Rare Earth Elements Distribution and Bacteriome to Assess and Characterize the Soil Landscapes of Old Olive Orchards
Abstract
:1. Introduction
2. Material and Methods
2.1. Soil Sampling Strategies and REE Analyses
2.2. Geological Settings
2.3. Extraction of Total DNA and 16S rRNA Gene Sequencing
2.4. Bioinformatic Analysis
2.4.1. Sequencing Data Processing
2.4.2. OTU Cluster and Taxonomic Annotation
2.5. Statistical Analysis
2.5.1. Multivariate Analysis
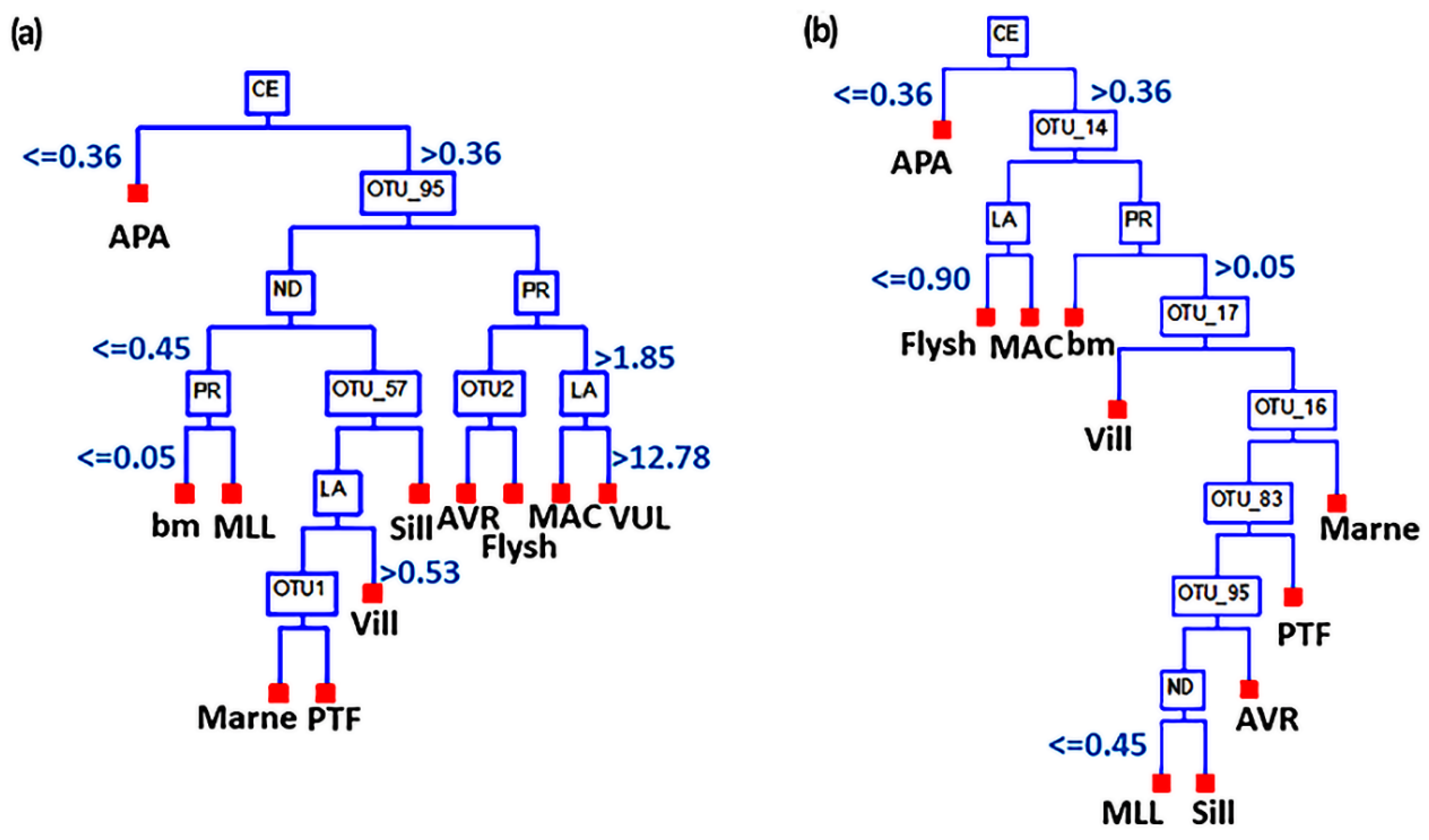
2.5.2. Supervised Machine Learning
3. Results
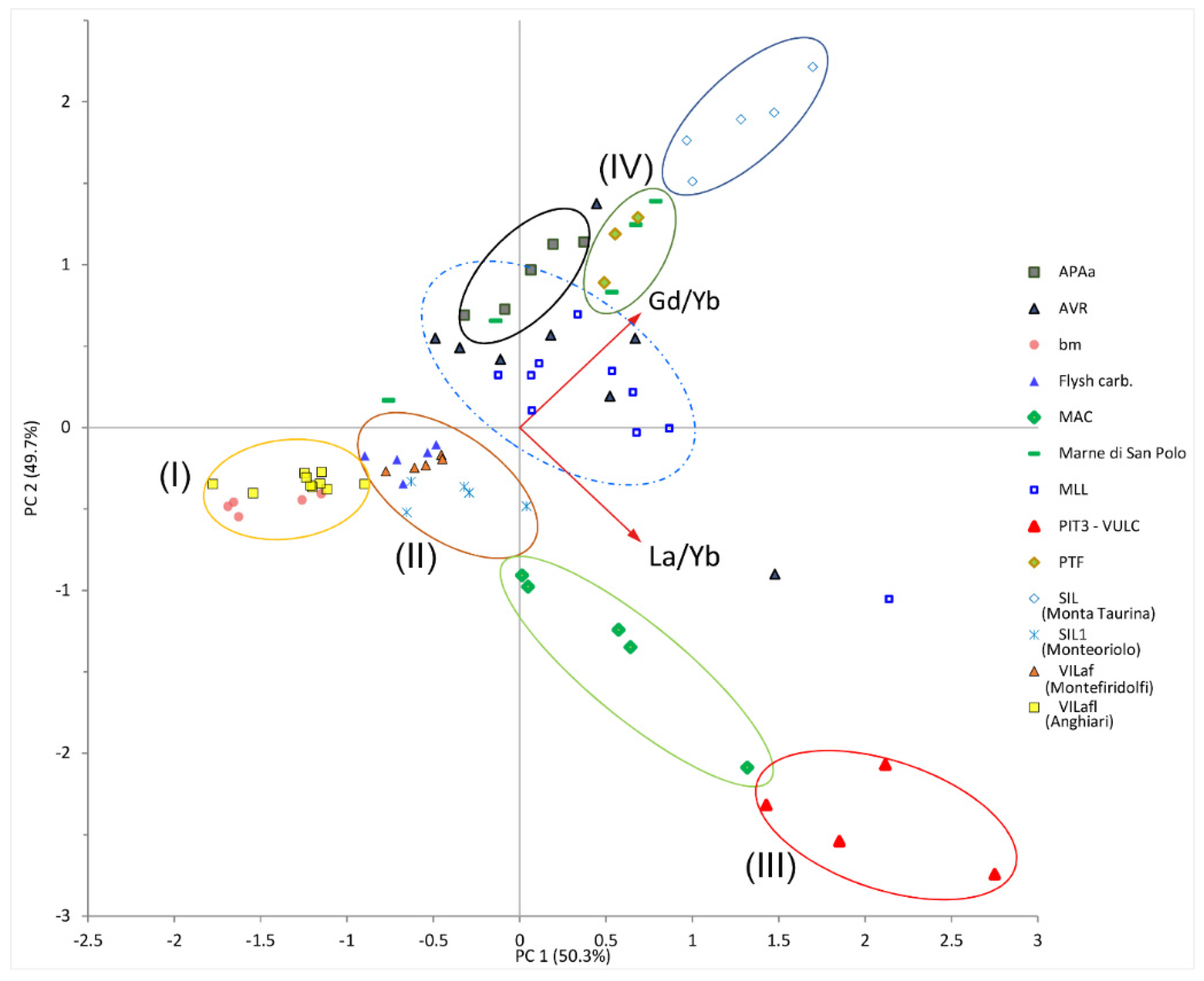
3.1. Geochemistry of REEs
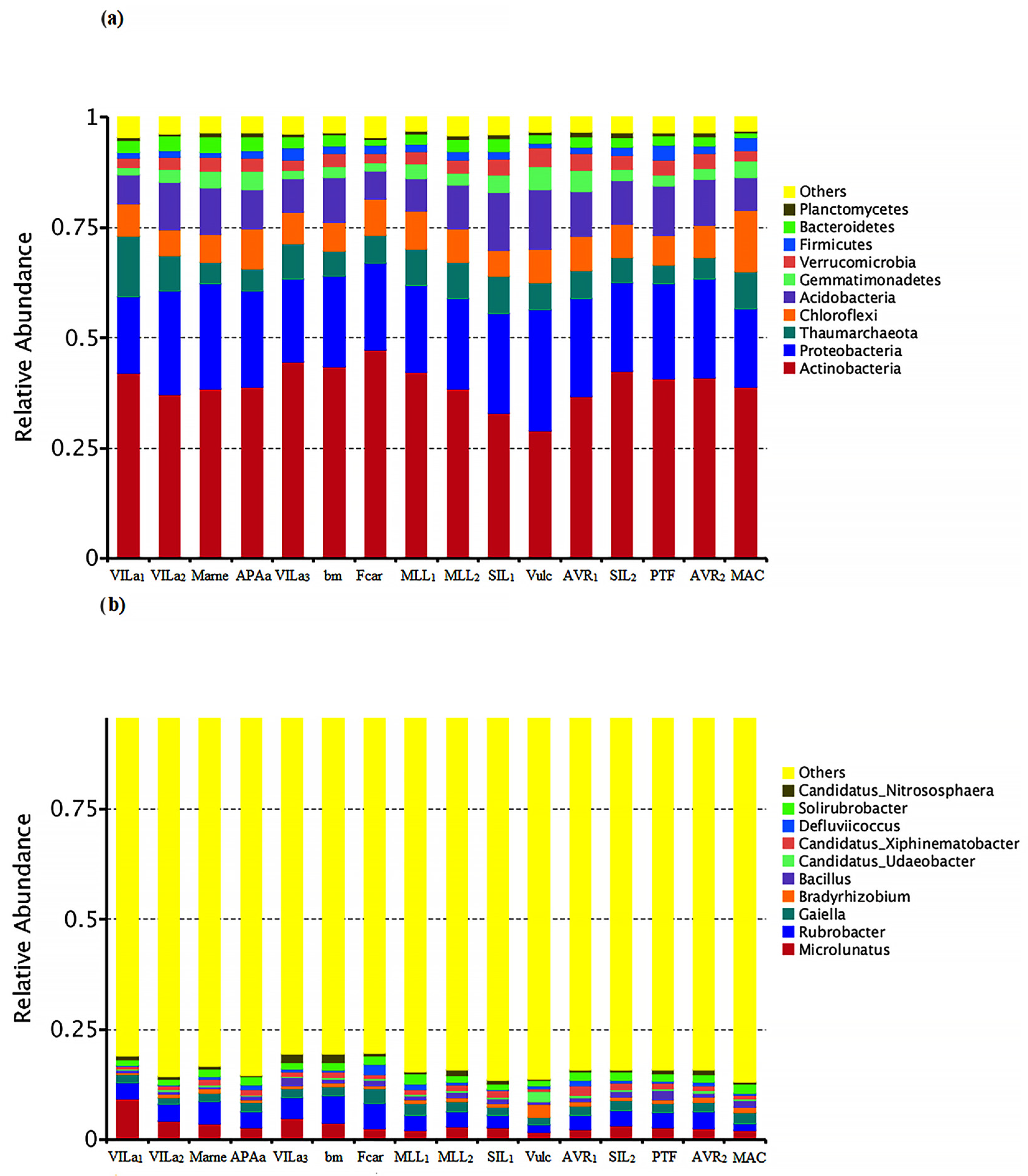
3.2. Bacteriome Sequencing
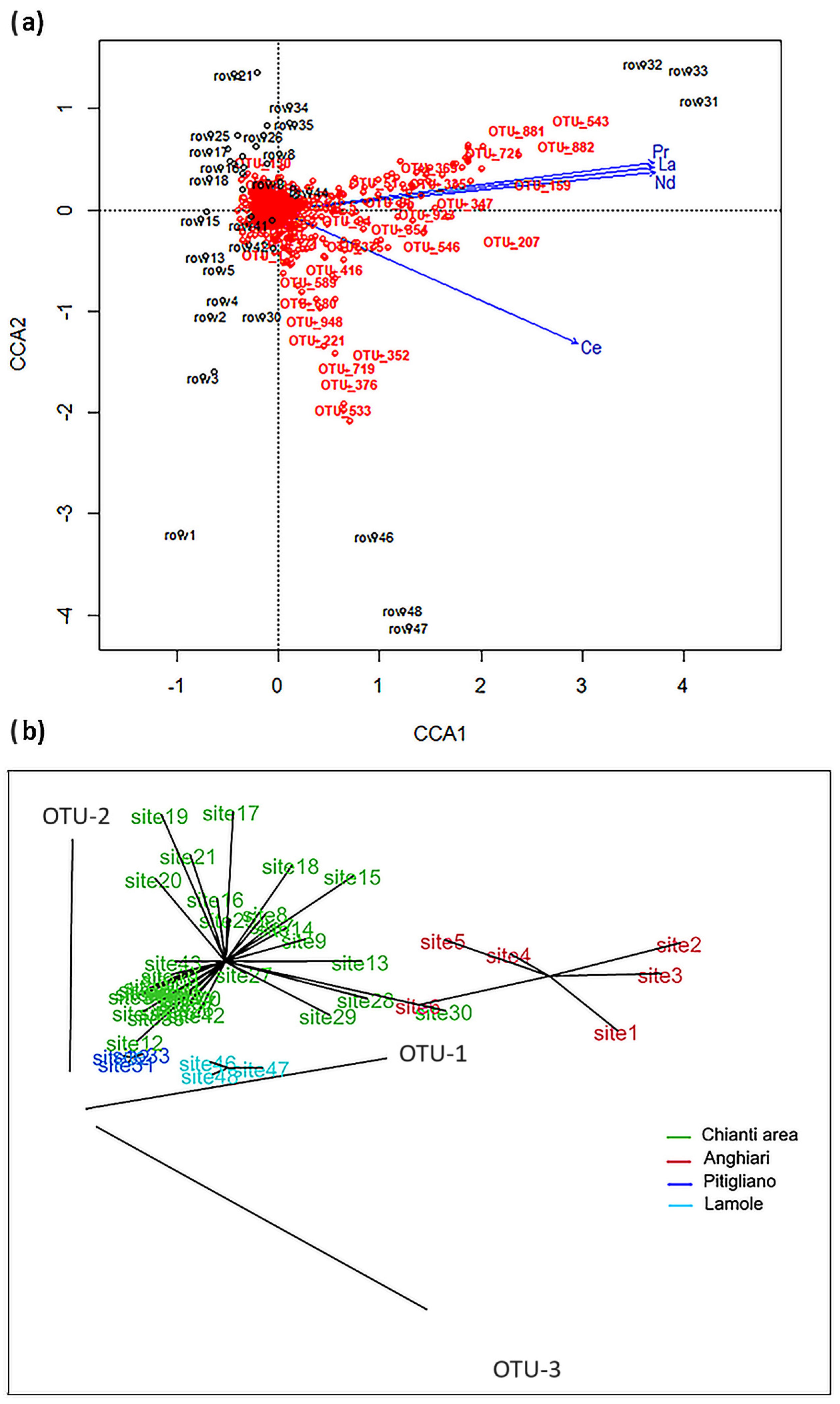
3.3. Correlation between Bacteriome and REEs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fausto, C.; Mininni, A.N.; Sofo, A.; Crecchio, C.; Scagliola, M.; Dichio, B.; Xiloyannis, C. Olive orchard microbiome: Characterisation of bacterial communities in soil-plant compartments and their comparison between sustainable and conventional soil management systems. Plant Ecol. Divers. 2018, 11, 597–610. [Google Scholar] [CrossRef]
- Ebrahimi-Zarandi, M.; Etesami, H.; Glick, B.R. Fostering plant resilience to drought with Actinobacteria: Unveiling perennial allies in drought stress tolerance. Plant Stress 2023, 10, 100242. [Google Scholar] [CrossRef]
- Pošćić, F.; Žanetić, M.; Fiket, Ž.; Furdek Turk, M.; Mikac, N.; Bačić, N.; Lučić, M.; Romić, M.; Bakić, H.; Jukić Špika, M. Accumulation and partitioning of rare earth elements in olive trees and extra virgin olive oil from Adriatic coastal region. Plant Soil 2020, 448, 133–151. [Google Scholar] [CrossRef]
- Festa, A.; Pini, G.A.; Dilek, Y.; Codegone, G. Mélanges and mélange-forming processes: A historical overview and new concepts. Int. Geol. Rev. 2010, 52, 1040–1105. [Google Scholar] [CrossRef]
- Lotti, B. Descrizione Geologica dell’Umbria; Istituto Poligrafico e Zecca dello Stato: Roma, Italy, 1926. [Google Scholar]
- Hibi, Y.; Asai, K.; Arafuka, H.; Hamajima, M.; Iwama, T.; Kawai, K. Molecular structure of La3+-induced methanol dehydrogenase-like protein in Methylobacterium radiotolerans. J. Biosci. Bioeng. 2011, 111, 547–549. [Google Scholar] [CrossRef]
