Arrested Succession on Fire-Affected Slopes in the Krummholz Zone and Subalpine Forest of the Northern Limestone Alps
Abstract
:1. Introduction
2. Materials and Methods
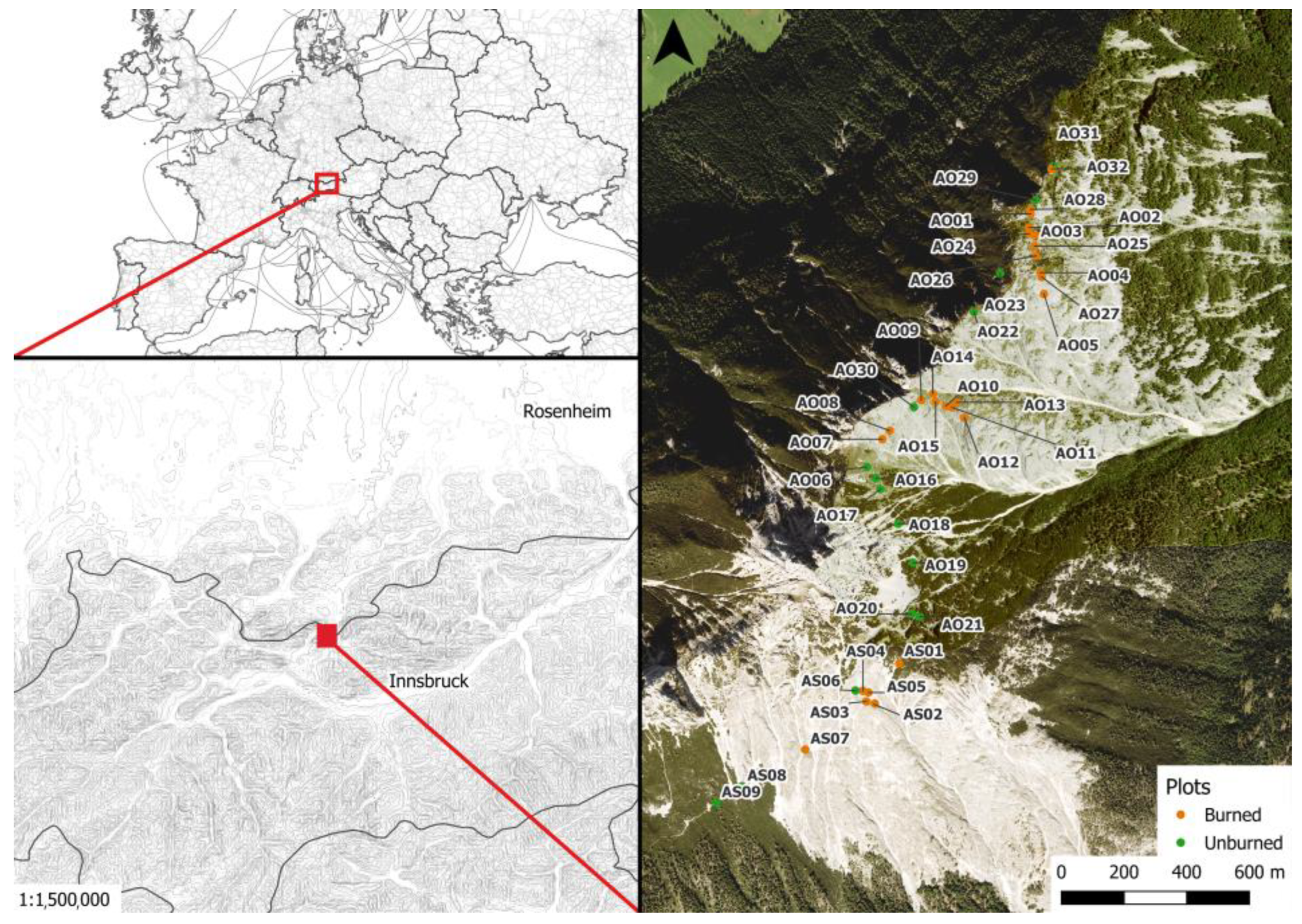
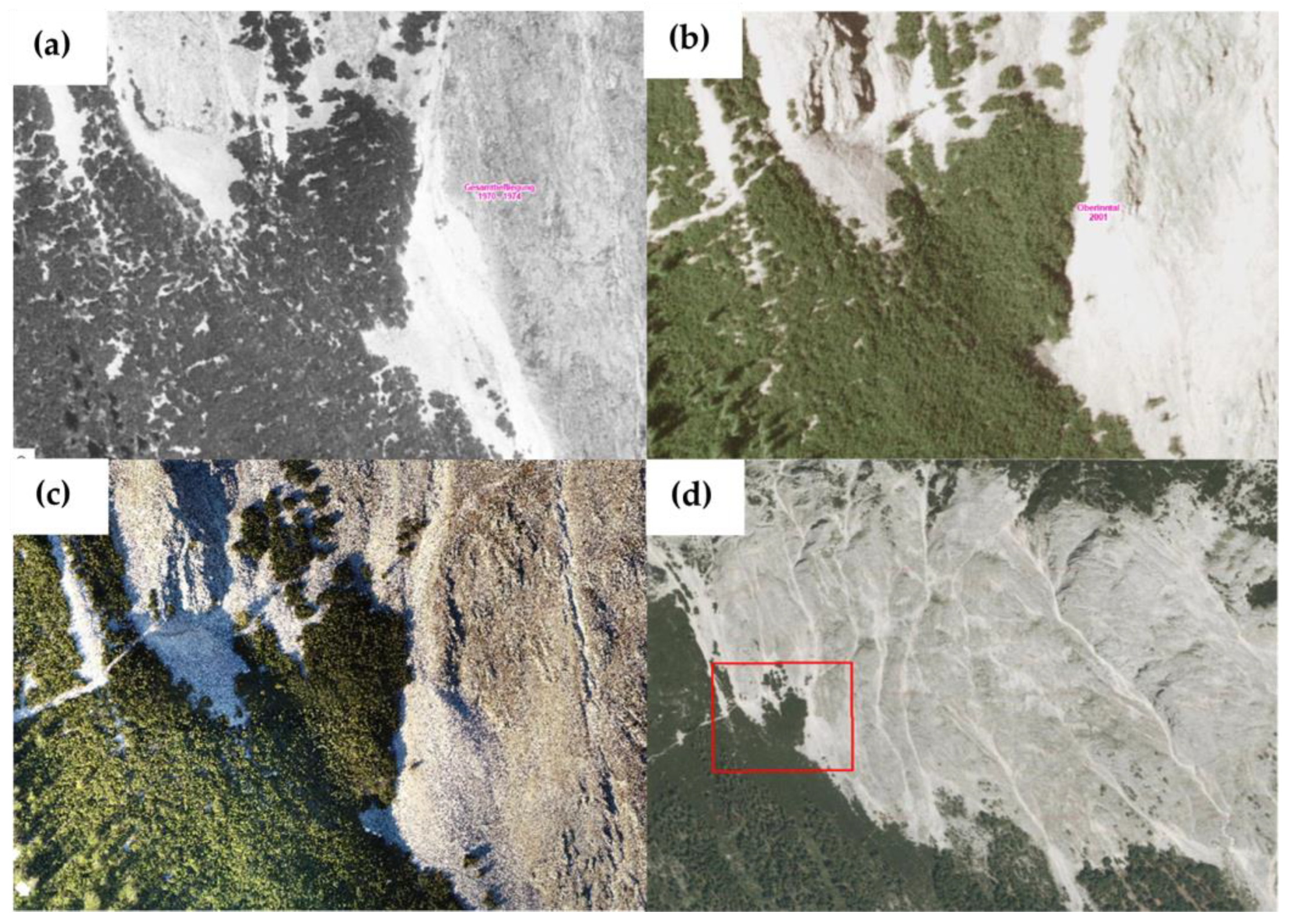
2.1. Study Site
2.2. Sampling Design
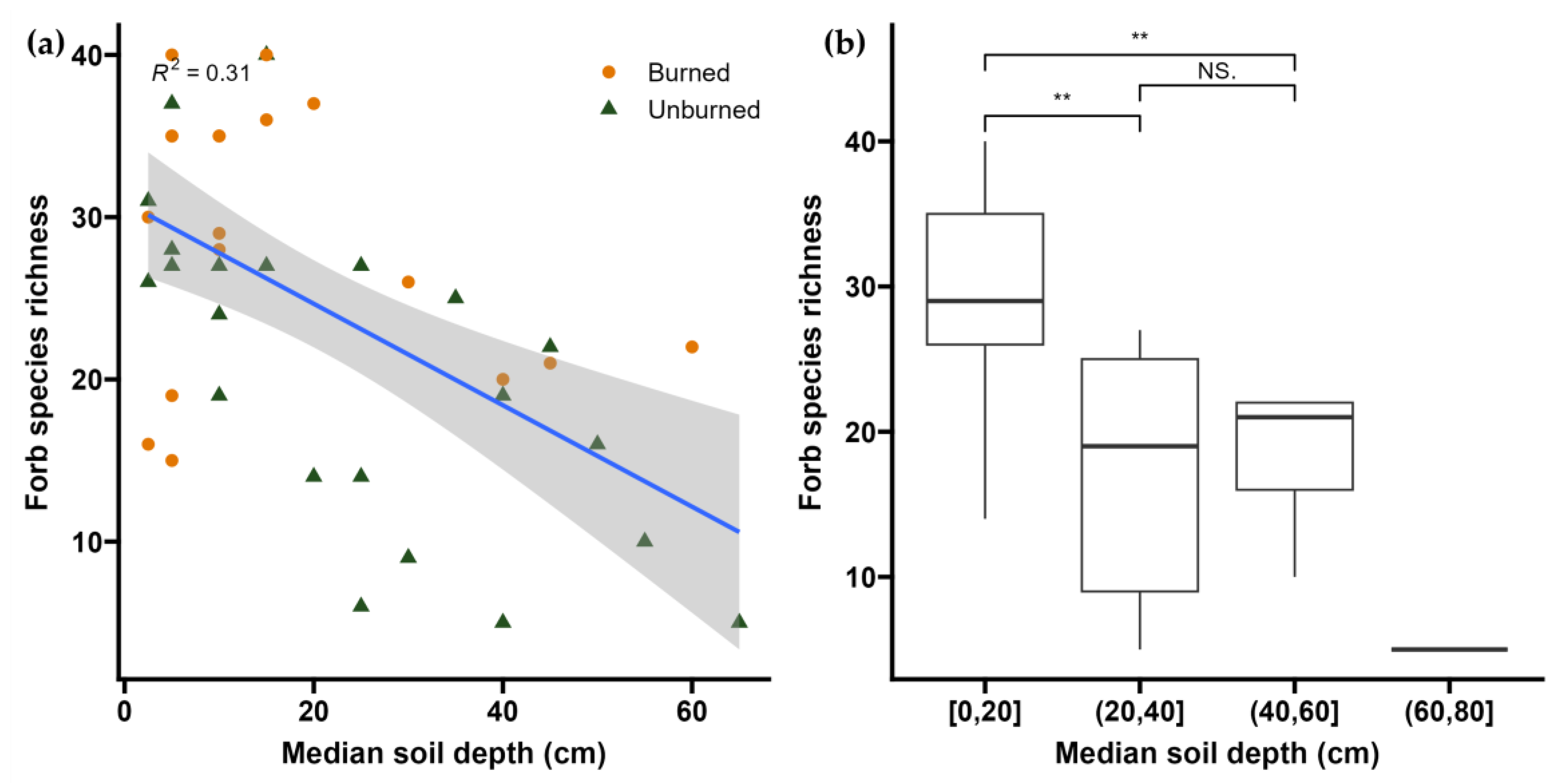
2.3. Soil Depth
2.4. Species Composition
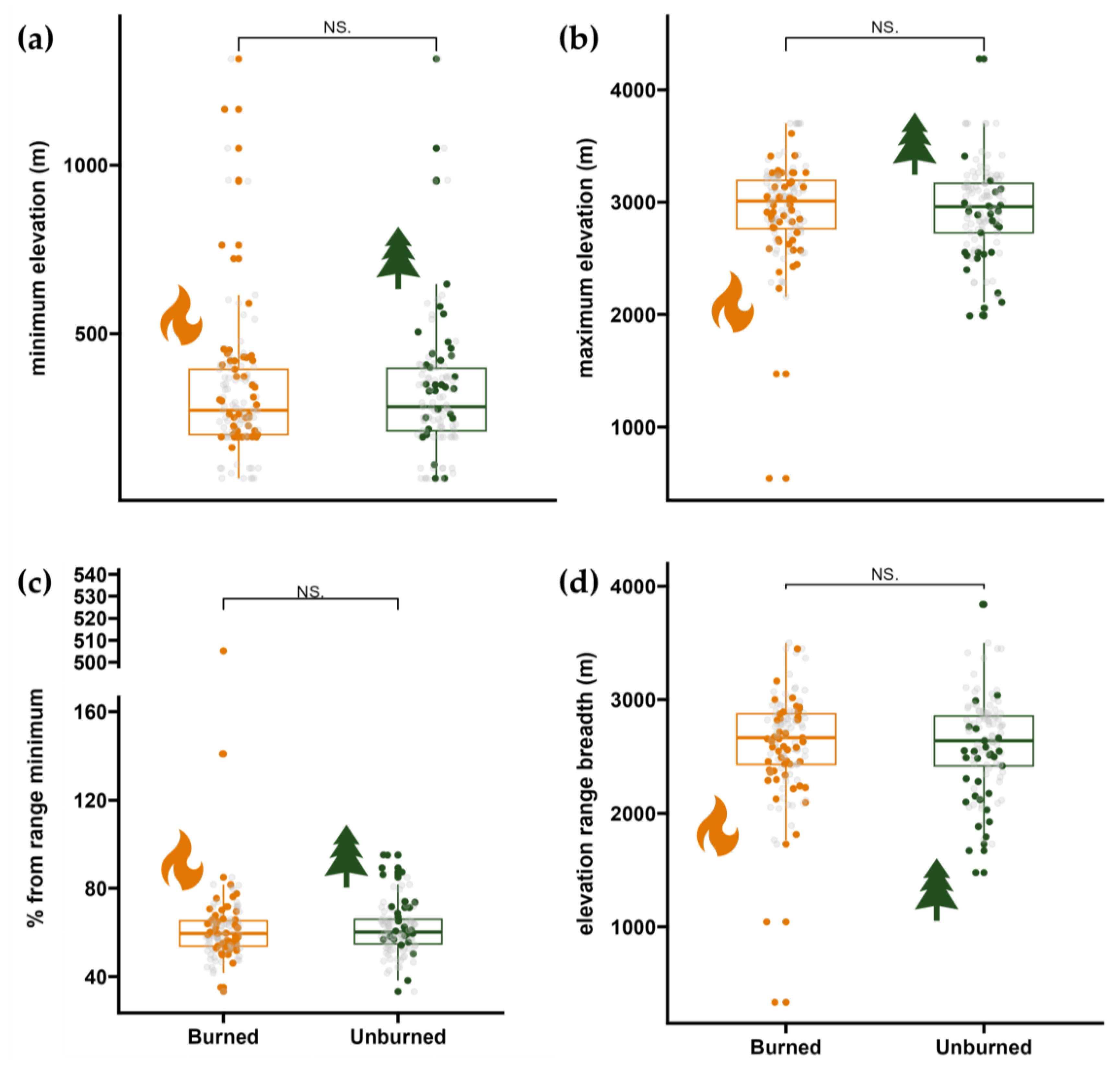
2.5. Species Elevational Ranges
2.6. Krummholz Species Recruitment
3. Results
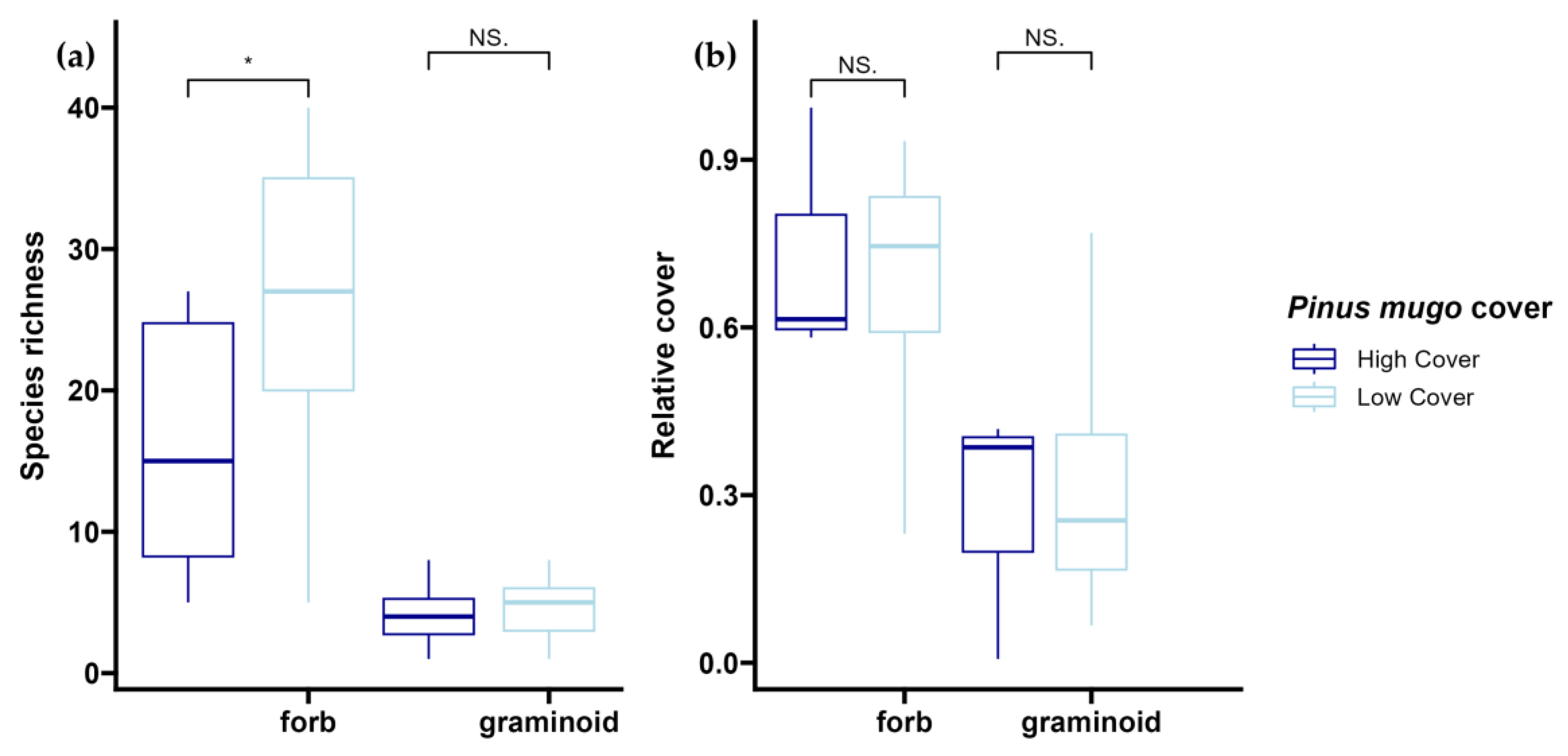
3.1. Effect of Fire on Species Composition and Plant Functional Types
3.2. Elevational Ranges of Colonizing Forbs and Graminoids
3.3. Effect of Fire on Community Ecologic Indicator Values
3.4. Krummholz Species Recruitment
3.5. Potential Drivers of Post-Fire Regime Shift
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Breckle, S.-W.; Lawlor, G.; Lawlor, D.W.; Walter, H.; Breckle, S.-W.; Walter, H. Walter’s Vegetation of the Earth: The Ecological Systems of the Geo-Biosphere, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 978-3-540-43315-6. [Google Scholar]
- Genries, A.; Muller, S.D.; Mercier, L.; Bircker, L.; Carcaillet, C. Fires Control Spatial Variability of Subalpine Vegetation Dynamics during the Holocene in the Maurienne Valley (French Alps). Écoscience 2009, 16, 13–22. [Google Scholar] [CrossRef]
- Sass, O.; Heel, M.; Leistner, I.; Stöger, F.; Wetzel, K.; Friedmann, A. Disturbance, Geomorphic Processes and Recovery of Wildfire Slopes in North Tyrol. Earth Surf. Process. Landf. 2012, 37, 883–894. [Google Scholar] [CrossRef]
- Carcaillet, C.; Ali, A.A.; Blarquez, O.; Genries, A.; Mourier, B.; Bremond, L. Spatial variability of fire history in subalpine forests: From natural to cultural regimes. Ecoscience 2009, 16, 1–12. [Google Scholar] [CrossRef]
- Zumbrunnen, T.; Menéndez, P.; Bugmann, H.; Conedera, M.; Gimmi, U.; Bürgi, M. Human impacts on fire occurrence: A case study of hundred years of forest fires in a dry alpine valley in Switzerland. Reg. Environ. Chang. 2012, 12, 935–949. [Google Scholar] [CrossRef]
