Relic Vergilius Oak (Quercus virgiliana Ten.) Trees Could Preserve Microhabitats of Pannonian Forest–Steppe Vegetation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methods of Selection and Description
- First Military Land-Survey of Hungarian Kingdom, 1782–1785 [https://maps.arcanum.com/hu/map/firstsurvey-hungary/?layers=147&bbox=2070390.0687322675%2C6004328.278894642%2C2173503.61988897%2C6041629.548697809 (accessed on 3 July 2024)]
- Second Military Land-Survey of Hungarian Kingdom, 1819–1869 [https://maps.arcanum.com/hu/map/secondsurvey-hungary/?layers=5&bbox=2114912.2490512794%2C6021329.6822395325%2C2127801.442945867%2C6025992.340964928 (accessed on 3 July 2024)]
- Military Land-Survey of Hungary, 1941 [https://maps.arcanum.com/hu/map/hungary1941/?layers=29&bbox=2110378.5778111913%2C6019224.854830895%2C2136156.965600367%2C6028550.172281686 (accessed on 3 July 2024)]
- Forestry Map of Hungary, 2022 [https://erdoterkep.nebih.gov.hu/ (accessed on 3 July 2024)]
2.2. Statistical Methods
3. Results
3.1. Taxonomic Evaluation of Relic Trees
3.2. Biocoenology of Microhabitats
3.3. Natural Succession and Landscape Dynamic
3.4. Effects of Land Use Dynamic
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bordács, S.; Popescu, F.; Slade, D.; Csaikl, U.M.; Lesur, I.; Borovics, A.; Kézdy, P.; König, A.O.; Gömöry, D.; Brewer, S.; et al. Chloroplast DNA variation of white oaks in northern Balkans and in the Carpathian Basin. For. Ecol. Manag. 2002, 156, 197–209. [Google Scholar] [CrossRef]
- Slade, D.; Kvorc, Z.S.; Ballian, D.; Gracan, J.; Papes, D. The Chloroplast DNA Polymorphisms of White Oaks of Section Quercus in The Central Balkans. Silvae Genet. 2008, 57, 227–234. [Google Scholar] [CrossRef]
- Curtu, A.L.; Gailing, O.; Finkeldey, F. Evidence for hybridization and introgression within a species-rich oak (Quercus spp.) community. BMC Evol. Biol. 2007, 7, 15. [Google Scholar] [CrossRef]
- Petit, R.J.; Brewer, S.; Bordács, S.; Burg, K.; Cheddadi, R.; Coart, E.; Cottrell, J.; Csaikl, U.M.; Deans, J.D.; Fineschi, S.; et al. Identification of refugia and postglacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence. For. Ecol. Manag. 2002, 156, 49–74. [Google Scholar] [CrossRef]
- Petit, R.J.; Csaikl, U.M.; Bordács, S.; Burg, K.; Coart, E.; Cottrell, J.; Deans, J.D.; Dumolin-Lapegue, S.; Fineschi, S.; Finkeldey, R.; et al. Chloroplast DNA variation in European white oaks. Phylogeography and patterns of diversity based on data from over 2600 populations. For. Ecol. Manag. 2002, 156, 5–26. [Google Scholar] [CrossRef]
- Bordács, S.; Zhelev, P.; Schirone, B. EUFORGEN Technical Guidelines for genetic conservation and use of pubescent oak (Quercus pubescens). In European Forest Genetic Resources Programme (Euforgen); European Forest Institute: Joensuu, Finland, 2019; 6p, ISBN 978-952-5980-63-9. [Google Scholar]
