The Roles of Alpha, Beta, and Functional Diversity Indices in the Ecological Connectivity between Two Sub-Antarctic Macrobenthic Assemblages
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Design
2.2.1. Field Work
2.2.2. Laboratory Work
2.3. Statistical Analysis
2.3.1. General Characteristics of Benthic Assemblages
2.3.2. Analysis of α-Diversity
2.3.3. Ecological Connectivity between BI and SA Assemblages
- (a)
- β-Diversity Analysis
- (i)
- Quantitative Data
- (ii)
- Qualitative Data
- (iii)
- Functional Diversity Analysis
3. Results
3.1. General Structure of the BI and SA Assemblages
3.1.1. Abundance
3.1.2. Taxonomic Composition
3.1.3. Pattern of α-Diversity
3.2. Ecological Connectivity between BI and SA Assemblages
- (a)
- Pattern of β-Diversity
- (b)
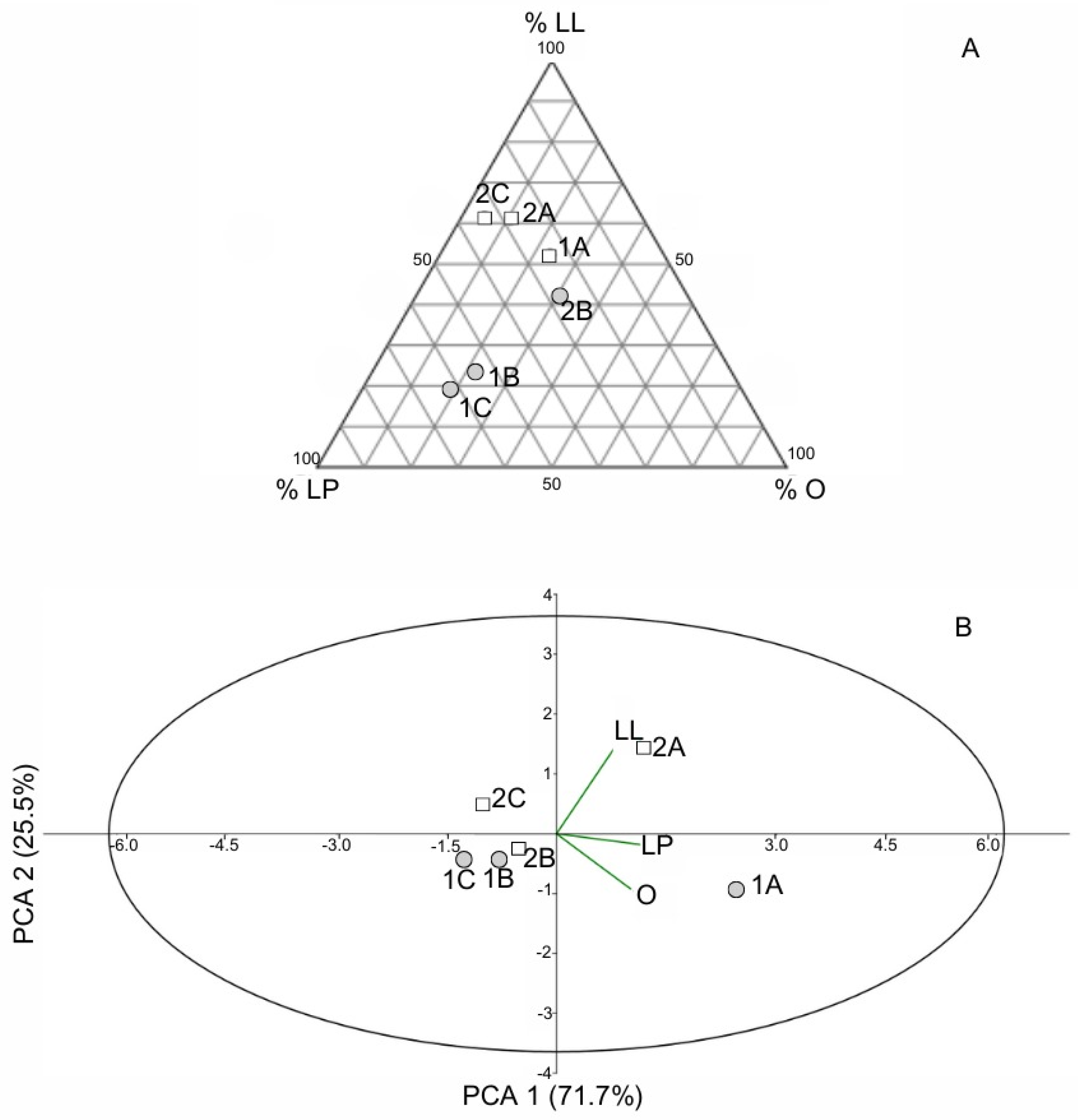
- Functional Diversity Pattern
4. Discussion
4.1. Abundance, Species Richness, and α-Diversity Patterns
4.2. Ecological Connectivity among Macrobenthic Assemblages
4.3. Ecological Connectivity and the Marine Protected Areas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cerdeira, J.O.; Pinto, L.S.; Cabeza, M.; Gaston, K.J. Species specific connectivity in reserve-network design using graphs. Biol. Conserv. 2010, 143, 408–415. [Google Scholar] [CrossRef]
- Socolar, J.B.; Gilroy, J.J.; Kunin, W.E.; Edwards, D.P. How should beta-diversity inform biodiversity conservation? Trends Ecol. Evol. 2016, 31, 67–80. [Google Scholar] [CrossRef]
- Córdova-Tapia, F.; Zambrano, L. La diversidad funcional en la ecología de comunidades. Ecosistemas 2015, 24, 78–87. [Google Scholar] [CrossRef]
- Virtanen, E.A.; Moilanen, A.; Viitasalo, M. Marine connectivity in spatial conservation planning: Analogues from the terrestrial realm. Landscape Ecol. 2020, 35, 1021–1034. [Google Scholar] [CrossRef]
- Treml, E.A.; Halpin, P.N. Marine population connectivity identifies ecological neighbors for conservation planning in the Coral Triangle. Conserv. Lett. 2012, 5, 441–449. [Google Scholar] [CrossRef]
- Neugarten, R.A.; Langhammer, P.F.; Osipova, E.; Bagstad, K.J.; Bhagabati, N.; Butchart, S.H.; Dudley, N.; Elliott, V.; Gerber, L.; Gutierrez, C.; et al. Tools for Measuring, Modelling, and Valuing Ecosystem Services: Guidance for Key Biodiversity Areas, Natural World Heritage Sites, and protected Areas; IUCN: Gland, Switzerland, 2018; 70p. [Google Scholar]
- Hilty, J.; Worboys, G.L.; Keeley, A.; Woodley, S.; Lausche, B.; Locke, H.; Carr, M.; Pulsford, I.; Pittock, J.; White, J.W.; et al. Lineamientos Para la Conservación de la Conectividad a Través de Redes y Corredores Ecológicos. Serie Directrices Para Buenas Prácticas en Áreas Protegidas; No. 30; UICN: Gland, Suiza, 2021; 146p. [Google Scholar]
- Fang, X.; Hou, X.; Li, X.; Hou, W.; Nakaoka, M.; Yu, X. Ecological connectivity between land and sea: A review. Ecol. Res. 2018, 33, 51–61. [Google Scholar] [CrossRef]
- Bishop, M.J.; Mayer-Pinto, M.; Airoldi, L.; Firth, L.B.; Morris, R.L.; Loke, L.H.; Hawkins, S.; Naylor, L.; Coleman, R.; Chee, S.; et al. Effects of ocean sprawl on ecological connectivity: Impacts and solutions. J. Exp. Mar. Biol. Ecol. 2017, 492, 7–30. [Google Scholar] [CrossRef]
- Kadoya, T. Assessing functional connectivity using empirical data. Popul. Ecol. 2009, 51, 5–15. [Google Scholar]
- Fletcher, R.J.; Burrell, N.S.; Reichert, B.E.; Vasudev, D.; Austin, J.D. Divergent perspectives on landscape connectivity reveal consistent effects from genes to communities. Curr. Landscape Ecol. Rep. 2016, 1, 67–79. [Google Scholar] [CrossRef]
- Endo, C.A.K.; Gherardi, D.F.M.; Pezzi, L.P.; Lima, L.N. Low connectivity compromises the conservation of reef fishes by marine protected areas in the tropical South Atlantic. Sci. Rep. 2019, 9, 8634. [Google Scholar] [CrossRef]
- Nagelkerken, I. Ecological Connectivity among Tropical Coastal Ecosystems, 1st ed.; Springer: Nijmegen, The Netherlands, 2009; 615p. [Google Scholar]
