Diversity and Distribution of Monocot Understory Herbs during Tropical Forest Succession in Northeastern Costa Rica
Abstract
:1. Introduction
2. Methods
2.1. Study Area
2.2. Data Collection
2.3. Statistical Analysis
3. Results
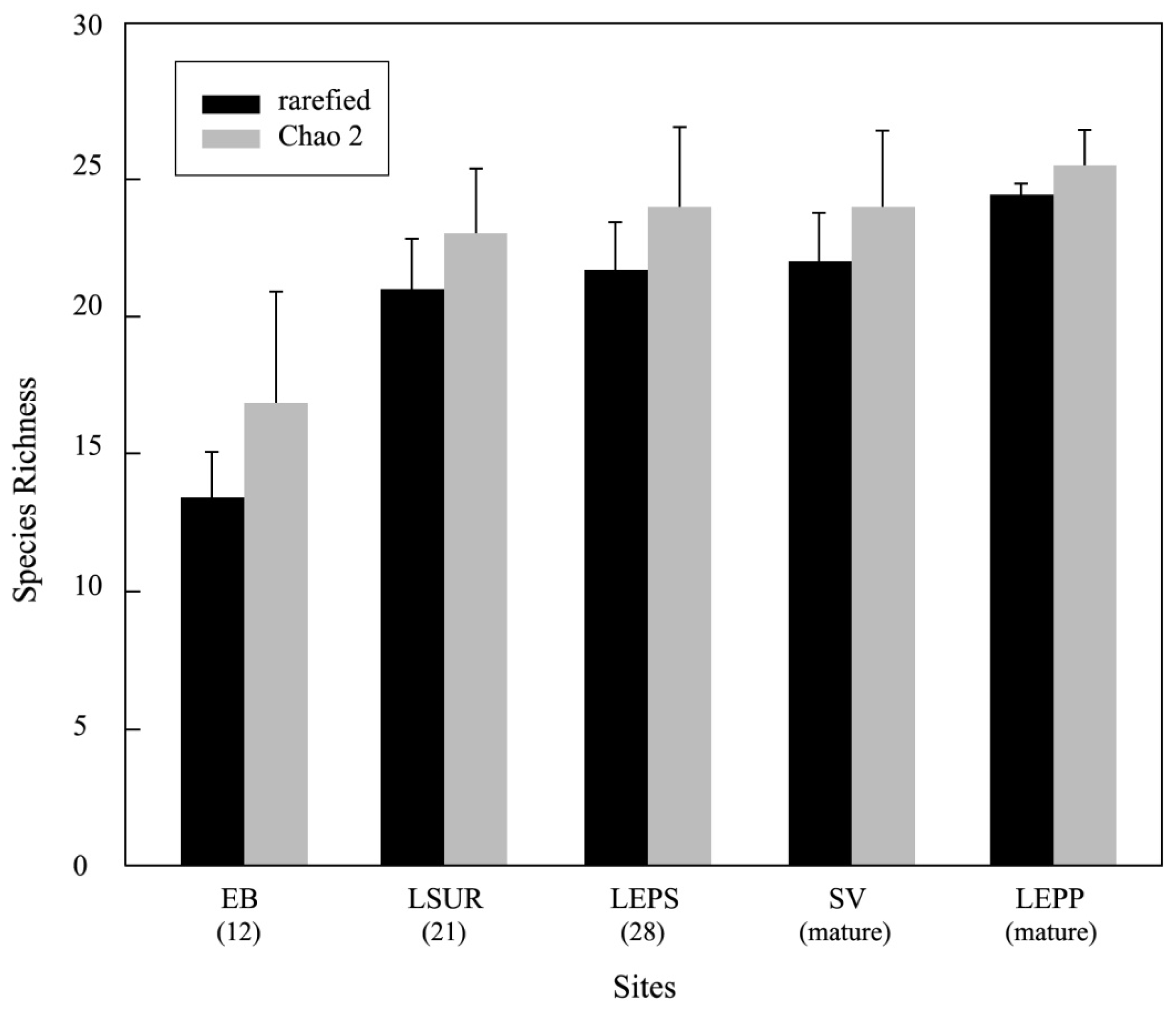
3.1. Distribution of Diversity
3.2. Density and Coverage
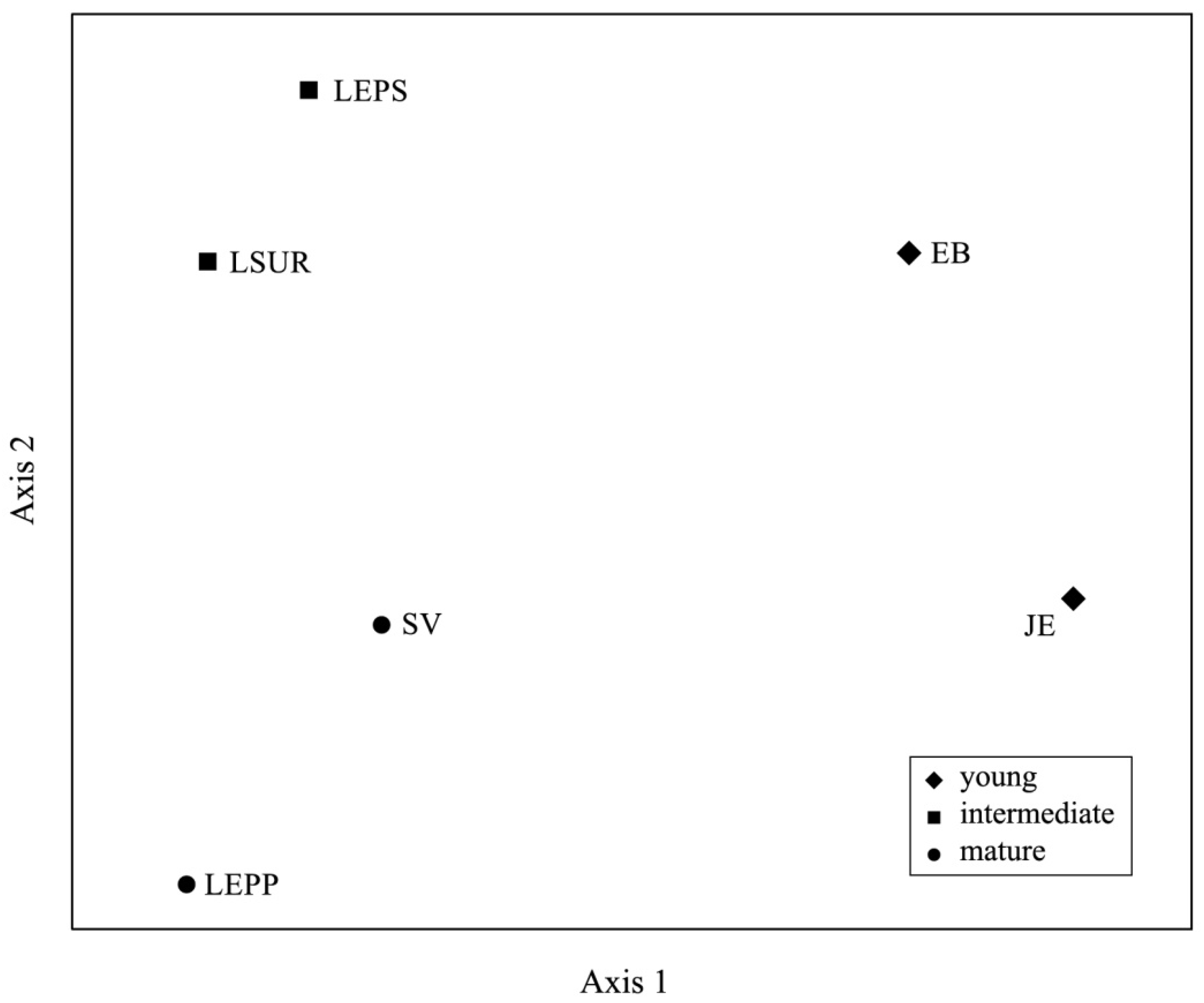
3.3. Floristic Similarity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chazdon, R.L. Second Growth: The Promise of Regeneration in an Age of Deforestation; University of Chicago Press: Chicago, IL, USA, 2014. [Google Scholar]
- Chazdon, R.L. Tropical forests–log ‘em or leave’ em? Science 1998, 281, 1295–1296. [Google Scholar] [CrossRef]
- Chazdon, R.L. Tropical forest recovery: Legacies of human impact and natural disturbances. Perspect. Plant Ecol. Evol. Syst. 2003, 6, 51–71. [Google Scholar] [CrossRef]
- Wright, S.J.; Muller-Landau, H.C. The future of tropical forest species. Biotropica 2006, 38, 287–301. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Coe, F.G. Ethnobotany of woody species in second-growth, old-growth, and selectively logged forests of northeastern Costa Rica. Conserv. Biol. 1999, 13, 1312–1322. [Google Scholar] [CrossRef]
- Silver, W.L.; Kueppers, L.M.; Lugo, A.E.; Ostertag, R.; Matzek, V. Carbon sequestration and plant community dynamics following reforestation of tropical pasture. Ecol. Appl. 2004, 14, 1115–1127. [Google Scholar] [CrossRef]
- Ticktin, T. The ecological implications of harvesting non-timber forest products. J. Appl. Ecol. 2004, 41, 11–21. [Google Scholar] [CrossRef]
- Grace, J. Understanding and managing the global carbon cycle. J. Ecol. 2004, 92, 189–202. [Google Scholar] [CrossRef]
- Feldpausch, T.R.; Riha, S.J.; Fernandes, E.C.M.; Wandell, E.V. Development of forest structure and leaf area in secondary forests regenerating on abandoned pastures in Central Amazonia. Earth Interact. 2005, 9, 1–22. [Google Scholar] [CrossRef]
- Hubbell, S.P.; Foster, R.B.; O’Brien, S.T.; Harms, K.E.; Condit, R.C.; Wechsler, B.; Wright, S.J.; Loo de Lao, S. Light-gap disturbances, recruitment limitation, and tree diversity in a neotropical rainforest. Science 1999, 283, 554–557. [Google Scholar] [CrossRef]
- Capers, R.S.; Chazdon, R.L.; Brenes, A.R. Successional dynamics of woody seedling communities in wet tropical secondary forests. J. Ecol. 2005, 93, 071–1084. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Finegan, B.; Capers, R.S.; Salgado-Negret, B.; Casanoves, F.; Boukili, V.; Norden, N. Composition and dynamics of functional groups of trees during tropical forest succession in northeastern Costa Rica. Biotropica 2010, 42, 31–40. [Google Scholar] [CrossRef]
