Abstract
The studies on the bioinvasion phenomenon focus mainly on the biological and ecological traits of a species, while scattered literature is addressed to a correct systematic assessment and an updated geographical distribution on the whole. It is noteworthy that non-indigenous species should be monitored on both sides of their range, i.e., towards the front of dispersal and beyond the original range. The backside range boundaries are not often monitored or well delimited; thus, the novel global distribution of an invasive taxon is not often accurately delimitated. A model species for highlighting criticisms about the scarce knowledge on the novel range was chosen. Brachidontes pharaonis is a mussel that has successfully colonised the Mediterranean Sea, following an immigration pathway through the Suez Canal from the Red Sea, presumably from a wider Indo-Pacific area. In this case, the Indo-Pacific boundaries have been overlooked and are still misunderstood due to diverse causes, principally as the synonymy with the congeneric B. variabilis has created confusion in the taxonomic identification. The present review demonstrates that the borders of the B. pharaonis range are still unclear and that the species should be analysed in a wider geographical area. Records of B. pharaonis from Southeast Asia should be treated with caution as this area is out of its range. The Indian and Pacific Oceans host the taxon B. variabilis, composed of two potential cryptic species. Data from the literature highlight the importance of the integrative taxonomic approach to solving various issues concerning the species group complex, morphological variations and cosmopolitan claims of species.
1. Introduction
Marine invasive species significantly alter the biodiversity of local ecosystems at fundamental levels. By introducing new competitive behaviours, dynamics and predatory practices into ecosystems where they are introduced [1], these species upset the natural equilibrium and consistency of native environments [2], contributing to the reduction of biodiversity. Human activities, particularly maritime transport and the discharge of ballast water, are often the primary means by which these non-native species cross biogeographic boundaries. Once these invasive organisms gain a foothold, they can dominate local species for resources, modify physical environments and spread new diseases, resulting in the decline or even the extinction of local species.
During the last century, many exotic species arrived in the Mediterranean Sea after the opening of the Suez Canal [3]. Most of these Lessepsian migrants established breeding populations. To evaluate potential risks and impacts on natural ecosystems, particularly in the rapidly changing Mediterranean Sea, a widely acknowledged aim is to explore biological traits and ecological plasticity, mainly of the most abundant invasive species [4,5,6,7]. It has been demonstrated that invertebrate invaders can tolerate high fluctuations in salinity and temperature [8]; an example is the skeleton shrimp Paracaprella pusilla, an alien invasive species in the Mediterranean, recorded abundantly in the vicinity of outfalls of desalination plants where hypersaline brine plume occur [9].
This review focuses on Brachidontes pharaonis, a frequent mussel originating from the Indo-Pacific area that has successfully colonised the Mediterranean through the Suez Canal from the Red Sea.
The presence of Brachidontes pharaonis has led to significant ecological effects, such as disruptions in food chains [10,11] and the displacement of indigenous mussels within aquaculture communities [11]. These changes not only diminish the variety and numbers of native species but also undermine essential ecosystem functionalities and services, including water purification and providing habitats for various species. The continuous threat from invasive species highlights the urgency for integrated management efforts focusing on prevention, early detection and swift action to lessen their environmental impacts. Addressing the rapid degradation of local biodiversity has become critical. Most of the current research is dedicated to understanding the biological characteristics and adaptability of predominant invasive species [4,5,6,7], starting with a thorough examination of bioinvasion phenomena. The phenomenon of bioinvasion also requires a comprehensive understanding across the boundaries of the species range, not only encompassing the forefront of dispersal but also extending beyond the species’ original range. This is crucial because the new distribution of invasive species is frequently challenging to precisely demarcate on both sides [12].
2. The Species Brachidontes pharaonis
Brachidontes pharaonis (P. Fisher, 1870), an umbonally non-septate marine Mytiloidea, is a small Lessepsian mussel (max length 40 mm) (Figure 1) found abundantly in mid-littoral habitats.

Figure 1.
External view of valves of Brachidontes pharaonis specimens, collected from the Mediterranean Sea (above) and the Red Sea (below).
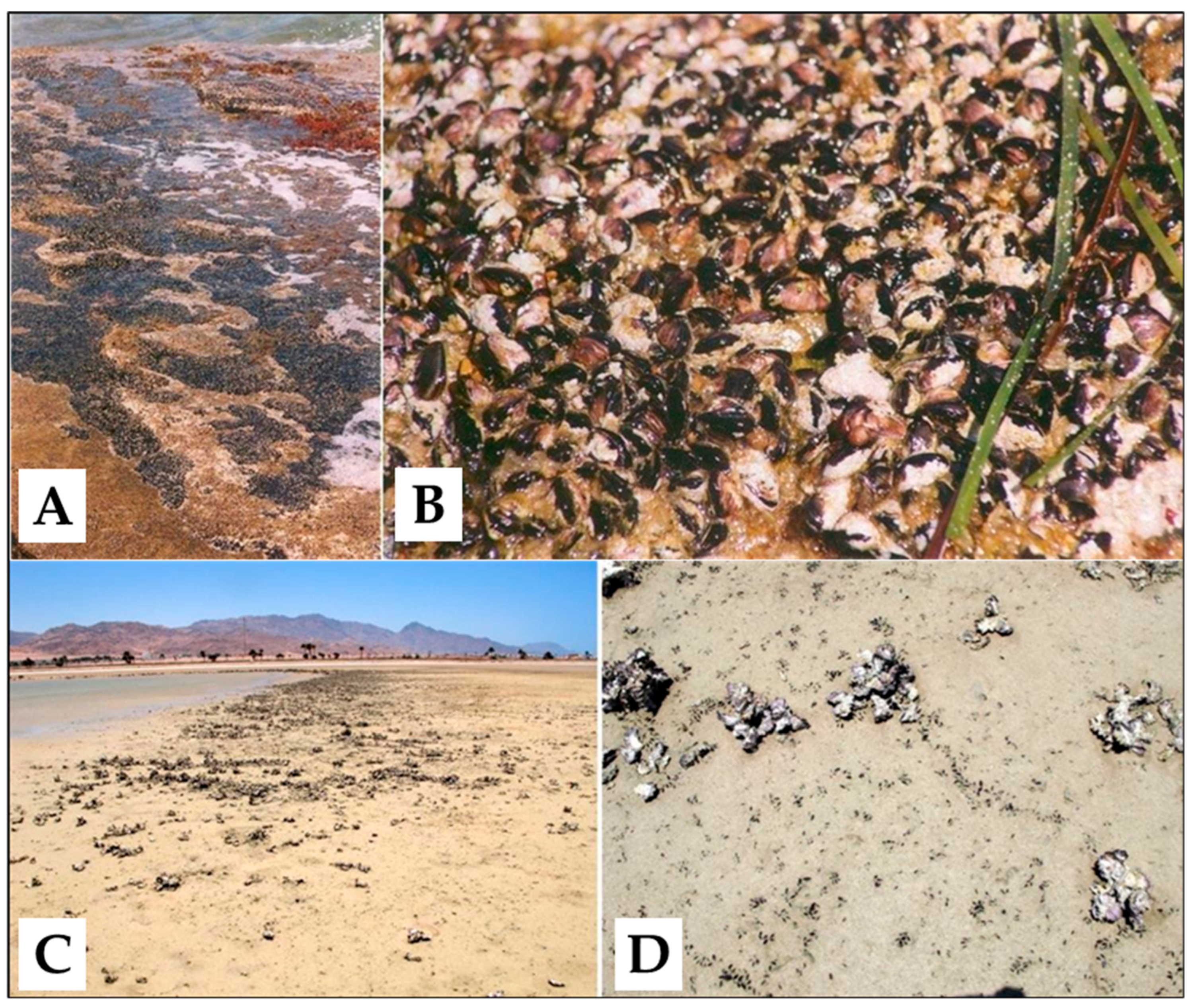
It is an euryhaline, eurythermal, general filter feeder, and its physiological plasticity has played an essential role in the colonisation of the Mediterranean Sea [13]. In the areas where wave exposure and sedimentation conditions are optimal [14,15], B. pharaonis forms beds, reaching high densities (>10,000 ind/m2) and covering entirely rocky shores (Figure 2).