- Laveuf, C.; Cornu, S. A review on the potentiality of rare earth elements to trace pedogenetic processes. Geoderma 2009, 154, 1–12. [Google Scholar] [CrossRef]
- Pang, X.; Li, D.; Peng, A. Application of rare-earth elements in the agriculture of China and its environmental behavior in soil. Environ. Sci. Pollut. Res. 2002, 9, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kalaimurugan, D.; Sivasankar, P.; Lavanya, K.; Shivakumar, M.S.; Venkatesan, S. Antibacterial and Larvicidal Activity of Fusarium proliferatum (YNS2) Whole Cell Biomass Mediated Copper Nanoparticles. J. Clust. Sci. 2019, 30, 1071–1080. [Google Scholar] [CrossRef]
- Li, X.; Qu, Z.; Zhang, Y.; Ge, Y.; Sun, H. Soil fungal community and potential function in different forest ecosystems. Diversity 2022, 14, 520. [Google Scholar] [CrossRef]
- Srikamwang, C.; Onsa, N.E.; Sunanta, P.; Sangta, J.; Chanway, C.P.; Thanakkasaranee, S.; Sommano, S.R. Role of microbial volatile organic compounds in promoting plant growth and disease resistance in horticultural production. Plant Signal. Behav. 2023, 18, 2227440. [Google Scholar] [CrossRef]
- Pastawan, V.; Suganuma, S.; Mizuno, K.; Wang, L.; Tani, A.; Mitsui, R.; Nakamura, K.; Shimada, M.; Hayakawa, T.; Fitriyanto, N.A. Regulation of lanthanide-dependent methanol oxidation pathway in the legume symbiotic nitrogen-fixing bacterium Bradyrhizobium sp. strain Ce-3. J. Biosci. Bioeng. 2020, 130, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Picone, N.; den Camp, H.J.M.O. Role of rare earth elements in methanol oxidation. Curr. Opin. Chem. Biol. 2019, 49, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Henderson, P. General geochemical properties and abundances of the rare earth elements. In Developments in Geochemistry; Elsevier: Amsterdam, The Netherlands, 1984; Volume 2, pp. 1–32. [Google Scholar]
- Kamenopoulos, S.N.; Agioutantis, Z.; Komnitsas, K. Framework for sustainable mining of rare earth elements. In Rare Earths Industry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 111–120. [Google Scholar]