- Dewalt, S.J.; Schnitzer, S.A.; Denslow, J.S. Density and diversity of lianas along a chronosequence in a central Panamanian lowland forest. J. Trop. Ecol. 2000, 16, 1–19. [Google Scholar] [CrossRef]
- Galeano, G.; Suárez, S.; Balslev, H. Vascular plant species count in a wet forest in the Chocó area on the pacific coast of Colombia. Biodivers. Conserv. 1998, 7, 1563–1575. [Google Scholar] [CrossRef]
- Costa, F.R.C. Structure and composition of the ground-herb community in a terra-firme central Amazonian forest. Acta Amaz. 2004, 34, 53–59. [Google Scholar] [CrossRef]
- Rundel, P.W.; Cooley, A.; Gerst, K.L.; Riordan, E.C.; Sharifi, M.R.; Sun, J.W.; Tower, J.A. Functional traits of broad-leaved monocot herbs in the understory and forest edges of a Costa Rican rainforest. PeerJ 2020, 8, e9958. [Google Scholar] [CrossRef]
- Benítez-Malvido, J. Effect of low vegetation on the recruitment of plants in successional habitat types. Biotropica 2006, 38, 171–182. [Google Scholar] [CrossRef]
- Rundel, P.W.; Sharifi, M.R.; Gibson, A.C.; Esler, K.J. Structural and physiological adaptation to light environments in neotropical Heliconia (Heliconiaceae). J. Trop. Ecol. 1998, 14, 789–801. [Google Scholar] [CrossRef]
- Cooley, A.M.; Reich, J.A.; Rundel, P.W. Petiole biomechanics of neotropical understory monocots. Am. J. Bot. 2004, 91, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Wunderle, J.M.; Willig, M.R.; Henriques, L.M.P. Avian distribution in treefall gaps and understory of terra firme forest in the lowland Amazon. Ibis 2005, 147, 109–129. [Google Scholar] [CrossRef]
- Horvitz, C.C.; Schemske, D.W. Effects of plant size, leaf herbivory, local competition and fruit production no survival, growth and future reproduction of a neotropical herb. J. Ecol. 2002, 90, 279–290. [Google Scholar] [CrossRef]
- Kay, K.M.; Schemske, D.W. Pollinator assemblages and visitation rates for 11 species of neotropical Costus (Costaceae). Biotropica 2003, 35, 198–207. [Google Scholar]
- Berry, F.; Kress, W.J. Heliconia: An Identification Guide; Smithsonian Institution Press: Washington, DC, USA, 1991. [Google Scholar]
- Salick, J.; Mejia, A.; Anderson, T. Non-timber forest products integrated with natural forest management, Río San Juan, Nicaragua. Ecol. Appl. 1995, 5, 878–895. [Google Scholar] [CrossRef]
- Castro, C.E.F.; Graziano, T.T. Espécies de Heliconia (Heliconiaceae) no Brasil. Rev. Bras. Hortic. Ornam. 1997, 3, 15–28. [Google Scholar]
- Lorenzi, H.; de Souza, H.M. Plantas Ornamentais No Brasil; Instituto Plantarum, Nova Odessa: São Paulo, Brazil, 1999. [Google Scholar]
