Disentangling the Effects of Climate and Land Uses on Small Mammals in Agroecosystems of NE Spain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Species Determination
2.3. Environmental Variables and Statistical Analyses
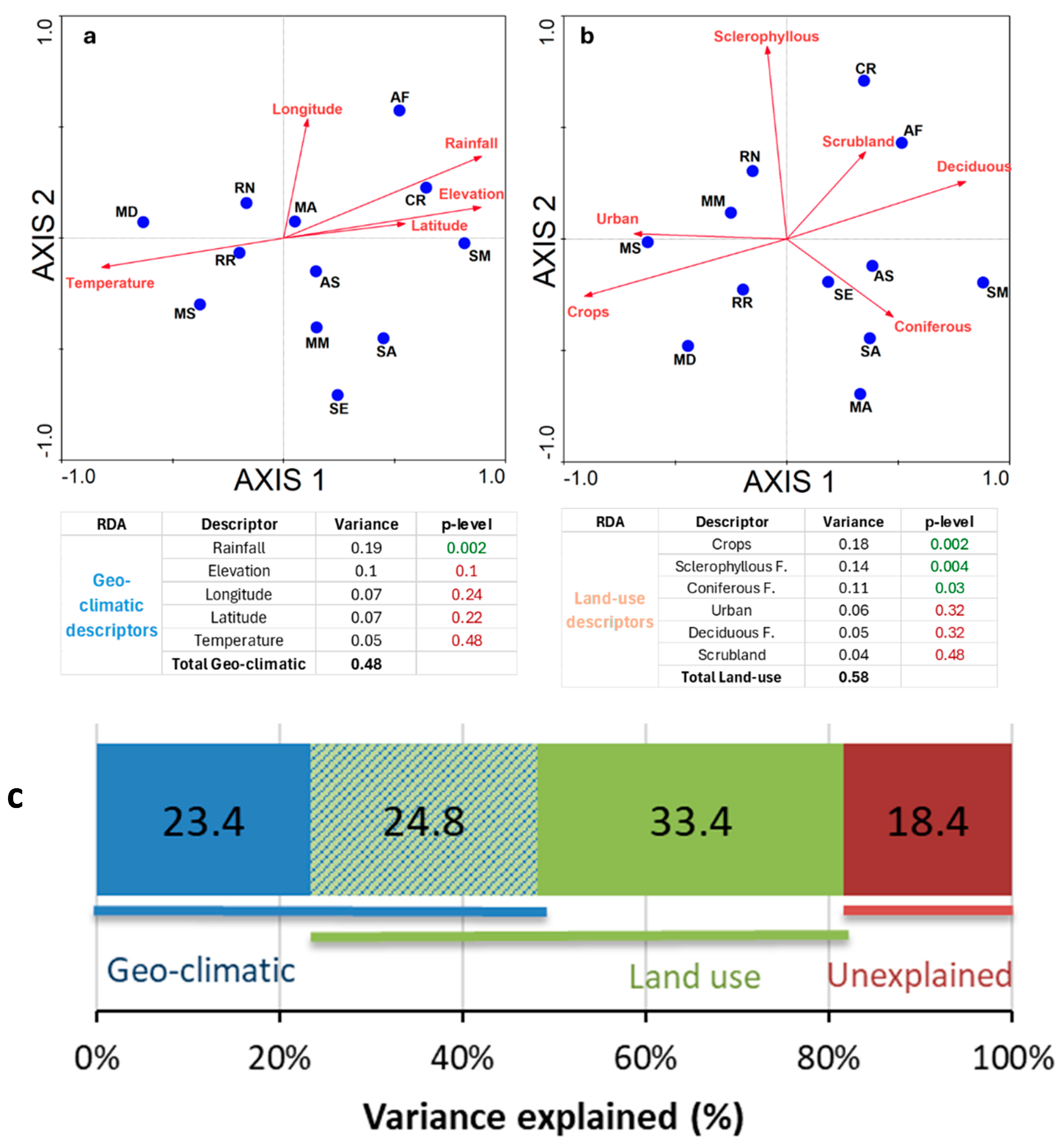
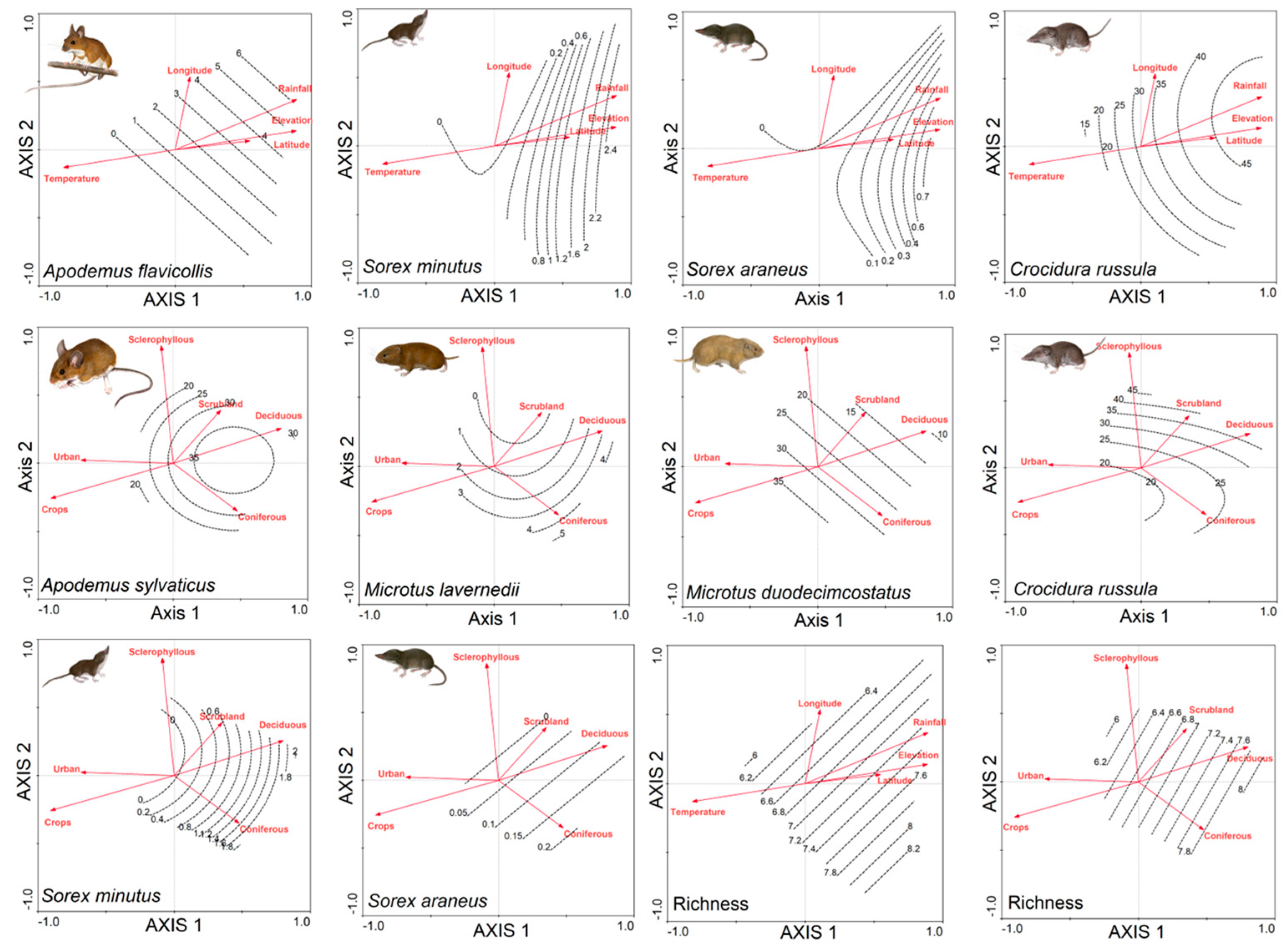
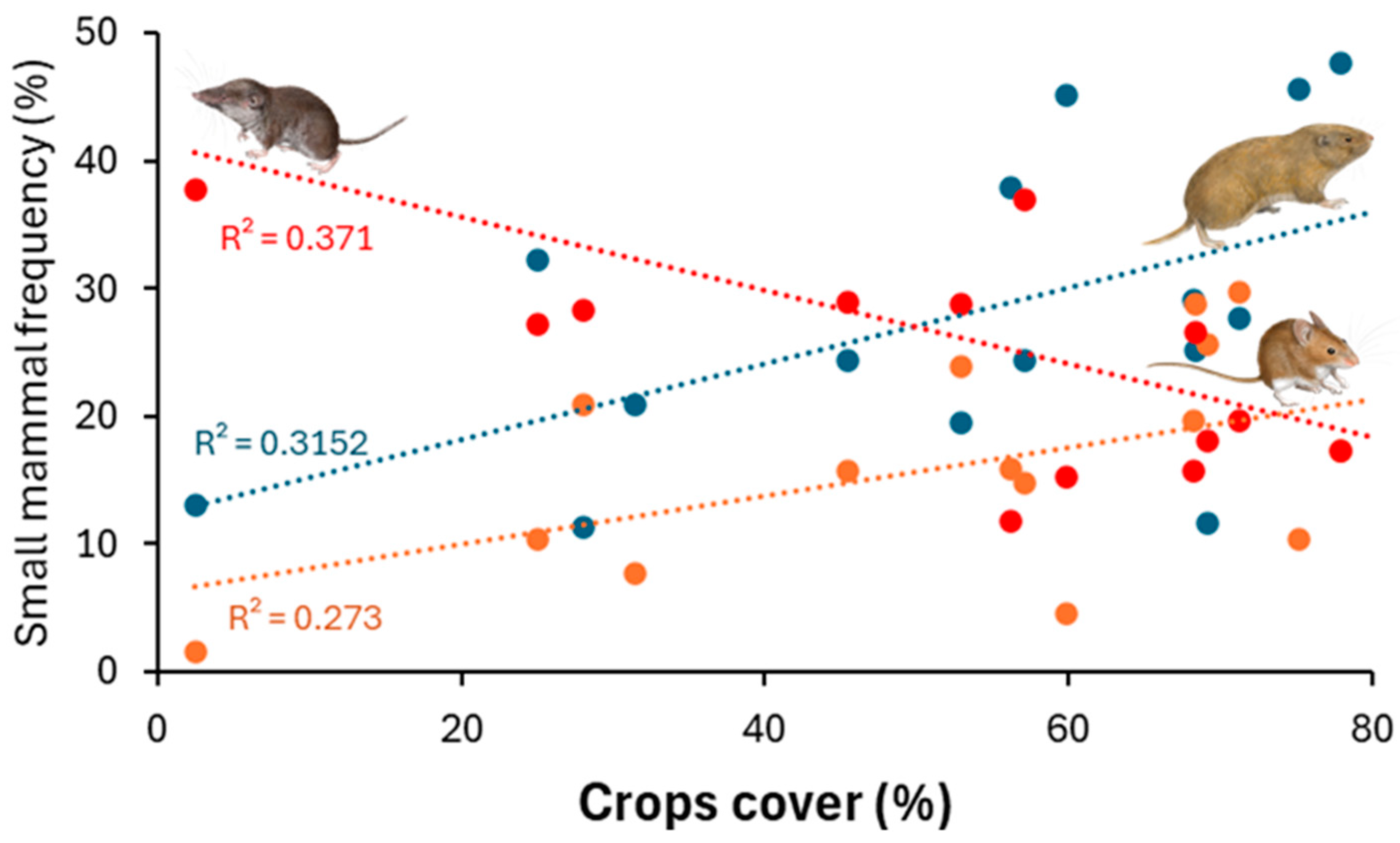
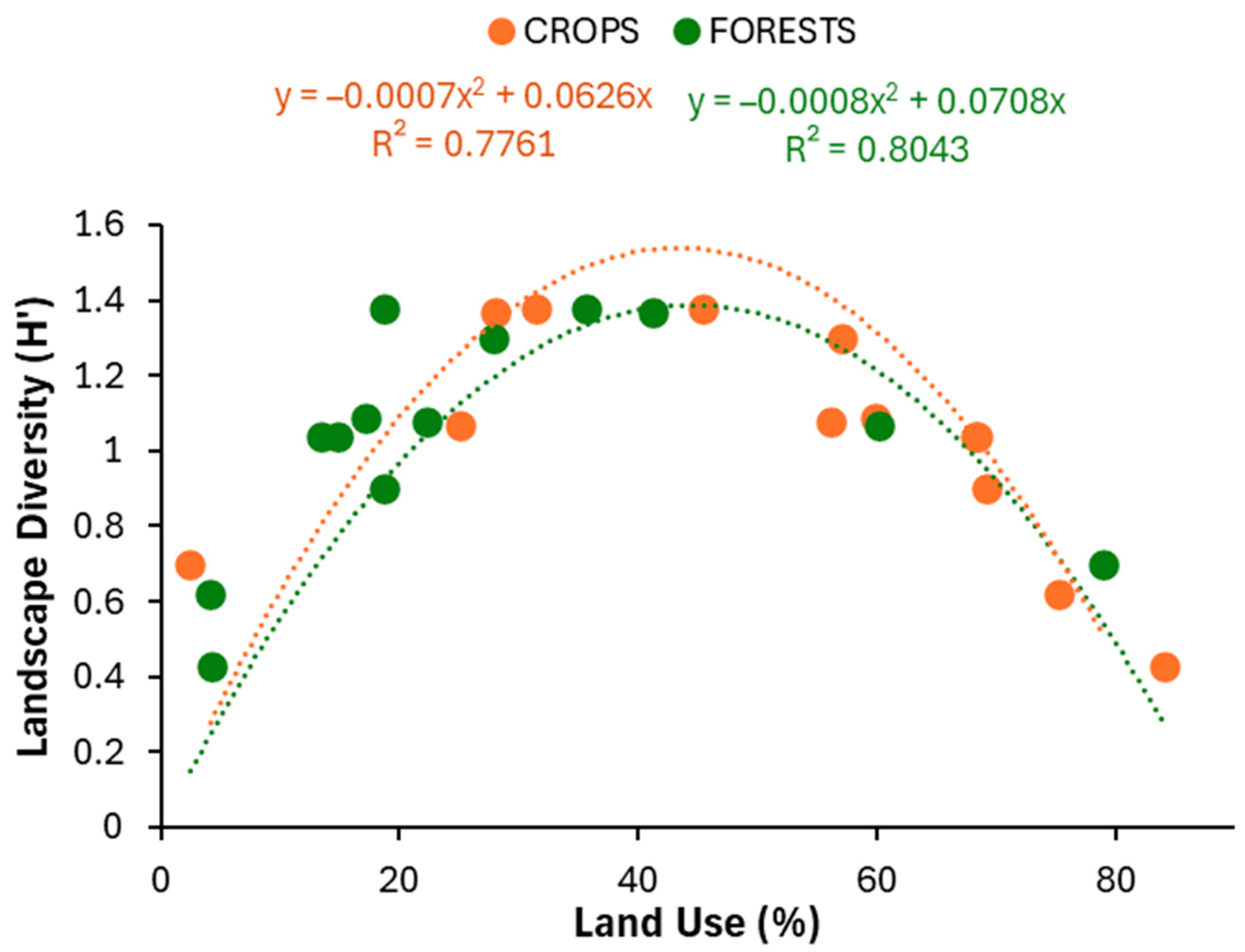
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hortal, J.; Rodríguez, J.; Nieto-Díaz, M.; Lobo, J.M. Regional and environmental effects on the species richness of mammal assemblages. J. Biogeogr. 2008, 35, 1202–1214. [Google Scholar] [CrossRef]
- Svenning, J.-C.; Flojgaard, C.; Baselga, A. Climate, history and neutrality as drivers of mammal beta diversity in Europe: Insights from multiscale deconstruction. J. Anim. Ecol. 2011, 80, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Q.; Wen, Z.; Xia, L.; Zhang, Q.; Zhou, H. What drives the species richness patterns of non-volant small mammals along a subtropical elevational gradient? Ecography 2013, 36, 185–196. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielborger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Pascual, L.-L.; Maiorano, L.; Alessandra, F.; Emilio, B.; Luigi, B. Hotspots of species richness, threat and endemism for terrestrial vertebrates in SW Europe. Acta Oecologica 2011, 37, 399–412. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Pausas, J.G. Does plant richness influence animal richness? the mammals of Catalonia (NE Spain). Divers. Distrib. 2004, 10, 247–252. [Google Scholar] [CrossRef]