- Bohn, H.L.; Myer, R.A.; O’Connor, G.A. Soil Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Li, X.; Chen, Z.; Chen, Z.; Zhang, Y. A human health risk assessment of rare earth elements in soil and vegetables from a mining area in Fujian Province, Southeast China. Chemosphere 2013, 93, 1240–1246. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, S.; Wang, L.; Kung, H.-T.; Wang, Y.; Hu, A.; Ding, S. Environmental biogeochemical behaviors of rare earth elements in soil–plant systems. Environ. Geochem. Health 2005, 27, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Albanese, S.; Morris, G.; Ladenberger, A.; Andersson, M.; Cannatelli, C.; Lima, A.; De Vivo, B. REE concentrations in agricultural soil in Sweden and Italy: Comparison of weak MMI® extraction with near total extraction data. Appl. Geochem. 2015, 63, 22–36. [Google Scholar] [CrossRef]
- Barbera, M.; Saiano, F.; Tutone, L.; Massenti, R.; Pisciotta, A. The pattern of rare earth elements like a possible helpful tool in traceability and geographical characterization of the soil-olive system (Olea europaea L.). Plants 2022, 11, 2579. [Google Scholar] [CrossRef]
- Pelacani, S.; Maerker, M.; Tommasini, S.; Moretti, S. Combining biodiversity and geodiversity on landscape scale: A novel approach using rare earth elements and spatial distribution models in an agricultural Mediterranean landscape. Ecol. Indic. 2024, 158, 111583. [Google Scholar] [CrossRef]
- Ramos, S.J.; Dinali, G.S.; de Carvalho, T.S.; Chaves, L.C.; Siqueira, J.O.; Guilherme, L.R.G. Rare earth elements in raw materials and products of the phosphate fertilizer industry in South America: Content, signature, and crystalline phases. J. Geochem. Explor. 2016, 168, 177–186. [Google Scholar] [CrossRef]
- Bispo, F.H.A.; de Menezes, M.D.; Fontana, A.; de Souza Sarkis, J.E.; Gonçalves, C.M.; de Carvalho, T.S.; Curi, N.; Guilherme, L.R.G. Rare earth elements (REEs): Geochemical patterns and contamination aspects in Brazilian benchmark soils. Environ. Pollut. 2021, 289, 117972. [Google Scholar] [CrossRef]
- Pelacani, S.; Tommasini, S.; Ungaro, F.; Falcone, E.; Saiano, F.R.E. Of Eafevooatcs, Tuscany Olive Groves IX, Italiana CNdSC (2017) Rare Earth Elements Analysis For Extra Virgin Olive Oil Assessment: The Case Study Of Tuscany Olive Groves, Italy. In Proceedings of the XXVI Congresso Nazionale della Società Chimica Italiana, Paestum, Italy, 10–14 September 2017. [Google Scholar]