- Arruda, R.; Carvalho, V.T.; Andrade, P.C.M.; Pinto, M.G. Heliconias of the Baixo Jurua Extractive Reserve: Economical potential for Amazonian traditional population. Acta Amazon. 2008, 38, 611–616. [Google Scholar] [CrossRef]
- Nakazono, E.M.; Bruna, E.M.; Mesquita, R.C.G. Experimental harvesting of the non-timber forest product Ischnosiphon polyphyllus in central Amazonia. For. Ecol. Manag. 2004, 190, 219–225. [Google Scholar] [CrossRef]
- Ticktin, T.; Johns, T.; Chapol Xoca, V. Patterns of growth in Aechmea magdalenae (Bromeliaceae) and its potential as a forest crop and conservation strategy. Agric. Ecosyst. Environ. 2003, 94, 123–139. [Google Scholar] [CrossRef]
- Royo, A.A.; Carson, W.P. The herb community of a tropical forest in central Panamá: Dynamics and impact of mammalian herbivores. Oecologia 2005, 145, 66–75. [Google Scholar] [CrossRef]
- Smith, A.P. Respuestas de hierbas del sotobosque tropical a claros ocasionados por la caída de árboles. Rev. Biol. Trop. 1987, 35, 111–118. [Google Scholar]
- Dirzo, R.; Horvitz, C.C.; Quevedo, H.; López, M.A. The effect of gap size and age on understory herb community of a tropical Mexican rain forest. J. Ecol. 1992, 80, 809–822. [Google Scholar] [CrossRef]
- Costa, F.R.C.; Magnusson, W. Selective logging effects on abundance, diversity, and composition of tropical understory herbs. Ecol. Appl. 2002, 12, 807–819. [Google Scholar] [CrossRef]
- Ribeiro, M.B.N.; Bruna, E.M.; Mantovani, W. Influence of post-clearing treatment on the recovery of herbaceous plant communities in Amazonian secondary forests. Restor. Ecol. 2010, 18, 50–58. [Google Scholar] [CrossRef]
- Letcher, S.G.; Chazdon, R.L. Rapid recovery of biomass, species richness, and species composition in a forest chronosequence in northeastern Costa Rica. Biotropica 2009, 41, 608–617. [Google Scholar] [CrossRef]
- Norden, N.; Chazdon, R.L.; Chao, A.; Jiang, Y.-H.; Vilchez-Alvarado, B. Resilience of tropical rain forests: Tree community reassembly in secondary forests. Ecol. Lett. 2009, 12, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Chazdon, R.L.; Alvarado, B.V.; Letcher, S.G.; Wendt, A.; Sezen, U.U. Effects of human activities on successional pathways: Case studies from lowland wet forests of northeastern Costa Rica. In The Social Lives of Forests: Past, Present, and Future of Woodland Resurgence Get Access Arrow 2014; University of Chicago Press: Chicago, IL, USA, 2014; pp. 129–142. [Google Scholar]
- Holdridge, L.R.; Grenk, W.G.; Hatheway, W.H.; Liang, T.; Tosi, J.A. Forest Environments in Tropical Life Zones; Pergamon Press: New York, NY, USA, 1975. [Google Scholar]
- Graham, E.A.; Hansen, M.; Kaiser, W.J.; Lam, Y.; Stealey, M.J.; Yuen, E.; Rundel, P.W. Dynamic microclimate boundaries across a sharp tropical rainforest clearing edge. Remote Sens. 2021, 13, 1646. [Google Scholar] [CrossRef]
