The Occurrence of the American Burying Beetle (Nicrophorus americanus) and Associated Silphid Beetle Community in South Dakota: Implications for Managed Relocation
Abstract
1. Introduction
2. Materials and Methods
2.1. Carrion Beetle Sampling
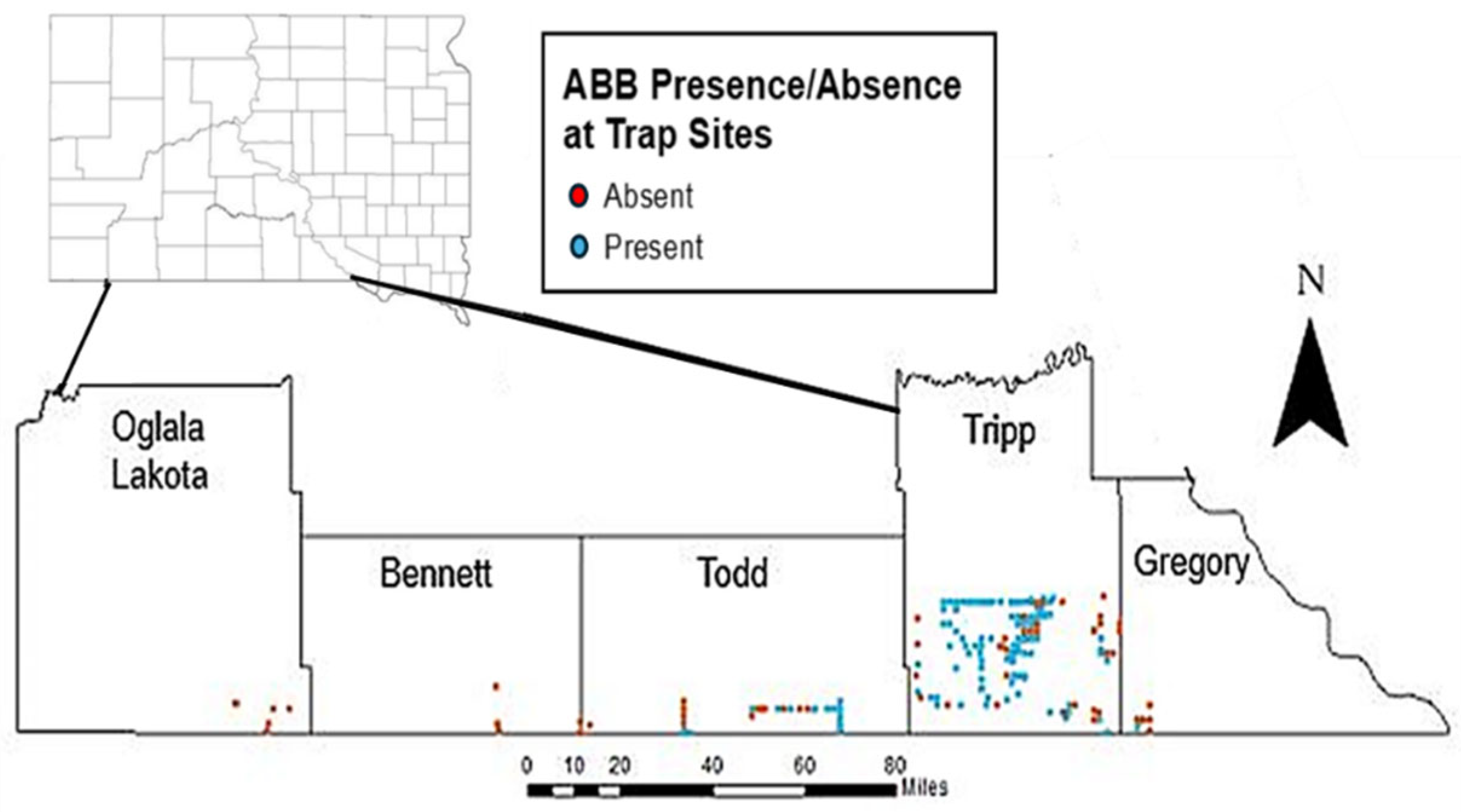
2.2. Site Selection
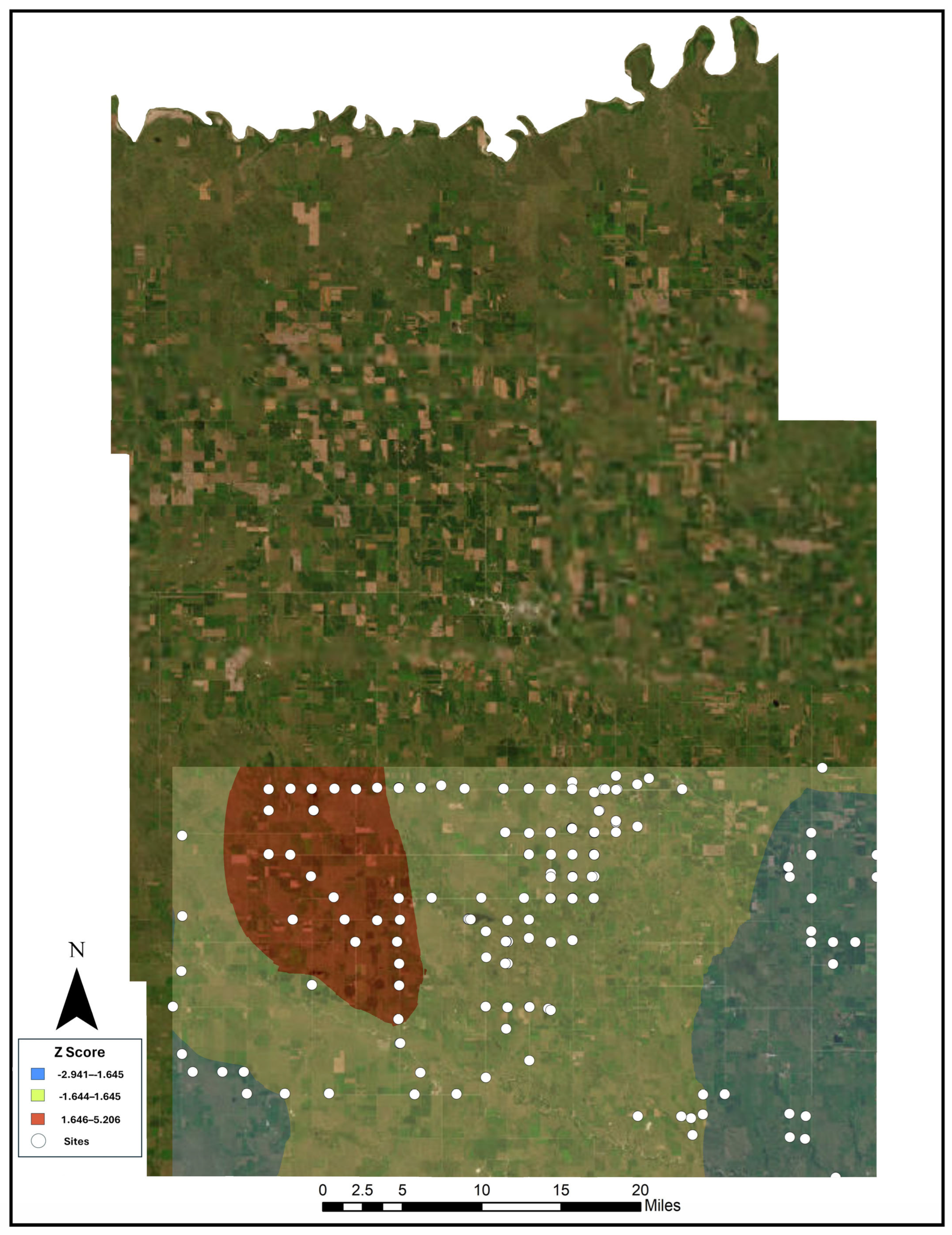
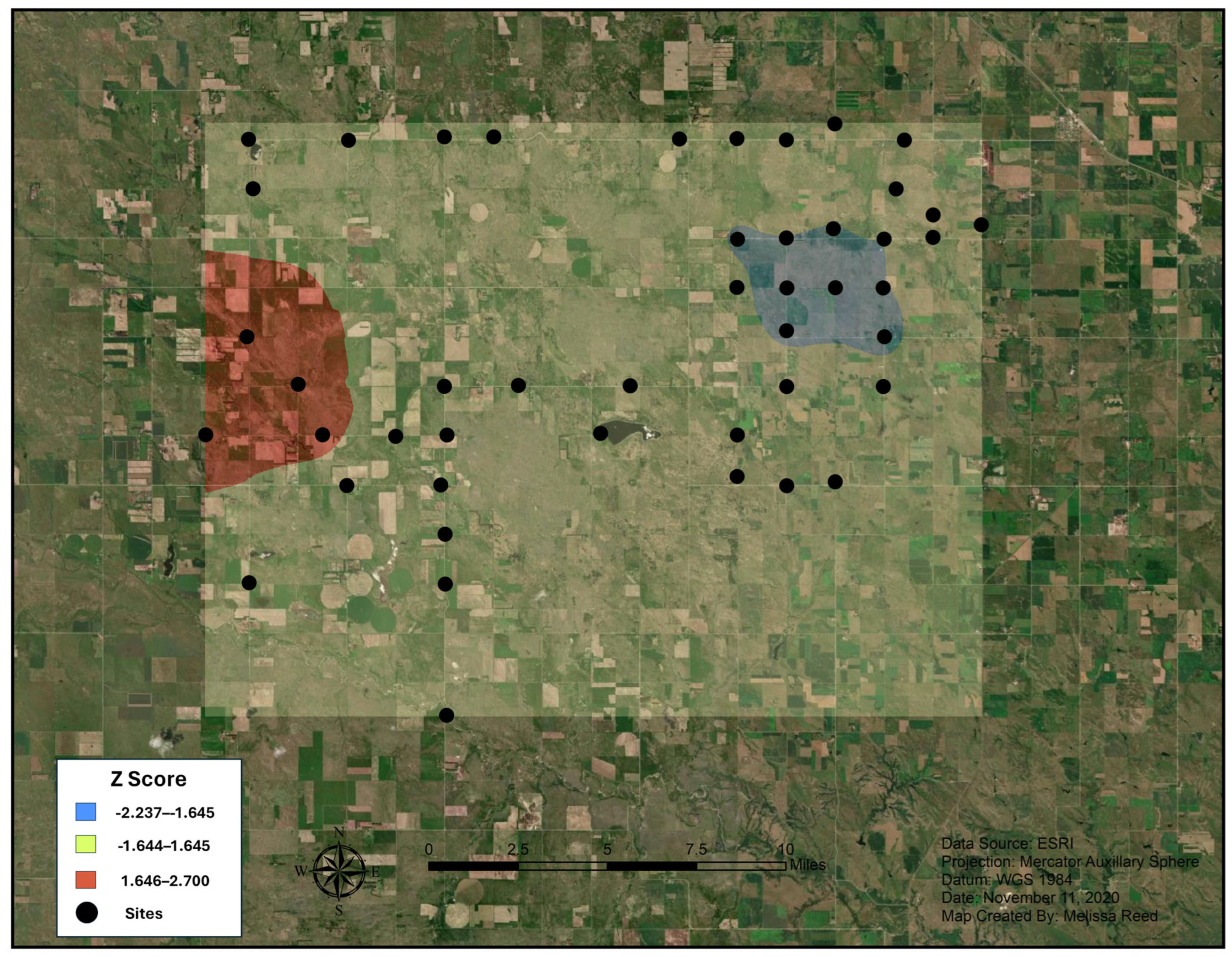
2.3. Hot Spot Analyses
2.4. Dominant Species
2.5. Community Data Analyses
2.6. Population Estimates
3. Results
3.1. Hot Spot Analyses
3.2. Dominant Species
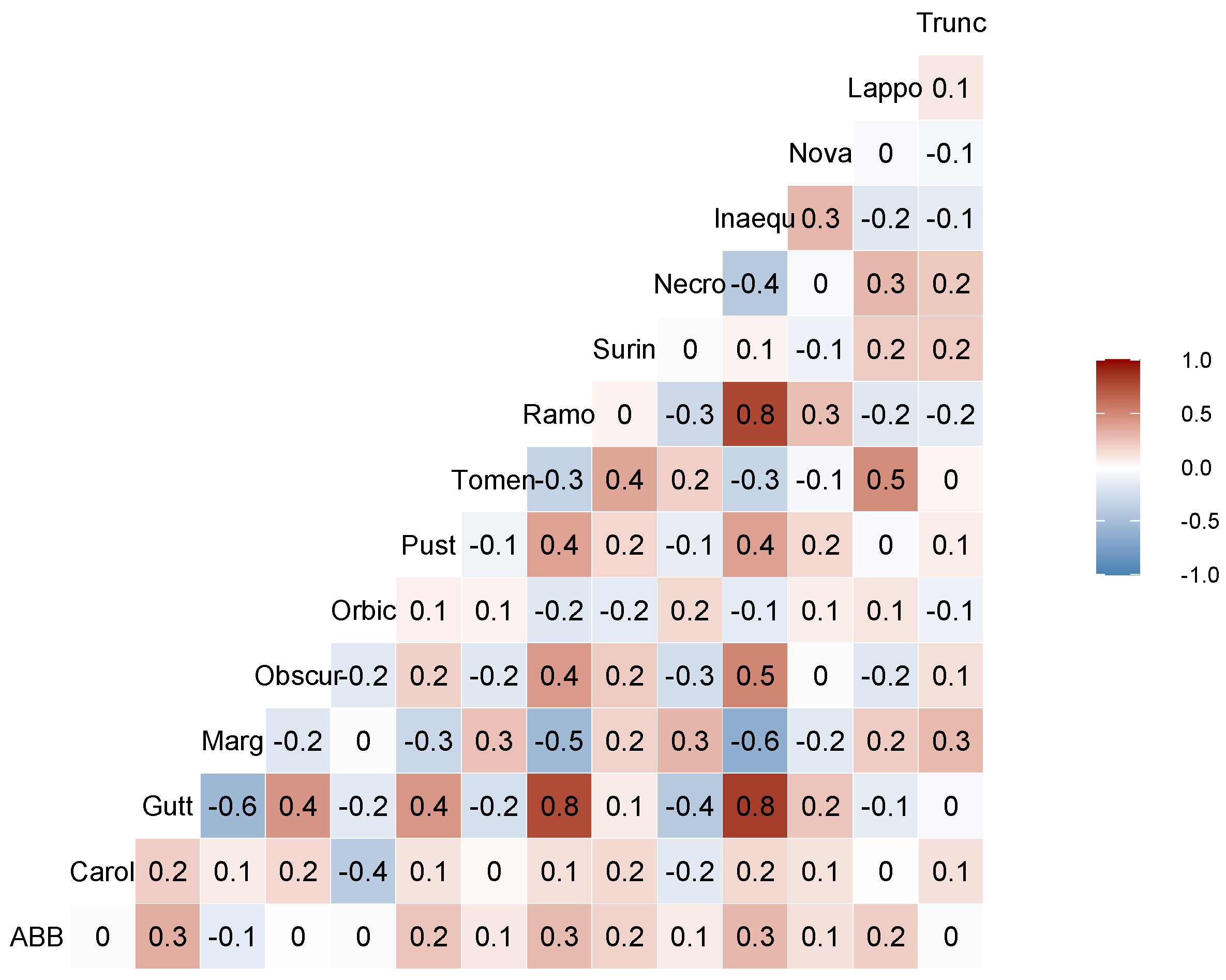
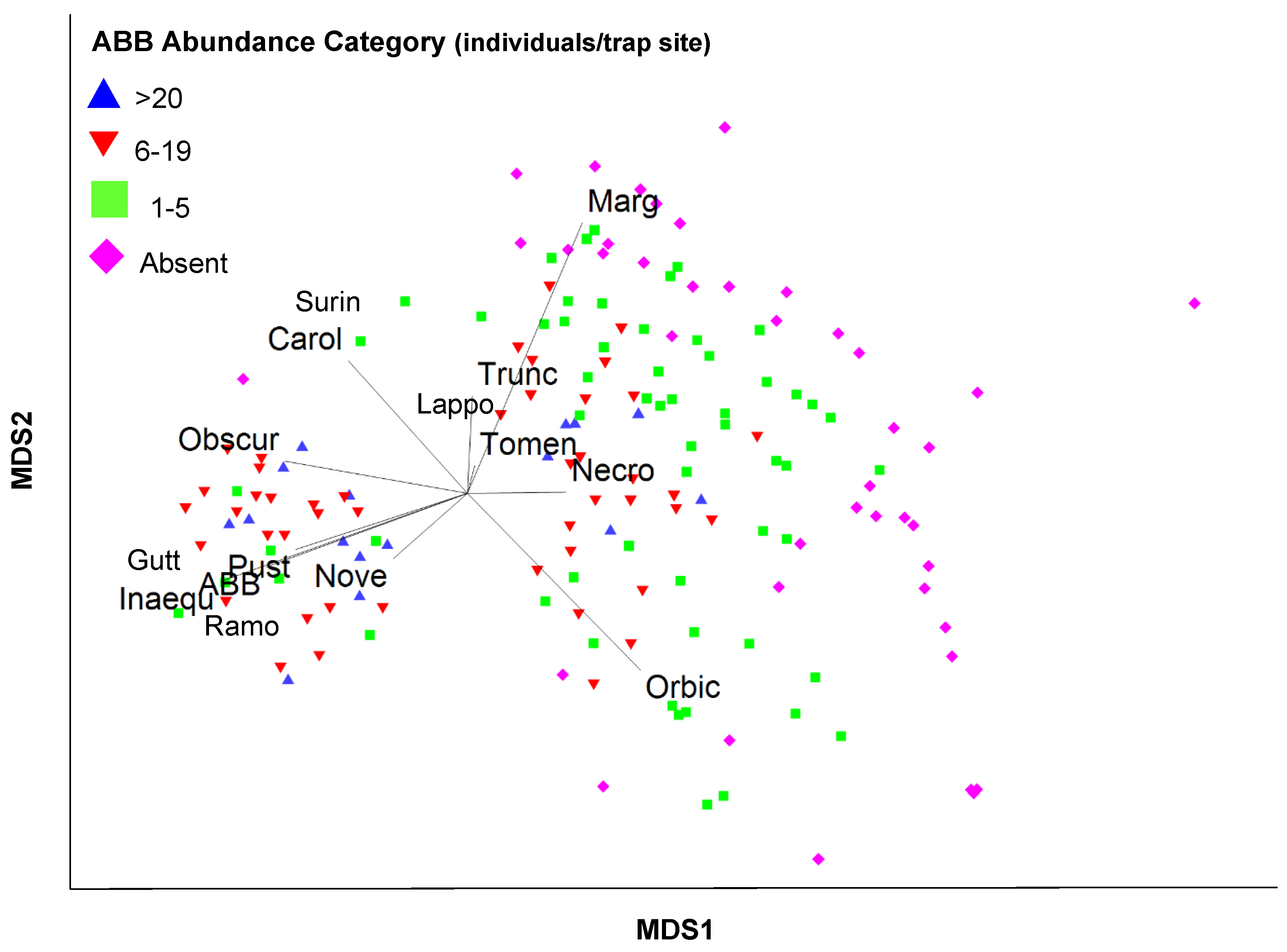
3.3. Community Data Analyses
4. Discussion
4.1. Nicrophorus americanus Range and Abundance
4.2. Nicrophorus americanus and Silphid Community Composition
4.3. Implications for Reintroduction and Translocation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinet, C.; Roques, A. Direct impacts of recent climate warming on insect populations. Integr. Zool. 2010, 5, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.L. Insect declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.J.; Maclean, I.M.D. Recent evidence for the climate change threat to Lepidoptera and other insects. J. Insect Conserv. 