- Barrat, J.-A.; Bayon, G.; Lalonde, S. Calculation of cerium and lanthanum anomalies in geological and environmental samples. Chem. Geol. 2023, 615, 121202. [Google Scholar] [CrossRef]
- Pol, A.; Barends, T.R.M.; Dietl, A.; Khadem, A.F.; Eygensteyn, J.; Jetten, M.S.M.; Op den Camp, H.J.M. Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ. Microbiol. 2014, 16, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Semrau, J.D.; DiSpirito, A.A.; Gu, W.; Yoon, S. Metals and methanotrophy. Appl. Environ. Microbiol. 2018, 84, e02289-17. [Google Scholar] [CrossRef] [PubMed]
- Anguita-Maeso, M.; Olivares-García, C.; Haro, C.; Imperial, J.; Navas-Cortés, J.A.; Landa, B.B. Culture-dependent and culture-independent characterization of the olive xylem microbiota: Effect of sap extraction methods. Front. Plant Sci. 2020, 10, 1708. [Google Scholar] [CrossRef] [PubMed]
- Zaharescu, D.G.; Burghelea, C.I.; Dontsova, K.; Presler, J.K.; Maier, R.M.; Huxman, T.; Domanik, K.J.; Hunt, E.A.; Amistadi, M.K.; Gaddis, E.E. Ecosystem composition controls the fate of rare earth elements during incipient soil genesis. Sci. Rep. 2017, 7, 43208. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, W.; Zhang, Y.; Chen, C.; Wu, W.; Zhang, T.C. Microbial communities in rare earth mining soil after in-situ leaching mining. Sci. Total Environ. 2021, 755, 142521. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Cohen, D.R.; Rutherford, N.F.; Zissimos, A.M.; Morisseau, E.G. Reflections of the geological characteristics of Cyprus in soil rare earth element patterns. Appl. Geochem. 2015, 56, 80–93. [Google Scholar] [CrossRef]
- Silva, C.M.C.A.C.; Barbosa, R.S.; Nascimento, C.W.A.D.; Silva, Y.J.A.B.D.; Silva, Y.J.A.B.D. Geochemistry and spatial variability of rare earth elements in soils under different geological and climate patterns of the Brazilian Northeast. Rev. Bras. Ciência Solo 2018, 42, e0170342. [Google Scholar] [CrossRef]
- Foley, N.; Ayuso, R. REE enrichment in granite-derived regolith deposits of the Southeastern United States: Prospective source rocks and accumulation processes. In British Columbia Geological Survey; Symposium on Strategic and Critical Materials Proceedings, 13–14 November 2015, Victoria, British Columbia, Ministry of Energy and Mines, 2015; Simandl, G.J., Neetz, M., Eds.; Volume 3, pp. 131–138.
