Abstract
Artemia is a genus of halophilic zooplanktons comprising bisexual and parthenogenetic forms, which is an important model for investigating adaption to hypersaline ecosystems. The genus Artemia in China comprises four species: A. sinica, A. tibetiana, A. franciscana and A. parthenogenetica. To investigate the evolutionary relationship of bisexual and parthenogenetic Artemia in China, we analyzed the morphometrics and phylogenetics among twenty-two geographical populations in China. We found significant morphological differentiation across different species and strains of Artemia in China, which exhibited a high level of intra-population variation. We also found overlaps in morphological characteristics between populations, which may raise challenges for the classification of Artemia species using traditional morphological methods. A. franciscana, which originated from various regions in America, was generally distributed along the Chinese coastlines through multiple human introductions. Additionally, native Asian clades split into Western and Eastern Lineages during the late Miocene due to the Himalayan orogeny. Within the Western Lineage, A. tibetiana can be grouped into three taxon units: A. tibeitiana, A. sorgeloosi and A. urmiana. We also found that the distribution and genetic structure of A. sinica were influenced by climate oscillations during the Pleistocene, which might play a pivotal role in driving the formation of parthenogenetic strains in the Eastern Lineage. Overall, our study provides new insight into invertebrate evolution under geographical and climatic impacts in hypersaline environments.
1. Introduction
The brine shrimp Artemia Leach, 1819, is a genus encompassing tiny invertebrates that belong to Crustacea, Anostraca. Artemia populations are highly valued for their wide use as fish and shrimp feeds in aquaculture [1], but scholars are also interested in their strong adaptation to extreme environments, especially hypersaline environments. As paradigmatic crustaceans, they are distributed in inland salt lakes and coastal salterns worldwide, except for Antarctica [2]. Due to the uniqueness of hypersaline environments compared to freshwater ecosystems, it is logical to use Artemia as a model to explore evolutionary processes in hypersaline ecosystems [2,3]. There are six recognized bisexual species in the genus Artemia: Artemia salina (Linnaeus, 1758); Artemia urmiana Günther, 1899; Artemia franciscana Kellogg, 1906; Artemia persimilis Piccinelli and Prosdocimi, 1968; Artemia sinica Cai, 1989; and Artemia tibetiana Abatzopoulos et al., 1998. A. franciscana and A. persimilis are native to the New World, while the other four species are native to the Old World. Recently, Asem [4] identified two new bisexual species from Kazakhstan and the Qinghai–Tibet Plateau, which were named as A. amati and A. sorgeloosi, respectively. However, little is known about these two species. In addition to bisexual species, there are a large number of obligate parthenogenetic populations termed Artemia parthenogenetica that are widely distributed over Eurasia with di-, tri-, tetra- and pentaploidy [5]. Therefore, it is critical to understand the morphological and genetic differences among Artemia species with various reproductive modes.
Progress has been made to demonstrate the biodiversity and genetic variation among Artemia species in Asia. A. sinica, A. tibetiana and A. urmiana have a restricted distribution, whereas A. franciscana has invaded a wide area along the southern and eastern coastal regions of Asia through human activities [6]. A. urmiana, an A.tibetiana Artemia. sp. from Kazakhstan, and the Eurasian Haplotype Complex (EHC; a group of parthenogenetic populations sharing the same basic haplotype from Eurasia) cluster together, forming a sister group with A. sinica. A. tibetiana is polyphyletic in the mitochondrial gene tree, with one single clade hosting samples from Lagkor Co. (Tibet, China), the type locality of A. tibetiana, and another clade that is mixed within A. urmiana. In addition, there are no clear boundaries in their genetic differentiation with the existence of a nuclear gene flow [6,7,8]. Due to the small sequence divergence [9] between A. urmiana and A. tibetiana, Hou [10] and Sainz-Escudero [11] treated A. tibetiana as synonymous with A. urmiana, and Li [12] mentioned them as forming an A. urmiana complex provisionally. Therefore, the taxonomic status and species identification of A. tibetiana should be re-evaluated. A. parthenogenetica was once thought to be a monophyletic [6,8], but phylogeny based on CO1, 16S and 12S suggested that parthenogenetic Artemia is a polyphyletic group, in which di- and triploid lineages share a common ancestor with A. urmiana, while tetra- and pentaploid lineages are closely related to A. sinica [13]. Taken together, the species identification of Artemia in the Qinghai–Tibet Plateau (QTP) and the speciation progress of tetra- and pentaploid A. parthenogenetica still need further study.
At present, morphological characterization is the most widely used approach to distinguish Artemia species [14]. Previous works on the morphometrics of Artemia have shown that morphological differences can effectively describe the differences among most bisexual populations [15,16,17,18,19], and even in asexual species, where the populations found in coastal China, inland China, Europe and Africa also exhibit significant morphological differences [20]. However, Asem [21] found no significant differentiation among the populations in China. Further, these studies did not perform a comparison between bisexual and asexual populations, which might make it challenging to determine if bisexual and parthenogenetic Artemia can be distinguished solely based on their morphological characteristics. In addition to morphological characteristics, molecular markers have also been applied to study Artemia taxonomy. Previous studies have found that nuclear gene markers, such as ITS and Na+/K+ ATPase, are unable to effectively identify different species. In contrast, mitochondrial markers including CO1, 16S and 12S exhibit a clear phylogenetic structure [6,13]. The complete mitochondrial genomes of Artemia species have been obtained through Next-Generation Sequence [4,11,22,23,24], but mitochondrial genome data have not been generally used for individual identification. The topology of phylogeny based on CO1 was found to be almost consistent with that based on mitochondrial genomes [25].
China is one of the countries with the richest Artemia locations and biodiversity. At present, four species have been found in the country, including A. sinica, A. tibetiana, A. parthenogenetica and the invasive species A. franciscana [26]. In past studies, samples from several localities were used to represent all of China, especially samples of A. sinica. To investigate the evolution of bisexual and parthenogenetic Artemia in China, we utilized samples from twenty-two geographical populations of the four cited species. In this paper, we will (1) describe the numerical morphological characteristics of female and male Artemia with different reproductive modes and localities; (2) compare the differences between morphological and molecular methods for identifying Artemia taxonomy; and (3) determine the phylogenetic relationship, divergence time and ancestral distribution of Artemia species in China. Our aim is to figure out the morphological differentiation and evolutionary history among different species and strains of Artemia in China.
2. Materials and Methods
2.1. Sample Collection and Culture Conditions
Artemia cyst samples were obtained from twenty-two sites in China, and an additional sample was obtained from Lake Urmia, Iran. The specific locality information and abbreviations are listed in Table 1. A map of the geographic distribution of Artemia populations, including the samples collected for this study and those retrieved from previous research studies, is shown in Figure 1. Commercial A. franciscana cysts with an unknown specific locality (population of AF) were also used in our study. To minimize the effect of environment on body form, all studied populations were cultured under standard conditions. The cysts from each population were hatched and maintained in artificial seawater (28 °C and 28‰ salinity) under a 12 h light/12 h dark (12:12 LD) cycle with a light intensity of 1000 Lux. After one day of incubation, newly born larvae were fed with Tetraselmis suecica once a day. After 35–40 days of cultivation, individuals reached the adult stage, with well-developed antennae on males and a well-developed ovisac on females.

Table 1.
Information on the Artemia samples used in this study for morphological or phylogenetic analysis.
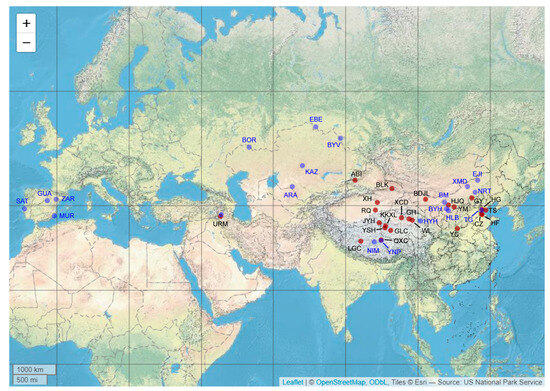
Figure 1.
