1. Introduction
The average annual increase in the world’s population is 1.1% [
1,
2], which is far faster than even the experts predicted [
3]. This is reflected in the increase in the rate of food consumption. Even though agricultural research has achieved great leaps forward in increasing the world’s food production, its achievements are being increasingly exposed to instability for various reasons, the most important being climate change [
4,
5]. The main effects of climate change, such as heat, drought, salinity, floods, humidity, decreasing groundwater table and resources, and unexpected high or low rainfall, hinder improvements in crop production, particularly under conditions of limited resources. Water is the most limiting abiotic factor for plant life through photosynthesis [
6,
7]. Water-deficit stress is a dominant climate factor that damages crop production, especially within the period of reproduction and grain filling. It is a danger to food security, and water-deficit stress affects, in an increasingly negative manner, the sustainable production of wheat. Reduced production has become more intense as global temperatures have risen, particularly around the Mediterranean basin [
4,
7,
8,
9]. Scientific studies have confirmed that drought stress in wheat causes changes in morpho-physio-biochemical characters and in the capacity for genetic reduction, causing negative changes in the final product, such as reduced grain weight, a shorter grain-filling period, a slower grain-filling rate, and reduced grain quality [
4,
6,
10].
Water-deficit-related tissue damage in crops is a substantial cause of yield loss in agricultural output worldwide owing to the strong negative relationship between water deficit and crop yields [
4,
10,
11]. Therefore, more research is necessary to promote the development/discovery of new factors that can improve drought tolerance in crops to meet the heightened food demands of the world’s population. To ensure global food security, global food policy must provide multiple priorities at the same time and stimulate greater cooperative investigation and the creation of new or improved wheat genotypes that are tolerant to drought stress [
12,
13]. A plant’s ability to overcome drought stress is due to appropriate environmental and agricultural conditions and/or genetic factors that improve its ability to save water as the flow of water from roots to leaves decreases and as the water through transpiration decreases when stomata open [
6]. A sustainable way to mitigate drought-stress damage is to provide/generate tolerant varieties via examining many genotypes to identify tolerant genotypes and then transferring the tolerance traits into commercially cultivated varieties to combine them into model varieties that are both high-yielding and drought-stress-tolerant [
7,
14,
15].
Evaluation of the genotypes under drought stress is important to identify the best ones, in terms of high quantity and quality, to select them as promising commercial varieties. These can be used for continuous improvement in prospective breeding programs for wheat tolerant to drought stress and for genetic stability, given that drought is a polygenic trait and more sensitive to environmental conditions [
16,
17]. Due to this, genotype performance in wheat may be different from one season to another (from strength to weakness or conversely). In this regard, more efforts should be made to determine which genotypes are stable across sites that are evaluated in many locations for a few seasons until satisfactory results are observed by farmers [
18,
19,
20]. Yield and yield components are affected by a multitude of factors during the growing season and are primarily impacted by water deficits and light periods. Thus, scientists are trying to understand interactions (GEN:ENV) between genotype (GEN) and environment (ENV) through genetic studies using a novel model, which became known as the WAAS biplot (WAASB, which combines in one unique index the advantages of AMMI and BLUP (best linear unbiased pre-diction)) to determine ideal genotypes based on a combination of high performance and stability, as suggested in [
21,
22]. The WAASB is more reliable and provides more comprehensible results. The WAASB evaluates the outperforming genotypes that combine high performance and stability in a two-dimensional plot of the model which considers all of the interaction principal component axes (IPCAs) of GEI effects [
8,
14,
22,
23,
24]. However, selecting the best drought-tolerant genotypes is the most challenging for breeders. Thus, many scholars have suggested several stress-tolerance indices, but few are most beneficial for the stress-tolerant genotype-selection process [
15,
25,
26]. These scholars have pointed out that the high values for MP (mean productivity index), STI (stress-tolerance index), GMP (geometric mean productivity), HM (harmonic mean), YSI (yield stability index), and YI (yield index), as well as low values for TOL (tolerance index), SSI (stress susceptibility index), RSI (relative stress index), and PYR (percent yield reduction) are preferable for discovering genotypes that are stable and tolerant [
25,
27,
28]. In this study, 20 wheat genotypes were evaluated for three seasons under non-stressful and drought-stress conditions, in a total of six environments. I observed that the genotypes’ production was reduced in cases under drought stress. The absolute and predicted values for yield and yield components coupled with drought-stress indices for grain yield traits were used for choosing and identifying drought-tolerant genotypes. Therefore, this study aimed the use different modern statistical methods that complement each other to (i) determine the best genotypes that combine high productivity and stability under drought stress, (ii) validate the competency of drought indices in screening tolerant genotypes, and (iii) assess the relationships between the various indices.