- Sollins, P.; Sancho, F.; Mata, R.; Sanford, R.L. Soils and soil process research. In La Selva: Ecology and Natural History of a Neotropical Rainforest; McDade, L.A., Bawa, K.S., Hespenheide, H., Hartshorn, G.S., Eds.; University of Chicago Press: Chicago, IL, USA, 1994; pp. 35–53. [Google Scholar]
- Butterfield, R.P. The regional context: Land colonization and conservation in Sarapiquí. In La Selva: Ecology and Natural History of a Neotropical Rain Forest; McDade, L.A., Bawa, K.S., Hespenheide, H.A., Hartshorn, G.S., Eds.; University of Chicago Press: Chicago, IL, USA, 1994; pp. 299–306. [Google Scholar]
- Guariguata, M.R.; Chazdon, R.L.; Denslow, J.S.; Dupuy, J.M.; Anderson, L. Structure and floristics of secondary and old-growth forest stands in lowland Costa Rica. Plant Ecol. 1997, 132, 107–120. [Google Scholar] [CrossRef]
- Read, J.M. Land-Cover Change Detection for the Tropics Using Remote Sensing and Geographic Information Systems. Ph.D. Dissertation, Louisiana State University, Baton Rouge, LA, USA, 1999. [Google Scholar]
- Pierce, S.M. Environmental history of La Selva biological Station: Colonization and deforestation of Sarapiquí Canton, Costa Rica. In Changing Tropical Forests: Historical Perspectives on Today’s Challenges in Central and South America; Steen, H.K., Rucker, R.P., Eds.; Forest History Society: Durham, NC, USA, 1992; pp. 40–57. [Google Scholar]
- Philips, O.; Miller, J.S. Global Patterns of Plant Diversity: Alwyn H. Gentry’s Forest Transect Data Set; Missouri Botanical Garden Press: St. Louis, MO, USA, 2002. [Google Scholar]
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. B 1994, 345, 101–118. [Google Scholar]
- Chazdon, R.L.; Colwell, R.K.; Denslow, J.S.; Guariguata, M.R. Statistical methods for estimating species richness of woody regeneration in primary and secondary rain forests of NE Costa Rica. In Forest Biodiversity Research, Monitoring and Modeling: Conceptual Background and Old World Case Studies; Dallmeier, F., Comiskey, J.A., Eds.; Parthenon Publishing: Paris, France, 1998; pp. 285–309. [Google Scholar]
- Gotelli, N.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Longino, J.T.; Coddington, J.; Colwell, R.K. The ant fauna of a tropical rain forest: Estimating species richness three different ways. Ecology 2002, 83, 689–702. [Google Scholar] [CrossRef]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.-J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 2005, 8, 148–159. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 8.00. 2007. Available online: http://purl.oclc.org/estimates (accessed on 1 January 2010).