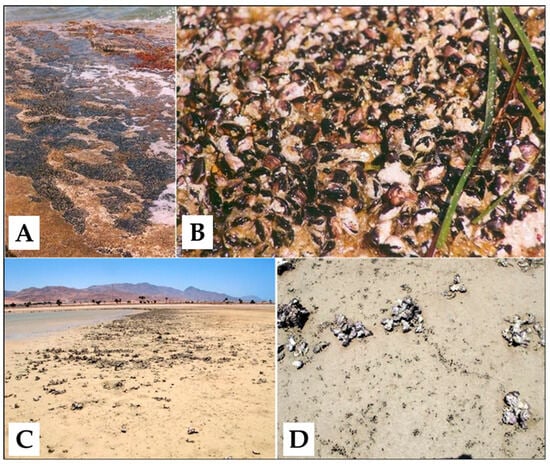
Figure 2.
Above: the mytilid beds (A) and a detail of the aggregated shells of Brachidontes pharaonis specimens (B) in Sicily, southern Italy, Mediterranean Sea. Below: a different mytilid aggregation (C), and a detail of individual specimens of Brachidontes pharaonis detectable by the very small black scattered shells (D) in the Dahab lagoon, Egypt, Red Sea.
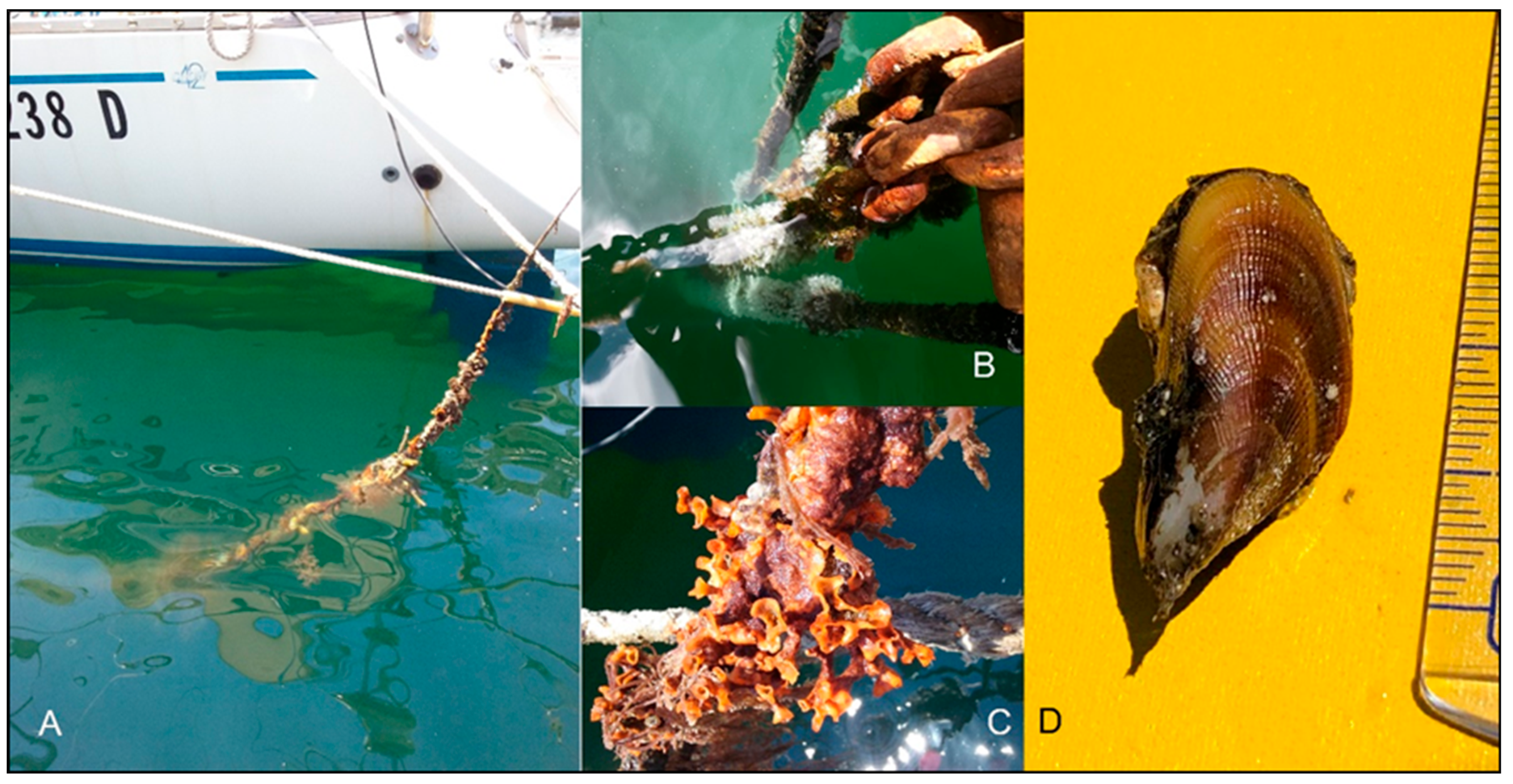
Many life-history traits, including high fecundity, planktonic larvae and early maturity, enable B. pharaonis to colonise several types of habitats across a wide geographic range [16,17,18,19]. Additionally, recent coastal monitoring, to assess the fouling biodiversity, has introduced new perspectives. A long-term qualitative survey has been conducted for 10 years in a locality of the central Mediterranean Sea on the northern coast of Sicily Island (Italy). The coastal epibenthic fouling communities were sampled at least once a year to monitor biodiversity changes in a specific site vulnerable to bioinvasions, i.e., a recreational marina (central Mediterranean Sea, 38°07′14.04″ N, 13°22′12.84″ E; Figure 3). The mussel was collected on ropes every year for the last decade. Specimens, not always aggregated in clusters or patches of different lengths, have been found; in some cases, mussels showed full gonads. The mooring ropes were regularly cleaned by the owners of the boats; thus, the mussels are thought to continuously recruit the substrate (direct observation).
