Advancing Conservation Strategies for Native Eastern Highlands-Strain Walleye Sander vitreus in West Virginia: Insights from Genomic Investigations and Broodstock Screening
Abstract
1. Introduction
2. Methods
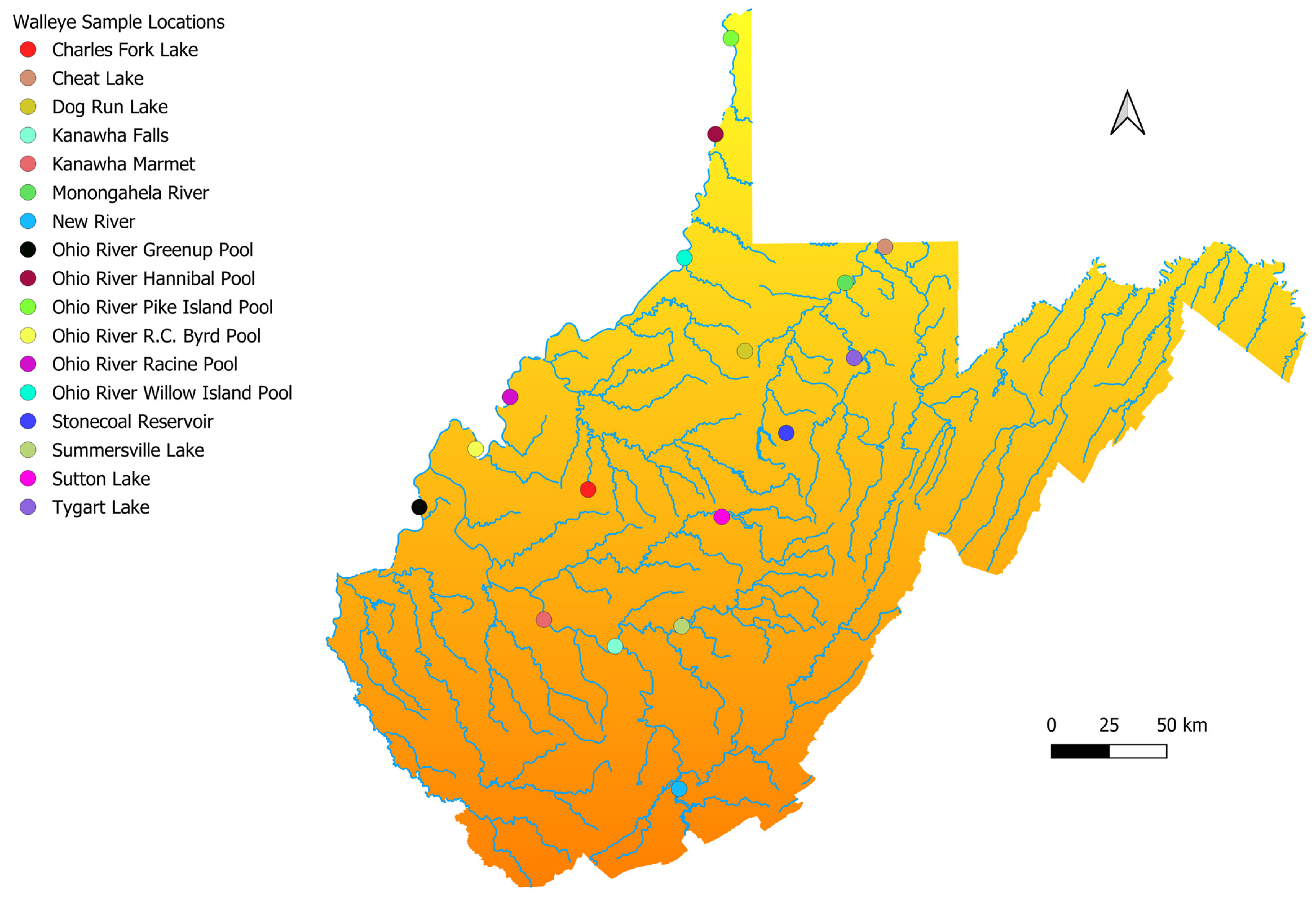
2.1. Sample Collection
2.2. Laboratory Methods
2.3. Genomic Analysis
3. Results
3.1. Comparison of Microsatellite and SNP-Assay Strain Identification Calls
3.2. Comparison of Two-SNP Strain Assignment to The Use of Additional Diagnostic SNPs
3.3. Ancestry Assignment of Sequenced Walleye
3.4. Evaluation of the Prevalence of Native Eastern Highlands Walleye in West Virginia
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halverson, M.A. Stocking Trends: A Quantitative Review of Governmental Fish Stocking in the United States, 1931 to 2004. Fisheries 2008, 33, 69–75. [Google Scholar] [CrossRef]
- Argent, D.G.; Kimmel, W.G.; Lorson, R.; Clancy, M. An Evaluation of Interstate Efforts to Re-Introduce Paddlefish to the Upper Ohio River Basin. Nena 2016, 23, 454–465. [Google Scholar] [CrossRef]
- Long, J.M.; Allen, M.S.; Porak, W.F.; Suski, C.D. A Historical Perspective of Black Bass Management in the United States. Am. Fish. Soc. Symp. 2015, 82, 99–122. [Google Scholar]
- Flowers, H.J.; Kwak, T.J.; Fischer, J.R.; Cope, W.G.; Rash, J.M.; Besler, D.A. Behavior and Survival of Stocked Trout in Southern Appalachian Mountain Streams. Trans. Am. Fish. Soc. 2019, 148, 3–20. [Google Scholar] [CrossRef]
- Oliver, D.C.; Mann, R.D.; Aymami, C.G.; Avenetti, L.D. An Evaluation of the Efficacy of a Targeted Supplemental Stocking Using a Multistate Capture–Recapture Model. N. Am. J. Fish. Manag. 2022, 42, 952–965. [Google Scholar] [CrossRef]
- Araki, H.; Schmid, C. Is Hatchery Stocking a Help or Harm? Aquaculture 2010, 308, S2–S11. [Google Scholar] [CrossRef]
- Laikre, L.; Schwartz, M.K.; Waples, R.S.; Ryman, N. Compromising Genetic Diversity in the Wild: Unmonitored Large-Scale Release of Plants and Animals. Trends Ecol. Evol. 2010, 25, 520–529. [Google Scholar] [CrossRef]
- Bruce, S.A.; Kutsumi, Y.; Van Maaren, C.; Hare, M.P. Stocked-Fish Introgression into Wild Brook Trout Populations Depends on Habitat. Trans. Am. Fish. Soc. 2020, 149, 427–442. [Google Scholar] [CrossRef]
- Almodóvar, A.; Leal, S.; Nicola, G.G.; Hórreo, J.L.; García-Vázquez, E.; Elvira, B. Long-Term Stocking Practices Threaten the Original Genetic Diversity of the Southernmost European Populations of Atlantic Salmon Salmo Salar. Endanger. Species Res. 2020, 41, 303–317. [Google Scholar] [CrossRef]