- Leys, B.; Carcaillet, C. Subalpine fires: The roles of vegetation, climate and, ultimately, land uses. Clim. Chang. 2016, 135, 683–697. [Google Scholar] [CrossRef]
- Higuera, P.E.; Brubaker, L.B.; Anderson, P.M.; Hu, F.S.; Brown, T.A. Vegetation mediated the impacts of postglacial climate change on fire regimes in the south-central Brooks Range, Alaska. Ecol. Monogr. 2009, 79, 201–219. [Google Scholar] [CrossRef]
- Leys, B.; Finsinger, W.; Carcaillet, C. Historical range of fire frequency is not the Achilles’ heel of the Corsican black pine ecosystem. J. Ecol. 2014, 102, 381–395. [Google Scholar] [CrossRef]
- Tinner, W.; Conedera, M.; Ammann, B.; Lotter, A.F. Fire Ecology North and South of the Alps since the Last Ice Age. Holocene 2005, 15, 1214–1226. [Google Scholar] [CrossRef]
- Sass, O.; Wetzel, K.-F.; Friedmann, A. Landscape Dynamics of Sub-Alpine Forest Fire Slopes in the Northern Alps-Preliminary Results. Z. Für Geomorphol. Suppl. 2006, 142, 207–227. [Google Scholar]
- Moser, B.; Gimmi, U.; Wohlgemuth, T. Ausbreitung Des Erdbeerspinats Blitum Virgatum Nach Dem Waldbrand von Leuk, Wallis (2003). Bot. Helv. 2006, 116, 179–207. [Google Scholar] [CrossRef]
- Moser, B.; Temperli, C.; Schneiter, G.; Wohlgemuth, T. Potential Shift in Tree Species Composition after Interaction of Fire and Drought in the Central Alps. Eur. J. For. Res. 2010, 129, 625–633. [Google Scholar] [CrossRef]
- Wohlgemuth, T.; Hester, C.; Jost, A.-R.; Wasem, U.; Moser, B. Dynamik Der Wiederbewaldung Im Waldbrandgebiet von Leuk (Wallis)|Recruitment Dynamics Following the Forest Fire near Leuk (Valais). Schweiz. Z. Forstwes. 2010, 161, 450–459. [Google Scholar] [CrossRef]
- Wohlgemuth, T.; Moser, B. Zehn Jahre Vegetationsdynamik Auf Der Waldbrandfläche von Leuk (Wallis). Schweiz. Z. Forstwes. 2018, 169, 279–289. [Google Scholar] [CrossRef]
- Shakesby, R.A. Post-Wildfire Soil Erosion in the Mediterranean: Review and Future Research Directions. Earth-Sci. Rev. 2011, 105, 71–100. [Google Scholar] [CrossRef]
- Mandrone, G.; Vacha, D.; Chicco, J. Post-Wildfire Erosion Rates and Triggering of Debris Flows: A Case Study in Susa Valley (Bussoleno). E3S Web Conf. 2023, 415, 1–4. [Google Scholar] [CrossRef]
