New Evidence of the Feeding Behaviors of Coronodon and the Origin of Filter Feeding in Mysticetes (Mammalia: Cetacea) Revisited
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dental Wear in Coronodon
2.1.1. Specimens Studied for Dental Wear
2.1.2. Measurement of Wear
2.1.3. Hypotheses and Statistical Tests for Wear Patterns
- (1)
- There is no association between apical wear and position in the toothrow (if Coronodon engaged in raptorial feeding, we predict that anterior teeth would display more apical wear because they would have more uncontrolled interactions with the hard parts of prey).
- (2)
- There is no association between apical wear and the height of the cusp (if Coronodon engaged in raptorial feeding, we predict that the higher cusps would impact prey more than lower cusps).
- (1)
- There is no association between apical wear and whether a cusp is on the mesial or distal side of the tooth (if Coronodon engaged in dental filtration, as outlined by Geisler et al. [11], the mesial cusps of the lower teeth should have more apical wear than the distal cusps of the lower teeth, as well as more wear than the mesial and distal cusps of the upper teeth).
- (1)
- There is no association between notch wear and the average apical wear of the two adjacent cusps (if notch wear formed by portions of prey being forced into the notch, we would expect that it would be associated with greater apical wear on adjacent cusps).
- (2)
- There is no association between notch wear and the height of the notch (if Coronodon engaged in raptorial feeding, we predict that the higher cusps would impact prey more than lower cusps).
- (3)
- There is no association between notch wear and whether the side it occurs on is occlusal or non-occlusal (if the notch wear formed when prey was wedged between the upper and lower teeth, we predict it would be more common on occlusal sides).
- (4)
