Occurrence of Novel and Legacy Per/Polyfluoroalkyl Substances (PFASs) in Scopoli’s Shearwater (Calonectris diomedea) Feathers
Abstract
:1. Introduction
2. Materials and Methods
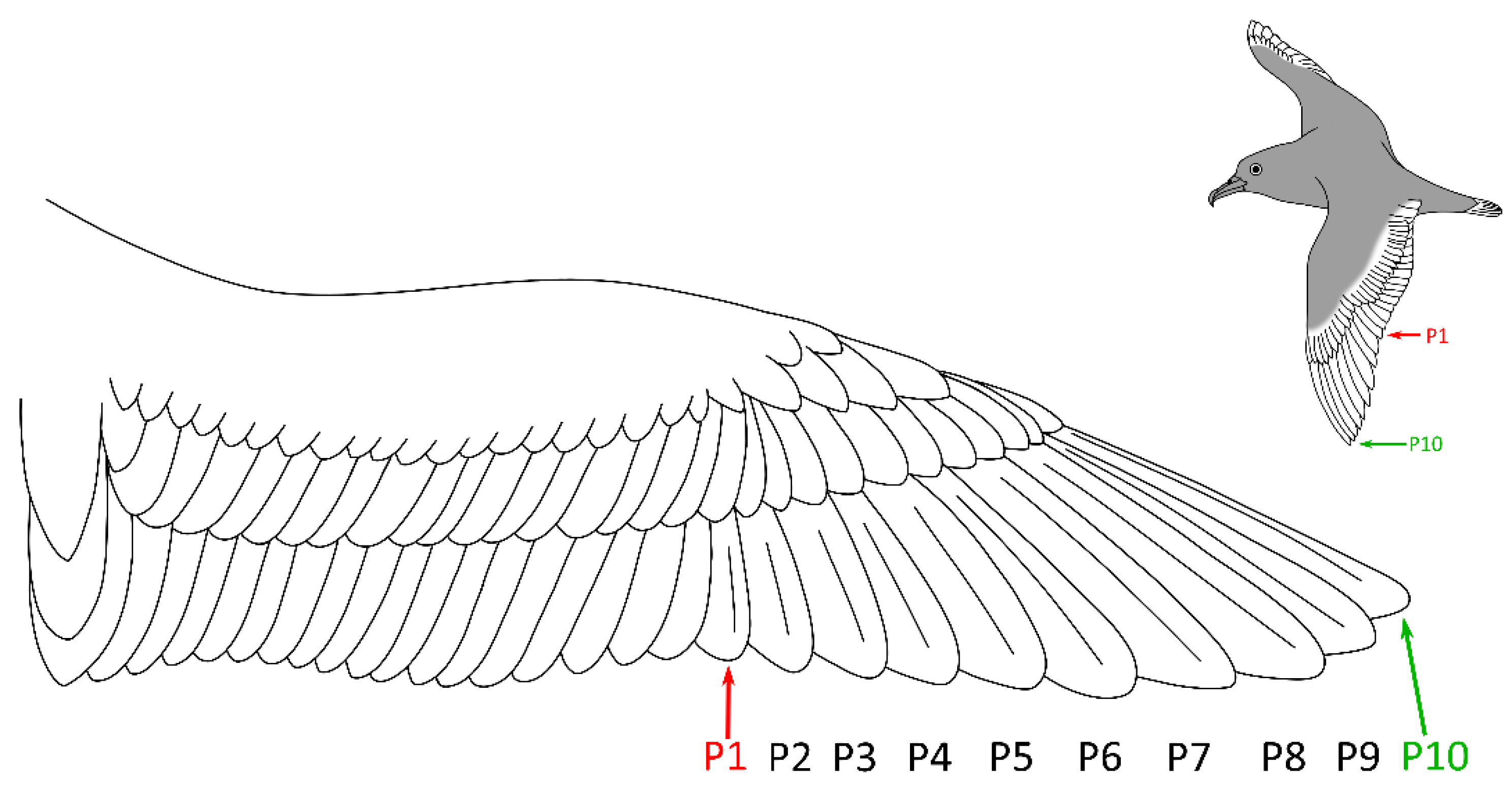
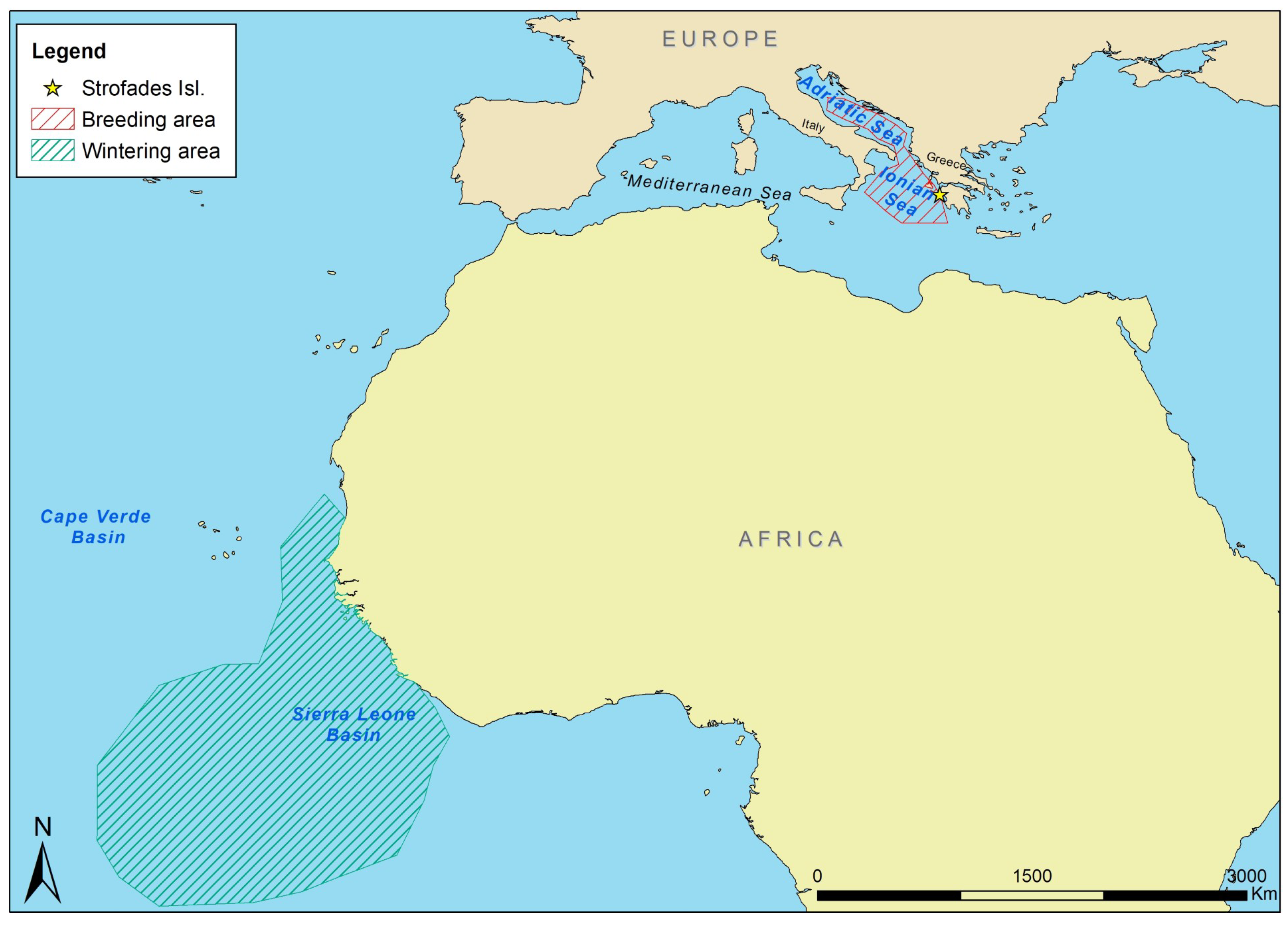
2.1. Study Area and Sampling
2.2. Pre-Treatment of the Feathers, Sample Extraction, and Analysis
2.3. Data Generation and Treatment
3. Results and Discussion
3.1. Occurrence of PFASs in Feathers
3.2. Comparison of PFAS Concentrations between P1 and P10 Feathers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buck, R.C.; Korzeniowski, S.H.; Laganis, E.; Adamsky, F. Identification and classification of commercially relevant per- and poly-fluoroalkyl substances (PFAS). Integr. Environ. Assess. Manag. 2021, 17, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Gans, O.; Weiss, S.; Sanz-Escribano, D.; Scharf, S.; Scheffknecht, C. Perfluorinated alkylated substances in the aquatic environment: An Austrian case study. Water Res. 2009, 43, 4760–4768. [Google Scholar] [CrossRef] [PubMed]
- AMAP. AMAP Assessment 2016: Chemicals of Emerging Arctic Concern; Arctic Monitoring and Assessment Programme (AMAP): Oslo, Norway, 2017; p. xvi+353. Available online: https://www.amap.no/documents/doc/AMAP-Assessment-2016-Chemicals-of-Emerging-Arctic-Concern/1624 (accessed on 15 July 2024)xvi+353pp.
- Stockholm Convention. Chemicals Listed in Annex A. 2019. Available online: https://www.pops.int/Implementation/Alternatives/AlternativestoPOPs/ChemicalslistedinAnnexA/tabid/5837/Default.aspx (accessed on 15 July 2024).
