Climate Change Threatens Barringtonia racemosa: Conservation Insights from a MaxEnt Model
Abstract
:1. Introduction
2. Materials and Methods
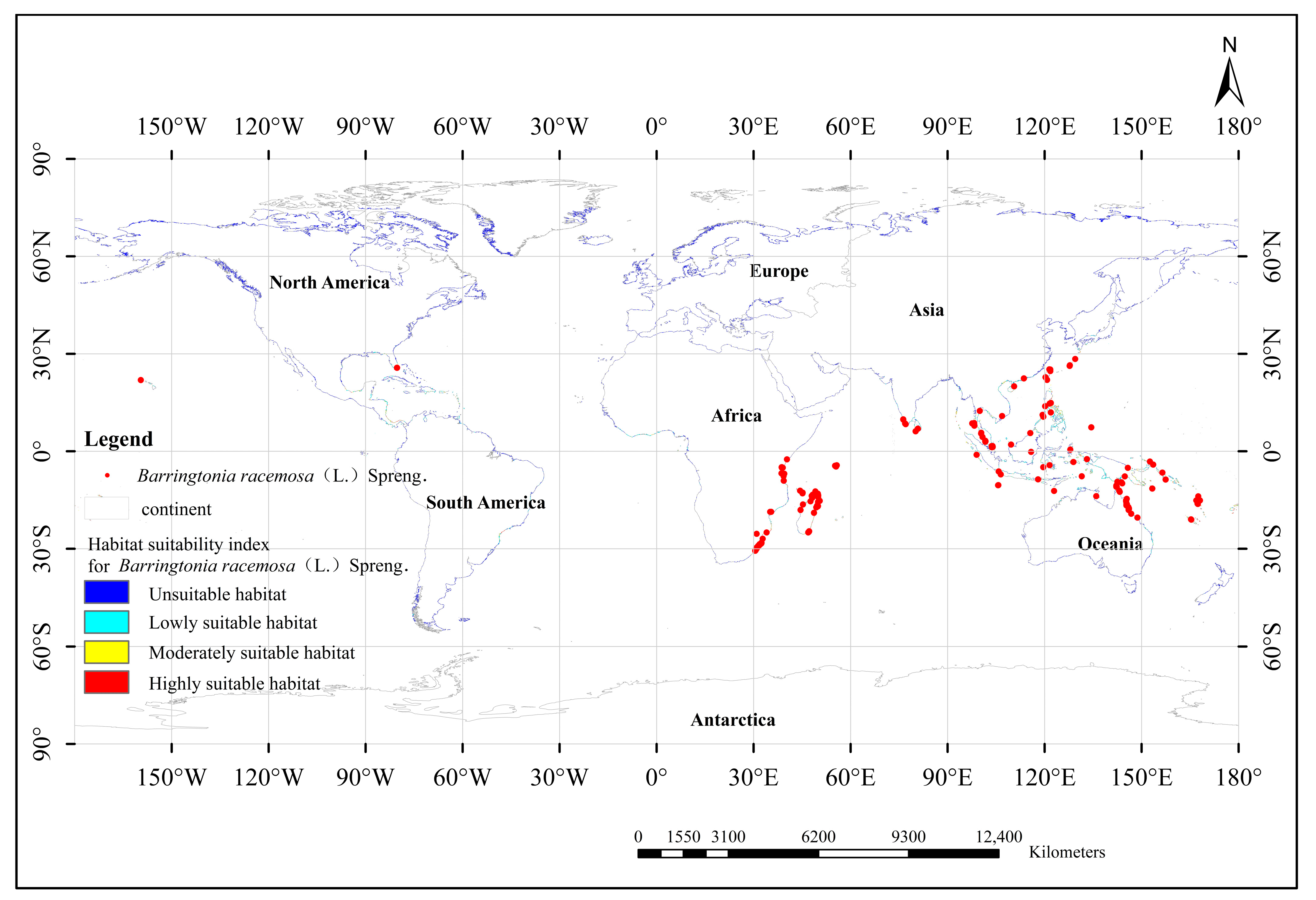
2.1. Data Collection of Barringtonia racemosa Distribution Points
2.2. Environmental Variables
2.3. Environmental Variable Screening
2.4. Species Distribution Modeling, Optimization, and Evaluation
3. Results
3.1. Model Optimization and Accuracy Evaluation Results
3.2. Dominant Environmental Variables of Potential Habitat Distribution of Barringtonia racemosa
3.3. Response of Potential Distribution of Barringtonia racemosa to Main Environmental Variables
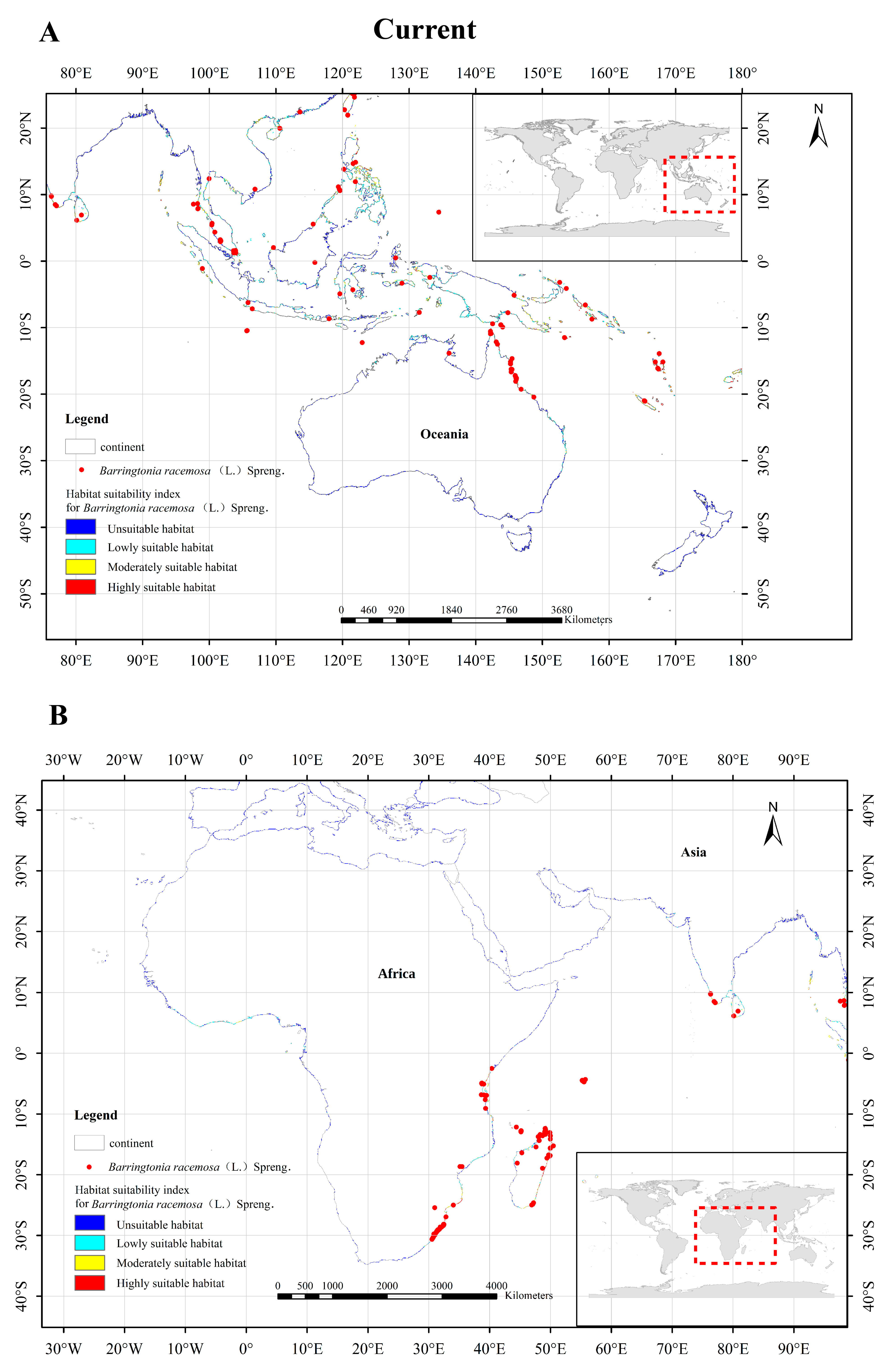
3.4. Distribution of Potential Suitable Growth Areas of Barringtonia racemosa in the Current Climate
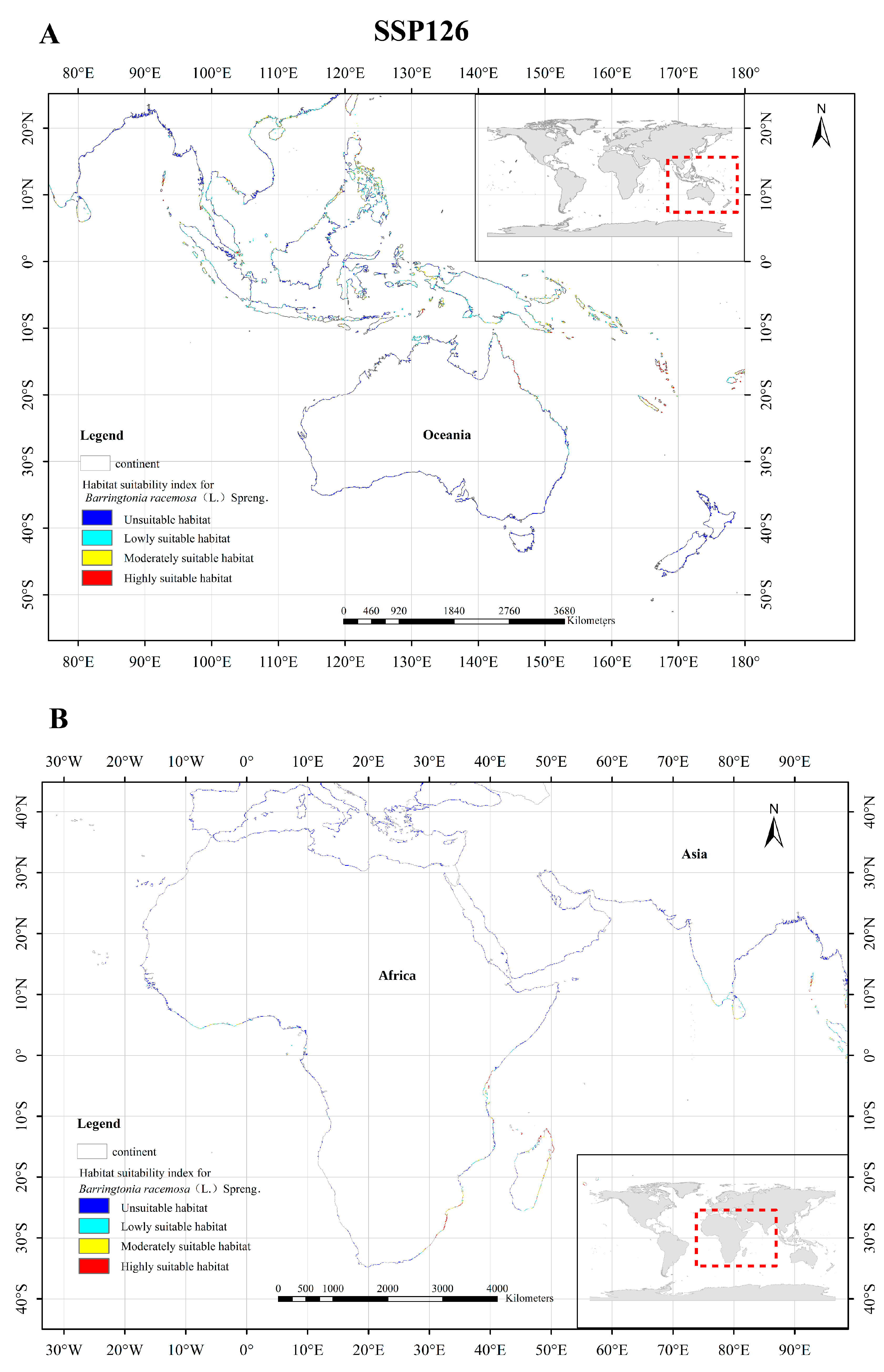
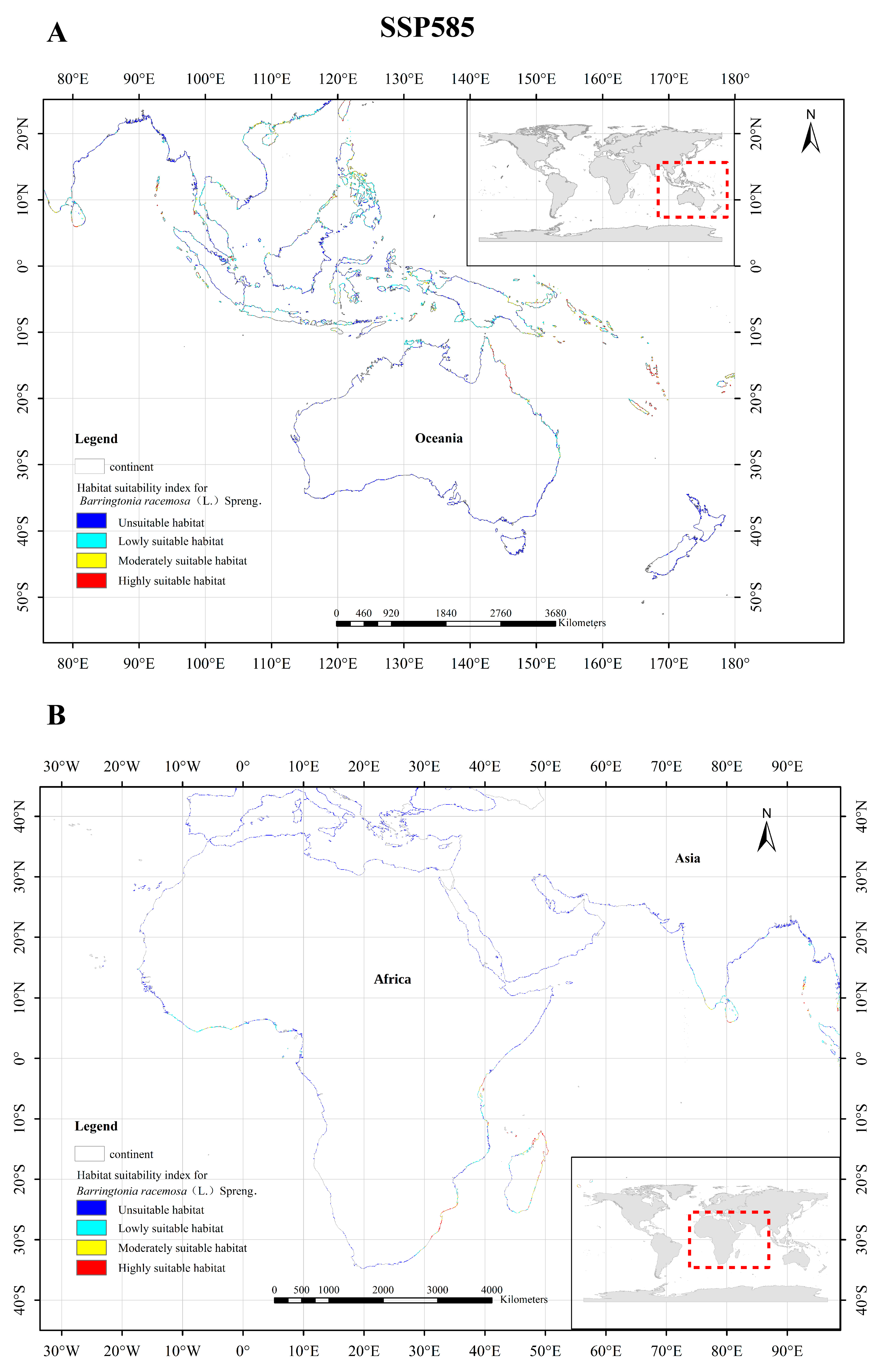
3.5. Changes of Potentially Suitable Zones of Barringtonia racemosa under Different Scenarios
4. Discussion
4.1. Dominant Environmental Factors Influencing the Suitability of Barringtonia racemosa
4.2. Possible Future Distribution of Barringtonia racemosa Based on Varying Climate Scenarios
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linhares, Y.; Kaganski, A.; Agyare, C.; Kurnaz, I.A.; Neergheen, V.; Kolodziejczyk, B.; Kędra, M.; Wahajuddin, M.; El-Youssf, L.; Cruz, T.E.D.; et al. Biodiversity: The overlooked source of human health. Trends Mol. Med. 2023, 29, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Tavilla, G.; Crisafulli, A.; Ranno, V.; Picone, R.M.; Redouan, F.Z.; del Galdo, G.G. First contribution to the ethnobotanical knowledge in the Peloritani Mounts (NE Sicily). Res. J. Ecol. Environ. Sci. 2022, 2, 1–34. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Booth, T.H. Biodiversity and Climate Change Adaptation in Australia: Strategy and Research Developments. Adv. Clim. Chang. Res. 2012, 3, 12–21. [Google Scholar] [CrossRef]
- Sorte, C.J.B.; Ibáñez, I.; Blumenthal, D.M.; Molinari, N.A.; Miller, L.P.; Grosholz, E.D.; Diez, J.M.; D’Antonio, C.M.; Olden, J.D.; Jones, S.J.; et al. Poised to prosper? A cross-system comparison of climate change effects on native and non-native species performance. Ecol. Lett. 2013, 16, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Aroloye, O.N. Mangrove Habitat Loss and the Need for the Establishment of Conservation and Protected Areas in the Niger Delta, Nigeria. In Habitats of the World; Carmelo Maria, M., Ana Cano, O., Ricardo Quinto, C., Eds.; IntechOpen: Rijeka, Croatia, 2019; p. Ch. 4. [Google Scholar]
- Cano, E.; Cano-Ortiz, A.; Veloz, A.; Alatorre, J.; Otero, R. Comparative analysis between the mangrove swamps of the Caribbean and those of the State of Guerrero (Mexico). Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2012, 146, 112–130. [Google Scholar] [CrossRef]