- Bartha, D. Quercus virgiliana Ten. In (Hrsg.): Enzyklopädie der Holzgewächse. Handbuch und Atlas der Dendrologie; Band III/2/25; Schutt, P., Weisgerber, H., Schuck, H.J., Lang, U.M., Roloff, A., Eds.; Ecomed Verlagsgesellschaft: Landsberg am Lech, Germany, 2001; pp. 1–5. [Google Scholar]
- Gencsi, I.; Vancsura, R. Dendrológia; Mezőgazda Kiadó: Budapest, Hungary, 1982. [Google Scholar]
- Majer, A. Magyarország Erdőtársulásai; Akadémiai Kiadó: Budapest, Hungary, 1968; 515p. [Google Scholar]
- Coldea, G.; Farcas, S.; Filipas, L.; Ursu, T.M.; Stoica, I.A. Syntaxonomic revision of Quercus Virgiliana Ten. and Quercus pedunculiflora K. Koch forests from Romania. Studia UBB. Biologia LV 2010, 2, 39–50. [Google Scholar]
- Enescu, C.M.; Curtu, A.L.; Șofletea, N. Is Quercus virgiliana a distinct morphological and genetic entity among European white oaks? Turk. J. Agric. For. 2013, 37, 632–641. [Google Scholar] [CrossRef]
- Trinajstić, I. About the Problem of Differentiation Between the Oaks Quercus pubescens Willd. and Quercus virgiliana. (Ten.) Šumar. List 2007, 131, 57–60. [Google Scholar]
- Bartha, D. Quercus dalechampii Ten. In (Hrsg.): Enzyklopädie der Holzgewächse. Handbuch und Atlas der Dendrologie; Band III/2/3; Schutt, P., Weisgerber, H., Schuck, H.J., Lang, U.M., Roloff, A., Eds.; Ecomed Verlagsgesellschaft: Landsberg am Lech, Germany, 2003; pp. 1–4. [Google Scholar]
- Ducosso, A.; Bordács, S. EUFORGEN Technical Guidelines for Genetic Conservation and Use for Pedunculate and Sessile Oaks (Quercus robur and Q. Petraea); International Plant Genetic Resources Institute: Rome, Italy, 2004; 6p, ISBN 92-9043-660-3. [Google Scholar]
- Schwarz, O. Monographie der Eichen Europas und des Mittelmeergebietes. In Feddes Repertorium; Sonderbeiheft D; Wageningen University and Research Library Catalog: Wageningen, The Netherlands, 1936. [Google Scholar]
- Majer, A. Beteiligung der Kleinarten von Traubeneiche (Quercus petraea) in den Populationen Ungarns. Folia Dendrologica 1989, 16, 179–194. [Google Scholar]
- Matula, R. Comparison of general tree characteristics of less known oak species Quercus dalechampii Ten. and Quercus polycarpa Schur. J. For. Sci. 2008, 54, 333–339. [Google Scholar] [CrossRef]
- Bouillon, P.; Hubert, J.; Bakkebø Fjellstad, K.; Rusanen, M.; Zavrl Bogataj, A.; Olrik, D.C.; Bordács, S.; Longauer, R.; Paitaridou, D.; Kõiv, K.; et al. The implications of global, European and national policies for the conservation and use of forest genetic resources in Europe. In European Forest Genetic Resources Programme (EUFORGEN); Bioversity International: Rome, Italy, 2015; Volume 12, 42p. [Google Scholar]
- Simeone, M.C.; Zhelev, P.; Kandemir, G. EUFORGEN Technical Guidelines for genetic conservation and use of Turkey oak (Quercus cerris). In European Forest Genetic Resources Programme (EUFORGEN); European Forest Institute: Joensuu, Finland, 2019; 6p, ISBN 978-952-5980-43-1. [Google Scholar]
- Gil, L.; Varela, M.C. EUFORGEN Technical Guidelines for Genetic Conservation and Use for Cork Oak (Quercus suber); Biodiversity International: Rome, Italy, 2008; 6p, ISBN 978-92-9043-790-1. [Google Scholar]
