Abstract
The association between two hydrozoans, Stylaster sp. and Millepora spp., has been described as a case of pseudo-auto-epizoism, and has only been reported from the Caribbean region of the Atlantic Ocean. Here, we report on the occurrence of this association in the Pacific Ocean on coral reefs around Iriomote-jima Island, Japan, suggesting the association to be more widespread than had previously been thought. Moreover, Stylaster sp. colonies were observed living healthily on bleached and dead branches of Millepora spp., indicating that this interaction is facultative. The interaction reported here differs from the relationship between the Caribbean Stylaster roseus and Millepora alcicornis by the connection points between the two partners, which is made evident by the whitening of the Millepora counterpart in Iriomote-jima Island, while being seamless in the Caribbean association. Further research is necessary to fully understand the nature of these relationships, comprehending under what conditions it occurs, and establishing which species are involved in the interactions.
1. Introduction
Species interactions play an important role in shaping coral reef ecosystems and diversity. Skeleton-forming (scleractinian) corals are known to be associated with a plethora of invertebrate fauna [1] and their three-dimensional structure provides shelter to other organisms. Similar to scleractinians, hydrozoan corals host a wide range of taxa, and in particular, fire corals of the genus Millepora host members of many marine phyla among their associated fauna [2], including cnidarians, crustaceans, molluscs, polychaetes, tunicates, sponges, and fish [3]. One notable epibiont of Millepora is another hydrozoan species, Stylaster roseus (Pallas, 1766), which has been found to live attached to Millepora alcicornis Linnaeus, 1758 in the Caribbean [4,5], despite it usually inhabiting small crevices and dim environments. This apparently rare interaction has been more extensively recorded in the coral reefs of Bonaire [6]. The relationship was described as a rare example of pseudo-auto-epizoism, a relationship in which a species lives on the surface of a closely related host, using it as a substratum [7]. Both organisms belong to groups of hydrozoans that calcify, namely the families Stylasteridae Gray, 