- Atwood, T.B.; Connolly, R.M.; Almahasheer, H.; Carnell, P.E.; Duarte, C.M.; Lewis, C.J.E.; Irigoien, X.; Kelleway, J.J.; Lavery, P.S.; Macreadie, P.I.; et al. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Chang. 2017, 7, 523–528. [Google Scholar] [CrossRef]
- Hoekstra, J.M.; Boucher, T.M.; Ricketts, T.H.; Roberts, C. Confronting a biome crisis: Global disparities of habitat loss and protection. Ecol. Lett. 2004, 8, 23–29. [Google Scholar] [CrossRef]
- Graham, E.M.; Reside, A.E.; Atkinson, I.; Baird, D.; Hodgson, L.; James, C.S.; VanDerWal, J.J. Climate change and biodiversity in Australia: A systematic modelling approach to nationwide species distributions. Australas. J. Environ. Manag. 2019, 26, 112–123. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, G. Predicting the Potential Distribution of Endangered Parrotia subaequalis in China. Forests 2022, 13, 1595. [Google Scholar] [CrossRef]
- Eiserhardt, W.L.; Svenning, J.-C.; Kissling, W.D.; Balslev, H. Geographical ecology of the palms (Arecaceae): Determinants of diversity and distributions across spatial scales. Ann. Bot. 2011, 108, 1391–1416. [Google Scholar] [CrossRef] [PubMed]
- Wasowicz, P.; Przedpelska-Wasowicz, E.M.; Kristinsson, H. Alien vascular plants in Iceland: Diversity, spatial patterns, temporal trends, and the impact of climate change. Flora 2013, 208, 648–673. [Google Scholar] [CrossRef]
- Lippmann, R.; Babben, S.; Menger, A.; Delker, C.; Quint, M. Development of Wild and Cultivated Plants under Global Warming Conditions. Curr. Biol. 2019, 29, R1326–R1338. [Google Scholar] [CrossRef] [PubMed]
- Selwood, K.E.; McGeoch, M.A.; Mac Nally, R. The effects of climate change and land-use change on demographic rates and population viability. Biol. Rev. 2015, 90, 837–853. [Google Scholar] [CrossRef] [PubMed]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Rai, I.D.; Singh, B.; Dutt, H.C.; Musarella, C.M. Predicting the suitable habitats of Elwendia persica in the Indian Himalayan Region (IHR). Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2023, 157, 769–778. [Google Scholar] [CrossRef]
- Nan, Q.; Li, C.; Li, X.; Zheng, D.; Li, Z.; Zhao, L. Modeling the Potential Distribution Patterns of the Invasive Plant Species Phytolacca americana in China in Response to Climate Change. Plants 2024, 13, 1082. [Google Scholar] [CrossRef]
- Kamyo, T.; Asanok, L. Modeling habitat suitability of Dipterocarpus alatus (Dipterocarpaceae) using MaxEnt along the Chao Phraya River in Central Thailand. For. Sci. Technol. 2020, 16, 1–7. [Google Scholar] [CrossRef]
- Abdelaal, M.; Fois, M.; Fenu, G.; Bacchetta, G. Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecol. Inform. 2019, 50, 68–75. [Google Scholar] [CrossRef]
- He, K.; Fan, C.; Zhong, M.; Cao, F.; Wang, G.; Cao, L. Evaluation of Habitat Suitability for Asian Elephants in Sipsongpanna under Climate Change by Coupling Multi-Source Remote Sensing Products with MaxEnt Model. Remote. Sens. 2023, 15, 1047. [Google Scholar] [CrossRef]