- Colwell, R.K.; Elsensohn, J.E. EstimateS turns 20: Statistical estimation of species richness and shared species from samples, with non-parametric extrapolation. Ecography 2014, 37, 609–613. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Lance, R.M.; Young, F.W. Introduction to Multidimensional Scaling: Theory, Methods, and Applications; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 1 January 2010).
- Chazdon, R.L.; Letcher, S.G.; van Breugel, M.; Martínez-Ramos, M.; Bongers, F.; Finegan, B. Rates of change in tree communities of secondary Neotropical forests following major disturbances. Philos. Trans. R. Soc. Lond. Ser. B 2007, 362, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Pickett, S.T.A.; Collins, S.L.; Armesto, J.J. Models, mechanisms and pathways of succession. Bot. Rev. 1987, 53, 335–371. [Google Scholar] [CrossRef]
- Pfitsch, W.A.; Smith, A.P. Growth and photosynthesis of Aechmea magdalenae, a terrestrial CAM plant in a tropical moist forest, Panama. J. Trop. Ecol. 1988, 4, 199–207. [Google Scholar] [CrossRef]
- Villegas, A.C. Spatial and temporal variability in clonal reproduction of Aechmea magdalenae, a tropical understory herb. Biotropica 2001, 33, 48–59. [Google Scholar] [CrossRef]
- Dupuy, J.M.; Chazdon, R.L. Effects of vegetation cover on seedling and sapling dynamics in secondary tropical wet forests in Costa Rica. J. Trop. Ecol. 2006, 22, 65–76. [Google Scholar] [CrossRef]
- Harms, K.E.; Powers, J.S.; Montgomery, R.A. Variation in small sapling density, understory cover, and resource availability in four neotropical forests. Biotropica 2004, 36, 40–51. [Google Scholar] [CrossRef]
- Uriarte, M.; Canham, C.D.; Thompson, J.; Zimmerman, J.K.; Brokaw, N. Seedling recruitment in a hurricane-drive tropical forest: Light limitation, density-dependence and the spatial distribution of parent trees. J. Ecol. 2005, 93, 291–304. [Google Scholar] [CrossRef]
- Wills, C.; Harms, K.E.; Condit, R.; King, D.; Thompson, J.; He, F.; Muller-Landau, H.C.; Ashton, P.; Losos, E.; Comita, L.; et al. Nonrandom processes maintain diversity in tropical forests. Science 2006, 27, 527–531. [Google Scholar] [CrossRef]
- Guevara, S.; Purata, S.E.; van der Maarl, E. The role of remnant forest trees in tropical secondary succession. Vegetatio 1986, 66, 77–84. [Google Scholar] [CrossRef]
- Slocum, M.G.; Horvitz, C.C. Seed arrival under different genera of trees in a neotropical pasture. Plant Ecol. 2000, 149, 51–62. [Google Scholar] [CrossRef]
- Guevara, S.; Laborde, J.; Sánchez-Rios, G. Rain forest regeneration beneath the canopy of fig trees isolated in pastures of Los Tuxtlas, Mexico. Biotropica 2004, 36, 99–108. [Google Scholar] [CrossRef]