- Johannesson, K.H.; Lyons, W.B.; Stetzenbach, K.J.; Byrne, R.H. The solubility control of rare earth elements in natural terrestrial waters and the significance of PO43− and CO32− in limiting dissolved rare earth concentrations: A review of recent information. Aquat. Geochem. 1995, 1, 157–173. [Google Scholar] [CrossRef]
- Zhou, W.; Han, G.; Liu, M.; Song, C.; Li, X. Geochemical distribution characteristics of rare earth elements in different soil profiles in Mun River Basin, Northeast Thailand. Sustainability 2020, 12, 457. [Google Scholar] [CrossRef]
- Brandl, H.; Barmettler, F.; Castelberg, C.; Fabbri, C. Microbial mobilization of rare earth elements (REE) from mineral solids: A mini review. AIMS Microbiol. 2016, 3, 190–204. [Google Scholar]
- Su, H.; Zhang, D.; Antwi, P.; Xiao, L.; Liu, Z.; Deng, X.; Asumadu-Sakyi, A.B.; Li, J. Effects of heavy rare earth element (yttrium) on partial-nitritation process, bacterial activity and structure of responsible microbial communities. Sci. Total Environ. 2020, 705, 135797. [Google Scholar] [CrossRef]
- Cantini, C.; Cimato, A.; Sani, G. Morphological evaluation of olive germplasm present in Tuscany region. Euphytica 1999, 109, 173–181. [Google Scholar] [CrossRef]
- Zorini, L.O.; Polidori, R. Economic and environmental aspects of the Tuscan olive production. Georgofili 2010, 7 (Suppl. S2), 17–47. [Google Scholar]
- Agnoletti, M.; Conti, L.; Frezza, L.; Santoro, A. Territorial analysis of the agricultural terraced landscapes of Tuscany (Italy): Preliminary results. Sustainability 2015, 7, 4564–4581. [Google Scholar] [CrossRef]
- Nanni, P. Vite e olivo nelle proprietà dei Medici nel Quattrocento. Medicea 2008, 1, 12–19. [Google Scholar]
- Rohdenburg, H. Methods for the analysis of agro-ecosystems in central Europe, with emphasis on geoecological aspects. Catena 1989, 16, 1–57. [Google Scholar] [CrossRef]
- Ruhe, R.V.; Walker, P.H. Hillslope models and soil formation. I. Open systems. Int. Soc. Soil. Sci. Trans. 1968, 4, 551–560. [Google Scholar]
- Ehlers, L.J.; Luthy, R.G. Peer reviewed: Contaminant bioavailability in soil and sediment. Environ. Sci. Technol. 2003, 37, 295A–302A. [Google Scholar] [CrossRef]
- Feng, M.H.; Shan, X.Q. A comparison of the rhizosphere-based method with DTPA, EDTA, CaCl2 and NaNO3 estraction methods for predition of bioavailability of metals in soil to barley. Environ. Pollut. 2005, 137, 231–240. [Google Scholar] [CrossRef]
- Bortolotti, V. Nota illustrativa della carta della distribuzione geografica della Formazione di Monte Morello (Alberese). Arti Graf. Pacini Mariotti 1964, 83, 155–190. [Google Scholar]
- Principi, G.; de Luca-Cardillo, M. Nuovi dati preliminari sulla coltre alloctona a Nord di Prato (Firenze). Boll. Della Soc. Geol. Ital. 1975, 94, 1199–1206. [Google Scholar]
- Merla, G. Macigno del Chianti. Studi Illustrativi della Carta Geologica d’Italia. Formazioni Geol. 1969, 2, 65–77. [Google Scholar]
- Cattuto, C.; Cencetti, C.; Fisauli, M.; Gregori, L. I bacini pleistocenici di Anghiari e Sansepolcro nell’alta valle del Tevere. Il Quat. 1995, 8, 119–128. [Google Scholar]
- Varekamp, J.C. The geology of the Vulsinian area, Lazio, Italy. Bull. Volcanol. 1980, 43, 489–503. [Google Scholar] [CrossRef]
- Ceccherini, M.T.; Ascher, J.; Pietramellara, G.; Mocali, S.; Viti, C.; Nannipieri, P. The effect of pharmaceutical waste-fungal biomass, treated to degrade DNA, on the composition of eubacterial and ammonia oxidizing populations of soil. Biol. Fertil. Soils 2007, 44, 299–306. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- White, J.R.; Nagarajan, N.; Pop, M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput. Biol. 2009, 5, e1000352. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’hara, B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. R package, version 1.15-1. Vegan: Community Ecology Package. 2008. Available online: http://vegan.r-forge.r-project.org/(accessed on 1 July 2024).
- Guyon, I.; Elisseeff, A. An introduction to variable and feature selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Ghannam, R.B.; Techtmann, S.M. Machine learning applications in microbial ecology, human microbiome studies, and environmental monitoring. Comput. Struct. Biotechnol. J. 2021, 19, 1092–1107. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution. Blackwell Scientific Publications: Hoboken, NJ, USA, 1985; 312p. [Google Scholar]
- Hotelling, H. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol. 1933, 24, 417. [Google Scholar] [CrossRef]