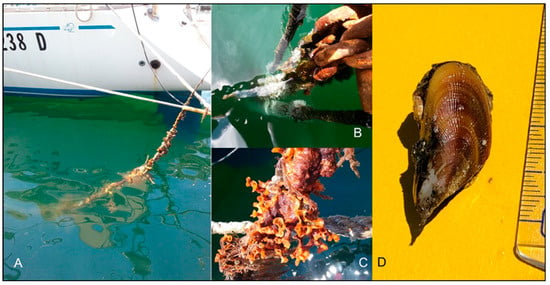
Figure 3.
The fouling community on the mooring ropes where scattered Brachidontes pharaonis individuals occurred (A–C). A specimen of the mussels sampled (D).
The features of Sicily have made this island a crossroads for marine species dispersal pathways, making it a sort of ‘trap’ for marine species carried by currents [3], thereby establishing local and scattered populations, such as the ones described above, on the Sicilian coast, which may guarantee the survival of the species in the Mediterranean Sea. Such observations add a further driver of species distribution, as some sites with “neglected specimens” can be a kind of sink-and-source site where settlement and growth are guaranteed (sink) and spawning and dispersal are assured (source). The environmental features of some sites and the high population connectivity conferred by the planktonic larvae can provide support for such a dispersal mechanism, not only based on the establishment of mytilid beds at high densities.
Recent observations from the Mediterranean Sea show that the range of B. pharaonis is still in a phase of expansion [3,14]. The species has spread from the eastern to the western Mediterranean basin, up to the northern Adriatic Sea, reaching the Venice lagoon and the coasts of Croatia, and westwards along the Tunisia, French and Spanish coasts [7,16] [20,21,22,23].
The pathway of dispersion has been demonstrated to be the result of the phenotypic and ecological traits of the species [8]. Curatolo [24] demonstrated a high differentiation in shell morphological features: the specimens grown in sand had a greater ventral concavity and a major broadened and angular dorsal margin (a kind of ‘triangular form’; see mussels shown below in Figure 1, in sharp contrast to specimens living on rocky shores. The development of strongly triangular morphs may have been triggered by competition for food, as individuals may have better access to the water column and have an advantage when competing for food in an environment with low hydrodynamics.
3. The Criticisms about B. pharaonis Distribution
The great differentiation detected by molecular markers was the cause of the no longer accepted synonymy of B. pharaonis with B. variabilis (Krauss), the last species distributed in the Indo-Pacific region. Different studies allocated B. pharaonis as the valid name to be used for the species occupying the Mediterranean and the northern Red Sea and considered B. variabilis a complex of two different taxonomic entities distributed in the Indo-Pacific waters [6,19,24]. However, recent assignments of the B. pharaonis taxon to samples collected in areas other than the Mediterranean and Red Sea [25,26,27,28] highlights the necessity for a deeper knowledge of the Brachidontes pharaonis–variabilis dichotomy.
Most pieces of literature report genetic patterns of B. pharaonis [6,16,17,18,19,20,24,26,29,30,31]. The analyses conducted on mitochondrial DNA [6,16,18,19,24,26,30,32] have shown a surprising variability within the species and the existence of separate clades. In particular, two co-existing mitochondrial forms, named L and M (according to the name of the substituted amino acids, leucine and methionine), seemed to be the result of paleo-vicariance processes that occurred in the Red Sea, of which traces remain in the sequence of the mtDNA [18]. In contrast with this ancient polymorphism, B. pharaonis samples collected in the Mediterranean and the Red Sea did not show a geographical structuring but showed an adequate level of genetic cohesion [6,18,24,30].
The identification of B. pharaonis is often complicated by the large variability of its morphological traits. Swennen et al. [27] identified samples as B. pharaonis collected in the Gulf of Thailand, with radial ribs bifurcating, inner margin crenulated on the posterior side, and colour yellowish-brown and internally shiny purplish. However, a recent paper by Wells et al. [33] stated that B. pharaonis in Thailand was misidentified with B. variabilis, corroborating the difficulties in taxonomic diagnosis [28].
It is noteworthy that several studies have listed B. pharaonis as present in Southeast Asia (Indonesia, Malaysia and Thailand) [24,25,28,34] and in the central Indian Ocean (Sri Lanka) [26] (these last referred to the sequences included in the tree of Figure 4: Sri Lanka Accession Number: AJ865780, AJ865781, AJ865786, AJ865782). Some authors have categorised the species in these zones as non-indigenous, originating from the western Indian Ocean and the Red Sea through shipping activities [25,33,35]. Unpredictably, the species has even considered native to the Mediterranean Sea [26].
The genus Brachidontes often occurs as one of the most common bivalve taxa in enclosed coastal marine habitats, such as Indonesian marine lakes [35]. The high degree of morphological variability of B. pharaonis has been evidenced for the variable valves in shape and height/length ratio [36] and the terminal umbone (often expanded posteriorly, sometimes arcuate and occasionally subcylindrical, with beaks being not quite terminal)—features which could also be ascribable to the sister species B. variabilis [37,38].