Map of geographic distribution of Artemia populations. Red circles represent populations sampled in this study, and blue circles represent sequences retrieved from NCBI. Detailed information is listed in Table 1.
2.2. Measuring Individuals
The cysts of 14 localities and A. franciscana successfully hatched and reached adulthood, which were used for the numerical morphological analysis. A. sinica from XCD, A. parthenogenetica from RQ and A.tibetiana from the other 6 localities have a low hatching rate and high mortality under standard conditions. We did not collect enough adult samples from these sites, so samples of XCD, RQ and A. tibetiana were not used for the morphological study. A random sample of at least 20 individuals from each population was removed from each culture (for each sex and population) and fixed in formalin after narcotizing with chloroform; their morphology was checked using a Leica M205A. After photographing the dorsal view and furca of each adult specimen, the following 14 morphometric parameters were measured with ImageJ according to Hontoria and Amat [14] and Triantaphyllidis et al. [15]: total length (TL), abdomen length (AL), length from the third abdominal segment to abdomen end (AL38), length of the eighth abdominal segment (AL8), width of the third abdominal segment (AW3), left furca length (LFL), right furca length (RFL), head width (HW), first antenna length (FAL), distance between compound eyes (DE), compound eye diameter (DCE), number of setae on the left furca (SLF), number of setae on the right furca (SRF), ovisac width (OW) for female and width of the second abdominal segment (AW2) for male. Ratio of abdomen length to total length (RA = AL/TL), ratio of the third abdominal segment to abdomen length (RAW3 = AW3/AL), and ratio of the ovisac (ROW = OW/AL for female) or second abdominal segment (RAW2 = AW2/AL for male) to abdomen length were also calculated for each sample.
2.3. Statistical Analysis
Discriminant analysis was performed separately on females and males using the 14 parameters mentioned above. The origin of each population was used as separation criterion. Due to the observation of mixed species in the HF and TS populations along China’s coastal regions, the data from these two populations were excluded from discriminant function construction and only used for sample prediction.
Differences among the populations were compared by means of one-way ANOVA and Tukey–Kramer test. If the data did not satisfy the normality assumption or homogeneity of variance, a non-parametric Kruskal–Wallis test was conducted with Dunn’s test. The Bonferroni method was used for adjustment of p-values. For groups with extremely unequal sample sizes, we divided the group with a larger sample size into several subsets through random sampling without replacement. Then, the subsets were compared with the other groups repeatedly to confirm the stability of the results. The significance level was set at p-value < 0.05. Hierarchical clustering based on Euclidean distance and principal component analysis (PCA) were also performed to check the differentiation among the populations of each species.
2.4. DNA Extract, PCR Amplification and Sequence Alignment
Total DNA was extracted from each individual adult specimen using an Omega E.Z.T.A. Insect DNA Kit following the manufacturer’s instructions. For populations that failed to hatch, their cysts were used for DNA extraction. All extracted DNA was stored at 80 °C for further genetic experiments. A degenerate primer was designed for amplifying the cytochrome oxidase subunit 1 (CO1) gene (F:5′-ACCTATTATCGGCCACTT-3′, R:5′-AGTTGTTCTATGAGRGGTG-3′) according to the mitochondrial genomes of A. sinica, A. tibetiana, A. franciscana and A. parthenogenetica. The PCR reaction (25 μL) included 12.5 μL of 2× TaqMix (Vazyme Biotech Co., Ltd.), 1 μL of the forward primer (10 μM), 1 μL of the reverse primer (10 μM), 2 μL of DNA and 8.5 μL of ddH2O. The PCR cycles included a first cycle at 95 °C for 5 min; 30 cycles at 94 °C for 60 s, 51.3 °C for 60 s and 72 °C for 90 s; and a final extension at 72 °C for 10 min. The process ended with incubation at 4 °C. Subsequently, the resultant PCR products were checked on 1% agarose gel and sequenced by TSINGKE Biological Technology Corporation (Beijing, China).
We obtained 188 CO1 sequences for this study (GenBank Accession Numbers PP093070-PP093156). The sequencing results were assembled using DNAstar SeqMan v7.1 and blasted on NCBI to determine the specific species of each individual. The blast results were used as the background information to verify the discriminant analysis results based on morphometrics. All sequences were aligned using MAFFTA v7.487.
2.5. Phylogenetic Study and Divergence Time Estimation
Phylogenetic analyses were carried out using both maximum likelihood (ML) and Bayesian inference (BI) methods, and Daphnia magna (NC_026914) was set as the outgroup. The best-fit nucleotide substitution model based on Akaike’s information criterion (AIC) was chosen using ModelFinder in IQ-tree v1.6.12. The best-fit model for the CO1 sequences was TIM2+F+R3. ML tree calculations were performed using IQ-tree with 1000 bootstrap replicates. BI tree reconstruction and divergence times were determined using BEAST 1.10.4 with the following parameters: nucleotide substitution model = GTR+F+G4 as a replacement for the suggested model, the molecular clock model = uncorrelated lognormal relaxed model and tree prior = birth–death process of speciation. To calibrate the molecular clock, the divergence time of A. franciscana and Asia species was set at 8.42 Ma (95% HPD: 9.55–7.32 Ma) based on Sainz-Escudero et al. [11]. The analyses were run twice independently for 100 million generations, with sampling every 10,000 generations. All runs were combined after a chain convergence check in TRACER 1.7.2 with a burn-in of 25% states. The maximum clade credibility tree using median heights was annotated using TreeAnnotator 1.10.4.
RASP 4 was applied to generalize the ancestral distributions of the Eastern Lineage species using statistical Dispersal–Vicariance analysis (S-DIVA) and Bayesian Binary MCMC (BBM) model. To clarify the vicariance and dispersal events, the geographic distributions of the Eastern lineage species were mapped onto 8 areas: (A) North China including the North China Plain and central Inner Mongolia; (B) the Ordos Basin in Inner Mongolia; (C) the Alxa area in Inner Mongolia including the Badanjilin Desert; (D) the Tsaidam Basin; (E) the Qinghai–Tibet Plateau (QTP); (F) Xinjiang; (G) Central and Western Asia; and (H) the Mediterranean. The maximum number of ancestor areas was limited to three.
2.6. Population Genetics Based on CO1 Datasets
All CO1 sequences obtained in this study with a length of 1492 bp were divided into three datasets based on the results of phylogeny. The genetic diversity of each population was measured using the number of polymorphic sites (S), total number of mutations (M), number of haplotypes (NH), haplotype diversity (Hd), nucleotide diversity (π) and average number of nucleotide differences (K) in DnaSP v.6.12.3. An analysis of molecular variance (AMOVA) and neutrality tests (i.e., Tajima’s d, Fu and Fu’s Fs) were computed using Arlequin v.3.5.1.2. Additional reference sequences of Artemia were retrieved from the NCBI to depict the interspecific genealogical relationships among the CO1 haplotypes using the TCS algorithm implemented in the PopART 1.7 software after alignment and trimming. Detailed information on the reference sequences used in the haplotype network analysis is listed in Table S1.
3. Results
3.1. Morphology
A total of 360 females from 15 populations and 239 males from 10 populations were analyzed. The two-dimensional plot of the discriminant analysis categorized the centroids of the Artemia populations into two groups, both for females and males (Figure 2). The first group containing the CZ, HG and AF populations belongs to A. franciscana, while the second group including the remaining populations belongs to A. sinica and A. parthenogenetica. The centroids of the GY, YM and HJ populations clustered with the YC population, which served as the type locality of A. sinica. However, the centroid of the BD population was situated near the parthenogenetic populations in females and isolated in males. The centroids of the HF and TS populations with mixed-species composition were distributed between the two groups. The first two discriminant functions describe 72.57% and 79.1% of the variance for females and males, respectively. There were 70.84% of females and 63.72% of males that could be correctly assigned to their origin populations using the discriminant approach. The CO1 blast results confirmed that each population was composed of a single species (A. franciscana, A. sinica or A. parthenogenetica), except for the HF and TS populations. Consequently, the proportion of assignment at the species level for each population was calculated based on the morphological results (Table S2). The discrimination accuracy of males at the species level was significantly higher than that of females (90.50% vs. 80.46%, p-value < 0.05).