1847 and Milleporidae Fleming, 1828 [7]. In the biodiverse marine ecosystems of the Ryukyu Archipelago, several unexpected interactions between different taxa have been reported [8,9,10], and some of these associations have been found to be widespread despite having remained unreported for a substantial amount of time [11]. Here we report our observations of the association between Millepora spp. and Stylaster sp. in the subtropical island of Iriomote-jima, Okinawa, Japan, constituting the first record of such an interaction between Millepora and Stylaster in the Pacific Ocean.
2. Materials and Methods
Three sites on the north of Iriomote-jima Island, Okinawa, Japan were visited by SCUBA diving where the association of Stylaster and Millepora was observed and photographed with an Olympus TG6 camera: at 5 to 7 m depths on a reef in front of Funauki Port lighthouse (24.35184° N, 123.70311° E) in September 2022, at approximately 5 m depth at Nishizaki-Higashi point (24.43884° N, 123.78771° E) in March 2023, and at 8 m depth in Nakano Beach (24.43151° N, 123.79237° E) in June 2023. Additionally, benthic transects (3 × 25 m/site) were conducted at four sites around Iriomote-jiima Island (Nakano Beach as mentioned above, and three additional sites: Nakano-oki (24.43522° N, 123.79934° E), Amitori (24.34577° N, 123.6899° E), and Sotopanari (24.38227° N, 123.71964° E), all depths 5 to 10 m). To evaluate the prevalence of the association, the number of Millepora spp. colonies were counted on each transect, and for each of them the presence or absence of Stylaster sp. was noted. Using R ver. 1.4 and package stats [12], we performed a one-way ANOVA to determine whether there were significant differences in the abundance of Millepora between sites, and to determine whether there were significant differences in abundance of Millepora hosting Stylaster between sites.
3. Results and Discussion
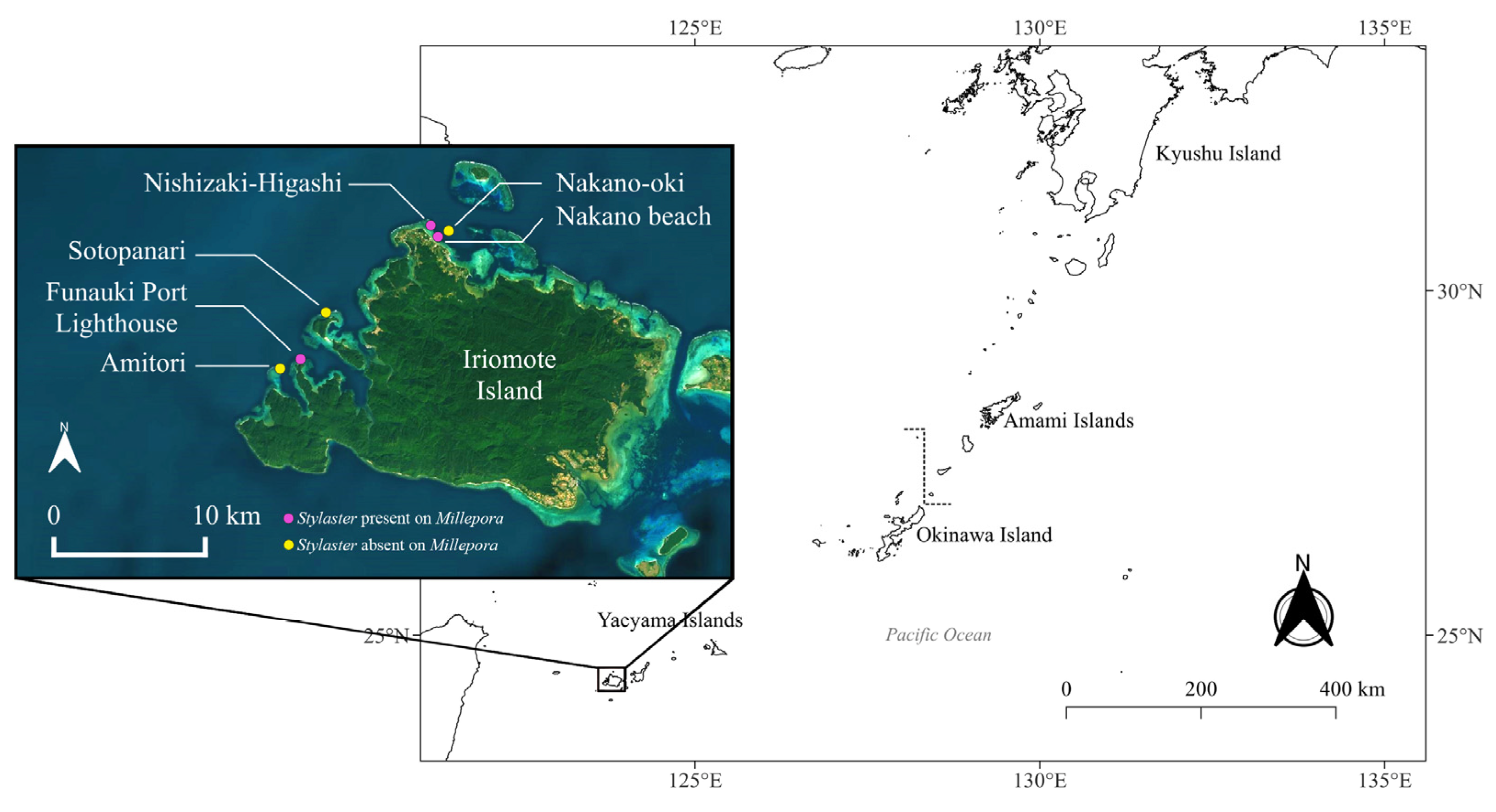
The association between Stylaster sp. and Millepora spp. was observed in three sites around Iriomote-jima Island: Funauki Port Lighthouse, Nishizaki-Higashi and Nakano Beach (Figure 1).
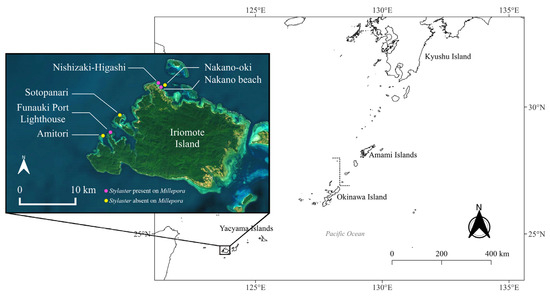
Figure 1.
Map of sites in Iriomote-jima Island, Japan, with sites where Stylaster sp. Was detected on Millepora spp. in pink, and sites where Stylaster sp. Was not observed on Millepora spp. in yellow.