- D’ Hollander, W.; Roosens, L.; Covaci, A.; Cornelis, C.; Reynders, H.; Campenhout, K.; Van Voogt, P.; de Bervoets, L. Brominated flame retardants and perfluorinated compounds in indoor dust from homes and offices in Flanders, Belgium. Chemosphere 2010, 81, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Giesy, J.P.; Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Groffen, T.; Wepener, V.; Malherbe, W.; Bervoets, L. Distribution of perfluorinated compounds (PFASs) in the aquatic environment of the industrially polluted Vaal River, South Africa. Sci. Total Environ. 2018, 627, 1334–1344. [Google Scholar] [CrossRef]
- Groffen, T.; Lasters, R.; Lopez-Antia, A.; Prinsen, E.; Bervoets, L.; Eens, M. Limited reproductive impairment in a passerine bird species exposed along a perfluoroalkyl acid (PFAA) pollution gradient. Sci. Total Environ. 2019, 652, 718–728. [Google Scholar] [CrossRef]
- Guigueno, M.F.; Fernie, K.J. Birds and flame retardants: A review of the toxic effects on birds of historical and novel flame retardants. Environ. Res. 2017, 154, 398–424. [Google Scholar] [CrossRef]
- Van den Eede, N.; Dirtu, A.C.; Neels, H.; Covaci, A. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ. Int. 2011, 37, 454–461. [Google Scholar] [CrossRef]
- Yamashita, N.; Kannan, K.; Taniyasu, S.; Horii, Y.; Petrick, G.; Gamo, T. A global survey of perfluorinated acids in oceans. Mar. Pollut. Bull. 2005, 51, 658–668. [Google Scholar] [CrossRef]
- European Chemicals Agency. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH). In Oj L (Volume 396, Issue 30.12.2006). 2006. Available online: https://eur-lex.europa.eu/eli/reg/2006/1907/oj (accessed on 15 July 2024).