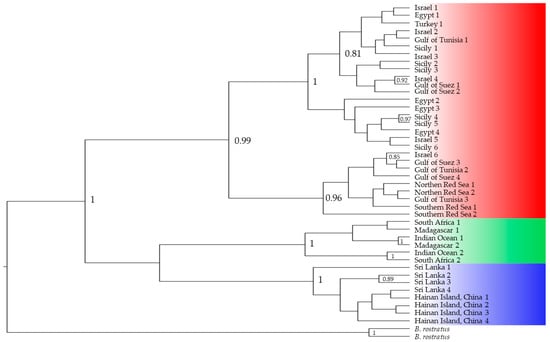
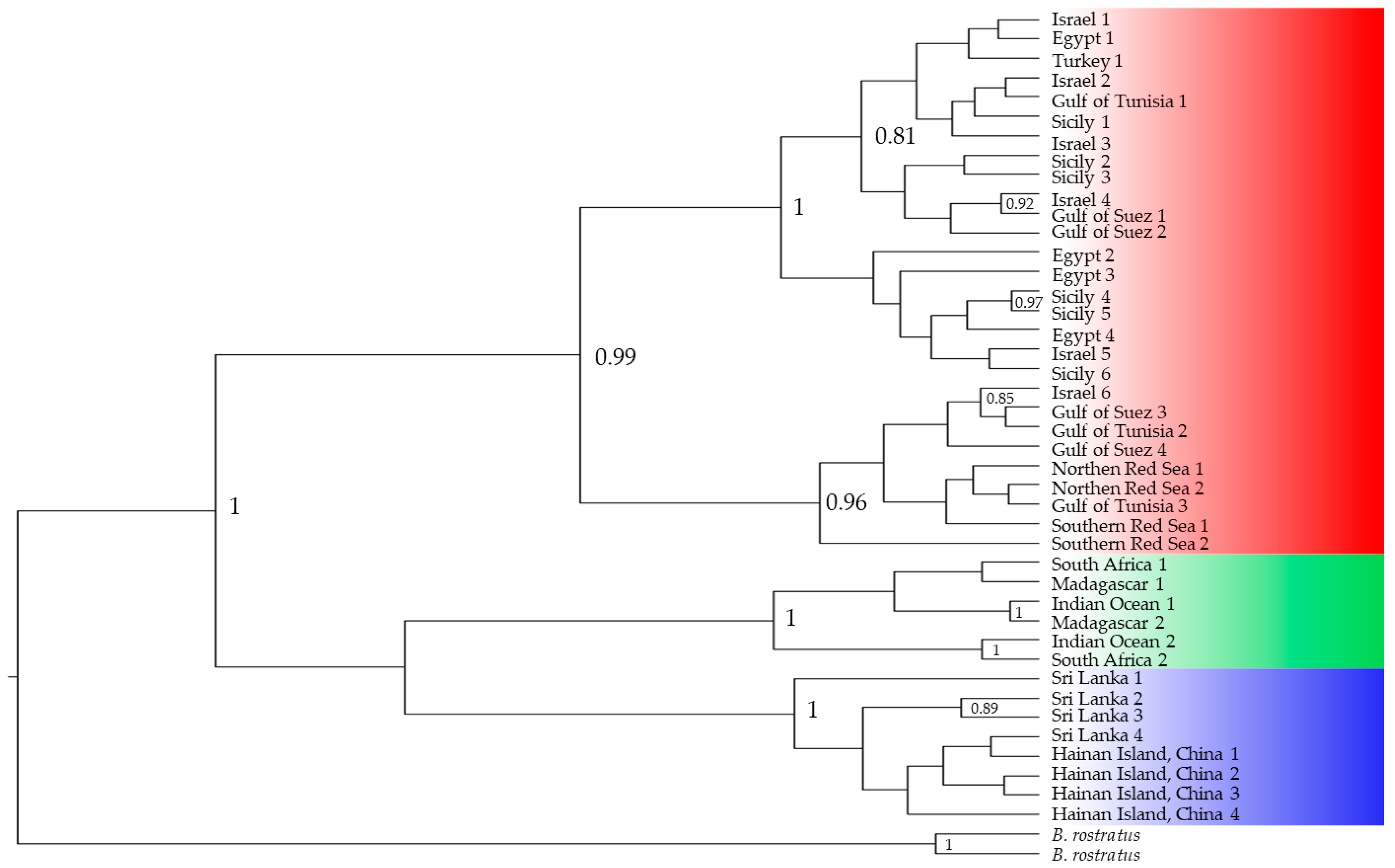
Figure 4.
Bayesian inference tree built using COI as a marker using published data [19,24,27,38]; a posterior value > 0.8 is shown; sequences from the Mediterranean and the Red Sea are highlighted in red, the southwestern Indian Ocean in green and the group from eastern Indian Ocean and western Pacific Ocean in blue.
Figure 4.
Bayesian inference tree built using COI as a marker using published data [19,24,27,38]; a posterior value > 0.8 is shown; sequences from the Mediterranean and the Red Sea are highlighted in red, the southwestern Indian Ocean in green and the group from eastern Indian Ocean and western Pacific Ocean in blue.
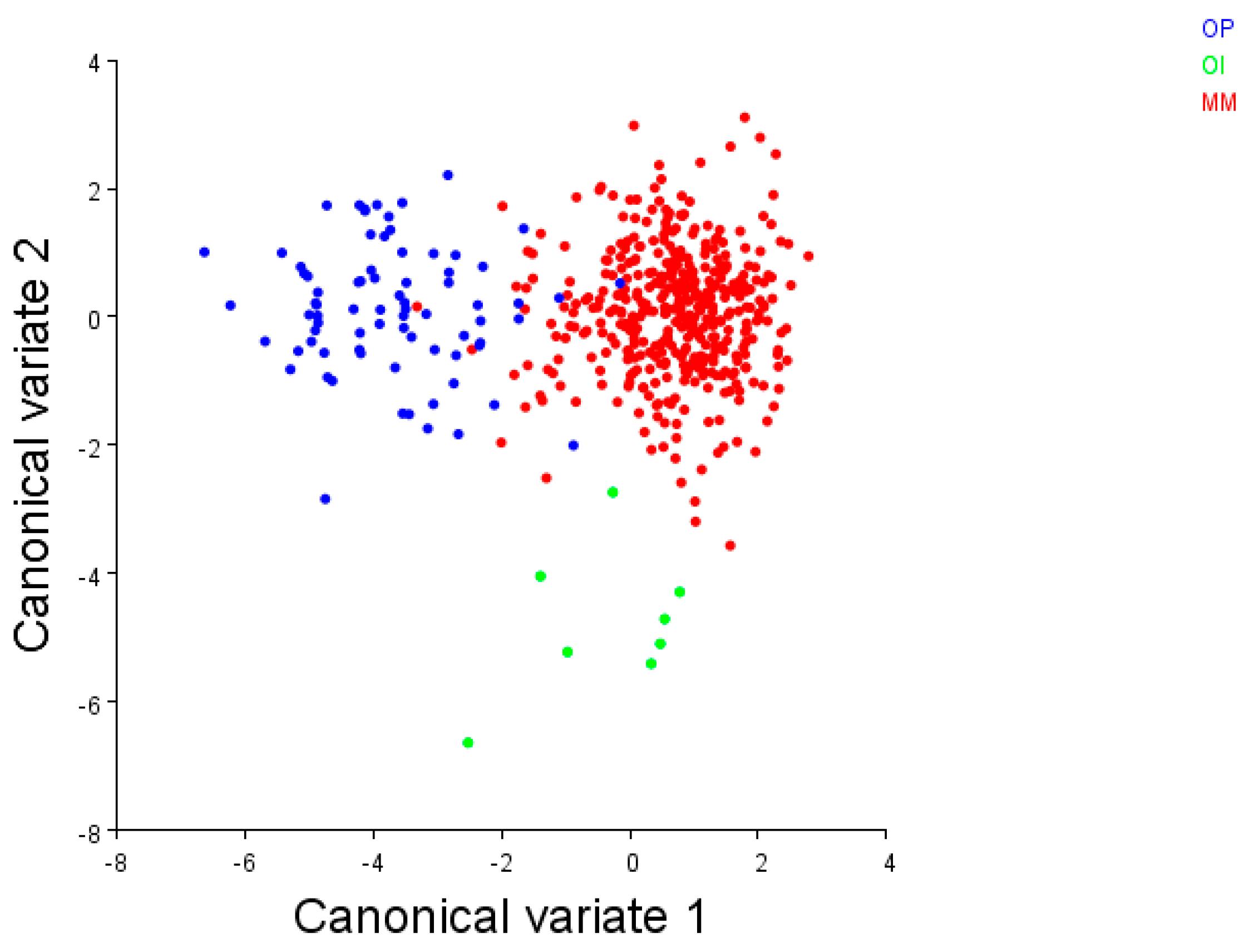
As a consequence, different studies [24,29,37] have applied an integrated approach by combining the data from genetics with geometric morphometrics on valves to reinforce the three lineages within the Brachidontes pharaonis + variabilis group, respectively, from the Mediterranean and Red Sea, from the Indian Ocean and from the Pacific Ocean. A further inferred phylogenetic tree has been built herein with the mitochondrial COI sequences (Figure 4) retrieved from the literature [19,24,38]. This was congruent with the existence of the three lineages, also supported by the previous geometric morphometric data of shells [24] (shown in the CVA scatterplot; Figure 5).