- Bergmeier, E.; Petermann, J.; Schroder, E. Geobotanical survey of wood-pasture habitats in Europe: Diversity, threats and conservation. Biodivers. Conserv. 2010, 19, 2995–3014. [Google Scholar] [CrossRef]
- Hartel, T.; Dorresteijn, I.; Klein, C.; Mathe, O.; Moga, C.I.; Ollerer, K.; Roellig, M.; von Wehrden, H.; Fischer, J. Wood-pastures in a traditional rural region of Eastern Europe: Characteristics, management and status. Biol. Conserv. 2013, 166, 267–275. [Google Scholar] [CrossRef]
- Plieninger, T.; Hartel, T.; Martín-López, B.; Beaufoy, G.; Bergmeier, E.; Kirby, K.; Montero, M.J.; Moreno, G.; Oteros-Rozas, E.; Van Uytvanck, J. Wood-Pastures of Europe: Geographic Coverage, Social–Ecological Values, Conservation Management, and Policy Implications. Biol. Conserv. 2015, 190, 70–79. [Google Scholar] [CrossRef]
- Mátyás, C.; Berki, I.; Bidló, A.; Csóka, G.; Czimber, K.; Führer, E.; Gálos, B.; Gribovszki, Z.; Illés, G.; Hirka, A.; et al. Sustainability of Forest Cover under Climate Change on the Temperate-Continental Xeric Limits. Forests 2018, 9, 489. [Google Scholar] [CrossRef]
- Mátyás, C.; Sun, G. Forests in a water limited world under climate change. Environ. Res. Lett. 2014, 9, 1–10. [Google Scholar] [CrossRef]
- Czúcz, B.; Gálhidy, L.; Mátyás, C. Present and forecasted xeric climatic limits of beech and sessile oak distribution at low altitudes in Central Europe. Ann. For. Sci. 2011, 68, 99–108. [Google Scholar] [CrossRef]
- Mátyás, V. A tölgyek dendrológiai ismertetése. In Szerk.: A Tölgyek; Keresztesi, B., Ed.; Akadémiai Kiadó: Budapest, Hungary, 1967; pp. 51–90. [Google Scholar]
- Manning, A.D.; Fischer, J.; Lindenmayer, D.B. Scattered Trees Are Keystone Structures—Implications for Conservation. Biol. Conserv. 2006, 132, 311–321. [Google Scholar] [CrossRef]
- Moreno, G.; Gonzalez-Bornay, G.; Pulido, F.; Lopez-Diaz, M.L.; Bertomeu, M.; Juárez, E.; Diaz, M. Exploring the Causes of High Biodiversity of Iberian Dehesas: The Importance of Wood Pastures and Marginal Habitats. Agrofor. Syst. 2016, 90, 87–105. [Google Scholar] [CrossRef]
- Dias, F.S.; Miller, D.L.; Marques, T.A.; Marcelino, J.; Caldeira, M.C.; Cerdeira, J.O.; Bugalho, M.N. Conservation zones promote oak regeneration and shrub diversity in certified Mediterranean oak woodlands. Biol. Conserv. 2016, 195, 226–234. [Google Scholar] [CrossRef]
- Arnolds, E.; Kuyper Th., W.; Noordeloos, M.E. (Eds.) Overzicht van de Paddestoelen in Nederland; Nederlandse Mycologische Vereniging: Wijster, The Netherlands, 1995; 872p. [Google Scholar]
- Krieglsteiner, G.J. (Ed.) Die Grosspilze Baden-Württembergs; Band 1; Ulmer: Stuttgart, Germany, 2000; 629p. [Google Scholar]
- Eduardo, N.; Florencia, S.; Nicolás, P.; József, G. Richness, species composition and functional groups in Agaricomycetes communities along a vegetation and elevational gradient in the Andean Yungas of Argentina. Biodivers. Conserv. 2018, 27, 1849–1871. [Google Scholar] [CrossRef]