- There is no association between the presence of side wear and whether a side is occlusal or non-occlusal (if Coronodon processed prey with its postcanines, we predict that side wear occurred as portions of the prey were wedged between the upper and lower teeth).
2.2. Evolution of Feeding Behaviors in Toothed Mysticetes
2.2.1. Taxa Included
2.2.2. Character Selection
2.2.3. Characters for Inferring Feeding Behaviors and Their Interpretations
2.2.4. Trees Used to Infer the Evolution of Feeding Behaviors
2.2.5. Calculating Support for Alternate Feeding Behavior in Extinct Taxa
3. Results
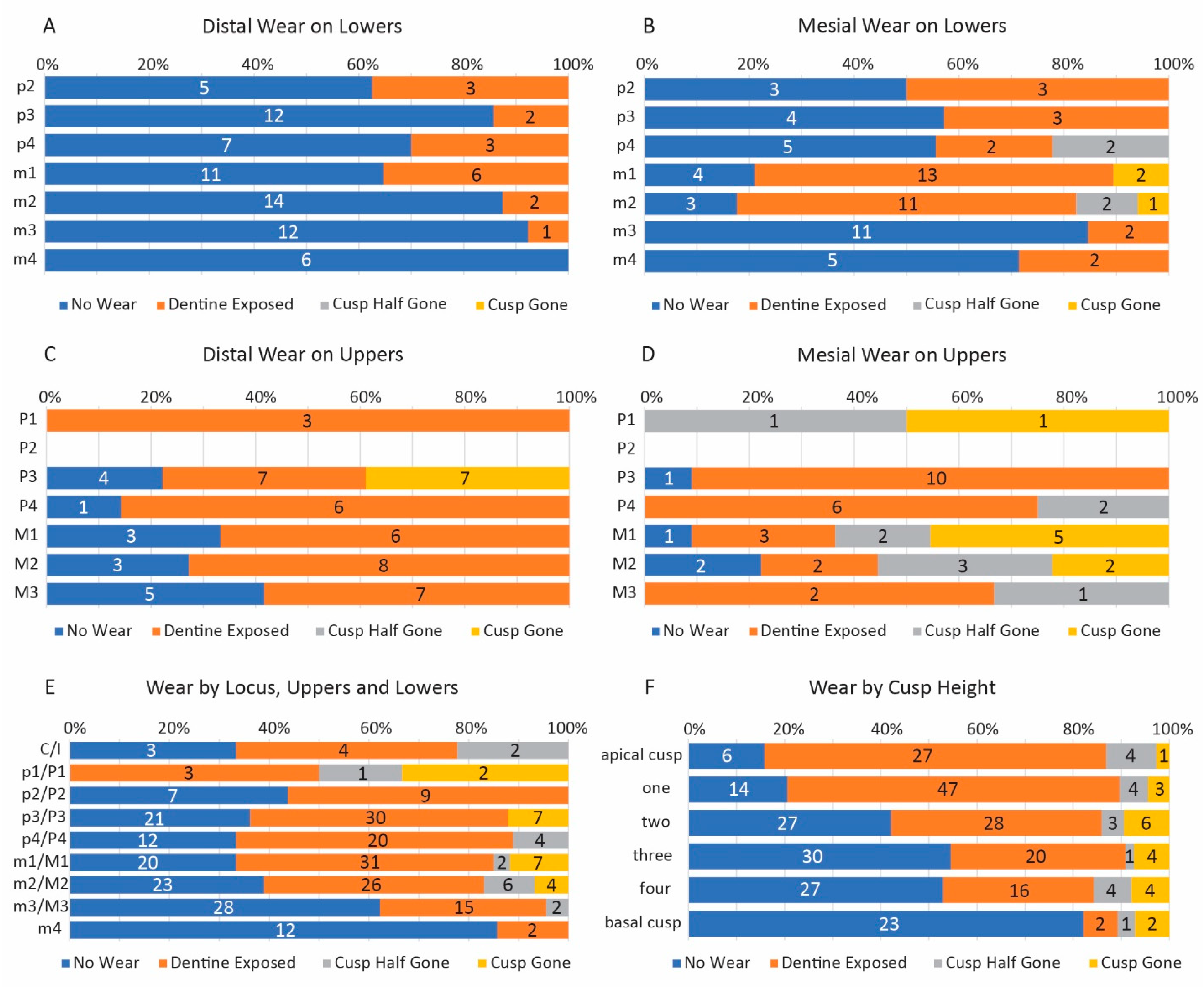
3.1. Dental Wear in Coronodon
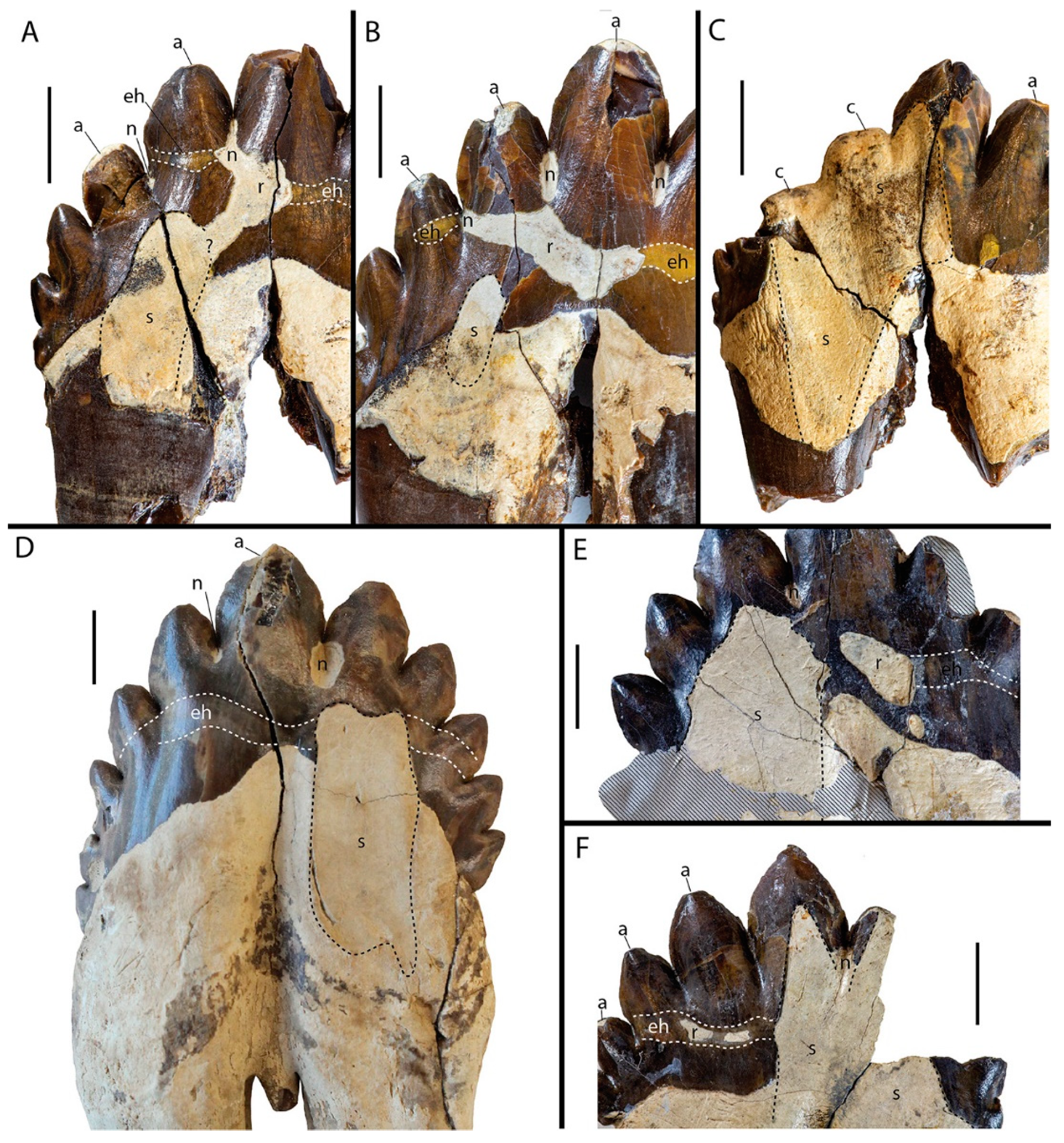
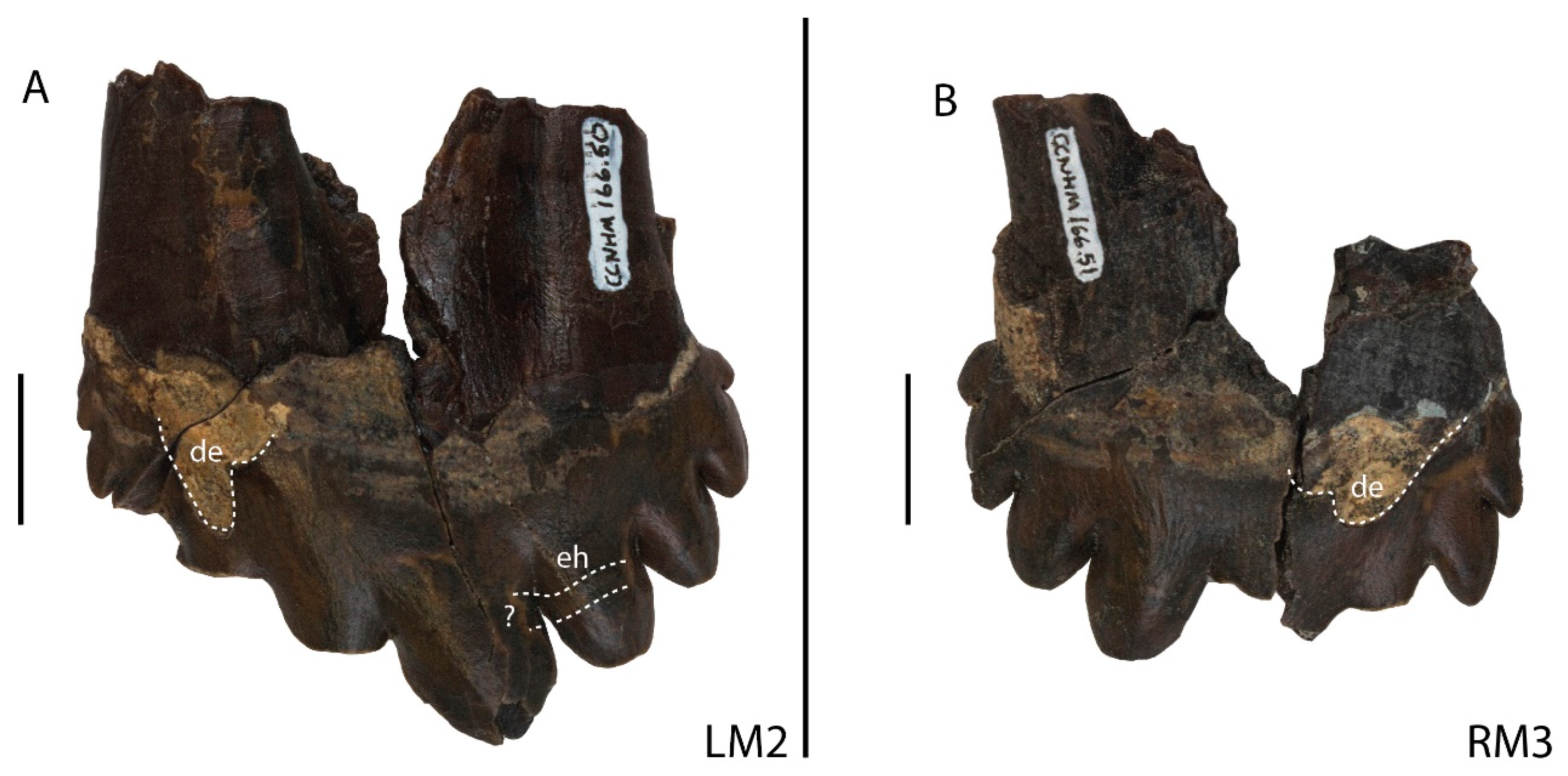
3.1.1. Types of Dental Wear in Coronodon
3.1.2. Statistical Analyses of Wear Patterns in Coronodon
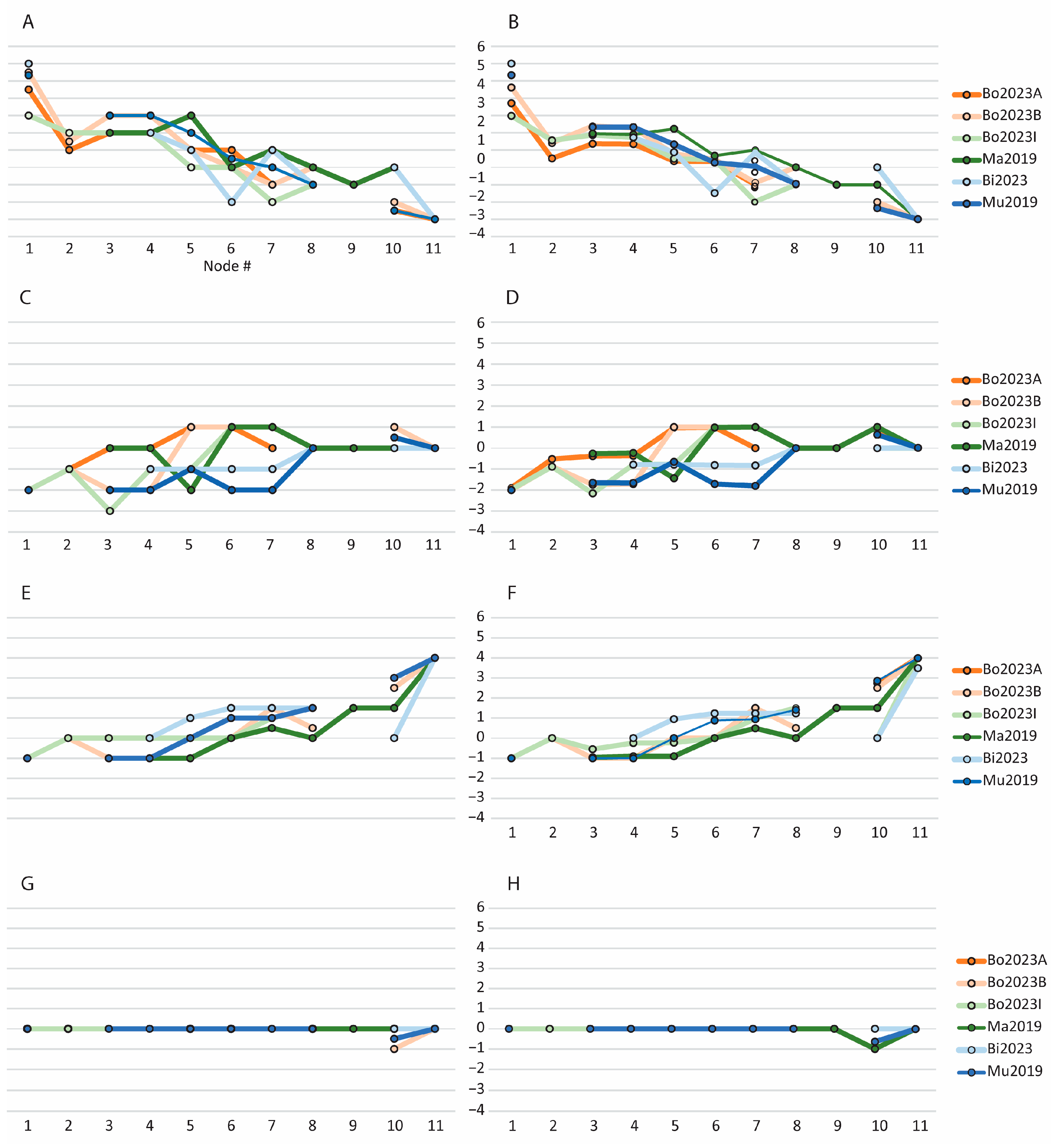
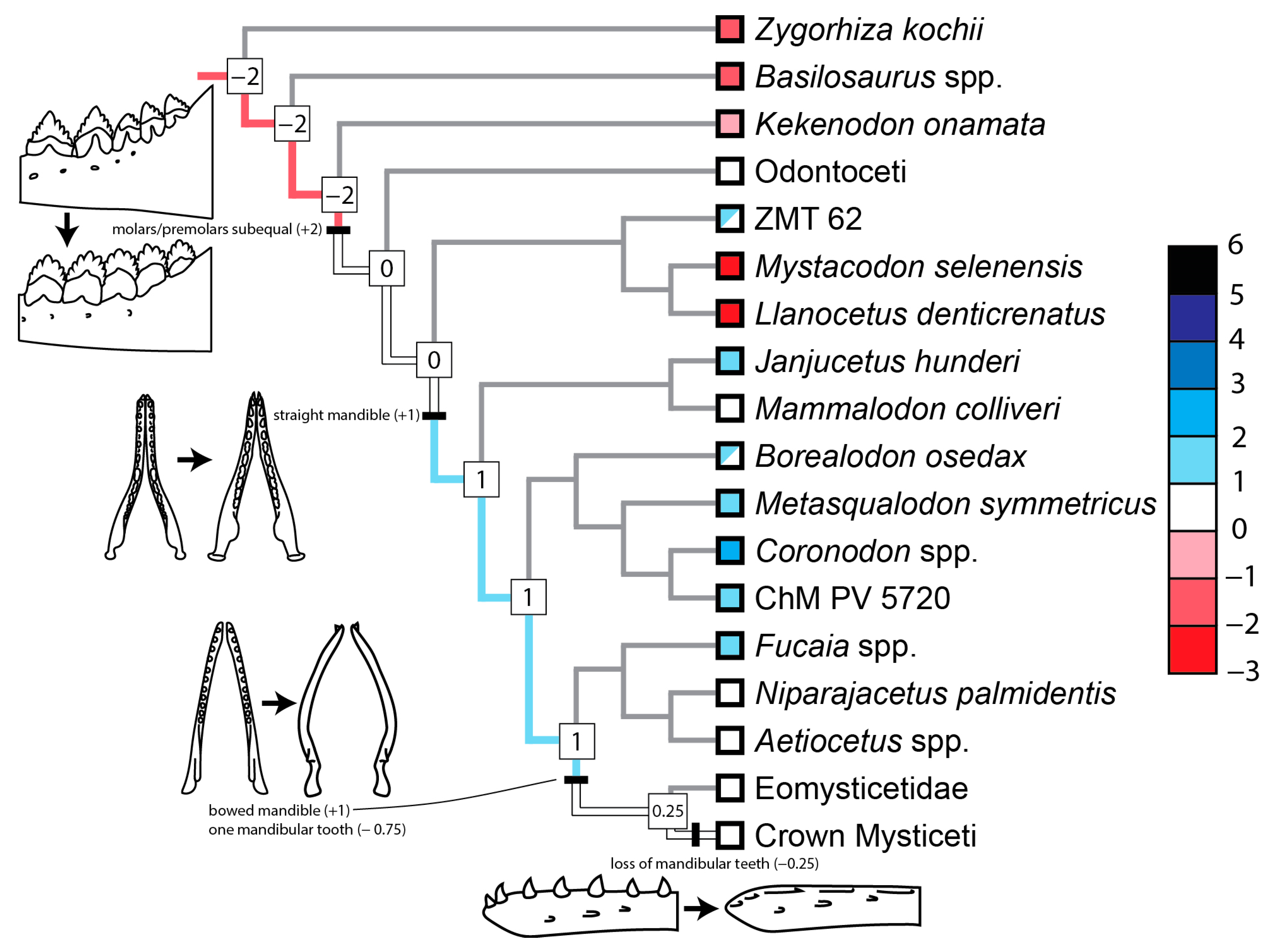
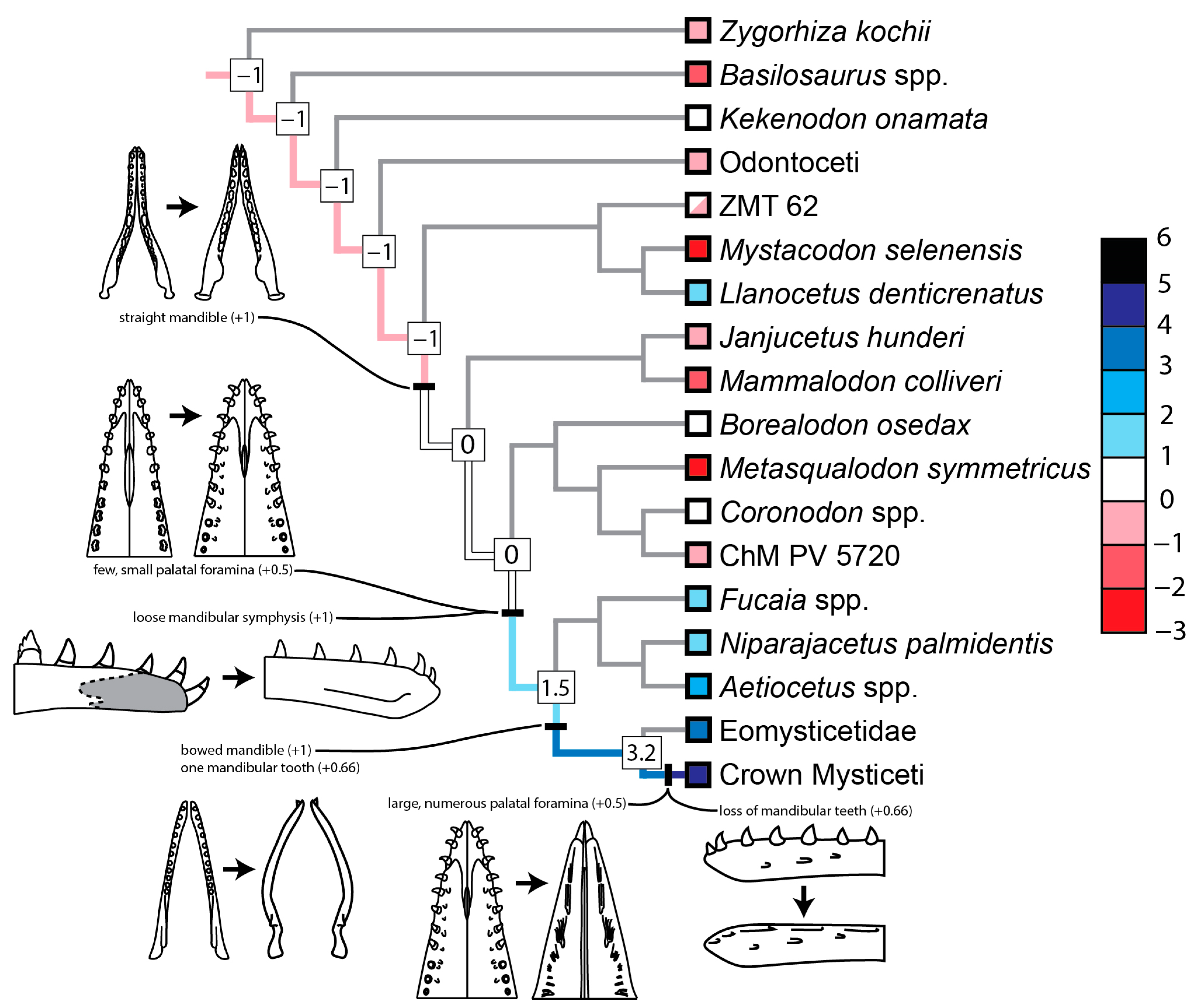
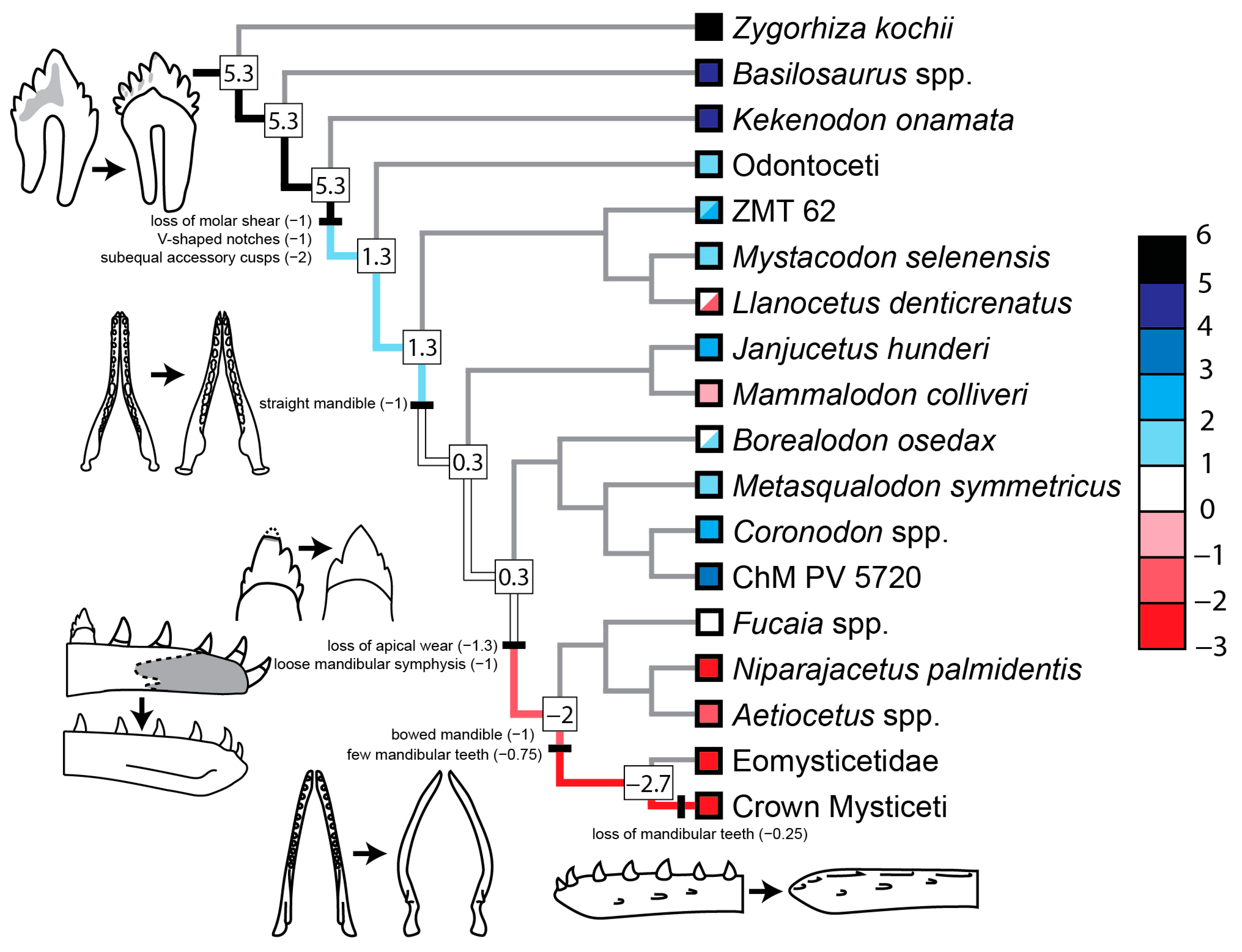
3.2. Evolution of Feeding Behaviors in Toothed Mysticetes