- Sass, O.; Heel, M.; Hoinkis, R.; Wetzel, K.-F. A Six-Year Record of Debris Transport by Avalanches on a Wildfire Slope (Arnspitze, Tyrol). Z. Geomorphol. 2010, 54, 181–193. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest Disturbances under Climate Change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Auer, I.; Böhm, R.; Jurkovic, A.; Lipa, W.; Orlik, A.; Potzmann, R.; Schöner, W.; Ungersböck, M.; Matulla, C.; Briffa, K.; et al. HISTALP—Historical Instrumental Climatological Surface Time Series of the Greater Alpine Region. Int. J. Climatol. 2007, 27, 17–46. [Google Scholar] [CrossRef]
- Gobiet, A.; Kotlarski, S.; Beniston, M.; Heinrich, G.; Rajczak, J.; Stoffel, M. 21st Century Climate Change in the European Alps—A Review. Sci. Total Environ. 2014, 493, 1138–1151. [Google Scholar] [CrossRef]
- Klein, G.; Vitasse, Y.; Rixen, C.; Marty, C.; Rebetez, M. Shorter Snow Cover Duration since 1970 in the Swiss Alps Due to Earlier Snowmelt More than to Later Snow Onset. Clim. Chang. 2016, 139, 637–649. [Google Scholar] [CrossRef]
- Cansler, C.A.; McKenzie, D.; Halpern, C.B. Area Burned in Alpine Treeline Ecotones Reflects Region-Wide Trends. Int. J. Wildland Fire 2016, 25, 1209. [Google Scholar] [CrossRef]
- Dupire, S.; Curt, T.; Bigot, S.; Fréjaville, T. Vulnerability of Forest Ecosystems to Fire in the French Alps. Eur. J. For. Res. 2019, 138, 813–830. [Google Scholar] [CrossRef]
- Moris, J.V.; Vacchiano, G.; Ravetto Enri, S.; Lonati, M.; Motta, R.; Ascoli, D. Resilience of European Larch (Larix decidua Mill.) Forests to Wildfires in the Western Alps. New For. 2017, 48, 663–683. [Google Scholar] [CrossRef]
- Maringer, J.; Ascoli, D.; Küffer, N.; Schmidtlein, S.; Conedera, M. What Drives European Beech (Fagus sylvatica L.) Mortality after Forest Fires of Varying Severity? For. Ecol. Manag. 2016, 368, 81–93. [Google Scholar] [CrossRef]