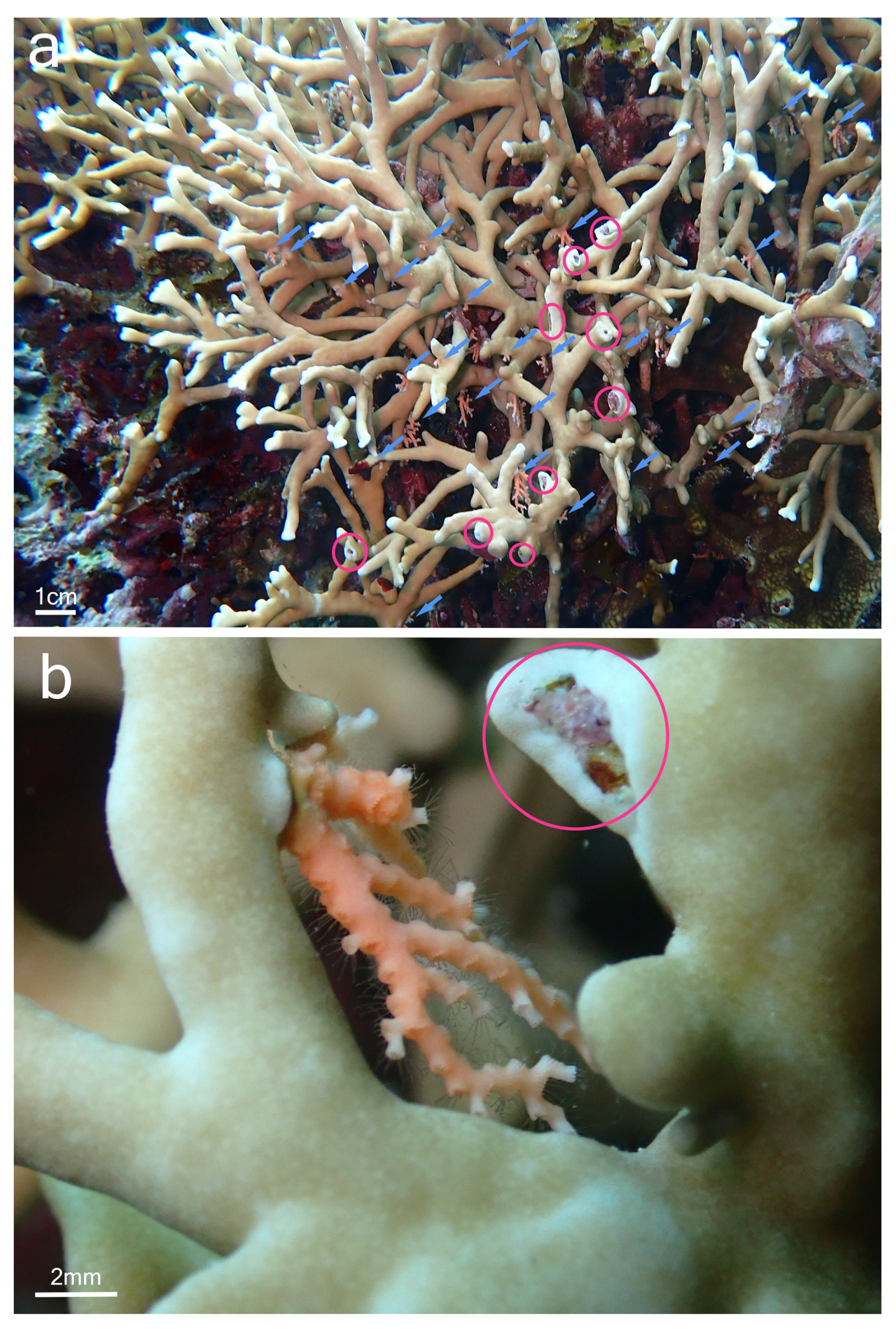
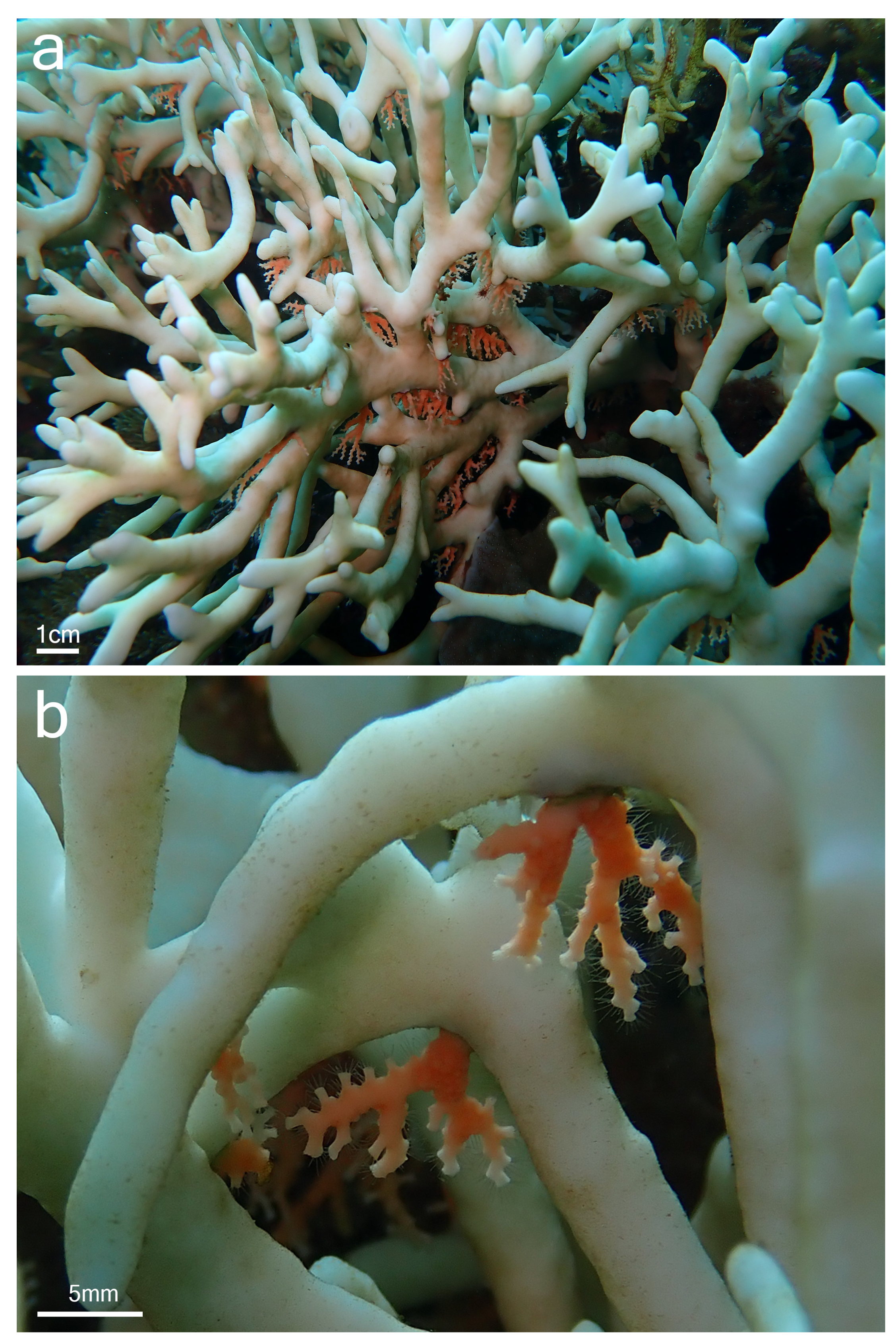
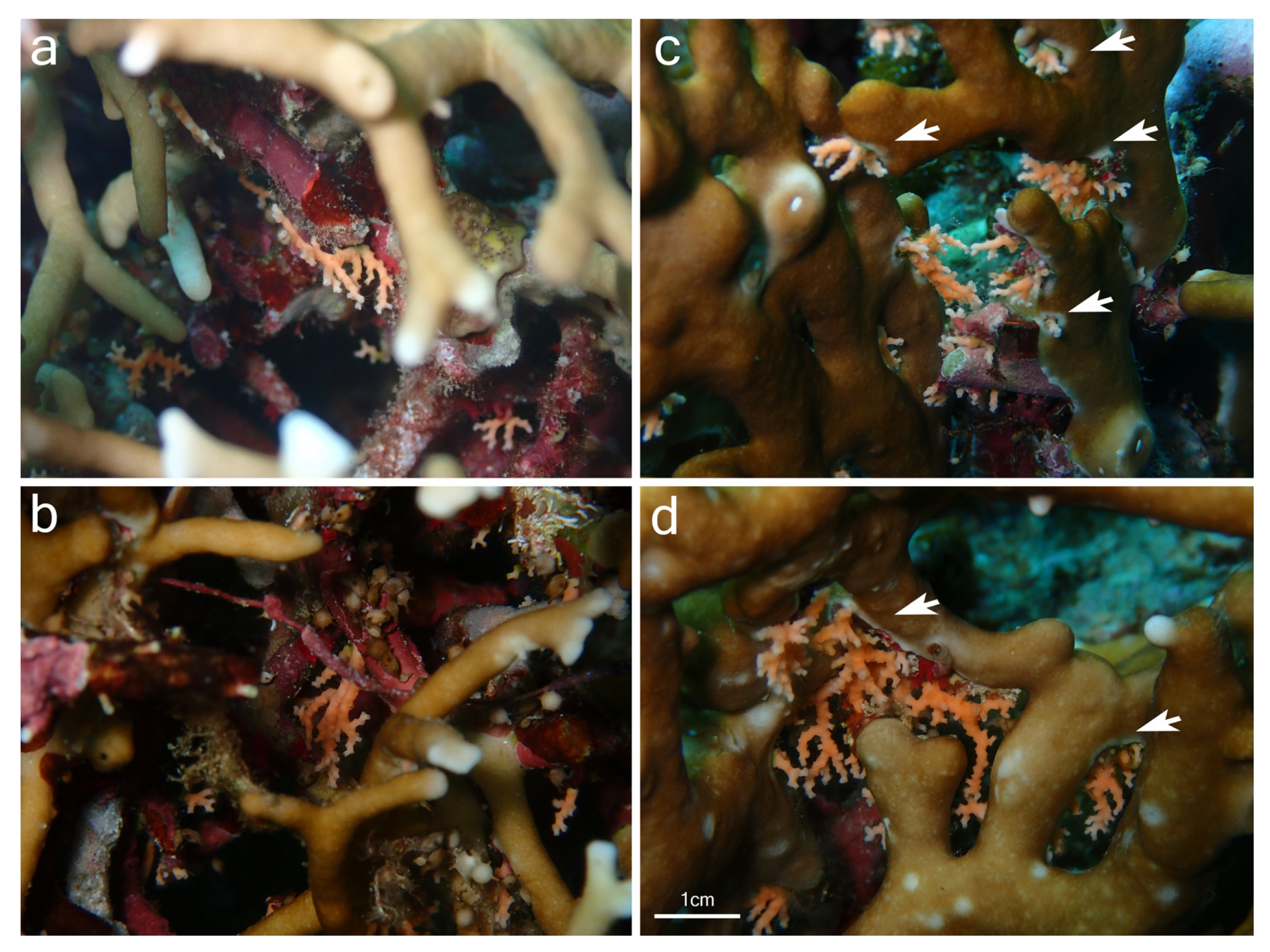
Although samples of Millepora spp. and Stylaster sp. could not be collected, the observation of the association in situ provided some insights into the relationship between the two partners and allowed for a comparison with the association between Millepora and Stylaster that has been reported in the Caribbean. In Iriomote-jima, the observed colonies of Stylaster sp. were growing downward from the underside of branches of Millepora (Figure 2a and Figure 3a). Numerous healthy Stylaster sp. colonies could be found on the same Millepora colonies (Figure 2a), and this interaction was present on multiple Millepora colonies at the three sites where the association was noticed. In addition, colonies of Stylaster sp. were observed on bleached Millepora (Figure 3) during an intense bleaching event in September 2022. Stylaster sp. colonies were seemingly unaffected by the conditions of their host, as live, open polyps could be observed (Figure 3b). Subsequently, colonies of Stylaster sp. were also observed in March 2023 (although at a different site), growing on dead skeletonized branches of Millepora (Figure 4a,b). Parts of these Millepora colonies showed growth of other benthic components such as crustose coralline algae, ascidians, and polychaete tubes (Figure 4a,b), suggesting that those branches may have been dead for some time. Therefore, it appears that the colonies of Stylaster sp. can maintain themselves on unhealthy or long dead Millepora colonies. This reinforces the idea that Stylaster sp. mainly uses Millepora as a substrate, that the species involved may also be found on other substrates, and that the association is facultative, much like Stylaster roseus in the Caribbean [6]. By using this additional niche, Stylaster sp. may be able to reduce intraspecific competition for crevices [6]. However, this does not discount the possibility that the association may have other benefits for Stylaster sp., such as an advantage in terms of planktivory by benefiting from the fire coral‘s position in the water column, as was previously hypothesized by Montano et al. [6]. A notable difference between the association reported herein