- Wang, Y.; Chao, B.; Dong, P.; Zhang, D.; Yu, W.; Hu, W.; Ma, Z.; Chen, G.; Liu, Z.; Chen, B. Simulating spatial change of mangrove habitat under the impact of coastal land use: Coupling MaxEnt and Dyna-CLUE models. Sci. Total Environ. 2021, 788, 147914. [Google Scholar] [CrossRef] [PubMed]
- Mousazade, M.; Ghanbarian, G.; Pourghasemi, H.R.; Safaeian, R.; Cerdà, A. Maxent Data Mining Technique and Its Comparison with a Bivariate Statistical Model for Predicting the Potential Distribution of Astragalus Fasciculifolius Boiss. in Fars, Iran. Sustainability 2019, 11, 3452. [Google Scholar] [CrossRef]
- Li, L.; Liu, W.; Ai, J.; Cai, S.; Dong, J. Predicting Mangrove Distributions in the Beibu Gulf, Guangxi, China, Using the MaxEnt Model: Determining Tree Species Selection. Forests 2023, 14, 149. [Google Scholar] [CrossRef]
- Çoban, H.O.; Örücü, Ö.K.; Arslan, E.S. MaxEnt Modeling for Predicting the Current and Future Potential Geographical Distribution of Quercus libani Olivier. Sustainability 2020, 12, 2671. [Google Scholar] [CrossRef]
- Li, M.; Li, Q.; Song, J.; Wang, Z.; Wang, Y.; Hu, M. Prediction of potential distribution areas of Chinese horseshoe crab and mangrove horseshoe crab in the Beibu Gulf of Guangxi based on MAXENT model and their population conservation strategies. Acta Ecol. Sin. 2019, 39, 3100–3109. [Google Scholar] [CrossRef]
- Wang, G.; Xie, C.; Wei, L.; Gao, Z.; Yang, H.; Jim, C. Predicting Suitable Habitats for China’s Endangered Plant Handeliodendron bodinieri (H. Lév.) Rehder. Diversity 2023, 15, 1033. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, W.-J.; Zhu, Y.-Z.; Cao, Y.-T.; Yang, X.-M.; Geng, Y.; Zhang, F.-J.; Sun, R.-Q.; Jia, R.-W.; Yan, C.-L.; et al. Impacts of Climate Changes on Geographic Distribution of Primula filchnerae, an Endangered Herb in China. Plants 2023, 12, 3561. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Luo, P.; Yi, H.; Yang, X.; Zhang, L.; Zeng, D.; Wang, L. Predicting Habitat Suitability and Adaptation Strategies of an Endangered Endemic Species, Camellia luteoflora Li ex Chang (Ericales: Theaceae) under Future Climate Change. Forests 2023, 14, 2177. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Y.; Zhang, D.; Yu, W.; Chen, G.; Xie, T.; Liu, Z.; Ma, Z.; Du, J.; Chao, B.; et al. Mapping the potential of mangrove forest restoration based on species distribution models: A case study in China. Sci. Total Environ. 2020, 748, 142321. [Google Scholar] [CrossRef]
- Cui, L.; Berger, U.; Cao, M.; Zhang, Y.; He, J.; Pan, L.; Jiang, J. Conservation and Restoration of Mangroves in Response to Invasion of Spartina alterniflora Based on the MaxEnt Model: A Case Study in China. Forests 2023, 14, 1220. [Google Scholar] [CrossRef]
