Abstract
The Lilac-crowned Amazon (Amazona finschi) is an endemic parrot from western Mexico with a historical distribution in the Pacific Slope from southern Sonora and southwestern Chihuahua to Oaxaca. However, a particularly worrying decline in the extension of its distribution range has been reported in the central and southern regions. Overall, the species is listed in CITES the IUCN Red List of Threatened Species, and the official Mexican standard NOM-059 as endangered. In this study, we aimed to obtain molecular information to support the planning of conservation strategies for A. finschi. For this purpose, we analyzed the genetic diversity and genealogical relationships between two groups of individuals from northern (Sinaloa) and central (Michoacan) portions of the species’ range based on mitochondrial DNA markers. In general agreement with the endangered status of the species, we found low genetic diversity values. However, at the regional level, the northern group showed high genetic diversity and the central group showed a lack of genetic diversity. Furthermore, in agreement with the proposal that A. finschi is monotypic, genealogical relationships revealed a haplotype distributed in the center and the north, although haplotypes exclusive to the north were also found. We suggest a differentiated management of northern and central populations to preserve evolutionary potential.
1. Introduction
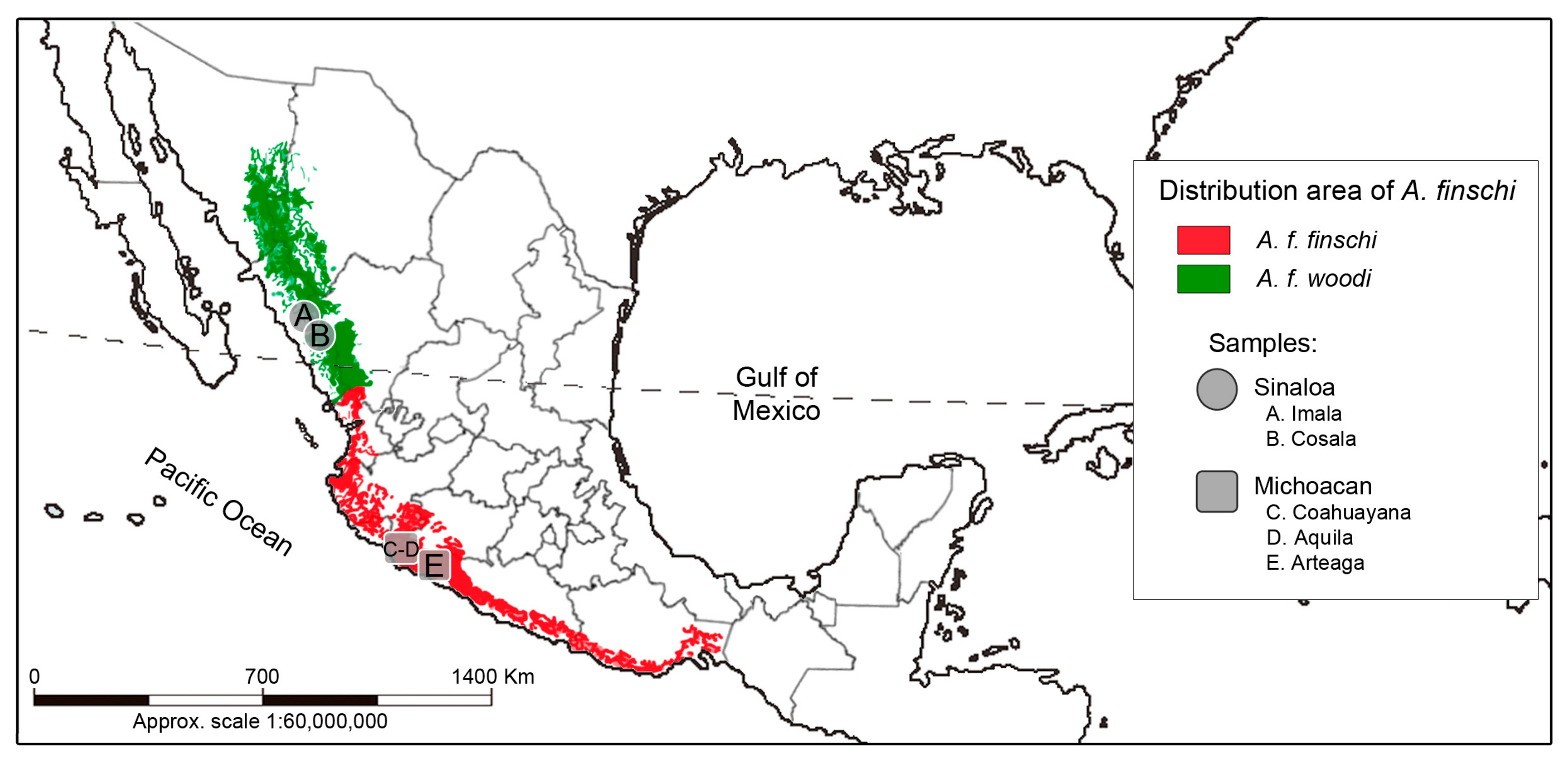
The Lilac-crowned Parrot (Amazona finschi, Sclater 1864) is a species endemic to Mexico that inhabits deciduous and semideciduous forests and edges, pine–oak forests, and mangroves. However, in local studies, records of the species have also been obtained in mountainous regions. It has been reported that during the dry season, this parrot undergoes seasonal altitudinal migrations from the deciduous forests on the coast to the medium sub-evergreen forest on the mountain slopes [1,2,3,4]. Until the last century, this species occupied the Pacific Slope from southern Sonora and southwestern Chihuahua to Oaxaca [1,2]. However, it has been reported recently that the historical range has been restricted to the states of Colima, Durango, Jalisco, Michoacan, and Sinaloa and that A. finschi has been virtually extirpated from the coastal areas of Nayarit, Guerrero, and Oaxaca (Figure 1) [5,6].
Figure 1.
The distribution range and sampling locations of the Lilac-crowned Parrot (Amazona finschi). The area occupied by the two subspecies is also shown [6,7,8].
For A. finschi, based on the geographical distribution of its populations and their morphological characters, two subspecies have been described. A. f. finschi, Scalter 1864, is distributed from southern Sinaloa and Durango to southern Oaxaca, and A. f. woodi, Moore 1937, is distributed in southeastern Sonora, southwestern Chihuahua, and east-central Sinaloa [9,10]. The morphological characteristics in A. f. finschi, according to Friedmann et al. [10] are, for males, the length of the wing ranges from 191 to 208 mm, the tail from 100 to 120 mm, the exposed culmen from 29 to 30 mm, and the tarsus from 21 to 23 mm. In females, the length of the wing ranges from 185 to 202 mm, the tail from 108 to 124 mm, the exposed culmen from 27 to 30 mm, and the tarsus from 21 to 23 mm [10]. Amazona f. woodi is similar to A. f. finschi, but the green plumage of its body is less yellowish and the brown of the crown and forehead is less intense and extensive. Its size is slightly larger than that of A. f. finschi. In males, the wing length ranges from 198 to 215 mm, the tail ranges from 109 to 124 mm, the exposed culmen from 29 to 32 mm, and the tarsus measures from 21 to 24 mm. In females, the wing length ranges from 193 to 208 mm, the tail from 104 to 124 mm, the exposed culmen from 27 to 31 mm, and the tarsus from 22 to 23 mm [10].