4. Discussion
Worldwide, and particularly in Arab regions, agriculture is confronted with a great number of challenges such as water crises, climate change, and a lack of production, despite many factors that enable agricultural production to achieve sustainable development [
36]. Universally, abiotic stress is the most damaging factor to the growth and production of crops. Drought stress is one of the outcomes of climate change that causes significant damage to the growth and productivity of wheat, especially when it occurs during a decisive phase in a plant’s life [
9,
11,
37]. The breeding of highly productive and highly stable drought-tolerant genotypes is the major objective and the primary concern of plant breeders who seek to help humanity achieve food security [
14,
38]. This study evaluated yield and yield component traits in 20 spring wheat genotypes under two conditions (non-stressful and drought stress) for three seasons and found that drought stress negatively affects the four traits (
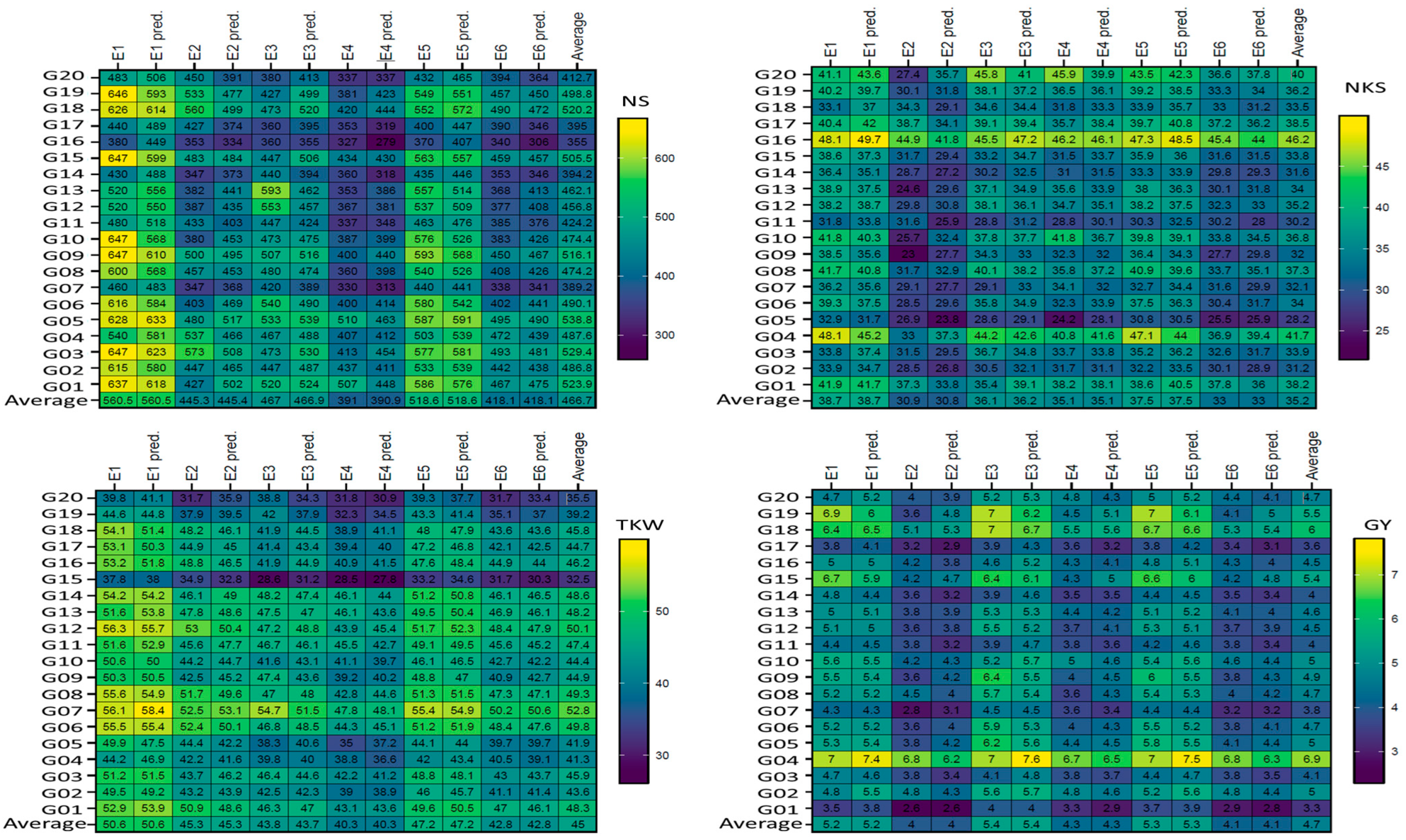
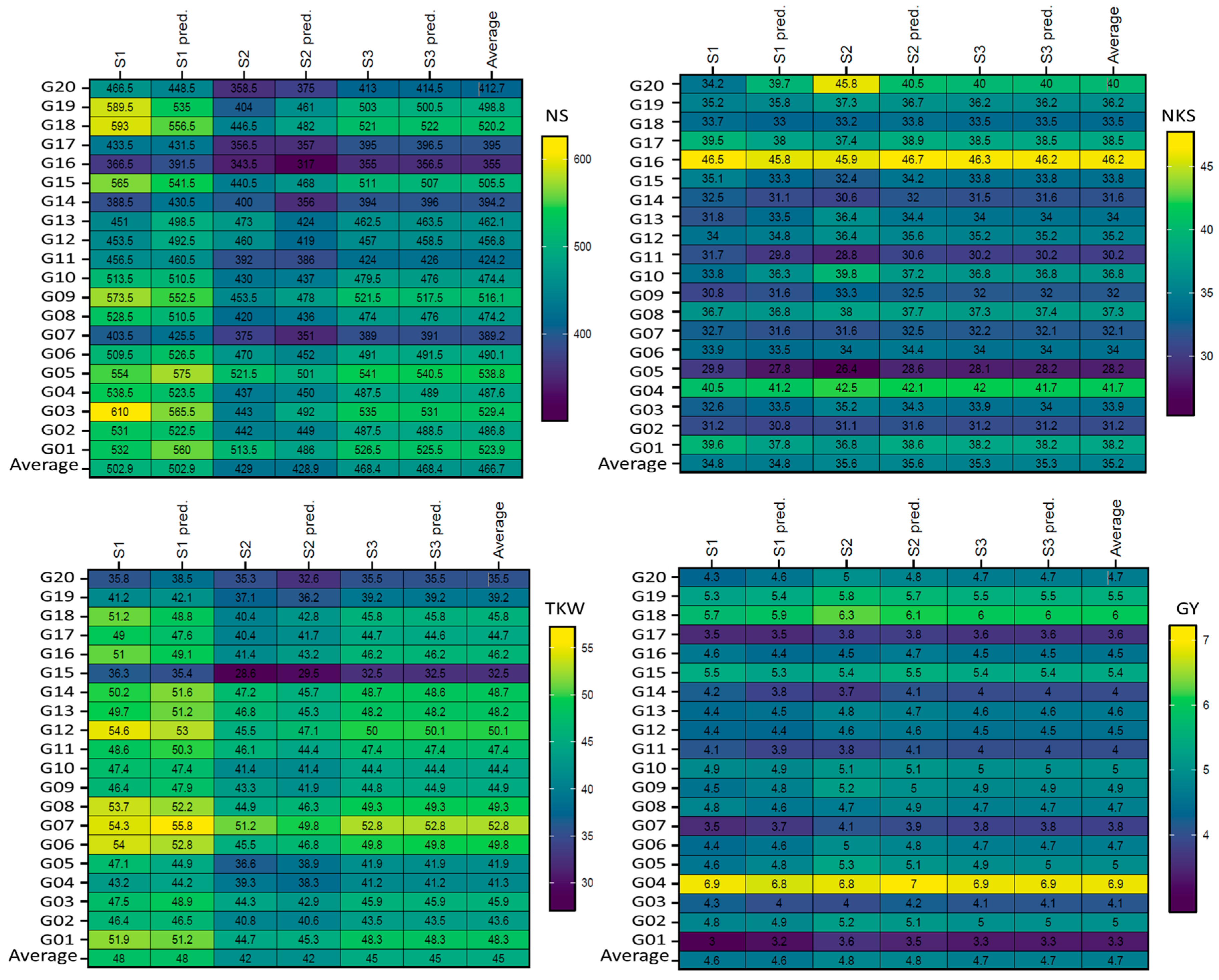
Figure 1 and
Figure 2). GEN:ENV can challenge the efficiency of selection due to varying genotype rankings in METs, notably for genetically complicated traits, to avoid bias and diminishing selection gains necessitates the use of METs [
31,
39]. This study highlighted the challenge of selecting wheat genotypes that balance stability and high performance through innovative statistical approaches that help us understand genetic parameters and detect genotypes less affected by climate changes. The
value (more than 53% from
total) exceeded the
value (less than 14% from
total) for yield and yield component traits, indicating the correct circumstances for genotype selection during the different stages of the breeding program. Approximately 46% of the
in the NS trait is attributed to
and
, reducing heritability (
Table 2). The r
gen-env value is relatively similar for NS (0.78) and GY (0.77), with studies suggesting that a high value points to simple interaction, while TKW shows lower correlations (0.44). Therefore, a low value is not preferred for choosing genotypes [
14,
40,
41]. The
h2ems values of the genotypes ranged from moderate to high, the
h2pm values were mixed and high,
h2gm values were high, and
h2piepho and
h2cullis values were very high, showing a dramatic increase in genetic variation (accuracy level of more than 0.94). This high accuracy points to a high predictive capacity for genetic value [
8,
31,
41]. The genotypic CVs were two to three times the residual CVs; therefore, the ratio was greater than 1. This is proof that there was little variation within the environment [
14,
21].