- Ying, B.; Tian, K.; Guo, H.; Yang, X.; Li, W.; Li, Q.; Luo, Y.C.; Zhang, X.M. Predicting potential suitable habitats of Kandelia obovata in China under future climatic scenarios based on MaxEnt model. Acta Ecol. Sin. 2024, 44, 224–234. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, P.; Hu, W.; Chen, G.; Zhang, D.; Chen, B.; Lei, G. Modeling the Climate Suitability of Northernmost Mangroves in China under Climate Change Scenarios. Forests 2022, 13, 64. [Google Scholar] [CrossRef]
- Jayathilake, D.R.; Costello, M.J. A modelled global distribution of the seagrass biome. Biol. Conserv. 2018, 226, 120–126. [Google Scholar] [CrossRef]
- Zellmer, A.J.; Claisse, J.T.; Williams, C.M.; Schwab, S.; Pondella, D.J. Predicting Optimal Sites for Ecosystem Restoration Using Stacked-Species Distribution Modeling. Front. Mar. Sci. 2019, 6, 3. [Google Scholar] [CrossRef]
- Aluri, J.S.R.; Palathoti, S.R.; Banisetti, D.K.; Samareddy, S.K. Pollination Ecology Characteristics of Barringtonia racemosa (L.) Spreng. (Lecythidaceae). Transylv. Rev. Syst. Ecol. Res. 2019, 21, 27–34. [Google Scholar] [CrossRef]
- Chantaranothai, P. Barringtonia (Lecythidaceae) in Thailand. Kew Bull. 1995, 50, 677. [Google Scholar] [CrossRef]
- Umaru, I.J.; Ahmed, F.B.; Umaru, H.A.; Umaru, K.I. Barringtonia racemosa: Phytochemical, pharmacological, biotechnological, botanical, traditional use and agronomical aspects. World J. Pharm. Pharm. Sci. 2018, 7, 78–121. [Google Scholar] [CrossRef]
- Lim, T.K. Barringtonia racemosa. In Edible Medicinal And Non Medicinal Plants: Volume 3, Fruits; Lim, T.K., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 114–121. [Google Scholar]
- Global Biodiversity Information Facility. Global Biodiversity Information Facility (GBIF) Occurrence Download. 2023. Available online: https://www.gbif.org/occurrence/download/0173717-210914110416597 (accessed on 29 October 2023).
- Chinese Virtual Herbarium. Chinese Virtual Herbarium(CVH) Occurrence Download. 2023. Available online: https://www.cvh.ac.cn/ (accessed on 2 November 2023).
- Royal Horticultural Society. The Royal Horticultural Society (RHS) Occurrence Download. 2023. Available online: https://www.rhs.org.uk/ (accessed on 2 November 2023).
- Yang, F.; Ai, X.; Wang, C.; Cheng, L.; Feng, Q.; Sheng, N. Investigation on the Population Status of Rare and Endangered Tree Species Barringtonia racemosa in Liuniutan Wetland, Zhanjiang. Trop. For. 2023, 51, 66–69. [Google Scholar] [CrossRef]
- WorldClim, Maps, Graphs, Tables, and DATA of the Globla Climate. Available online: http://www.worldclim.org/ (accessed on 16 July 2020).
- World Soil Information. World Soil Information (ISRIC) Occurrence Download. 2023. Available online: https://data.isric.org/ (accessed on 29 October 2023).
- Bio-ORACLE. Bio-ORACLE Global Marine Life Model Environment Database Occurrence Download. 2023. Available online: https://www.bio-oracle.org/ (accessed on 27 October 2023).
- National Centers for Environmental Information. National Geophysical Data Center (NGDC) Occurrence Download. 2023. Available online: https://www.ncei.noaa.gov/products/etopo-global-relief-model (accessed on 17 November 2023).
