R.; Magalhães, T.; Tamburus, A.; Rossi, N.; Miyazaki, M. DNA Sequence Database as a Tool to Identify Decapod Crustaceans on the São Paulo Coastline. Mitochondrial DNA Parte A 2018, 29, 805–815. [Google Scholar] [CrossRef]
- Maurin, C. Les Crustaces Captures Par La “Thalassa” Au Large Des Cotes Nord-Ouest Africaines. Rev. Roum. Biol. Ser. Zool. 1968, 13, 479–493. [Google Scholar]
- Pastore, M.A. Decapoda Crustacea in the Gulf of Taranto and the Gulf of Catania with a Discussion of a New Species of Dromidae (Decapoda Brachyoura) in the Mediterranean Sea. Thalass. Jugosl. 1972, 8, 105–117. [Google Scholar]
- Matzen da Silva, J.; Creer, S.; dos Santos, A.; Costa, A.C.; Cunha, M.R.; Costa, F.O.; Carvalho, G.R. Systematic and Evolutionary Insights Derived from mtDNA COI Barcode Diversity in the Decapoda (Crustacea: Malacostraca). PLoS ONE 2011, 6, e19449. [Google Scholar] [CrossRef] [PubMed]
- Barnard, K.H. Descriptive Catalogue of South African Decapod Crustacea (Crabs and Shrimps). Ann. S. Afr. Mus. 1950, 38, 1–837. [Google Scholar]
- Gordon, I. Crustacea: Dromiacea, Part I: Systematic Account of the Dromiacea Collected by the “John Murray” Expedition. Part II: The Morphology of the Spermatheca in Certain Dromiacea. Sci. Rep. John Murray Exped. 1950, 9, 201–253. [Google Scholar]
- Zariquiey Álvarez, R. Crustáceos Decápodos Ibéricos. Investig. Pesq. 1968, 32, 1–510. [Google Scholar]
- Abelló, P.; Carbonell, A.; Torres, P. Biogeography of Epibenthic Crustaceans on the Shelf and Upper Slope off the Iberian Peninsula Mediterranean Coasts: Implications for the Establishment of Natural Management Areas. Sci. Mar. 2002, 66, 183–198. [Google Scholar] [CrossRef]
- González, J.A.; Triay-Portella, R.; Martins, A.; Lopes, E. Checklist of Brachyuran Crabs (Crustacea: Decapoda) from the Cape Verde Islands, with a Biogeographic Comparison with the Canary Islands (Eastern Atlantic). Cah. Biol. Mar. 2017, 58, 137–151. [Google Scholar] [CrossRef]
- Studer, T. Verzeichnis Der Während Der Crustaceen, Welche Während Der Reise S.M.S. “Gazelle” an Der Westküste von Afrika, Ascension Un Dem Cap Der Guten Hoffnung Gesammelten Crustaceen. Abh. K. Akad. Wiss. Berl. 1883, 2, 1–32. [Google Scholar]
- Longhurst, A.R. An Ecological Survey of the West African Marine Benthos. Colon. Off. Fish. Publ. 1958, 11, 1–102. [Google Scholar]
- Voss, G.L. Narrative of the Cruises: The R/V Pillsbury DeepSea Biological Expedition to the Gulf of Guinea, 1964–1965; Studies In Tropical Oceanography; University of Miami: Miami, FL, USA, 1966; pp. 1–60. [Google Scholar]
- Guinot, D. Recherches Préliminaires Sur Les Groupements Naturels Chez Les Crustacés. Décapodes Brachyoures. I. Les Affinités Des Genres Aethra, Osachila, Hepatus. Bull. Mus. Natl. Hist. Nat. 1967, 38, 828–845. [Google Scholar]
- Neves, K.; Susana, S.; Ramil, F.; Ramos, A. Contribution to the Knowledge of the Decapod Fauna from Cabo Verde Islands. Front. Mar. Sci. 2016, 3. [Google Scholar] [CrossRef]
- Monod, T. Sur La Présence Du Genre Acanthocarpus Dans l’Atlantique Oriental. Publicações Inst. Zool. Porto 1946, 32, 7–10. [Google Scholar]
- Ewers-Saucedo, C.; Wares, J.P.; Hanel, R.; Brandis, D. Evolution of Male Copulatory Organs in Box Crabs (Decapoda: Eubrachyura: Calappidae De Haan, 1833). J. Crust. Biol. 2016, 36, 804–814. [Google Scholar] [CrossRef]
- Galil, B.S. 9. Crustacea Decapoda: A revision of the Indo-Pacific species of the genus Calappa Weber, 1795 (Calappidae). In Résultats des Campagnes MUSORSTOM 18; Crosnier, A., Ed.; Mémoires du Muséum national d’histoire naturelle, Série A: Zoologie; Paris, Éd. du Muséum: Paris, France, 1993; Volume 176, pp. 271–335. [Google Scholar]
- Manning, R.B.; Chace, F.A. Decapod and stomatopod Crustacea from Ascension Island, South Atlantic Ocean. Smithson. Contr. Zool. 1990, 503, 1–91. [Google Scholar] [CrossRef]
- Tavares, M.; Mendoza, J.B.J. Brachyuran crabs (Crustacea, Decapoda) from the remote oceanic Archipelago Trindade and Martin Vaz, South Atlantic Ocean. Zootaxa 2022, 5146, 1–129. [Google Scholar] [CrossRef]
- WoRMS Editorial Board. World Register of Marine Species. 2024. Available online: https://www.marinespecies.org/imis.php?dasid=1447&doiid=170 (accessed on 11 January 2024).