- Baquero, R.A.; Telleria, J.L. Species richness, rarity and endemicity of European mammals: A biogeographical approach. Biodivers. Conserv. 2001, 10, 29–44. [Google Scholar] [CrossRef]
- Brown, J.H.; Lomolino, M.V. Biogeography, 2nd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 1998. [Google Scholar]
- Araújo, M.B.; Peterson, A.T. Uses and misuses of bioclimatic envelope modeling. Ecology 2012, 93, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, L.; Falcucci, A.; Zimmermann, N.E.; Psomas, A.; Pottier, J.; Baisero, D.; Rondinini, C.; Guisan, A.; Boitani, L. The future of terrestrial mammals in the Mediterranean basin under climate change. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2681–2692. [Google Scholar] [CrossRef]
- Flojgaard, C.; Normand, S.; Skov, F.; Svenning, J.-C. Deconstructing the mammal species richness pattern in Europe—Towards an understanding of the relative importance of climate, biogeographic history, habitat heterogeneity and humans. Glob. Ecol. Biogeogr. 2011, 20, 218–230. [Google Scholar] [CrossRef]
- Mehr, M.; Brandl, R.; Hothorn, T.; Dziock, F.; Foerster, B.; Mueller, J. Land use is more important than climate for species richness and composition of bat assemblages on a regional scale. Mamm. Biol. 2011, 76, 451–460. [Google Scholar] [CrossRef]
- Holland, G.J.; Bennett, A.F. Occurrence of small mammals in a fragmented landscape: The role of vegetation heterogeneity. Wildl. Res. 2007, 34, 387–397. [Google Scholar] [CrossRef]