Psittacine species in the Neotropics are usually threatened by habitat transformation and illegal trade [6,11,12,13]. In a recent study, for A. finschi, a significant loss in its distribution of 37.6% was estimated with respect to the historical range of 332,597 km2, presenting a modest protected area equivalent to 6.6% of the current distribution [6]. Information concerning the species abundance of local populations and global size is relatively unknown. In 2003, Renton and Iñigo-Elias [5] estimated the global population at 7000–10,000 individuals, based on surveys covering most of the species’ global range. Furthermore, in their study, the highest relative abundance was found in the northern (Sinaloa) and central (Jalisco and Michoacan) regions, which had greater availability of high and medium forests. A local population density estimation for A. finschi is not available to date [5]. Currently, it has been observed that the species suffered from significant population declines in many areas of its original range [4,5,6,14]. Indeed, approximately 5000 individuals are extracted annually for illegal trade purposes on domestic and international markets [13,15].
Another demographic parameter that increases the species’ vulnerabilities to habitat degradation and loss is low nesting success and high fluctuation in reproductive success over the years. A study carried out between 1996 and 2003 reported that only 42% of nests observed had young that fledged and that the reproductive output averaged 0.99 fledglings from an initial investment of 2.6 eggs [16]. Furthermore, the same study reported that the clutch size and chick survival varied significantly over the years, thus leading to large interannual fluctuations in reproductive output [16]. Based on the threatening factors above reported, A. finschi is considered a priority species for the conservation of psittacines in Mexico and it is currently listed in the Mexican Official Law (NOM-059) as in danger of extinction. Furthermore, it is included in Appendix I of CITES, and its status was recently assessed by the International Union for Conservation of Nature and Natural Resources (IUCN) as an endangered species [17,18,19].
In conservation biology, the subspecies taxonomic level has been applied successfully to the identification of priorities in management and conservation plans within species populations in their distributions [20,21]. On this topic, Ryder [22] proposed the concept of Evolutionarily Significant Units (ESUs) to guide its application in the conservation of populations within species. Since then, the concept has generated extensive debates and other definitions have been added considering molecular and/or ecological data [23,24,25,26,27,28,29]. In general, concepts based on molecular data propose that the historical information reflected by molecular characters should be considered in designing management strategies [23,25,26,30,31]. In particular, the ESU definition proposed by Moritz [25] considers that, through phylogenetic analyses, groups must be identified via reciprocal monophyly to be recognized as ESUs. One of the main objectives of this concept is to ensure that the heritage and evolutionary potential of groups are recognized and protected. On the other hand, the author proposed using Management Units (MUs) to identify groups that have not reached monophyly. The concept is based on significant divergence of allele frequencies, where, if very low levels of gene flow are observed, groups are functionally independent [25,32]. However, in birds, Zink and Barrowclough [33] stated that to identify whether a species has one or several groups that are evolving independently (phylogroups), mtDNA is widely recognized as the ideal marker because of its high variability and rapid coalescence time.
Supported by the information stored in DNA sequences, molecular phylogeny has made it possible to document the diversity of evolutionary trajectories/paths within species and to infer the historical processes that sustained them [34]. These processes are fundamental in the design of conservation strategies with the aim of preserving the system and allowing it to evolve [25,31,35]. Species conservation programs require the evaluation of intraspecific diversity levels, which helps in the decision to prioritize populations for their conservation from a phylogenetic point of view [25,31,34,35,36,37].
In this study, an analysis of the genetic diversity of A. finschi was carried out by using mtDNA sequences of the small subunit ribosomal RNA (12S), large subunit ribosomal RNA (16S), cytochrome oxidase 1 (COI), and NADH nitrogen dehydrogenase 2 (ND2) genes from two groups of individuals, one from the north (Sinaloa) and the other from the center (Michoacan) of Mexico, belonging to the two recognized subspecies A. f. finschi and A. f. woodi.
2. Materials and Methods
2.1. Biological Samples, DNA Extraction, PCR Amplification, and Sequencing
The samples used in the present study were collected in 2005, 2006, and 2007 under collection permit SGPA/DGVS/06387. The samples came from specimens found in 5 locations in the north and center of the Pacific Slope in Mexico (Sinaloa and Michoacan states) (Table 1, Figure 1). The geographic coordinates of each collection location were recorded. Twenty-one biological samples of blood or feathers were collected from nests without harming the individuals. The samples were preserved by using the method described by Padilla-Jacobo et al. [38] and deposited in the wildlife samples collection at Centro Multidisciplinario de Estudios en Biotecnología (CMEB), at the Universidad Michoacana de San Nicolás de Hidalgo (UMSNH), in Morelia, Michoacan, Mexico.

Table 1.
Sample list of Lilac-crowned Parrot (Amazona finschi) individuals from this study. The sample sizes for the species and each subspecies are indicated, as well as the code assigned to each individual and the type of biological sample. The collection locations indicated with letters (A, B, C, D, and E) can be seen on the map in Figure 1. Additionally, the list of mtDNA sequences and their sizes (base pairs) obtained in this study and those published for the species in GenBank that were used in our analyses are included. The GenBank accession numbers of all sequences are listed in Supplementary Table S1.
DNA was extracted by using the phenol-free method described by FitzSimmons [39]. DNA quality and concentration were verified by agarose gel electrophoresis and on a Nanodrop (Thermo Scientific, Waltham, MA, USA). Fragments of the 12S (12), 16S (14), COI (15), and ND2 mitochondrial genes (18) were amplified (Table 1). The 12S and 16S fragments were amplified by using the primers and PCR conditions described by Miyaki et al. [40], the COI fragment was amplified by using the primers and PCR conditions described by Palumbi et al. [41], and the ND2 fragment was amplified by using the PCR conditions and primers described by Hackett [42]. PCR product quality and concentration were verified by agarose gel electrophoresis and on a Nanodrop (Thermo Scientific, Waltham, MA, USA). The concentrations of the PCR products were adjusted to 30 ng/μL (total volume of 20 μL) for the 12S, 16S, and COI fragments and to 50 ng/μL (total volume of 20 μL) for the ND2 fragments. DNA sequencing was performed by using the Sanger technique on both strands [43] with the commercial service Psomagen (Psomagen Inc., Rockville, MD, USA).