- Bosso, L.; Lacatena, F.; Varlese, R.; Nocerino, S.; Cristinzio, G.; Russo, D. Plant pathogens but not antagonists change in soil fungal communities across a land abandonment gradient in a Mediterranean landscape. Acta Oecologica 2017, 78, 1–6. [Google Scholar] [CrossRef]
- Parveen, A.; Khataniar, L.; Goswami, G.; Hazarika, D.I.; Das, P.; Gautom, T.; Madhumita Barooah, M.; Boro, R.C. A Study on the Diversity and Habitat Specificity of Macrofungi of Assam, India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 275–297. [Google Scholar] [CrossRef]
- Rudolf, K.; Pál-Fám, F.; Morschhauser, T. Macrofungi records from a wood pasture in the Belső-Cserehát (NE Hungary). Stud. Bot. Hung. 2016, 47, 29–40. [Google Scholar] [CrossRef]
- Bugalho, M.N.; Caldeira, M.C.; Pereira, J.S.; Aronson, J.; Pausas, J.G. Mediterranean cork oak savannas require human use to sustain biodiversity and ecosystem services. Front. Ecol. Environ. 2011, 9, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Bíró, M.; Czúcz, B.; Horváth, F.; Révész, A.; Csatári, B.; Molnár, Z. Drivers of grassland loss in Hungary during the post-socialist transformation (1987–1999). Landsc. Ecol. 2013, 28, 789–803. [Google Scholar] [CrossRef]
- Moga, C.I.; Samoilă, C.; Öllerer, K.; Băncilă, R.I.; Réti, K.O.; Craioveanu, C.; Poszet, S.; Rákosy, L.; Hartel, T. Environmental determinants of the old oaks in wood-pastures from a changing traditional social–ecological system of Romania. Ambio 2016, 45, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Mihók, B.; Bíró, M.; Molnár, Z.; Kovács, E.; Bölöni, J.; Erős, T.; Báldi, A. Biodiversity on the waves of history: Conservation in a changing social and institutional environment in Hungary, a post-soviet EU member state. Biol. Conserv. 2017, 211, 67–75. [Google Scholar] [CrossRef]
- Varga, A.; Ódor, P.; Molnár, Z.; Bölöni, J. The history and natural regeneration of a secondary oak-beech woodland on a former wood-pasture in Hungary. Acta Soc. Bot. Pol. 2015, 84, 215–225. [Google Scholar] [CrossRef]
- Leroy, T.; Louvet, J.M.; Lalanne, C.; Le Provost, G.; Labadie, K.; Aury, J.M.; Delzon, S.; Plomion, C.; Kremer, A. Adaptive introgression as a driver of local adaptation to climate in European white oaks. New Phytol. 2020, 226, 1171–1182. [Google Scholar] [CrossRef]
- Leroy, T.; Rougemont, Q.; Dupouey, J.L.; Bodénès, C.; Lalanne, C.; Belser, C.; Labadie, K.; Le Provost, G.; Aury, J.M.; Kremer, A.; et al. Massive postglacial gene flow between European white oaks uncovered genes underlying species barriers. New Phytol. 2020, 226, 1183–1197. [Google Scholar] [CrossRef]
- Leroy, T.; Roux, C.; Villate, L.; Bodénès, C.; Romiguier, J.; Paiva, J.; Azema-Dossat, C.; Aury, J.M.; Plomion, C.; Kremer, A. Extensive recent secondary contacts between four European white oak species. New Phytol. 2017, 214, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Horak, J.; Vodka, S.; Kout, J.; Halda, J.P.; Bogusch, P.; Pech, P. Biodiversity of most dead wood-dependent organisms in thermophilic temperate oak woodlands thrives on diversity of open landscape structures. For. Ecol. Manag. 2014, 315, 80–85. [Google Scholar] [CrossRef]