- Bacci, G.; Ceccherini, M.T.; Bani, A.; Bazzicalupo, M.; Castaldini, M.; Galardini, M.; Giovannetti, L.; Mocali, S.; Pastorelli, R.; Pantani, O.L.; et al. Exploring the dynamics of bacterial community composition in soil: The panbacteriome approach. Antonie Van Leeuwenhoek 2015, 107, 785–797. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher-Jenull, J.; Ceccherini, M.T.; Pietramellara, G.; Renella, G.; Schloter, M. Beyond microbial diversity for predicting soil functions: A mini review. Pedosphere 2020, 30, 5–17. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.M. Chapter Six—Chatting With a Tiny Belowground Member of the Holobiome: Communication Between Plants and Growth-Promoting Rhizobacteria. In Advances in Botanical Research; Becard, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 82, pp. 135–160. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Z.; Pan, H.; Bai, Y.; Hu, Y.; Jin, S. Effects of rare earth elements on bacteria in rhizosphere, root, phyllosphere and leaf of soil–rice ecosystem. Sci. Rep. 2022, 12, 2089. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, S.; Coelho, L.P.; Chaffron, S.; Kultima, J.R.; Labadie, K.; Salazar, G.; Djahanschiri, B.; Zeller, G.; Mende, D.R.; Alberti, A. Structure and function of the global ocean microbiome. Science 2015, 348, 1261359. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Brioschi, L.; Steinmann, M.; Lucot, E.; Pierret, M.-C.; Stille, P.; Prunier, J.; Badot, P.-M. Transfer of rare earth elements (REE) from natural soil to plant systems: Implications for the environmental availability of anthropogenic REE. Plant Soil 2013, 366, 143–163. [Google Scholar] [CrossRef]
- Fedele, L.; Plant, J.A.; De Vivo, B.; Lima, A. The rare earth element distribution over Europe: Geogenic and anthropogenic sources. Geochemistry: Exploration, Environment. Analysis 2008, 8, 3–18. [Google Scholar]
- Loell, M.; Reiher, W.; Felix-Henningsen, P. Contents and bioavailability of rare earth elements in agricultural soils in Hesse (Germany). J. Plant Nutr. Soil Sci. 2011, 174, 644–654. [Google Scholar] [CrossRef]
- Luo, Y.; Yuan, H.; Zhao, J.; Qi, Y.; Cao, W.-W.; Liu, J.-M.; Guo, W.; Bao, Z.-H. Multiple factors influence bacterial community diversity and composition in soils with rare earth element and heavy metal co-contamination. Ecotoxicol. Environ. Saf. 2021, 225, 112749. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Liu, W.; Chen, Y.; Chen, W.; Zhao, L.; Ding, Q.; Wang, S.; Tang, Y.-T.; Zhang, T.; Qiu, R.-L. Structure, Variation, and Co-occurrence of Soil Microbial Communities in Abandoned Sites of a Rare Earth Elements Mine. Environ. Sci. Technol. 2016, 50, 11481–11490. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, W.; Yang, Y.; Ma, J.; Li, S.; Wen, Z. Soil characteristics and microbial community response in rare earth mining areas in southern Jiangxi Province, China. Environ. Sci. Pollut. Res. 2021, 28, 56418–56431. [Google Scholar] [CrossRef]
- Battistuzzi, F.U.; Hedges, S.B. A Major Clade of Prokaryotes with Ancient Adaptations to Life on Land. Mol. Biol. Evol. 2009, 26, 335–343. [Google Scholar] [CrossRef]
- Prathyusha, A.M.V.N.; Bramhachari, P.V. Chapter 16—Novel Perspectives of Biotic and Abiotic Stress Tolerance Mechanism in Actinobacteria. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, B.P., Gupta, V.K., Passari, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 235–244. [Google Scholar] [CrossRef]
- Gupta, A.; Dutta, A.; Sarkar, J.; Panigrahi, M.K.; Sar, P. Low-abundance members of the Firmicutes facilitate bioremediation of soil impacted by highly acidic mine drainage from the Malanjkhand copper project, India. Front. Microbiol. 2018, 9, 2882. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zheng, C.; Chen, M.; Du, W.; Xu, X. Geobiochemistry characteristics of rare earth elements in soil and ground water: A case study in Baotou, China. Sci. Rep. 2020, 10, 11740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ullah, F.; Ahmad, R.; Shah, S.U.A.; Khan, A.; Adnan, M. Response of soil proteobacteria to biochar amendment in sustainable agriculture-a mini review. J. Soil Plant Environ. 2022, 1, 16–30. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent understanding of soil acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Bu, L.; Zhang, M.; Yuan, J.; Zhang, Y.; Wei, G.; Wang, H. Soil bacteria with distinct diversity and functions mediates the soil nutrients after introducing leguminous shrub in desert ecosystems. Glob. Ecol. Conserv. 2021, 31, e01841. [Google Scholar] [CrossRef]
- Baliyarsingh, B.; Dash, B.; Nayak, S.; Nayak, S.K. Soil verrucomicrobia and their role in sustainable agriculture. In Advances in Agricultural and Industrial Microbiology; Microbial Diversity and Application in Agroindustry; Springer: Berlin/Heidelberg, Germany, 2022; Volume 1, pp. 105–124. [Google Scholar]
- Dash, B.; Nayak, S.; Pahari, A.; Nayak, S.K. Verrucomicrobia in soil: An agricultural perspective. In Frontiers in Soil and Environmental Microbiology; CRC Press: Boca Raton, FL, USA, 2020; pp. 37–46. [Google Scholar]