- Araújo, M.B.; Guilhaumon, F.; Rodrigues Neto, D.; Pozo Ortego, I.; Calmaestra, R.G. Impactos, Vulnerabilidad y Adaptación al Cambio Climático de la Biodiversidad Española 2. Fauna de Vertebrados; Dirección General de Medio Natural y Política Forestal, Ministerio de Medio Ambiente, y Medio Rural y Marino: Madrid, Spain, 2011. [Google Scholar]
- Rowe, R.J.; Finarelli, J.A.; Rickart, E.A. Range dynamics of small mammals along an elevational gradient over an 80-year interval. Glob. Chang. Biol. 2010, 16, 2930–2943. [Google Scholar] [CrossRef]
- Moritz, C.; Patton, J.L.; Conroy, C.J.; Parra, J.L.; White, G.C.; Beissinger, S.R. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 2008, 322, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Krystufek, B.; Donev, N.R.; Skok, J. Species richness and distribution of non-volant small mammals along an elevational gradient on a Mediterranean mountain. Mammalia 2011, 75, 3–11. [Google Scholar] [CrossRef]
- Moreno, E.; Barbosa, A. Distribution patterns of small mammal fauna along gradients of latitude and altitude in Northern Spain. Zeischrift für Säugetierkd. 1992, 57, 169–175. [Google Scholar]
- Torre, I.; Tella, J.; Arrizabalaga, A. Environmental and geographic factors affecting the distribution of small mammals in an isolated Mediterranean mountain. Z. Fur Saugetierkd. 1996, 61, 365–375. [Google Scholar]