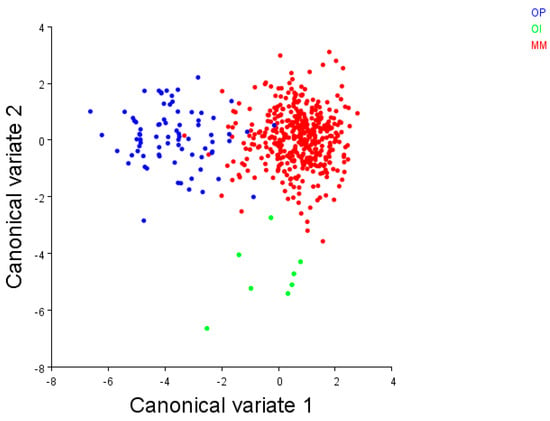
Figure 5.
The CVA scatterplot of the Brachidontes pharaonis–variabilis complex from the three biogeographical regions analysed by [24]. Mediterranean Sea + Red Sea (MM): red circles; Indian Ocean (OI): green circles; and Pacific Ocean (OP): blue circles. (Image courtesy T. Curatolo)
The sequences were aligned in BioEdit [39] using ClustaW [40] and default parameters; then, they were handled in Beauti 2 2.6.0 and analysed in Beast 2 2.6.6 [41,42,43]. The Bayesian analysis was conducted for 100,000,000 generations in Cipres Portal [44] and HKY and Yule as models. The molecular clock used was 1.21 × 10−8 according to Marko [45].
COI sequences representative of the different B. pharaonis/variabilis samples worldwide were analysed. Twenty-eight sequences from the Mediterranean Sea (Sicily, Turkey, Tunisia, Egypt) and Red Sea (Northern Red Sea, Southern Red Sea, Gulf of Suez), six from the Southwestern Indian Ocean (Madagascar and South Africa) and eight from the Eastern Indian Ocean/Western Pacific Ocean (Sri Lanka and Hainan Island, China) were retrieved from previous studies [19,24,27,38]. The COI molecular marker has attracted some criticism regarding the current taxonomic identification. The sequences from the Mediterranean and Red Sea cluster in a single group with a solid posterior value (0.99), which corresponds to the Brachidontes pharaonis taxon. The Southwestern Indian Ocean and the Eastern Indian Ocean + Western Pacific Ocean sequences are two additional well-identified clusters (posterior value of 1) corresponding to the two cryptic Brachidontes cf. variabilis entities.
Despite the present phylogenetic pattern, which mirrors the past literature, individuals from Sri Lanka have been identified as B. pharaonis [27] and samples from both lineages, Brachidontes cf. variabilis, are still identified indistinctly as B. variabilis (Figure 6), though previous results have demonstrated the dichotomy within the Indo-Pacific area [19].
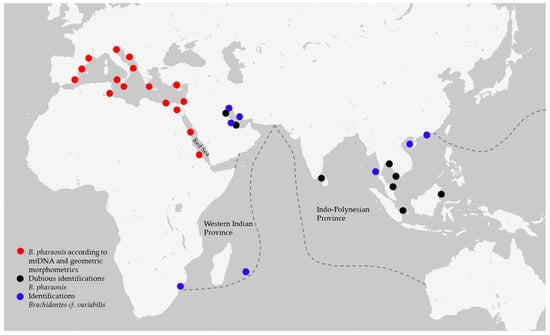
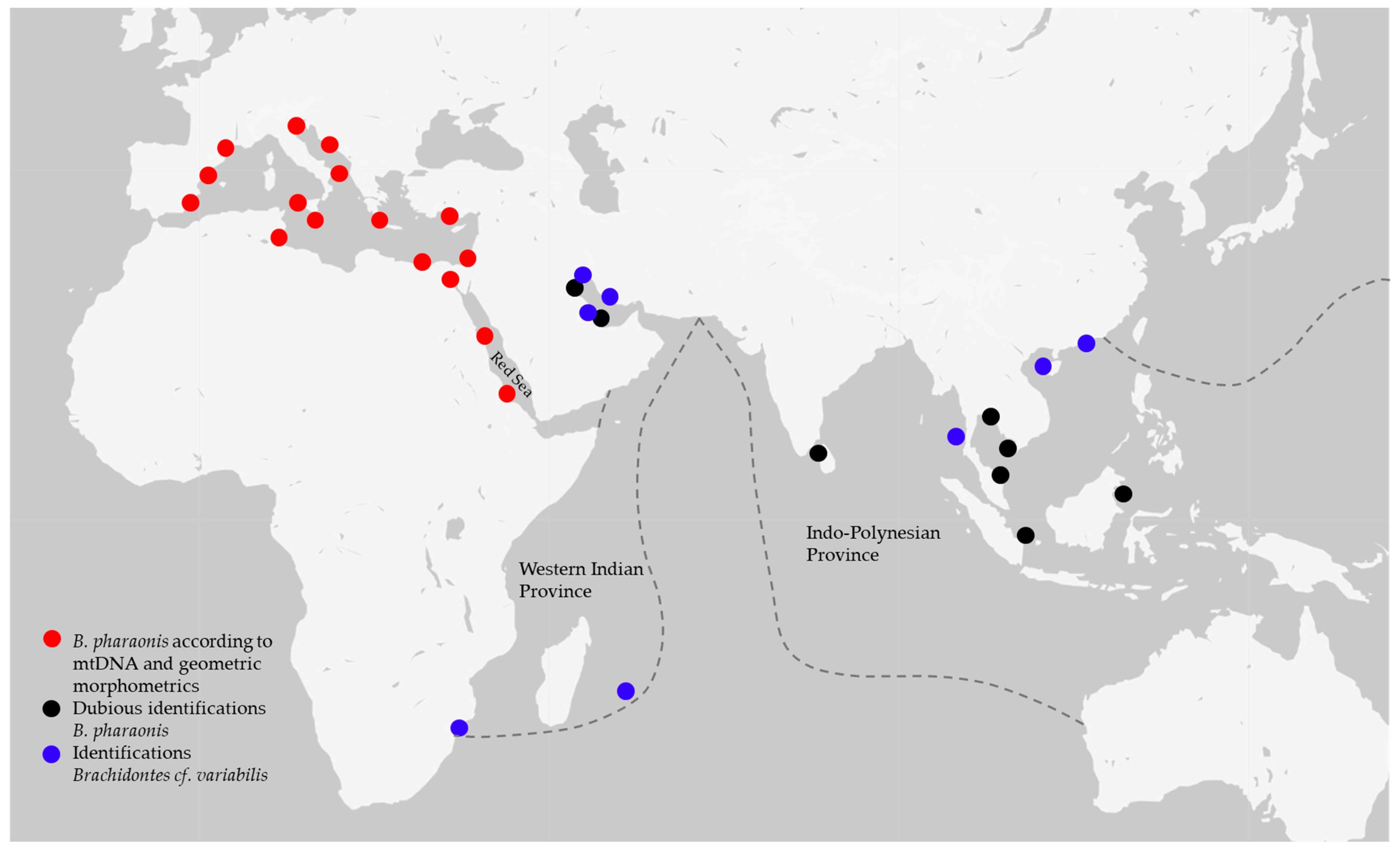
Figure 6.