- Thrush, S.F.; Hewitt, J.E.; Lohrer, A.M.; Chiaroni, L.D. When small changes matter: The role of cross-scale interactions between habitat and ecological connectivity in recovery. Ecol. Appl. 2013, 23, 226–238. [Google Scholar] [CrossRef]
- Balbar, A.C.; Metaxas, A. The current application of ecological connectivity in the design of marine protected areas. Glob. Ecol. Conserv. 2019, 17, e00569. [Google Scholar] [CrossRef]
- Purvis, A.; Hector, A. Getting the measure of biodiversity. Nature 2000, 405, 212–219. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988; 79p. [Google Scholar]
- Koleff, P.; Gaston, K.J.; Lennon, J.J. Measuring beta diversity for presence–absence data. J. Anim. Ecol. 2003, 72, 367–382. [Google Scholar] [CrossRef]
- Schroeder, P.J.; Jenkins, D.G. How robust are popular beta diversity indices to sampling error? Ecosphere 2018, 9, e02100. [Google Scholar] [CrossRef]
- Roughgarden, J.; Gaines, S.; Possingham, H. Recruitment dynamics in complex life cycles. Science 1988, 241, 1460–1466. [Google Scholar] [CrossRef]
- Cowen, R.K.; Paris, C.B.; Srinivasan, A. Scaling of connectivity in marine populations. Science 2006, 311, 522–527. [Google Scholar] [CrossRef]
- Christie, M.R.; Tissot, B.N.; Albins, M.A.; Beets, J.P.; Jia, Y.; Ortiz, D.M.; Stephen, E.; Thompson, M.A.; Hixon, M.A. Larval connectivity in an effective network of marine protected areas. PLoS ONE 2010, 5, e15715. [Google Scholar] [CrossRef]
- Puckett, B.J.; Eggleston, D.B.; Kerr, P.C.; Luettich, J.R.A. Larval dispersal and population connectivity among a network of marine reserves. Fish. Oceanogr. 2014, 23, 342–361. [Google Scholar] [CrossRef]
- Colson, I.; Hughes, R.N. Rapid recovery of genetic diversity of dogwhelk (Nucella lapillus L.) populations after local extinction and recolonization contradicts predictions from life-history characteristics. Mol. Ecol. 2004, 13, 2223–2233. [Google Scholar] [CrossRef]
- Moritz, C.; Meynard, C.N.; Devictor, V.; Guizien, K.; Labrune, C.; Guarini, J.M.; Mouquet, N. Disentangling the role of connectivity, environmental filtering, and spatial structure on metacommunity dynamics. Oikos 2013, 122, 1401–1410. [Google Scholar] [CrossRef]
- Pineda-Metz, S.E.; Montiel, A. Seasonal dynamics of meroplankton in a sub-Antarctic fjord (Southern Patagonia, Chile). Polar Biol. 2021, 44, 875–886. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity (FD), species richness and community composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Schratzberger, M.; Warr, K.; Rogers, S.I. Functional diversity of nematode communities in the southwestern North Sea. Mar. Environ. Res. 2007, 63, 368–389. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, P.; Li, G.; Zhou, D. Relationships between functional diversity and ecosystem functioning: A review. Acta Ecol. Sin. 2014, 34, 85–91. [Google Scholar] [CrossRef]
- Delfan, N.; Shojaei, M.G.; Naderloo, R. Patterns of structural and functional diversity of macrofaunal communities in a subtropical mangrove ecosystem. Estuar. Coast. Shelf Sci. 2021, 252, 107288. [Google Scholar] [CrossRef]
- Muller, A.; Dubois, S.F.; Boyé, A.; Becheler, R.; Droual, G.; Chevalier, M.; Pasquier, M.; Roudaut, L.; Fournier-Sowinski, J.; Auby, I.; et al. Environmental filtering and biotic interactions act on different facets of the diversity of benthic assemblages associated with eelgrass. Ecol. Evol. 2023, 13, e10159. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Pérez, L.; Rodríguez-Zaragoza, F.A.; Ortiz, M.; Cupul-Magaña, A.L.; Carriquiry, J.D.; Ríos-Jara, E.; Rodríguez-Troncoso, A.; García-Rivas, M.D.C. Coral reef health indices versus the biological, ecological and functional diversity of fish and coral assemblages in the Caribbean Sea. PLoS ONE 2016, 11, e0161812. [Google Scholar]
- Briceño-Méndez, M.; Naranjo, E.; Pérez-Irineo, G.; Contreras-Perera, Y.; Sandoval-Serés, E.; Hidalgo-Mihart, M.G. Richness and trophic guilds of carnivorous mammals in ejido Nuevo Becal, Calakmul, Campeche, Mexico. Therya 2017, 8, 145–150. [Google Scholar] [CrossRef]