from Iriomote-jima and the association reported from the Caribbean resides in the appearance of the connecting points between colonies of the two hydrozoan partners. In Bonaire, S. roseus was observed in intimately close contact with M. alcicornis at the base of the colony, with almost no visible boundary between their tissues [6]. This was interpreted as a sign that the presence of S. roseus on M. alcicornis did not cause stress to the host. In contrast, the tissue of Millepora spp. surrounding Stylaster sp. colonies observed in Iriomote-jima appeared to be whitened, and sometimes other benthic components such as calcifying algae, filamentous algae, ascidians, and polychaete tubes could be observed growing on branches of Millepora in the vicinity of Stylaster sp. (Figure 2a and Figure 4c,d). It is possible that those Stylaster sp. colonies have grown from points where branches of Millepora had been broken. Another possibility is that Millepora spp., while overgrowing dead coral skeletons, may have encountered previously settled Stylaster sp. and surrounded these colonies without coming into direct contact with them. Such scenarios may be indicated by parts of the Millepora colony seemingly growing on dead corals, highlighted by red circles in Figure 2. Alternatively, direct settlement of Stylaster sp. on Millepora spp. may be possible and accompanied by a harmful effect on the Millepora tissue. In any case, the small distance observed between tissues of the two partners suggests that Stylaster sp. is not as well tolerated by Millepora in Iriomote-jima as in the case of the Caribbean species duet. In a remarkable case of behavioural adaptation, it has been shown that cyprid larvae of Wanella milleporae—a species of barnacle living on Millepora—are able to settle directly on the fire coral and inactivate or withstand the corals’ nematocysts [13], showing that symbionts develop complex strategies in order to settle on Millepora hosts. As there seem to be differences between the Stylaster–Millepora interactions from Iriomote-jima and those from the Caribbean, these pairs of species represent interesting models to study the interactions and settlement of symbiotic organisms. Further investigations are required to understand the circumstances of the settlement of Stylaster sp. on Millepora spp.