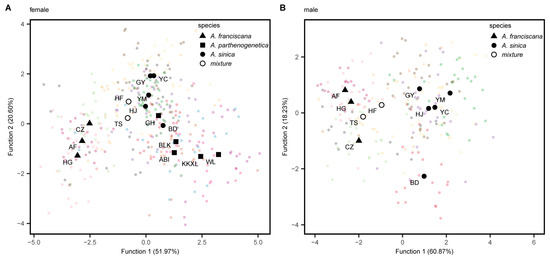
Figure 2.
Scatterplot of the first two functions obtained from the discriminant analysis of Artemia (A) females and (B) males using 14 morphological parameters. The colored dots represent individuals from each population. The abbreviations for populations are explained in Table 1. ▲ represents the centroid of A. franciscana populations, ■ represents the centroid of A. parthenogenetica populations, ● represents the centroid of A. sinica populations and ○ represents the centroid of populations with mixed species.
The statistical results of the morphological parameters (mean ± sd) are provided in Table 2 and Table 3 for females and males, respectively. Compared to the other two species, A. franciscana displayed a thicker abdominal segment and a shorter first antenna in both females and males. Although no statistically significant differences in body shape were observed between female A. sinica (n = 148) and A. parthenogenetica (n = 124), the parthenogenetic populations exhibited a more developed head and a longer first antenna, as well as significantly fewer setae on the furca (Figures S1 and S2). Within A. franciscana, specimens in the HF population (n = 16 for females and n = 15 for males) exhibited a longer abdominal segment and a narrower abdominal width compared to other groups (Figures S3 and S4). Moreover, the body shape characteristics of females in the HF population closely resembled those of A. sinica (p-value > 0.05, Figure 3). The HF and TS populations are geographically close to each other. Due to the limited sample size of A. parthenogenetica in these two populations, we merged the parthenogenetic samples of the TS and HF populations into one group and compared their differences with the other parthenogenetic populations. High variations were detected in the A. sinica (Figures S5 and S6) and A. parthenogenetica (Figure S7) populations. Females from the BD population (n = 24) had the longest first antenna (1.05 ± 0.13 mm) and the fewest setae on the furcae (10.59 ± 2.4, 11.09 ± 2.18 mm for the left and the right furca, respectively) among the A. sinica populations. This pattern of characteristics was comparable to that of the parthenogenetic populations, but there were still significant differences between BD females and A. parthenogenetica (p-value < 0.05, Figure S8). Males from the BD population (n = 24) displayed substantial variations compared to the other A. sinica populations, being characterized by a smaller and wider body size with a longer first antenna and a longer furca with more setae.

Table 2.
Morphometric characteristics of the fourteen female populations of Artemia in China (mean ± Sd).

Table 3.
Morphometric characteristics of the ten male populations of Artemia in China (mean ± Sd).

Figure 3.
Comparison of morphological differences between female A. franciscana in the HF population and female A. sinica. All A. sinica samples were divided into nine subsets with a sample size of 16–20 individuals in each subset via random sampling. The variance analysis was repeated nine times using the nine subsets with the same results. The graphs show the result of one of the subsets. A. fran is the abbreviation of A. franciscana. *: 0.01 < p-value < 0.05; ****: p-value < 0.001; ns: p-value > 0.05.
The hierarchical clustering results were consistent with the discriminant and variance analysis. Female A. franciscana from the HG, CZ and TS populations clustered together and could be distinguished from other populations, even though female A. franciscana of the HF population clustered with the A. sinica populations. Furthermore, the A. sinica and A. parthenogenetica populations clustered in the same clade, which indicates a lack of easily identifiable morphological features between these two species. As for males, the A. franciscana and A. sinica populations clustered into two groups. In addition, the branch representing the BD population was separated from the rest of the A. sinica populations (Figure 4). The PCA plot also illustrates the distinctiveness of the male BD population in A. sinica and the female HF population in A. franciscana, as they formed separate clusters from the others (Figure S9).

Figure 4.
Hierarchical clustering of Artemia populations in (A) females and (B) males. Abbreviations: HF_f = A. franciscana in HF population; HF_p = A. parthenogenetica in HF population; HF_s = A. sinica in HF population; TS_f = A. franciscana in TS population; TS_p = A. parthenogenetica in TS population; and TS_s = A. sinica in TS population.
3.2. Mitochondrial Phylogeny of Artemia in China
The phylogenetic trees generated by means of ML and BI showed consistent topologies with three distinct and well-supported clades (the American Lineage, Eastern Lineage and Western Lineage; Figure 5). The American Lineage was composed of A. franciscana from the Bohai Bay (CZ, HF, HG and TS). The samples from Eurasia were split into two lineages: the Eastern and Western Lineages. The Eastern Lineage had two well-defined clades, including A. sinica from China, and partially parthenogenetic individuals from Europe, Iran, the QTP, the Tsaidam Basin, Xinjiang and the Bohai Bay. A. sinica that originated mainly from North China (Clade 1) and the Ordos Basin in Inner Mongolia (Clade 2) clustered in a group at the base of the Eastern Lineage. The other samples were split into three groups, including bisexual populations from the Tsaidam Basin (Clade 3), bisexual populations from the Alxa area (Clade 4) and parthenogenetic populations (Clade 5). However, the parthenogenetic samples in the Eastern Lineage did not form a monophyletic group. The A. parthenogenetica haplotypes (H40 and H41) from Lake Urmia, Iran, clustered within the BD group, forming a sister branch to all the other parthenogenetic specimens in the Eastern Lineage.
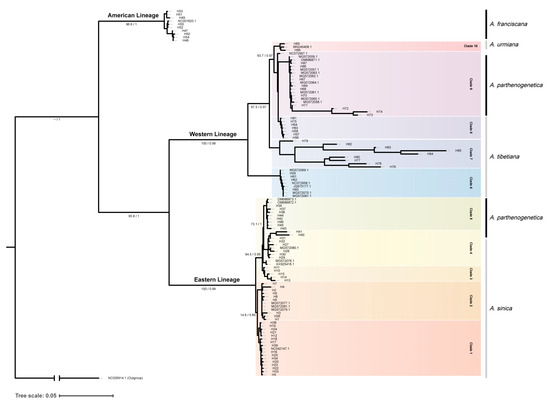
Figure 5.
COI phylogeny based on ML and BI for 87 unique haplotypes of Artemia in China. The numbers along the nodes indicate bootstrap supports and the posterior probability for the ML and BI approaches. Daphnia magna is used as an outgroup.
The Western Lineage contained A. urmiana from Iran, A. tibetiana and the rest of the A. parthenogenetica specimens from Eurasia. As previous studies have found, A. tibetiana with traditional definition was a polyphyletic group. The samples from QXC, Yangnapeng Co and Nima Lake formed a basal clade together with the newly identified taxon A. sorgeloosi (NC072958.1). The LGC population, the type locality of A. tibetiana, clustered with samples from the GLC population into an independent branch. The rest of the A. tibetiana samples from the JYH, XH and YSH populations clustered with the parthenogenetic samples in the Western Lineage and bisexual species from Kazakhstan (A. amati, NC072957.1), forming a sister clade to A. urmiana.
The results of the divergence time estimation are shown in Figure 6A. The diversification of the Western and Eastern Lineages began in the late Miocene (6.33 Ma, 95% HPD: 5.21–7.44 Ma). The Western Lineage diverged in the late Pliocene (2.87 Ma, 95% HPD: 2.14–3.56 Ma), and A. tibetiana composed of the LGC and GLC populations (Clade 7) split at ~2.22 Ma (95% HPD: 1.36–3.03 Ma). The parthenogenetic variants of the Western Lineage occurred during the Pleistocene (0.87 Ma, 95% HPD: 0.23–1.59 Ma). The time to the most recent common ancestor of the two main clades within the Eastern Lineage was placed at ~1.4 Ma (95% HPD: 0.36–2.87 Ma). The Tsaidam bisexual populations split at ~0.74 Ma (95% HPD: 0.21–1.48 Ma), while the North China populations and the Ordos Basin populations diverged 0.18 million years later (mean = 0.49 Ma, 95% HPD: 0.14–1.14 Ma). For the Eastern Lineage, the historical isolation of the parthenogenetic populations started at 0.58 Ma (95% HPD: 0.15–1.12 Ma).