- González, H.E.; Graeve, M.; Kattner, G.; Silva, N.; Castro, L.; Iriarte, J.L.; Osmán, L.; Daneri, G.; Vargas, C.A. Carbon flow through the pelagic food web in southern Chilean Patagonia: Relevance of Euphausia vallentini as a key species. Mar. Ecol. Prog. Ser. 2016, 557, 91–110. [Google Scholar] [CrossRef]
- Silva, N.; Vargas, C.A. Hypoxia in Chilean patagonian fjords. Prog. Oceanogr. 2014, 129, 62–74. [Google Scholar] [CrossRef]
- Sanderson, E.W.; Jaiteh, M.; Levy, M.A.; Redford, K.H.; Wannebo, A.V.; Woolmer, G. The human footprint and the last of the wild: The human footprint is a global map of human influence on the land surface, which suggests that human beings are stewards of nature, whether we like it or not. BioScience 2002, 52, 891–904. [Google Scholar] [CrossRef]
- Tecklin, D.; Farías, A.; Peña, M.; Gélvez, X.; Castilla, J.C.; Sepúlveda, M.; Viddi, F.; Hucke-Gaete, R. Protección Costero-Marina en la Patagonia Chilena: Situación presente, avances y desafíos. In Conservación en la Patagonia Chilena: Evaluación del Conocimiento, Oportunidades y Desafíos; Castilla, J., Armesto, J., Marínez-Harms, M.J., Eds.; Ediciones Universidad Católica: Santiago, Chile, 2021; 600p. [Google Scholar]
- Montiel, A.; Gerdes, D.; Ríos, C. Distribución y abundancia del Macrozoobentos en una microcuenca marina submareal del Estrecho de Magallanes, Chile. An. Inst. Patagon. 2001, 29, 117–133. [Google Scholar]
- Montiel, A.; Gerdes, D.; Arntz, W.E. Distributional patterns of shallow-water polychaetes in the Magellan region: A zoogeographical and ecological synopsis. Sci. Mar. 2005, 69 (Suppl. S2), 123–133. [Google Scholar] [CrossRef][Green Version]
- Montiel, A.; Quiroga, E.; Gerdes, D. Diversity and spatial distribution patterns of polychaete assemblages in the Paso Ancho, Straits of Magellan Chile. Cont. Shelf Res. 2011, 31, 304–314. [Google Scholar] [CrossRef]
- Ríos, C.; Mutschke, E.; Morrison, E. Biodiversidad bentónica sublitoral en el estrecho de Magallanes, Chile. Rev. Biol. Mar. Oceanogr. 2003, 38, 1–12. [Google Scholar]
- Ríos, C.; Mutschke, E.; Montiel, A. Estructura de la comunidad macrofaunística bentónica en la boca oriental del estrecho de Magallanes, Chile austral. An. Inst. Patagon. 2010, 38, 83–96. [Google Scholar]
- Thatje, S.; Brown, A. The macrobenthic ecology of the straits of Magellan and the beagle channel. An. Inst. Patagon. 2009, 37, 17–27. [Google Scholar] [CrossRef][Green Version]
- Quiroga, E.; Ortiz, P.; González, R.; Tapia, F.; Pérez-Santos, I.; Rebolledo, L.; Mansilla, R.; Pineda, C.; Cari, I.; Salinas, N.; et al. Seasonal patterns in the benthic realm of a glacial fjord (Martinez Channel, Chilean Patagonia): The role of suspended sediment and terrestrial organic matter. Mar. Ecol. Prog. Ser. 2016, 56, 31–50. [Google Scholar] [CrossRef]
- Aldea, C.; Rosenfeld, S.; Cárdenas, J. Caracterización de la diversidad de moluscos bentónicos sublitorales en Isla Carlos III y áreas adyacentes, Estrecho de Magallanes, Chile. An. Inst. Patagon. 2011, 39, 73–89. [Google Scholar] [CrossRef][Green Version]
- Villalobos, V.I.; Valdivia, N.; Försterra, G.; Ballyram, S.; Espinoza, J.P.; Wadham, J.L.; Burgos-Andrade, K.; Häussermann, V. Depth-dependent diversity patterns of rocky subtidal macrobenthic communities along a temperate fjord in Northern Chilean Patagonia. Fronti. Mar. Sci. 2021, 8, 635855. [Google Scholar] [CrossRef]
- Rolls, R.J.; Deane, D.C.; Johnson, S.E.; Heino, J.; Anderson, M.J.; Ellingsen, K.E. Biotic homogenisation and differentiation as directional change in beta diversity: Synthesising driver–response relationships to develop conceptual models across ecosystems. Biol. Rev. 2023, 98, 1388–1423. [Google Scholar] [CrossRef]
- General Bathymetric Chart of the Oceans. Gridded Bathymetry Data. Available online: https://www.opendem.info/download_bathymetry.html (accessed on 22 April 2021).