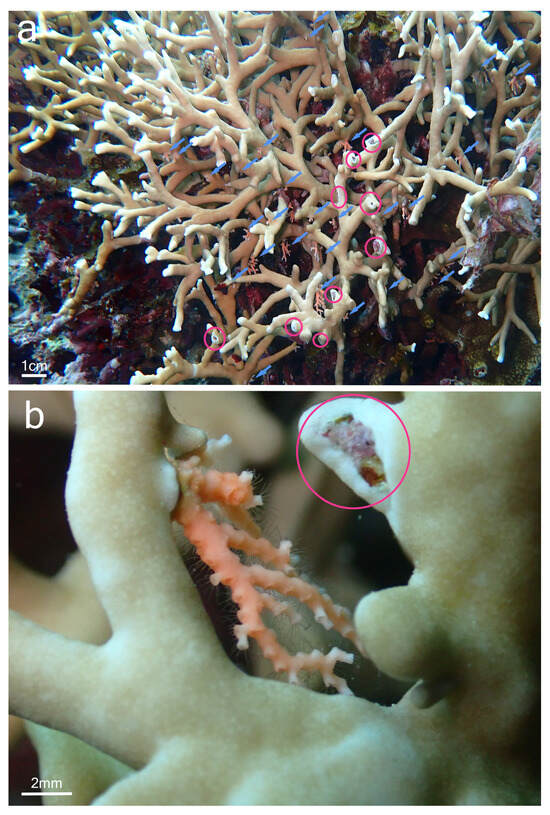
Figure 2.
Stylaster sp. on Millepora sp. colony, observed at Nishizaki-Higashi, Iriomote-jima, Japan. (a) View of the colony, with blue arrows pointing to Stylaster sp. colonies and red circles showing the regions where Millepora sp. tissue surrounds benthic component; (b) close-up view of a Stylaster sp. colony with open polyps close to Millepora sp. tissue.
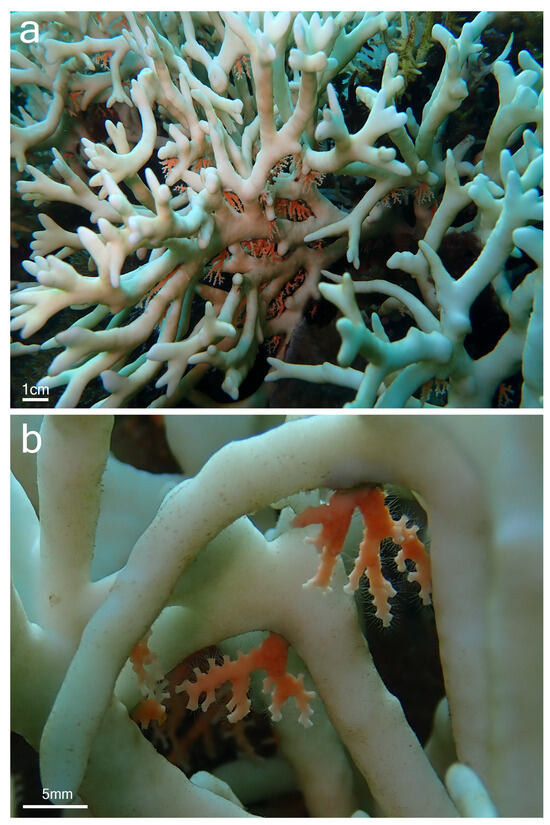
Figure 3.
Stylaster sp. on bleached Millepora sp. colony, observed at Funauki Port Lighthouse, Iriomote-jima, Japan. (a) View of the colony; (b) close-up view at Stylaster sp. colonies with open polyps close to Millepora sp. tissue.
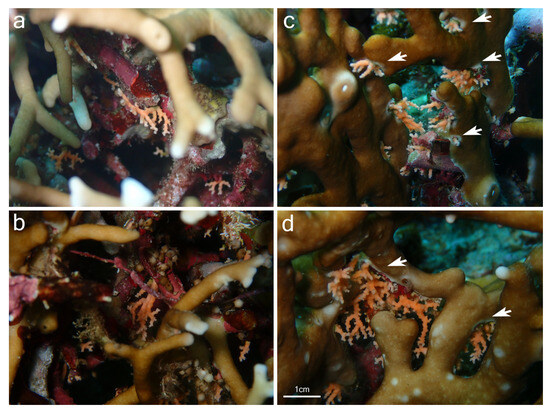
Figure 4.
Stylaster sp. growing (a,b) on benthic substrate on dead Millepora sp. branches; (c,d) on live Millepora branches with a mix of benthic substrate present at the base of Stylaster sp. colonies. Bleached Millepora tissue surrounding Stylaster sp. is indicated by white arrows. Photographs were taken at Nishizaki-Higashi, Iriomote-jima, Japan.