2.2. Sequence Analysis, Genetic Diversity, and Differentiation
To review each electropherogram or chromatogram, Sequencher v.4.1 [44] software was used and, given that the fragments obtained were sequenced in both directions of the chain, the software was used to perform alignments for each pair of sequences and obtain a consensus sequence for each individual. Data matrices were constructed and reviewed using Phyde v0.9971 [45]. Analyses were carried out on sequences generated in this study, along with three sequences available for the species in GenBank-NCBI (Table 1) (see accession numbers in Supplementary Table S1). The number of haplotypes (H) and polymorphic sites (S) and the nucleotide diversity (Pi), haplotype diversity (Hd), and Tajima‘s D [46] were estimated by using ARLEQUIN v3.1 [47]. To estimate genetic diversity and differentiation by group, the sampled individuals were assigned to one of the two recognized subspecies according to their geographical distribution (Michoacan—A. f. finschi—and Sinaloa—A. f. woodi). Pairwise comparisons of FST values were obtained with ARLEQUIN v3.1 [47]. The FST analyses were run with 1000 permutations.
2.3. Haplotype Relationships and Genealogical Analyses
To visualize the frequency and relationships among haplotypes we built haplotype networks with the median-joining method by using NETWORK v5 [48]. The genealogical relationships among haplotypes were built according to the maximum likelihood (ML) criterion and utilizing Bayesian inference (BI), with RaxML [49] and Mr. Bayes v3.1 [50] software, respectively. The data matrix included 2263 concatenated characters (12S, 16S, COI, and ND2 markers) of unique haplotypes. Sequences of the Red-crowned Parrot (Amazona viridigenalis) were included as the outgroup (see accession numbers in Supplementary Table S1). The molecular evolution model was constructed by using jModelTest v2.1.1 [51], where the data were considered as a single matrix and analyzed under the best-fit model selected using the corrected Akaike Information Criterion (cAIC) [52]. The best model obtained by using this criterion was Hasegawa–Kishino–Yano (HKY); this model allows transitions and transversions to occur at different rates and base frequencies to vary as well [53]. Mr. Bayes runs were performed with the following parameters: two independent runs of four chains each, one cold chain, and three hot chains for 10 million generations, sampling one tree every 1000 generations. The branch-support values were estimated by performing a bootstrap analysis (BP) on 500 replicates for the ML criterion and posterior probabilities (PP) for BI. Trees and parameters were summarized after discarding 25% of the data (burn-in). The remaining trees were summarized as a majority consensus tree. The trees were visualized by using FigTree v1.4.0 [54].
3. Results and Discussion
3.1. Genetic Diversity and Differentiation
In this study, 59 sequences were obtained (Table 2) and deposited in GenBank-NCBI (See accession numbers in Supplementary Table S1). For the analysis of genetic diversity using ARLEQUIN v3.1 [47], the data matrix included our sequences of the 12S, 16S, ND2, and COI mitochondrial genes and those available for the species in GenBank-NCBI.

Table 2.
Indices of genetic diversity in individuals of Lilac-crowned Parrot (Amazona finschi). N = number of sequences; Nt = number of characters; H = number of haplotypes; S = polymorphic sites; Hd = haplotype diversity; Pi = nucleotide diversity. Values +/− SD; Tajima’s D value and corresponding p-values, where p > 0.1 means not significant.
The results revealed a lack of genetic diversity for the 12S and ND2 markers; therefore, one haplotype with these sequences was detected in all individuals (Table 2). For the 16S and COI markers, three and two haplotypes were identified, respectively. Few polymorphic sites were observed, one for COI and two for the 16S, and the mutations identified were transitions. According to the values proposed by Grant and Bowen [55], a low haplotypic (below 0.5) and nucleotide diversity (below 0.005) for both markers were detected (Hd = 0.4476, Pi = 0.002358 for 16S and Hd = 0.1250, Pi = 0.000259 for COI) (Table 2). However, these values should be considered with caution mainly because of sampling. Although, in a comparable study on A. albifrons, greater genetic diversity was observed in 16 individuals from Mexico (12S, N = 8, Hd = 0.46, Pi = 0.0018; 16S, N = 16, Hd = 0.66, Pi = 0.0031; COI, N = 16, Hd = 0.85, Pi = 0.0034; ND2, N = 16, Hd = 0.95, Pi = 0.0039) [56]. The above suggests considering whether there are other conditions such as (1) a low mutational rate in the mtDNA of the species (as happens in M. gallopavo [57]); (2) population processes not detected through mtDNA, such as a recent separation, whether it is a metapopulation, etc.; or (3) the effect of subtraction. To resolve them, it is essential, to increase the sampling effort and include markers such as microsatellites that allow observing recent population processes. Given that the population of the species is in decline, we direct attention to a process that may be impacting genetic diversity. It is known that harvesting can alter the sex ratio, effective population size, reproductive system, and gene flow and lead to inbreeding and the loss of genetic diversity [58]. Furthermore, especially when species with a large involvement in the illegal pet market are considered, one has also to consider that selective harvesting for desirable morphological characteristics by humans can change the genetic composition and phenotypes of a population. However, in the case of psittacines in Mexico, the random poaching of chicks in their nests without the selection of morphological characteristics has been documented [13]. Nontheless, subtraction removes rare haplotypes from populations, resulting in a loss of haplotype diversity. For example, in the Orange-fronted Parakeet (Eupsittula canicularis), it was shown that out of 50 poached individuals, 12 were carriers of unique haplotypes, causing their frequency to decrease in the population from which the carriers were poached [59].
Grant and Bowen [55] suggested that Hd and Pi values such as those found in A. finschi (Table 2) can be interpreted as a “Recent population bottleneck or founder event by single or a few mtDNA lineages”; however, because of the size of the sample analyzed, the data are not conclusive. It should be highlighted that the genetic diversity values observed warn about the condition of the species. As is well known, the loss of genetic diversity may cause a reduction in the ability to cope with environmental changes by natural selection in a population; in other words, the ability of a population to evolve depends on genetic diversity [60]. The low genetic diversity detected in individuals of A. finschi contrasts with the results obtained for mitochondrial markers in other psittacine species with which it shares its historical distribution on the Pacific Slope. For instance, for the White-fronted Parrot (Amazona albifrons), the haplotypic diversity is moderate to high, with values ranging from 0.4643 (+/−0.200) to 0.925 (+/−0.038) (N = 16) [56]; for the Orange-fronted Parakeet (E. canicularis), the values are high, with a haplotype diversity from 0.799 to 0.894 (N = 53) [59]; and for the Military Macaw (Ara militaris), the values are also high, with a haplotype diversity of 0.7760 (+/−0.0002) (N = 53) [61]. The populations of these species are under similar threats, such as habitat loss and illegal trade; however, the high genetic deterioration of A. finschi suggests an important effect on their biology.