- Kazyak, D.C.; Lubinski, B.A.; Rash, J.M.; Johnson, T.C.; King, T.L. Development of Genetic Baseline Information to Support the Conservation and Management of Wild Brook Trout in North Carolina. N. Am. J. Fish. Manag. 2021, 41, 626–638. [Google Scholar] [CrossRef]
- Hargrove, J.S.; Kazyak, D.C.; Lubinski, B.A.; Rogers, K.M.; Bowers, O.K.; Fesenmyer, K.A.; Habera, J.W.; Henegar, J. Landscape and Stocking Effects on Population Genetics of Tennessee Brook Trout. Conserv. Genet. 2022, 23, 341–357. [Google Scholar] [CrossRef]
- Erdman, B.; Mitro, M.G.; Griffin, J.D.T.; Rowe, D.; Kazyak, D.C.; Turnquist, K.; Siepker, M.; Miller, L.; Stott, W.; Hughes, M.; et al. Broadscale Population Structure and Hatchery Introgression of Midwestern Brook Trout. Trans. Am. Fish. Soc. 2022, 151, 81–99. [Google Scholar] [CrossRef]
- Currens, K.P.; Hemmingsen, A.R.; French, R.A.; Buchanan, D.V.; Schreck, C.B.; Li, H.W. Introgression and Susceptibility to Disease in a Wild Population of Rainbow Trout. N. Am. J. Fish. Manag. 1997, 17, 1065–1078. [Google Scholar] [CrossRef]
- Naish, K.A.; Taylor, J.E.; Levin, P.S.; Quinn, T.P.; Winton, J.R.; Huppert, D.; Hilborn, R. An Evaluation of the Effects of Conservation and Fishery Enhancement Hatcheries on Wild Populations of Salmon1. In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 2007; Volume 53, pp. 61–194. [Google Scholar]
- Gossieaux, P.; Lavoie, É.; Sirois, P.; Thibault, I.; Bernatchez, L.; Garant, D. Effects of Genetic Origin on Phenotypic Divergence in Brook Trout Populations Stocked with Domestic Fish. Ecosphere 2020, 11, e03119. [Google Scholar] [CrossRef]
- Hoyle, J.A.; Holden, J.P.; Yuille, M.J. Diet and Relative Weight in Migratory Walleye (Sander vitreus) of the Bay of Quinte and Eastern Lake Ontario, 1992–2015. J. Great Lakes Res. 2017, 43, 846–853. [Google Scholar] [CrossRef]
- Uphoff, C.S.; Schoenebeck, C.W.; Koupal, K.D.; Pope, K.L.; Wyatt Hoback, W. Age-0 Walleye Sander vitreus Display Length-Dependent Diet Shift to Piscivory. J. Freshw. Ecol. 2019, 34, 27–36. [Google Scholar] [CrossRef]
- Elliott, C.W.; Ridgway, M.S.; Brown, E.; Tufts, B.L. Spatial Ecology of Bay of Quinte Walleye (Sander vitreus): Annual Timing, Extent, and Patterns of Migrations in Eastern Lake Ontario. J. Great Lakes Res. 2022, 48, 159–170. [Google Scholar] [CrossRef]
- Embke, H.S.; Douglas Beard Jr, T.; Lynch, A.J.; Vander Zanden, M.J. Fishing for Food: Quantifying Recreational Fisheries Harvest in Wisconsin Lakes. Fisheries 2020, 45, 647–655. [Google Scholar] [CrossRef]