- GBIF.org, GBIF Home Page. 2024. Available online: https://www.gbif.org (accessed on 11 January 2024).
- Venera-Pontón, D.; Driskell, A.; Grave, S.D.; Felder, D.; Scioli, J.; Collin, R. Documenting Decapod Biodiversity in the Caribbean from DNA Barcodes Generated during Field Training in Taxonomy. Biodivers. Data J. 2020, 8, e47333. [Google Scholar] [CrossRef]
- Pastore, M. The genus Calappa in the Ionian Sea. Oebalia 1995, 21, 187–196. [Google Scholar]
- Gauld, D.T. Brachyura: An Annotated Check-List of the Crustacea of Ghana, IV. J. West Afr. Sci. Assoc. 1960, 6, 68–72. [Google Scholar]
- Johnston, H. List of Invertebrate Animals of Liberia Founded on the Collections of Bittikofer, Reynolds, Whicker, H.H. Johnston, A. White, Etc. In Liberia with Anappendix on the Flora of Libera by Dr. O. Stapf.; Johnston, H., Ed.; Hutchinson & Co: London, UK, 1906; Volume 2, pp. 860–883. [Google Scholar]
- Le Loeuff, P.; Intes, A. Premieres Observations Sur La Faune Benthique Du Plateau Continental de Cote d’lvoire. Cah. ORSTOM Ser. Oceanogr. 1969, 7, 61–66. [Google Scholar]
- Uschakov, P.V. Observations Sur La Repartition de La Faune Benthique Du Littoral Guineen. Cah. Biol. Mar. 1970, 11, 435–457. [Google Scholar]
- Bouvier, E.-L. Crustacés et Pycnogonides. Act. Soc. Linn. Bordeaux 1910, 64, 221–226. [Google Scholar]
- Sin, Y.; Lai, J.; Ng, P.; Chu, K.H. Phylogeny of Dorippoidea (Crustacea: Decapoda: Brachyura) Inferred from Three Mitochondrial Genes. Invertebr. Syst. 2009, 23, 223–230. [Google Scholar] [CrossRef]
- Milne-Edwards, A.; Bouvier, E.-L. Crustacés Nouveaux Provenant Des Campagnes Du Travailleur et Du Talisman. Bull. Mus. Natl. Hist. Nat. 1897, 3, 364–367. [Google Scholar]
- Bouvier, E.-L. Observations Complémentaires Sur Les Crustacés Décapodes (Abstraction Faite Les Carides) Provenant Des Campagnes de S. A. S. Le Prince de Monaco. Rés. Camp. Sci. Accompl. Sur Son Yacht Par Albert 1er Prince Souver. Monaco 1922, 62, 1–106. [Google Scholar]
- González, J.A. Catálogo de Los Crustáceos Decápodos de Las Islas Canarias; Publicaciones Turquesa: St. Cruz Tenerife, Spain, 1995; 282p. [Google Scholar]
- Doflein, F. Brachyura. In Wissenschaftliche Ergebnisse Der Deutschen Tiefsee-Expedition Auf Dem Dampfer “Valdivia” 1898–1899; Band VI. Gustav Fischer: Jena, German, 1904; 314p. [Google Scholar]
- Castro, P.; Ng, P.K.L. Revision of the Family Euryplacidae Stimpson, 1871 (Crustacea: Decapoda: Brachyura: Goneplacoidea). Zootaxa 2010, 2375, 1. [Google Scholar] [CrossRef]
- Crosnier, A. Fonds de pêche le long des cotes de la République Fédérale du Cameroun. Cahiers ORSTOM.Série Océanographie (Nº Sp.) 1964, 1–133. Available online: https://www.documentation.ird.fr/hor/fdi:19465 (accessed on 11 January 2024).