- Aislabie, J.; Deslippe, J.R.; Dymond, J. Soil microbes and their contribution to soil services. In Ecosystem Services in New Zealand; Conditions and Trends Manaaki Whenua Press: Lincoln, New Zealand, 2013; Volume 1, pp. 143–161. [Google Scholar]
- Murindangabo, Y.T.; Kopecký, M.; Perná, K.; Nguyen, T.G.; Konvalina, P.; Kavková, M. Prominent use of lactic acid bacteria in soil-plant systems. Appl. Soil Ecol. 2023, 189, 104955. [Google Scholar] [CrossRef]
- Crits-Christoph, A.; Robinson, C.K.; Barnum, T.; Fricke, W.F.; Davila, A.F.; Jedynak, B.; McKay, C.P.; DiRuggiero, J. Colonization patterns of soil microbial communities in the Atacama Desert. Microbiome 2013, 1, 28. [Google Scholar] [CrossRef] [PubMed]
- Bachar, A.; Soares, M.I.M.; Gillor, O. The effect of resource islands on abundance and diversity of bacteria in arid soils. Microb. Ecol. 2012, 63, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, Y.; Valverde, A.; Pierneef, R.E.; Cowan, D.A. Differences in Precipitation Regime Shape Microbial Community Composition and Functional Potential in Namib Desert Soils. Microb. Ecol. 2022, 83, 689–701. [Google Scholar] [CrossRef]
- Salinger, M.J.; Baldi, M.; Grifoni, D.; Jones, G.; Bartolini, G.; Cecchi, S.; Messeri, G.; Dalla Marta, A.; Orlandini, S.; Dalu, G.A.; et al. Seasonal differences in climate in the Chianti region of Tuscany and the relationship to vintage wine quality. Int. J. Biometeorol. 2015, 59, 1799–1811. [Google Scholar] [CrossRef]
- Mocali, S.; Kuramae, E.E.; Kowalchuk, G.A.; Fornasier, F.; Priori, S. Microbial functional diversity in vineyard soils: Sulfur metabolism and links with grapevine plants and wine quality. Front. Environ. Sci. 2020, 8, 75. [Google Scholar] [CrossRef]
- Noisangiam, R.; Nuntagij, A.; Pongsilp, N.; Boonkerd, N.; Denduangboripant, J.; Ronson, C.; Teaumroong, N. Heavy metal tolerant Metalliresistens boonkerdii gen. nov., sp. nov., a new genus in the family Bradyrhizobiaceae isolated from soil in Thailand. Syst. Appl. Microbiol. 2010, 33, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, M.; Sun, X.; Wang, Y.; Yuan, C.; Sun, H. Nationwide distribution of polycyclic aromatic hydrocarbons in soil of China and the association with bacterial community. J. Environ. Sci. 2023, 128, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Avontuur, J.R.; Wilken, P.M.; Palmer, M.; Coetzee, M.P.A.; Stępkowski, T.; Venter, S.N.; Steenkamp, E.T. Complex evolutionary history of photosynthesis in Bradyrhizobium. Microb. Genom. 2023, 9, 001105. [Google Scholar] [CrossRef] [PubMed]
- Certini, G.; Campbell, C.D.; Edwards, A.C. Rock fragments in soil support a different microbial community from the fine earth. Soil Biology and Biochemistry 2004, 36, 1119–1128. [Google Scholar] [CrossRef]
- Huang, L.; Bao, W.; Hu, H.; Eissenstat, D.M.; Li, F. Influences of rock fragment content and vegetation on soil microbial communities. Catena 2023, 225, 107018. [Google Scholar] [CrossRef]
- Willms, I.M.; Bolz, S.H.; Yuan, J.; Krafft, L.; Schneider, D.; Schöning, I.; Schrumpf, M.; Nacke, H. The ubiquitous soil verrucomicrobial clade ‘Candidatus Udaeobacter’ shows preferences for acidic pH. Environ. Microbiol. Rep. 2021, 13, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Finley, B.K.; Mau, R.L.; Hayer, M.; Stone, B.W.; Morrissey, E.M.; Koch, B.J.; Rasmussen, C.; Dijkstra, P.; Schwartz, E.; Hungate, B.A. Soil minerals affect taxon-specific bacterial growth. ISME J. 2022, 16, 1318–1326. [Google Scholar] [CrossRef]
- Mikutta, R.; Kleber, M.; Jahn, R. Poorly crystalline minerals protect organic carbon in clay subfractions from acid subsoil horizons. Geoderma 2005, 128, 106–115. [Google Scholar] [CrossRef]
- Lal, R. Digging deeper: A holistic perspective of factors affecting soil organic carbon sequestration in agroecosystems. Glob. Chang. Biol. 2018, 24, 3285–3301. [Google Scholar] [CrossRef]
- Hamer, U.; Marschner, B. Priming effects in different soil types induced by fructose, alanine, oxalic acid and catechol additions. Soil Biol. Biochem. 2005, 37, 445–454. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Aguilera-Huertas, J.; Lozano-García, B.; González-Rosado, M.; Parras-Alcántara, L. Effects of management and hillside position on soil organic carbon stratification in Mediterranean centenary olive grove. Agronomy 2021, 11, 650. [Google Scholar] [CrossRef]
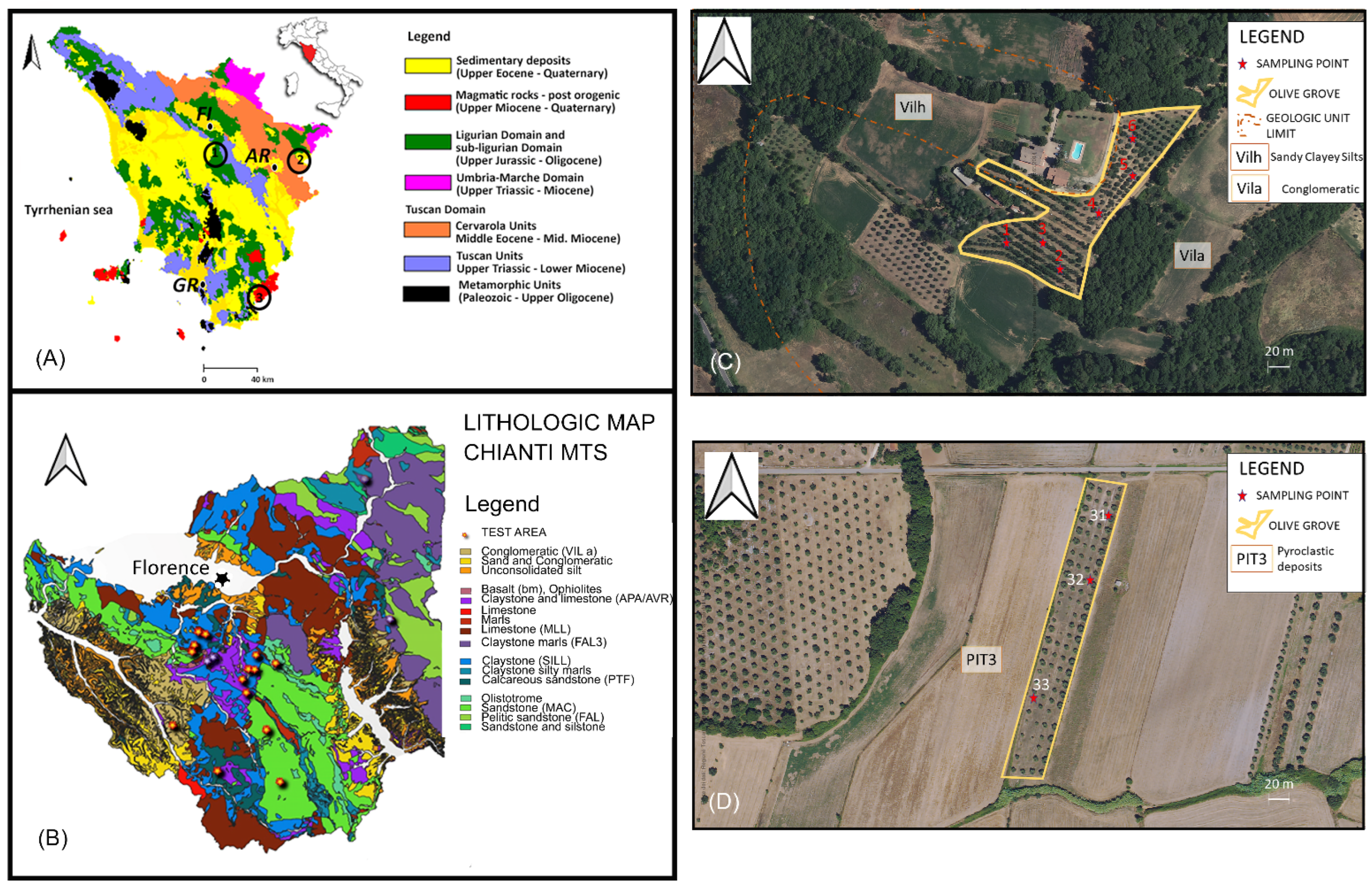
| Landform | Site Location | Zone | Soil Samples | Soil Classification WRB | Lithology | Aspect | Sand (2 mm) | Silt (50 µm) | Clay (2 µm) | pH | SOM (%) | ΣREE [mg/kg] | (La/Yb)n | (La/Sm)n | (La/Gd)n | (Gd/Yb)n | (Pr/Ce)n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low gradient slope | Tosteto–Pitigliano | Maremma | 31-32-33 | Eutric Andosols | Pyroclastic deposits (Vulc–PIT3) | SE | 80.6 | 1.3 | 18.0 | 6.28 | 1.2 | 69.1 | 2.45 | 0.95 | 1.02 | 2.39 | 0.96 |