- Melstrom, R.T.; Lupi, F.; Esselman, P.C.; Stevenson, R.J. Valuing Recreational Fishing Quality at Rivers and Streams. Water Resour. Res. 2015, 51, 140–150. [Google Scholar] [CrossRef]
- Hansen, G.J.A.; Winslow, L.A.; Read, J.S.; Treml, M.; Schmalz, P.J.; Carpenter, S.R. Water Clarity and Temperature Effects on Walleye Safe Harvest: An Empirical Test of the Safe Operating Space Concept. Ecosphere 2019, 10, e02737. [Google Scholar] [CrossRef]
- Raabe, J.K.; VanDeHey, J.A.; Zentner, D.L.; Cross, T.K.; Sass, G.G. Walleye Inland Lake Habitat: Considerations for Successful Natural Recruitment and Stocking in North Central North America. Lake Reserv. Manag. 2020, 36, 335–359. [Google Scholar] [CrossRef]
- Berkman, L.K.; Titus, C.L.; Thomas, D.R.; Fluker, B.L.; Cieslewicz, P.; Knuth, D.; Koppelman, J.B.; Eggert, L.S. Genetic Differences among the Interior Highlands Walleye (Sander vitreus) with Mitochondrial and Nuclear Markers Indicate the Need for Updated Stocking Practices. Conserv. Genet 2023, 24, 1–13. [Google Scholar] [CrossRef]
- Mandrak, N.; Crossman, E.J. Postglacial Dispersal of Freshwater Fishes into Ontario. Can. J. Zool. 1992, 70, 2247–2259. [Google Scholar] [CrossRef]
- Wilson, C.C.; Hebert, P.D.N. Phylogeography and Postglacial Dispersal of Lake Trout (Salvelinus Namaycush) in North America. Can. J. Fish. Aquat. Sci. 1998, 55, 1010–1024. [Google Scholar] [CrossRef]
- Palmer, G.; Culver, M.; Dutton, D.; Murphy, B.; Hallerman, E.M.; Billington, N.; Williams, J. Genetic Distinct Walleye Stocks in Claytor Lake and the Upper New River, Virginia. Proc. Southeast. Assoc. Fish Wildl. Agencies 2006, 60, 125–131. [Google Scholar]
- White, M.M.; Kassler, T.W.; Philipp, D.P.; Schell, S.A. A Genetic Assessment of Ohio River Walleyes. Trans. Am. Fish. Soc. 2005, 134, 661–675. [Google Scholar] [CrossRef]
- White, M.M.; Faber, J.E.; Zipfel, K.J. Genetic Identity of Walleye in the Cumberland River. Am. Midl. Nat. 2012, 167, 373–383. [Google Scholar] [CrossRef]
- Stepien, C.A.; Murphy, D.J.; Lohner, R.N.; Sepulveda-Villet, O.J.; Haponski, A.E. Signatures of Vicariance, Postglacial Dispersal and Spawning Philopatry: Population Genetics of the Walleye Sander vitreus—STEPIEN—2009—Molecular Ecology—Wiley Online Library. Mol. Ecol. 2009, 18, 3411–3428. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.; Williams, J.; Scott, M.; Finne, K.; Johnson, N.; Dutton, D.; Murphy, B.; Hallerman, E.M. Genetic Marker-Assisted Restoration of the Presumptive Native Walleye Fishery in the New River, Virginia and West Virginia. Proc. Annu. Conf. Southeast. Assoc. Fish Wildl. Agencies 2007, 61, 17–22. [Google Scholar]