- Frejaville, T.; Curt, T.; Carcaillet, C. Bark Flammability as a Fire-Response Trait for Subalpine Trees. Front. Plant Sci. 2013, 4, 466. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. The Cold Range Limit of Trees. Trends Ecol. Evol. 2021, 36, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, A.; White, P. A Theory of Pulse Dynamics and Disturbance in Ecology. Ecology 2019, 100, e02734. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.W.; Ballard, T.M. Effects of Fire on Alpine Plant Communities in the North Cascades, Washington. Ecology 1971, 52, 1058–1064. [Google Scholar] [CrossRef]
- Tomback, D.F. Post-Fire Regeneration of Krummholz Whitebark Pine: A Consequence Of Nutcracker Seed Caching. Madroño 1986, 33, 100–110. [Google Scholar]
- Takahashi, K. Damage of Alpine Vegetation by the 2009 Fire on Mt. Shirouma, Central Japan: Comparison between Herbaceous Vegetation and Pinus Pumila Scrub. Landsc. Ecol. Eng. 2012, 8, 123–128. [Google Scholar] [CrossRef]
- Kalas, M.M.; Berg, C. Regeneration of High Montane Plant Communities in the “Nationalpark Kalkalpen” (Northern Alps) after Fire Events. In Proceedings of the 5th Symposium for Research in Protected Areas, Mittersill, Austria, 10–12 June 2013. [Google Scholar]
- Gauye, C.; Bernard, C.; Moser, B.; Rigling, A.; Wohlgemuth, T. L’importance de l’exposition Sur Le Reboisement Suite à Deux Incendies de Forêt En Valais. Schweiz. Z. Forstwes. 2023, 174, 238–242. [Google Scholar] [CrossRef]
- Geppert, C.; Bertolli, A.; Prosser, F.; Marini, L. Red-Listed Plants Are Contracting Their Elevational Range Faster than Common Plants in the European Alps. Proc. Natl. Acad. Sci. USA 2023, 120, e2211531120. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, A.-K.; Nopp-Mayr, U.; Zohmann, M. Small-Scale Habitat Use of Black Grouse (Tetrao tetrix L.) and Rock Ptarmigan (Lagopus Muta Helvetica Thienemann) in the Austrian Alps. Eur. J. Wildl. Res. 2012, 58, 35–45. [Google Scholar] [CrossRef]
- Scridel, D.; Tenan, S.; Brambilla, M.; Celva, R.; Forti, A.; Fracasso, I.; Volcan, G.; Dorigatti, E.; Anderle, M.; Marchesini, A. Early-Succession Secondary Forests Following Agropastoral Abandonment Are Key Winter Habitats for the Conservation of a Priority Bird in the European Alps. Eur. J. For. Res. 2022, 141, 1029–1043. [Google Scholar] [CrossRef]