Distribution map of specimens identified as Brachidontes pharaonis using genetic and geometric data (red dots), dubious specimens identified as B. pharaonis using morphological approach (black dots) and specimens of Brachidontes cf. variabilis (blue dots); all records are from published data [3,6,7,8,11,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,46,47,48,49,50,51]. Brachidontes pharaonis extends its range in the Red Sea Province and from there to the Mediterranean Sea. At the same time, the identification of specimens from the Western Indian Province and Indo-Polynesian Province is still somewhat unclear and causes misunderstandings, implying a deep revision of the genus.
It is noteworthy that the tree lineages have a congruity in the marine biogeographical provinces [52]. The Red Sea, the Western Indian Ocean and the Indo-Polynesian areas are identified as provinces for the presence of a high level of endemism and the isolation from other provinces due to barriers [52], as often revealed in other species distributed worldwide (e.g., [53]). The three lineages detected depict these three provinces: the B. pharaonis originated from the Red Sea and invaded the Mediterranean Sea; the Brachidontes cf. variabilis from Madagascar and South Africa belong to the Western Indian Ocean Province; and the B. cf. variabilis samples from Sri Lanka and China are included in the Indo-Polynesian Province. In particular, the clustering of these two last populations may be explained by a recent colonization (less than 100,000 years before present) of the Eastern India ancestral population after the Sunda Shelf Barrier disappeared at the end of the last glaciation and the Indo-Polynesian Province was born [52].
Regarding the Red Sea Province, it should be highlighted that previous genetic data [26] had revealed a point break in the southern Red Sea, between B. pharaonis samples from the northern Red Sea (i.e., sequences from [30]) and Eritrea; curiously, it has not been herein confirmed as northern and southern Red Sea samples clustered together (Figure 4).
Furthermore, records from the Persian-Arabian Gulf, embedded in the Western Indian Ocean Province, report both B. variabilis (from Iraq [46]; Iran [50]; Qatar [51]; Saudi Arabia [47]) and B. pharaonis (Kuwait [48]; United Arab Emirates [26]), generating great taxonomic confusion.
As such, records of Brachidontes pharaonis or B. cf. variabilis from the Indian Ocean and western Pacific regions should be treated with caution; the three entities that resulted in being discriminated against (Figure 4 and Figure 5) should be deeply investigated and the borders of the B. pharaonis range delineated as they are not clear yet (Figure 6).
4. Conclusions
The present paper could be considered as a baseline for future evaluations, and it also echoes the need for an integrative taxonomic framework for the proper identification of the species. The phenomenon of marine bioinvasions is certainly the most challenging to monitor. Unrestricted by scientific and sampling tracking, the species move freely under the sea, and often, they are found in new geographical areas after they have already established themselves. This is particularly true for small-sized invertebrates; their detection occurs when specialised taxonomists identify them, whereas large-sized animals or plant detection is easier and nowadays also supported and assisted by citizens’ involvement.
Among the various challenges in drawing the diffusion and dispersal models of an invasive alien species, the delimitation of the boundaries of the maximum range that the species can reach is a major task, along with the characterization of the morpho-anatomical and physiological traits that enable the species to survive in a new environment. In addition to this consideration, errors in taxonomic identification create confusion and limit the accurate monitoring of a species. Consequently, assessing the state of the art in cases where there is a rich bibliography on a species becomes a crucial step in the study of specific taxa.
Author Contributions
Conceptualization, S.L.B. and G.S.; methodology, M.B. and T.C.; formal analysis, M.B. and T.C.; data curation, M.B. and M.D.N.; writing—original draft preparation, M.B., G.S. and S.L.B.; writing—review and editing, S.L.B. and G.S.; funding acquisition, S.L.B. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the University of Palermo (FFR–Anno 2024) and National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4—Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of the Italian Ministry of University and Research funded by the European Union—NextGenerationEU; Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP D33C22000960007 and CUP B73C22000790001, Project title “National Biodiversity Future Center—NBFC”.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to M. Sirna Terranova and Gerhard Steiner for providing the images.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ruiz, G.M.; Carlton, J.T. (Eds.) Invasive Species: Vectors and Management Strategies; Island Press: Washington, DC, USA, 2003; ISBN 978-1-55963-902-6. [Google Scholar]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the Global Threat of Invasive Species to Marine Biodiversity. Front. Ecol. Environ. 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Servello, G.; Andaloro, F.; Azzurro, E.; Castriota, L.; Catra, M.; Chiarore, A.; Crocetta, F.; D’Alessandro, M.; Denitto, F.; Froglia, C.; et al. Marine Alien Species in Italy: A Contribution to the Implementation of Descriptor D2 of the Marine Strategy Framework Directive. Mediterr. Mar. Sci. 2019, 1–48. [Google Scholar] [CrossRef]