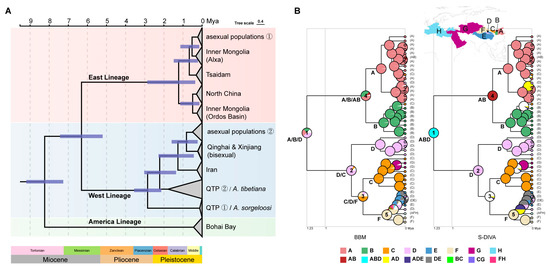
Figure 6.
(A) A chronogram of the divergence time of Artemia lineages constructed under a relaxed clock model using BEAST. Time is indicated in million years (Ma). Blue horizontal bars represent 95% HPD. (B) Ancestral distributions of Artemia in the Eastern Lineage were reconstructed by means of RASP based on BBM and the S-DIVA model. The distribution areas of Artemia are marked in capital letters A–H (A: North China including the North China Plain and central Inner Mongolia; B: the Ordos Basin in Inner Mongolia; C: Alxa area in Inner Mongolia including the Badanjilin Desert; D: Tsaidam Basin; E: Qinghai–Tibet Plateau (QTP); F: Xinjiang; G: Central and Western Asia; H: the Mediterranean).
The S-DIVA and BBM analyses illustrated that the most likely ancestral range of Eastern Asia Artemia (Figure 6B) originated in (A) North China; (B) the Ordos Basin in Inner Mongolia; and (D) the Tsaidam Basin (100% ABD for S-DIVA and 24.28% A, 22.53% B and 39.36% D for BBM). The S-DIVA analysis postulated 22 dispersal and 13 vicariance events, while the BBM analysis postulated 15 dispersal and 10 vicariance events in the Eastern Lineage. The first vicariance occurred at node 1 with a possible event route ABD->D|AB. The species remaining in the Tsaidam Basin spread to Xinjiang and the Alxa area and then split into parthenogenetic populations in Northwest China.
3.3. Genetic Diversity and Population Structure
The CO1 sequences in the Eastern Lineage were divided into seven groups (North China, Ordos, Alxa, Tsaidam bisexual, Tsaidam parthenogenetic, QTP parthenogenetic and Xinjiang parthenogenetic) to calculate genetic diversity. The A. parthenogenetica specimens from the TS and HF populations in the Western Lineage were merged into the Bohai population. The statistics of the sequence polymorphisms are detailed in Table 4. The Eastern Lineage species had high levels of Hd and π, which might have resulted from the long-term evolution of large and stable populations. Although the nucleotide diversity of the parthenogenetic populations in QTP (π = 0.0203) was the highest among the Eastern Lineage, the genetic diversity of bisexual species (Hd = 0.967, π = 0.0109) was still higher than that of parthenogenetic species (Hd = 0.733, π = 0.00834). There was only one haplotype in the JYH population with an extremely low genetic diversity. The XH and YSH populations, which clustered with the JYH population in Clade 8, were not high in genetic diversity either. The highest Hd and π of the Western Lineage were found in the LGC/GLC populations (Clade 7), which is the clade that is composed of samples from the type locality of A. tibetiana. Within the three datasets, only the Eastern Lineage had a neutrality test that significantly deviated from 0. The AMOVA results (Table 5) showed that the main contribution of genetic variation in A. franciscana from the Bohai Bay occurred among populations (54.27% among populations vs. 45.73% within populations). For the Eastern Lineage, 47.49% of the total genetic variation originated from variability among groups, 44.66% originated from variability within populations, and 7.85% was attributed to variability among populations within groups. Moreover, we divided the Western Lineage into three groups when calculating the variance contribution: Clade 6 was group 1, Clade 7 was group 2, and Clade 8 and Clade 9 were combined into group 3. Most of the genetic variation was partitioned among groups in the Western Lineage (52.78% among groups, 18.66% within populations and 28.56% within groups among populations).

Table 4.
Summary of CO1 genetic statistics for Artemia populations in China.

Table 5.
Analysis of molecular variance in Artemia from China.
All A. franciscana sequences from the Bohai Bay and the American continent were composed of six haplotypes (Figure 7). Hap1, Hap2 and Hap4 were the most abundant haplotypes, with a share of 34.4%, 37.5% and 23.8%, respectively. Hap1 was mainly composed of samples from the San Francisco Bay (SFB) in the USA (47.3%) and Mexico (27.3%,), and all samples from the HG population were distributed in Hap1. Hap2 was mainly composed of samples from Chile (40%) and Jamaica (25%), with over 70% of the samples from the TS and HF populations distributed in this haplotype. Hap4 included samples from the Great Salt Lake (GSL) in the USA (55.3%) and Mexico (21.1%). The CZ and HF populations had their distinct haplotypes, H5 and H6, respectively.
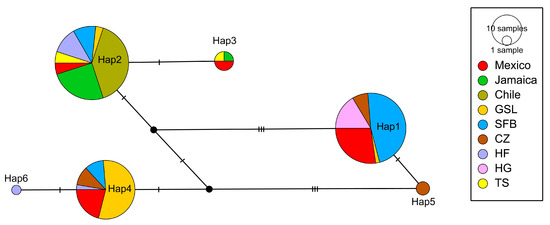
Figure 7.
The relationship network of CO1 haplotypes constructed from 159 sequences for the Artemia populations in the American Lineage. The size of each circle is proportional to the frequency of specimens. Black dots indicate intermediate or unsampled haplotypes. Dashes represent the number of nucleotide substitutions.
The Eastern Lineage presented 67 haplotypes within the 166 individuals (Figure 8), which could be gathered into three main groups. Group 1 was constituted by Hap1–30, which contained bisexual populations from the North China Plain, central Inner Mongolia and the Ordos Basin in Inner Mongolia. There was no haplotype shared between the samples from the Ordos Basin and the North China Plain. Group 2 (Hap31–45) included bisexual populations from the Tsaidam Basin and the Alxa area in Inner Mongolia, with three parthenogenetic samples from Russia and Iran. Group 3 (Hap46–67) corresponded to the parthenogenetic populations around Eurasia, from the coast of China to the west of the Mediterranean. Group 2 (Hap31–45) and group 3 (Hap46–67) were connected by two mutations, while the parthenogenetic samples from KKXL and Yinggehai (Hainan Province in China) differed in more than 25 mutations from the other parthenogenetic haplotypes.

Figure 8.
The relationship network of CO1 haplotypes constructed from 172 sequences for the Artemia populations in the Eastern Lineage. The size of each circle is proportional to the frequency of specimens. Black dots indicate intermediate or unsampled haplotypes. Dashes represent the number of nucleotides substitutions.
The Western Lineage presented 118 haplotypes within the 667 individuals (Figure 9), which could be gathered into four groups. Group 1 (Hap1–32) was composed of parthenogenetic populations from Asia (including China, Kazakhstan, Russia, Iran, Pakistan, Ukraine, Israel and India), the Mediterranean (including southern Europe and northern Africa) and other locations in southern Africa (HM998995, Swakopmund, Namibia) and eastern Africa (HM998999, Ankiembe, Madagascar). These parthenogenetic populations shared a major haplotype among 43.03% of the total individuals. Group 2 (Hap33–91) was composed of bisexual populations from Iran (A. urmiana), QTP (XH, YSH and JYH populations) and Kazakhstan. Group 3 (Hap92–101) was also composed of parthenogenetic populations from inland China, Central and Western Asia, and the Mediterranean. These three haplotype groups were connected by one or two mutations, while group 4 (Hap102–118), which was formed by samples from the LGC, GLC and QXC populations, was genetically distant from them.
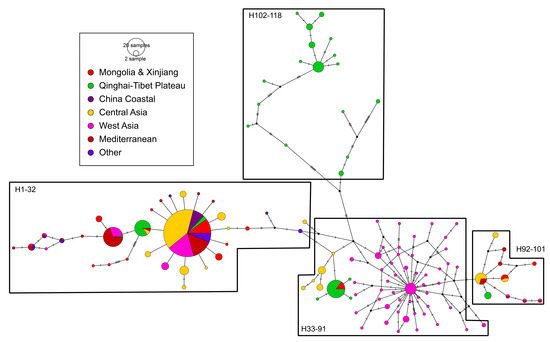
Figure 9.