- UNEP. Annual Report: Seizing the Green Opportunity; UNEP: Osaka, Japan, 2010. [Google Scholar]
- Bertolero, A.; Vicente, J.; Meyer, J.; Lacorte, S. Accumulation and maternal transfer of perfluorooctane sulphonic acid in yellow-legged (Larus michahellis) and Audouin’s gull (Larus audouinii) from the Ebro Delta Natural Park. Environ. Res. 2015, 137, 208–214. [Google Scholar] [CrossRef]
- Løseth, M.E.; Briels, N.; Flo, J.; Malarvannan, G.; Poma, G.; Covaci, A.; Herzke, D.; Nygård, T.; Bustnes, J.O.; Jenssen, B.M.; et al. White-tailed eagle (Haliaeetus albicilla) feathers from Norway are suitable for monitoring of legacy, but not emerging contaminants. Sci. Total Environ. 2019, 647, 525–533. [Google Scholar] [CrossRef]
- van der Schyff, V.; Kwet Yive, N.S.C.; Polder, A.; Cole, N.C.; Bouwman, H. Perfluoroalkyl substances (PFAS) in tern eggs from St. Brandon’s Atoll, Indian Ocean. Mar. Pollut. Bull. 2020, 154, 111061. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Bertolero, A.; Meyer, J.; Viana, P.; Lacorte, S. Distribution of perfluorinated compounds in Yellow-legged gull eggs (Larus michahellis) from the Iberian Peninsula. Sci. Total Environ. 2012, 416, 468–475. [Google Scholar] [CrossRef]
- Gebbink, W.A.; Letcher, R.J. Comparative tissue and body compartment accumulation and maternal transfer to eggs of perfluoroalkyl sulfonates and carboxylates in Great Lakes herring gulls. Environ. Pollut. 2012, 162, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Verboven, N.; Verreault, J.; Letcher, R.J.; Gabrielsen, G.W.; Evans, N.P. Differential investment in eggs by arctic-breeding Glaucous Gulls (Larus hyperboreus) exposed to persistent organic pollutants. Auk 2009, 126, 123–133. [Google Scholar] [CrossRef]
- Verreault, J.; Houde, M.; Gabrielsen, G.W.; Berger, U.; Haukås, M.; Letcher, R.J.; Muir, D.C.G. Perfluorinated alkyl substances in plasma, liver, brain, and eggs of glaucous gulls (Larus hyperboreus) from the Norwegian Arctic. Environ. Sci. Technol. 2005, 39, 7439–7445. [Google Scholar] [CrossRef]
- Eulaers, I.; Jaspers, V.L.B.; Halley, D.J.; Lepoint, G.; Nygård, T.; Pinxten, R.; Covaci, A.; Eens, M. Brominated and phosphorus flame retardants in White-tailed Eagle Haliaeetus albicilla nestlings: Bioaccumulation and associations with dietary proxies (δ13C, δ15N and δ34S). Sci. Total Environ. 2014, 478, 48–57. [Google Scholar] [CrossRef]
- Gauthier, L.T.; Hebert, C.E.; Weseloh, D.V.C.; Letcher, R.J. Current-use flame retardants in the eggs of herring gulls (Larus argentatus) from the Laurentian Great Lakes. Environ. Sci. Technol. 2007, 41, 4561–4567. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramírez, P.; Bustnes, J.O.; Eulaers, I.; Herzke, D.; Johnsen, T.V.; Lepoint, G.; Pérez-García, J.M.; García-Fernández, A.J.; Jaspers, V.L.B. Per- and polyfluoroalkyl substances in plasma and feathers of nestling birds of prey from northern Norway. Environ. Res. 2017, 158, 277–285. [Google Scholar] [CrossRef]
- Burger, J. Metals in avian feathers: Bioindicators of environmental pollution. Rev. Env. Toxicol. 1993, 5, 203–311. [Google Scholar]
- Sun, J.; Bossi, R.; Bustnes, J.O.; Helander, B.; Boertmann, D.; Dietz, R.; Herzke, D.; Jaspers, V.L.B.; Labansen, A.L.; Lepoint, G.; et al. White-Tailed Eagle (Haliaeetus albicilla) Body Feathers Document Spatiotemporal Trends of Perfluoroalkyl Substances in the Northern Environment. Environ. Sci. Technol. 2019, 53, 12744–12753. [Google Scholar] [CrossRef]