- Baumgartner, A.; Reichel, E.; Weber, G. Der Wasserhaushalt Der Alpen: Niederschlag, Verdunstung, Abfluß u. Gletscherspende Im Gesamtgebiet d. Alpen Im Jahresdurchschnitt Für d. Normalperiode 1931–1960; Mit 68 Tab; R. Oldenbourg: München, Germany, 1983; ISBN 978-3-486-27251-2. [Google Scholar]
- Schiechtl, H.; Stern, R.; Meisel, K. Karte Der Aktuellen Vegetation von Tirol 1:100,000. XI. Teil: Blatt 2 Lechtaler Alpen-Wetterstein. Grenoble 1987, XXX, 25–48. [Google Scholar]
- Hüttl, C. Steuerungsfaktoren und Quantifizierung der Chemischen Verwitterung auf dem Zugspitzplatt (Wettersteingebirge, Deutschland): Mit 77 Tabellen; Münchener Geographische Abhandlungen Reihe B; Geobuch-Verl: München, Germany, 1999; ISBN 978-3-925308-51-2. [Google Scholar]
- Sass, O.; Haas, F.; Schimmer, C.; Heel, M.; Bremer, M.; Stöger, F.; Wetzel, K. Impact of Forest Fires on Geomorphic Processes in the Tyrolean Limestone Alps. Geogr. Ann. Ser. Phys. Geogr. 2012, 94, 117–133. [Google Scholar] [CrossRef]
- Korch, O.; Friedmann, A. Vegetation und Vegetationsdynamik auf dem Zugspitzplatt (Bayerische Alpen) Natur- und Kulturlandschaft im hochalpinen Raum als Produkt natürlicher, anthropogener und zoogener Einflüsse. Polarforschung 2016, 86, 35–45. [Google Scholar] [CrossRef]
- Sass, O. Waldbrände in den Nordtiroler Kalkalpen: Verbreitung-Geschichte-Regeneration; Innsbrucker Geographische Studien; Geographie Innsbruck: Innsbruck, Austria, 2019; ISBN 978-3-901182-44-0. [Google Scholar]
- Heel, M. Waldbrände in den Nördlichen Kalkalpen: Raumzeitliche Verteilung und Beispiele Lokaler Auswirkungen. Ph.D. Thesis, Universität Augsburg, Augsburg, Germany, 2016. [Google Scholar]
- Stine, M.B.; Butler, D.R. Effects of Fire on Geomorphic Factors and Seedling Site Conditions within the Alpine Treeline Ecotone, Glacier National Park, MT. Catena 2015, 132, 37–44. [Google Scholar] [CrossRef]
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 17 May 2024).
- Londo, G. The decimal scale for releves of permanent quadrats. Vegetatio 1976, 33, 61–64. [Google Scholar] [CrossRef]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W. Zeigerwerte von Pflanzen in Mitteleuropa; Scripta Geobotanica; 3. Durchgesehene Auflage.; Verlag Erich Goltze GmbH & Co KG: Göttingen, Germany, 2001; ISBN 978-3-88452-518-0. [Google Scholar]