Concerning evolution population process inference, Tajima‘s D value was significantly negative for 16S and COI markers. Since the value of Tajima‘s D is estimated from Pi and S, if the population is growing, then S will grow faster than Pi, and the difference between the estimates will be negative [62]. In the present analysis, the negative value obtained can be attributed to the presence of haplotypes separated by a single mutation detected in individuals from Sinaloa (Table 2), which would be expected in a growing population. Despite the above, it is worth mentioning that the value was determined by analyzing a few individuals, which should be taken under consideration.
Considering that the 16S and COI markers were the best represented with more sequences among the analyzed individuals, these sequences were concatenated (16S + COI) to estimate the genetic diversity of the northern group (Sinaloa) and the central group (Michoacan) (Table 3).

Table 3.
Indices of genetic diversity in groups of Lilac-crowned Parrot (Amazona finschi) individuals from Sinaloa (A. f. woodi) and Michoacan (A. f. finschi). N = number of sequences; Nt = number of characters; H = number of haplotypes; S = polymorphic sites; Hd = haplotype diversity; Pi = nucleotide diversity; Tajima’s D value and corresponding p-values, p > 0.1 means not significant.
The genetic diversity indices showed high haplotypic and low nucleotide diversities in the Sinaloa group, while the Michoacan group did not reveal genetic diversity (Table 3). It should be considered that this lack of genetic diversity could be the result of the loss of haplotypes due to poaching in Michoacan. Although the sampling was modest and this result should be taken with caution, the lack of genetic diversity in individuals from Michoacan has serious implications for the conservation of the species in this region.
Concerning the Tajima’s D values by group (Sinaloa and Michoacan), the values of zero in the population of Michoacan are highlighted; far from representing a population in equilibrium, this is the result of the evaluation of a single haplotype, with null haplotypic and nucleotide diversity. The Sinaloa group’s value (Tajima’s D = −1.04849) reflects the relationship between Hd and Pi detected in the sample. In general, it can be mentioned that the groups analyzed have gone through different processes, which may be long-term evolutionary processes or recent events, such as poaching.
Some authors have proposed that A. finschi is monotypic because the subspecies A. f. finschi and A. f. woodi show poorly differentiated morphological characteristics, particularly in plumage color. Furthermore, it has been argued that individuals of this species make extensive large-scale movements, which reduces the probability of subspecies formation [1,63,64]. In this study, the presence of a haplotype identified in all individuals with the 12S and ND2 markers is consistent with the proposal of A. finschi being monotypic. However, in the results of the concatenated 16S + COI analysis, the FST value obtained (FST = 0.6, p = 0.0595) (Table 3) shows genetic differentiation between the Sinaloa and Michoacan groups. Nevertheless, this result is influenced by the high genetic diversity among individuals from Sinaloa and the lack of diversity among individuals from Michoacan. We recommend expanding the sampling to identify the possible undetected haplotypes in Michoacan.
3.2. Haplotype Relationships and Genealogical Analyses
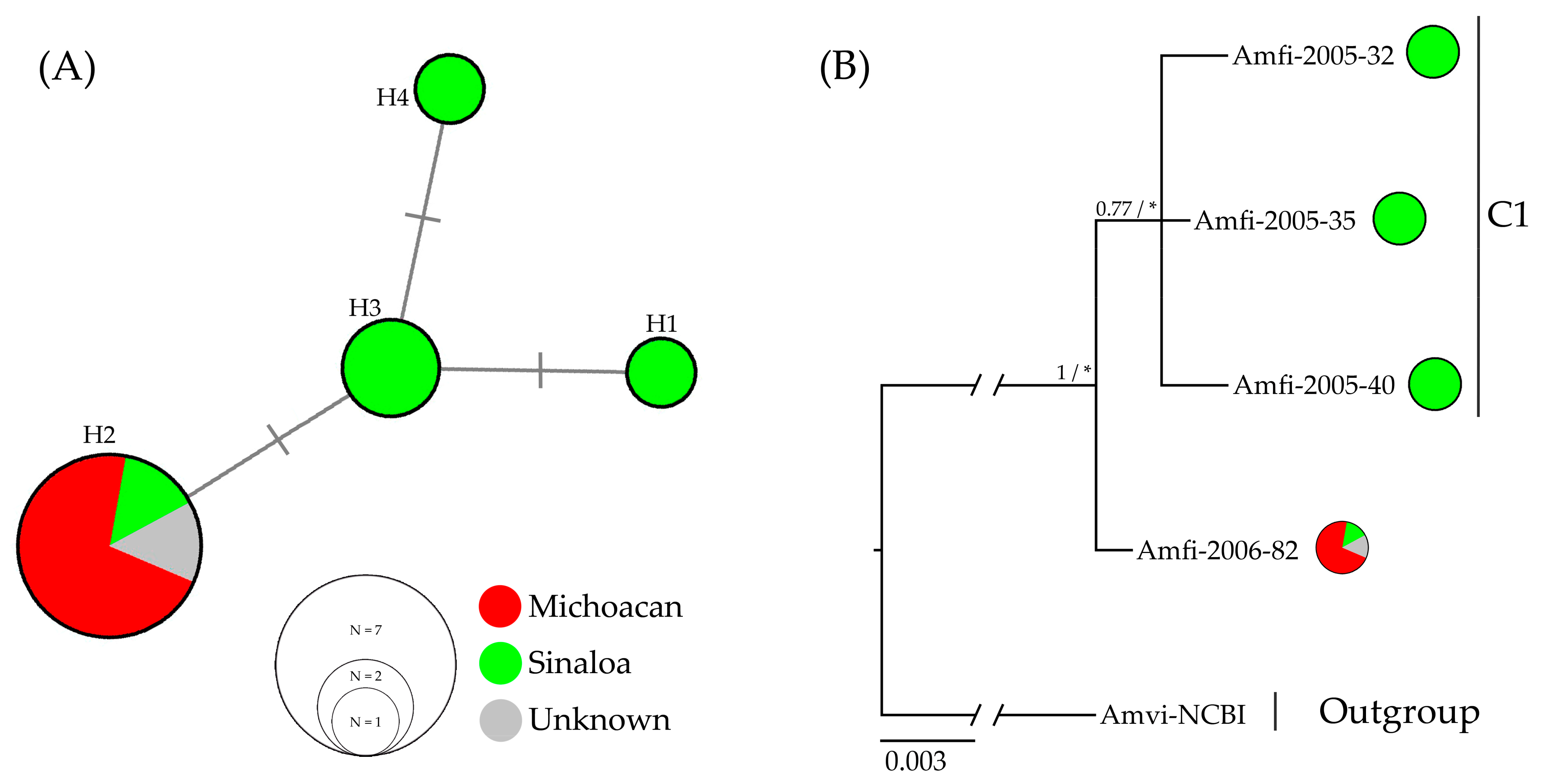
The ND2 and 12S markers were not considered for the haplotype network analysis because they showed a single haplotype; therefore, they do not provide polymorphic sites for estimating networks based on median-joining analysis. We used the 16S and COI markers in a concatenated matrix of eleven individuals, including one reported in GenBank (NCBI). In the resulting network, four haplotypes are observed, separated by a single mutation (Figure 2).
