Resilience of Aboveground Biomass of Secondary Forests Following the Abandonment of Gold Mining Activity in the Southeastern Peruvian Amazon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design and Vegetation Sampling
2.3. Aboveground Biomass (AGB)
2.4. Stand Structure and Alpha and Beta-Diversity Calculations
2.5. Data Analysis
3. Results
3.1. Effects of Mining Type, Stand Age, and Distance from the Forest on AGB
Distance from the Forest Edge Strongly Mediates the Effects of Species Diversity on AGB According to Mining Type
3.2. Recovery of AGB, Tree Diversity and Forest Structure
3.3. Tree Species Dominance and Composition
4. Discussion
4.1. Effects of Mining Type, Stand Age, and Distance from the Forest on AGB
4.2. Recovery of AGB, Tree Diversity, and Forest Structure
4.3. Tree Species Dominance and Composition
4.4. Limitations and Suggestions for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, D.L.M.; Lewis, S.L.; Sullivan, M.J.P.; Prado, P.I.; ter Steege, H.; Barbier, N.; Slik, F.; Sonké, B.; Ewango, C.E.N.; Adu-Bredu, S.; et al. Consistent patterns of common species across tropical tree communities. Nature 2024, 625, 728–734. [Google Scholar] [CrossRef]
- Houghton, R.A.; Skole, D.L.; Nobre, C.A.; Hackler, J.L.; Lawrence, K.T.; Chomentowski, W.H. Annual fluxes of carbon from deforestation and regrowth in the Brazilian Amazon. Nature 2000, 403, 301–304. [Google Scholar] [CrossRef]
- Mitchard, E.T.A. The tropical forest carbon cycle and climate change. Nature 2018, 559, 527–534. [Google Scholar] [CrossRef]
- Tito, R.; Salinas, N.; Cosio, E.G.; Boza Espinoza, T.E.; Muñiz, J.G.; Aragón, S.; Nina, A.; Roman-Cuesta, R.M. Secondary forests in Peru: Differential provision of ecosystem services compared to other post-deforestation forest transitions. Ecol. Soc. 2022, 27, art12. [Google Scholar] [CrossRef]
- Asner, G.P.; Powell, G.V.N.; Mascaro, J.; Knapp, D.E.; Clark, J.K.; Jacobson, J.; Kennedy-Bowdoin, T.; Balaji, A.; Paez-Acosta, G.; Victoria, E.; et al. High-resolution forest carbon stocks and emissions in the Amazon. Proc. Natl. Acad. Sci. USA 2010, 107, 16738–16742. [Google Scholar] [CrossRef]
- Hall, J.S.; Plisinski, J.S.; Mladinich, S.K.; van Breugel, M.; Lai, H.R.; Asner, G.P.; Walker, K.; Thompson, J.R. Deforestation scenarios show the importance of secondary forest for meeting Panama’s carbon goals. Landsc. Ecol. 2022, 37, 673–694. [Google Scholar] [CrossRef]
- Aragón, S.; Salinas, N.; Nina-Quispe, A.; Qquellon, V.H.; Paucar, G.R.; Huaman, W.; Porroa, P.C.; Olarte, J.C.; Cruz, R.; Muñiz, J.G.; et al. Aboveground biomass in secondary montane forests in Peru: Slow carbon recovery in agroforestry legacies. Glob. Ecol. Conserv. 2021, 28, e01696. [Google Scholar] [CrossRef]
- Cherlet, M.; Hutchinson, C.; Reynolds, J.; Hill, J.; Sommer, S.; von Maltitz, G. World Atlas of Desertification, Mapping Land Degradation and Sustainable Land Management Opportunities, 3rd ed.; Publication Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Walker, L.R.; del Moral, R. Primary Succession and Ecosystem Rehabilitation; Cambridge University Press: New York, NY, USA, 2003; ISBN 9780521800761. [Google Scholar]
- Prach, K.; Walker, L.R. Differences between primary and secondary plant succession among biomes of the world. J. Ecol. 2019, 107, 510–516. [Google Scholar] [CrossRef]
- Rocha-Nicoleite, E.; Campos, M.L.; Colombo, G.T.; Overbeck, G.E.; Müller, S.C. Forest restoration after severe degradation by coal mining: Lessons from the first years of monitoring. Brazilian J. Bot. 2018, 41, 653–664. [Google Scholar] [CrossRef]
- Kuramoto, J. Artisanal and Informal Mining in Peru; International Institute for Enviroment and Development, and World Business Council for Sustainable Development: London, UK, 2001. [Google Scholar]
- Stoll, E.; Roopsind, A.; Maharaj, G.; Velazco, S.; Caughlin, T.T. Detecting gold mining impacts on insect biodiversity in a tropical mining frontier with SmallSat imagery. Remote Sens. Ecol. Conserv. 2022, 8, 379–390. [Google Scholar] [CrossRef]