- Sun, J.; Bustnes, J.O.; Helander, B.; Bårdsen, B.J.; Boertmann, D.; Dietz, R.; Jaspers, V.L.B.; Labansen, A.L.; Lepoint, G.; Schulz, R.; et al. Temporal trends of mercury differ across three northern white-tailed eagle (Haliaeetus albicilla) subpopulations. Sci. Total Environ. 2019, 687, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Covaci, A.; Bustnes, J.O.; Jaspers, V.L.; Helander, B.; Bårdsen, B.J.; Boertmann, D.; Dietz, R.; Labansen, A.-L.; Lepoint, G.; et al. Temporal trends of legacy organochlorines in different white-tailed eagle (Haliaeetus albicilla) subpopulations: A retrospective investigation using archived feathers. Environ. Int. 2020, 138, 105618. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, V.L.B.; Covaci, A.; Van den Steen, E.; Eens, M. Is external contamination with organic pollutants important for concentrations measured in bird feathers? Environ. Int. 2007, 33, 766–772. [Google Scholar] [CrossRef]
- Jaspers, V.L.B.; Voorspoels, S.; Covaci, A.; Lepoint, G.; Eens, M. Evaluation of the usefulness of bird feathers as a non-destructive biomonitoring tool for organic pollutants: A comparative and meta-analytical approach. Environ. Int. 2007, 33, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gin, K.Y.H.; Chang, V.W.C.; Goh, B.P.L.; Reinhard, M. Novel perspectives on the bioaccumulation of PFCs—The concentration dependency. Environ. Sci. Technol. 2011, 45, 9758–9764. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Li, X.; Jin, Y.H.; Sasaki, K.; Saito, N.; Sato, I.; Tsuda, S. Human nails analysis as biomarker of exposure to perfluoroalkyl compounds. Environ. Sci. Technol. 2011, 45, 8144–8150. [Google Scholar] [CrossRef]
- Jaspers, V.L.B.; Herzke, D.; Eulaers, I.; Gillespie, B.W.; Eens, M. Perfluoroalkyl substances in soft tissues and tail feathers of Belgian barn owls (Tyto alba) using statistical methods for left-censored data to handle non-detects. Environ. Int. 2013, 52, 9–16. [Google Scholar] [CrossRef]
- Jaspers, V.L.B.; Covaci, A.; Herzke, D.; Eulaers, I.; Eens, M. Bird feathers as a biomonitor for environmental pollutants: Prospects and pitfalls. Trends Anal. Chem. 2019, 118, 223–226. [Google Scholar] [CrossRef]
- Padilha, J.; de Carvalho, G.O.; Willems, T.; Lepoint, G.; Cunha, L.; Pessoa, A.R.L.; Eens, M.; Prinsen, E.; Costa, E.; Torres, J.P.; et al. Perfluoroalkylated compounds in the eggs and feathers of resident and migratory seabirds from the Antarctic Peninsula. Environ. Res. 2022, 214, 114157. [Google Scholar] [CrossRef]
- Renzoni, A.; Focardi, S.; Fossi, C.; Leonzio, C.; Mayol, J. Comparison between concentrations of mercury and other contaminants in eggs and tissues of Cory’s shearwater Calonectris diomedea collected on Atlantic and Mediterranean islands. Environ. Pollut. A 1986, 40, 17–35. [Google Scholar] [CrossRef]
- Voulgaris, M.D.; Karris, G.; Xirouchakis, S.; Zaragoza Pedro, P.; Asimakopoulos, A.G.; Grivas, K.; Bebianno, M.J. Trace metal blood concentrations in Scopoli’s shearwaters (Calonectris diomedea) during 2007–2014: A systematic analysis of the largest species colony in Greece. Sci. Total Environ. 2019, 691, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Rodríguez, B.; Nazaret Carrasco, M. High prevalence of parental delivery of plastic debris in Cory’s shearwaters (Calonectris diomedea). Mar. Pollut. Bull. 2012, 64, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Roscales, J.L.; Muñoz-Arnanz, J.; González-Solís, J.; Jiménez, B. Geographical PCB and DDT Patterns in Shearwaters (Calonectris sp.) breeding across the NE Atlantic and the Mediterranean Archipelagos. Environ. Sci. Technol. 2010, 44, 2328–2334. [Google Scholar] [CrossRef]
- Escoruela, J.; Garreta, E.; Ramos, R.; González-Solís, J.; Lacorte, S. Occurrence of Per- and Polyfluoroalkyl substances in Calonectris shearwaters breeding along the Mediterranean and Atlantic colonies. Mar. Pollut. Bull. 2018, 131, 335–340. [Google Scholar] [CrossRef]
- Hazen, E.L.; Abrahms, B.; Brodie, S.; Carroll, G.; Jacox, M.G.; Savoca, M.S.; Scales, K.L.; Sydeman, W.J.; Bograd, S.J. Marine top predators as climate and ecosystem sentinels. Front. Ecol. Environ. 2019, 17, 565–574. [Google Scholar] [CrossRef]