- Fodor, E.; Hâruța, O.; Dorog, S. Nurse plants and the regeneration niche of tree seedlings in wood-pastures from Western and North-Western Romania. Reforesta 2018, 6, 41–59. [Google Scholar] [CrossRef]
- Lőrincz, Á.; Hábenczyus, A.A.; Kelemen, A.; Ratkai, B.; Tölgyesi, C.; Lőrinczi, G.; Frei, K.; Bátori, Z.; Maák, I.E. Wood-pastures promote environmental and ecological heterogeneity on a small spatial scale. Sci. Total Environ. 2024, 906, 167510. [Google Scholar] [CrossRef] [PubMed]
- Járdi, I.; Saláta, D.; S.-Falusi, E.; Stilling, F.; Pápay, G.; Zachar, Z.; Falvai, D.; Csontos, P.; Péter, N.; Penksza, K. Habitat Mosaics of Sand Steppes and Forest-Steppes in the Ipoly Valley in Hungary. Forests 2021, 12, 135. [Google Scholar] [CrossRef]
- Thomas, E.; Jalonen, R.; Loo, J.; Boshier, D.; Gallo, L.; Cavers, S.; Bordács, S.; Smith, P.; Bozzano, M. Genetic considerations in ecosystem restoration using native tree species. For. Ecol. Manag. 2014, 333, 66–75. [Google Scholar] [CrossRef]
- Soó, R. A Magyar Flóra és Vegetáció Rendszertani-Növényföldrajzi Kézikönyve I–VI; Akadémiai Kiadó: Budapest, Hungary, 1980. [Google Scholar]
- Borhidi, A. Magyarország Növénytársulásai; Akadémiai Kiadó: Budapest, Hungary, 2003; 610p. [Google Scholar]
- Zólyomi, B.; Horváth, A.; Kevey, B.; Lendvai, G. Steppe woodlands with Tatarian maple (Aceri tatarici-Quercetum pubescentis-roboris) on the Great Hungarian Plain and its neighborhood. An unfinished synthesis with supplementary notes. Acta Bot. Hung. 2013, 55, 167–189. [Google Scholar] [CrossRef]





| Site Plot | Locality | Plant Origin | Taxonomic Status Shortly | Position | 1782 | 1819 | 1941 | 2022 |
|---|---|---|---|---|---|---|---|---|
| 1 | Dúzs | seedling | virg × dal × pet | 4 additional younger tree(s) | f | al | pa | hm |
| 2 | Gyönk | root-shoot | dal × virg | 1 additional sapling | f | fb | fb | fb |
| 3 | Gyönk | root-shoot | virg × dal × pet | 1 additional tree | fb | fb | pa | fb |
| 4 | Kalaznó | stump shoot | virg × dal | 6 additional tree(s) | pa | fb | pa | fb |
| 5 | Kalaznó | root-shoot | virg × dal × pet | 2 additional saplings | pa | pa | pa | hm |
| 6 | Kalaznó | root-shoot | virg × dal | solitaire tree | pa | al | al | hm |
| 7 | Kalaznó | seedling | dal × virg | solitaire tree | fb | pa | pa | f |
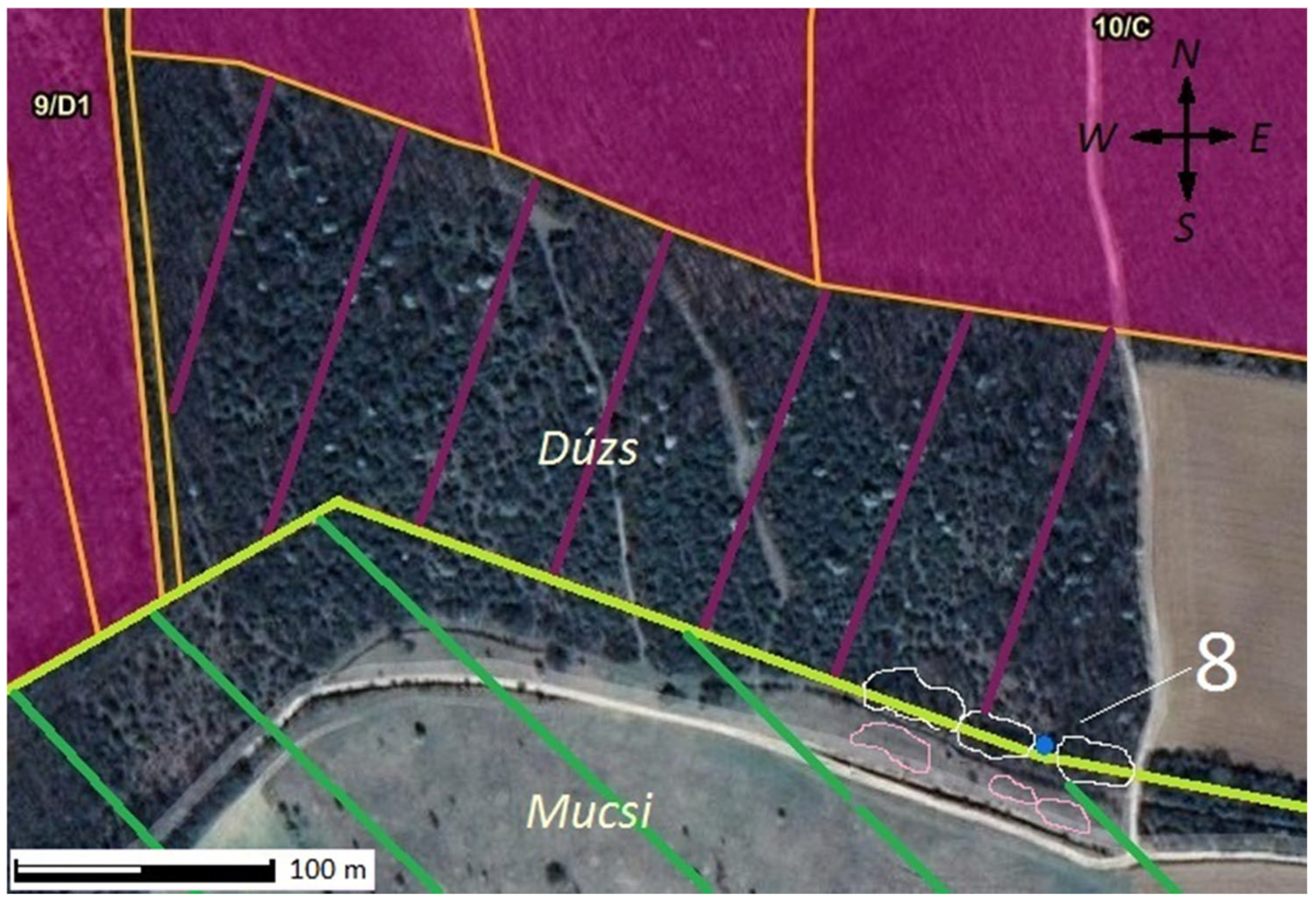
| 8 | Mucsi | stump shoot | virg × dal | 12 additional tree(s) | pa | al | al | hm |
| 9 | Gyönk | stump shoot | virg × dal | solitaire tree | f | fb | fb | hm |
| 10 | Mucsi | stump shoot | virg × dal | solitaire tree | pa | al | al | fb |
| 11 | Mucsi | stump shoot | virg × dal | solitaire tree | pa | al | al | fb |
| 14 | Gyönk | seedling | dal × virg | border of a new forest | f | fb | fb | hm |
| 15 | Gyönk | seedling | virg × dal | border of a new forest | f | fb | fb | hm |