- Álvarez-Berríos, N.; L’Roe, J.; Naughton-Treves, L. Does formalizing artisanal gold mining mitigate environmental impacts? Deforestation evidence from the Peruvian Amazon. Environ. Res. Lett. 2021, 16, 064052. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Martínez-Ramos, M.; Bongers, F. Biomass is the main driver of changes in ecosystem process rates during tropical forest succession. Ecology 2015, 96, 1242–1252. [Google Scholar] [CrossRef]
- Csillik, O.; Asner, G.P. Aboveground carbon emissions from gold mining in the Peruvian Amazon. Environ. Res. Lett. 2020, 15, 014006. [Google Scholar] [CrossRef]
- Alvarez-Berríos, N.L.; Mitchell Aide, T. Global demand for gold is another threat for tropical forests. Environ. Res. Lett. 2015, 10, 014006. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F.; Aide, T.M.; Almeyda Zambrano, A.M.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; Broadbent, E.N.; Chazdon, R.L.; et al. Biomass resilience of Neotropical secondary forests. Nature 2016, 530, 211–214. [Google Scholar] [CrossRef]
- Alarcon-Aguirre, G.; Canahuire Robles, R.R.; Guevara Duarez, F.M.; Rodríguez Achata, L.; Gallegos Chacón, L.E.; Garate-Quispe, J. Dynamics of forest loss in the southeast of the Peruvian Amazon: A case study in Madre de Dios. Ecosistemas 2021, 30, 2175. [Google Scholar] [CrossRef]
- Chambi-Legoas, R.; Ortega Rodriguez, D.R.; Figueiredo, F.d.M.d.; Peña Valdeiglesias, J.; Zevallos Pollito, P.A.; Marcelo-Peña, J.L.; Rother, D.C. Natural Regeneration After Gold Mining in the Peruvian Amazon: Implications for Restoration of Tropical Forests. Front. For. Glob. Chang. 2021, 4, 594627. [Google Scholar] [CrossRef]
- Kalamandeen, M.; Gloor, E.; Johnson, I.; Agard, S.; Katow, M.; Vanbrooke, A.; Ashley, D.; Batterman, S.A.; Ziv, G.; Holder-Collins, K.; et al. Limited biomass recovery from gold mining in Amazonian forests. J. Appl. Ecol. 2020, 57, 1730–1740. [Google Scholar] [CrossRef]
- Ploton, P.; Mortier, F.; Barbier, N.; Cornu, G.; Réjou-Méchain, M.; Rossi, V.; Alonso, A.; Bastin, J.F.; Bayol, N.; Bénédet, F.; et al. A map of African humid tropical forest aboveground biomass derived from management inventories. Sci. Data 2020, 7, 221. [Google Scholar] [CrossRef]
- Liu, C.; Chen, D.; Zou, C.; Liu, S.; Li, H.; Liu, Z.; Feng, W.; Zhang, N.; Ye, L. Modeling Biomass for Natural Subtropical Secondary Forest Using Multi-Source Data and Different Regression Models in Huangfu Mountain, China. Sustainability 2022, 14, 13006. [Google Scholar] [CrossRef]
- Abbasi, U.A.; Gilani, H.; Hussain, K.; Ali, A. Increasing stand stature weakens the positive effects of tree richness and structural imbalance on aboveground biomass in temperate forests: The stand stature hypothesis. For. Ecol. Manag. 2023, 539, 121040. [Google Scholar] [CrossRef]
- Fawcett, D.; Sitch, S.; Ciais, P.; Wigneron, J.P.; Silva-Junior, C.H.L.; Heinrich, V.; Vancutsem, C.; Achard, F.; Bastos, A.; Yang, H.; et al. Declining Amazon biomass due to deforestation and subsequent degradation losses exceeding gains. Glob. Chang. Biol. 2023, 29, 1106–1118. [Google Scholar] [CrossRef]
- Messinger, M.; Asner, G.P.; Silman, M. Rapid assessments of amazon forest structure and biomass using small unmanned aerial systems. Remote Sens. 2016, 8, 615. [Google Scholar] [CrossRef]
- Requena Suarez, D.; Rozendaal, D.M.A.; De Sy, V.; Phillips, O.L.; Alvarez-Dávila, E.; Anderson-Teixeira, K.; Araujo-Murakami, A.; Arroyo, L.; Baker, T.R.; Bongers, F.; et al. Estimating aboveground net biomass change for tropical and subtropical forests: Refinement of IPCC default rates using forest plot data. Glob. Chang. Biol. 2019, 25, 3609–3624. [Google Scholar] [CrossRef]
- Cardozo, E.G.; Celentano, D.; Rousseau, G.X.; Silva, H.R.E.; Muchavisoy, H.M.; Gehring, C. Agroforestry systems recover tree carbon stock faster than natural succession in Eastern Amazon, Brazil. Agrofor. Syst. 2022, 96, 941–956. [Google Scholar] [CrossRef]
- Sharma, S.B.; Kumar, S.; Hegde, N. Biomass and carbon recovery of secondary forest in a Montane Subtropical Forest of North Eastern India. Trop. Ecol. 2023, 64, 114–121. [Google Scholar] [CrossRef]
- Oberleitner, F.; Egger, C.; Oberdorfer, S.; Dullinger, S.; Wanek, W.; Hietz, P. Recovery of aboveground biomass, species richness and composition in tropical secondary forests in SW Costa Rica. For. Ecol. Manag. 2021, 479, 118580. [Google Scholar] [CrossRef]