- Guinot, D. Recherches Préliminaires Sur Les Groupements Naturels Chez Les Crustacés Décapodes Brachyoures. VII. Les Goneplacidae. Bull. Mus. Natl. Hist. Nat. 1969, 41, 241–265. [Google Scholar]
- Galil, B.S. An Examination of the Genus Philyra Leach, 1817 (Crustacea, Decapoda, Leucosiidae) with Descriptions of Seven New Genera and Six New Species. Zoosystema 2009, 31, 279–320. [Google Scholar] [CrossRef]
- Loeuff, P.L.; Intès, A. La Faune Benthique Du Plateau Continental de Côte d’Ivoire: Récoltes Au Chalut-Abondance-Répartition-Variations Saisonnières Mars 1966-Février 1967; Doc. Sci. provisoire—Cent. Rech. Océanograph; ORSTOM: Abidjan, Côte d’Ivoire, 1968; Volume 25, pp. 1–32. [Google Scholar]
- Milne-Edwards, A.; Bouvier, E.L. Crustacés Nouveaux Provenant Des Campagnes Du Travailleur et Du Talisman. Bull. Mus. Natl. Hist. Nat. 1898, 4, 32–35. [Google Scholar]
- Milne-Edwards, A.; Bouvier, E.-L. Crustacés Décapodes Provenant Des Campagnes Du Yacht l’Hirondelle (1886, 1887, 1888). I. Brachyures et Anomoures. Rés. Camp. Sci. Accompl. Sur Son Yacht Par Albert Ier Prince Souver Monaco 1894, 7, 3–112. [Google Scholar]
- Milne-Edwards, A.; Bouvier, E.-L. Crustacés Décapodes. Ptie. 1: Brachyures et Anomoures; Masson: Paris, France, 1900; 366p. [Google Scholar]
- Ng, P.K.L.; Guinot, D.; Davie, P. Systema Brachyurorum: Part I. An Annotated Checklist of Extant Brachyuran Crabs of the World. Raffles Bull. Zool. 2008, 17, 1–296. [Google Scholar]
- Lee, B.Y.; Richer De Forges, B.; Ng, P.K.L. Revision of the Deep-Water Spider Crab Genus, Scyramathia A. Milne-Edwards, 1880, with the Description of a New Species from the Mediterranean and Notes on Rochinia A. Milne-Edwards, 1875, and Anamathia Smith, 1885 (Crustacea, Decapoda, Brachyura, Epialtidae). Zoosystematics Evol. 2020, 96, 537–569. [Google Scholar] [CrossRef]
- Muñoz, I.; García-Raso, J.E.; Gónzalez, J.; Lopes, E.; Santos, A.; Cuesta, J. Taxonomic Revision and Molecular Phylogeny of Pisa (Decapoda: Majoidea: Epialtidae), Including the Description of a New Genus of Pisinae. Sci. Mar. 2023, 87, 1–23. [Google Scholar] [CrossRef]
- Colavite, J.; Windsor, A.; Santana, W. Three New Species and a New Genus of Majoid Crabs from the Eastern Pacific (Decapoda, Brachyura). ZooKeys 2019, 825, 1–24. [Google Scholar] [CrossRef]
- Miers, E.J. Descriptions of New or Little Known Species of Maioid Crustacea (Oxyrhyncha) in the Collection of the British Museum. Ann. Mag. Nat. Hist. Zool. Bot. Geol. 1879, 4, 1–28. [Google Scholar] [CrossRef]
- González-Gordillo, J.; Cuesta, J.; Pablos, J.A. Adiciones al Conocimiento de Los Crustáceos Decápodos de Las Zonas Mediolitoral e Infralitoral de Las Costas Suratlánticas Andaluzas (S.O. España). Cah. Biol. Mar. 1990, 31, 417–429. [Google Scholar]
- Anadón, R. Crustáceos Decápodos (Excl. Paguridea) Recogidos Durante La Campaña «Atlor VII» En Las Costas Noroccidentales de África (Noviembre 1975). Res. Exp. Cient. 1981, 9, 151–159. [Google Scholar]
- Rathbun, M.J. A Revision of the Nomenclature of the Brachyura. Proc. Biol. Soc. Wash. 1897, 11, 153–167. [Google Scholar]