- Anselme, L.; Durand, J. The Cory’s Shearwater Calonectris diomedea diomedea, Updated State of Knowledge and Conservation of the Nesting Populations of the Small Mediterranean Islands; Initiative PIM: Marseille, France, 2012; 23p. [Google Scholar]
- Cramp, S. The Birds of the Western Palearctic. In The Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1985; Volume IV, p. 960. [Google Scholar]
- Afán, I.; Navarro, J.; Cardador, L.; Ramírez, F.; Kato, A.; Rodríguez, B.; Ropert-Coudert, Y.; Forero, M.G. Foraging movements and habitat niche of two closely related seabirds breeding in sympatry. Mar. Biol. 2014, 161, 657–668. [Google Scholar] [CrossRef]
- Alonso, H.; Granadeiro, J.P.; Paiva, V.H.; Dias, A.S.; Ramos, J.A.; Catry, P. Parent-offspring dietary segregation of Cory’s shearwaters breeding in contrasting environments. Mar. Biol. 2012, 159, 1197–1207. [Google Scholar] [CrossRef]
- Monteiro, L.R.; Ramos, J.A.; Furness, R.W.; del Nevo, A.J. Movements, Morphology, Breeding, Molt, Diet and Feeding of Seabirds in the Azores. Col. Waterbirds 1996, 19, 82–97. [Google Scholar] [CrossRef]
- Neves, V.; Nolf, D.; Clarke, M. Spatio-temporal variation in the diet of Cory’s shearwater Calonectris diomedea in the Azores archipelago, northeast Atlantic. Deep-Sea Res. I Oceanogr. Res. Pap. 2012, 70, 1–13. [Google Scholar] [CrossRef]
- Karris, G.; Ketsilis-Rinis, V.; Kalogeropoulou, A.; Xirouchakis, S.; Machias, A.; Maina, I.; Kavadas, S. The use of demersal trawling discards as a food source for two scavenging seabird species: A case study of an eastern Mediterranean oligotrophic marine ecosystem. Avian Res. 2018, 9, 26. [Google Scholar] [CrossRef]
- Karris, G.; Xirouchakis, S.; Grivas, C.; Voulgaris, M.D.; Sfenthourakis, S.; Giokas, S. Estimating the population size of Scopoli’s Shearwaters (Calonectris diomedea) frequenting the Strofades islands (Ionian Sea, western Greece) by raft counts and surveys of breeding pairs. North-West. J. Zool. 2017, 13, 101–108. [Google Scholar]
- Keller, V.; Herrando, S.; Voříšek, P.; Franch, M.; Kipson, M.; Milanesi, P.; Martí, D.; Anton, M.; Klvaňová, A.; Kalyakin, M.V.; et al. European Breeding Bird Atlas 2: Distribution, Abundance and Change; European Bird Census Council & Lynx Edicions: Barcelona, Spain, 2020. [Google Scholar]
- Ramos, R.; Militão, T.; González-Solís, J.; Ruiz, X. Moulting strategies of a long-distance migratory seabird, the Mediterranean Cory’s Shearwater Calonectris diomedea diomedea. Ibis 2009, 151, 151–159. [Google Scholar] [CrossRef]
- Bretagnolle, V.; Thibault, J.C. Method for Sexing Fledglings in Cory’s Shearwaters and Comments on Sex-ratio Variation. Auk 1995, 112, 785–790. [Google Scholar]
- Cure, C.; Aubin, T.; Mathevon, N. Acoustic convergence and divergence in two sympatric burrowing nocturnal seabirds. Biol. J. Linn. Soc. 2009, 96, 115–134. [Google Scholar] [CrossRef]
- Κarris, G.; Thanou, E.; Xirouchakis, S.; Voulgaris, M.D.; Fraguedakis-Tsolis, S.; Sfenthourakis, S.; Giokas, S. Sex Determination of Scopoli’s Shearwater (Calonectris diomedea) Juveniles: A Combined Molecular and Morphometric Approach. Waterbirds 2013, 36, 240–246. [Google Scholar] [CrossRef]
- Zhang, J.; Jaspers, V.L.B.; Røe, J.; Castro, G.; Kroglund, I.B.; Gonzalez, S.V.; Østnes, J.E.; Asimakopoulos, A.G. Per- and poly-fluoroalkyl substances in Tawny Owl (Strix aluco) feathers from Trøndelag, Norway. Sci. Total Environ. 2023, 903, 166213. [Google Scholar] [CrossRef]
- Tartu, S.; Gabrielsen, G.W.; Blévin, P.; Ellis, H.; Bustnes, J.O.; Herzke, D.; Chastel, O. Endocrine and fitness correlates of long-chain perfluorinated carboxylates exposure in arctic breeding black-legged kittiwakes. Environ. Sci. Technol. 2014, 48, 13504–13510. [Google Scholar] [CrossRef]