Although Stylasteridae is considered to be especially diverse in the Central Indo-Pacific region [7], very scant information is available about shallow water Indo-Pacific stylasterids, particularly those from Japan. Without detailed morphological analyses, the identification of the Stylaster species was not possible. On the other hand, five species of Millepora have been reported in the Archipelago of the Ryukyus and their genetic diversity has been studied, together with aspects of their colony morphology [14]. The Millepora colonies that we observed harbouring Stylaster had a morphology that was most similar to that of Millepora intricata Edwards, 1857 (Figure 2, Figure 3 and Figure 4), with spaced branches growing in all directions, although the branches appeared thicker than in a recent description of the species [15]. However, in molecular diversity analyses of Ryukyuan Millepora spp. made by Takama et al. [14], one clade (Clade 1) harboured most of the branching specimens, lumping the three Millepora species with branching growth forms present in the Ryukyus into one group (M. intricata, M. dichotoma Forskål, 1775 and M. tenera Boschma, 1949), suggesting the taxonomy of Millepora present in the Ryukyus requires further consideration. Based on this information and our data being limited to photographs, we cannot establish the identity of the Millepora species we observed associating with Stylaster species with certainty. Although we could not identify Millepora to species level, it is clear that the set of species involved here are different than in the previously reported relationship, as both M. alcicornis and S. roseus are Caribbean species [16,17]. Species with similar habits of symbiosis with Millepora have been found to exhibit profound genetic differentiation across different regions. Such cases have been documented in several different barnacles associated with Millepora [18,19], for which the species are also closely related and morphologically similar.
The fact that the association between Stylaster and Millepora occurs between different species across two oceans suggests that the physiological and/or genetic background allowing them to associate with one another has been present for a long period of time, or that, alternatively, this type of relationship may have evolved separately in both the Atlantic and Pacific Oceans as a case of parallel evolution. Either way, as the relationship between Millepora and Stylaster has been defined as a case of pseudo-auto-epizoism, the emergence of such an interaction is likely facilitated by the relatively close phylogenetic relatedness of the two members [20]. The use of Millepora as a substratum for Stylaster highlights the adaptation capabilities of stylasterids. Stylasteridae is especially known to be diversified in the deep sea [21] and thought to have conquered shallow-water environments multiple times [21]. The Millepora–Stylaster association may be one of the strategies that has helped stylasterids to maintain themselves in highly competitive coral reef environments. Finally, it is notable that, according to a past reconstruction of the group’s distribution [22], members of the family Stylasteridae appeared to be primarily distributed in oceanic islands, archipelagos, atolls, or seamounts, and were found to not be as prevalent in waters surrounding large land masses. In the Pacific Ocean, the fact that an association between Stylaster and Millepora is presently only known to exist around Iriomote-jima in the Ryukyus Islands offers some support to the theory that island or archipelago environments promote the diversity and abundance of Stylasteridae.