- Hou, J.; Xiang, J.; Li, D.; Liu, X. Prediction of Potential Suitable Distribution Areas of Quasipaa spinosa in China Based on MaxEnt Optimization Model. Biology 2023, 12, 366. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- O’neill, B.C.; Carter, T.R.; Ebi, K.; Harrison, P.A.; Kemp-Benedict, E.; Kok, K.; Kriegler, E.; Preston, B.L.; Riahi, K.; Sillmann, J.; et al. Achievements and needs for the climate change scenario framework. Nat. Clim. Chang. 2020, 10, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Wang, C.; Li, Z.; Mihara, K.; Kuramochi, K.; Toma, Y.; Hatano, R. Climate change multi-model projections in CMIP6 scenarios in Central Hokkaido, Japan. Sci. Rep. 2023, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Ebi, K.L.; Hallegatte, S.; Kram, T.; Arnell, N.W.; Carter, T.R.; Edmonds, J.; Kriegler, E.; Mathur, R.; O’Neill, B.C.; Riahi, K.; et al. A new scenario framework for climate change research: Background, process, and future directions. Clim. Chang. 2014, 122, 363–372. [Google Scholar] [CrossRef]
- Guo, L.; Guo, Z.; Jia, H.; Fan, R. Residents electric larceny detection based on Pearson correlation coefficient and SVM. J. Hebei Univ. 2023, 43, 357–363. [Google Scholar] [CrossRef]
- Wang, R.; Yang, H.; Luo, W.; Wang, M.; Lu, X.; Huang, T.; Zhao, J.; Li, Q. Predicting the potential distribution of the Asian citrus psyllid, Diaphorina citri(Kuwayama), in China using the MaxEnt model. PeerJ 2019, 7, e7323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Qiao, H. Effect of the Maxent model’s complexity on the prediction of species potential distributions. Biodivers. Sci. 2016, 24, 1189–1196. [Google Scholar] [CrossRef]
- Cobos, M.E.; Townsend Peterson, A.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Wang, R.; Li, Q.; Wang, M. Climatic suitability regionalization of Actinidia chinensis in China. Acta Agric. Zhejiangensis 2018, 30, 1504–1512. [Google Scholar] [CrossRef]
- Merckx, B.; Steyaert, M.; Vanreusel, A.; Vincx, M.; Vanaverbeke, J. Null models reveal preferential sampling, spatial autocorrelation and overfitting in habitat suitability modelling. Ecol. Model. 2011, 222, 588–597. [Google Scholar] [CrossRef]
- North, M.A. A Method for Implementing a Statistically Significant Number of Data Classes in the Jenks Algorithm. In Proceedings of the 2009 Sixth International Conference on Fuzzy Systems and Knowledge Discovery, Tianjin, China, 14–16 August 2009; Volume 1, pp. 35–38. [Google Scholar]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2013, 41, 629–643. [Google Scholar] [CrossRef]
- Zhang, Q.; Sui, S.; Zhang, Y.; Yu, H.; Sun, Z.; Wen, X. Marine environmental indexes related to mangrove growth. Acta Ecol. Sin. 2001, 21, 1427–1437. [Google Scholar] [CrossRef]
- Wang, Y. Impacts, challenges and opportunities of global climate change on mangrove ecosystems. J. Trop. Oceanogr. 2021, 40, 1–14. [Google Scholar] [CrossRef]
- Easterling, D.R.; Horton, B.; Jones, P.D.; Peterson, T.C.; Karl, T.R.; Parker, D.E.; Salinger, M.J.; Razuvayev, V.; Plummer, N.; Jamason, P.; et al. Maximum and Minimum Temperature Trends for the Globe. Science 1997, 277, 364–367. [Google Scholar] [CrossRef]