- Oliveira, L.Z.; de Maçaneiro, J.P.; da Silva, D.A.; Uller, H.F.; de Britto, P.C.; Correia, J.; Piazza, G.E.; Zambiazi, D.C.; Vibrans, A.C.; Fantini, A.C. Structure, Biomass and Diversity of a Late-Successional Subtropical Atlantic Forest in Brazil. Floresta E Ambient. 2022, 29, e20210095. [Google Scholar] [CrossRef]
- Wallis, C.I.B.; Crofts, A.L.; Inamdar, D.; Arroyo-Mora, J.P.; Kalacska, M.; Laliberté, É.; Vellend, M. Remotely sensed carbon content: The role of tree composition and tree diversity. Remote Sens. Environ. 2023, 284, 113333. [Google Scholar] [CrossRef]
- Mora, F.; Jaramillo, V.J.; Bhaskar, R.; Gavito, M.; Siddique, I.; Byrnes, J.E.K.; Balvanera, P. Carbon Accumulation in Neotropical Dry Secondary Forests: The Roles of Forest Age and Tree Dominance and Diversity. Ecosystems 2018, 21, 536–550. [Google Scholar] [CrossRef]
- Rebola, L.C.; Paz, C.P.; Gamarra, L.V.; Burslem, D.F. Land use intensity determines soil properties and biomass recovery after abandonment of agricultural land in an Amazonian biodiversity hotspot. Sci. Total Environ. 2021, 801, 149487. [Google Scholar] [CrossRef]
- Rozendaal, D.; Bongers, F.; Aide, T.; Alvarez-Dávila, E.; Ascarrunz, N.; Balvanera, P.; Becknell, J.M.; Bentos, T.V.; Brancalion, P.H.S.; Cabral, G.A.L.; et al. Biodiversity recovery of Neotropical secondary forests. Sci. Adv. 2019, 5, eaau3114. [Google Scholar] [CrossRef]
- Brienen, R.J.W.; Phillips, O.L.; Feldpausch, T.R.; Gloor, E.; Baker, T.R.; Lloyd, J.; Lopez-Gonzalez, G.; Monteagudo-Mendoza, A.; Malhi, Y.; Lewis, S.L.; et al. Long-term decline of the Amazon carbon sink. Nature 2015, 519, 344–348. [Google Scholar] [CrossRef]
- Ojoatre, S.; Barlow, J.; Jacobs, S.R.; Rufino, M.C. Recovery of aboveground biomass, soil carbon stocks and species diversity in tropical montane secondary forests of East Africa. For. Ecol. Manag. 2024, 552, 121569. [Google Scholar] [CrossRef]
- Chazdon, R.; Letcher, S.G.; van Breugel, M.; Martinez-Ramos, M.; Bongers, F.; Finegan, B. Rates of change in tree communities of secondary Neotropical forests following major disturbances. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 273–289. [Google Scholar] [CrossRef]
- Garate-Quispe, J.S.; Canahuire-Robles, R.; Surco-Huacachi, O.; Alarcón-Aguirre, G. Desarrollo estructural y composición florística arbórea en áreas afectadas por minería aurífera en la Amazonía peruana: A 20 años de su reforestación. Rev. Mex. Biodivers. 2021, 92, 923437. [Google Scholar] [CrossRef]
- Alarcón-Aguirre, G.; Sajami Quispe, E.; Vásquez Zavaleta, T.; Ponce Tejada, L.V.; Ramos Enciso, D.; Rodríguez Achata, L.; Garate-Quispe, J. Vegetation dynamics in lands degraded by gold mining in the southeastern Peruvian Amazon. Trees For. People 2023, 11, 100369. [Google Scholar] [CrossRef]
- Garate-Quispe, J.; Velásquez Ramírez, M.; Becerra-Lira, E.; Baez-Quispe, S.; Abril-Surichaqui, M.; Rodriguez-Achata, L.; Muñoz-Ushñahua, A.; Nascimento Herbay, P.; Fernandez-Mamani, Y.; Alarcon-Aguirre, G.; et al. Influence of Distance from Forest Edges on Spontaneous Vegetation Succession Following Small-Scale Gold Mining in the Southeast Peruvian Amazon. Diversity 2023, 15, 793. [Google Scholar] [CrossRef]
- Garate-Quispe, J.S.; de Leon, R.P.; Machaca, M.H.; Laime, E.J.; Ramos, C.N. Growth and survivorship of Vetiveria zizanioides in degraded soil by gold-mining in the Peruvian Amazon. J. Degrad. Min. Lands Manag. 2021, 9, 3219–3225. [Google Scholar] [CrossRef]
- Velásquez Ramírez, M.G.; del Castillo Torres, D.; Guerrero Barrantes, J.A.; Vásquez Bardales, J.; Thomas, E.; Cusi Auca, E.; Chinen Gushiken, M.; Muñoz Diaz, B.; Russo, R.; Corvera Gomringer, R. Soil recovery of alluvial gold mine spoils in the Peruvian Amazon using Stylosanthes guianensis, a promising cover crop. L. Degrad. Dev. 2021, 32, 5143–5153. [Google Scholar] [CrossRef]
- Román-Dañobeytia, F.; Cabanillas, F.; Lefebvre, D.; Farfan, J.; Alferez, J.; Polo-Villanueva, F.; Llacsahuanga, J.; Vega, C.M.; Velasquez, M.; Corvera, R.; et al. Survival and early growth of 51 tropical tree species in areas degraded by artisanal gold mining in the Peruvian Amazon. Ecol. Eng. 2021, 159, 106097. [Google Scholar] [CrossRef]