- Johnson, A.; Zipfel, K.J.; Hallerman, E.M.; Massure, W.; Euclide, P.; Welsh, A.B. Genomic Evaluation of Native Walleye in the Appalachian Region and the Effects of Stocking. Johns. Trans. Am. Fish. Soc. Wiley Online Libr. 2023, 153, 3. [Google Scholar] [CrossRef]
- Page, K.S.; Zweifel, R.D.; Stott, W. Spatial and Temporal Genetic Analysis of Walleyes in the Ohio River. Trans. Am. Fish. Soc. 2017, 146, 1168–1185. [Google Scholar] [CrossRef]
- Borer, S.O.; Miller, L.M.; Kapuscinski, A.R. Microsatellites in Walleye Stizostedion Vitreum. Molecular Ecology 1999, 8, 336–338. [Google Scholar]
- Poland, J.; Brown, P.; Sorrells, M.; Jannink, J.-L. Development of High-Density Genetic Maps for Barley and Wheat Using a Novel Two-Enzyme Genotyping-by-Sequencing Approach. PLoS ONE 2012, 7, e32253. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Catchen, J.; Hohenlohe, P.A.; Bassham, S.; Amores, A.; Cresko, W.A. Stacks: An Analysis Tool Set for Population Genomics. Mol. Ecol. 2013, 22, 3124–3140. [Google Scholar] [CrossRef]
- Hall, T. BioEdit: An User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Anderson, E.C.; Thompson, E.A. A Model-Based Method for Identifying Species Hybrids Using Multilocus Genetic Data. Genetics 2002, 160, 1217–1229. [Google Scholar] [CrossRef]
- Zhao, H.; Beck, B.; Fuller, A.; Peatman, E. EasyParallel: A GUI Platform for Parallelization of STRUCTURE and NEWHYBRIDS Analyses. PLoS ONE 2020, 15, e0232110. [Google Scholar] [CrossRef]
- Zipfel, K.J. The Distribution and Status of Native Walleye (Sander vitreus) Stocks in West Virginia. Master’s Thesis, Ohio University, Athens, OH, USA, 2006. [Google Scholar]
- Harris, S.; Palmer, G.; Stepien, C.A.; Hallerman, E.M. Population Genetic Differentiation of Walleye (Sander vitreus) across the Eastern Highlands of the United States. Fishes 2024, 9, 15. [Google Scholar] [CrossRef]
- Prunier, J.G.; Saint-Pé, K.; Tissot, L.; Poulet, N.; Marselli, G.; Veyssière, C.; Blanchet, S. Captive-Bred Ancestry Affects Spatial Patterns of Genetic Diversity and Differentiation in Brown Trout (Salmo Trutta) Populations. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 1529–1543. [Google Scholar] [CrossRef]