- Schlawin, J.R.; Zahawi, R.A. ‘Nucleating’ succession in recovering neotropical wet forests: The legacy of remnant trees. J. Veg. Sci. 2008, 19, 485–492. [Google Scholar] [CrossRef]
- Aide, T.M.; Cavalier, J. Barriers to tropical lowland forest restoration in the Sierra Nevada de Santa Marta, Colombia. Restor. Ecol. 1994, 2, 219–229. [Google Scholar] [CrossRef]
- Walker, L.R. Effects of fern thickets on woodland development on landslides in Puerto Rico. J. Veg. Sci. 1995, 5, 425–532. [Google Scholar] [CrossRef]
- Suazo Ortuño, I. Aspectos Ecológicos de Pteridium aquilinum (Polypodiaceae) en la Región de Chajul, Chiapas. Master’s Thesis, Facultad de Biología, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Mexico, 1998. [Google Scholar]
- George, L.O.; Bazzaz, F.A. The fern understory as an ecological filter: Emergence and establishment of canopy tree seedlings. Ecology 1999, 80, 833–845. [Google Scholar] [CrossRef]
- Matlaga, D.P.; Horvitz, C. Growth and survival across a gap– understory gradient: Contrast in performance of sexually vs. clonally produced offspring. Am. J. Bot. 2009, 96, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Matlaga, D.P.; Horvitz, C.C. Large size and high light do not lower the cost of reproduction for the Neotropical herb Goeppertia marantifolia. Am. J. Bot. 2015, 102, 350–357. [Google Scholar] [CrossRef]
- Matlaga, D.P.; Snyder, R.K.; Horvitz, C.C. Dispersal of Goeppertia marantifolia clonal offspring increases with greater canopy openness and larger plant size. J. Trop. Ecol. 2017, 33, 107–113. [Google Scholar] [CrossRef]
- van Breugel, M.; Bongers, F.; Martínez-Ramos, M. Species dynamics during early secondary forest succession: Recruitment, mortality and species turnover. Biotropica 2007, 35, 610–619. [Google Scholar] [CrossRef]
- Hanson, T.; Brunsfeld, S.; Finegan, B. Variation in seedling density and seed predation indicators for the emergent tree Dipteryx panamensis in continuous and fragmented rain forest. Biotropica 2006, 38, 770–774. [Google Scholar] [CrossRef]
- Franklin, J.; Rey, S.J. Spatial patterns of tropical forest trees in Western Polynesia suggest recruitment limitations during secondary succession. J. Trop. Ecol. 2007, 23, 1–12. [Google Scholar] [CrossRef]
- Beck, H. Seed predation and dispersal by peccaries throughout the Neotropics and its consequences: A review and synthesis. In Seed Fate; Forget, P.M., Lambert, J.E., Hulme, P.E., Wall, S.B.V., Eds.; CABI Publishing: Cambridge, MA, USA, 2005; pp. 77–100. [Google Scholar]
- Wang, Y.H.; Augspurger, C. Dwarf palms and cyclanth strongly reduce Neotropical seedling recruitment. Oikos 2004, 107, 619–633. [Google Scholar] [CrossRef]