- Lefebvre, D.; Román-Dañobeytia, F.; Soete, J.; Cabanillas, F.; Corvera, R.; Ascorra, C.; Fernandez, L.E.; Silman, M. Biochar Effects on Two Tropical Tree Species and Its Potential as a Tool for Reforestation. Forests 2019, 10, 678. [Google Scholar] [CrossRef]
- Román-Dañobeytia, F.; Huayllani, M.; Michi, A.; Ibarra, F.; Loayza-Muro, R.; Vázquez, T.; Rodríguez, L.; García, M. Reforestation with four native tree species after abandoned gold mining in the Peruvian Amazon. Ecol. Eng. 2015, 85, 39–46. [Google Scholar] [CrossRef]
- Lefebvre, D.; Williams, A.G.; Kirk, G.J.D.; Paul; Burgess, J.; Meersmans, J.; Silman, M.R.; Román-Dañobeytia, F.; Farfan, J.; Smith, P. Assessing the carbon capture potential of a reforestation project. Sci. Rep. 2021, 11, 19907. [Google Scholar] [CrossRef]
- Garate-Quispe, J.S.; Roca, M.R.G.; Aguirre, G.A. Survival and Growth of Brazil-Nut Seedlings in Tree-Fall Gaps and Forest Understory. Floresta E Ambient. 2020, 27, e20171168. [Google Scholar] [CrossRef]
- MINAM. Mapa Nacional de Cobertura Vegetal; Ministerio del Ambiente: Lima, Peru, 2015. [Google Scholar]
- Shoo, L.; Freebody, K.; Kanowski, J.; Catterall, C. Slow recovery of tropical old-field rainforest regrowth and the value and limitations of active restoration. Conserv. Biol. 2016, 30, 121–132. [Google Scholar] [CrossRef]
- Araújo, F.; Martins, S.; Meira, J.; Lani, J.; Pires, I. Florística da vegetação arbustivo-arbórea colonizadora de uma área degradada por mineração de caulim, em Brás Pires, MG. Rev. Árvore 2005, 29, 983–992. [Google Scholar] [CrossRef]
- Leiva, J.D. Appropriate technologies and the geosocial evolution of informal, small-scale gold mining in Madre de Dios, Peru. Extr. Ind. Soc. 2022, 12, 101165. [Google Scholar] [CrossRef]
- Caballero, J.; Messinger, M.; Román-Dañobeytia, F.; Ascorra, C.; Fernandez, L.; Silman, M. Deforestation and Forest Degradation Due to Gold Mining in the Peruvian Amazon: A 34-Year Perspective. Remote Sens. 2018, 10, 1903. [Google Scholar] [CrossRef]
- Velásquez-Ramírez, M.; Vega Ruiz, C.; Gomringer, R.; Pillaca, M.; Thomas, E.; Stewart, P.M.; Gamarra Miranda, L.A.; Dañobeytia, F.R.; Guerrero Barrantes, J.A.; Gushiken, M.C.; et al. Mercury in soils impacted by alluvial gold mining in the Peruvian Amazon. J. Environ. Manag. 2021, 288, 112364. [Google Scholar] [CrossRef]
- Wardius, Y.; Hein, S. Terrestrial laser scanning vs. manual methods for assessing complex forest stand structure: A comparative analysis on plenter forests. Eur. J. For. Res. 2024, 143, 635–649. [Google Scholar] [CrossRef]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- APG IV (Angiosperm Phylogeny Group); Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Su, L.; Heydari, M.; Omidipour, R.; Soheili, F.; Cheraghi, J.; Manuel Villa, P.; Prévosto, B. Stand structural diversity and elevation rather than functional diversity drive aboveground biomass in historically disturbed semiarid oak forests. For. Ecol. Manag. 2023, 543, 121139. [Google Scholar] [CrossRef]
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L.; Miller, R.B.; Swenson, N.G.; Wiemann, M.C.; Chave, J. Data from: Towards a worldwide wood economics spectrum [Dataset]. Dryad. 2009. [CrossRef]
- Aryal, D.R.; De Jong, B.H.J.; Sánchez-Silva, S.; Haas-Ek, A.; Esparza-Olguin, L.; Ochoa-Gaona, S.; Ghimire, R.; Morales-Ruiz, D.E. Biomass recovery along a tropical forest succession: Trends on tree diversity, wood traits and stand structure. For. Ecol. Manag. 2024, 555, 121709. [Google Scholar] [CrossRef]
- Galván-Cisneros, C.M.; Sánchez Montaño, L.R.; Ojeda-Rodríguez, A.E.; Meira-Neto, J.A.A. Structures of tropical dry forests in the Andes: Forest conservation, composition and the role of fabaceae and myrtaceae. Cerne 2023, 29, e-1033189. [Google Scholar] [CrossRef]
- Curtis, J.; McIntosh, R. An upland forest continuum in the prairie-forest border region of Wisconsin. Ecology 1951, 32, 476–496. [Google Scholar] [CrossRef]
- Kindt, R.; Coe, R. Tree Diversity Analysis. A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies; World Agroforestry Centre: Nairobi, Kenia, 2005. [Google Scholar]