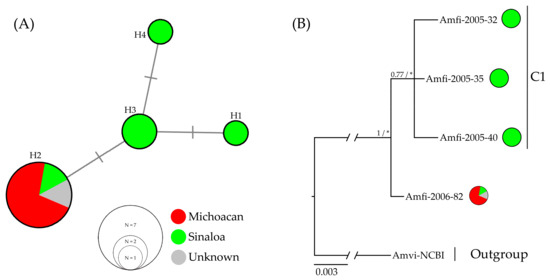
Figure 2.
Haplotype relationships and genealogical analyses: (A) The haplotype network of the A. finschi, median-joining network based on 16S (412 bp) and COI (483 bp) molecular markers; the size of the circles is proportional to the frequency of each haplotype, and transversal lines on the branches represent the mutation. (B) Genealogical relationships built with 2263 characters of unique haplotypes identified in the analyzed samples of A. finschi; a consensus tree obtained with ML and BI analyses is shown; Amvi = Amazona viridigenalis was used as the outgroup; The values at the nodes represent posterior probabilities and bootstrap values (PP/BP); (*) values < 50%. The scale bar under the tree refers to the length of the branches (proportional to the amount of evolutionary change).
The presence of haplotypes exclusive to Sinaloa in the haplotype network explains the genetic differentiation observed between the Sinaloa and Michoacan groups (FST = 0.6, p = 0.0595) (Table 3). Thus, among the individuals from Sinaloa, three exclusive haplotypes were identified, while the individuals from Michoacan revealed a dominant haplotype (H2) shared with the individual Amfi-2005-33 from Sinaloa and that from GenBank (NCBI) of unknown origin (Figure 2). The presence of the dominant H2 haplotype indicates that the species has a genetic lineage with a geographical distribution from the center to the north of the Pacific Slope (Figure 3). Furthermore, considering the Neotropical origin of the species, the common and widespread H2 haplotype between the central and northern Pacific Slope is probably the ancestral condition from which the H1, H2, and H4 haplotypes were recently derived (Figure 3) [65]. To verify this, we performed genealogical tree analyses to identify the ancestor–descendant relationships among haplotypes.
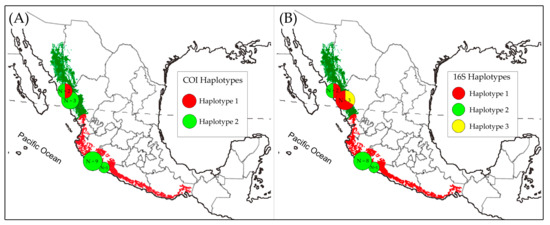
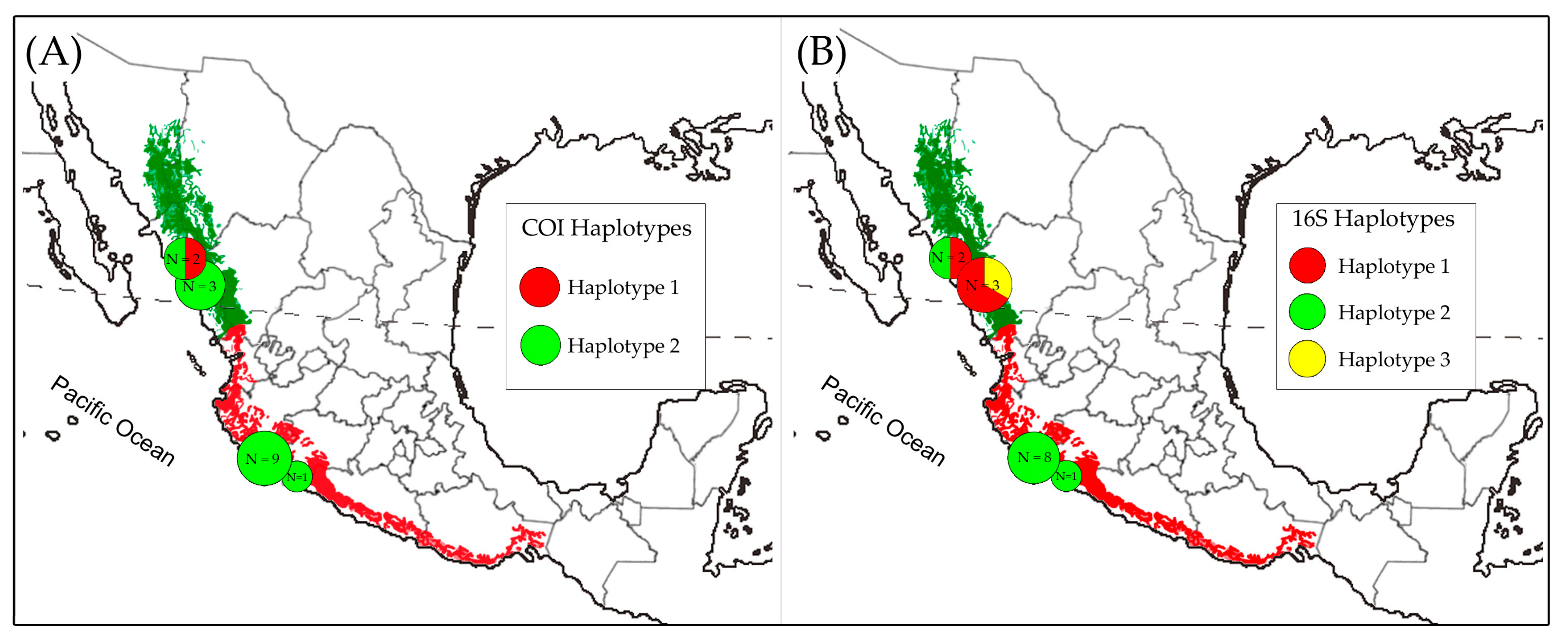
Figure 3.
Geographic distributions of the haplotypes of the Lilac-crowned Parrot (Amazona finschi). The identified (A) COI and (B) 16S haplotypes. The circles indicate the frequency of each haplotype per locality, and the associated colors indicate the different haplotypes identified in this study.