- Jaspers, V.L.B.; Covaci, A.; Deleu, P.; Eens, M. Concentrations in bird feathers reflect regional contamination with organic pollutants. Sci. Total Environ. 2009, 407, 1447–1451. [Google Scholar] [CrossRef]
- Roscales, J.; Vicente, A.; Ryan, P.; González-Solís, J.; Jiménez, B. Spatial and Interspecies Heterogeneity in Concentrations of Perfluoroalkyl Substances (PFASs) in Seabirds of the Southern Ocean. Environ. Sci. Technol. 2019, 53, 9855–9865. [Google Scholar] [CrossRef]
- Lescord, G.L.; Kidd, K.A.; De Silva, A.O.; Williamson, M.; Spencer, C.; Wang, X.; Muir, D.C. Perfluorinated and polyfluorinated compounds in lake food webs from the Canadian high arctic. Environ. Sci. Technol. 2015, 49, 2694–2702. [Google Scholar] [CrossRef]
- Leat, E.H.K.; Bourgeon, S.; Magnusdottir, E.; Gabrielsen, G.W.; Grecian, J.; Hanssen, S.A.; Olafsdottir, K.; Petersen, A.; Phillips, R.A.; Strøm, H.; et al. Influence of wintering area on persistent organic pollutants in a breeding migratory seabird. Mar. Ecol. Prog. Ser. 2013, 491, 277–293. [Google Scholar] [CrossRef]
- Briels, N.; Ciesielski, T.M.; Herzke, D.; Jaspers, V.L.B. Developmental Toxicity of Perfluorooctanesulfonate (PFOS) and Its Chlorinated Polyfluoroalkyl Ether Sulfonate Alternative F-53B in the Domestic Chicken. Environ. Sci. Technol. 2018, 52, 12859–12867. [Google Scholar] [CrossRef]
- Herzke, D.; Jaspers, V.L.B.; Boertman, D.; Rasmussen, L.; Sonne, C.; Dietz, R.; Covaci, A.; Eens, M.; Bustnes, J.O. PFCs in feathers of white tailed eagles Haliaeetus albicilla from Greenland and Norway; useful for non-destructive monitoring? Organohalogen Compd. 2011, 73, 1337–1339. [Google Scholar]
- Monclús, L.; Løseth, M.E.; Dahlberg Persson, M.J.; Eulaers, I.; Kleven, O.; Covaci, A.; Benskin, J.P.; Awad, R.; Zubrod, J.P.; Schulz, R.; et al. Legacy and emerging organohalogenated compounds in feathers of Eurasian eagle-owls (Bubo bubo) in Norway: Spatiotemporal variations and associations with dietary proxies (δ13C and δ15N). Environ. Res. 2022, 204, 112372. [Google Scholar] [CrossRef]
- Braune, B.M.; Letcher, R.J. Perfluorinated Sulfonate and Carboxylate Compounds in Eggs of Seabirds Breeding in the Canadian Arctic: Temporal Trends (1975–2011) and Interspecies Comparison. Environ. Sci. Technol. 2013, 47, 616–624. [Google Scholar] [CrossRef]
- Sciancalepore, G.; Pietroluongo, G.; Centelleghe, C.; Milan, M.; Bonato, M.; Corazzola, G.; Mazzariol, S. Evaluation of per- and poly-fluorinated alkyl substances (PFAS) in livers of bottlenose dolphins (Tursiops truncatus) found stranded along the northern Adriatic Sea. Environ. Pollut. 2021, 291, 118186. [Google Scholar] [CrossRef]
- Zafeiraki, E.; Gebbink, W.A.; van Leeuwen, S.P.J.; Dassenakis, E.; Megalofonou, P. Occurrence and tissue distribution of perfluoroalkyl substances (PFASs) in sharks and rays from the eastern Mediterranean Sea. Environ. Pollut. 2019, 252, 379–387. [Google Scholar] [CrossRef]
- Armitage, J.M.; MacLeod, M.; Cousins, I.T. Modeling the Global Fate and Transport of Perfluorooctanoic Acid (PFOA) and Perfluorooctanoate (PFO) Emitted from Direct Sources Using a Multispecies Mass Balance Model. Environ. Sci. Technol. 2009, 43, 1134–1140. [Google Scholar] [CrossRef]
- Armitage, J.M.; MacLeod, M.; Cousins, I.T. Comparative Assessment of the Global Fate and Transport Pathways of Long-Chain Perfluorocarboxylic Acids (PFCAs) and Perfluorocarboxylates (PFCs) Emitted from Direct Sources. Environ. Sci. Technol. 2009, 43, 5830–5836. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.M.; Schenker, U.; Scheringer, M.; Martin, J.W.; MacLeod, M.; Cousins, I.T. Modeling the Global Fate and Transport of Perfluorooctane Sulfonate (PFOS) and Precursor Compounds in Relation to Temporal Trends in Wildlife Exposure. Environ. Sci. Technol. 2009, 43, 9274–9280. [Google Scholar] [CrossRef]