- Vargas, C.A.; Cuevas, L.A.; Silva, N.; González, H.E.; De Pol-Holz, R.; Narváez, D.A. Influence of glacier melting and river discharges on the nutrient distribution and DIC recycling in the southern Chilean Patagonia. J. Geophys. Res. G Biogeosci. 2018, 123, 256–270. [Google Scholar] [CrossRef]
- Aracena, C.; Kilian, R.; Lange, C.B.; Bertrand, S.; Lamy, F.; Arz, H.W.; De Pol-Holz, R.; Baeza, O.; Pantoja, S.; Kissel, C. Holocene variations in productivity associated with changes in glacier activity and freshwater flux in the central basin of the Strait of Magellan. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 436, 112–122. [Google Scholar] [CrossRef]
- Brambati, A.; Fontolan, G.; Simeoni, U. Recent sediments and sedimentological processes in the Strait of Magellan. Boll. Oceanol. Teor. Appl. 1991, 9, 217–259. [Google Scholar]
- Valdenegro, A.; Silva, N. Caracterización física y química de la zona de canales y fiordos australes de Chile entre el Estrecho de Magallanes y Cabo de Hornos (CIMAR 3 Fiordo). Cienc. Tecnol. Mar. 2003, 26, 19–60. [Google Scholar]
- MHNRS’s Report. First Expedition of the Rio Seco’s Natural History Museum in the Magellanic Region (MHNRS/MAG-1); Rio Seco’s Natural History Museum Press: Punta Arenas, Chile, 2019. [Google Scholar]
- Böggemann, M. Revision of the Glyceridae Grube 1850 (Annelida: Polychaeta). Abh. Senckenb. Naturforsch. Ges. 2002, 555, 1–249. [Google Scholar]
- Hartman, O. Polychaeta Errantia of Antarctica. Antarct. Res. Ser. 1964, 3, 1–131. [Google Scholar]
- Hartman, O. Polychaeta Myzostomidae and Sedentaria of Antarctica. Antarct. Res. Ser. 1966, 7, 1–158. [Google Scholar]
- Hartmann-Schröder, G. Zur Kenntnis des Sublitorals der chilenischen Küste unter besonderer Berücksichtigung der Polychaeten und Ostracoden. Tl. II. Die Polychaeten des Sublitorals. Mitt. Zool. Mus. Inst. 1965, 62, 59–305. [Google Scholar]
- Hartmann-Schröder, G. Annelida, borstenwurmer, polychaeta. In Die Tierwelt Deutschlands und der Angrenzenden Meeresteile Nach Ihren Merkmalen und Nach Ihrer Lebensweise; G. Fischer: Riverwood, Australia, 1971; Volume 58, pp. 1–594. [Google Scholar]
- Kornicker, L.S. (Ed.) Biology of the Antarctic Seas XXI; American Geophysical Union: Washington, DC, USA, 1991; Volume 52. [Google Scholar]
- Orensanz, J.M. Los anelidos poliquetos de la provincia biogeografica Argentina: 4. Lumbrineridae. Physis/Sección A Los Océanos Y Sus Org. 1973, 32, 343–393. [Google Scholar]
- Schüller, M. Biodiversity and Zoogeography of the Polychaeta (Annelida) in the Deep Weddell Sea (Southern Ocean, Antarctica) and Adjacent Deep-Sea Basins. Ph.D. Dissertation, University Bochum, Bochum, Germany, 2007. [Google Scholar]
- Rozbaczylo, N. Clave para el reconocimiento de familias de anélidos poliquetos del mar chileno. Stud. Neotrop. Fauna Environ. 1980, 15, 167–196. [Google Scholar] [CrossRef]
- Linse, K. Mollusca of the Magellan region. A cheklist of the species and their distribution. Sci. Mar. 1999, 63, 399–407. [Google Scholar] [CrossRef]
- Linse, K. The shelled magellanic Mollusca: With special reference to biogeographic relations in the Southern Ocean. Theses Zool. 2002, 34, 1–252. [Google Scholar]
- Reid, D.G.; Osorio, C. The shallow-water marine mollusca of the Estero Elefantes and Laguna San Rafael, southern Chile. Bull. Nat. Hist. Mus. Lond. Zool. 2000, 66, 109–146. [Google Scholar]
- Zelaya, D.; Ituarte, C. The genus Neolepton Monterosato, 1875 in southern South America (Bivalvia: Neoleptonidae). J. Molluscan Stud. 2004, 70, 123–137. [Google Scholar] [CrossRef]
- Menzies, R.J. The zoogeography, ecology and systematics of the chilean marine isopods. Rep. Lund Uni. Chile Exped. 1962, 11, 162. [Google Scholar]
- Retamal, M.A. Contribución al conocimiento de los crustáceos decápodos de la región magallánica. Gayana. Zool. 1974, 31, 23. [Google Scholar]
- Haussermann, V.; Forsterra, G. Fauna Marina Bentónica de la Patagonia Chilena. Guía de identificación ilustrada. In Guía de Identificación Ilustrada; Nature in Focus: Santiago, Chile, 2009; 1000p, ISBN 978-956-332-244-6. [Google Scholar]
- Zagal, C.; Hermosilla, C. Guía de Invertebrados Marinos del sur de Chile; FantásticoSur: Punta Arenas, Chile, 2007. [Google Scholar]
- Spitzer, M.; Wildenhain, J.; Rappsilber, J.; Tyers, M. BoxPlotR: A web tool for generation of box plots. Nat. Methods 2014, 11, 121–122. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chao, A.; Ma, K.H.; Hsieh, T.C. iNEXT (iNterpolation and EXTrapolation) Online: Software for Interpolation and Extrapolation of Species Diversity, 2016; Program and User’s Guide. Available online: http://chao.stat.nthu.edu.tw/wordpress/software_download/inext-online/ (accessed on 22 April 2021).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeont. Electr. 2001, 4, 9. [Google Scholar]
- Baselga, A. Separating the two components of abundance-based dissimilarity: Balanced changes in abundance vs. abundance gradients. Methods Ecol. Evol. 2013, 4, 552–557. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; 256p. [Google Scholar]
- Carvalho, J.C.; Cardoso, P.; Gomes, P. Determining the relative roles of species replacement and species richness differences in generating beta-diversity patterns. Glob. Ecol. Biogeogr. 2012, 21, 760–771. [Google Scholar] [CrossRef]
- Macdonald, T.A.; Burd, B.J.; Macdonald, V.I.; Van Roodselaar, A. Taxonomic and Feeding Guild Classification for the Marine Benthic Macroinvertebrates of the Strait of Georgia, British Columbia; Fisheries and Oceans Canada = Pêches et oceans Canada: Ottawa, ON, Canada, 2010; 63p.