2011, 15, 259–268. [Google Scholar] [CrossRef]
- Larson, E.L.; Tinghitella, R.M.; Taylor, S.A. Insect hybridization and climate change. Front. Ecol. Evol. 2019, 7, 348. [Google Scholar] [CrossRef]
- Nufio, C.R.; McGuire, C.R.; Bowers, M.D.; Guralnick, R.P. Grasshopper community response to climatic change: Variation along an elevational gradient. PLoS ONE 2010, 5, e12977. [Google Scholar] [CrossRef] [PubMed]
- Breed, G.A.; Stichter, S.; Crone, E.E. Climate-driven changes in northeastern US butterfly communities. Nat. Clim. Chang. 2013, 3, 142–145. [Google Scholar] [CrossRef]
- Dortel, E.; Thuiller, W.; Lobo, J.M.; Bohbot, H.; Lumaret, J.P.; Jay-Robert, P. Potential effects of climate change on the distribution of Scarabaeidae dung beetles in Western Europe. J. Insect Conserv. 2013, 17, 1059–1070. [Google Scholar] [CrossRef]
- Menéndez, R.; González-Megías, A.; Jay-Robert, P.; Marquéz-Ferrando, R. Climate change and elevational range shifts: Evidence from dung beetles in two European mountain ranges. Glob. Ecol. Biogeogr. 2014, 23, 646–657. [Google Scholar] [CrossRef]
- Betzholtz, P.-E.; Pettersson, L.B.; Ryrholm, N.; Franzén, M. With that diet, you will go far: Trait-based analysis reveals a link between rapid range expansion and a nitrogen-favoured diet. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122305. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.S.; Peck, S.B. The Carrion Beetles of Canada and Alaska. Coleoptera: Silphidae and Agyrtidae; Canadian Government Pub. Centre, Supply and Services: Ottawa, ON, Canada, 1985; ISBN 0662117522. [Google Scholar]
- Scott, M.P. The ecology and behavior of burying beetles. Annu. Rev. Entomol. 1998, 43, 595–618. [Google Scholar] [CrossRef] [PubMed]
- Milne, L.J.; Milne, M. The social behavior of burying beetles. Sci. Am. 1976, 235, 84–89. [Google Scholar] [CrossRef]
- Bedick, J.C.; Ratcliffe, B.C.; Hoback, W.W.; Higley, L.G. Distribution, ecology and population dynamics of the American burying beetle [Nicrophorus americanus Olivier (Coleoptera, Silphidae)] in south-central Nebraska, USA. J. Insect Conserv. 1999, 3, 171–181. [Google Scholar] [CrossRef]