- Davy, R.; Esau, I.; Chernokulsky, A.; Outten, S.; Zilitinkevich, S. Diurnal asymmetry to the observed global warming. Int. J. Climatol. 2017, 37, 79–93. [Google Scholar] [CrossRef]
- Xu, L.; Myneni, R.B.; Iii, F.S.C.; Callaghan, T.V.; Pinzon, J.E.; Tucker, C.J.; Zhu, Z.; Bi, J.; Ciais, P.; Tømmervik, H.; et al. Temperature and vegetation seasonality diminishment over northern lands. Nat. Clim. Chang. 2013, 3, 581–586. [Google Scholar] [CrossRef]
- Xia, J.; Chen, J.; Piao, S.; Ciais, P.; Luo, Y.; Wan, S. Terrestrial carbon cycle affected by non-uniform climate warming. Nat. Geosci. 2014, 7, 173–180. [Google Scholar] [CrossRef]
- Tan, J.; Piao, S.; Chen, A.; Zeng, Z.; Ciais, P.; Janssens, I.A.; Mao, J.; Myneni, R.B.; Peng, S.; Peñuelas, J.; et al. Seasonally different response of photosynthetic activity to daytime and night-time warming in the Northern Hemisphere. Glob. Chang. Biol. 2014, 21, 377–387. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Li, X.; Liang, E.; Beck, P.S.A.; Huang, Y. Seasonal divergence in the interannual responses of Northern Hemisphere vegetation activity to variations in diurnal climate. Sci. Rep. 2016, 6, 19000. [Google Scholar] [CrossRef]
- Cleland, E.E.; Allen, J.M.; Crimmins, T.M.; Dunne, J.A.; Pau, S.; Travers, S.E.; Zavaleta, E.S.; Wolkovich, E.M. Phenological tracking enables positive species responses to climate change. Ecology 2012, 93, 1765–1771. [Google Scholar] [CrossRef]
- Wei, X.; Shi, F.; Fan, J.; Yang, Q. Climate Change Impacts on Marine Lives and Ecosystems. Adv. Mar. Sci. 2011, 29, 241–252. [Google Scholar] [CrossRef]
- Yan, X.; Cai, R.; Guo, H.; Xu, W.; Tan, H. Vulnerability of Hainan Dongzhaigang mangrove ecosystem to the climate change. J. Appl. Oceanogr. 2019, 38, 338–349. [Google Scholar] [CrossRef]
- Ellison, J.C.; Stoddart, D.R. Mangrove ecosystem collapse during predicted sea-level rise: Holocene analogues and implications. J. Coast. Res. 1991, 7, 151–165. [Google Scholar]
- Fang, L.; Pan, Y.J.; Deng, X.; Wu, Y.; Liang, Z.; Zhao, S.; Tan, X. Responses of Barringtonia racemosa to Tidal Flooding. Fujian J. Agric. Sci. 2020, 35, 1346–1356. [Google Scholar] [CrossRef]
- Jagtap, T.G.; Nagle, V.L. Response and Adaptability of Mangrove Habitats from the Indian Subcontinent to Changing Climate. AMBIO 2007, 36, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, Y.; Mo, W.; Fan, H.; Liu, W.; Sun, M.; Xie, M.; Xu, S.M. Climate change and its influence in Beibu Gulf mangrove biome of Guangxi in past 60 years. Acta Ecol. Sin. 2021, 41, 5026–5033. [Google Scholar] [CrossRef]
- Liao, B. The Adaptability of Seedlings of Three Mangrove Species to Tide-Flooding and Water Salinity; Chinese Academy of Forestry: Beijing, China, 2010. [Google Scholar]
- Chen, C.; Wang, W.; Lin, P. Influences of salinity on the growth and some ecophysiological characteristics of mangrove species, Sonneratia apetala seedlings. Chin. Bull. Bot. 2000, 17, 457–461. [Google Scholar]
- Tan, X.H.; Liang, F.; Liu, B.; Deng, X.; Zhao, S.H.; Zeng, Q.Q.; Qu, Z.Y. Effects of salt stress on growth of Barringtonia racemosa and absorption and translocation of mineral elements in it. J. South. Agric. 2021, 52, 1887–1898. [Google Scholar] [CrossRef]