- Krystufek, B.; Griffiths, H.I. Species richness and rarity in European rodents. Ecography 2002, 25, 120–128. [Google Scholar] [CrossRef]
- Torre, I.; Gracia-Quintas, L.; Arrizabalaga, A.; Baucells, J.; Díaz, M. Are recent changes in the terrestrial small mammal communities related to land use change? A test using pellet analyses. Ecol. Res. 2015, 30, 813–819. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef]
- Heisler, L.M.; Somers, C.M.; Poulin, R.G. Owl pellets: A more effective alternative to conventional trapping for broad-scale studies of small mammal communities. Methods Ecol. Evol. 2016, 7, 96–103. [Google Scholar] [CrossRef]
- Baśnou, C.; Álvarez, E.; Bagaria, G.; Guardiola, M.; Isern, R.; Vicente, P.; Pino, J. Spatial patterns of land use changes across a mediterranean metropolitan landscape: Implications for biodiversity management. Environ. Manage. 2013, 52, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Peres-Neto, P.R.; Legendre, P.; Dray, S.; Borcard, D. Variation partitioning of species data matrices: Estimation and comparison of fractions. Ecology 2006, 87, 2614–2625. [Google Scholar] [CrossRef] [PubMed]
- Baucells, J.; Camprodon, J.; Ordeix, M. La Fauna Vertebrada d’Osona; Lynx Edicions: Barcelona, Spain, 1998. [Google Scholar]
- Vigués, J.; Grajera, J.; Arrizabalaga, A.; Torre, I. Long-term human-induced landscape changes and small mammal communities in a Mediterranean place. Galemys 2018, 30, 37–47. [Google Scholar] [CrossRef]