- Landolt, E. Flora Indicativa: Ökologische Zeigerwerte und Biologische Kennzeichen zur Flora der Schweiz und der Alpen; 2. Völlig Neu Bearbeitete und Erweiterte Aufl.; Haupt: Bern, Switzerland, 2010; ISBN 978-3-258-07461-0. [Google Scholar]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–473. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Jutzi, M.; Vilpert, M.; Juillerat, P.; Eggenberg, S. Swiss National Databank of Vascular Plants. Version 1.15. Swiss National Biodiversity Data and Information Centres—Infospecies.ch. 2023. Occurrence Dataset. Accessed via GBIF.org. Available online: https://doi.org/10.15468/7jffp3 (accessed on 1 December 2023).
- Xu, S.; Chen, M.; Feng, T.; Zhan, L.; Zhou, L.; Yu, G. Use Ggbreak to Effectively Utilize Plotting Space to Deal with Large Datasets and Outliers. Front. Genet. 2021, 12, 774846. [Google Scholar] [CrossRef]
- Hess, H.E.; Landolt, E.; Hirzel, R. Flora der Schweiz und Angrenzender Gebiete; 1: Pteridophyta bis Caryophyllaceae; 2. Durchges. Aufl.; Birkhäuser: Basel Stuttgart, Germany, 1976; ISBN 978-3-7643-0843-8. [Google Scholar]
- Wohlgemuth, T.; Jentsch, A.; Seidl, R. Disturbance Ecology; Springer Nature: Berlin/Heidelberg, Germany, 2022; Volume 32, ISBN 3-030-98756-6. [Google Scholar]
- Wastl, C.; Schunk, C.; Leuchner, M.; Pezzatti, G.B.; Menzel, A. Recent Climate Change: Long-Term Trends in Meteorological Forest Fire Danger in the Alps. Agric. For. Meteorol. 2012, 162, 1–13. [Google Scholar] [CrossRef]
- Daskalova, G.N.; Myers-Smith, I.H.; Bjorkman, A.D.; Blowes, S.A.; Supp, S.R.; Magurran, A.E.; Dornelas, M. Landscape-Scale Forest Loss as a Catalyst of Population and Biodiversity Change. Science 2020, 368, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Palm, S.; Bruelheide, H.; de Villemereuil, P.; Menzel, A.; Lachmuth, S. Disturbance and Indirect Effects of Climate Warming Support a Plant Invader in Mountains. Oikos 2022, 2022, e08783. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; Pauchard, A.; Lenoir, J.; Nuñez, M.A.; Geron, C.; Ven, A.; Bravo-Monasterio, P.; Teneb, E.; Nijs, I.; Milbau, A. Disturbance is the key to plant invasions in cold environments. Proc. Natl. Acad. Sci. USA 2016, 113, 14061–14066. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.R.; Wardle, D.A.; Bardgett, R.D.; Clarkson, B.D. The Use of Chronosequences in Studies of Ecological Succession and Soil Development. J. Ecol. 2010, 98, 725–736. [Google Scholar] [CrossRef]
- Šenfeldr, M.; Treml, V. Which Generative Reproduction Characteristics Determine Successful Establishment of the Subalpine Shrub Pinus mugo? J. Veg. Sci. 2020, 31, 403–415. [Google Scholar] [CrossRef]
- Simon, A.; Katzensteiner, K.; Gratzer, G. Drivers of Forest Regeneration Patterns in Drought Prone Mixed-Species Forests in the Northern Calcareous Alps. For. Ecol. Manag. 2019, 453, 117589. [Google Scholar] [CrossRef]
- Beniston, M. The 2003 heat wave in Europe: A shape of things to come? An analysis based on Swiss climatological data and model simulations. Geophys. Res. Lett. 2004, 31, L02202. [Google Scholar] [CrossRef]
- Beniston, M. August 2005 intense rainfall event in Switzerland: Not necessarily an analog for strong convective events in a greenhouse climate. Geophys. Res. Lett. 2006, 33, L05701. [Google Scholar] [CrossRef]
- Beniston, M.; Keller, F.; Goyette, S. Snow pack in the Swiss Alps under changing climatic conditions: An empirical approach for climate impacts studies. Theoret. Appl. Climatol. 2003, 74, 19–31. [Google Scholar] [CrossRef]





| Forb | Graminoid | Shrub | Tree | |
|---|---|---|---|---|
| Burned | D. octopetala (14.4%) | Nardus stricta L. (11.7%) | Rhododendron hirsutum L. (2%) | P. abies (5.2%) |
| Crepis foetida L. (11%) | Carex humilis Leyss. (5.75%) | Vaccinium vitis-idea L. (1.3%) | L. decidua (3%) | |
| Gymnocarpium robertianum (Hoffm.) Newman (10%) | C. curvula (4.85%) | Vaccinium myrtillus L. (0.7%) | P. mugo (1%) | |
| Unburned | Viola biflora L. (9.1%) | Alpagrostis alpina (Scop.) P.M. Peterson, Romasch., Soreng & Sylvester (20.0%) | Rhododendron ferrugineum L. (15.2%) | P. mugo (54.2%) |