- Montiel San Martin, A. Biodiversity, zoogeography and ecology of polychaetes from the Magellan region and adjacent areas = Diversität, Zoogeographie und Ökologie von Polychaeten der Magellanregion und angrenzender Gebiete. Ber. Polarforsch. 2005, 505, 1–103. [Google Scholar]
- Arntz, W.E.; Thatje, S.; Gerdes, D.; Gili, J.M.; Gutt, J.; Jacob, U.T.E.; Montiel, A.; Orejas, C.; Teixidó, N. The Antarctic-Magellan connection: Macrobenthos ecology on the shelf and upper slope, a progress report. Scie. Mar. 2005, 69, 237–269. [Google Scholar] [CrossRef]
- Thrush, S.F.; Hewitt, J.E.; Cummings, V.J.; Norkko, A.; Chiantore, M. β-diversity and species accumulation in Antarctic coastal benthos: Influence of habitat, distance and productivity on ecological connectivity. PLoS ONE 2010, 5, e11899. [Google Scholar] [CrossRef] [PubMed]
- Wren, J.L.; Kobayashi, D.R. Exploration of the “larval pool”: Development and ground-truthing of a larval transport model off leeward Hawai ‘i. PeerJ 2016, 4, e1636. [Google Scholar] [CrossRef][Green Version]
- Gaines, S.D.; Gaylor, D.B.; Gerber, L.R.; Hastings, A.; Kinlan, B.P. Connecting places: The ecological consequences of dispersal in the sea. Oceanography 2007, 20, 90–99. [Google Scholar] [CrossRef]
- Villéger, S.; Ramos Miranda, J.; Flores Hernández, D.; Mouillot, D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol. Appl. 2010, 20, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Cari, I.; Andrade, C.; Quiroga, E.; Mutschke, E. Benthic trophic structure of a Patagonian fjord (47 S): The role of hydrographic conditions in the food supply in a glaciofluvial system. Estuar. Coast. Shelf Sci. 2020, 233, 106536. [Google Scholar] [CrossRef]