- Rossignoli, M. Crustacés Décapodes Marins de La Région de Pointe-Noire. In Mollusques, Crustacés, Poissons Marins Des Côtes d’A.E.F. [= Afrique Equatoriale Française] En Collection Au Centre d’Océanographie de l’Institut d’Études Centrafricaines de Pointe-Noire; Collignon, J., Rossignol, M., Roux, C., Eds.; Larose: Paris, France, 1957; pp. 71–136. [Google Scholar]
- Crosnier, A. Crustacés Décapodes Brachyoures et Macroures Recueillis Par l’«Undaunted » Au Sud de l’Angola. Description de Scyllarus subarctus sp. nov. Bull. Mus. Natl. Hist. Nat. 1970, 41, 1214–1227. [Google Scholar]
- Forest, J.; Gantès, H. Sur une collection de Crustacés Décapodes Marcheurs du Maroc. Bull. Mus. Natl. Hist. Nat. 1960, 32, 346–358. [Google Scholar]
- García-Raso, J.E.; González-Ortegón, E.; Palero, F.; Cuesta, J.A. A New Cryptic Species of Inachus (Decapoda: Brachyura: Inachidae) from European Waters and an Updated Identification Key to the Species of Inachus with Two Protogastric Tubercles. J. Crust. Biol. 2022, 42, ruac035. [Google Scholar] [CrossRef]
- Cuesta, J.; Almón, B.; Pérez, J.; Trigo, J.; Bañón, R. Role of Ships’ Hull Fouling and Tropicalization Process on European Carcinofauna: New Records in Galician Waters (NW Spain). Biol. Invasions 2016, 18, 619–630. [Google Scholar] [CrossRef]
- Forest, J. Le Genre Macropodia Leach dans les eaux Atlantiques Européennes (Crustacea Brachyura Majidae). Cah. Biol. Mar. 1978, 19, 323–342. [Google Scholar]
- Henriksen, C.S. Investigation of Crustaceans from Shelf Areas in the Gulf of Guinea, with Special Emphasis on Brachyura. Master Thesis, University of Bergen, Bergen, Norway, 2009; 163p. [Google Scholar]
- Udekem D’Acoz, C. D’. Inventaire et Distribution des Crustacés Décapodes de l’Atlantique nord-Oriental, de la Méditerranée et des eaux Continentales Adjacentes au nord de 25°N; Patrimoines naturels, 40; Muséum National d’Histoire Naturelle: Paris, France, 1999; 383p. [Google Scholar]
- Yang, W.T. A Study of Zoeal, Megalopal, and Early Crab Stages of Some Oxyrhynchous Crabs (Crustacea: Decapoda). Ph.D. Thesis, University of Miami, Miami, FL, USA, 1967. [Google Scholar]
- García-Raso, J.E. Resultados de La Segunda Campaña Del LE.O. Para La Exploración de Los Fondos de Coral Rojo En El Mar de Alborán. Crustáceos Decápodos. Bol. Inst. Esp. Ocenogr. 1989, 5, 2–36. [Google Scholar]
- Kensley, B. On the Zoogeography of Southern African Decapod Crustacea, with a Distributional Checklist of the Species. Smithson. Contrib. Zool. 1981, 338, 1–64. [Google Scholar] [CrossRef]
- Türkay, M. Decapoda Reptantia von der portugisischen und marokkanischen Küste: Auswertung der Fahrten 8, 9c (1967), 19 (1970), 23 (1971) und 36 (1975) von F.S. “Meteor”. Meteor Forschungsergebnisse Reihe Biol. 1976, 23, 23–44. [Google Scholar]
- Tan, S.H.; Ng, P.K.L. Descriptions of New Genera from the Subfamily Parthenopinae (Crustacea: Decapoda: Brachyura: Parthenopidae). Raffles Bull. Zool. 2007, 16, 95–119. [Google Scholar]
- Sourie, R. Contribution à l’étude Écologique Des Côtes Rocheuses Du Sénégal; Mémoires de l’Institut française d’Afrique noire; IFAN: Dakar, Senegal, 1954; 342p. [Google Scholar]