- Chao, A.; Kubota, Y.; Zelený, D.; Chiu, C.H.; Li, C.F.; Kusumoto, B.; Yasuhara, M.; Thorn, S.; Wei, C.L.; Costello, M.J.; et al. Quantifying sample completeness and comparing diversities among assemblages. Ecol. Res. 2020, 35, 292–314. [Google Scholar] [CrossRef]
- Li, D. hillR: Taxonomic, functional, and phylogenetic diversity and similarity through Hill Numbers. J. Open Source Softw. 2018, 3, 1041. [Google Scholar] [CrossRef]
- Melo, A. CommEcol: Community Ecology Analyses, R package version 1.7.1; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Niku, J.; Warton, D.I.; Hui, F.K.C.; Taskinen, S. Generalized Linear Latent Variable Models for Multivariate Count and Biomass Data in Ecology. J. Agric. Biol. Environ. Stat. 2017, 22, 498–522. [Google Scholar] [CrossRef]
- Rosseel, Y. lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Muthén, L.; Muthén, B. Mplus User’s Guide, 8th ed.; Muthén & Muthén: Los Angeles, CA, USA, 2017. [Google Scholar]
- Jain, A.; Li, X.H.; Chen, W.N. Similarities and differences in gut microbiome composition correlate with dietary patterns of Indian and Chinese adults. AMB Express 2018, 8, 104. [Google Scholar] [CrossRef]
- Liu, F.; Tan, C.; Yang, Z.; Li, J.; Xiao, H.; Tong, Y. Regeneration and growth of tree seedlings and saplings in created gaps of different sizes in a subtropical secondary forest in southern China. For. Ecol. Manag. 2022, 511, 120143. [Google Scholar] [CrossRef]
- Van Der Sande, M.T.; Powers, J.S.; Kuyper, T.W.; Norden, N.; Salgado-Negret, B.; Silva De Almeida, J.; Bongers, F.; Delgado, D.; Dent, D.H.; Derroire, G.; et al. Soil resistance and recovery during neotropical forest succession. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20210074. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cuervo, A.M.; Santos, L.; Dallmeier, F.; Garate, P.; Bravo, A.; Vanthomme, H. Twenty years of land cover change in the southeastern Peruvian Amazon: Implications for biodiversity conservation. Reg. Environ. Chang. 2020, 20, 8. [Google Scholar] [CrossRef]
- de Almeida Valente, F.D.; de Castro, M.F.; Lustosa Filho, J.F.; de Carvalho Gomes, L.; Neves, J.C.L.; da Silva, I.R.; de Oliveira, T.S. Native multispecies and fast-growing forest root biomass increase C and N stocks in a reclaimed bauxite mining area. Environ. Monit. Assess. 2023, 195, 129. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.C.; Martins, F.R.; Souza, B.C.; de Sa Barretto Sampaio, E.V.; Loiola, M.I.B.; Soares, A.A. Resilience and successional trends of woody vegetation in seasonally dry tropical forests. For. Int. J. For. Res. 2023, 96, 740–753. [Google Scholar] [CrossRef]
- Kim, S.; Zang, H.; Mortimer, P.; Shi, L.; Li, Y.; Xu, J.; Ostermann, A. Tree species and recovery time drives soil restoration after mining: A chronosequence study. L. Degrad. Dev. 2018, 29, 1738–1747. [Google Scholar] [CrossRef]
- Damptey, F.; Birkhofer, K.; de la Riva, E.G. Soil Properties and Biomass Attributes in a Former Forest Restoration. Land 2020, 9, 209. [Google Scholar] [CrossRef]
- Poorter, L.; Craven, D.; Jakovac, C.C.; Van Der Sande, M.T.; Amissah, L.; Bongers, F.; Chazdon, R.L.; Farrior, C.E.; Kambach, S.; Meave, J.A. Multidimensional tropical forest recovery. Science 2021, 374, 1370–1376. [Google Scholar] [CrossRef]
- Uscanga, A.; Bartlein, P.J.; Silva, L.C.R. Local and Regional Effects of Land-Use Intensity on Aboveground Biomass and Tree Diversity in Tropical Montane Cloud Forests. Ecosystems 2023, 26, 1734–1752. [Google Scholar] [CrossRef]
- Li, T.; Yang, H.; Yang, X.; Guo, Z.; Fu, D.; Liu, C.; Li, S.; Pan, Y.; Zhao, Y.; Xu, F.; et al. Community assembly during vegetation succession after metal mining is driven by multiple processes with temporal variation. Ecol. Evol. 2022, 12, e8882. [Google Scholar] [CrossRef]
- Velasco-Murguía, A.; del Castillo, R.F.; Rös, M.; Rivera-García, R. Successional pathways of post-milpa fallows in Oaxaca, Mexico. For. Ecol. Manag. 2021, 500, 119644. [Google Scholar] [CrossRef]
- Mensah, S.; Noulèkoun, F.; Salako, V.K.; Lokossou, C.S.M.J.; Akouété, P.; Seifert, T.; Glèlè Kakaï, R. Structural and taxonomic diversity predict above-ground biomass better than functional measures of maximum height in mixed-species forests. Appl. Veg. Sci. 2023, 26, e12732. [Google Scholar] [CrossRef]