- Corlett, R.T. The ecological transformation of Singapore, 1819–1990. J. Biogeogr. 1992, 19, 411–420. [Google Scholar] [CrossRef]
- Clark, D.B. Abolishing virginity. J. Trop. Ecol. 1996, 12, 735–739. [Google Scholar] [CrossRef]
- Finegan, B. Pattern and process in neotropical secondary rain forests: The first 100 years of succession. Trends Ecol. Evol. 1996, 11, 119–124. [Google Scholar] [CrossRef]
- Dewalt, S.J.; Maliakal, S.K.; Denslow, J.S. Changes in vegetation structure and composition along a tropical forest chronosesquence: Implications for wildlife. For. Ecol. Manag. 2003, 182, 139–151. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Ostertag, R. Neotropical secondary succession: Changes in structural and functional characteristics. For. Ecol. Manag. 2001, 148, 185–206. [Google Scholar] [CrossRef]
- Letcher, S.G.; Chazdon, R.L. Lianas and self-supporting plants during tropical forest succession. For. Ecol. Manag. 2009, 257, 2150–2156. [Google Scholar] [CrossRef]
- Gleason, H.A. The individualistic concept of the plant association. Bull. Torrey Bot. Club 1926, 53, 7–26. [Google Scholar] [CrossRef]
- Chazdon, R.L. Chance and determinism in tropical forest succession. In Tropical Forest Community Ecology; Carson, W., Schnitzer, S.A., Eds.; Wiley-Blackwell Publishing: Oxford, UK, 2008; pp. 384–408. [Google Scholar]
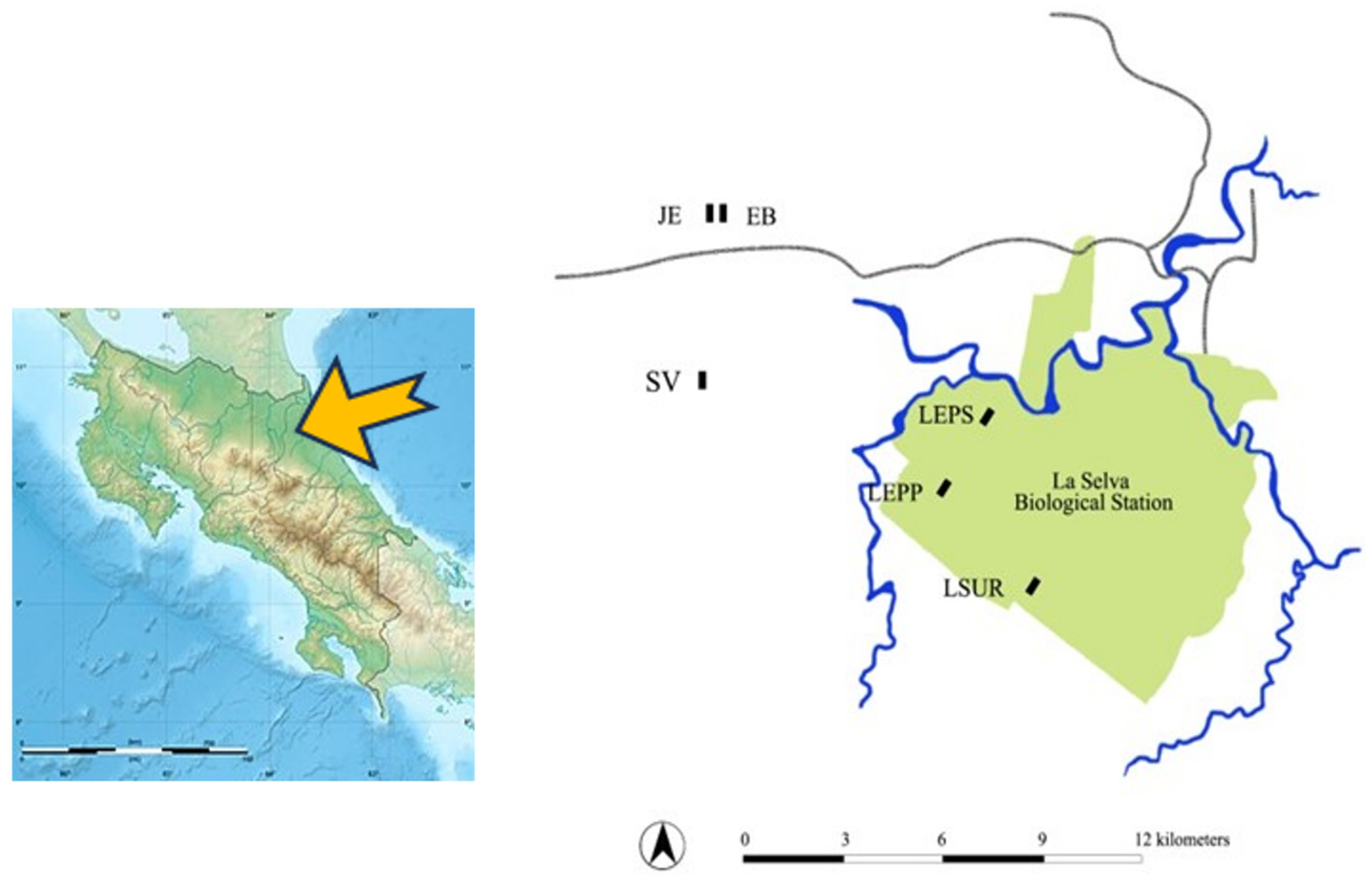


| Site | Latitude, Longitude | Location | Age in 2006 (yrs) | Prior Land-Use | Landscape Matrix |
|---|---|---|---|---|---|
| Juan Enriquez (JE) | 10.46° N, 84.07° W | Chilamate | 12 | Pasture (ca. 12 yr) | Pasture, secondary, and mature forests |
| El Bejuco(EB) | 10.46° N, 84.67° W | Chilamate | 12 | Pasture (ca. 10 yr) | Pasture, secondary, and mature forests |
| Lindero Sur (LSUR) | 10.41° N, 84.03° W | La Selva | 21 | Pasture (ca. 5 yr) | Mature and secondary forests |
| Peje Second- growth (LEPS) | 10.43° N, 84.03° W | La Selva | 29 | Pasture (ca. 5 yr) | Mature and secondary forests |
| Selva Verde (SV) | 10.44° N, 84.07° W | Chilamate | – | Mature forest | Pasture, secondary, and mature forests |
| Peje Old-growth (LEPP) | 10.42° N, 84.04° W | La Selva | – | Mature Forest | Mature and secondary forests |
| YOUNG | INTERMEDIATE | MATURE | CLONALITY | |||||
|---|---|---|---|---|---|---|---|---|
| FAMILY | SPECIES | JE | EB | LSUR | LEPS | SV | LEPP | |
| Araceae | Anthurium lancifolium | 0.8 | 0.1 | NC | ||||
| Anthurium ochranthum | 1.7 | 5.8 | 11.7 | 10 | 5 | NC | ||
| Dieffenbachia grayumiana | 17.5 | 26.7 | 5.8 | 0.8 | 0.8 | 2.5 | WC | |
| Dieffenbachia hammelii | 3.3 | NC | ||||||
| Dracontium gigas | 0.8 | NC | ||||||