The relationship network of CO1 haplotypes constructed from 634 sequences for the Artemia populations in the Western Lineage. The size of each circle is proportional to the frequency of specimens. Black dots indicate intermediate or unsampled haplotypes. Dashes represent the number of nucleotide substitutions.
4. Discussion
Morphometrical traits have been used as the basis for describing species and strains. Male Artemia species could be identified by observing their gonopods and second antennal frontal knobs under a scanning electron microscope (SEM) [16,27,28,29,30,31]. In females, the phenotypic pattern of ovisac is thought to be useful for distinction [27,32], but it is not as commonly used as morphological traits in males. Since the study by Hontoria [14] on the variation in morphology of Artemia females from the Iberian Peninsula and adjacent areas, discriminant analyses have been used for species and strain identification. In this study, we chose 14 parameters, following the studies by Hontoria [14] and Triantaphyllidis [15]. The discriminant analysis results classified the 14 Chinese Artemia populations into two separate groups: A. franciscana from the Bohai bay and another group including A. sinica and A. parthenogentica. The invasion of A. franciscana in China has been documented since 1989, and it was initially introduced to coastal salterns for commercial interests [26]. This exotic bisexual species co-occurs with indigenous parthenogenetic Artemia and even gradually replaces the native species. The morphological analysis and subsequent validation through the use of the CO1 marker confirmed the invasion, as evidenced by the fact that all samples from the CZ and HG populations were A. franciscana, while the HF and TS populations contained both Chinese native species and A. franciscana. However, female A. franciscana in the HF population did not exhibit typical characteristics, showing a thick and short abdomen. Instead, the body shape of female A. franciscana in the HF population was similar to that of A. sinica, leading to a centroid shift toward the A. sinica group in the discriminant analysis and aggregation with the A. sinica populations in the hierarchical clustering analysis.
Previous studies have focused on the morphological patterns of bisexual and parthenogenetic populations in the Old World, respectively [15,20,33], but few studies have combined them for analysis. Our results showed that although the parthenogenetic populations were close to each other, they could not be completely separated from the A. sinica group as A. franciscana was in the discriminant analysis. An analysis of interspecific differences revealed that these two species could not be easily identified based on body shape. The variables that were most conducive to distinguishing between A. sinica and A. parthenogenetica were the width of the head, the length of the first antenna, the diameter of the eyes, the distance between the eyes and the number of setae on the furca. The results of hierarchical clustering also illustrated the overlap in morphological characteristics between bisexual and parthenogenetic populations. The evolution of asexual lineages is a complicated issue. Both previous studies and our molecular results indicated that A. parthenogenetica populations are closely related with bisexual species in the Old World, including A. sinica, A. urmiana and A. tibetiana [7,13]. Rare males produced by asexual lineages can hybridize with these bisexual females and transmit asexuality to their offspring [34]. The similarity in body shape between parthenogenetic and A. sinica populations might be due to their close relationship. The main reason for a higher discrimination accuracy at the strain level but a lower accuracy at the species level among females is the misclassification between A. sinica and A. parthenogenetica. In contrast, the misclassification of males at the strain level quite often occurs within species. Zheng [19] also found that rare males of A. parthenogenetica from Shandong and GH diverged from bisexual species. The inconsistency between the male and female results could be attributed to the lower selection pressure on female A. parthenogenetica. Therefore, we postulate that the traditional classification method for Artemia based on morphological features has limitations and is more applicable to males than females.
Compared to discriminant analysis, PCA is another method for classification through dimensionality reduction that could preserve the most relevant information from the original data [35]. Besides female A. franciscana in the HF population, male A. sinica from the BD population clustered separately in the PCA results, showing a uniqueness that was consistent with the ANOVA results. Male individuals from the BD population exhibited significant differences in body size, first antennae and furcal shape compared to individuals from the other populations. Moreover, females in the BD population exhibited a similar pattern of first antennae and furca to A. parthenogenetica and clustered with the parthenogenetic populations in the discriminant analysis. Based on the above results, we speculate that the Badanjinlin Desert region might play an important role in the evolution of A. sinica. However, despite the existence of intraspecific differences among populations, most samples were still mixed in the PCA plot and were difficult to distinguish. This suggests that it is insufficient to complete the Artemia taxonomy solely based on classical morphometrics. Zheng [30] proposed a new set of parameters for male taxonomy based on the frontal knobs and gonopods that could support the validity of A. tibetiana status, but the problem of female taxonomy remained. Thus, the use of molecular markers could be the most effective approach for Artemia species identification.
The benefits of employing a mitochondrial CO1 fragment for species identification and evolutionary studies have been validated through extensive research studies [36,37,38,39]. Our CO1 phylogeny showed that the Artemia samples clustered into three groups: the American Lineage, Eastern Lineage and Western Lineage. Most of the bisexual samples from the Bohai Bay were A. franciscana located in the American Lineage. GSL and SFB are two primary commercialized sources of A. franciscana invasion into Eurasia [6,40], and the common haplotypes shared with the Bohai Bay samples contained sequences from both of these two localities. Additionally, the samples from Chile, Jamaica and Mexico also accounted for a major proportion in shared haplotypes. The genetic evidence suggests that, in addition to human introductions from GSL and SFB, these sites also exhibit signs of natural distribution, probably due to bird migration [41]. Therefore, we do not rule out the possibility that A. franciscana in the Bohai Bay originated from different regions in America. Wang [42] once observed that all specimens that were collected from different salterns in the Bohai Bay with multiple sampling over time were A. franciscana, which indicates that the introduction of A. franciscana into the Bohai Bay, or even the coastal areas of China, was not a single event. The high adaptive potential and plasticity of A. franciscana allowed this species to successfully colonize along China’s coastal regions, followed by a rapid displacement of native parthenogenetic populations.
Regarding the Eurasian species, the confirmation of the multiple independent origins of A. parthenogenetica [13,25,43] was established, as the parthenogenetic populations did not form a monophyletic group. These parthenogenetic populations clustered within the Eastern and Western Lineages without a significant geographic structure. The split between the Eastern and Western Lineage occurred at 6.31 Ma during the late Miocene. Much of the world’s present landscape dates from the Miocene period due to a series of geological events [44,45]. The uplift of the Himalayas and the Tibetan region began at about 50 Ma, but the Tibetan Plateau did not form until the Neogene, which occurred concurrently with the Himalaya rising above 5 km by ~15 Ma. Further elevation of the Tibetan Plateau occurred around 10–8 Ma. The uplift of the Himalayas and topographic changes in the Tibetan Plateau drove the formation of the South Asian monsoon and modern East Asian monsoon, exacerbating the aridification of inland Asia [46,47]. The divergences between the Western and Eastern Lineages that were caused by climatic and geographic variations have been reported in multiple species [48,49,50]. Therefore, we speculate that the separation of the Asian Lineage in Artemia is related to the Himalayan orogeny and accompanying climate change. In studies as early as those in the 2010s, it was discovered that the mitochondrial sequences of A. tibetiana clustered into two non-sister clades [6,7,51]. Thus, the taxonomic status of bisexual populations in the Tibetan region remains to be elucidated. However, in the Western Lineage of our phylogeny, A. tibetiana with traditional defination clustered into three non-sister clades. The sequences from QXC, Yangnapeng Co and Nima Lake in Tibet clustered with A. sorgeloosi (NC072958.1) as the basal clade of the Western Lineage. The GLC population clustered with the samples from LGC, the type locality of A. tibetiana. The other samples from the JYH, XH and YSH populations clustered with A. amati and an asexual lineage, forming a sister group with A. urmiana. A. sorgeloosi and A. amati are two new bisexual species, identified by Asem [4]. The specimens of A. sorgeloosi and A. amati came from Haiyan Lake in Qinghai, China, and an unknow locality in Kazakhstan. The haplotype network showed a distant relationship between the Hap102–118 group (bisexual samples from the LGC, GLC and QXC populations) and the other groups, while the bisexual samples from Clade 8 and Kazakhstan clustered with A. urimiana. Meanwhile, the haplotypes of the parthenogenetic populations diverged into two distinct groups, but both were genetically related to A. urmiana. Considering the high contribution of genetic variation among groups with frequent gene flows that were observed between the JYH population and A. urmiana [12], we agree with Asem that A. sorgeloosi is a new species, but we remain skeptical about the taxonomic status of A. amati. These bisexual samples from Kazakhstan and Tibet could be intermediates in the evolution of the A. urmiana lineage from sexual reproduction to parthenogenesis.