- Hammen, J.J.; Sloss, B.L. Walleye Genetic Characterization in the Northern Ceded Territory of Wisconsin: Implications for Stocking Using Conservation Strategies. N. Am. J. Fish. Manag. 2019, 39, 693–704. [Google Scholar] [CrossRef]
- Zhao, H.; Silliman, K.; Lewis, M.; Johnson, S.; Kratina, G.; Rider, S.J.; Stepien, C.A.; Hallerman, E.M.; Beck, B.; Fuller, A.; et al. SNP Analyses Highlight a Unique, Imperiled Southern Walleye (Sander vitreus) in the Mobile River Basin. Can. J. Fish. Aquat. Sci. 2020, 77, 1366–1378. [Google Scholar] [CrossRef]
- Euclide, P.T.; Robinson, J.; Faust, M.; Ludsin, S.A.; MacDougall, T.M.; Marschall, E.A.; Chen, K.-Y.; Wilson, C.; Bootsma, M.; Stott, W.; et al. Using Genomic Data to Guide Walleye Management in the Great Lakes. In Yellow Perch, Walleye, and Sauger: Aspects of Ecology, Management, and Culture; Bruner, J.C., DeBruyne, R.L., Eds.; Springer International Publishing: Cham, Swizterland, 2021; pp. 115–139. ISBN 978-3-030-80678-1. [Google Scholar]
| Assay | Forward Primer | Reverse Primer | Great Lakes Probe | Native Probe |
|---|---|---|---|---|
| 5164 | TGCAGCCTCAAATACCTTGGG | TCTGCTGCGCCGATTCTC | CAATCTCCCACTCCATTG | ATCTCCCACACCATTG |
| 14,317 | GCGGTTGGCCATCAGTGA | TCCTGGACGCCTGGGA | CTCAGGAGATCAGATGC | TCAGGAGACCAGATGC |
| Eastern Highlands | Hybrid | Great Lakes | |
|---|---|---|---|
| Microsatellite panel | 208 (87.0%) | 171 (21.0%) | 9 (77.7%) |
| SNP assay | 312 (58.0%) | 61 (59.0%) | 15 (46.6%) |
| Strain | Probability | Eastern Highlands | Great Lakes | F1 Hybrid | F2 Hybrid | EH × Bx | GL × Bx |
|---|---|---|---|---|---|---|---|
| Eastern Highlands (181) | >99.999 | 81.2 | 0.0 | 0.0 | 0.5 | 2.2 | 0.0 |
| >95.00 | 87.2 | 0.0 | 0.0 | 1.1 | 5.5 | 0.0 | |
| >80.00 | 89.5 | 0.0 | 0.0 | 1.1 | 6.6 | 0.0 | |
| F1 hybrid (37) | >99.999 | 21.6 | 0.0 | 0.0 | 35.1 | 0.0 | 0.0 |
| >95.00 | 29.7 | 0.0 | 0.0 | 35.1 | 8.1 | 0.0 | |
| >80.00 | 32.4 | 0.0 | 2.7 | 40.5 | 13.5 | 0.0 | |
| Great Lakes (21) | >99.999 | 0.0 | 90.4 | 0.0 | 4.7 | 0.0 | 0.0 |
| >95.00 | 0.0 | 90.4 | 0.0 | 4.7 | 0.0 | 0.0 | |
| >80.00 | 0.0 | 90.4 | 0.0 | 4.7 | 0.0 | 0.0 |
| Location | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | Total |
|---|---|---|---|---|---|---|---|
| Charles Fork Lake | 94.1% (17) | 90.0% (30) | 88.2% (17) | 100% (18) | 81.8% (11) | N/A | 91.4% (93) |
| Cheat Lake | N/A | 20% (20) | N/A | N/A | N/A | N/A | 20.0% (20) |
| Dog Run Lake | 100% (25) | N/A | 100% (11) | 100% (24) | 100% (9) | 100% (41) | 100% (110) |