- Planes, S. Biogeography and larval dispersal inferred from population genetic analysis. In Chapter 9. Coral Reef Fishes; Sale, P.F., Ed.; Academic Press: New York, NY, USA, 2002; pp. 201–220. [Google Scholar]
- Cowen, R.K.; Sponaugle, S. Larval dispersal and marine population connectivity. Ann. Rev. Mar. Sci. 2009, 1, 443–466. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Cabido, M. Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Shanks, A.L.; Grantham, B.A.; Carr, M.H. Propagule dispersal distance and the size and spacing of marine reserves. Ecol. Appl. 2003, 13 (Suppl. S1), 159–169. [Google Scholar] [CrossRef]
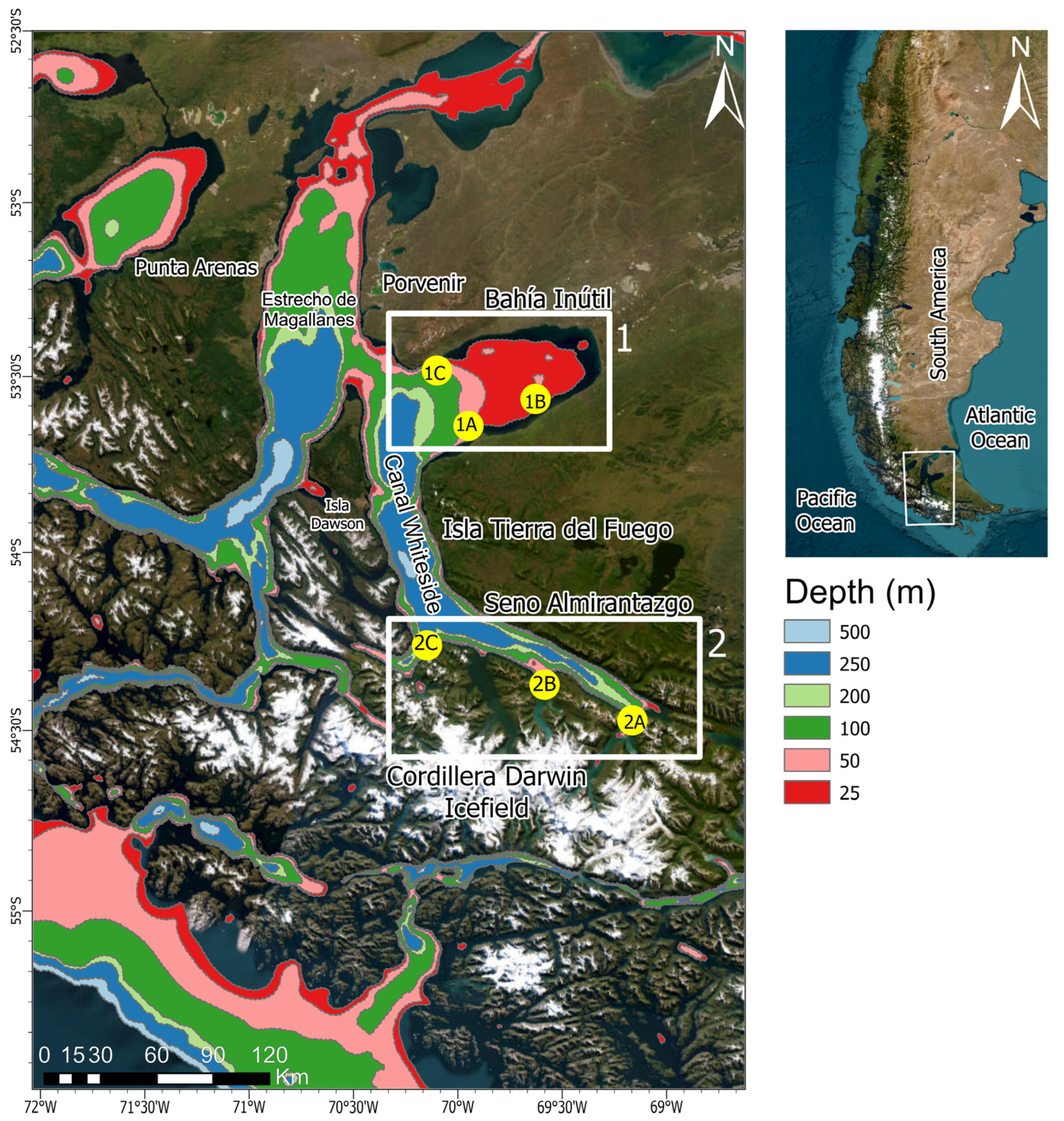
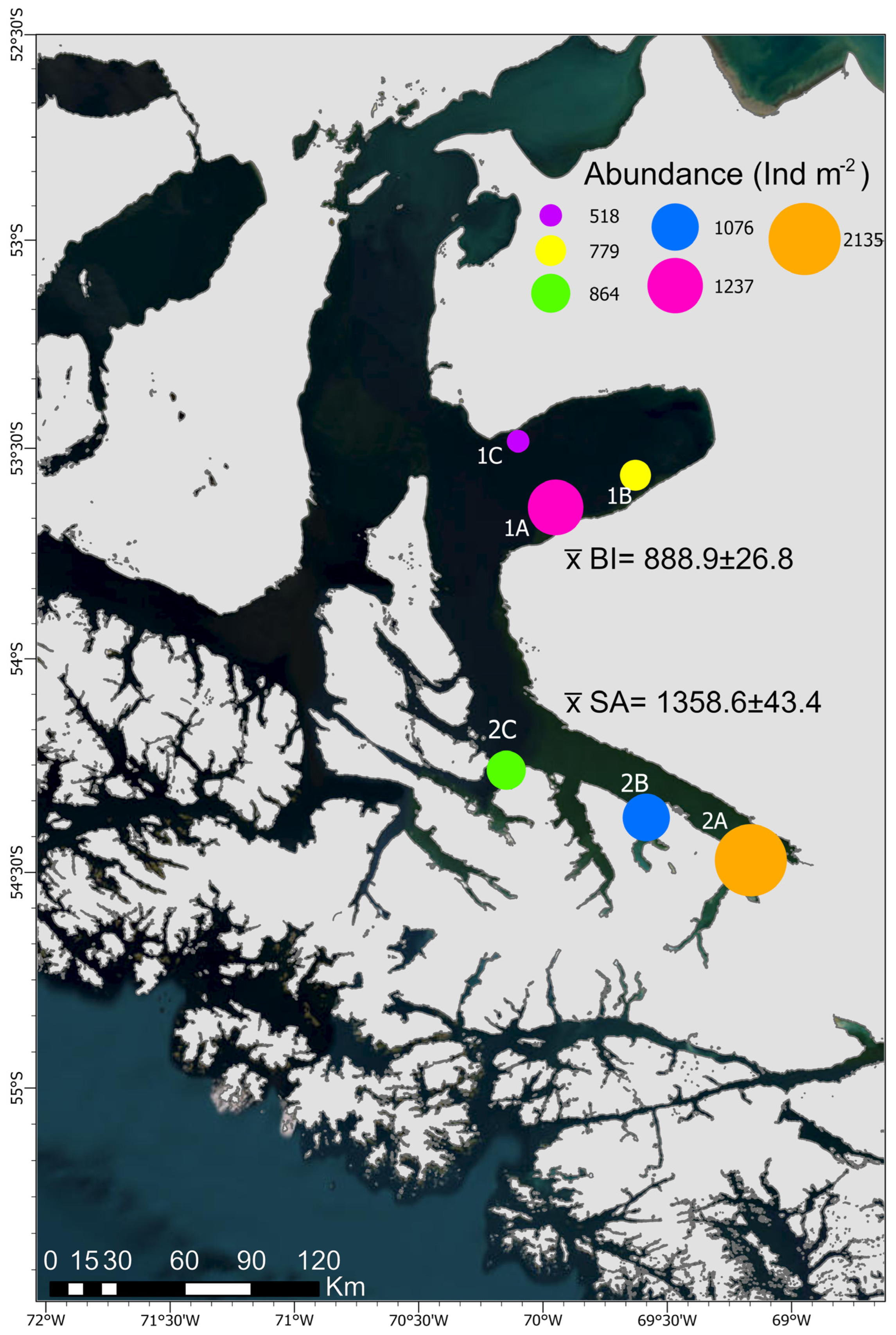
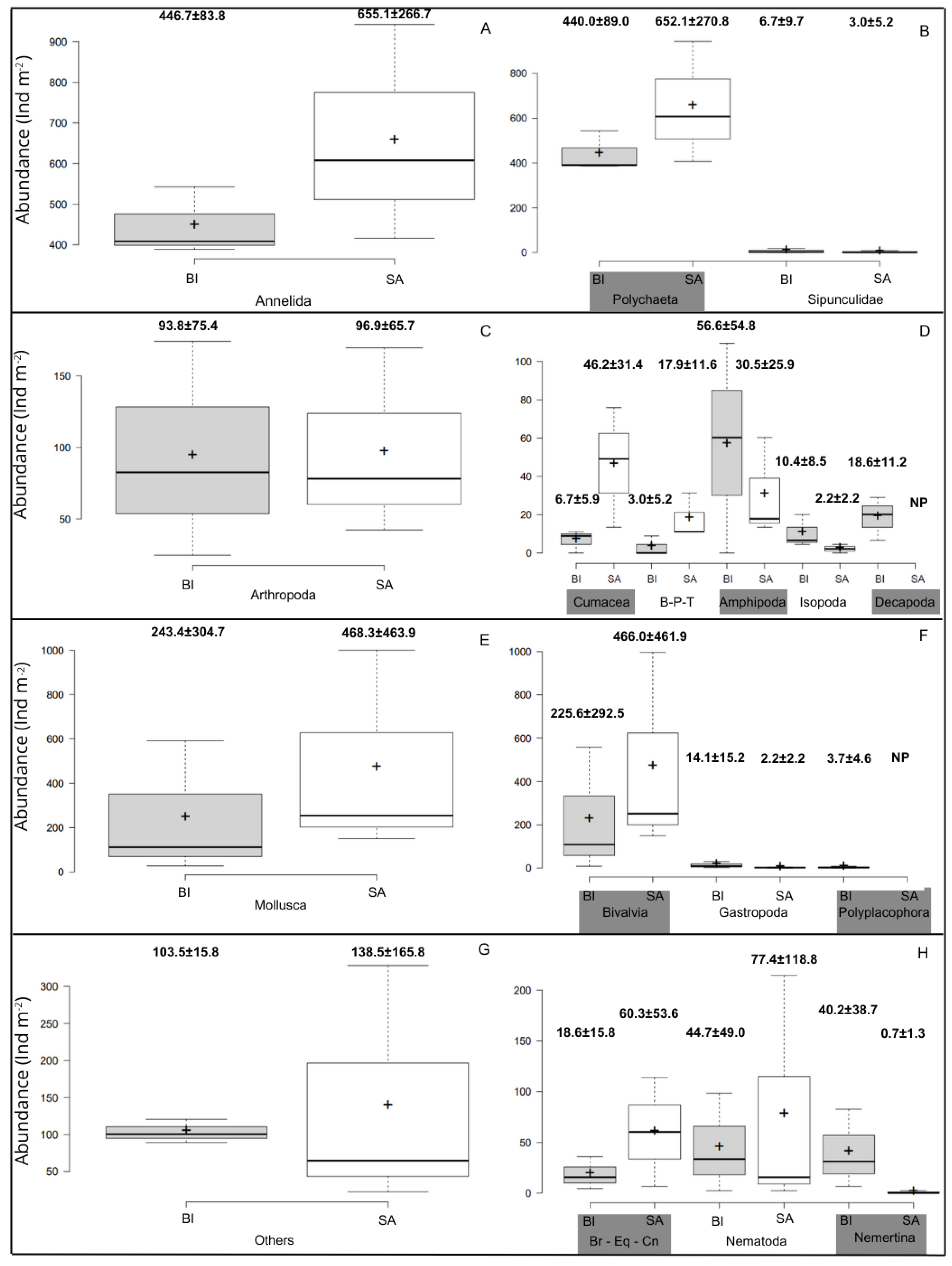
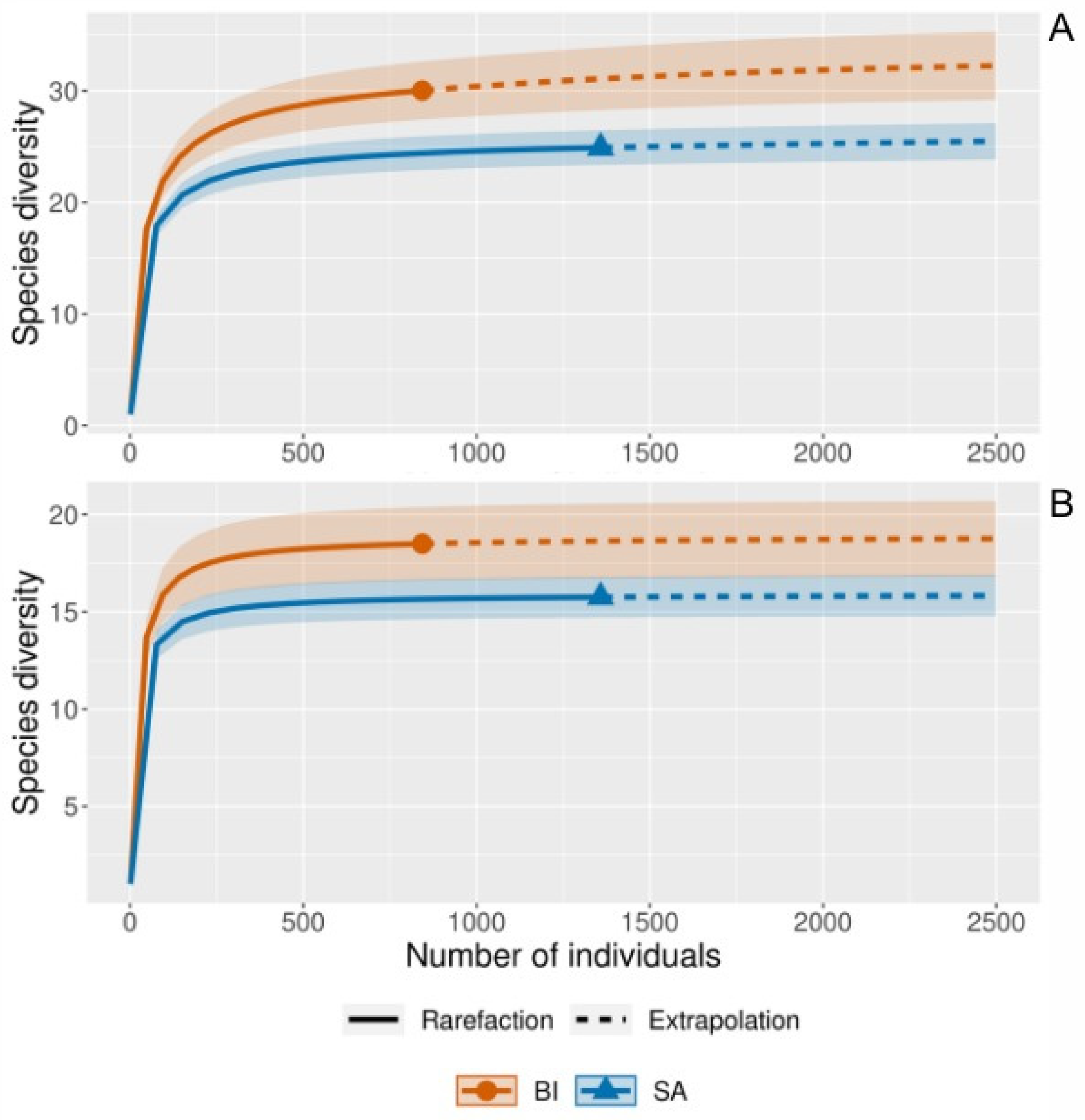


| Station N° (N° Samples) | Date (November 2018) | Depth (X) | Lat (°S) | Long (°W) |
|---|---|---|---|---|
| 1A (3) | 20 | 30 | 53°39′00″ | 69°56.3′ |
| 1B (3) | 20 | 30 | 53°35′00″ | 69°36.1′ |
| 1C (3) | 20 | 30 | 53°28′00″ | 70°07.0′ |
| 2A (3) | 21 | 36 | 54°33′00″ | 69°10.7′ |
| 2B (3) | 23 | 45 | 54°23′33″ | 69°37.7′ |
| 2C (3) | 24 | 48 | 54°23′00″ | 70°12.9′ |
| Permutation N | 9999 |
| Total sum of squares: | 1.321 |
| Within-group sum of squares: | 0.9573 |
| F: | 1.519 |
| p (same): | 0.1976 |
| No | Share Species | Phylum | Spp. BI | Spp. SA |
|---|---|---|---|---|
| 1 | Capitella capitata | An | 127.30 | 446.65 |