| Philodendron grandipes | 0.8 | 4.2 | 32.5 | 41.7 | 0.8 | 40.8 | SC | |
| Spathiphyllum friedrichsthalii | 0.8 | SC | ||||||
| Spathiphyllum fulvovirens | 0.8 | 4.2 | 12.5 | 58.3 | 24.2 | 66.7 | NC | |
| Spathiphyllum laeve | 1.7 | 3.3 | 17.5 | 20 | 33.3 | 39.2 | NC | |
| Spathiphyllum phryniifolium | 1.7 | 8.3 | 37.5 | 5.8 | 28.3 | 5 | WC | |
| Costaceae | Costus bracteatus | 0.8 | 0.8 | 13.3 | 11.7 | 6.7 | 6.7 | C |
| Costus laevis | 10 | 4.2 | 2.5 | C | ||||
| Costus malortieanus | 5 | 4.2 | 30.0 | 8.3 | 0.8 | 3.3 | SC | |
| Costus pulverulentus | 5.0 | 1.7 | WC | |||||
| Costus scaber | 8.3 | 20.8 | 15 | 14.2 | WC | |||
| Cyclanthaceae | Asplundia longipetula | 0.8 | 1.7 | NC | ||||
| Asplundia sleeperae | 1.7 | NC | ||||||
| Asplundia uncinata | 6.7 | 5.0 | 0.8 | 13.3 | C | |||
| Cyclanthus bipartitus | 11.7 | 19.2 | 7.5 | 29.2 | 33.3 | 20.8 | SC | |
| Carludovica sulcata | 0.8 | SC | ||||||
| Dicranopygium umbrophilum | 14.2 | 70.8 | NC | |||||
| Heliconiaceae | Heliconia hursuita | 6.7 | ||||||
| Heliconia imbricata | 0.8 | WC | ||||||
| Heliconia irrasa | 46.7 | 17.5 | 18.3 | 11.7 | NC | |||
| Heliconia latispatha | 8.3 | 5.8 | 1.7 | 7.5 | C | |||
| Heliconia mathiasiae | 1.7 | 14.2 | 9.2 | 2.5 | C | |||
| Heliconia pogonantha | 1.7 | C | ||||||
| Heliconia umbrophila | 1.7 | 2.5 | 5.0 | NC | ||||
| Marantaceae | Calathea lasiostachya | 1.7 | 15.0 | 12.5 | 12.5 | 2.5 | NC | |
| Goeppertia cleistantha | 65.0 | 52.5 | 15.0 | 7.5 | C | |||
| Goeppertia hammelii | 0.1 | C | ||||||
| Goeppertia inocephala | 30.8 | 17.5 | 0.8 | NC | ||||
| Goeppertia leucostachys | 0.8 | WC | ||||||
| Goeppertia micans | 9.2 | 87.5 | 80 | 78.3 | 43.3 | 35 | SC | |
| Goeppertia venusta | 0.1 | 4.2 | NC | |||||
| Ischnosiphon inflatus | 0.8 | 0.8 | 0.8 | 5.3 | 13.3 | 11.7 | WC | |
| Pleiostachya pruinosa | 0.8 | 0.1 | WC | |||||
| Zingiberaceae | Renealmia cernua | 25.8 | 3.3 | 1.7 | WC | |||
| Renealmia pluriplicata | 0.8 | 9.2 | 8.3 | 5.8 | 2.5 | WC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.W.C.; Chazdon, R.L.; Rundel, P.W. Diversity and Distribution of Monocot Understory Herbs during Tropical Forest Succession in Northeastern Costa Rica. Diversity 2024, 16, 439. https://doi.org/10.3390/d16080439
Sun JWC, Chazdon RL, Rundel PW. Diversity and Distribution of Monocot Understory Herbs during Tropical Forest Succession in Northeastern Costa Rica. Diversity. 2024; 16(8):439. https://doi.org/10.3390/d16080439
Chicago/Turabian StyleSun, Jennifer W. C., Robin L. Chazdon, and Philip W. Rundel. 2024. "Diversity and Distribution of Monocot Understory Herbs during Tropical Forest Succession in Northeastern Costa Rica" Diversity 16, no. 8: 439. https://doi.org/10.3390/d16080439