- Lo Brutto, S.; Iaciofano, D.; Lubinevsky, H.; Galil, B.S. Grandidierella Bonnieroides Stephensen, 1948 (Amphipoda, Aoridae)—First Record of an Established Population in the Mediterranean Sea. Zootaxa 2016, 4092, 518–528. [Google Scholar] [CrossRef]
- Lo Brutto, S.; Iaciofano, D. A Taxonomic Revision Helps to Clarify Differences between the Atlantic Invasive Ptilohyale Littoralis and the Mediterranean Endemic Parhyale Plumicornis (Crustacea, Amphipoda). ZooKeys 2018, 754, 47–62. [Google Scholar] [CrossRef]
- Mohammed-Geba, K.; Sheir, S.K.; El-Aziz Hamed, E.A.; Galal-Khallaf, A. Molecular and Morphological Signatures for Extreme Environmental Adaptability of the Invasive Mussel Brachidontes Pharaonis (Fischer, 1870). Mol. Cell. Probes 2020, 53, 101594. [Google Scholar] [CrossRef]
- Jelić Mrčelić, G.; Nerlović, V.; Doğan, A. Sustainable Management of High-Impact Non-Native Molluscs and Their Potential Commercial Importance in the Eastern Adriatic Sea. Sustainability 2023, 15, 11384. [Google Scholar] [CrossRef]
- Sarà, G.; Romano, C.; Widdows, J.; Staff, F.J. Effect of Salinity and Temperature on Feeding Physiology and Scope for Growth of an Invasive Species (Brachidontes Pharaonis—MOLLUSCA: BIVALVIA) within the Mediterranean Sea. J. Exp. Mar. Biol. Ecol. 2008, 363, 130–136. [Google Scholar] [CrossRef]
- Lo Brutto, S.; Iaciofano, D.; Guerra García, J.M.; Lubinevsky, H.; Galil, B.S. Desalination effluents and the establishment of the non-indigenous skeleton shrimp Paracaprella pusilla Mayer, 1890 in the south-eastern Mediterranean. BioInvasions Rec. 2019, 8, 661–669. [Google Scholar] [CrossRef]
- Sarà, G.; Giommi, C.; Giacoletti, A.; Conti, E.; Mulder, C.; Mangano, M.C. Multiple Climate-Driven Cascading Ecosystem Effects after the Loss of a Foundation Species. Sci. Total Environ. 2021, 770, 144749. [Google Scholar] [CrossRef] [PubMed]
- Sarà, G.; Lo Martire, M.; Buffa, G.; Mannino, A.M.; Badalamenti, F. The Fouling Community as an Indicator of Fish Farming Impact in Mediterranean. Aquac. Res. 2007, 38, 66–75. [Google Scholar] [CrossRef]
- Tiralongo, F.; Pappalardo, A.M.; Ignoto, S.; Lombardo, B.M.; Ferrito, V.; Campos Sosa, A.; Spinelli, A. The African Striped Grunt, Parapristipoma Octolineatum (Valenciennes, 1833), in the Mediterranean Sea: The Third Record with Biological and Ecological Notes, and Identification Key for Haemulidae Recorded in the Mediterranean. J. Mar. Sci. Eng. 2023, 11, 1688. [Google Scholar] [CrossRef]
- Sarà, G.; Milanese, M.; Prusina, I.; Sarà, A.; Angel, D.L.; Glamuzina, B.; Nitzan, T.; Freeman, S.; Rinaldi, A.; Palmeri, V.; et al. The Impact of Climate Change on Mediterranean Intertidal Communities: Losses in Coastal Ecosystem Integrity and Services. Reg. Environ. Chang. 2014, 14, 5–17. [Google Scholar] [CrossRef]
- Cilia, D.P.; Deidun, A. Branching out: Mapping the Spatial Expansion of the Lessepsian Invader Mytilid Brachidontes Pharaonis around the Maltese Islands. Mar. Biodivers. Rec. 2012, 5, e28. [Google Scholar] [CrossRef]
- Safriel, U.N.; Gilboa, A.; Felsenburg, T. Distribution of Rocky Intertidal Mussels in the Red Sea Coasts of Sinai, the Suez Canal and the Mediterranean Coast of Israel, with Special Reference to Recent Colonizers. J. Biogeogr. 1980, 7, 39. [Google Scholar] [CrossRef]
- Antit, M.; Amor, N.; Urra, J.; Alagaili, A.; Farjallah, S. Genetic Variability of the Lessepsian Migrant Mussel Brachidontes Pharaonis (Bivalvia: Mytilidae) in Tunisia. Afr. J. Mar. Sci. 2018, 40, 211–217. [Google Scholar] [CrossRef]
- El-Deeb, R.S.; Abdel Razek, F.A.; Omar, H.A.; Khafage, A.R.; Abdul-Aziz, K.K. The Gametogenic Cycle and Spawning of the Mussel Brachidontes Pharaonis (Fischer, 1876) (Bivalvia: Mytilidae) from Alexandria Coast, Egypt. Egypt. J. Aquat. Res. 2018, 44, 353–359. [Google Scholar] [CrossRef]
- Sirna Terranova, M.; Lo Brutto, S.; Arculeo, M.; Mitton, J.B. Population Structure of Brachidontes Pharaonis (P. Fisher, 1870) (Bivalvia, Mytilidae) in the Mediterranean Sea, and Evolution of a Novel mtDNA Polymorphism. Mar. Biol. 2006, 150, 89–101. [Google Scholar] [CrossRef]
- Terranova, M.S.; Lo Brutto, S.; Arculeo, M.; Mitton, J.B. A Mitochondrial Phylogeography of Brachidontes Variabilis (Bivalvia: Mytilidae) Reveals Three Cryptic Species. J. Zool. Syst. Evol. Res. 2007, 45, 289–298. [Google Scholar] [CrossRef]
- Doğan, A.; Önen, M.; Öztürk, B. A New Record of the Invasive Red Sea Mussel Brachidontes Pharaonis (Fischer P., 1870) (Bivalvia: Mytilidae) from the Turkish Coasts. Aquat. Invasions 2007, 2, 461–463. [Google Scholar] [CrossRef]
- Quiñonero-Salgado, S. Poblaciones Consolidadas de Brachidontes Pharaonis (Fischer, 1870)(Bivalvia: Mytilidae) En El Levante Peninsular. Elona Rev. Malacol. Ibérica 2022, 3, 68–71. [Google Scholar]
- Murcia Requena, J.; Verdejo Guirao, J.F.; Quiñonero-Salgado, S.; López-Soriano, J. Final Del Trayecto: Llegada Del Bivalvo Lessepsiano Brachidontes Pharaonis (Fischer 1870)(Bivalvia: Mytilidae) a La Península Ibérica. Elona Rev. Malacol. Ibérica 2020, 2, 114–117. [Google Scholar]
- Lipej, L.; Acevedo, I.; Akel, E.H.K.; Anastasopoulou, A.; Angelidis, A.; Azzurro, E.; Castriota, L.; Çelik, M.; Cilenti, L.; Crocetta, F.; et al. “New Mediterranean Biodiversity Records” (March 2017). Mediterr. Mar. Sci. 2017, 18, 179. [Google Scholar] [CrossRef]
- Curatolo, T. Filogenesi e Filogeografia Del Sistema Brachidontes Pharaonis-Variabilis (Bivalvia, Mytiloida, Mytilidae). Ph.D. Thesis, Università degli Studi di Palermo, Palermo, Italy, 2011. [Google Scholar]
- Yahya, N.; Idris, I.; Rosli, N.S.; Bachok, Z. Mangrove-Associated Bivalves in Southeast Asia: A Review. Reg. Stud. Mar. Sci. 2020, 38, 101382. [Google Scholar] [CrossRef]
- Belmaker, J.; Abelson, A.; Haddas-Sasson, M.; Yamaguchi, N.; Shefer, S.; Geffen, E. Potential Pitfalls in the Definition of Lessepsian Migrants: The Case of Brachidontes. In The Arabian Seas: Biodiversity, Environmental Challenges and Conservation Measures; Jawad, L.A., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1293–1307. ISBN 978-3-030-51505-8. [Google Scholar]