| 2 | Aricidia (Acmira) finitima | An | 67.00 | 305.97 |
| 3 | Aphelochaeta sp. 1 | An | 238.97 | 67.00 |
| 4 | Lumbrineris sp. | An | 127.30 | 178.67 |
| 5 | Notomastus latericeus | An | 122.83 | 2.23 |
| 6 | Spiophanes sp. | An | 11.17 | 104.97 |
| 7 | Cirriformia nasuta | An | 60.30 | 96.03 |
| 8 | Prionospio patagonica | An | 6.70 | 87.10 |
| 9 | Monticellina sp. | An | 80.40 | 75.93 |
| 10 | Hemipodia sp. | An | 67.00 | 6.70 |
| 11 | Caulleriella sp. | An | 46.90 | 2.23 |
| 12 | Sipunculida indet. | An | 20.10 | 8.93 |
| 13 | Cistenides ehlersi | An | 20.10 | 2.23 |
| 14 | Melinna cristata | An | 2.23 | 20.10 |
| 15 | Eteone aurantiaca | An | 6.70 | 15.63 |
| 16 | Eulalia subulifera | An | 6.70 | 15.63 |
| 17 | Brania sp. | An | 2.23 | 11.17 |
| 18 | Nicon maculata | An | 2.23 | 8.93 |
| 19 | Magelona sp. | An | 8.93 | 2.23 |
| 20 | Harmothoe ciliata | An | 2.23 | 8.93 |
| 21 | Augeneria tentaculata | An | 4.47 | 6.70 |
| 22 | Trichobranchus glacialis | An | 6.70 | 2.23 |
| 23 | Harmothoe spp. | An | 4.47 | 6.70 |
| 24 | Hauchiella sp. | An | 6.70 | 2.23 |
| 25 | Paraninoe sp. | An | 4.47 | 4.47 |
| 26 | Nucula pisum | Mo | 335.10 | 491.33 |
| 27 | Yoldiella sp. 1 | Mo | 2.23 | 504.73 |
| 28 | Bivalvia indet. 1 | Mo | 158.57 | 20.10 |
| 29 | Neilonella sulculata | Mo | 53.60 | 93.80 |
| 30 | Xymenopsis muriciformis | Mo | 24.57 | 2.23 |
| 31 | Tawera elliptica | Mo | 8.93 | 2.23 |
| 32 | Pareuthria atrata | Mo | 4.47 | 4.47 |
| 33 | Cumacea spp. | Ar | 17.87 | 138.47 |
| 34 | Amphipoda spp. | Ar | 129.53 | 73.70 |
| 35 | Tanaidacea spp. | Ar | 4.47 | 51.37 |