For the genealogical trees construction, the data matrix included concatenated sequences of the 12S (390 bp), 16S (412 bp), COI (483 bp), and ND2 (978 bp) markers (2263 characters in total), with five of the individuals from the one with 43.22% missing data and four having complete sequences. The genealogical relationships among haplotypes were the same in the BI and ML analyses; however, the bootstrap values were not reliable for ML (BP < 50), and in the BI analysis, the posterior probability values were greater than 0.7 (PP > 0.7). The consensus tree of the ML and BI analyses (Figure 2), whose topology is consistent with the haplotype network (Figure 2) and the FST values (Table 3), shows a clade composed exclusively of haplotypes belonging to individuals from Sinaloa (C1). This clade, C1, is related to the haplotype represented by the individual Amfi-2006-82, which corresponds to the dominant H2 haplotype shared by the 15 individuals from Michoacan, the individual from Sinaloa, and the individual from GenBank (NCBI) of unknown origin (Figure 2). The topologies of the tree and the haplotype network suggest that the dominant H2 haplotype is the ancestor of the rest of the haplotypes. Therefore, it is possible that in the north of the species’ geographical distribution, there has been a process of genetic diversification after the arrival and establishment of populations, as has occurred with the Orange-fronted Parakeet (E. canicularis), the White-fronted Parrot (A. albifrons), and the Military Macaw (A. militaris) [56,59,61]. The geographical distribution of the haplotypes identified according to the 16S and COI markers shows the genetic diversity of individuals located in the north of the Pacific Slope (Figure 3).
3.3. Implications for Conservation
Among the psittacines in Mexico, the Lilac-crowned Parrot is notable, as this species has recently been recognized as endangered based on a decreasing population trend and with the loss of its historical distribution range, particularly in Nayarit, Guerrero, and Oaxaca [5,6]. The threats to its populations are well known and have been reported previously [6,11,12,13]. For the conservation of this species endemic to Mexico, data must be gathered from different sources. One of these sources is molecular data, through which fundamental characteristics that help prioritize populations for conservation are determined. For example, variety in the demographic and phylogenetic histories of species can be distinguished [65]. The use of different molecular markers has allowed us to observe various degrees of differentiation among populations, which is fundamental in the determination and proposal of population units for their conservation [66]. Furthermore, molecular data are important in the identification and conservation of the genetic diversity of populations since they constitute the evolutionary potential of the species [60]. To identify whether a species has one or several groups that are evolving independently (phylogroups), mtDNA is widely recognized as the ideal marker because of its high variability and rapid coalescence time [33]. Although it is recommended to include nuclear loci, according to Zink and Barrowclough [33], they are not indispensable since a structured mtDNA gene tree indicates lineage divergence. On the other hand, markers such as microsatellites are useful for observing recent processes in populations such as gene flow, sex-based flow, paternity, isolation, dispersal, introgression, etc. However, they are limited in identifying historical entities because they present homoplasy and difficulty rooting a network. In addition, the clustered objects could be polyphyletic or paraphyletic groupings of genotypes, not monophyletic entities that have unique patterns of ancestry and descent [33].
In this study, the estimation of the genetic diversity of two groups of A. finschi individuals with mtDNA molecular markers revealed low genetic diversity of the species, which has serious implications that may contribute to the rapid decline in its population size [58]. Furthermore, the estimation of genetic diversity for each group revealed high diversity for the northern group (Sinaloa), while the central group (Michoacan) lacked genetic diversity. The loss of genetic variation may have a variety of effects on demographic parameters (survival, reproductive rate, etc.), which can lead to large fluctuations in population size, thus increasing the probability of extinction [60]. Endangered species, by definition, have small or declining populations; these populations are more likely to suffer from inbreeding processes, lower reproductive fitness, lower genetic diversity, and reduced capacity to evolve in response to environmental changes [58]. Particularly, in previous reviews, a correlation has been identified between small population sizes and low levels of genetic diversity [58], which coincides with the reported population decline and estimated genetic values for A. finschi. A large population normally has greater genetic diversity, which generates differences among individuals, allowing them to face various environmental conditions, such as diseases, parasites, competition, predators, pollution, and changes generated by humans [58]. Therefore, the main problem with the loss of genetic diversity in populations is that the ability to face environmental changes is reduced, which increases the risk of extinction [58,60].
Another aspect to highlight derives from the genealogical analysis. We analyzed individuals that belong to the two recognized subspecies of A. finschi (A. f. finschi and A. f. woodi). However, with the molecular data, we found a haplotype (maternal line) distributed in the center and the north (Michoacan and Sinaloa), which does not fit with subspeciation and haplotypes exclusive to the north (Sinaloa) (Figure 2 and Figure 3). From a conservation point of view, if two subspecies exist, two ESUs can be proposed for the species. Several definitions of ESUs can be cited; however, in strict adherence to these concepts, in A. finschi, ESUs could not be identified. According to the ESU concept proposed by Ryder [22], in addition to molecular data, the use of information on the natural history, morphometry, and distribution of populations is required. However, we agree with some authors who believe that the designation of ESUs should be flexible and analyzed on a case-by-case basis [67]. In the case of A. finschi, a decline in its population and a reduction in its distribution have been observed. This, together with the low genetic diversity for the species and the different diversity values for each group observed in the present analysis, provides arguments to suggest differentiated management of the northern and central populations to preserve evolutionary potential [60]. It is worth mentioning that we recommend expanding the analyses by considering additional ecological and molecular data.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d16080435/s1, Table S1: List of GenBank accession numbers of the sequences.
Author Contributions
Conceptualization, G.P.-J., T.C.M.-R. and M.G.Z.-P.; data curation, G.P.-J.; formal analysis, G.P.-J. and M.G.Z.-P.; funding acquisition, T.C.M.-R., H.C.-C. and M.G.Z.-P.; investigation, G.P.-J.; project administration, T.C.M.-R., H.C.-C. and M.G.Z.-P.; resources, T.C.M.-R.; supervision, M.G.Z.-P.; visualization, G.P.-J.; writing—original draft preparation, G.P.-J. and M.G.Z.-P.; writing—review and editing, G.P.-J., T.C.M.-R., H.C.-C. and M.G.Z.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fondos Mixtos, Consejo Nacional de Humanidades, Ciencias y Tecnologías-Michoacán, grant number 41168 to M.G.Z.-P., H.C.-C., and T.C.M.-R., Consejo Nacional de Humanidades, Ciencias y Tecnologías, grant number 2002-C01-00021 to T.C.M.-R., and Coordinación de la Investigación Científica de la Universidad Michoacana de San Nicolás de Hidalgo, grant number Proyect-2016-2017 to M.G.Z.-P.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All sequences were deposited in GenBank-NCBI.