- Türkay, M. Krebse Erzählen Eine Klimageschichte. Senckenberg. Nat. Forsch. Mus. 2015, 145, 202–211. [Google Scholar]
- Miers, E.J. On a Collection of Crustacea Made by Baron Hermann Maltzam [Sic] at Goree Island, Senegambia. Ann. Mag. Nat. Hist. 1881, 8, 204–220. [Google Scholar] [CrossRef]
- Monod, T. Sur Quelques Crustacés de l’Afrique Occidentale (Liste Des Décapodes Mauritaniens et Des Xanthidés Ouest-Africains). Bull. Com. Étud. Hist. Sci. Afr. Occ. Fr. 1933, 15, 456–548. [Google Scholar]
- Marco-Herrero, E.; González-Gordillo, J.; Cuesta, J. Larval Morphology of the Family Parthenopidae, with the Description of the Megalopa Stage of Derilambrus angulifrons (Latreille, 1825) (Decapoda: Brachyura), Identified by DNA Barcode. J. Mar. Biol. Assoc. UK 2014, 95, 1–9. [Google Scholar] [CrossRef]
- Crosnier, A. Remarques Sur Quelques Crustacés Décapodes Benthiques Ouest-Africains. Description de Heteropanope acanthocarpus et Medaeus rectifrons spp. nov. Bull. Mus. Natl. Hist. Nat. 1967, 39, 320–344. [Google Scholar]
- Hernández, M.; Martín, M.V.; Herrador-Gómez, P.M.; Jiménez, S.; Hernández-González, C.; Barreiro, S.; Sarralde, R.; van Zyl, B.J.; Gamatham, J.C.; Almeida, T.; et al. Mitocondrial COI and 16S rDNA Sequences Support Morphological Identification and Biogeography of Deep-Sea Red Crabs of the Genus Chaceon (Crustacea, Decapoda, Geryonidae) in the Eastern Central and South Atlantic Ocean. PLoS ONE 2019, 14, e0211717. [Google Scholar] [CrossRef]
- Manning, R.B.; Holthuis, L.B. Two New Genera and Nine New Species of Geryonid Crabs (Crustacea, Decapoda, Geryonidae). Proc. Biol. Soc. Wash. 1989, 102, 50–77. [Google Scholar]
- Christiansen, M.E. Crustacea Decapoda Brachyura; Marine invertebrates of Scandinavia; Universitetsforlaget: Oslo, Norway, 1969. [Google Scholar]
- Forest, J. Sur Une Crevette Recueillie Au Cours de La Campagne de Chalutage Dans Le Golfe de Guinée, Plesionika williamsi sp. nov. Bull. Mus. Natl. Hist. Nat. 1963, 35, 620–629. [Google Scholar]
- Rathbun, M.J. The Oxystomatous and Allied Crabs of America. Bull. U. S. Natl. Mus. 1937, 166, 1–272. [Google Scholar] [CrossRef]
- Evans, N. Molecular Phylogenetics of Swimming Crabs (Portunoidea Rafinesque, 1815) Supports a Revised Family-Level Classification and Suggests a Single Derived Origin of Symbiotic Taxa. PeerJ 2018, 6, e4260. [Google Scholar] [CrossRef] [PubMed]
- Schubart, C.; Reuschel, S. A Proposal for a New Classification of Portunoidea and Cancroidea (Brachyura: Heterotremata) Based on Two Independent Molecular Phylogenies. In Decapod Crustacean Phylogenetics; Crustac. Issues; Martin, J., Crandall, K., Felder, D., Eds.; CRC Press: Boca Raton, FL, USA, 2009; Volume 18, pp. 533–549. [Google Scholar]
- Plagge, C.; Son, N.T.; Ng, P.L.K.; Türkay, M.; Streit, B. Liocarcinus corrugatus (Pennant, 1777) (Crustacea: Brachyura: Portunidae): A Cosmopolitan Brachyuran Species? RAFFLES Bull. Zool. 2016, 374–388. [Google Scholar]
- Guinot, D. Caractères et affinités de Macropipus australis sp. nov., Crustacé Décapode Brachyoure de la côte sud-ouest Africaine. Bull. Inst. R. Sci. Nat. Belg. 1961, 37, 1–13. [Google Scholar]