- Rodriguez, C.; Peris, S.J. Habitat associations of small mammals in farmed landscapes: Implications for agri-environmental schemes. Anim. Biol. 2007, 57, 301–314. [Google Scholar] [CrossRef]
- van Strien, A.J.; Bekker, D.L.; La Haye, M.J.J.; van der Meij, T. Trends in small mammals derived from owl pellet data using occupancy modelling. Mamm. Biol. 2015, 80, 340–346. [Google Scholar] [CrossRef]
- Gosálbez, J. Insectívors i Rosegadors de Catalunya. Metodologia D’estudi i Catàleg Faunístic; Ketres Editora, S.A.: Barcelona, Spain, 1987. [Google Scholar]
- Culí, J.; Riera, S.; Solà, E. Les Egagròpiles. Concepte, Tractament i Utilitat.Treballs de Camp i de Laboratori. Aplicacions Escolars; EUMO Editorial: Cataluna, Spain, 1989. [Google Scholar]
- Torre, I.; Fernández, L.; Arrizabalaga, A. Using barn owl Tyto alba pellet analyses to monitor the distribution patterns of the yellow-necked mouse (Apodemus flavicollis Melchior 1834) in a transitional Mediterranean mountain. Mammal Study 2015, 40, 133–142. [Google Scholar] [CrossRef]
- Sans-Fuentes, M.A.; Ventura, J. Distribution patterns of the small mammals (Insectivora and Rodentia) in a transitional zone between the Eurosiberian and the Mediterranean regions. J. Biogeogr. 2000, 27, 755–764. [Google Scholar] [CrossRef]
- Martínez, J.A.; Zuberogoitia, Í. Habitat preferences and causes of population decline for Barn Owls Tyto alba: A multi-scale approach. Ardeola 2004, 51, 303–317. [Google Scholar]