Acknowledgments
The authors thank Secretaría del Medio Ambiente y Recursos Naturales de México for issuing the collection permit (number SGPA/DGVS/06387 to T.C.M.-R.).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
References
- Forshaw, J.M. Parrots of the World; Lansdowne Editions: Sydney, Australia, 1989. [Google Scholar]
- Howell, S.N.G.; Webb, S. A Guide to the Birds of Mexico and Northern Central America; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Renton, K.; Salinas-Melgoza, A. Amazona finschi (Sclater 1864) (Loro Corona Lila). In Historia Natural de Chamela Instituto de Biología; Noguera, F.A., Vega, R.J.H., García, A.A.N., Quesada, A.M., Eds.; Instituto de Biología, Universidad Nacional Autónoma de México: Ciudad de México, México, 2002; pp. 343–344. [Google Scholar]
- Marín-Togo, M.C.; Monterrubio-Rico, T.C.; Renton, K.; Rubio-Rocha, Y.; Macías-Caballero, C.; Ortega-Rodríguez, J.M.; Cancino-Murillo, R. Reduced current distribution of Psittacidae on the Mexican Pacific coast: Potential impacts of habitat loss and capture for trade. Biodivers. Conserv. 2012, 21, 451–473. [Google Scholar] [CrossRef]
- Renton, K.; Iñigo Elías, E. Evaluación del Estado de Conservación de las Poblaciones de loro Corona lila (Amazona finschi) en México; Universidad Nacional Autónoma de México, Instituto de Biología: Ciudad de México, México, 2003. [Google Scholar]
- Monterrubio-Rico, T.C.; Charre-Medellín, J.F.; Pacheco-Figueroa, C.; Arriaga-Weiss, S.; de Dios Valdez-Leal, J.; Cancino-Murillo, R.; Escalona-Segura, G.; Bonilla-Ruz, C.; Rubio-Rocha, Y. Distribución potencial histórica y contemporánea de la familia Psittacidae en México. Rev. Mex. Biodivers. 2016, 87, 1103–1117. [Google Scholar] [CrossRef]
- Forshaw, J.M. Parrots of the World; Princeton University Press: Princeton, NJ, USA, 2010; p. 328. [Google Scholar]
- BLI. The BirdLife Checklist of the Birds of the World (Version 81). Available online: https://datazone.birdlife.org/species/taxonomy (accessed on 24 February 2024).
- Moore, R.T. A New Race of Finsch’s Parrot. Auk 1937, 54, 528–529. [Google Scholar] [CrossRef]
- Friedmann, H.; Griscom, L.; Moore, R.T. Distributional Check-List of the Birds of Mexico, Part I. Pacific Coast Avifauna 1950, 29, 1–202. [Google Scholar]
- Berkunsky, I.; Quillfeldt, P.; Brightsmith, D.J.; Abbud, M.; Aguilar, J.; Alemán-Zelaya, U.; Aramburú, R.M.; Arias, A.A.; McNab, R.B.; Balsby, T.J. Current threats faced by Neotropical parrot populations. Biol. Conserv. 2017, 214, 278–287. [Google Scholar] [CrossRef]
- Collar, N.J.; Juniper, A.T. Dimensions and causes of the parrot conservation crisis. In New World Parrots in Crisis: Solutions from Conservation Biology; Beissinger, S.R., Snyder, N.F.R., Eds.; Smithsonian Institute Press: Washington, DC, USA, 1992; pp. 1–24. [Google Scholar]
- Cantú-Guzmán, J.C.; Sánchez-Saldaña, M.; Grosselet, M.; Silva-Gámez, J. Tráfico Ilegal de Ppericos en México: Una Evaluación Detallada; Defenders of Wildlife: Washington, DC, USA, 2007. [Google Scholar]
- Macías Caballero, C.; Elías, E.I.; Hoeflich, E.E. Proyecto de Recuperación de Especies Prioritarias: Proyecto Nacional Para la Conservación, Manejo y Aprovechamiento Sustentable de los Psitácidos de México; Instituto Nacional de Ecología: Ciudad de México, México, 2000. [Google Scholar]
- UNODC. World Wildlife Crime Report: Trafficking in Protected Species; United Nations: New York, NY, USA, 2016. [Google Scholar]
- Renton, K.; Salinas-Melgoza, A. Nesting behavior of the Lilac-crowned Parrot. Wilson Bull. 1999, 111, 488–493. Available online: https://www.jstor.org/stable/4164133 (accessed on 24 April 2024).
- CITES. The CITES Appendices. Available online: https://cites.org/eng/app/appendices.php (accessed on 21 January 2024).
- IUCN. The IUCN Red List of Threatened Species. Version 2023-1. Available online: https://www.iucnredlist.org (accessed on 21 February 2024).
- DOF. Norma Oficial Mexicana NOM-059-SEMARNAT-2010 Protección Ambiental—Especies Nativas de México de Flora y Fauna Silvestres—Categorías de Riesgo y Especificaciones Para su Inclusión, Exclusión o Cambio—Lista de Especies en Riesgo; Secretaría de Medio Ambiente y Recursos Naturales: Ciudad de México, México, 2010. [Google Scholar]
- Zink, R.M. The role of subspecies in obscuring avian biological diversity and misleading conservation policy. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Phillimore, A.B.; Owens, I.P. Are subspecies useful in evolutionary and conservation biology? Proc. R. Soc. Lond. B Biol. Sci. 2006, 273, 1049–1053. [Google Scholar] [CrossRef]
- Ryder, O.A. Species conservation and systematics: The dilemma of subspecies. Trends Ecol. Evol. 1986, 1, 9–10. [Google Scholar] [CrossRef]
- Avise, J.C. Molecular Markers, Natural History, and Evolution; Chapman & Hall: New York, NY, USA, 1994. [Google Scholar]
- Dizon, A.E.; Lockyer, C.; Perrin, W.F.; Demaster, D.P.; Sisson, J. Rethinking the Stock Concept: A Phylogeographic Approach. Conserv. Biol. 1992, 6, 24–36. [Google Scholar] [CrossRef]
- Moritz, C. Defining “Evolutionarily Significant Units” for conservation. Trends Ecol. Evol. 1994, 9, 373–375. [Google Scholar] [CrossRef]
- Bowen, B. What is wrong with ESUs? The gap between evolutionary theory and conservation principles. J. Shellfish Res. 1998, 17, 1355–1358. [Google Scholar]
- Vogler, A.P.; Desalle, R. Diagnosing Units of Conservation Management. Conserv. Biol. 1994, 8, 354–363. [Google Scholar] [CrossRef]
- Crandall, K.A.; Bininda-Emonds, O.R.P.; Mace, G.M.; Wayne, R.K. Considering evolutionary processes in conservation biology. Trends Ecol. Evol. 2000, 15, 290–295. [Google Scholar] [CrossRef]
- de Guia, A.P.O.; Saitoh, T. The gap between the concept and definitions in the Evolutionarily Significant Unit: The need to integrate neutral genetic variation and adaptive variation. Ecol. Res. 2007, 22, 604–612. [Google Scholar] [CrossRef]