| 16 | Dúzs | seedling | dal × virg × pub | solitaire tree | fb | al | al | hm |
| 17 | Dúzs | seedling | pet × virg × dal | solitaire tree | fb | al | al | hm |
| 18 | Szakály | seedling | dal × virg × pet | solitaire tree | vi | vi | al | f |
| 19 | Szakály | stupm shoot | dal × virg | solitaire tree | vi | vi | al | f |
| 20 | Szakály | root-shoot | virg × dal | solitaire tree | vi | vi | al | f |
| 21 | Gyönk | stump-shoot | virg × dal | solitaire tree | f | fb | pa | fb |
| 22 | Gyönk | seedling | pub × virg | solitaire tree | f | fb | pa | fb |
| 23 | Gyönk | seedling | virg × dal | solitaire tree | f | pa | pa | hm |
| 24 | Diósberény | root-shoot | virg × pet | solitaire tree | pa | pa | fb | pa |
| 25 | Diósberény | root-shoot | virg × pub | solitaire tree | pa | pa | fb | pa |
| 26 | Diósberény | seedling | virg × pet × dal | solitaire tree | pa | pa | fb | pa |
| 27 | Diósberény | seedling | virg × pet × dal | solitaire tree | pa | pa | fb | pa |
| 28 | Diósberény | stump-shoot | virg × pet × dal | solitaire tree | pa | pa | fb | pa |
| 29 | Diósberény | root-shoot | virg × dal × pet | solitaire tree | pa | fb | fb | pa |
| 30 | Diósberény | root-shoot | pet × virg × dal | solitaire tree | pa | fb | fb | pa |
| 31 | Diósberény | root-shoot | virg × pet × dal | solitaire tree | pa | pa | fb | pa |
| 32 | Diósberény | root-shoot | virg × dal | solitaire tree | pa | pa | fb | pa |
| 33 | Diósberény | seedling | dal × virg | solitaire tree | pa | pa | fb | pa |
| 34 | Diósberény | seedling | pet × virg × dal | 1 additional tree | pa | pa | fb | pa |
| 35 | Diósberény | roor-shoot | virg | 1 additional tree | pa | fb | fb | pa |
| 36 | Diósberény | stump-shoot | virg | solitaire tree | pa | fb | fb | pa |
| 37 | Diósberény | seedling | virg × pet × dal | solitaire tree | pa | fb | fb | pa |
| 38 | Diósberény | root-shoot | virg × dal | solitaire tree | pa | fb | fb | pa |
| 39 | Diósberény | stump-shoot | virg | solitaire tree | pa | fb | fb | pa |
| 40 | Diósberény | root-shoot | virg × dal | solitaire tree | pa | pa | fb | pa |
| 41 | Diósberény | stump-shooot | virg × dal × pet | solitaire tree | pa | pa | fb | pa |
| Species | Character Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acer campestre | F | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||||||
| Acer tataricum | F | x | ||||||||||||||||||||||||||||||||||||||
| Berberis vulgare | F | x | x | x | ||||||||||||||||||||||||||||||||||||
| Carpinus betulus | F | x | x | x | x | |||||||||||||||||||||||||||||||||||
| Clematis vitalba | F | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||||||||||||||
| Colutea arborescens | F | x | x | |||||||||||||||||||||||||||||||||||||
| Cornus sanguinea | F | x | x | x | x | x | x | |||||||||||||||||||||||||||||||||
| Corylus avellana | F | x | ||||||||||||||||||||||||||||||||||||||
| Crataegus monogyna | F/WS | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |||||||||||
| Chamaecystus hirsutus | F | x | x | |||||||||||||||||||||||||||||||||||||