- Szpunar, G.; Aloise, G.; Mazzotti, S.; Nieder, L.; Cristaldi, M. Effects of global climate change on terrestrial small mammal communities in Italy. Fresenius Environ. Bull. 2008, 17, 1526–1533. [Google Scholar]
- Torre, I.; Arrizabalaga, A.; Freixas, L.; Ribas, A.; Flaquer, C.; Diaz, M. Using scats of a generalist carnivore as a tool to monitor small mammal communities in Mediterranean habitats. Basic Appl. Ecol. 2013, 14, 155–164. [Google Scholar] [CrossRef]
- Bontzorlos, V.; Vlachopoulos, K.; Xenos, A. Distribution of Four Vole Species through the Barn Owl Tyto alba Diet Spectrum: Pattern Responses to Environmental Gradients in Intensive Agroecosystems of Central Greece. Life 2023, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Leps, J.; Smilauer, P. Multivariate Analysis of Ecological Data using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the spatial component of ecological variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical estimation of species richness and shared species from samples. In Software and User’s Guide. 2012. Available online: https://www.robertkcolwell.org/pages/1407 (accessed on 5 June 2024).
- Jiménez-Valverde, A.; Hortal, J. Las curvas de acumulación de especies y la necesidad de evaluar la calidad de los inventarios biológicos. Rev. Ibérica Aracnol. 2003, 8, 151–161, 1576–9518. [Google Scholar]
- Gotelli, N.J.; Entsminger, G. ECOSIM: Null Models Software for Ecology; Acquired Intelligence Inc., Kesey-Bear: Vancouver, WA, Canada, 2005; Available online: http://www.garyentsminger.com (accessed on 5 June 2024).