| Erica carnea L. (7.3%) | Carex sempervirens Vill. (19.6%) | R. hirsutum (12.3%) | P. abies (23.1%) | |
| D. octopetala (5.4%) | Luzula campestris (L.) DC. (15.0%) | Vaccinium uliginosum L. (6.8%) | L. decidua (14.3%) |
| Indicator | Source | Mean Burned | Mean Unburned | Statistic | p-Value | Significance |
|---|---|---|---|---|---|---|
| Aeration | Landolt | 3.07 | 2.61 | 5.09 | <<0.001 | **** |
| Dominance in situ | Landolt | 2.36 | 2.59 | −3.99 | <<0.001 | **** |
| Humus content | Landolt | 3.00 | 3.53 | −6.53 | <<0.001 | **** |
| Light | Ellenberg | 7.44 | 6.91 | 5.50 | <<0.001 | **** |
| Landolt | 3.80 | 3.46 | 5.74 | <<0.001 | **** | |
| Moisture | Ellenberg | 4.71 | 4.93 | −2.44 | 0.015 | * |
| Landolt | 2.75 | 2.85 | −2.02 | 0.043 | * | |
| Moisture variability | Landolt | 1.50 | 1.57 | −1.45 | 0.148 | ns |
| Nutrients | Ellenberg | 2.96 | 3.02 | −0.57 | 0.571 | ns |
| Landolt | 2.29 | 2.28 | 0.12 | 0.902 | ns | |
| Reaction/Soil pH | Ellenberg | 6.61 | 6.12 | 2.81 | 0.005 | ** |
| Landolt | 3.45 | 3.28 | 2.27 | 0.024 | * | |
| Root depth | Landolt | 1.82 | 1.90 | −1.08 | 0.279 | ns |
| Temperature | Ellenberg | 2.91 | 3.05 | −1.64 | 0.102 | ns |
| Landolt | 2.35 | 2.26 | 2.03 | 0.043 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Giuli, M.; Winkler, M.; Deola, T.; Henschel, J.; Sass, O.; Wolff, P.; Jentsch, A. Arrested Succession on Fire-Affected Slopes in the Krummholz Zone and Subalpine Forest of the Northern Limestone Alps. Diversity 2024, 16, 366. https://doi.org/10.3390/d16070366
De Giuli M, Winkler M, Deola T, Henschel J, Sass O, Wolff P, Jentsch A. Arrested Succession on Fire-Affected Slopes in the Krummholz Zone and Subalpine Forest of the Northern Limestone Alps. Diversity. 2024; 16(7):366. https://doi.org/10.3390/d16070366
Chicago/Turabian StyleDe Giuli, Marta, Markus Winkler, Thomas Deola, Julia Henschel, Oliver Sass, Peter Wolff, and Anke Jentsch. 2024. "Arrested Succession on Fire-Affected Slopes in the Krummholz Zone and Subalpine Forest of the Northern Limestone Alps" Diversity 16, no. 7: 366. https://doi.org/10.3390/d16070366