The AMMI-ANOVA findings showed significant variations in the GEN:ENV for the four traits under the six ENVs (three seasons × two treatments), and the genotypes’ performance in the three seasons was uneven for both treatments but converged under the same treatment (
Table 3). In most of the genotypes, the reduction was very clear in yield and yield component traits under drought stress compared to these traits under non-stressful conditions (
Figure 1). Earlier studies indicated that wheat genotype performance varied dramatically under stressful condition [
8,
20,
42]. Genotype performance is determined by genetic and environmental factors, and the high ability of multi-environment trials (METs) to demonstrate statistical differences is the critical factor in the selection of genotypes that are the most convenient and reliable in modern breeding programs [
42,
43]. To achieve this goal, it is necessary to use statistically appropriate models that have high prediction abilities to understand this GEI complexity [
14,
20,
21,
43,
44,
45], which was the focus of our study.
The reliability of predictions for genotype performance (the predicted and absolute values are close together) is decisive in recommending the appropriate genotypes for mega-environments [
14,
21,
46]. The results for the non-stressful (ENV1, ENV3, and ENV5) environments were comparable, and similar findings were observed for the drought stress (ENV2, ENV4, and ENV6) environments. However, the differences were most clearly observed for genotypes in drought-stress environments compared to non-stressful (
Figure 1) environments. AMMI-ANOVA for ENVs, GENs, and GEN:ENV indicated highly significant disparities for yield and yield components, suggesting that the GEN performance varies depending on the ENV type (
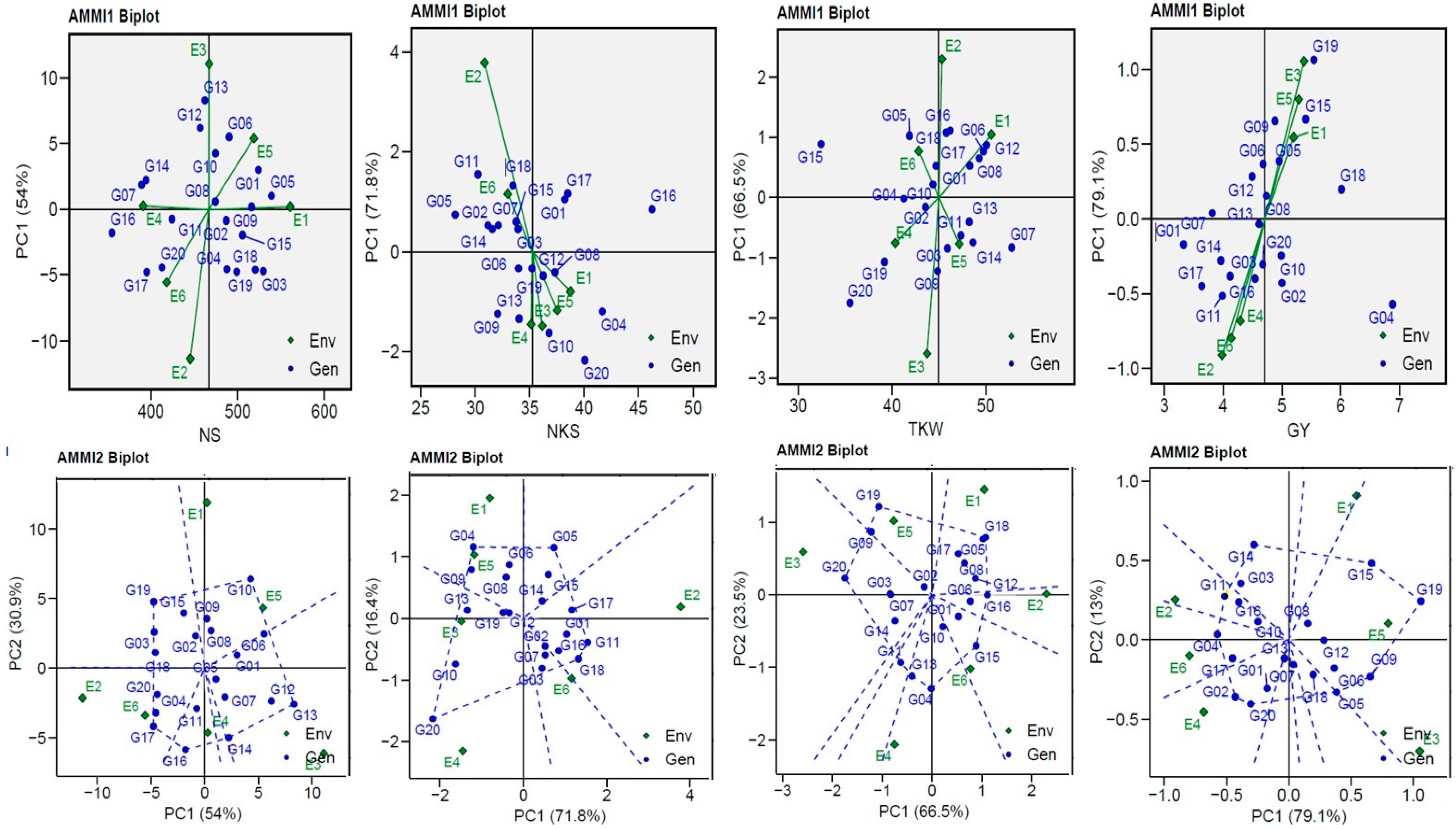
Table 3). The GEN:ENV relationship was of a crossover type and exerted a large influence on GEN performance, so plant breeders are still searching for a genotype with genetic stability. Biplots have the advantage of using all IPCA axes, thus allowing GEN:ENV not maintained in IPCA1 to be included in the genotypes’ ranking [
14,
21]. In this study, AMMI indicated that the environment SS was partitioned into three significant components (IPCA1–IPCA3) with a percentage of 100.00%.
In this study, I have shown a comparison between the predicted (AMMI model) and absolute values, indicating the greater reliability of MET analysis. Han et al. [