- Moreno, C.E.; Halffter, G. Assessing the completeness of bat biodiversity inventories using species accumulation curves. J. Appl. Ecol. 2000, 37, 149–158. [Google Scholar] [CrossRef]
- Torre, I.; Arrizabalaga, A.; Flaquer, C. Three methods for assessing richness and composition of small mammal communities. J. Mammal. 2004, 85, 524–530. [Google Scholar] [CrossRef]
- Balestrieri, A.; Gazzola, A.; Formenton, G.; Canova, L. Long-term impact of agricultural practices on the diversity of small mammal communities: A case study based on owl pellets. Environ. Monit. Assess. 2019, 191, 725. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.; Nagle, A.; Smiddy, P.; Mria, J.F.; Muircheartaigh, I.Ó. The diet of the barn owl Tyto alba in county Cork in relation to land use. Proc. R. Ir. Acad. 1996, 96, 97–111. [Google Scholar]
- Millán de la Peña, N.; Butet, A.; Delettre, Y.; Paillat, G.; Morant, P.; Le Du, L.; Burel, F. Response of the small mammal community to changes in western French agricultural landscapes. Landsc. Ecol. 2003, 18, 265–278. [Google Scholar] [CrossRef]
- Love, R.A.; Webbon, C.; Glue, D.E.; Harris, S. Changes in the food of British Barn Owls (Tyto alba) between 1974 and 1997. Mamm. Rev. 2000, 30, 107–129. [Google Scholar] [CrossRef]
- Meek, W.; Burman, P.; Sparks, T.; Nowakowski, M.; Burman, N. The use of Barn Owl Tyto alba pellets to assess population change in small mammals. Bird Study 2012, 59, 166–174. [Google Scholar] [CrossRef]
- Torre, I.; Tella, J.L.; Ballesteros, T. Predation trends of the barn owl (Tyto alba) in the Ebro Valley (NE Spain). Hist. Anim. 1997, 3, 35–44. [Google Scholar]
- Torre, I.; Raspall, A.; Arrizabalaga, A.; Díaz, M. SEMICE: An unbiased and powerful monitoring protocol for small mammals in the Mediterranean Region. Mamm. Biol. 2018, 88, 161–167. [Google Scholar] [CrossRef]
- Munguía, M.; Trejo, I.; González-Salazar, C.; Pérez-Maqueo, O. Human impact gradient on mammalian biodiversity. Glob. Ecol. Conserv. 2016, 6, 79–92. [Google Scholar] [CrossRef]
- Serafini, V.N.; Priotto, J.W.; Gomez, M.D. Effects of agroecosystem landscape complexity on small mammals: A multi-species approach at different spatial scales. Landsc. Ecol. 2019, 34, 1117–1129. [Google Scholar] [CrossRef]
- Benedek, A.M.; Sîrbu, I. Responses of small mammal communities to environment and agriculture in a rural mosaic landscape. Mamm. Biol. 2018, 90, 55–65. [Google Scholar] [CrossRef]
- Fischer, C.; Thies, C.; Tscharntke, T. Small mammals in agricultural landscapes: Opposing responses to farming practices and landscape complexity. Biol. Conserv. 2011, 144, 1130–1136. [Google Scholar] [CrossRef]
- Gomes, V.; Ribeiro, R.; Carretero, M.A. Effects of urban habitat fragmentation on common small mammals: Species versus communities. Biodivers. Conserv. 2011, 20, 3577–3590. [Google Scholar] [CrossRef]
- Chace, J.F.; Walsh, J.J. Urban effects on native avifauna: A review. Landsc. Urban Plan. 2006, 74, 46–69. [Google Scholar] [CrossRef]
- Torre, I.; Bastardas-Llabot, J.; Arrizabalaga, A.; Díaz, M. Population dynamics of small endotherms under global change: Greater white-toothed shrews Crocidura russula in Mediterranean habitats. Sci. Total Environ. 2020, 705, 135799. [Google Scholar] [CrossRef] [PubMed]
- Torre, I.; Jaime-González, C.; Díaz, M. Habitat Suitability for Small Mammals in Mediterranean Landscapes: How and Why Shrubs Matter. Sustainability 2022, 14, 1562. [Google Scholar] [CrossRef]
- Torre, I.; Díaz, M.; Arrizabalaga, A. Additive effects of climate and vegetation structure on the altitudinal distribution of greater white-toothed shrews Crocidura russula in a Mediterranean mountain range. Acta Theriol. 2014, 59, 139–147. [Google Scholar] [CrossRef]
- Palomo, L.J.; Justo, E.R.; Vargas, J.M. Mus spretus (Rodentia: Muridae). Mamm. Species 2009, 840, 1–10. [Google Scholar] [CrossRef]
- Torre, I.; Puig-Montserrat, X.; Díaz, M. Global Change Effects on Mediterranean Small Mammal Population Dynamics: Demography of Algerian Mice (Mus spretus) Along Land Use and Climate Gradients. Sci. Total Environ. 2023, 863, 160875. [Google Scholar] [CrossRef] [PubMed]