- D’eon, J.C.; Hurley, M.D.; Wallington, T.J.; Mabury, S.A. Atmospheric chemistry of N-methyl perfluorobu-tane sulfonamidoethanol, C4F9SO2N(CH3)CH2CH2OH: Kinetics and mechanism of reaction with OH. Environ. Sci. Technol. 2006, 40, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.A.; Martin, J.W.; De Silva, A.O.; Mabury, S.A.; Hurley, M.D.; Sulbaek Andersen, M.P.; Wallington, T.J. Degradation of Fluorotelomer Alcohols: A Likely Atmospheric Source of Perfluorinated Carboxylic Acids. Environ. Sci. Technol. 2004, 38, 3316–3321. [Google Scholar] [CrossRef] [PubMed]
- Karris, G.; Xirouchakis, S.; Maina, I.; Grivas, K.; Kavadas, S. Home range and foraging habitat preference of Scopoli’s Shearwater Calonectris diomedea during the early chick-rearing phase in the eastern Mediterranean. Wildl. Biol. 2018, 2018, wlb-00388. [Google Scholar] [CrossRef]
- Newsted, J.L.; Beach, S.A.; Gallagher, S.P.; Giesy, J.P. Pharmacokinetics and Acute Lethality of Perfluorooctanesulfonate (PFOS) to Juvenile Mallard and Northern Bobwhite. Arch. Environ. Contam. Toxicol. 2006, 50, 411–420. [Google Scholar] [CrossRef]
- Meyer, J.; Jaspers, V.L.B.; Eens, M.; de Coen, W. The relationship between perfluorinated chemical levels in the feathers and livers of birds from different trophic levels. Sci. Total Environ. 2009, 407, 5894–5900. [Google Scholar] [CrossRef]
- González-Solís, J.; Croxall, J.P.; Oro, D.; Ruiz, X. Trans-equatorial migration and mixing in the wintering areas of a pelagic seabird. Front. Ecol. Environ. 2007, 5, 297–301. [Google Scholar] [CrossRef]
- Morera-Pujol, V.; Catry, P.; Magalhães, M.; Péron, C.; Reyes-González, J.M.; Granadeiro, J.P.; Militão, T.; Dias, M.P.; Oro, D.; Dell’Omo, G.; et al. Methods to detect spatial biases in tracking studies caused by differential representativeness of individuals, populations, and time. Divers. Distrib. 2023, 29, 19–38. [Google Scholar] [CrossRef]
- Karris, G. Breeding Ecology of Calonectris diomedea (Aves, Procellariiformes) on Strofades Island Group. Ph.D. Thesis, University of Patras, Patras, Greece, 2014. [Google Scholar]
- Zango, L.; Reyes-González, J.M.; Militão, T.; Zajková, Z.; Álvarez-Alonso, E.; Ramos, R.; González-Solís, J. Year-round individual specialization in the feeding ecology of a longlived seabird. Sci. Rep. 2019, 9, 11812. [Google Scholar] [CrossRef]
- Keller, J.M.; Kannan, K.; Taniyasu, S.; Yamashita, N.; Day, R.D.; Arendt, M.D.; Segars, A.L.; Kucklick, J.R. Perfluorinated Compounds in the Plasma of Loggerhead and Kemp’s Ridley Sea Turtles from the Southeastern Coast of the United States. Environ. Sci. Technol. 2005, 39, 9101–9108. [Google Scholar] [CrossRef]
- Bustnes, J.O.; Erikstad, K.E.; Skaare, J.U.; Bakken, V.; Mehlum, F. Ecological effects of organochlorine pollutants in the Arctic: A study of the Glaucous Gull. Ecol. Appl. 2003, 13, 504–515. [Google Scholar] [CrossRef]
- Sagerup, K.; Henriksen, E.O.; Skorping, A.; Skaare, J.U.; Gabrielsen, G.W. Intensity of parasitic nematodes increases with organochlorine levels in the glaucous gull. J. Appl. Ecol. 2000, 37, 532–539. [Google Scholar] [CrossRef]
- Bustnes, J.O.; Folstad, I.; Erikstad, K.E.; Fjeld, M.; Miland, Ø.O.; Skaare, J.U. Blood concentration of organochlorine pollutants and wing feather asymmetry in Glaucous Gulls. Funct. Ecol. 2002, 16, 617–622. [Google Scholar] [CrossRef]
- Newsted, J.L.; Jones, P.D.; Coady, K.; Giesy, J.P. Avian Toxicity Reference Values for Perfluorooctane Sulfonate. Environ. Sci. Technol. 2005, 39, 9357–9362. [Google Scholar] [CrossRef]
- Molina, E.D.; Balander, R.; Fitzgerald, S.D.; Giesy, J.P.; Kannan, K.; Mitchell, R.; Bursian, S.J. Effects of air cell injection of perfluorooctane sulfonate before incubation on development of the white leghorn chicken (Gallus domesticus) embryo. Environ. Toxicol. Chem. 2006, 25, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.; Huber, N.; Bustnes, J.O.; Herzke, D.; Bårdsen, B.-J.; Eulaers, I.; Johnsen, T.V.; Bourgeon, S. A novel use of the leukocyte coping capacity assay to assess the immunomodulatory effects of organohalogenated contaminants in avian wildlife. Environ. Int. 2020, 142, 105861. [Google Scholar] [CrossRef]