- Liang, F.; Tan, X.H.; Deng, X.; Wu, Y.S.; Wu, M.; Yang, X.C.; Li, J.L. Growth and physiological responses of semi-mangrove plant Barringtonia racemosa to waterlogging and salinity stress. Guihaia 2021, 41, 872–882. [Google Scholar] [CrossRef]
- Cerasoli, F.; D’Alessandro, P.; Biondi, M. Worldclim 2.1 versus Worldclim 1.4: Climatic niche and grid resolution affect between-version mismatches in Habitat Suitability Models predictions across Europe. Ecol. Evol. 2022, 12, e8430. [Google Scholar] [CrossRef]
- Merkenschlager, C.; Bangelesa, F.; Paeth, H.; Hertig, E. Blessing and curse of bioclimatic variables: A comparison of different calculation schemes and datasets for species distribution modeling within the extended Mediterranean area. Ecol. Evol. 2023, 13, e10553. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Chen, Q.; Xu, S.; Chen, H.; Li, W.; Pan, T. The Disturbance Mechanism and Potential Ecological Loss of the Barringtonia racemosa Community on the East Coast of Leizhou Peninsula. J. South China Norm. Univ. 2019, 51, 67–75. [Google Scholar] [CrossRef]
- Zhong, J.; Cheng, X.; Mo, Y.; Li, H.; Chen, Y.; Chen, Y. Dynamic of Barringtonia racemosa polulation in Jiulongshan mangrove national wetland Park, Leizhou. Wetl. Sci. 2018, 16, 231–237. [Google Scholar] [CrossRef]
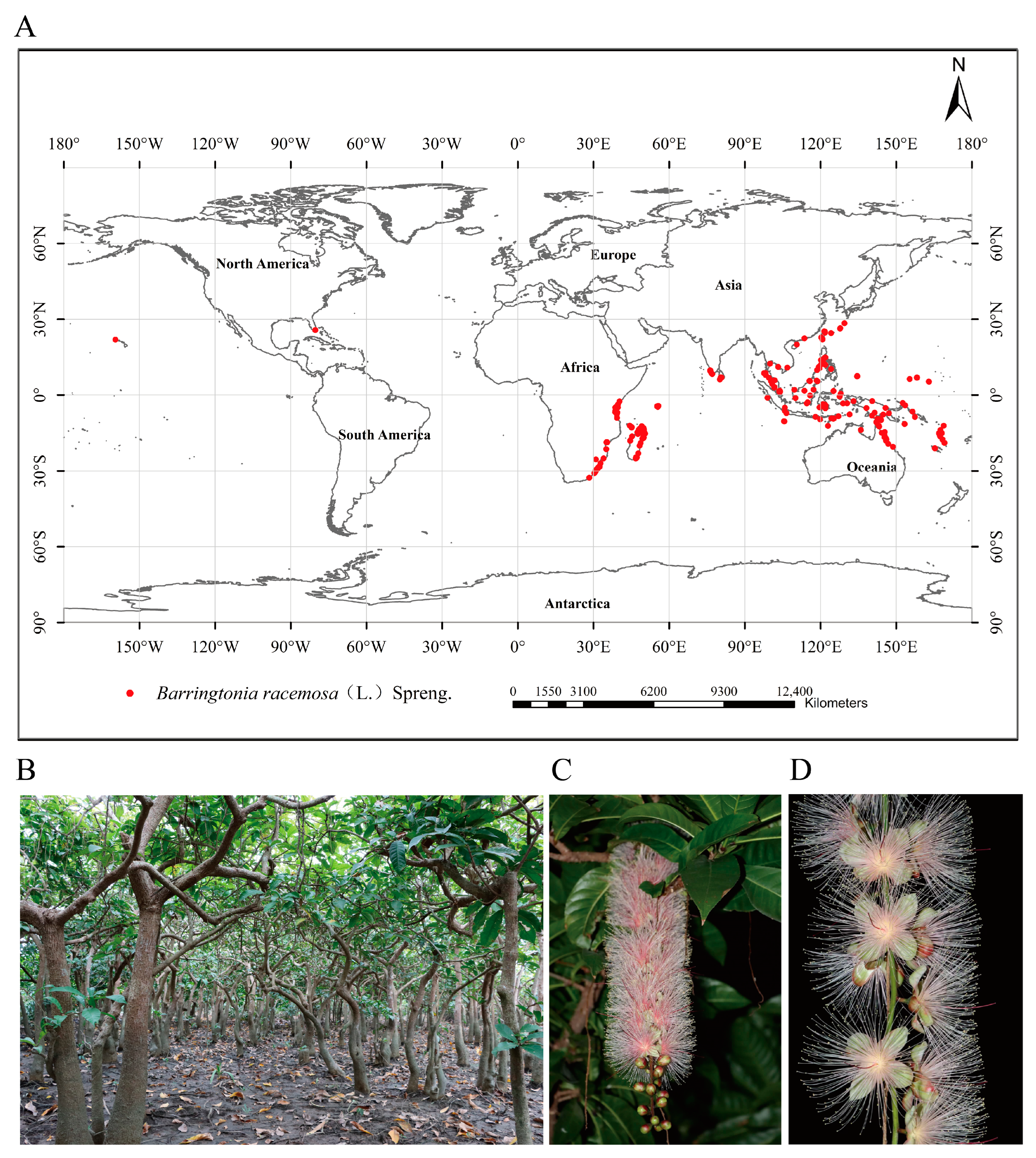
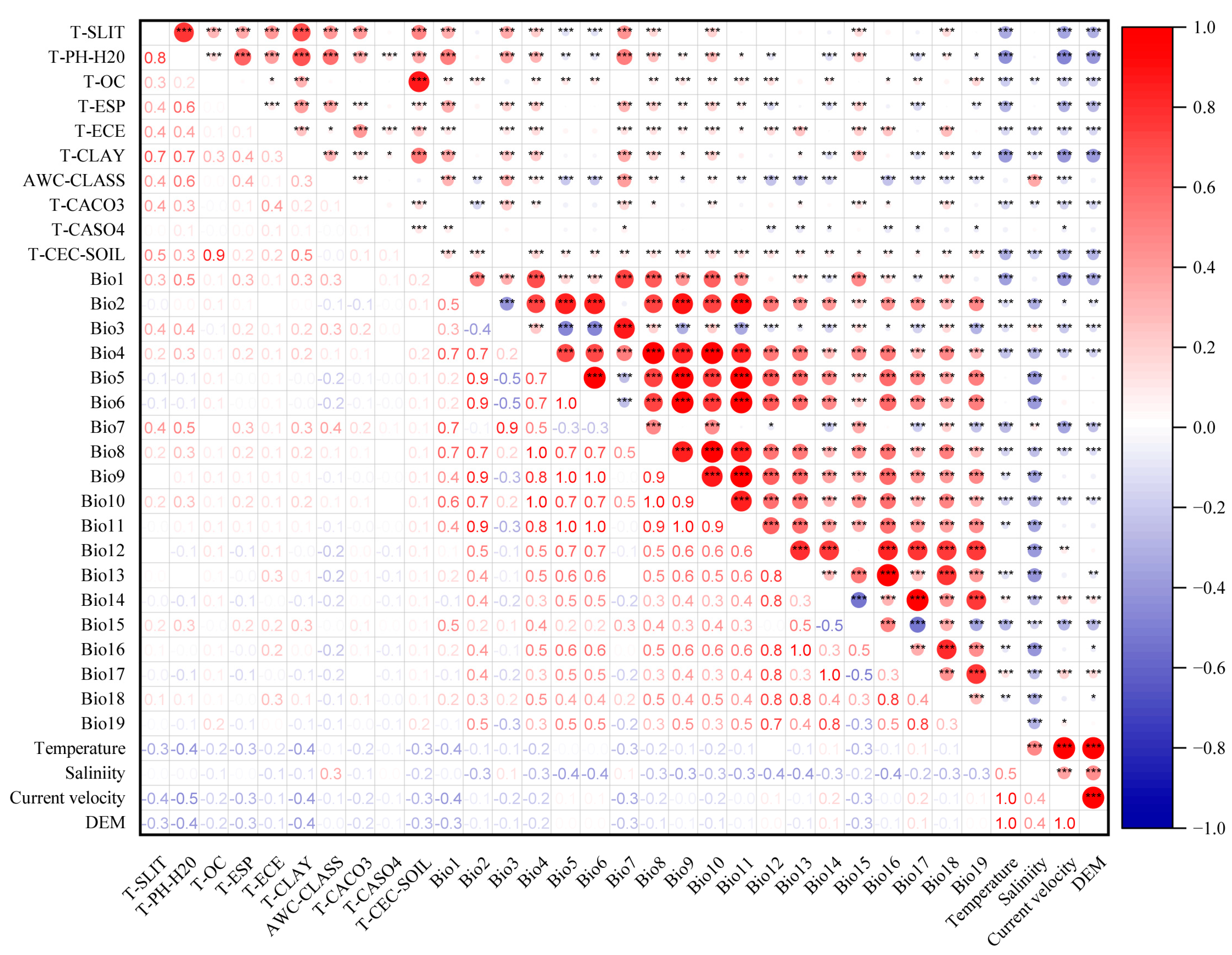
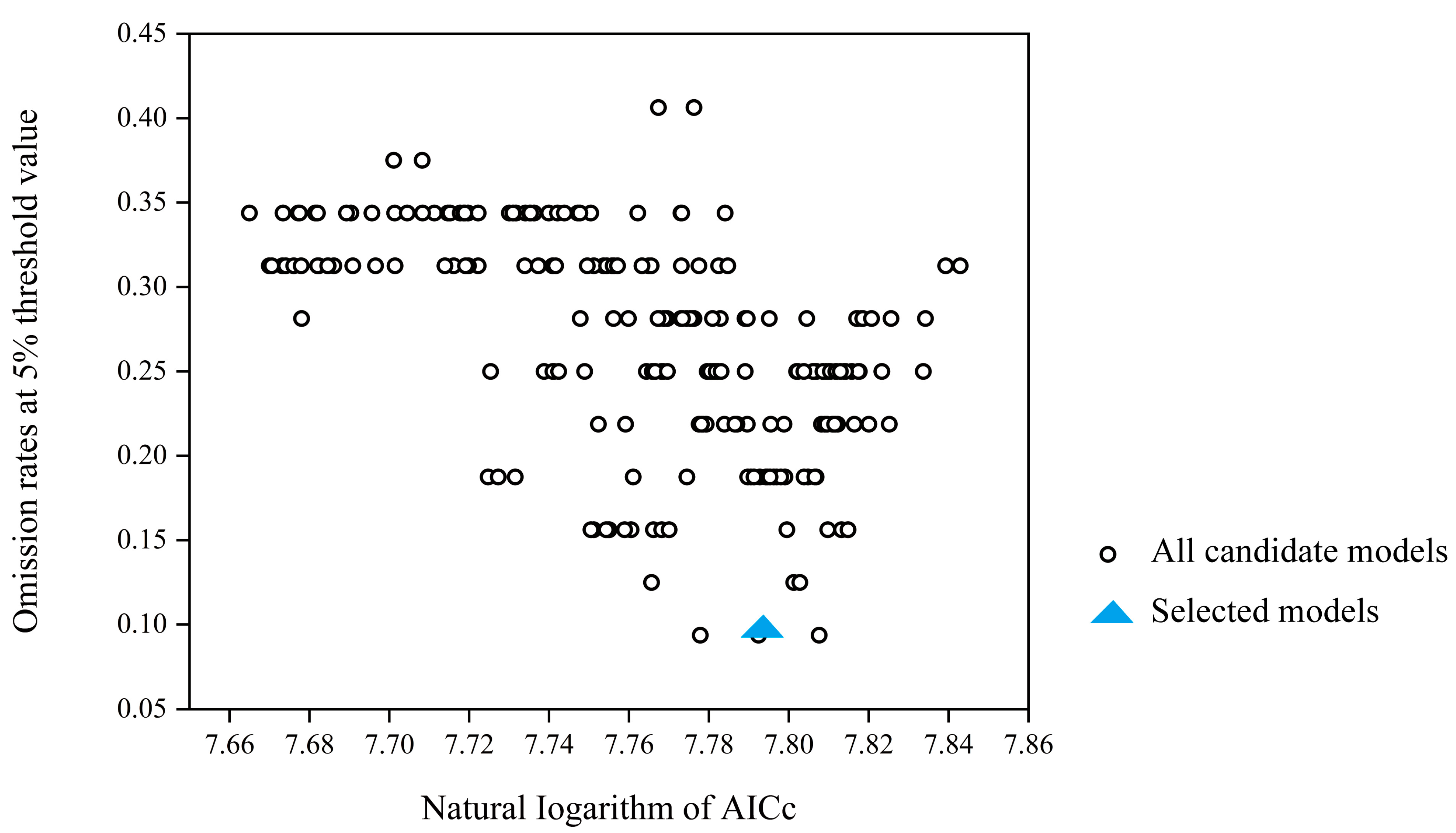

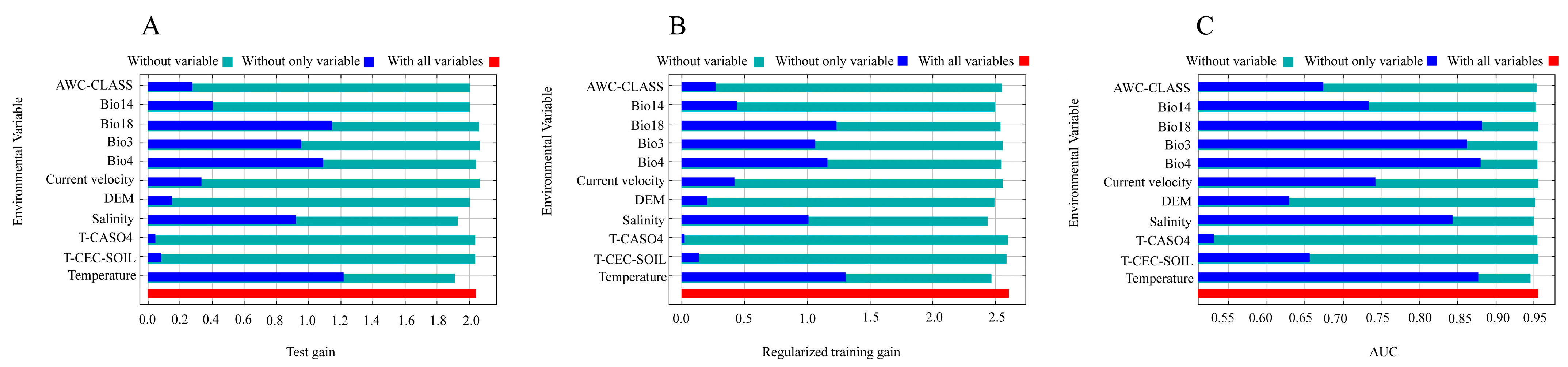


| Variables | Variable Description/Unit | Percent Contribution (PC)/% | Permutation Importance (PI)/% |
|---|---|---|---|
| Bio1 | Annual mean temperature/°C | 0 | 0 |
| Bio2 | Mean diurnal range/°C | 0.3 | 0 |
| Bio3 | Isothermality | 2.1 | 3.1 |
| Bio4 | Seasonal variation coefficient of temperature | 2.7 | 8 |
| Bio5 | Maximum temperature of the warmest month/°C | 0 | 0 |
| Bio6 | Minimum temperature of the coldest month/°C | 0.2 | 0.4 |
| Bio7 | Temperature annual range/°C | 0.5 | 1.2 |
| Bio8 | Mean temperature of the wettest quarter/°C | 0.4 | 1.7 |
| Bio9 | Mean temperature of the driest quarter/°C | 0 | 0 |
| Bio10 | Mean temperature of the warmest quarter/°C | 0 | 0 |
| Bio11 | Mean temperature of the coldest quarter/°C | 0 | 1.7 |
| Bio12 | Annual precipitation/mm | 0 | 0 |
| Bio13 | Precipitation of the wettest month/mm | 0.1 | 1.9 |
| Bio14 | Precipitation of the driest month/mm | 7.7 | 11.1 |
| Bio15 | Precipitation seasonality | 0 | 0.3 |
| Bio16 | Precipitation of the wettest quarter/mm | 0 | 0 |
| Bio17 | Precipitation of the driest quarter/mm | 0.2 | 0.1 |
| Bio18 | Precipitation of the warmest quarter/mm | 20.1 | 8.4 |
| Bio19 | Precipitation of the coldest month/mm | 0.3 | 0.5 |
| AWC-CLASS | Topsoil available water content/% | 1.1 | 2.1 |
| T-ECE | Topsoil conductivity/% | 0.5 | 1 |
| T-ESP | Topsoil exchangeable sodium salt/% | 0 | 0 |
| T-CASO4 | Upper soil sulfate content/% | 2.9 | 3.1 |
| T-CACO3 | Topsoil carbonate or lime content/% | 0 | 0 |
| T-CEC-SOIL | Cation exchange capacity of the topsoil/% | 3.8 | 0.1 |