- Swennen, C.; Moolenbeek, R.G.; Ruttanadakul, N.; Hobbelinkl, H.; Dekker, H. The Molluscs of the Southern Gulf of Thailand. In The Biodiversity Research and Training Program (BRT); Biodiversity Research and Training Program (BRT) The Molluscs of the southern Gulf of Thailand (uva.nl): Bangkok, Thailand, 2001; pp. 76–94. [Google Scholar]
- Yahya, N.; Zakaria, N.Z.; Mohd Taufeq, Z.; Bachok, Z. Diversity and Abundance of Bivalvia (Phylum Mollusca) in Lagoon of Setiu Wetlands. In Invertebrates of Setiu Wetlands; Mohamad, F., Ibrahim, Y.S., Baharuddin, N., Azmi, N.A.A.A.R., Borkhanuddin, M.F., Eds.; Universiti Malaysia Terengganu: 2130, Kuala Nerus: Terengganu, Malaysia, 2017; pp. 69–76. [Google Scholar]
- Becking, L.E.; De Leeuw, C.A.; Knegt, B.; Maas, D.L.; De Voogd, N.J.; Abdunnur; Suyatna, I.; Peijnenburg, K.T.C.A. Highly Divergent Mussel Lineages in Isolated Indonesian Marine Lakes. PeerJ 2016, 4, e2496. [Google Scholar] [CrossRef] [PubMed]
- Shefer, S.; Abelson, A.; Mokady, O.; Geffen, E. Red to Mediterranean Sea Bioinvasion: Natural Drift through the Suez Canal, or Anthropogenic Transport? Mol. Ecol. 2004, 13, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Çinar, M.E.; Bakir, K.; Öztürk, B.; Katağan, T.; Doğan, A.; Açik, S.; Kurt-Sahin, G.; Özcan, T.; Dağli, E.; Bitlis-Bakir, B.; et al. Macrobenthic Fauna Associated with the Invasive Alien Species Brachidontes Pharaonis (Mollusca: Bivalvia) in the Levantine Sea (Turkey). J. Mar. Biol. Assoc. United Kingd. 2017, 97, 613–628. [Google Scholar] [CrossRef]
- Radwan, N.; Morsy, Z.; Ahmed, M.; Ibrahim, N.; Mohammed, S. Molecular Analysis of Brachidonte Spharaonis (Fischer P., 1870) in Egypt Reveals Cryptic Species Complex. Egypt. J. Aquat. Biol. Fish. 2014, 18, 37–46. [Google Scholar] [CrossRef][Green Version]
- Wells, F.E.; Sanpanich, K.; Tan, S.K.; Duangdee, T. The Marine and Estuarine Molluscs of Thailand; Lee Kong Chian Natural History Museum, National University of Singapore: Singapore, 2021; Conservatory Drive Singapore 117377 REPUBLIC OF SINGAPORE. [Google Scholar]
- Printrakoon, C.; Tëmkin, I. Comparative Ecology of Two Parapatric Populations of Isognomon (Bivalvia: Isognomonidae) of Kungkrabaen Bay, Thailand. Raffles Bull. Zool. 2008, 18, 75–94. [Google Scholar]
- Aji, L.P.; Goud, J.; Van Der Steeg, S.; Tapilatu, R.; Maas, D.L.; Becking, L.E. The Diversity of Molluscan Faunas in Marine Lakes of Raja Ampat, West Papua, Indonesia. Contrib. Zool. 2023, 92, 391–430. [Google Scholar] [CrossRef]
- El-Sayed, A.; Ibrahim, N.; Nassef, A.; El-Hammady, M.; El-Mekawy, H. Biometric Relationships for the Bivalve Mussel, Brachidontes Pharaonis Populations from the North-Western Coast of Suez Gulf, Egypt. Egypt. Acad. J. Biol. Sci. B Zool. 2016, 8, 61–73. [Google Scholar] [CrossRef]
- Goto, T.V.; Tamate, H.B.; Hanzawa, N. Phylogenetic Characterization of Three Morphs of Mussels (Bivalvia, Mytilidae) Inhabiting Isolated Marine Environments in Palau Islands. Zoolog. Sci. 2011, 28, 568–579. [Google Scholar] [CrossRef]
- Morton, B.; Leung, P.T.Y.; Wei, J.; Lee, G.Y. A Morphological and Genetic Comparison of Septifer Bilocularis, Mytilisepta Virgata and Brachidontes Variabilis (Bivalvia: Mytiloidea) from Hong Kong and Erection of the Mytiliseptiferinae Sub-Fam. Nov. Reg. Stud. Mar. Sci. 2020, 34, 100981. [Google Scholar] [CrossRef]
- Hall, T.A. Bioedit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.-H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Suchard, M.A.; Rambaut, A. Many-Core Algorithms for Statistical Phylogenetics. Bioinformatics 2009, 25, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phyloge-Netic Trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Marko, P.B. Fossil Calibration of Molecular Clocks and the Divergence Times of Geminate Species Pairs Separated by the Isthmus of Panama. Mol. Biol. Evol. 2002, 19, 2005–2021. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M. Systematic Study on Mollusca from Arabian Gulf and Shatt Al-Arab. Center for Arab Gulf Studies, Basrah University: Basrah, Iraq, 1975; Volume 75. [Google Scholar]
- Hasan, A.K.H. A Taxonomic Review of the Bivalve and Gastropod Mollusc Fauna along the Saudi Intertidal Zone of the Arabian Gulf. Mar. Scienes-Ceased Lssuerg 1994, 17, 1–2. [Google Scholar] [CrossRef]
- Al-Kandari, M.; Oliver, P.G.; Chen, W.; Skryabin, V.; Raghu, M.; Yousif, A.; Al-Jazzaf, S.; Taqi, A.; AlHamad, A. Diversity and Distribution of the Intertidal Mollusca of the State of Kuwait, Arabian Gulf. Reg. Stud. Mar. Sci. 2020, 33, 100905. [Google Scholar] [CrossRef]
- Graham Oliver, P.; Al-Kandari, M.; Behbehani, M.; Dekker, H. An IllustrAted CheCklIst of the IntertIdAl BIvAlvIA of the stAte of kuwAIt. Journal of Conchology. J. Conchol. 2023, 44, 483–528. [Google Scholar] [CrossRef]
- Papahn, F.; Ghajari, T. Identification and Classification of Bivalvia in Northwestern of the Persian Gulf Coastal Water (from Deylam to Bahmanshir River). Exp. Anim. Biol. 2018, 6, 41–55. [Google Scholar]
- Mohamed, S.; Al-Khayat, J. A Preliminary Check-List of Benthic Mollusca on the Qatari Coasts, Arabian Gulf. Qatar Univ. Sci. J. 1994, 14, 201–206. [Google Scholar]
- Toonen, R.J.; Bowen, B.W.; Iacchei, M.; Briggs, J.C. Biogeography, Marine. In Encyclopedia of Evolutionary Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 166–178. ISBN 978-0-12-800426-5. [Google Scholar]
- Battiata, M.; Serena, F.; Lo Brutto, S. Genetic and Distribution Data of the Bramble Shark Echinorhinus Brucus (Bonnaterre, 1788) and the Prickly Shark Echinorhinus Cookei Pietschmann, 1928 to Better Reconstruct Their Conservation Status. Animals 2024, 14, 993. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).