- Dennis, N.M.; Subbiah, S.; Karnjanapiboonwong, A.; Dennis, M.L.; McCarthy, C.; Salice, C.J.; Anderson, T.A. Species- and Tissue-Specific Avian Chronic Toxicity Values for Perfluorooctane Sulfonate (PFOS) and a Binary Mixture of PFOS and Perfluorohexane Sulfonate. Environ. Toxicol. Chem. 2021, 40, 899–909. [Google Scholar] [CrossRef]

| Chemical Type | n | Detection Rate (%) | Mean ± Stdv | Median | Min | Max |
|---|---|---|---|---|---|---|
| 6:2 FTSA | 51 | 98 | 14.08 ± 10.01 | 12.16 | 1.74 | 36.96 |
| FOSA | 22 | 42 | 8.03 ± 9.62 | 5.00 | 0.76 | 37.56 |
| PFTeDeA | 14 | 27 | 26.85 ± 12.17 | 25.65 | 13.07 | 54.19 |
| 7H-PFHpA | 12 | 23 | 3.29 ± 3.12 | 2.62 | 1.18 | 13.23 |
| PFNA | 10 | 19 | 109.10 ± 80.00 | 77.56 | 13.71 | 248.43 |
| PFHpA | 9 | 17 | 15.12 ± 8.39 | 15.29 | 5.14 | 28.08 |
| PFTrDA | 7 | 13 | 29.60 ± 18.06 | 25.34 | 17.15 | 72.83 |
| EtFOSA | 5 | 10 | 10.24 ± 6.83 | 7.36 | 3.03 | 22.60 |
| PFHxA | 5 | 10 | 7.69 ± 2.28 | 6.89 | 4.52 | 10.84 |
| PFHpS | 5 | 10 | 10.32 ± 3.77 | 10.38 | 5.26 | 14.83 |
| PFUnDA | 5 | 10 | 16.63 ± 6.78 | 13.51 | 11.39 | 29.88 |
| ADONA | 5 | 10 | 10.63 ± 9.82 | 5.59 | 3.41 | 29.95 |
| diSAMPAP | 5 | 10 | 14.77 ± 3.33 | 13.40 | 11.80 | 20.98 |
| PFPeA | 4 | 8 | 32.13 ± 6.45 | 31.24 | 23.97 | 42.05 |
| PFOS | 3 | 6 | 20.53 ± 6.18 | 23.69 | 11.90 | 26.01 |
| P37DMOA | 3 | 6 | 3.03 ± 0.21 | 3.08 | 2.76 | 3.26 |
| MeFOSAA | 3 | 6 | 14.81 ± 5.22 | 17.29 | 7.54 | 19.59 |
| PFBS | 3 | 6 | 2.35 ± 1.13 | 2.15 | 1.08 | 3.82 |
| PFDA | 3 | 6 | 11.44 ± 1.60 | 11.72 | 9.36 | 13.25 |
| PFDoDA | 1 | 2 | 15.37 | 15.37 | 15.37 | 15.37 |
| PFHxS | 1 | 2 | 7.79 | 7.79 | 7.79 | 7.79 |
| GenX | 1 | 2 | 61.94 | 61.94 | 61.94 | 61.94 |
| MeFOSA | 8 | 15 | n.q. | n.q. | n.q. | n.q. |
| 8:2 FTSA | 2 | 4 | n.q. | n.q. | n.q. | n.q. |
| 9Cl-PF3ONS | 1 | 2 | n.q. | n.q. | n.q. | n.q. |
| PFAS | n | Mean | Stdv | St. Error Mean | Mann–Whitney U Test Asymp. Sig. (2-Tailed) | Independent Samples t-Test Sig. (2-Tailed) |
|---|---|---|---|---|---|---|
| 6:2 FTS–P1 | 26 | 16.03 | 11.66 | 2.29 | 0.356 | - |
| 6:2 FTS–P10 | 25 | 12.06 | 7.94 | 1.59 | ||
| FOSAA–P1 | 14 | 10.15 | 10.42 | 2.78 | 0.306 | - |
| FOSAA–P10 | 8 | 4.32 | 2.66 | 0.94 | ||
| 7H-PFHpA–P1 | 7 | 2.50 | 1.12 | 0.42 | 1.000 | - |
| 7H-PFHpA–P10 | 5 | 4.38 | 4.98 | 2.23 | ||
| PFTeDA–P1 | 5 | 26.27 | 13.40 | 6.00 | - | 0.904 |
| PFTeDA–P10 | 9 | 27.17 | 13.00 | 4.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trypidaki, E.; Gudmundsen, S.M.B.; Karris, G.; Xirouchakis, S.; Gonzalez, S.V.; Zhang, J.; Jaspers, V.L.B.; Ciesielski, T.M.; Tsangaris, C.; Asimakopoulos, A.G. Occurrence of Novel and Legacy Per/Polyfluoroalkyl Substances (PFASs) in Scopoli’s Shearwater (Calonectris diomedea) Feathers. Diversity 2024, 16, 541. https://doi.org/10.3390/d16090541
Trypidaki E, Gudmundsen SMB, Karris G, Xirouchakis S, Gonzalez SV, Zhang J, Jaspers VLB, Ciesielski TM, Tsangaris C, Asimakopoulos AG. Occurrence of Novel and Legacy Per/Polyfluoroalkyl Substances (PFASs) in Scopoli’s Shearwater (Calonectris diomedea) Feathers. Diversity. 2024; 16(9):541. https://doi.org/10.3390/d16090541
Chicago/Turabian StyleTrypidaki, Eirini, Silje Marie Bøe Gudmundsen, Georgios Karris, Stavros Xirouchakis, Susana V. Gonzalez, Junjie Zhang, Veerle L. B. Jaspers, Tomasz Maciej Ciesielski, Catherine Tsangaris, and Alexandros G. Asimakopoulos. 2024. "Occurrence of Novel and Legacy Per/Polyfluoroalkyl Substances (PFASs) in Scopoli’s Shearwater (Calonectris diomedea) Feathers" Diversity 16, no. 9: 541. https://doi.org/10.3390/d16090541