33] stated that assessments using the AMMI model are as close as possible to the “true” value; therefore, predictive precision deserves our special attention for model profiling in MET analysis [
14,
21,
47]. I found that the NS trait showed distinct differences compared with NKS, TKW, and GY, which showed minor variations (
Figure 1) that were attributed to heritability (
Table 2). The TKW and GY traits showed very low variations between predicted and observed values compared to the NS and NKS traits (as a result of heritability). The biplots visualized the genotypes’ ranking [
21,
23,
48]. The AMMI1 biplot explained the interaction between environments, which made it clear that when far away from its origin and when possessing a longer vector, higher interaction was indicated (as in the case of ENV2 and ENV3 for the NS trait; ENV2 for the NKS trait; ENV2 and ENV3 for the TKW trait; and ENV1, ENV3, ENV5, and ENV6 for the GY trait). On the other hand, in environments that were close to their origin and possessed shorter vectors, lower interaction was indicated [
13,
19,
20,
42,
43,
49]. The three non-stressful (ENV1, ENV3, and ENV5) environments had a correlation that was mostly positive (the angle among the vectors was less than 90°), and the three drought-stress (ENV2, ENV4, and ENV6) environments provided similar findings (
Figure 3). This suggests that the GEI effects tend to be in the same range and independent when applying them under comparable circumstances, except for ENV1 and ENV3 for the NS trait and ENV2 and ENV4 for the NKS and TKW traits, which demonstrated negative correlations (the angle between the vectors was greater than 90°). The GGE biplot polygon was used to describe the discriminativeness and representativeness (DR) of genotypes in METs, which fall in the top right quartile of the polygon and have high DR. They are considered the most desirable genotypes. A vertical projection from the GEN to the ENV vector indicates the extent of the interaction with the ENVs [
14,
19,
20]. Thus, the eight genotypes for NS, five genotypes for NKS, nine genotypes for TKW, and nine genotypes for GY are considered as unstable with the six environments used, as shown in
Figure 3. The G20 genotype was observed to be unstable with the four (NS, NKS, TKW, and GY) traits, the G19 genotype was observed to be unstable with three (NS, TKW, and GY) traits, the G16 was observed to be unstable with the NS and NKS traits, and the G15 genotype was observed to be unstable with the TKW and GY traits.