Similar to other species, the divergence of A. sinica occurred during the Pleistocene with a clear phylogeographic structure. Compared to Europe and North America, the East Asian continent underwent a mild Quaternary climate due to its complex terrain and strong influence from monsoons. However, the cold–warm climate oscillations that were caused by the alternation of ice ages and interglacial periods still affected many species in terms of diversity and distributions [52]. According to the results of the divergence time and ancestral distributions, we propose the most likely scenario to have occurred to depict the evolutionary route of A. sinica. In the early Pleistocene, A. sinica diverged into eastern and western groups along the northeastern margin of the Tibetan Plateau, resulting in a deep mitochondrial lineage split. The North China Plain could be a refuge for Artemia, which expanded northward into central Inner Mongolia after the glacial periods. The importance of northern refuges has been emphasized in plants, including aquatic and terrestrial species [53,54]. Due to the unique sedimentary characteristics in geography [55], the samples from the Ordos Basin have a set of private haplotypes, illustrating a narrow genetic structure with limited distribution. The populations of the western group might have spread from the Tsaidam Basin to the northwest and the hinterland of the QTP. A. parthenogenetica in the Eastern Lineage diverged from Northwestern China during the Middle–Upper Pleistocene and spread throughout Eurasia, which was supported by the results showing significantly different morphological characteristics of the BD population from the other A. sinica populations. An expansion of asexual lineages was not a rare phenomenon during the Pleistocene [56,57,58,59]. This phenomenon is often explained by the hypothesis that asexual species were able to colonize faster than sexual species after the glacial periods, as parthenogenetic reproduction was beneficial over mating to cope with short suitable seasons [60], which means that the de-glaciation periods during the Pleistocene could have provided great opportunities for the evolution of asexual organisms. The significant negative values of the neutrality test also imply a historical population expansion of asexual lineages. Moreover, considering the extremely close relationship in haplotypes between asexual and bisexual species from the northwestern area, as well as the parthenogenetic samples from central and western Asia that were found in the bisexual Alxa clade and the morphological evidence, we agree to not include A. parthenogenetica in the Eastern Lineage as an independent taxon [8,11,43]. This taxon should be considered a subspecies of A. sinica, which evolved asexual reproduction in response to climate oscillations during the Pleistocene.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d16030144/s1: Figure S1. Comparison of morphological differences between females of A. franciscana, A. parthenogenetica and A. sinica. Figure S2. Comparison of morphological differences between males of A. franciscana and A. sinica. Figure S3. Comparison of morphological differences between females of A. franciscana populations. Figure S4. Comparison of morphological differences between males of A. franciscana populations. Figure S5. Comparison of morphological differences between females of A. sinica populations. Figure S6. Comparison of morphological differences between males of A. sinica populations. Figure S7. Comparison of morphological differences between A. parthenogenetica populations. Figure S8. Comparison of morphological differences between A. parthenogenetica and female A. sinica in the BD population. Figure S9. Scatterplot of the first two functions obtained from PCA of (A) female A. franciscana populations; (B) female A. sinica populations; (C) A. parthenogenetica populations; (D) male A. franciscana populations; and (E) male A. sinica populations. Table S1. CO1 reference sequences used in haplotype network analysis. Table S2. Accuracy of assignment at the species level for each population. References [4,6,7,8,9,10,13,24,25,41,42,43,61,62,63,64,65,66] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, D.Z. and Y.Z.; investigation, H.P., K.Z., W.W. and M.Z.; visualization: H.P.; writing—original draft preparation, H.P.; writing—review and editing: D.Z and Y.Z.; supervision, D.Z. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31872274) and the Hebei Provincial Innovation Capacity Enhancement Program Special Project for High-level Talent Team Building (No. 225A2904D).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The CO1 sequences obtained from this study were deposited into the NCBI GenBank (PP093070-PP093156).
Acknowledgments
We thank Xiangchu Yin and Hong Yin for their contributions to the Artemia cyst collection. We also thank Shichun Sun, Alireza Asem and Tangbin Huo for providing Artemia cysts from the YM, BD, URM and ABI populations.
Conflicts of Interest
We declare that we have no financial or personal relationships with other people or organizations that can inappropriately influence our work. There is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript.
References
- Dhont, J.; Sorgeloos, P. Applications of Artemia. In Artemia: Basic and Applied Biology; Abatzopoulos, T.J., Beardmore, J.A., Clegg, J.S., Sorgeloos, P., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 251–277. [Google Scholar]
- Van Stappen, G. Zoogeography. In Artemia: Basic and Applied Biology; Abatzopoulos, T.J., Beardmore, J.A., Clegg, J.S., Sorgeloos, P., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 171–224. [Google Scholar]
- Kappas, I.; Baxevanis, A.D.; Abatzopoulos, T.J. Phylogeographic Patterns in Artemia: A Model Organism for Hypersaline Crustaceans, in Phylogeography and Population Genetics in Crustacea; Held, C., Koenemann, S., Christoph, D., Schubart, C.D., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2011; pp. 233–255. [Google Scholar]
- Asem, A.; Yang, C.; Eimanifar, A.; Hontoria, F.; Varó, I.; Mahmoudi, F.; Fu, C.-Z.; Shen, C.-Y.; Rastegar-Pouyani, N.; Wang, P.-Z.; et al. Phylogenetic analysis of problematic Asian species of Artemia Leach, 1819 (Crustacea, Anostraca), with the descriptions of two new species. J. Crustac. Biol. 2023, 43, ruad002. [Google Scholar] [CrossRef]
- Gajardo, G.; Abatzopoulos, T.J.; Kappas, I.; Beardmore, J.A. Evolution and Speciation. In Artemia: Basic and Applied Biology; Abatzopoulos, T.J., Beardmore, J.A., Clegg, J.S., Sorgeloos, P., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 225–250. [Google Scholar]
- Eimanifar, A.; Van Stappen, G.; Marden, B.; Wink, M. Artemia biodiversity in Asia with the focus on the phylogeography of the introduced American species Artemia franciscana Kellogg, 1906. Mol. Phylogenetics Evol. 2014, 79, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Maccari, M.; Amat, F.; Gómez, A. Origin and Genetic Diversity of Diploid Parthenogenetic Artemia in Eurasia. PLoS ONE 2013, 8, e83348. [Google Scholar] [CrossRef] [PubMed]