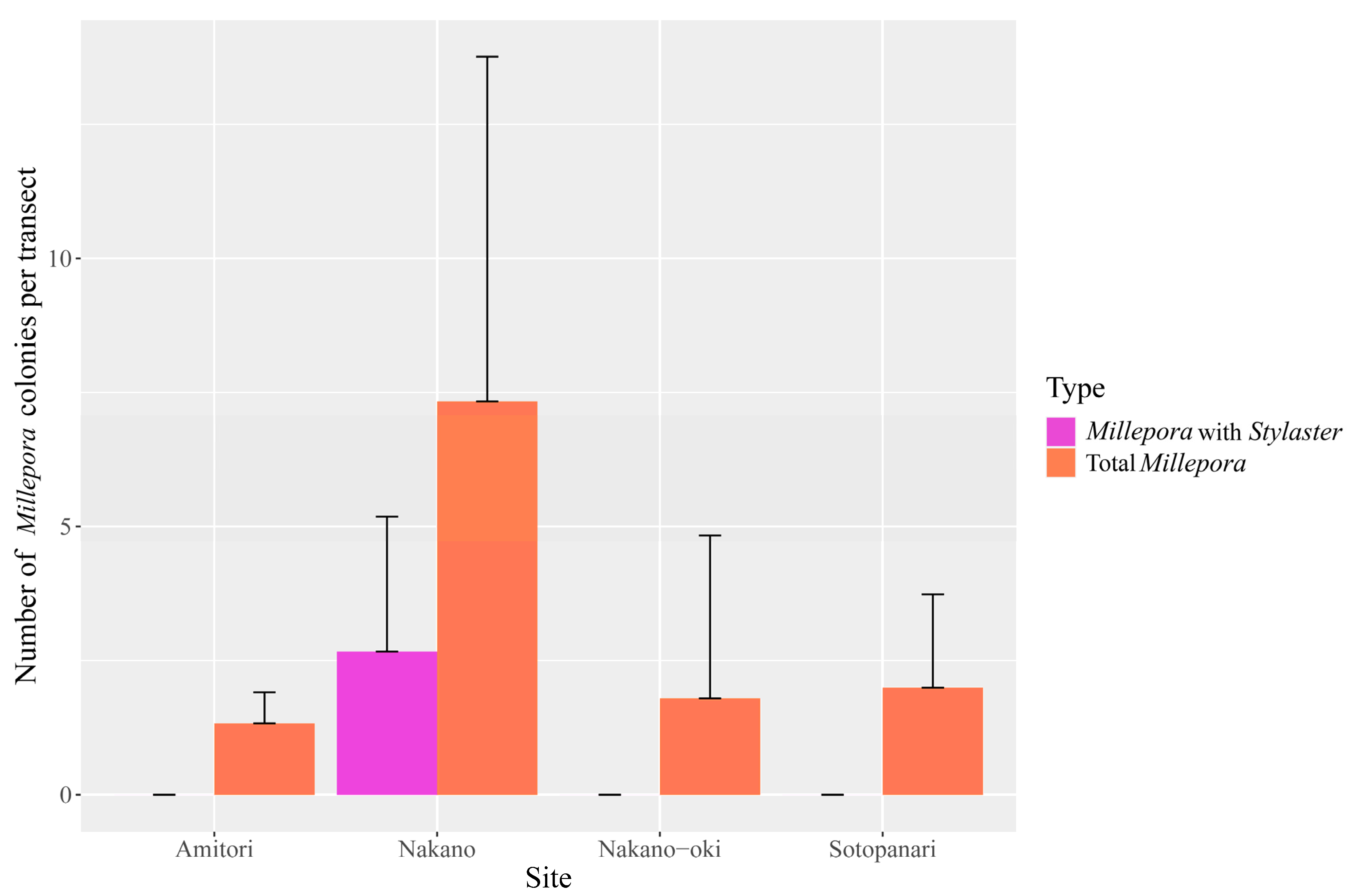
Finally, we also laid transects at four sites to obtain estimates of the prevalence of this association. Although Millepora was present at all four sites, where transect data could be obtained, Stylaster was only observed at Nakano Beach, where it was hosted by 23.8 ± 21.4% of Millepora colonies (n = 7.3 ± 6.4 colonies per transect including 2.6 ± 2.5 colonies inhabited by Stylaster, Figure 5). Millepora colonies appeared to be more abundant in Nakano Beach (Figure 5). While Stylaster inhabiting Millepora could potentially be more frequently detected at Nakano Beach due to the abundance of the host; the ANOVA test found no significant differences in the abundance of Millepora between sites. Although we also observed the Millepora and Stylaster relationship in Funauki Port Lighthouse and Nishizaki-Higashi sites, we could not obtain transect data from those sites. The high abundance of Stylaster on Millepora colonies at Nakano Beach, in contrast with its apparent absence at other nearby sites, suggests that the association may be constrained to certain environments and that it is patchy in distribution. This is in line with the observations of Montano et al. [6], who recorded the Caribbean association between Millepora and Stylaster in locations that were very close to other sites where it was not detected. While Stylaster has been reported from the Yaeyama Islands [23], their range of habitats in the region are not well documented. Surveys including Stylaster potentially living in crevices are required to better understand the context of Millepora and Stylaster relationship.
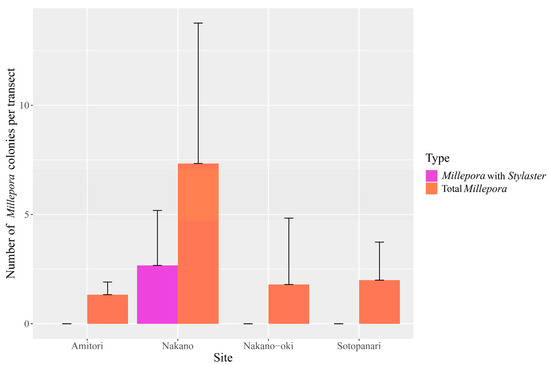
Figure 5.
Mean abundance of Millepora colonies at each site and Millepora hosting Stylaster at each site, with error bars showing standard deviation.
Our observation of Stylaster sp. using Millepora spp. as a substrate represents the first record of such an association in the Pacific Ocean. This raises multiple questions regarding the evolutionary implications and the exact meaning of the relationship for both partners. The ecological and physiological conditions in which it can occur, which Millepora and Stylaster species are involved, and how are they related to their counterparts in the Caribbean, M. alcicornis, and S. roseus, also remain to be established.
Author Contributions
Conceptualization, C.J.L.F. and J.D.R.; Methodology, C.J.L.F. and J.D.R.; Formal Analysis, C.J.L.F., D.P. and J.D.R.; Investigation, C.J.L.F., D.P., G.M.C., E.A.J., I.M. and J.D.R.; Resources, C.J.L.F., G.M.C., E.A.J., I.M. and J.D.R.; Data Curation, C.J.L.F. and J.D.R.; Writing—Original Draft Preparation, C.J.L.F.; Writing—Review and Editing, C.J.L.F., D.P., G.M.C., E.A.J., I.M. and J.D.R.; Visualization, C.J.L.F. and D.P.; Supervision, J.D.R.; Funding Acquisition, J.D.R. All authors have read and agreed to the published version of the manuscript.
Funding
C.J.L.F. and E.A.J. are thankful to the 100 Island Challenge Project, Scripps Institution of Oceanography for travel funding and to MEXT for university scholarships. Field work in 2023 was partially supported by a JSPS Grant-in-Aid for Transformative Research Areas entitled “Environmental, ecological, and genetic observations of coral reef Symbiodiniaceae-host holobiont symbioses” (23H03821) to J.D.R.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The transect data obtained in this study are available upon request to the corresponding author.