- Calderón-Balcázar, A.; Cárdenas, C.D.; Díaz-Vasco, O.; Fandiño, E.; Márquez, T.; Pizano, C. Biomass and carbon stocks of four vegetation types in the Llanos Orientales of Colombia (Mapiripán, Meta). Trees For. People 2023, 12, 100380. [Google Scholar] [CrossRef]
- Kohyama, T.I.; Sheil, D.; Sun, I.-F.; Niiyama, K.; Suzuki, E.; Hiura, T.; Nishimura, N.; Hoshizaki, K.; Wu, S.-H.; Chao, W.-C.; et al. Contribution of tree community structure to forest productivity across a thermal gradient in eastern Asia. Nat. Commun. 2023, 14, 1113. [Google Scholar] [CrossRef]
- Sousa, T.R.; Schietti, J.; Ribeiro, I.O.; Emílio, T.; Fernández, R.H.; ter Steege, H.; Castilho, C.V.; Esquivel-Muelbert, A.; Baker, T.; Pontes-Lopes, A.; et al. Water table depth modulates productivity and biomass across Amazonian forests. Glob. Ecol. Biogeogr. 2022, 31, 1571–1588. [Google Scholar] [CrossRef]
- Santoro, M.; Cartus, O.; Carvalhais, N.; Rozendaal, D.M.A.; Avitabile, V.; Araza, A.; De Bruin, S.; Herold, M.; Quegan, S.; Rodríguez-Veiga, P.; et al. The global forest above-ground biomass pool for 2010 estimated from high-resolution satellite observations. Earth Syst. Sci. Data 2021, 13, 3927–3950. [Google Scholar] [CrossRef]
- Alarcon-Aguirre, G.; Mamani Mamani, M.; Canahuire-Robles, R.R.; Vasquez Zavaleta, T.; Peña Valdeiglesias, J.; Diaz Revoredo, J.; Rodríguez Achata, L.; Ramos Enciso, D.; Garate-Quispe, J. Forest Loss Related to Brazil Nut Production in Non-Timber Forest Product Concessions in a Micro-Watershed in the Peruvian Amazon. Remote Sens. 2023, 15, 5438. [Google Scholar] [CrossRef]
- Asner, G.; Knapp, D.; Martin, R.; Tupayachi, R.; Anderson, C.; Mascaro, J.; Sinca, F.; Chadwick, D.; Sousan, S.; Higgins, M.; et al. La Geografía del Carbono en Alta Resolución del Perú; Stanford: Lima, Peru, 2014. [Google Scholar]
- Gutierrez, N.; Alvarez, C.; Riveros, J. Aguilar-Amuchastegui Estimación del Carbono en la Biomasa aérea de los bosques de la Región de Madre de Dios; WWF: Lima, Peru, 2014. [Google Scholar]
- Noulèkoun, F.; Birhane, E.; Mensah, S.; Kassa, H.; Berhe, A.; Gebremichael, Z.M.; Adem, N.M.; Seyoum, Y.; Mengistu, T.; Lemma, B.; et al. Structural diversity consistently mediates species richness effects on aboveground carbon along altitudinal gradients in northern Ethiopian grazing exclosures. Sci. Total Environ. 2021, 776, 145838. [Google Scholar] [CrossRef]
- Yuan, Z.; Ali, A.; Ruiz-Benito, P.; Jucker, T.; Mori, A.S.; Wang, S.; Zhang, X.; Li, H.; Hao, Z.; Wang, X.; et al. Above- and below-ground biodiversity jointly regulate temperate forest multifunctionality along a local-scale environmental gradient. J. Ecol. 2020, 108, 2012–2024. [Google Scholar] [CrossRef]
- Silva, J.L.d.S.; Araujo, R.A.d.; Esteves, V.P.P.; Loebmann, D.G.d.S.W.; Vicente, L.E.; Paschoal, J.P.; Morgado, C.d.R.V. Analysis of vegetation recovery in areas impacted by bauxite mining in the Amazon Forest. Clean Technol. Environ. Policy 2021, 23, 1617–1640. [Google Scholar] [CrossRef]
- Martínez-garcía, E.; Nilsson, M.B.; Laudon, H.; Lundmark, T.; Fransson, J.E.S.; Wallerman, J.; Peichl, M. Drought response of the boreal forest carbon sink is driven by understorey—Tree composition. Nat. Geosci. 2024, 17, 197–204. [Google Scholar] [CrossRef]
- Gris, D.; Casagrande, J.C.; Marques, M.R.; Oldeland, J.; Damasceno-Júnior, G.A. Periodic flooding and edaphic factors shape Erythrina fusca dominance in riparian forests in the Pantanal wetland. Trop. Ecol. 2024, 2024, 1–15. [Google Scholar] [CrossRef]
- Makelele, I.A.; Verheyen, K.; Boeckx, P.; Cizungu Ntaboba, L.; Mujinya Bazirake, B.; Ewango, C.; Bauters, M. Afrotropical secondary forests exhibit fast diversity and functional recovery, but slow compositional and carbon recovery after shifting cultivation. J. Veg. Sci. 2021, 32, e13071. [Google Scholar] [CrossRef]
- Poorter, L.; Amissah, L.; Bongers, F.; Hordijk, I.; Kok, J.; Laurance, S.G.W.; Lohbeck, M.; Martínez-Ramos, M.; Matsuo, T.; Meave, J.A.; et al. Successional theories. Biol. Rev. 2023, 98, 2049–2077. [Google Scholar] [CrossRef]
- Staude, I.R.; Weigelt, A.; Wirth, C. Biodiversity change in light of succession theory. Oikos 2023, 2023, e09883. [Google Scholar] [CrossRef]