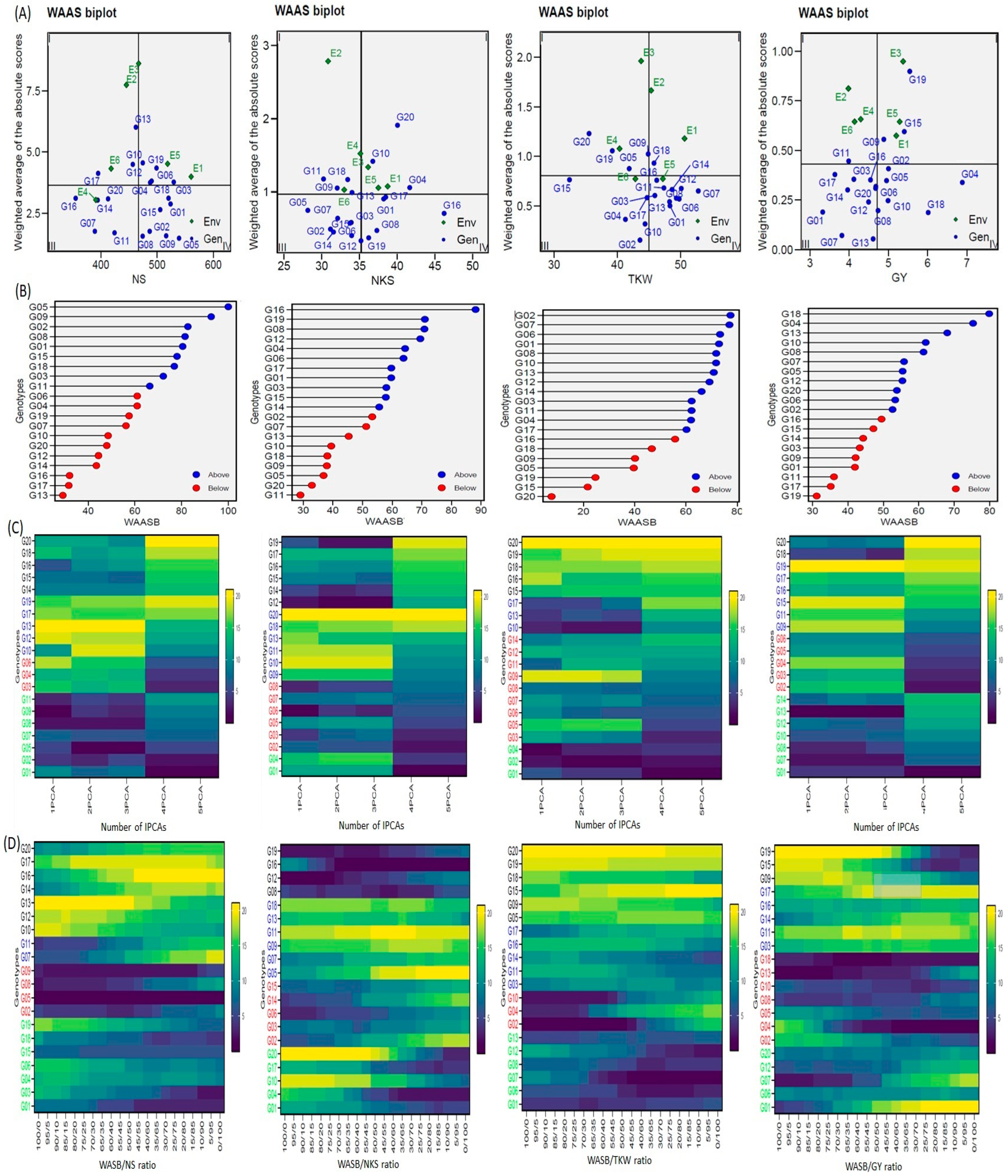
The novel WAAS biplot (WAASB) model combines the AMMI and BLUP models to demonstrate the GEI, which has a unique way of selecting the genotypes with the best performance and stability by taking into account all IPCAs from the singular value decomposition (SVD) of the matrix of GEI effects, resulting from a linear mixed-effect model [
8,
13,
21,
24,
40]. Depending on the WAAS and trait values, a WAAS biplot was used to determine high-performing and stable genotypes. These fell in the bottom right quartile (Sector IV) of the biplot (
Figure 4). The (trait × WAAS) biplot provides a full explanation of combining the high-performance and stable genotypes in a two-dimensional plot, keeping in mind the five IPCAs (
Figure 4C). I believe that this is a promising way to discover high-performance and stable genotypes in prospective studies. Consequently, this method can be utilized in future hybridization programs to choose the donors’ parents. For example, in a future study, I expect to crossbreed the genotype G16 (which demonstrates high performance and is stable for NKS and TKW) with any of the G01, G02, G05, G09, G15, and G18 (high-performance and stable for NS) genotypes to obtain new genotypes combining the three traits into an ideotype (which results in high performance and stable genotypes for GY). This method can lead to minimized superfluous accounts and will make it easier to make recommendations for ideotype cultivars [
8,
40,
50]. The (GY × WAAS) biplot also revealed high-yield and stable genotypes, i.e., G02, G04, G05, G08, G10, and G18 (
Figure 4C). The heatmap also revealed stable genotypes based on IPCAs and the WAASB × trait, with darker colors reflecting higher rankings and lighter colors reflecting lower rankings (
Figure 4C). The genotype groups were identified easily, according to those that had the same performance levels and stability, through the use of genotype colors [
13]. The heatmap (
Figure 4D) for genotype ranking (WAASB × trait) based on the weights of the stability (WAASB) and mean performance (trait) were consistent or largely converged with the (WAASB × trait) results [
8,
13,
21]. This is very important in the breeding program, as the breeder may want to confer preference to a high-performance genotype at the cost of stability or, conversely, confer preference to a stable genotype at the cost of performance. Thus,
Figure 4 should help breeders and agronomists with decision making and recommendations on the selection of genotypes that have the same mean performance and stability [
21]. The stable and high-performance genotypes can be used in the ongoing development of varieties and in prospective breeding programs for the development of high-performance and drought-stress-tolerant varieties.