- Eimanifar, A.; Van Stappen, G.; Wink, M. Geographical distribution and evolutionary divergence times of Asian populations of the brine shrimp Artemia (Crustacea, Anostraca). Zool. J. Linn. Soc. 2015, 174, 447–458. [Google Scholar] [CrossRef]
- Abatzopoulos, T.J.; Amat, F.; Baxevanis, A.D.; Belmonte, G.; Hontoria, F.; Maniatsi, S.; Moscatello, S.; Mura, G.; Shadrin, N.V. Updating Geographic Distribution of Artemia urmiana Günther, 1890 (Branchiopoda: Anostraca) in Europe: An Integrated and Interdisciplinary Approach. Int. Rev. Hydrobiol. 2009, 94, 560–579. [Google Scholar] [CrossRef]
- Hou, L.; Bi, X.; Zou, X.; He, C.; Yang, L.; Qu, R.; Liu, Z. Molecular systematics of bisexual Artemia populations. Aquac. Res. 2006, 37, 671–680. [Google Scholar] [CrossRef]
- Sainz-Escudero, L.; López-Estrada, E.K.; Rodríguez-Flores, P.C.; García-París, M. Settling taxonomic and nomenclatural problems in brine shrimps, Artemia (Crustacea: Branchiopoda: Anostraca), by integrating mitogenomics, marker discordances and nomenclature rules. PeerJ 2021, 9, e10865. [Google Scholar] [CrossRef]
- Li, W.-J.; Guo, Y.; Sun, S.-C. Population genetics of Artemia urmiana species complex (Crustacea, Anostraca): A group with asymmetrical dispersal and gene flow mediated by migratory waterfowl. Gene 2024, 894, 147957. [Google Scholar] [CrossRef]
- Asem, A.; Eimanifar, A.; Sun, S.-C. Genetic variation and evolutionary origins of parthenogenetic Artemia (Crustacea: Anostraca) with different ploidies. Zool. Scr. 2016, 45, 421–436. [Google Scholar] [CrossRef]
- Hontoria, F.; Amat, F. Morphological characterization of adult Artemia (Crustacea, Branchiopoda) from different geographical origin. Mediterranean populations. J. Plankton Res. 1992, 14, 949–959. [Google Scholar] [CrossRef]
- Triantaphyllidis, G.V.; Criel, G.R.J.; Abatzopoulos, T.J.; Sorgeloos, P. International study on Artemia. LIII. Morphological study of Artemia with emphasis to Old World strains. I. Bisexual populations. Hydrobiologia 1997, 357, 139–153. [Google Scholar] [CrossRef]
- Triantaphyllidis, G.V.; Criel, G.R.J.; Abatzopoulos, T.J.; Thomas, K.M.; Peleman, J.; Beardmore, J.A.; Sorgeloos, P. International Study on Artemia. LVII. Morphological and molecular characters suggest conspecificity of all bisexual European and North African Artemia populations. Mar. Biol. 1997, 129, 477–487. [Google Scholar] [CrossRef]
- Baxevanis, A.D.; Triantaphyllidis, G.V.; Kappas, I.; Triantafyllidis, A.; Triantaphyllidis, C.D.; Abatzopoulos, T.J. Evolutionary assessment of Artemia tibetiana (Crustacea, Anostraca) based on morphometry and 16S rRNA RFLP analysis. J. Zool. Syst. Evol. Res. 2005, 43, 189–198. [Google Scholar] [CrossRef]
- Hachem Ben, N.; Amel Ben Rejeb, J.; Salah, R.M. Morphometric Characterization of Adult Artemia (Crustacea: Branchiopoda) Populations from Costal and Inland Tunisian Salt Lakes. Afr. Invertebr. 2013, 54, 543–555. [Google Scholar]
- Zheng, B.; Sun, S.-C. Morphology and biometry of two Chinese diploid parthenogenetic Artemia populations with a special emphasis on the gonopods and frontal knobs of rare males. Zool. Anz. 2023, 303, 80–89. [Google Scholar] [CrossRef]
- Triantaphyllidis, G.V.; Criel, G.R.J.; Abatzopoulos, T.J.; Sorgeloos, P. International study on Artemia. LIV. Morphological study of Artemia with emphasis to Old World strains. II. Parthenogenetic populations. Hydrobiologia 1997, 357, 155–163. [Google Scholar] [CrossRef]
- Asem, A. Comparative Morphology and Molecular Phylogeny of Parthenogenetica Artemia (Crustacea: Anostraca) from China. Ph.D. Thesis, Degree-Ocean University of China, Qiangdao, China, 2016. [Google Scholar]
- De Vos, S.; Rombauts, S.; Coussement, L.; Dermauw, W.; Vuylsteke, M.; Sorgeloos, P.; Clegg, J.S.; Nambu, Z.; Van Nieuwerburgh, F.; Norouzitallab, P.; et al. The genome of the extremophile Artemia provides insight into strategies to cope with extreme environments. BMC Genom. 2021, 22, 635. [Google Scholar] [CrossRef] [PubMed]
- Deji, G.; Zhang, C.; Sui, L.; Han, X. The complete mitochondrial genome of Artemia salina Leach, 1819 (Crustacea: Anostraca). Mitochondrial DNA Part B 2021, 6, 3255–3256. [Google Scholar] [CrossRef]
- Asem, A.; Eimanifar, A.; Li, W.; Shen, C.-Y.; Shikhsarmast, F.M.; Dan, Y.-T.; Lu, H.; Zhou, Y.; Chen, Y.; Wang, P.-Z.; et al. Reanalysis and Revision of the Complete Mitochondrial Genome of Artemia urmiana Günther, 1899 (Crustacea: Anostraca). Diversity 2021, 13, 14. [Google Scholar] [CrossRef]
- Sainz-Escudero, L.; López-Estrada, E.K.; Rodríguez-Flores, P.C.; García-París, M. Brine shrimps adrift: Historical species turnover in Western Mediterranean Artemia (Anostraca). Biol. Invasions 2022, 24, 2477–2498. [Google Scholar] [CrossRef]
- Zheng, B.; Sun, S.-C. Review of the biogeography of Artemia Leach, 1819 (Crustacea: Anostraca) in China. Int. J. Artemia Biol. 2013, 3, 20–50. [Google Scholar]
- Torrentera, L.; Dodson, S.I. Morphological Diversity of Populations of Artemia (Branchiopoda) in Yucatan. J. Crustac. Biol. 1995, 15, 86–102. [Google Scholar] [CrossRef]
- Torrentera, L.; Belk, D. New penis characters to distinguish between two American Artemia species. Hydrobiologia 2002, 470, 149–156. [Google Scholar] [CrossRef]
- Mura, G.; Brecciaroli, B. Use of morphological characters for species separation within the genus Artemia (Crustacea, Branchiopoda). Hydrobiologia 2004, 520, 179–183. [Google Scholar] [CrossRef]
- Zheng, B.; Sun, S.-C. Taxonomic consideration of eight Chinese bisexual Artemia populations, based on the morphology of frontal knob and gonopod and the result of cross-breeding tests. Zootaxa 2008, 1919, 25–44. [Google Scholar] [CrossRef]
- Mura, G.; Gajardo, G. The highly divergent New World Artemia species (Branchiopoda, Anostraca), A. franciscana and A. persimilis, show subtle differences in morphological traits involved in mating. Zootaxa 2011, 2912, 37–48. [Google Scholar] [CrossRef]
- Amat, F. Differentiation in Artemia strains from Spain. In The Brine Shrimp Artemia; Universa Press Wetteren: Belgium, Switzerland, 1980; pp. 19–39. [Google Scholar]
- Asem, A.; Sun, S.-C. Morphological differentiation of seven parthenogenetic Artemia (Crustacea: Branchiopoda) populations from China, with special emphasis on ploidy degrees. Microsc. Res. Tech. 2016, 79, 258–266. [Google Scholar] [CrossRef]
- Boyer, L.; Jabbour-Zahab, R.; Mosna, M.; Haag, C.R.; Lenormand, T. Not so clonal asexuals: Unraveling the secret sex life of Artemia parthenogenetica. Evol. Lett. 2021, 5, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Sun, M.; Lian, J.; Hou, S. Feature dimensionality reduction: A review. Complex Intell. Syst. 2022, 8, 2663–2693. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Gholamzadeh, S.; Incekara, Ü. Review of molecular taxonomy studies on Coleoptera aquatic insects. Int. J. Entomol. Res. 2016, 4, 25–36. [Google Scholar]
- Rach, J.; Bergmann, T.; Paknia, O.; DeSalle, R.; Schierwater, B.; Hadrys, H. The marker choice: Unexpected resolving power of an unexplored CO1 region for layered DNA barcoding approaches. PLoS ONE 2017, 12, e0174842. [Google Scholar] [CrossRef] [PubMed]