| Euonymus europaeus | F | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||||||||||||||||||
| Frangula alnus | F | x | ||||||||||||||||||||||||||||||||||||||
| Fraxinus ornus | F | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |||||||||||||||||||||||
| Ligustrum vulgare | F | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||
| Prunus spinosa | F/WS | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||||||||||||||||
| Quercus cerris | F | x | x | |||||||||||||||||||||||||||||||||||||
| Rosa canina | F | x | x | x | x | x | x | x | x | x | x | |||||||||||||||||||||||||||||
| Rosa gallica | F | x | ||||||||||||||||||||||||||||||||||||||
| Rubus caesius | F | x | ||||||||||||||||||||||||||||||||||||||
| Ruscus aculeatus | F | x | ||||||||||||||||||||||||||||||||||||||
| Ulmus minor | F | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||||||||||||
| Viburnum lantana | F | x | x | x | x | x | x |
| Species Character | Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aegonychon purpurea-coeruleum | WS | x | x | |||||||||||||||||||||||||||||||||||||
| Ajuga reptans | F | x | x | |||||||||||||||||||||||||||||||||||||
| Anthriscus cerefolium | F | x | x | x | x | |||||||||||||||||||||||||||||||||||
| Anthriscus sylvestris | F | x | x | x | x | |||||||||||||||||||||||||||||||||||
| Galium odoratum | F | x | ||||||||||||||||||||||||||||||||||||||
| Astragalus glycyphyllos | F | x | ||||||||||||||||||||||||||||||||||||||
| Campanula persicifolia | F | x | x | |||||||||||||||||||||||||||||||||||||
| Corydalis solida | WS | x | ||||||||||||||||||||||||||||||||||||||
| Digitalis grandiflora | F | x | x | |||||||||||||||||||||||||||||||||||||
| Euphorbia amygdaloides | F | x | x | x | ||||||||||||||||||||||||||||||||||||
| Galium schultesii | WS | x | ||||||||||||||||||||||||||||||||||||||
| Galium sylvaticum | F | x | ||||||||||||||||||||||||||||||||||||||
| Geum urbanum | WS | x | x | x | x | x | x | x | ||||||||||||||||||||||||||||||||
| Glechoma hederacea | F | x | ||||||||||||||||||||||||||||||||||||||
| Helleborus odorus | F | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||||||||||||||||
| Hypericum perforatum | F/WS | x | x | |||||||||||||||||||||||||||||||||||||
| Knautia drymeia | F | x | ||||||||||||||||||||||||||||||||||||||
| Vinca herbacea | WS | x | x | |||||||||||||||||||||||||||||||||||||
| Viola silvestris | F | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x |
| Species | Character Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arum maculatum | F | x | x | x | x | |||||||||||||||||||||||||||||||||||
| Brachipodium sylvaticum | F | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||||||||||
| Bromus sterilis | F/WS | x | x | x | x | x | x | x | x | x | ||||||||||||||||||||||||||||||
| Calamagrostris epigeios | F | x | ||||||||||||||||||||||||||||||||||||||
| Carex humilis | WS | x | x | x | ||||||||||||||||||||||||||||||||||||