The AMMI-ANOVA findings showed significant variations in the GEI in this study, and the genotype performance varied under both conditions (non-stressful and drought stress), which revealed that each genotype responded differently to yield and yield components under the two treatments. Based on the variability in genotypes, several ways are used by plant breeders to select high-productivity genotypes under conditions of stress, which are referred to as “selection indices” or stress-tolerance index (STI), utilized in numerous studies to identify genotypes that can stand up to drought stress [
25,
26,
34,
51]. Lower values in TOL, SSPI, SSI, ATI, and RDI indicate more tolerant genotypes, while the opposite is true for sensitive genotypes (
Table S4). The tolerance level increases with a deficiency in these values, but the results of these values cannot distinguish between genotypes that have higher productivity [
25,
34]. Consequently, the G02 and G04 genotypes are more tolerant (the difference between their performance under non-stressful conditions and drought-stress conditions is minimal), while the G15 and G19 genotypes are more sensitive. According to Shabani et al. [
52] and Erdemcı [
53], STI is the most suitable variable for choosing tolerant genotypes. A better genotype performance under non-stressful and drought-stress conditions is indicated by higher values for MP, GMP, and STI. The genotypes G04 and G15 had the highest values. The genotype G04 had the highest productivity under drought stress among all the genotypes. Kamrani et al. [
54] and Basavaraj et al. [
55] also reported comparable findings and proposed that genotypes that were more productive and more drought-tolerant could be selected using high values of STI, MRP, and GMP as indices. Scientists need access to the most suitable indices for drought-stress tolerance; therefore, I carried out a correlation coefficient analysis for GY under non-stressful (Yp) and drought-stress (Ys) conditions and for drought-stress indices. Yp and Ys both had a positive correlation, so high-performance genotypes were easily identifiable from Yp and Ys (the outcome of Yp is effective for choosing Ys indirectly). The SSI, RDE, and PYR had a negative correlation with Ys but a positive correlation with Yp; thus, selection based on these indices will strengthen productivity in non-stressful environments but decrease productivity in stressful environments (
Table 4). In total, 10 indices showed positive and significant association with Yp and Ys. As a result, these indices can be utilized for detecting genotypes that are highly productive in both cases. Our results were compatible with those reported in [