| Kanawha River Marmet Pool | 88.9% (36) | 83.9% (62) | 77.8% (81) | 89.3% (47) | 82.8% (35) | 83.9% (62) | 83.3% (323) |
| Kanawha River Kanawha Falls | 83.7% (43) | 87.5% (40) | 86.4% (37) | 84.5% (58) | 100% (10) | 92.6% (27) | 86.9% (215) |
| Monongahela River | N/A | 30.0% (20) | N/A | N/A | N/A | N/A | 30.0% (20) |
| New River | 78.8% (33) | 92.1% (38) | 100% (30) | 94.2% (35) | 93.0% (57) | 100% (36) | 93.0% (229) |
| Stonecoal Reservoir | N/A | N/A | N/A | 1.5% (64) | N/A | N/A | 1.5% (64) |
| Summersville Lake | N/A | 54.5% (11) | 66.6% (12) | N/A | N/A | N/A | 60.1% (23) |
| Sutton Lake | N/A | N/A | 61.5% (26) | N/A | N/A | N/A | 61.5% (26) |
| Tygart Lake | N/A | 0% (20) | N/A | N/A | N/A | N/A | 0% (20) |
| Ohio River Pike Island Pool | N/A | 66.6% (24) | 88.9% (18) | N/A | N/A | N/A | 76.2% (42) |
| Ohio River Hannibal Pool | N/A | 58.3% (12) | 60.0% (10) | 66.6% (6) | N/A | N/A | 60.7% (28) |
| Ohio River Willow Island Pool | 55.9% (34) | 20.0% (5) | 69.7% (43) | 65.2% (23) | 28.6% (7) | 86.6% (15) | 63.8% (127) |
| Ohio River Racine Pool | N/A | 54.5% (11) | N/A | 68.9% (29) | 57.1% (7) | 75% (12) | 67.8% (59) |
| Ohio River R.C. Byrd Pool | 50.0% (30) | 63.1% (19) | 100% (1) | 50.0% (2) | 42.8% (7) | N/A | 54.2% (59) |
| Ohio River Greenup Pool | 53.8% (13) | 33.3% (12) | 82.3% (17) | 50.0% (10) | 68.1% (22) | N/A | 60.8% (74) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, A.; Zipfel, K.; Welsh, A. Advancing Conservation Strategies for Native Eastern Highlands-Strain Walleye Sander vitreus in West Virginia: Insights from Genomic Investigations and Broodstock Screening. Diversity 2024, 16, 371. https://doi.org/10.3390/d16070371
Johnson A, Zipfel K, Welsh A. Advancing Conservation Strategies for Native Eastern Highlands-Strain Walleye Sander vitreus in West Virginia: Insights from Genomic Investigations and Broodstock Screening. Diversity. 2024; 16(7):371. https://doi.org/10.3390/d16070371
Chicago/Turabian StyleJohnson, Andrew, Katherine Zipfel, and Amy Welsh. 2024. "Advancing Conservation Strategies for Native Eastern Highlands-Strain Walleye Sander vitreus in West Virginia: Insights from Genomic Investigations and Broodstock Screening" Diversity 16, no. 7: 371. https://doi.org/10.3390/d16070371
APA StyleJohnson, A., Zipfel, K., & Welsh, A. (2024). Advancing Conservation Strategies for Native Eastern Highlands-Strain Walleye Sander vitreus in West Virginia: Insights from Genomic Investigations and Broodstock Screening. Diversity, 16(7), 371. https://doi.org/10.3390/d16070371