- Williams, A.B. The swimming crabs of the genus Callinectes. Fish. Bull. 1974, 72, 685–798. [Google Scholar]
- de Rochebrune, A.T. Diagnoses d’Arthropodes Nouveaux Propres à La Sénégambie. Bull. Soc. Phil. Paris Sér 1883, 7, 167–182. [Google Scholar]
- Longhurst, A.R. The Food of the Demersal Fish of a West African Estuary. J. Anim. Ecol. 1957, 26, 369–387. [Google Scholar] [CrossRef]
- Ribeiro, A. Crustáceos Decapodos Braquiuros Do Arquipélago (Coleção Do Centro de Biologia Piscatoria). Nolas Mimeografadas Cent. Biot. Piscatória Lisb. 1964, 38, 1–27. [Google Scholar]
- Marco-Herrero, E.; Cuesta, J.A.; González-Gordillo, J.I. DNA Barcoding Allows Identifcation of Undescribed Crab Megalopas from the Open Sea. Sci. Rep. 2021, 11, 20573. [Google Scholar] [CrossRef]
- Bayer, F.M. The R/V Pillsbury Deep-Sea Biological Expedition to the Gulf of Guinea, 1964–1965: 3. Dredging and Trawlling Records of R/V John Elliott Pillsbury for 1964 and 1965; Studies In Tropical Oceanography; University of Miami: Miami, FL, USA, 1966; Volume 4, pp. 82–105. [Google Scholar]
- Gruvel, A. L’industrie Des Pêches Sur La Côte Occidentale d’Afrique (Du Cap Blanc Au Cap de Bonne-Espérance); Emile Laros: Paris, France, 1913; 193p. [Google Scholar]
- Irvine, F.R. Gold Coast Crabs and Lobsters; Nature Study Leaflets; Director of Education, Accra, Gold Coast Colony: Accra, Ghana, 1932; 20p. [Google Scholar]
- Robles, R.; Schubart, C.D.; Conde, J.E.; Carmona-Suárez, C.; Alvarez, F.; Villalobos, J.L.; Felder, D.L. Molecular Phylogeny of the American Callinectes Stimpson, 1860 (Brachyura: Portunidae), Based on Two Partial Mitochondrial Genes. Mar. Biol. 2007, 150, 1265–1274. [Google Scholar] [CrossRef]
- Ejike, C. Macrofibres in the Cuticle of the Crab Callinectes gladiator (Benedict). Zool. J. Linn. Soc. 1973, 53, 253–255. [Google Scholar] [CrossRef]
- Frade, F. Notas de Zoogeografia e de Historia das Explorajoes Faunisticas da Guine Portuguesa. Junta Investig. Colon. Estud. Ens. Doc. 1950, 8, 7–32. [Google Scholar]
- Cuesta, J.A.; Drake, P.; Arias, A.M. First Record of the Blue Crab Callinectes exasperatus (Decapoda, Brachyura, Portunidae) for European Waters. Mar. Biodivers. Rec. 2015, 8, e36. [Google Scholar] [CrossRef]
- Negri, M.; Schubart, C.D.; Mantelatto, F.L. Tracing the Introduction History of the Invasive Swimming Crab Charybdis hellerii (A. Milne-Edwards, 1867) in the Western Atlantic: Evidences of High Genetic Diversity and Multiple Introductions. Biol. Invasions 2018, 20, 1771–1798. [Google Scholar] [CrossRef]
- Windsor, A.M.; Moore, M.K.; Warner, K.A.; Stadig, S.R.; Deeds, J.R. Evaluation of Variation within the Barcode Region of Cytochrome c Oxidase I (COI) for the Detection of Commercial Callinectes sapidus Rathbun, 1896 (Blue Crab) Products of Non-US Origin. PeerJ 2019, 7, e7827. [Google Scholar] [CrossRef]
- Baron, J.-C. Note Préliminaire sur le Sérum du Crabe Portunus validus (Herklots, 1851). Cah. ORSTOM Sér. Océanogr. 1976, 13, 103–106. [Google Scholar]
- Büttikofer, J. Reisebilder aus Liberia: Resultate Geographischer, Naturwissenschaftlicher und Ethnographischer Untersuchungen Während der jahre 1879–1882 und 1886–1887; Leiden, E.J., Ed.; Brill: Leiden, The Netherlands, 1890; 510p. [Google Scholar]