| T-PH-H2O | Topsoil pH | 0.1 | 1.4 |
| T-CLAY | Clay content in the upper soil/% | 0.9 | 0.1 |
| T-OC | Topsoil organic carbon content/% | 0.5 | 1.1 |
| T-SILT | Upper soil silt content/% | 0.4 | 3.3 |
| Temperature | Average temperature of ocean surface/°C | 49.7 | 39.2 |
| Salinity | Average salinity of ocean surface/‰ | 2.9 | 4.1 |
| Current velocity | Ocean current velocity/ m·s−1 | 1.4 | 0.4 |
| DEM | Altitude/m | 1.1 | 5.6 |
| Sequence Number | Variables | Percent Contribution (PC)/% | Permutation Importance (PI)/% |
|---|---|---|---|
| 1 | Temperature | 49.7 | 39.2 |
| 2 | Bio18 | 20.1 | 8.4 |
| 3 | Bio14 | 7.7 | 11.1 |
| 4 | T-CEC-SOIL | 3.8 | 0.1 |
| 5 | Salinity | 2.9 | 4.1 |
| 6 | T-CASO4 | 2.9 | 3.1 |
| 7 | Bio4 | 2.7 | 8 |
| 8 | Bio3 | 2.1 | 3.1 |
| 9 | Current velocity | 1.4 | 0.4 |
| 10 | AWC-CLASS | 1.1 | 2.1 |
| 11 | DEM | 1.1 | 5.6 |
| Variables | Current | SSP126 | SSP245 | SSP585 | ||||
|---|---|---|---|---|---|---|---|---|
| PC/% | PI/% | PC/% | PI/% | PC/% | PI/% | PC/% | PI/% | |
| Temperature | 39.1 | 59.9 | 38.3 | 46.3 | 44 | 48.5 | 40.1 | 52.8 |
| Salinity | 17.9 | 10.1 | 17.3 | 7.6 | 16 | 6.5 | 15.5 | 8.5 |
| Bio18 | 16.1 | 3.1 | 17.1 | 3.6 | 11.7 | 2.7 | 17.7 | 3.4 |
| Bio14 | 7.2 | 6.2 | 7.8 | 7.1 | 8.7 | 7.4 | 7.2 | 4.6 |
| DEM | 5 | 3.1 | 6 | 4.9 | 4.4 | 5.4 | 4.9 | 3.6 |
| Bio3 | 4.2 | 4 | 2.5 | 3.6 | 3.7 | 3.9 | 2.5 | 5.2 |
| Bio4 | 4.1 | 8.1 | 3.4 | 19.7 | 3.9 | 16.1 | 4.6 | 13.4 |
| T-CEC-SOIL | 2.4 | 0.8 | 3 | 1.3 | 2.8 | 1.5 | 3.3 | 1.8 |
| AWC-CLASS | 1.7 | 2.7 | 1.6 | 4.2 | 1.8 | 6.5 | 1.8 | 4.5 |
| Current velocity | 1.5 | 1.2 | 2.5 | 1.6 | 1.9 | 1.3 | 2 | 1 |
| T-CASO4 | 0.7 | 0.8 | 0.5 | 0.1 | 1 | 0.3 | 0.6 | 1.1 |
| Habitat Grade | Period | |||||||
|---|---|---|---|---|---|---|---|---|
| Current | Percent (%) | Future (2021–2040) | ||||||
| SSP126 | Percent (%) | SSP245 | Percent (%) | SSP585 | Percent (%) | |||
| Unsuitable | 216.63 | 88.05 | 215.81 | 87.14 | 216.39 | 87.38 | 215.06 | 86.84 |
| Lowly suitable | 16.94 | 6.88 | 19.54 | 7.89 | 18.38 | 7.42 | 18.89 | 7.63 |
| Moderately suitable | 8.57 | 3.48 | 8.53 | 3.45 | 8.44 | 3.41 | 9.33 | 3.77 |
| Highly suitable | 3.90 | 1.58 | 3.76 | 1.52 | 4.44 | 1.79 | 4.37 | 1.76 |
| Total suitable | 246.03 | 100.00 | 247.65 | 100.00 | 247.65 | 100.00 | 247.65 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Tan, X.; Yu, Y.; Zeng, X.; Xie, X.; Dong, Z.; Wei, Y.; Song, J.; Li, W.; Liang, F. Climate Change Threatens Barringtonia racemosa: Conservation Insights from a MaxEnt Model. Diversity 2024, 16, 429. https://doi.org/10.3390/d16070429
Tan Y, Tan X, Yu Y, Zeng X, Xie X, Dong Z, Wei Y, Song J, Li W, Liang F. Climate Change Threatens Barringtonia racemosa: Conservation Insights from a MaxEnt Model. Diversity. 2024; 16(7):429. https://doi.org/10.3390/d16070429
Chicago/Turabian StyleTan, Yanfang, Xiaohui Tan, Yanping Yu, Xiaping Zeng, Xinquan Xie, Zeting Dong, Yilan Wei, Jinyun Song, Wanxing Li, and Fang Liang. 2024. "Climate Change Threatens Barringtonia racemosa: Conservation Insights from a MaxEnt Model" Diversity 16, no. 7: 429. https://doi.org/10.3390/d16070429