| Graded | Faggeto–Anghiari (Arezzo) | Alta Valtiberina | 1-2-3 | Calcari Epileptic Cambisols | Fluvial-lacustrine deposits (VILa1) | NE | 46.1 | 42.6 | 11.3 | 7.41 | 1.5 | 13.5 | 0.56 | 0.40 | 0.27 | 2.0 | 0.87 |
| Faggeto–Anghiari | Alta Valtiberina | 4-5-6 | Calcari Epileptic Cambisols | Fluvial-lacustrine deposits (VILa2) | NW | 46.1 | 42.6 | 11.3 | 7.41 | 1.5 | 13.5 | 0.56 | 0.40 | 0.27 | 2.0 | 0.87 | |
| Lamole-Greve in Chianti | Chianti | 46-47-48 | Eutric Cambisols | Macigno Formation (MAC) | S-SW | 76.8 | 15.1 | 8.1 | 7.46 | 0.5 | 21.9 | 1.51 | 0.82 | 0.74 | 2.03 | 0.74 | |
| Midslope ridges | Torriano–Montefiridolfi, San Casciano | Chianti | 13-14-15 | Endoskeleti Calcaric Cambisols | Fluvial deposits (VILa3) | NW | 52.6 | 27.1 | 20.3 | 7.97 | 2.2 | 7.0 | 0.37 | 0.41 | 0.24 | 1.5 | 0.85 |
| Pruneti–Chiocchio, | Chianti | 10-11-12 | Calcari Endoleptic Cambisols | Palombini Shales (APAa) | NE | 26.4 | 42.4 | 31.2 | 8.15 | 1.6 | 2.1 | 0.36 | 0.19 | 0.11 | 3.30 | 2.80 | |
| Pruneti-S. Polo in Chianti | Chianti | 7-8-9 | Calcari Endoleptic Cambisols | San Polo Marls (Marne) | SE | 64.0 | 18.5 | 17.5 | 7.59 | 2.1 | 4.0 | 0.45 | 0.20 | 0.14 | 3.31 | 0.78 | |
| Upper slopes | Rignana-Greve in Chianti | Chianti | 40-41-42 | Eutri Epileptic Regosol | Pietraforte Formation (PTF) | S-SW | 39.8 | 51.8 | 8.4 | 7.78 | 1.2 | 2.0 | 0.48 | 0.17 | 0.13 | 3.74 | 1.18 |
| Rignana-Greve in Chianti | Chianti | 43-44-45 | Calcaric Regosols | Varicolori Shales (AVR2) | S-SW | 29.5 | 66.0 | 4.5 | 7.62 | 1.3 | 5.9 | 0.54 | 0.23 | 0.17 | 3.10 | 0.90 | |
| Castel Ruggero Monta Taurina | Chianti | 37-38-39 | Calcaric Regosols | Claystone (SIL2) | S-SW | 33.1 | 42.6 | 24.3 | 7.95 | 1.1 | 3.2 | 0.47 | 0.10 | 0.10 | 0.17 | 3.50 | |
| Monteoriolo, Impruneta | Chianti | 28-28-30 | Calcaric Regosols | Claystone (SIL1) | E | 61.3 | 22.5 | 16.2 | 7.05 | 2.6 | 6.9 | 0.68 | 0.34 | 0.34 | 0.09 | 1.22 | |
| Pruneti-I Tinti, Strada in Chianti | Chianti | 16-17-18 | Calcari Endoleptic Cambisols | Basalts (bm) | N-NW | 61.9 | 31.5 | 6.6 | 7.90 | 2.3 | 1.2 | 0.33 | 0.46 | 0.27 | 1.23 | 0.95 | |
| Open slopes | Pruneti–Lizzano | Chianti | 22-23-24 | Calcaric Regosols | Monte Morello Formation (MLL1) | N-NW | 36.7 | 38.9 | 24.4 | 8.07 | 1.2 | 2.5 | 0.81 | 0.32 | 0.26 | 3.2 | 0.93 |
| La Querce-Impruneta | Chianti | 25-26-27 | Calcaric Regosols | Monte Morello Formation (MLL2) | W | 22.7 | 68.4 | 8.9 | 8.12 | 1.1 | 2.4 | 0.65 | 0.50 | 0.23 | 2.9 | 0.86 | |
| Erta di Quintole-Impruneta | Chianti | 19-20-21 | Endoskeleti Calcaric Cambisols | Carbonatic flysch (Fcar) | S | 57.6 | 22.4 | 20.0 | 7.54 | 2.8 | 3.5 | 0.51 | 0.38 | 0.26 | 1.98 | 0.77 | |
| Castel Ruggero Poggio Fontaccia | Chianti | 34-35-36 | Calcaric Regosols | Varicolori Shales (AVR1) | S-SW | 34.5 | 51.3 | 14.2 | 8.09 | 1.1 | 2.8 | 0.68 | 0.31 | 0.18 | 3.0 | 1.30 |
| Pair | Pearson’s r | Fisher 95% CI | p-Value | |
|---|---|---|---|---|
| Sand, REE | 0.614 | 0.148 | to 0.857 | 0.0149 |
| Silt, REE | −0.591 | 0.847 | to −0.113 | 0.0203 |
| Clay, REE | −0.013 | 0.522 | to 0.503 | 0.9643 |
| Sand, (La/Yb)n | 0.587 | 0.107 | to 0.845 | 0.0214 |
| Sand, (La/Sm)n | 0.675 | 0.250 | to 0.882 | 0.0057 |
| Sand, (La/Gd)n | 0.741 | 0.369 | to 0.909 | 0.0016 |
| Clay, (Pr/Ce)n | 0.584 | 0.102 | to 0.844 | 0.0223 |
| Silt, (La/Yb)n | −0.547 | 0.827 | to −0.048 | 0.0348 |
| Silt, (La/Sm)n | −0.552 | 0.829 | to −0.055 | 0.0330 |
| Silt, (La/Gd)n | −0.665 | 0.878 | to −0.055 | 0.0069 |
| pH, (La/Yb)n | −0.755 | 0.914 | to −0.396 | 0.0011 |
| pH, (La/Gd)n | −0.784 | 0.925 | to −0.455 | 0.0005 |
| pH, (Gd/Yb)n | 0.226 | 0.323 | to 0.662 | 0.4173 |
| pH, (Pr/Ce)n | 0.293 | 0.258 | to 0.700 | 0.2888 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roccotelli, A.; Tommasini, S.; Ceccherini, M.T.; Calamai, L.; Ferrari, M.; Ghiotto, M.; Riccio, R.; Bonciani, L.; Pietramellara, G.; Moretti, S.; et al. Rare Earth Elements Distribution and Bacteriome to Assess and Characterize the Soil Landscapes of Old Olive Orchards. Diversity 2024, 16, 427. https://doi.org/10.3390/d16070427
Roccotelli A, Tommasini S, Ceccherini MT, Calamai L, Ferrari M, Ghiotto M, Riccio R, Bonciani L, Pietramellara G, Moretti S, et al. Rare Earth Elements Distribution and Bacteriome to Assess and Characterize the Soil Landscapes of Old Olive Orchards. Diversity. 2024; 16(7):427. https://doi.org/10.3390/d16070427
Chicago/Turabian StyleRoccotelli, Angela, Simone Tommasini, Maria Teresa Ceccherini, Luca Calamai, Mattia Ferrari, Matthias Ghiotto, Roberto Riccio, Lisa Bonciani, Giacomo Pietramellara, Sandro Moretti, and et al. 2024. "Rare Earth Elements Distribution and Bacteriome to Assess and Characterize the Soil Landscapes of Old Olive Orchards" Diversity 16, no. 7: 427. https://doi.org/10.3390/d16070427