| Carex michellii | WS | x | x | x | ||||||||||||||||||||||||||||||||||||
| Cephalantera damasonium | F | x | x | x | ||||||||||||||||||||||||||||||||||||
| Cephalantera longifolia | F | x | ||||||||||||||||||||||||||||||||||||||
| Dactylis polygama | WS | x | x | x | x | x | x | x | x | x | x | x | x | |||||||||||||||||||||||||||
| Iris variegata | WS | x | ||||||||||||||||||||||||||||||||||||||
| Limodorum abortivum | F | x | ||||||||||||||||||||||||||||||||||||||
| Melica uniflora | F | x | x | |||||||||||||||||||||||||||||||||||||
| Orchis purpurea | F | x | x | |||||||||||||||||||||||||||||||||||||
| Poa nemoralis | F | x | x | x | ||||||||||||||||||||||||||||||||||||
| Polygonatum latifolium | F | x | x | |||||||||||||||||||||||||||||||||||||
| Scilla vindobonensis | F | x |
| Species | Functional Spectrum | Substrate Specificity | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aleurodiscus disciformis | Sh | only Quercus spp. | x | ||||||||||
| Antrodia albida | Sh | polyphagous | x | ||||||||||
| Auricularia mesenterica | Sh | polyphagous | x | x | x | ||||||||
| Exidia nigricans | Sh | polyphagous | x | ||||||||||
| Fuscoporia torulosa | Pn | polyphagous | x | ||||||||||
| Hypholoma fasciculare | Sh | polyphagous | x | ||||||||||
| Peniophora quercina | Sh | dominantly Quercus spp. | x | ||||||||||
| Perenniporia fraxinea | Pn | polyphagous | x | ||||||||||
| Polyporus arcularius | Sh | polyphagous | x | ||||||||||
| Stereum hirsutum | Sh | polyphagous | x | x | |||||||||
| Vuilleminia comedens | Sh | dominantly Quercus spp. | x |
| Species Types | Land-Use Types | Mean ± Standard Deviation | N |
|---|---|---|---|
| forest woody plant | forest | 2.75 ± 1.26 a | 4 |
| boundary line of forested land | 6.20 ± 1.10 b | 5 | |
| pasture | 5.17 ± 1.95 ab | 18 | |
| hay meadow | 5.60 ± 1.84 b | 10 | |
| forest dicot herbs | forest | 1.00 ± 1.41 a | 4 |
| boundary line of forested land | 1.80 ± 0.45 a | 5 | |
| pasture | 1.89 ± 0.83 a | 18 | |
| hay meadow | 2.40 ± 2.12 a | 10 | |
| forest monocot herbs | forest | 1.00 ± 1.15 a | 4 |
| boundary line of forested land | 3.80 ± 2.77 b | 5 | |
| pasture | 1.33 ± 0.69 a | 18 | |
| hay meadow | 2.00 ± 1.25 ab | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordács, S.; Pintér, B.; Horváth, C.; Benedek, L.; Ladányi, M. Relic Vergilius Oak (Quercus virgiliana Ten.) Trees Could Preserve Microhabitats of Pannonian Forest–Steppe Vegetation. Diversity 2024, 16, 401. https://doi.org/10.3390/d16070401
Bordács S, Pintér B, Horváth C, Benedek L, Ladányi M. Relic Vergilius Oak (Quercus virgiliana Ten.) Trees Could Preserve Microhabitats of Pannonian Forest–Steppe Vegetation. Diversity. 2024; 16(7):401. https://doi.org/10.3390/d16070401
Chicago/Turabian StyleBordács, Sándor, Beáta Pintér, Csaba Horváth, Lajos Benedek, and Márta Ladányi. 2024. "Relic Vergilius Oak (Quercus virgiliana Ten.) Trees Could Preserve Microhabitats of Pannonian Forest–Steppe Vegetation" Diversity 16, no. 7: 401. https://doi.org/10.3390/d16070401