- Ratcliffe, B.C. The carrion beetles (Coleoptera: Silphidae) of Nebraska. Ann. Entomol. Soc. Am. 1997, 90, 399. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Creighton, J.C. Habitat selection, breeding success and conservation of the endangered American burying beetle Nicrophorus americanus. Biol. Conserv. 1996, 77, 235–241. [Google Scholar] [CrossRef]
- Wilson, D.S.; Fudge, J. Burying beetles: Intraspecific interactions and reproductive success in the field. Ecol. Entomol. 1984, 9, 195–203. [Google Scholar] [CrossRef]
- Creighton, J.C. Population density, body size, and phenotypic plasticity of brood size in a burying beetle. Behav. Ecol. 2005, 16, 1031–1036. [Google Scholar] [CrossRef]
- Hopwood, P.E.; Moore, A.J.; Tregenza, T.; Royle, N.J. Niche variation and the maintenance of variation in body size in a burying beetle. Ecol. Entomol. 2016, 41, 96–104. [Google Scholar] [CrossRef]
- Vangenne, Y.D.; Sheppard, B.; Martin, P.R. Behavioral dominance interactions between two species of burying beetles (Nicrophorus orbicollis and Nicrophorus pustulatus). PeerJ 2023, 11, e16090. [Google Scholar] [CrossRef]
- Sikes, D.S.; Raithel, C.J. A review of hypotheses of decline of the endangered American burying beetle (Silphidae: Nicrophorus americanus Olivier). J. Insect Conserv. 2002, 6, 103–113. [Google Scholar] [CrossRef]
- Leasure, D.R.; Hoback, W.W. Distribution and habitat of endangered American burying beetle in northern and southern regions. J. Insect Conserv. 2017, 21, 75–86. [Google Scholar] [CrossRef]
- Howard, D.R.; Hall, C.L. Examining the management of rare insects through the lens of biotic interactions: A comparative case study of Nicrophorus americanus (Coleoptera: Silphidae) and Gryllotalpa major (Orthoptera: Gryllotalpidae). Ann. Entomol. Soc. Am. 2019, 112, 158–168. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service. American Burying Beetle (Nicrophorus americanus) 5 Year Review: Summary and Evaluation. 2008. Available online: https://ecos.fws.gov/ecp/species/66 (accessed on 20 May 2020).