- Adinugroho, W.C.; Prasetyo, L.B.; Kusmana, C.; Krisnawati, H.; Weston, C.J.; Volkova, L. Recovery of Carbon and Vegetation Diversity 23 Years after Fire in a Tropical Dryland Forest of Indonesia. Sustainability 2022, 14, 6964. [Google Scholar] [CrossRef]
- Bombino, G.; D’Agostino, D.; Marziliano, P.A.; Pérez Cutillas, P.; Praticò, S.; Proto, A.R.; Manti, L.M.; Lofaro, G.; Zimbone, S.M. A Nature-Based Approach Using Felled Burnt Logs to Enhance Forest Recovery Post-Fire and Reduce Erosion Phenomena in the Mediterranean Area. Land 2024, 13, 236. [Google Scholar] [CrossRef]
- Liu, H.; Ye, Q.; Lundgren, M.R.; Young, S.N.R.; Liu, X.; Luo, Q.; Lin, Y.; Ye, N.; Hao, G. Phylogeny and climate explain contrasting hydraulic traits in different life forms of 150 woody Fabaceae species. J. Ecol. 2024, 112, 741–754. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Y.; Xian, W.; Li, X.; Feng, Y.; Bu, F.; Shi, Y.; Chen, S.; van Velzen, R.; Battenberg, K.; et al. Comparative phylogenomics and phylotranscriptomics provide insights into the genetic complexity of nitrogen-fixing root-nodule symbiosis. Plant Commun. 2024, 5, 100671. [Google Scholar] [CrossRef]
- Balieiro, F.d.C.; Costa, C.A.; de Oliveira, R.B.; de Oliveira, R.; Donagemma, G.K.; de Andrade, A.G.; Capeche, C.L. Carbon stocks in mined area reclaimed by leguminous trees and sludge. Rev. Arvore 2017, 41, e410610. [Google Scholar] [CrossRef]
- Saatchi, S.; Marlier, M.; Chazdon, R.L.; Clark, D.B.; Russell, A.E. Impact of spatial variability of tropical forest structure on radar estimation of aboveground biomass. Remote Sens. Environ. 2011, 115, 2836–2849. [Google Scholar] [CrossRef]
- Otypková, Z.; Chytry, M. Effects of plot size on the ordination of vegetation samples. J. Veg. Sci. 2006, 17, 465–472. [Google Scholar] [CrossRef]
- Chai, Y.; Yue, M.; Wang, M.; Xu, J.; Liu, X.; Zhang, R. Plant functional traits suggest a change in novel ecological strategies for dominant species in the stages of forest succession. Oecologia 2016, 180, 771–783. [Google Scholar] [CrossRef]
- Chen, X.; Luo, M.; Larjavaara, M. Effects of climate and plant functional types on forest above-ground biomass accumulation. Carbon Balance Manag. 2023, 18, 5. [Google Scholar] [CrossRef]
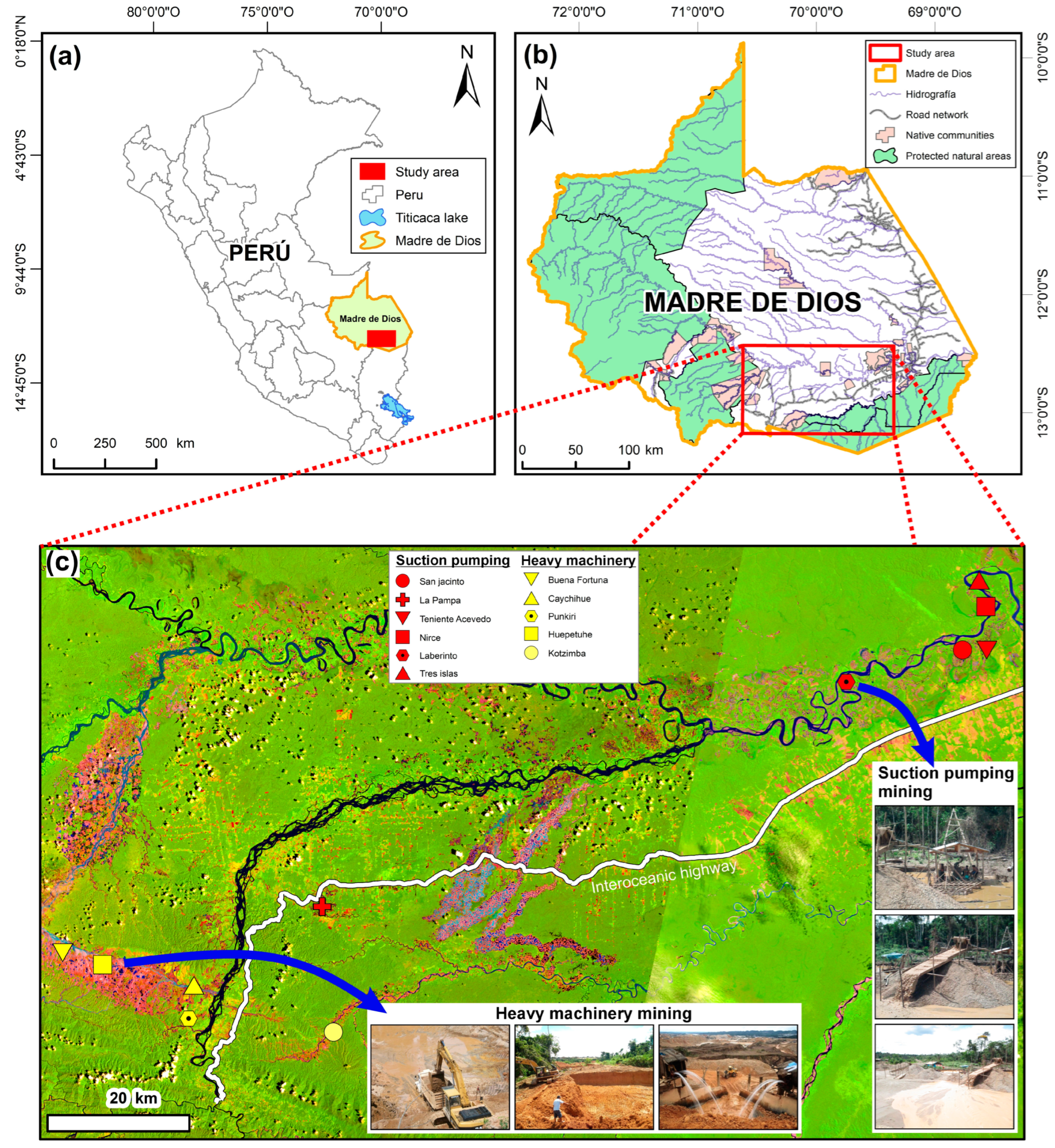
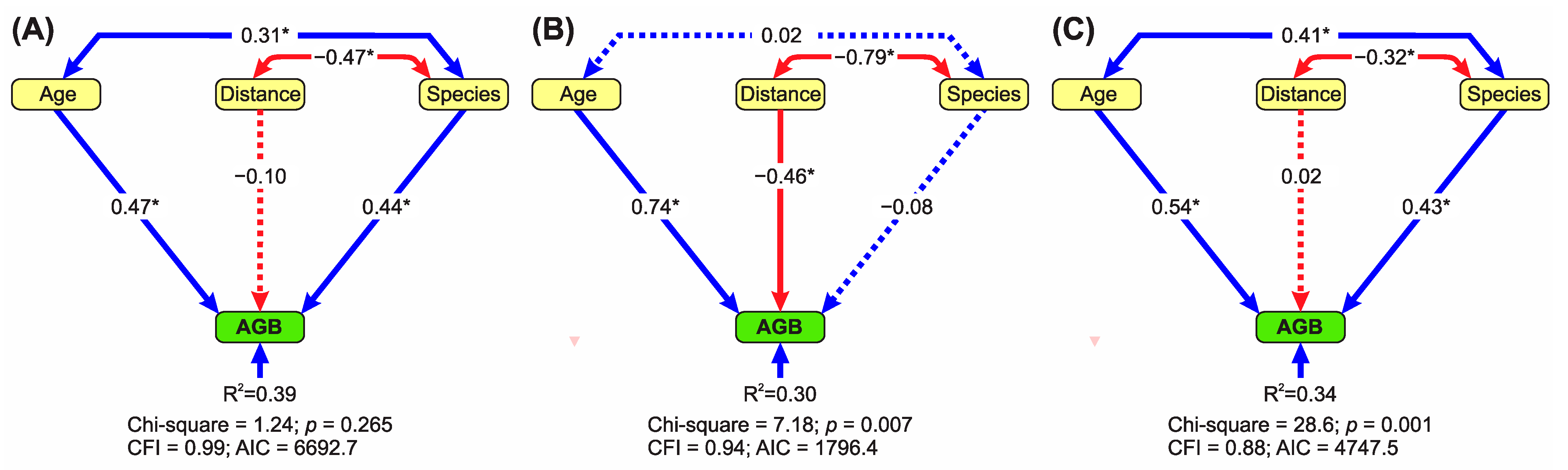
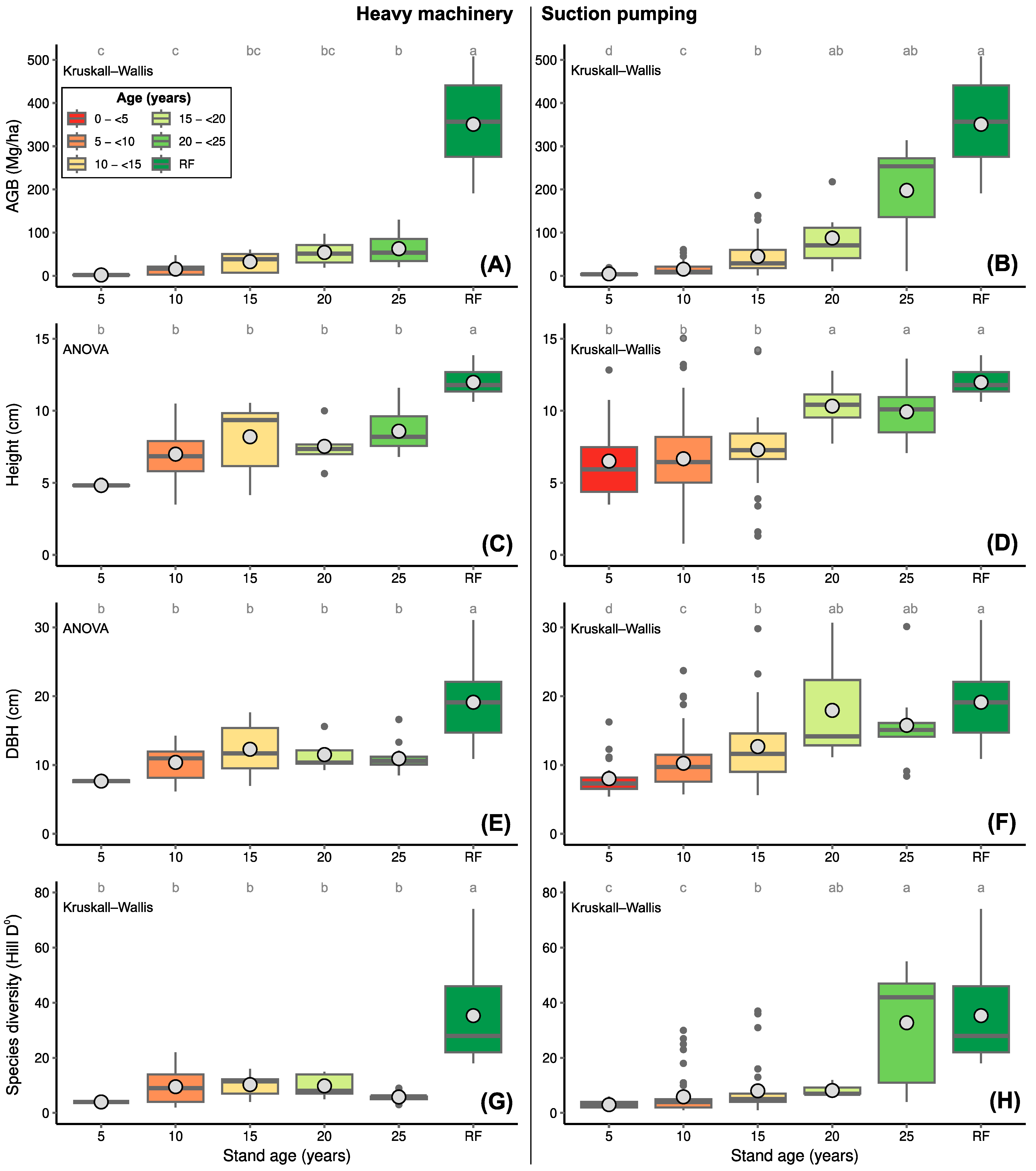

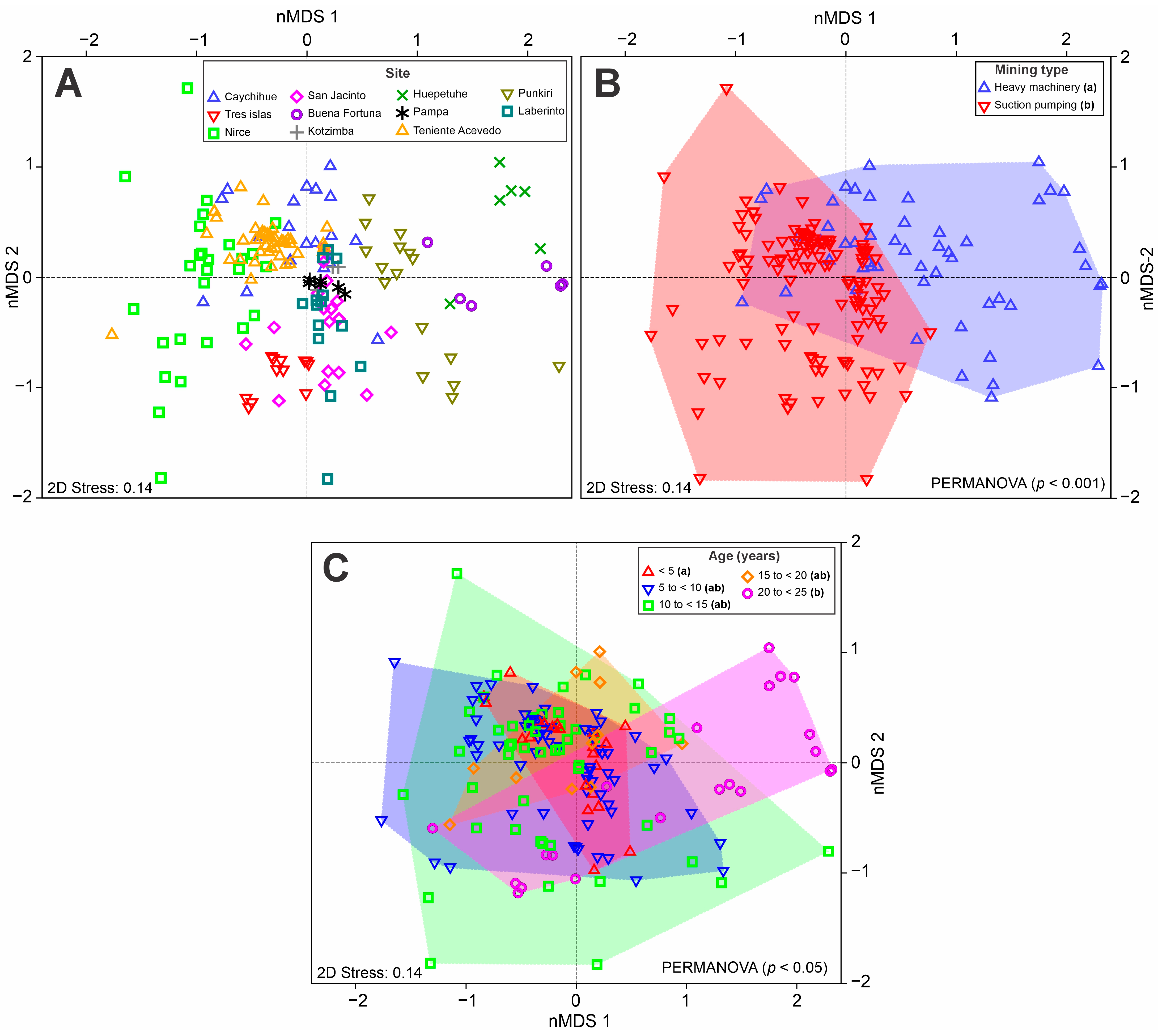
| N | Site | Mining Type | Number of Plots in Secondary Forest | Distance from the Forest Edge (m) | Stand Age (Years) | Number of Plots in Reference Forest | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Min | Max | Mean | Min | Max | |||||
| 1 | Buena Fortuna | Heavy machinery | 6 | 733 | 550 | 900 | 18 | 17 | 20 | |
| 2 | Kotzimba | 3 | 333 | 230 | 500 | 8 | 4 | 9 | ||
| 3 | Caychihue | 19 | 607 | 340 | 943 | 10 | 4 | 16 | ||
| 4 | Huepetuhe | 8 | 675 | 600 | 900 | 24 | 24 | 16 | 4 | |
| 5 | Punkiri | 16 | 91 | 25 | 280 | 10 | 7 | 15 | 8 | |
| 6 | Laberinto | Suction pumping | 14 | 64 | 22 | 152 | 9 | 2 | 19 | |
| 7 | Nirce | 27 | 272 | 95 | 517 | 10 | 4 | 22 | ||
| 8 | Teniente Acevedo | 50 | 617 | 180 | 940 | 7 | 2 | 14 | ||
| 9 | Tres islas | 12 | 35 | 15 | 70 | 15 | 5 | 23 | 3 | |
| 10 | La Pampa | 6 | 82 | 10 | 176 | 8 | 6 | 12 | 2 | |
| 11 | San Jacinto | 18 | 175 | 29 | 520 | 8 | 2 | 23 | ||
| Total | 179 | - | - | - | 17 | |||||
| (a) AGB | (d) Hill 2D | ||||||||
| Source of variation | Estimate | Std. Error | p | D2 | Source of variation | Estimate | Std. Error | p | D2 |
| Intercept (heavy machinery) | 2.0086 | 0.1986 | <0.001 | 73.87 | Intercept (heavy machinery) | 2.0650 | 0.1040 | <0.001 | 71.57 |
| Mining type (Suction pumping) | 0.2972 | 0.1291 | 0.023 | Mining type (Suction pumping) | −0.5382 | 0.0731 | <0.001 | ||
| Age | 0.1592 | 0.0100 | <0.001 | Age | 0.0441 | 0.0056 | <0.001 | ||
| Distance from forest edge | −0.0021 | 0.0002 | <0.001 | Distance from forest edge | −0.0020 | 0.0001 | <0.001 | ||
| (b) Hill 0D | (e) Mean DBH | ||||||||
| Source of variation | Estimate | Std. Error | p | D2 | Source of variation | Estimate | Std. Error | p | D2 |
| Intercept (heavy machinery) | 2.3214 | 0.0848 | <0.001 | 68.52 | Intercept (heavy machinery) | 2.2480 | 0.0823 | <0.001 | 57.38 |