- Moritz, C. Strategies to Protect Biological Diversity and the Evolutionary Processes That Sustain It. Syst. Biol. 2002, 51, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C. Uses of molecular phylogenies for conservation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1995, 349, 113–118. [Google Scholar] [CrossRef]
- Palsboll, P.; Bérubé, M.; Allendorf, F. Identification of management units using population genetic data. Trends Ecol. Evol. 2007, 22, 11–16. [Google Scholar] [CrossRef]
- Zink, R.M.; Barrowclough, G.F. Mitochondrial DNA under siege in avian phylogeography. Mol. Ecol. 2008, 17, 2107–2121. [Google Scholar] [CrossRef] [PubMed]
- Avise, J.C. Phylogeography: The History and Formation of Species; Harvard University Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Moritz, C.; Patton, J.; Schneider, C.; Smith, T. Diversification of Rainforest Faunas: An Integrated Molecular Approach. Annu. Rev. Ecol. Syst. 2000, 31, 533–563. [Google Scholar] [CrossRef]
- Haig, S.M. Molecular contributions to conservation. Ecology 1998, 79, 413–425. [Google Scholar] [CrossRef]
- Yuan, J.H.; Cheng, F.Y.; Zhou, S.L. The phylogeographic structure and conservation genetics of the endangered tree peony, Paeonia rockii (Paeoniaceae), inferred from chloroplast gene sequences. Conserv. Genet. 2011, 12, 1539–1549. [Google Scholar] [CrossRef]
- Padilla-Jacobo, G.; Monterrubio-Rico, T.C.; Camacho, H.C.; Zavala-Páramo, M.G. Use of phylogenetic analysis to identify evolutionarily significant units for the Orange-fronted Parakeet (Eupsittula canicularis) in Mexico. Ornitol. Neotrop. 2016, 26, 325–335. [Google Scholar] [CrossRef]
- FitzSimmons, N.N. Male Marine Turtles: Gene Flow, Philopatry and Mating Systems of the Green Turtle (Chelonia mydas). Ph.D. Thesis, University of Queensland, Brisbane, Australia, 1997. [Google Scholar]
- Miyaki, C.Y.; Matioli, S.R.; Burke, T.; Wajntal, A. Parrot Evolution and Paleogeographical Events: Mitochondrial DNA Evidence. Mol. Biol. Evol. 1998, 15, 544–551. [Google Scholar] [CrossRef]
- Palumbi, S.; Martin, A.; Romano, S.; McMillan, W.; Stice, L.; Grabowski, G. The Simple Fool’s Guide to PCR, Version 2.0; University of Hawaii: Honolulu, HI, USA, 1991. [Google Scholar]
- Hackett, S.J. Molecular Phylogenetics and Biogeography of Tanagers in the Genus Ramphocelus (Aves). Mol. Phylogenet. Evol. 1996, 5, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Genes Codes. Software Sequencher. Available online: http://www.genecodes.com/sequencher (accessed on 6 March 2015).
- Müller, J.; Müller, K.; Neinhuis, C.; Quandt, D. PhyDE-Phylogenetic Data Editor. Available online: http://www.phyde.de/ (accessed on 13 March 2015).
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polimorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 2005, 1, 47–50. [Google Scholar] [CrossRef]
- Fluxus Technology Ltd. Network v5.0 Software: Network Publisher and DNA Alignment Software. Available online: https://www.fluxus-engineering.com/sharenet.htm (accessed on 25 January 2017).
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic Model Averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Alfaro, M.E.; Huelsenbeck, J.P. Comparative Performance of Bayesian and AIC-Based Measures of Phylogenetic Model Uncertainty. Syst. Biol. 2006, 55, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Kishino, H.; Yano, T.A. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Molec. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.4.0. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 16 March 2015).
- Grant, W.; Bowen, B. Shallow population histories in deep evolutionary lineages of marine fishes: Insights from sardines and anchovies and lessons for conservation. J. Hered. 1998, 89, 415–426. [Google Scholar] [CrossRef]
- Cano-Zavala, E.T.; Monterrubio-Rico, T.C.; Zavala-Páramo, M.G.; Cano-Camacho, H.; Padilla-Jacobo, G. Genetic diversity and structure of the White-Fronted Parrot (Amazona albifrons) in Mexico. Ornitol. Neotrop. 2023, 33, 192–201. [Google Scholar] [CrossRef]
- Padilla-Jacobo, G.; Cano-Camacho, H.; López-Zavala, R.; Cornejo-Pérez, M.E.; Zavala-Páramo, M.G. Evolutionary history of Mexican domesticated and wild Meleagris gallopavo. Genet. Sel. Evol. 2018, 50, 19. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R.; Ballou, J.D.; Briscoe, D.A.; McInnes, K.H. A Primer of Conservation Genetics; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Padilla-Jacobo, G.; Monterrubio-Rico, T.; Cano-Camacho, H.; Zavala-Páramo, M.G. Demographic history of the Orange-fronted Parakeet (Eupsittula canicularis) in Mexico. Ornitol. Neotrop. 2018, 29, 323–336. [Google Scholar] [CrossRef]
- Allendorf, F.W.; Luikart, G.H. Conservation and the Genetics of Populations; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Rivera-Ortíz, F.A.; Sanabria-Urbán, S.; Prieto-Torres, D.A.; Navarro-Singüenza, A.G.; Arizmendi, M.d.C.; Oyama, K. Phylogeography of Ara militaris (Military Macaw): Implications for Conservation. Diversity 2023, 15, 1035. [Google Scholar] [CrossRef]
- Hedrick, P.W. Genetics of Populations; Jones & Bartlett Learning: Sudbury, MA, USA, 2011. [Google Scholar]
- Collar, N.J. Family Psittacidae (Parrots). In Handbook of the Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Eds.; Lynx Edicions: Barcelona, Spain, 1997; pp. 280–477. [Google Scholar]
- Salinas-Melgoza, A.; Renton, K. Postfledging Survival and Development of Juvenile Lilac-Crowned Parrots. J Wildl Manag. 2007, 71, 43–50. [Google Scholar] [CrossRef]
- Avise, J.C. Phylogeography: Retrospect and prospect. J. Biogeogr. 2009, 36, 3–15. [Google Scholar] [CrossRef]
- Freeland, J.R. Molecular Ecology; John Wiley & Sons, Ltd.: Chichester, UK, 2005. [Google Scholar]
- Fraser, D.J.; Bernatchez, L. Adaptive evolutionary conservation: Towards a unified concept for defining conservation units. Mol. Ecol. 2001, 10, 2741–2752. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).