25,
54,
55].
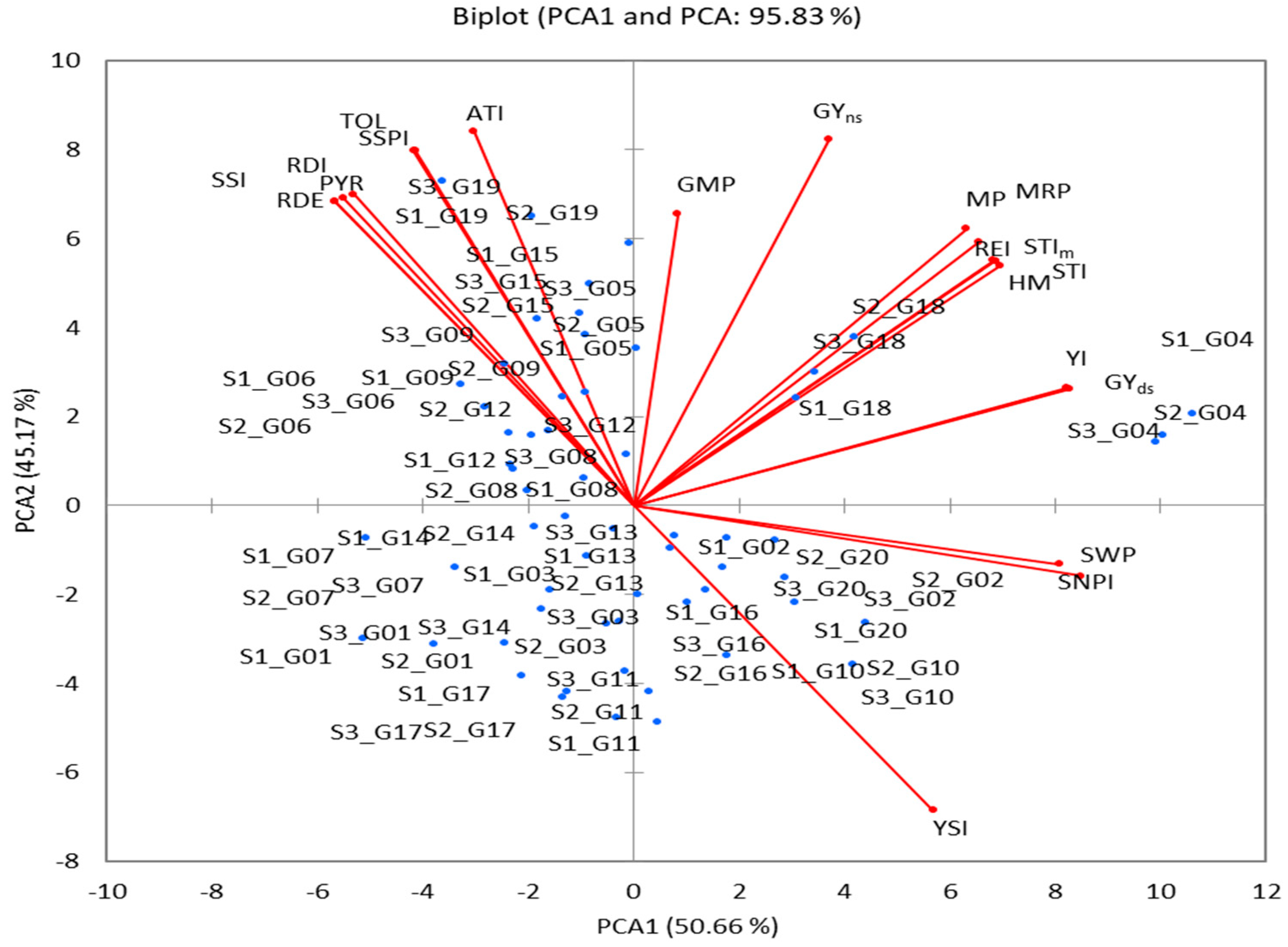
PCA can be used for the selection of the best-yielding genotypes under non-stressful and drought-stress conditions. PCA reveals the links between all variables at once, decreases the number of variables (choosing the best ones), and addresses the problems of multicollinearity [
10,
56,
57]. The first two PCAs showed an eigenvalue of >1 (the basis for the selection of the components) and contributed 95.83% of the total variation (
Table 5). PCA1 was positively associated with 13 variables (including major Yp and Ys) which are used to select genotypes of high-yield and drought-tolerant varieties, and PCA2 was positively associated with 17 variables used to select genotypes of drought-sensitive varieties (
Table 5 and
Figure 5). A PCA1 and PA2 biplot was used to compare genotypes with variables. This biplot illustrated the fact that the genotypes G02, G10, G16, and G20 were stable under Yp and Ys conditions; the genotypes G05, G06, G08, G09, G12, G15, and G19 were drought-sensitive; and the genotypes G04 and G18 were the most drought-tolerant. Based on the drought-tolerance indices (GMP, STI, MP, HM, MRP, REI), the 20 genotypes were grouped into five clusters. The highest value of indices was found in two genotypes (G04 and G18) of cluster HT, followed by two genotypes (G05 and G19) of cluster T (
Figure 6). Several researchers have used hierarchical clustering to rank the drought-tolerant genotypes [
7,
25,
58,
59,
60] without verifying the accuracy of their rankings. To validate the categories, I used discriminant analysis, which showed that previously, (M) differed in terms of the posterior (S) in the genotype (G08), and the identical ratio was 95% (
Table 6). However, in cross-validation ranking, previous differences were found in the posterior in six genotypes (a proportion of 30%), and these were compatible in fourteen genotypes (% correct = 70.00%). Therefore, discriminant analysis is regarded as an important statistical instrument for verifying genotype categories [
10,
12,
61]. However, when comparing our previous study [
7] with the current one, I found differences in some outputs due to several reasons, including statistical methods, traits used, and the number of seasons. So, the study suggests that combining statistical approaches that consider both stability and high performance can significantly enhance the reliability of recommendations for new wheat genotypes. This information is crucial for breeding programs aiming to develop wheat genotypes that can withstand drought-stress conditions while maintaining high yield and stability.