| 36 | Epimeriidae spp. | Ar | 20.10 | 17.87 |
| 37 | Isopoda spp. | Ar | 4.47 | 6.70 |
| 38 | Ophiuroidea indet. | Ec | 15.63 | 4.47 |
| 39 | Holothuroidea indet. | Ec | 13.40 | 4.47 |
| 40 | Nemertina indet. | Na | 134.00 | 232.27 |
| 41 | Nematoda indet. | Ne | 120.60 | 2.23 |
| BI | SA | |
|---|---|---|
| Diet type | ||
| H’ He | 1.718 | 1.248 |
| H’ Om | 3.147 | 2.954 |
| H’ Ca | 2.083 | 2.248 |
| Larval development | ||
| H’ PL | 2.322 | 2.304 |
| H’ LL | 2.585 | 2.508 |
| H’ O | 2.231 | 1.745 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nykol, J.; Americo, M.; Benjamin, C. The Roles of Alpha, Beta, and Functional Diversity Indices in the Ecological Connectivity between Two Sub-Antarctic Macrobenthic Assemblages. Diversity 2024, 16, 430. https://doi.org/10.3390/d16070430
Nykol J, Americo M, Benjamin C. The Roles of Alpha, Beta, and Functional Diversity Indices in the Ecological Connectivity between Two Sub-Antarctic Macrobenthic Assemblages. Diversity. 2024; 16(7):430. https://doi.org/10.3390/d16070430
Chicago/Turabian StyleNykol, Jara, Montiel Americo, and Cáceres Benjamin. 2024. "The Roles of Alpha, Beta, and Functional Diversity Indices in the Ecological Connectivity between Two Sub-Antarctic Macrobenthic Assemblages" Diversity 16, no. 7: 430. https://doi.org/10.3390/d16070430
APA StyleNykol, J., Americo, M., & Benjamin, C. (2024). The Roles of Alpha, Beta, and Functional Diversity Indices in the Ecological Connectivity between Two Sub-Antarctic Macrobenthic Assemblages. Diversity, 16(7), 430. https://doi.org/10.3390/d16070430