- Górecki, A.; Meczeva, R.; Pis, T.; Gerasimov, S.; Walkowa, W. Geographical variation of thermoregulation in wild populations of Mus musculus and Mus spretus. Acta Theriol. 1990, 35, 209–214. [Google Scholar] [CrossRef]
- Borghi, C.E.; Giannoni, S.M.; Martinez-Rica, J.P. Habitat segregation of three sympatric fossorial rodents in the Spanish Pyrenees. Zeitschrift fuer Saeugetierkd. 1994, 59, 52–57. [Google Scholar]
- Santos, S.M.; da Luz Mathias, M.; Mira, A.P. Local coexistence and niche differences between the Lusitanian and Mediterranean pine voles (Microtus lusitanicus and M. duodecimcostatus). Ecol. Res. 2010, 25, 1019–1031. [Google Scholar] [CrossRef]
- Oro, D.; Sanz-Aguilar, A.; Carbonell, F.; Grajera, J.; Torre, I. Multi-species prey dynamics influences local survival in resident and wintering generalist predators. Oecologia 2021, 197, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Paniccia, C.; Laura Carranza, M.; Frate, L.; Di Febbraro, M.; Rocchini, D.; Loy, A. Distribution and functional traits of small mammals across the Mediterranean area: Landscape composition and structure definitively matter. Ecol. Indic. 2022, 135, 108550. [Google Scholar] [CrossRef]
- Torre, I.; Raspall, A.; Arrizabalaga, A.; Díaz, M. Weasel (Mustela nivalis) decline in NE Spain: Prey or land use change? Mammal Res. 2018, 63, 501–505. [Google Scholar] [CrossRef]
- Torre, I.; Grajera, J.; Amat, F.; Oro, D.; Mañosa, S. Prey dynamics and breeding performance in a generalist predator: The differential role of prey density, biomass, and effective consumption rates. Acta Oecologica 2024, 123, 103999. [Google Scholar] [CrossRef]
- Johnson, M.; Matthew, D.; George, S. Estimating the Number of Rodents Removed by Barn Owls Nesting in Boxes on Winegrape Vineyards UC Agriculture & Natural Resources Proceedings of the Vertebrate Pest Conference Authors University of California. Proc. Vertebr. Pest Conf. 2020, 29, 29. [Google Scholar]
- Jareño, D.; Luna, A.P.; Viñuela, J. Local Effects of Nest-Boxes for Avian Predators over Common Vole Abundance during a Mid-Density Outbreak. Life 2023, 13, 1963. [Google Scholar] [CrossRef] [PubMed]



| Environmental Gradients | Model Selection | GLM Results | ||
|---|---|---|---|---|
| Geography-Climate (Axis 1 + Axis 2) | Linear | Quadratic | F | p |
| Sorex araneus | √ | √ * | 27.41 | <0.0001 |
| Sorex minutus | √ * | √ | 37.7 | <0.0001 |
| Crocidura russula | √ * | √ | 8.08 | 0.002 |
| Suncus etruscus | √ | 14.16 | 0.0002 | |
| Apodemus flavicollis | √ | 12.49 | 0.0009 | |
| Microtus duodecimcostatus | √ | √ * | 20.3 | <0.0001 |
| Species Richness | √ | 14.56 | 0.0004 | |
| Land uses (Axis 1 + Axis 2) | ||||
| Sorex araneus | √ | √ * | 11.93 | 0.0005 |
| Sorex minutus | √ * | √ | 40.55 | <0.0001 |
| Crocidura russula | √ * | √ | 17.03 | 0.0001 |
| Suncus etruscus | √ | 4.78 | 0.017 | |
| Apodemus flavicollis | √ * | √ | 11.05 | 0.0007 |
| Apodemus sylvaticus | √ | 5.41 | 0.01 | |
| Microtus duodecimcostatus | √ | 6.73 | 0.009 | |
| Microtus lavernedii | √ * | √ | 7.33 | 0.003 |
| Species Richness | √* | √ | 9.49 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torre, I.; Requejo, A.; Arrizabalaga, A.; Baucells, J. Disentangling the Effects of Climate and Land Uses on Small Mammals in Agroecosystems of NE Spain. Diversity 2024, 16, 343. https://doi.org/10.3390/d16060343
Torre I, Requejo A, Arrizabalaga A, Baucells J. Disentangling the Effects of Climate and Land Uses on Small Mammals in Agroecosystems of NE Spain. Diversity. 2024; 16(6):343. https://doi.org/10.3390/d16060343
Chicago/Turabian StyleTorre, Ignasi, Andrés Requejo, Antoni Arrizabalaga, and Jordi Baucells. 2024. "Disentangling the Effects of Climate and Land Uses on Small Mammals in Agroecosystems of NE Spain" Diversity 16, no. 6: 343. https://doi.org/10.3390/d16060343
APA StyleTorre, I., Requejo, A., Arrizabalaga, A., & Baucells, J. (2024). Disentangling the Effects of Climate and Land Uses on Small Mammals in Agroecosystems of NE Spain. Diversity, 16(6), 343. https://doi.org/10.3390/d16060343