- Crosnier, A.; Berrit, G.R.; Marteau, J. Fonds de Pêche Le Long Des Côtes Des Républiques Du Dahomey et Du Togo. Cah. ORSTOM 1966, 4 (Suppl. 1), 1–144. [Google Scholar]
- Rathbun, M.J. The Spider Crabs of America. Bull. U. S. Natl. Mus. 1925, 129, 1–613. [Google Scholar] [CrossRef]
- Mantelatto, F.L.; Robles, R.; Wehrtmann, I.S.; Schubart, C.D.; Felder, D.L. New Insights into the Molecular Phylogeny of the Swimming Crabs of the Genera Portunus Weber, 1795 and Achelous De Haan, 1833 (Brachyura: Portunidae) of the Americas. J. Crust. Biol. 2018, 38, 190–197. [Google Scholar] [CrossRef]
- Dessouassi, C.E.; Laleye, P.; d’Udekem d’Acoz, C. First Record of the Globally Invasive Crab, Charybdis hellerii (A. Milne-Edwards, 1867), in Benin, with Notes on Its Taxonomy (Crustacea, Decapoda, Brachyura, Portunidae). Zootaxa 2019, 4576, 201. [Google Scholar] [CrossRef] [PubMed]
- Naderloo, R. Atlas of Crabs of the Persian Gulf; Springer: Cham, Switzerland, 2017; ISBN 978-3-319-49372-5. [Google Scholar]
- Yokes, M.B.; Galil, B. New Records of Alien Decapods (Crustacea) from the Mediterranean Coast of Turkey, with a Description of a New Palaemonid Species. Zoosystema 2006, 28, 747–755. [Google Scholar]
- Yokes, B. Alien Crustacean Decapods from the Aegean Coast of Turkey. Aquat. Invasions 2007, 2, 162–168. [Google Scholar] [CrossRef]
- Milne-Edwards, A. Recherches sur la faune carcinologique de la Nouvelle-Calédonie. Nouv. Arch. Mus. Hist. Nat. 1872, 8, 229–267. [Google Scholar]
- Buchanan, J.B. The Bottom Fauna Communities Across the Continental Shelf Off Accra, Ghana (Gold Coast). Proc. Zool. Soc. Lond. 1958, 130, 1–56. [Google Scholar] [CrossRef]
- González, J.; Triay-Portella, R.; Escribano, A.; Cuesta, J. Northernmost Record of the Pantropical Portunid Crab Cronius ruber in the Eastern Atlantic (Canary Islands): Natural Range Extension or Human-Mediated Introduction? Sci. Mar. 2017, 81, 81–89. [Google Scholar] [CrossRef]
- Mendoza, J.C.E.; Chan, K.O.; Lai, J.C.Y.; Thoma, B.P.; Clark, P.F.; Guinot, D.; Felder, D.L.; Ng, P.K.L. A Comprehensive Molecular Phylogeny of the Brachyuran Crab Superfamily Xanthoidea Provides Novel Insights into Its Systematics and Evolutionary History. Mol. Phylogenet. Evol. 2022, 177, 107627. [Google Scholar] [CrossRef] [PubMed]
- Osorio, B. Crustaceos Da Africa Occidental Portugueza. Extr. J. Sci. Math. Phys. E Naturaes 1895, 13, 54. [Google Scholar]
- Vilela, H. Crustáceos Decápodes e Estomatópodes Da Guiné Portuguesa. An. Junta Investig. Colonais 1949, 4, 47–70. [Google Scholar]
- Shahdadi, A.; Ndongo, P.A.M.; Suess, T.; Schubart, C.D. Reappraisal and Redescription of the Three Species of the Recently Defined Genus Guinearma Shahdadi & Schubart, 2017, with a Key to the West African Sesarmidae (Decapoda, Brachyura). Crustaceana 2019, 92, 307–334. [Google Scholar]
- Maccagno, T. Crostacei Decapodi. Le Specie Del Genere Uca Leach Conservate Nel Regio Museo Zoologico Di Torino. Boll. Dei Musei Zool. E Anat. Comp. Della R Univ. Torino 1928, 41, 1–52. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).