- Girard, E.B.; Langerak, A.; Jompa, J.; Wangensteen, O.S.; Macher, J.N.; Renema, W. Mitochondrial Cytochrome Oxidase Subunit 1: A Promising Molecular Marker for Species Identification in Foraminifera. Front. Mar. Sci. 2022, 9, 809659. [Google Scholar] [CrossRef]
- Horváth, Z.; Lejeusne, C.; Amat, F.; Sánchez-Fontenla, J.; Vad, C.F.; Green, A.J. Eastern spread of the invasive Artemia franciscana in the Mediterranean Basin, with the first record from the Balkan Peninsula. Hydrobiologia 2018, 822, 229–235. [Google Scholar] [CrossRef]
- Muñoz, J.; Amat, F.; Green, A.J.; Figuerola, J.; Gomez, A. Bird migratory flyways influence the phylogeography of the invasive brine shrimp Artemia franciscana in its native American range. Peerj 2013, 1, e200. [Google Scholar] [PubMed]
- Wang, W.; Luo, Q.; Guo, H.; Bossier, P.; Van Stappen, G.; Sorgeloos, P.; Xin, N.; Sun, Q.; Hu, S.; Yu, J. Phylogenetic analysis of brine shrimp (artemia) in China using DNA barcoding. Genom. Proteom. Bioinform. 2008, 6, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Maniatsi, S.; Baxevanis, A.D.; Kappas, I.; Deligiannidis, P.; Triantafyllidis, A.; Papakostas, S.; Bougiouklis, D.; Abatzopoulos, T.J. Is polyploidy a persevering accident or an adaptive evolutionary pattern? The case of the brine shrimp Artemia. Mol. Phylogenet. Evol. 2011, 58, 353–364. [Google Scholar] [CrossRef]
- Potter, P.E.; Szatmari, P. Global Miocene tectonics and the modern world. Earth-Sci. Rev. 2009, 96, 279–295. [Google Scholar] [CrossRef]
- Steinthorsdottir, M.; Coxall, H.K.; De Boer, A.M.; Huber, M.; Barbolini, N.; Bradshaw, C.D.; Burls, N.J.; Feakins, S.J.; Gasson, E.; Henderiks, J.; et al. The Miocene: The Future of the Past. Paleoceanogr. Paleoclimatol. 2021, 36, e2020PA004037. [Google Scholar] [CrossRef]
- Zhisheng, A.; Kutzbach, J.E.; Prell, W.L.; Porter, S.C. Evolution of Asian monsoons and phased uplift of the Himalaya–Tibetan plateau since Late Miocene times. Nature 2001, 411, 62–66. [Google Scholar] [CrossRef]
- Spicer, R.A.; Su, T.; Valdes, P.J.; Farnsworth, A.; Wu, F.X.; Shi, G.; Spicer, T.E.V.; Zhou, Z. Why ‘the uplift of the Tibetan Plateau’ is a myth. Natl. Sci. Rev. 2020, 8, nwaa091. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.-L.; Wang, M.-M.; Cun, Y.-Z.; Yang, F.-S.; Wang, S.-S.; Ran, J.-H.; Wang, X.-Q. Phylogeographic Evidence for a Link of Species Divergence of Ephedra in the Qinghai-Tibetan Plateau and Adjacent Regions to the Miocene Asian Aridification. PLoS ONE 2013, 8, e56243. [Google Scholar] [CrossRef]
- Zhu, L.; Song, J.; Zhou, J.-L.; Si, J.; Cui, B.-K. Species Diversity, Phylogeny, Divergence Time, and Biogeography of the Genus Sanghuangporus (Basidiomycota). Front. Microbiol. 2019, 10, 812. [Google Scholar] [CrossRef]
- Othman, S.N.; Litvinchuk, S.N.; Maslova, I.; Dahn, H.; Messenger, K.R.; Andersen, D.; Jowers, M.J.; Kojima, Y.; Skorinov, D.V.; Yasumiba, K.; et al. From Gondwana to the Yellow Sea, evolutionary diversifications of true toads Bufo sp. in the Eastern Palearctic and a revisit of species boundaries for Asian lineages. eLife 2022, 11, e70494. [Google Scholar] [CrossRef]
- Asem, A.; Eimanifar, A.; Rastegar-Pouyani, N.; Hontoria, F.; De Vos, S.; Van Stappen, G.; Sun, S.-C. An overview on the nomenclatural and phylogenetic problems of native Asian brine shrimps of the genus Artemia Leach, 1819 (Crustacea, Anostraca). ZooKeys 2020, 902, 1–15. [Google Scholar] [CrossRef]
- Fu, J.; Wen, L. Impacts of Quaternary glaciation, geological history and geography on animal species history in continental East Asia: A phylogeographic review. Mol. Ecol. 2023, 32, 4497–4514. [Google Scholar] [CrossRef]
- Li, J.K.; Zhou, E.X.; Li, D.X.; Huang, S.Q. Multiple northern refugia for Asian sacred lotus, an aquatic plant with characteristics of ice-age endurance. Aust. J. Bot. 2010, 58, 463–472. [Google Scholar] [CrossRef]
- Hao, Q. Glacial Refugia and the Postglacial Migration of Dominant Tree Species in Northern China. In The LGM Distribution of Dominant Tree Genera in Northern China’s Forest-Steppe Ecotone and Their Postglacial Migration; Springer: Singapore, 2018; pp. 31–56. [Google Scholar]
- Yang, R.; van Loon, A.J. Chapter 1—Sedimentary and tectonic development of the Ordos Basin and its hydrocarbon potential. In The Ordos Basin; Yang, R., Van Loon, A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–29. [Google Scholar]
- Little, T.J.; Hebert, P.D.N. Abundant asexuality in tropical freshwater ostracodes. Heredity 1994, 73, 549–555. [Google Scholar] [CrossRef][Green Version]
- Neiman, M.; Jokela, J.; Lively, C.M. Variation in asexual lineage age in Potamopyrgus antipodarum, a New Zealand snail. Evolution 2005, 59, 1945–1952. [Google Scholar]
- Kearney, M.; Blacket, M.J. The evolution of sexual and parthenogenetic Warramaba: A window onto Plio–Pleistocene diversification processes in an arid biome. Mol. Ecol. 2008, 17, 5257–5275. [Google Scholar] [CrossRef] [PubMed]
- Borissov, S.B.; Hristov, G.H.; Chobanov, D.P. Phylogeography of the Poecilimon ampliatus species group (Orthoptera: Tettigoniidae) in the context of the Pleistocene glacial cycles and the origin of the only thelytokous parthenogenetic phaneropterine bush-cricket. Arthropod Syst. Phylogeny 2021, 79, 401–418. [Google Scholar] [CrossRef]
- Schön, I. Did Pleistocene glaciations shape genetic patterns of European ostracods? A phylogeographic analysis of two species with asexual reproduction. Hydrobiologia 2007, 575, 33–50. [Google Scholar] [CrossRef]
- Maccari M, Gómez A, Hontoria F, Amat, F. Functional rare males in diploid parthenogenetic Artemia. Journal of Evolutionary Biology. 2013, 26, 1934–1948.
- Zuo, J.; Xu, Q.; Deng, Y.; Liang, X.; Han, X.; Sui, L. Comparative study of Artemia taxonomic and evolution using three DNA barcodes (in Chinese). J. Tianjin Univ. Sci. Technol. 2022, 37, 28–36. [Google Scholar]
- Naganawa, H.; Mura, G. Two new cryptic species of Artemia (Branchiopoda, Anostraca) from Mongolia and the possibility of invasion and disturbance by the aquaculture industry in East Asia. Crustaceana 2017, 90, 1679–1698. [Google Scholar] [CrossRef]
- Muñoz, J.; Gomez, A.; Green, A.J.; Figuerola, J.; Amat, F.; Rico, C. Evolutionary origin and phylogeography of the diploid obligate parthenogen Artemia parthenogenetica (Branchiopoda: Anostraca). PLoS ONE 2010, 5, e11932. [Google Scholar] [CrossRef]
- Asem, A.; Li, W.; Wang, P.Z.; Eimanifar, A.; Shen, C.Y.; De Vos, S.; Van Stappen, G. The complete mitochondrial genome of Artemia sinica Cai, 1989 (Crustacea: Anostraca) using next-generation sequencing. Mitochondrial DNA Part B 2019, 4, 746–747. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Q.; Sun, J.; Liu, F.; Wu, G.; Yu, J.; Wang, W. Mitochondrial genome sequences of Artemia tibetiana and Artemia urmiana: Assessing molecular changes for high plateau adaptation. Sci. China Life Sci. 2013, 56, 440–452. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).