Acknowledgments
We thank T. Naruse (U. Ryukyus), N. Holloway, G. Turner and S. Kodera (all Scripps Institute) for field support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stella, J.; Pratchett, M.; Hutchings, P.; Jones, G. Coral-associated invertebrates: Diversity, ecological importance and vulnerability to disturbance. Oceanogr. Mar. Biol. Annu. Rev. 2011, 49, 43–104. [Google Scholar]
- Garcia, T.; Matthews-Cascon, H.; Franklin-Júnior, W. Macrofauna associated with the branching fire coral Millepora alcicornis (Cnidaria: Hydrozoa). Thalassas 2009, 24, 11–19. [Google Scholar]
- Pereira, P.H.C.; Leal, I.C.S.; de Araújo, M.E.; Souza, A.T. Feeding association between reef fishes and the fire coral Millepora spp. (Cnidaria: Hydrozoa). Marine Biodiver Rec. 2012, 5, e42. [Google Scholar] [CrossRef]
- Sanchez, J.; Navas, S.G. Notes on the distribution, habitat and morphology of Stylaster roseus (Pallas, 1766) (Hydrozoa: Stylasterina) in the Colombian Caribbean. Bol. Investig. Mar. Costeras—INVEMAR 1994, 23, 193–197. [Google Scholar] [CrossRef]
- Hoeksema, B.W.; van Moorsel, G.W.N.M. Stony corals of St. Eustatius. In Marine Biodiversity Survey of St. Eustatius; Hoeksema, B.W., Ed.; Dutch Caribbean, Naturalis Biodiversity Center: Leiden, The Netherlands, 2016; pp. 32–37. [Google Scholar]
- Montano, S.; Reimer, J.D.; Ivanenko, V.N.; García-Hernández, J.E.; van Moorsel, G.W.N.M.; Galli, P.; Hoeksema, B.W. Widespread occurrence of a rarely known association between the hydrocorals Stylaster roseus and Millepora alcicornis at Bonaire, Southern Caribbean. Diversity 2020, 12, 218. [Google Scholar] [CrossRef]
- Cairns, S.D. Global diversity of the Stylasteridae (Cnidaria: Hydrozoa: Athecatae). PLoS ONE 2011, 6, e21670. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Jimi, N.; Goto, R. Widening the host range of the ectosymbiotic scale-worm Asterophilia culcitae (Annelida: Polynoidae) to three echinoderm classes, with data on its body color variation. Plankton Benthos Res. 2020, 15, 289–295. [Google Scholar] [CrossRef]
- Hamamoto, K.; Reimer, J. Ascidians observed associating epizoically on holothurians in waters in the Kerama Islands, Okinawa, Japan. Plankton Benthos Res. 2022, 17, 338–342. [Google Scholar] [CrossRef]
- Kushida, Y.; Kunihiro, S.; Reimer, J.D. First observation of Waminoa sp. on Dendronephthya aff. rigida. Plankton Benthos Res. 2023, 18, 52–54. [Google Scholar] [CrossRef]
- Reimer, J.D.; Fourreau, C.J.L.; Fujii, T.; Ise, Y.; Kushida, Y.; Mizukami, I.; Nakano, M. Records and distribution of the coral-killing sponge Chalinula nematifera in the Ryukyu Islands, Japan. Plankton Benthos Res. 2022, 17, 249–254. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 19th February 2023).
- Yap, F.-C.; Høeg, J.T.; Chan, B.K.K. Living on fire: Deactivating fire coral polyps for larval settlement and symbiosis in the fire coral-associated barnacle Wanella milleporae (Thoracicalcarea: Wanellinae). Ecol. Evol. 2022, 12, e9057. [Google Scholar] [CrossRef] [PubMed]
- Takama, O.; Fernandez-Silva, I.; López, C.; Reimer, J.D. Molecular phylogeny demonstrates the need for taxonomic reconsideration of species diversity of the hydrocoral genus Millepora (Cnidaria: Hydrozoa) in the Pacific. Zool. Sci. 2018, 35, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Razak, T.B.; Hoeksema, B.W. The hydrocoral genus Millepora (Hydrozoa: Capitata: Milleporidae) in Indonesia. Zool. Verh. 2003, 345, 313–336. [Google Scholar]
- Dubé, C.E.; Bourmaud, C.A.F.; Mercière, A.; Planes, S.; Boissin, E. Ecology, Biology and Genetics of Millepora Hydrocorals on Coral Reefs; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Gnecco, M.; Nunes, F.L.D.; González-Zapata, F.L.; Dueñas, L.; Zilberberg, C.; Lindner, A.; Sánchez, J.A. Remarkable population structure in the tropical Atlantic lace corals Stylaster roseus (Pallas, 1766) and Stylaster blatteus (Boschma, 1961). Coral Reefs 2023, 42, 181–194. [Google Scholar] [CrossRef]
- Yu, M.C.; Gnmanee, M.; Tsao, Y.F.; Chan, B.K.K. Genetic differentiation and host usage of coral and fire coral-associated barnacles (Cirripedia: Pyrgomatinae and Wanellininae) across the Indian and Pacific Oceans. Zool. J. Linn. Soc. 2023, 199, 871–888. [Google Scholar] [CrossRef]
- Casartelli, M.; Terraneo, T.I.; Anker, A.; Vimercati, S.; Maggioni, D.; Paulay, G.; Benzoni, F. New ecological and phylogenetic insights in the boring barnacle Berndtia Utinomi, 1950 (Acrothoracica: Lithoglyptidae) reveal higher diversity, new hosts, and range extension to the Western Indian Ocean. Mar. Bioidivers. 2023, 53, 74. [Google Scholar] [CrossRef]
- Cartwright, P.; Nawrocki, A.M. Character evolution in Hydrozoa (phylum Cnidaria). Integr. Comp. Bio. 2010, 50, 456–472. [Google Scholar] [CrossRef] [PubMed]
- Lindner, A.; Cairns, S.D.; Cunningham, C.W. From offshore to onshore: Multiple origins of shallow-water corals from deep-sea ancestors. PLoS ONE 2008, 3, e2429. [Google Scholar] [CrossRef] [PubMed]
- Cairns, S.D. Worldwide distribution of the Stylasteridae (Cnidaria: Hydrozoa). Sci. Marina 1992, 56, 125–130. [Google Scholar]
- Eguchi, M. Notes on coral genera of the Yaeyama insland group, with descriptions of a new species, Cladocora kabiraensis n. sp. Proc. JPN. Soci. Syst. Zool. 1975, 11, 1–4. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).