- Keller, M.L.; Howard, D.R.; Hall, C.L. The thermal ecology of burying beetles: Temperature influences reproduction and daily activity in Nicrophorus marginatus. Ecol. Entomol. 2021, 46, 1266–1272. [Google Scholar] [CrossRef]
- Schnell, G.D.; Hiott, A.E.; Creighton, J.C.; Smyth, V.L.; Komendat, A. Factors affecting overwinter survival of the American burying beetle, Nicrophorus americanus (Coleoptera: Silphidae). J. Insect Conserv. 2008, 12, 483–492. [Google Scholar] [CrossRef]
- Trumbo, S.T. Reproductive success, phenology and biogeography of burying beetles (Silphidae, Nicrophorus). Am. Midl. Nat. 1990, 124, 1–11. [Google Scholar] [CrossRef]
- Keller, M.L.; Howard, D.R.; Hall, C.L. Spatiotemporal niche partitioning in a specious silphid community (Coleoptera: Silphidae Nicrophorus). Sci. Nat. 2019, 106, 57. [Google Scholar] [CrossRef] [PubMed]
- McCain, C.M. Another rejection of the more-individuals-hypothesis: Carrion beetles (Silphidae, Coleoptera) in the Southern Rocky Mountains. Front. Biogeogr. 2021, 13. [Google Scholar] [CrossRef]
- Engasser, E.L.; Stone, R.L.; Jameson, M.L. Habitat associations of carrion beetles (Coleoptera: Silphidae) across a full annual cycle. Environ. Entomol. 2021, 50, 605–614. [Google Scholar] [CrossRef]
- Schrempf, S.D.; Burke, K.W.; Wettlaufer, J.D.; Martin, P.R. Behavioral dominance interactions between Nicrophorus orbicollis and N. tomentosus burying beetles (Coleoptera: Silphidae). PeerJ 2021, 9, e10797. [Google Scholar] [CrossRef] [PubMed]
- Wettlaufer, J.D.; Burke, K.W.; Beresford, D.V.; Martin, P.R. Partitioning resources through the seasons: Abundance and phenology of carrion beetles (Silphidae) in southeastern Ontario, Canada. Can. J. Zool. 2021, 99, 961–973. [Google Scholar] [CrossRef]
- Bishop, A.A.; Hoback, W.W.; Albrecht, M.; Skinner, K.M. A comparison of an ecological model and GIS spatial analysis to describe niche partitioning amongst carrion beetles in Nebraska. Trans. GIS 2002, 6, 457–470. [Google Scholar] [CrossRef]
- Backlund, D.C.; Marrone, G.M. New records of the endangered American burying beetle, Nicrophorus americanus Olivier, (Coleoptera: Silphidae) in South Dakota. Coleopt. Bull. 1997, 51, 53–58. [Google Scholar]
- Backlund, D.C.; Marrone, G.M.; Williams, C.K.; Tilmon, K. Population estimate of the endangered American burying beetle, Nicrophorus americanus Olivier (Coleoptera: Silphidae) in South Dakota. Coleopt. Bull. 2008, 62, 9–15. [Google Scholar] [CrossRef]
- Jurzenski, J.D.; Jorgensen, C.F.; Bishop, A.; Grosse, R.; Riens, J.; Hoback, W.W. Identifying priority conservation areas for the American burying beetle, Nicrophorus americanus (Coleoptera: Silphidae), a habitat generalist. Syst. Biodivers. 2014, 12, 149–162. [Google Scholar] [CrossRef]
- Bedick, J.C.; Ratcliffe, B.C.; Higley, L.G. A new sampling protocol for the endangered American burying beetle, Nicrophorus americanus Olivier (Coleoptera: Silphidae). Coleopt. Bull. 2004, 58, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, M.C.; Barnhart, M.C.; Hoback, W.W. Causes of rapid carrion beetle (Coleoptera: Silphidae) death in flooded pitfall traps, response to soil flooding, immersion tolerance, and swimming behavior. Environ. Entomol. 2017, 46, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.M.; Hoback, W.W.; Mulder, P.G. Elytron-branding as a permanent marking technique for Nicrophorus Fabricius (Coleoptera: Silphidae). Coleopt. Bull. 2016, 70, 249–254. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service. American Burying Beetle Nicrophorus Americanus Oklahoma Presence/Absence Live-Trapping Survey Guidance; United States Department of Interior, Fish and Wildlife Service: Tulsa, OK, USA, 2018. [Google Scholar]
- Butler, S.R.; Harms, R.; Farnsworth-Hoback, K.; Koupal, K.; Jurzenski, J.; Hoback, W.W. Standardized capture rates of the endangered American burying beetle, Nicrophorus americanus Olivier (Coleoptera: Silphidae) using different trap protocols. J. Insect Conserv. 2013, 17, 607–613. [Google Scholar] [CrossRef]
- ESRI. ArcGIS 10.2 for Desktop; Esri Inc.: Redlands, CA, USA, 2013. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Barton, K.; Barton, M.K. Package ‘mumin’. Version 2015, 1, 439. [Google Scholar]