| Mining type (Suction pumping) | −0.2468 | 0.0598 | <0.001 | Mining type (Suction pumping) | 0.0820 | 0.0540 | 0.129 | ||
| Age | 0.0550 | 0.0043 | <0.001 | Age | 0.0265 | 0.0039 | <0.001 | ||
| Distance from forest edge | −0.0024 | 0.0001 | <0.001 | Distance from forest edge | −0.0005 | 0.0001 | <0.001 | ||
| (c) Hill 1D | (f) Mean height | ||||||||
| Source of variation | Estimate | Std. Error | p | D2 | Source of variation | Estimate | Std. Error | p | D2 |
| Intercept (heavy machinery) | 2.0786 | 0.0970 | <0.001 | 71.65 | Intercept (heavy machinery) | 2.0645 | 0.0977 | <0.001 | 58.03 |
| Mining type (Suction pumping) | −0.3930 | 0.0680 | <0.001 | Mining type (Suction pumping) | −0.0531 | 0.0647 | 0.412 | ||
| Age | 0.0537 | 0.0050 | <0.001 | Age | 0.0187 | 0.0048 | <0.001 | ||
| Distance from forest edge | −0.0022 | 0.0001 | <0.001 | Distance from forest edge | −0.0006 | 0.0001 | <0.001 | ||
| Forest Stand Age (Years) | Mining Type | |||
|---|---|---|---|---|
| Suction Pumping | Heavy Machinery | |||
| Species | IVI (%) | Species | IVI (%) | |
| (a) <5 | Ochroma pyramidale | 38.0 | Ochroma pyramidale | 35.3 |
| Cecropia engleriana | 18.6 | Trema micrantha | 26.6 | |
| Cecropia membranacea | 10.2 | Piper aduncum | 10.9 | |
| Trema micrantha | 3.6 | Vismia macrophylla | 10.0 | |
| Erythrina poeppigiana | 3.4 | Coccoloba mollis | 8.6 | |
| (b) 5 to <10 | Ochroma pyramidale | 18.3 | Ochroma pyramidale | 36.1 |
| Cecropia membranacea | 12.1 | Cecropia membranacea | 6.0 | |
| Inga marginata | 8.4 | Bauhinia sp1 | 2.6 | |
| Cecropia engleriana | 6.5 | Erythrina ulei | 2.0 | |
| Tessaria integrifolia | 3.7 | Cecropia polystachya | 1.9 | |
| (c) 10 to <15 | Ficus insipida | 12.2 | Ochroma pyramidale | 9.9 |
| Ochroma pyramidale | 12.2 | Cecropia engleriana | 4.3 | |
| Cecropia engleriana | 7.6 | Apeiba tibourbou | 3.4 | |
| Inga sertulifera | 7.3 | Piper aduncum | 2.8 | |
| Cecropia membranacea | 5.3 | Bellucia pentamera | 2.8 | |
| (d) 15 to <20 | Ochroma pyramidale | 25.8 | Piper aduncum | 10.0 |
| Ficus insipida | 13.1 | Miconia minutiflora | 9.5 | |
| Inga marginata | 8.8 | Acalypha macrostachya | 9.2 | |
| Inga thibaudiana | 6.5 | Cecropia engleriana | 7.3 | |
| Cecropia membranacea | 6.4 | Apeiba membranacea | 5.9 | |
| (e) 20 to <25 | Sapium marmieri | 9.4 | Tachigali sp1 | 26.2 |
| Erythrina poeppigiana | 5.8 | Miconia tococa | 11.9 | |
| Cecropia membranacea | 5.3 | Vismia macrophylla | 10.0 | |
| Pourouma cecropiifolia | 3.3 | Vismia baccifera | 8.0 | |
| Guazuma ulmifolia | 3.2 | Miconia poeppigii | 7.4 | |
| (e) Reference forest | Virola surinamensis | 3.0 | ||
| Astrocaryum murumuru | 2.8 | |||
| Guarea macrophylla | 2.4 | |||
| Brosimum lactescens | 2.2 | |||
| Spondias mombin | 2.1 | |||
| Source | df 1 | Sum of Squares | Pseudo-F | p (Permutation) |
|---|---|---|---|---|
| Age | 1 | 5573.4 | 1.12 | <0.001 |
| Distance from forest edge (Dist) | 1 | 5637.6 | 1.13 | <0.001 |
| Mining type (Min) | 1 | 5300.7 | 1.07 | <0.001 |
| Age × Dist | 1 | 5193.6 | 1.04 | <0.001 |
| Age × Min | 1 | 5009.9 | 1.01 | 0.412 |
| Dist × Min | 1 | 5210.0 | 1.04 | <0.001 |
| Age × Dist × Min | 1 | 5066.4 | 1.02 | 0.163 |
| Residuals | 172 | 845,150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garate-Quispe, J.; Herrera-Machaca, M.; Pareja Auquipata, V.; Alarcón Aguirre, G.; Baez Quispe, S.; Carpio-Vargas, E.E. Resilience of Aboveground Biomass of Secondary Forests Following the Abandonment of Gold Mining Activity in the Southeastern Peruvian Amazon. Diversity 2024, 16, 233. https://doi.org/10.3390/d16040233
Garate-Quispe J, Herrera-Machaca M, Pareja Auquipata V, Alarcón Aguirre G, Baez Quispe S, Carpio-Vargas EE. Resilience of Aboveground Biomass of Secondary Forests Following the Abandonment of Gold Mining Activity in the Southeastern Peruvian Amazon. Diversity. 2024; 16(4):233. https://doi.org/10.3390/d16040233
Chicago/Turabian StyleGarate-Quispe, Jorge, Marx Herrera-Machaca, Victor Pareja Auquipata, Gabriel Alarcón Aguirre, Sufer Baez Quispe, and Edgar Eloy Carpio-Vargas. 2024. "Resilience of Aboveground Biomass of Secondary Forests Following the Abandonment of Gold Mining Activity in the Southeastern Peruvian Amazon" Diversity 16, no. 4: 233. https://doi.org/10.3390/d16040233