- Team, R.C. R: A language and environment for statistical computing. Suppl. Inf. Ref. S 2021, 1, 371–378. [Google Scholar]
- Schumacher, F.X. The estimation of fish populations in lakes and ponds. J. Tenn. Acad. Sci. 1999, 18, 228. [Google Scholar]
- Dexter, E.; Rollwagen-Bollens, G.; Bollens, S.M. The trouble with stress: A flexible method for the evaluation of nonmetric multidimensional scaling. Limnol. Oceanogr. Methods 2018, 16, 434–443. [Google Scholar] [CrossRef]
- Jenkins, T.; Hoback, W.W.; Leasure, D.; Mulder, P.; Davis, C. Distribution of the endangered American burying beetle at the northwestern limit of its range. Insect Syst. Divers. 2018, 2, ixx011. [Google Scholar] [CrossRef]
- McMurry, R.S.; Cavallaro, M.C.; Shufran, A.; Hoback, W.W. Establishing age-based color changes for the american burying beetle, Nicrophorus americanus Olivier, with implications for conservation efforts. Insects 2023, 14, 844. [Google Scholar] [CrossRef] [PubMed]
- Potticary, A.L.; Otto, H.W.; McHugh, J.V.; Moore, A.J. Spatiotemporal variation in the competitive environment, with implications for how climate change may affect a species with parental care. Ecol. Evol. 2023, 13, e9972. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Hanula, J.L.; Horn, S.; Kilgo, J.C.; Moorman, C.E. Spatial and temporal patterns of beetles associated with coarse woody debris in managed bottomland hardwood forests. For. Ecol. Manag. 2004, 199, 259–272. [Google Scholar] [CrossRef]
- Dyer, N.W.; Price, D.L. Notes on the diversity and foraging height of carrion beetles (Coleoptera: Silphidae) of the Nassawango Creek Preserve, Maryland, USA. Coleopt. Bull. 2013, 67, 397–400. [Google Scholar] [CrossRef]
- Owings, C.G.; Picard, C.J. Temporal survey of a carrion beetle (Coleoptera: Silphidae) community in Indiana. Indiana Acad. Sci. 2015, 124, 124–128. [Google Scholar]
- Ringrose, J.L.; Langer, S.V.; Fleming, K.J.; Burt, T.O.; Bourne, D.R.; Brand, R.; Beresford, D.V. Burying beetles of the genus Nicrophorus Fabricius (Coleoptera: Silphidae) from northern Ontario and Akimiski Island, Nunavut. J. Entomol. Soc. Ont. 2019, 150, 1–10. [Google Scholar]
- Burke, K.W.; Wettlaufer, J.D.; Beresford, D.V.; Martin, P.R. Habitat use of co-occurring burying beetles (genus Nicrophorus) in southeastern Ontario, Canada. Can. J. Zool. 2020, 98, 591–602. [Google Scholar] [CrossRef]
- Cook, L.M.; Smith, A.N.; Meyers, P.J.; Creighton, J.C.; Belk, M.C. Evidence for differential diel activity patterns in two co-occurring species of burying beetles (Coleoptera: Silphidae: Nicrophorinae). West. N. Am. Nat. 2019, 79, 270–274. [Google Scholar] [CrossRef]
- Kadlec, J.; Mikatova, S.; Maslo, P.; Sipkova, H.; Sipek, P.; Sladecek, F.X.J. Delaying insect access alters community composition on small carrion: A quantitative approach. Entomol. Exp. Appl. 2019, 167, 729–740. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Rubenstein, D.R.; Fan, Y.-M.; Yuan, T.-N.; Chen, B.-F.; Tang, Y.; Chen, I.-C.; Shen, S.-F. Locally-adapted reproductive photoperiodism determines population vulnerability to climate change in burying beetles. Nat. Commun. 2020, 11, 1398. [Google Scholar] [CrossRef]
- Bedick, J.C.; Hoback, W.W.; Albrecht, M.C. High water-loss rates and rapid dehydration in the burying beetle, Nicrophorus marginatus. Physiol. Entomol. 2006, 31, 23–29. [Google Scholar] [CrossRef]
- Walker, T.L.; Hoback, W.W. Effects of invasive eastern redcedar on capture rates of Nicrophorus americanus and other Silphidae. Environ. Entomol. 2007, 36, 297–307. [Google Scholar] [CrossRef]
- Conley, A.L.; Jorde, E.K.; Jorde, R.E.; Yares, L.K.; Lee, K.K.; Hall, C.L.; Howard, D.R. Habitat-related differences in Necrophilous species composition: Implications for resource competition. Prairie Nat. 2015; 47, 45–49. [Google Scholar]
- Anderson, R.S. On the decreasing abundance of Nicrophorus americanus Olivier (Coleoptera: Silphidae) in eastern North America. Coleopt. Bull. 1982, 36, 362–365. [Google Scholar]
- Trumbo, S.T.; Bloch, P.L. Habitat fragmentation and burying beetle abundance and success. J. Insect Conserv. 2000, 4, 245–252. [Google Scholar] [CrossRef]
- Bajomi, B.; Pullin, A.S.; Stewart, G.B.; TakAcs-SAnta, A. Bias and dispersal in the animal reintroduction literature. Oryx 2010, 44, 358–365. [Google Scholar] [CrossRef]
- Drag, L.; Cizek, L. Successful reintroduction of an endangered veteran tree specialist: Conservation and genetics of the Great Capricorn beetle (Cerambyx cerdo). Conserv. Genet. 2015, 16, 267–276. [Google Scholar] [CrossRef]
- Pires, M.M. Rewilding ecological communities and rewiring ecological networks. Perspect. Ecol. Conserv. 2017, 15, 257–265. [Google Scholar] [CrossRef]
- Dunwiddie, P.W.; Martin, R.A. Microsites matter: Improving the success of rare species reintroductions. PLoS ONE 2016, 11, e0150417. [Google Scholar] [CrossRef] [PubMed]
- Hunter-Ayad, J.; Ohlemuller, R.; Recio, M.R.; Seddon, P.J. Reintroduction modelling: A guide to choosing and combining models for species reintroductions. J. Appl. Ecol. 2020, 57, 1233–1243. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service. Endangered and threatened wildlife and plants; reclassification of the American burying beetle from endangered to threatened with a section 4 (d) rule. Fed. Regist. 2020, 85, 65241–65261. [Google Scholar]
- Amaral, M.; Kozol, A.; French, T. Conservation status and reintroduction of the endangered American burying beetle. Northeast. Nat. 1997, 4, 121–132. [Google Scholar] [CrossRef]
- Mckenna-Foster, A.; Perrotti, L.; Blyth, J.; LoPresti, E.; Kennedy, R.S. Measuring success of a reintroduced population of the American burying beetle (Nicrophorus americanus Olivier) to Nantucket Island, MA. J. Insect Conserv. 2016, 20, 895–904. [Google Scholar] [CrossRef]
| Burying Beetle | Number | % of Total | % of Subfamily |
|---|---|---|---|
| Nicrophorus americanus | 1479 | 5.71% | 7.50% |
| Nicrophorus carolinus | 492 | 1.90% | 2.49% |
| Nicrophorus guttula | 3984 | 15.37% | 20.20% |
| Nicrophorus marginatus | 12,053 | 46.50% | 61.12% |
| Nicrophorus obscurus | 424 | 1.64% | 2.15% |
| Nicriophorus orbicollis | 794 | 3.06% | 4.03% |
| Nicrophorus pustulatus | 137 | 0.53% | 0.69% |
| Nicrophorus tomentosus | 358 | 1.38% | 1.82% |
| Carrion beetle | |||
| Necrophila americana | 1281 | 4.94% | 20.65% |
| Necrodes surinamensis | 1820 | 7.02% | 29.35% |
| Heterosilpha ramosa | 1230 | 4.74% | 19.83% |
| Oiceoptoma inaequale | 416 | 1.60% | 6.71% |
| Oiceoptoma noveboracense | 43 | 0.17% | 0.69% |
| Thanatophilus lapponicus | 1341 | 5.17% | 21.62% |
| Thanatophilus truncatus | 71 | 0.27% | 1.14% |
| TOTAL | 25,923 |
| 2018 | |||
|---|---|---|---|
| Sex | Mean | S.E. | N |
| Male | 10.06 | 0.06 | 215 |
| Female | 9.53 | 0.08 | 222 |
| 2020 | |||
| Sex | Mean | S.E. | N |
| Male | 10.12 | 0.05 | 344 |
| Female | 9.93 | 0.06 | 365 |
| N. americanus Abundance (Response Variable) a | Model Parameters b | |||
|---|---|---|---|---|
| logLik | AICc | ∆AICc | AICwt | |
| O. inaequale + N. marginatus | −648.98 | 1310.3 | 0 | 0.223 |
| O. inaequale + N. marginatus + O. noveboracense | −649.31 | 1311.0 | 0.67 | 0.160 |
| N. marginatus | −648.32 | 1311.1 | 0.80 | 0.150 |
| O. inaequale + N. marginatus + N. pustulatus | −650.70 | 1311.7 | 1.33 | 0.115 |
| Intercept-only (null) | −670.40 | 1349.0 | 38.63 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoback, W.W.; Snethen, D.G.; Reed, M.; Cavallaro, M.C. The Occurrence of the American Burying Beetle (Nicrophorus americanus) and Associated Silphid Beetle Community in South Dakota: Implications for Managed Relocation. Diversity 2024, 16, 232. https://doi.org/10.3390/d16040232
Hoback WW, Snethen DG, Reed M, Cavallaro MC. The Occurrence of the American Burying Beetle (Nicrophorus americanus) and Associated Silphid Beetle Community in South Dakota: Implications for Managed Relocation. Diversity. 2024; 16(4):232. https://doi.org/10.3390/d16040232
Chicago/Turabian StyleHoback, William Wyatt, Daniel G. Snethen, Melissa Reed, and Michael C. Cavallaro. 2024. "The Occurrence of the American Burying Beetle (Nicrophorus americanus) and Associated Silphid Beetle Community in South Dakota: Implications for Managed Relocation" Diversity 16, no. 4: 232. https://doi.org/10.3390/d16040232
APA StyleHoback, W. W., Snethen, D. G., Reed, M., & Cavallaro, M. C. (2024). The Occurrence of the American Burying Beetle (Nicrophorus americanus) and Associated Silphid Beetle Community in South Dakota: Implications for Managed Relocation. Diversity, 16(4), 232. https://doi.org/10.3390/d16040232