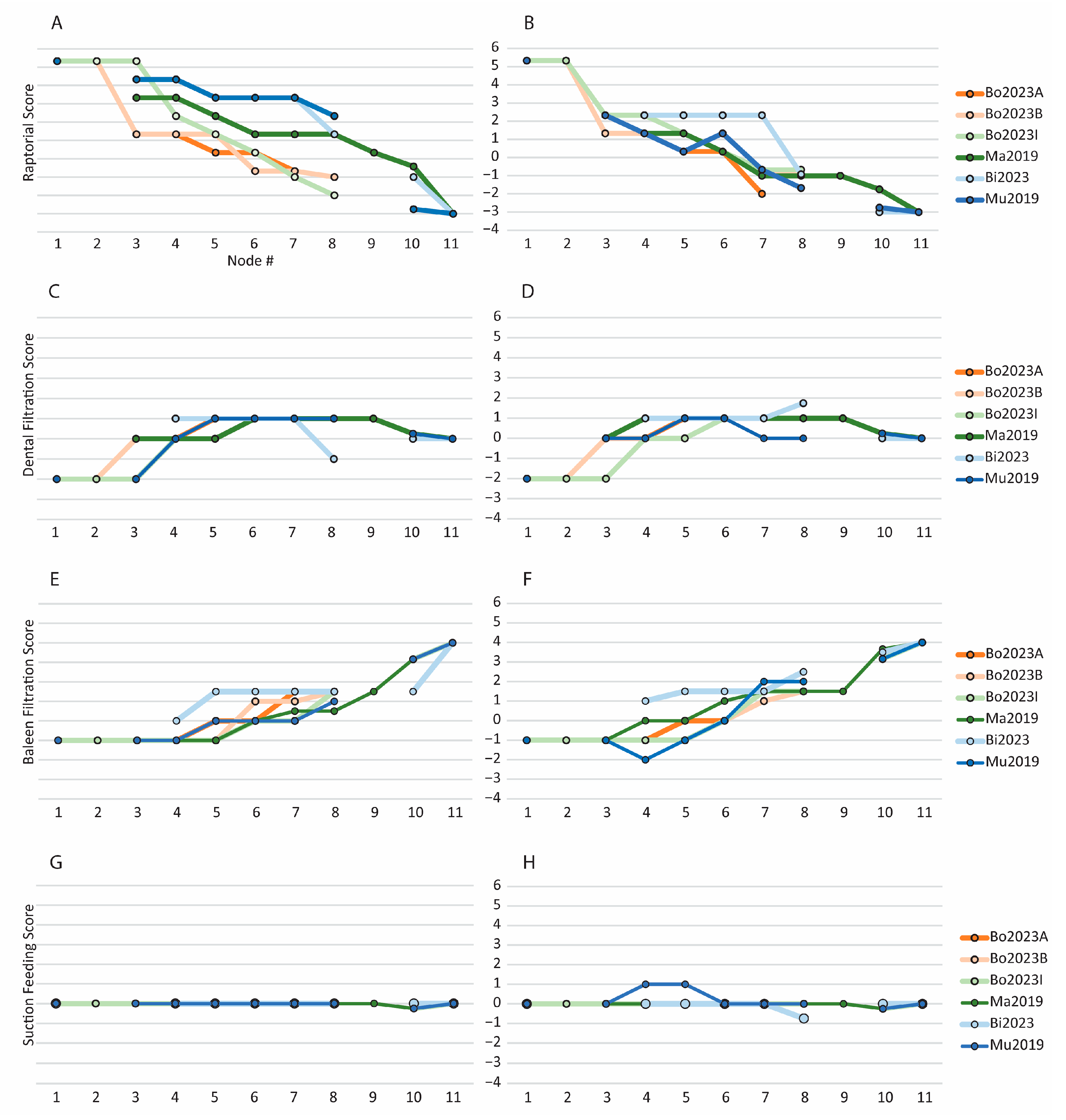
3.2.1. Scores for Extant and Extinct Taxa
3.2.2. Scores of Reconstructed Ancestral Toothed Mysticetes
4. Discussion
4.1. Feeding Behaviors of Coronodon
4.1.1. Suction Feeding Hypothesis for Coronodon
4.1.2. Dental Filtration Hypothesis for Coronodon
4.1.3. Raptorial Feeding Hypothesis for Coronodon
4.2. Origin and Evolution of Filter Feeding in Mysticeti
4.3. Future Tests and Avenues of Investigation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnes, L.G.; Sanders, A.E. The transition from archaeocetes to mysticetes: Late Oligocene toothed mysticetes from near Charleston, South Carolina. Paleontol. Soc. Spec. Publ. 1996, 8, 24. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Fordyce, R.E. A new genus and species of eomysticetid (Cetacea: Mysticeti) and a reinterpretation of “Mauicetus” lophocephalus Marples, 1956: Transitional baleen whales from the upper Oligocene of New Zealand. Zool. J. Linnaean Soc. 2015, 175, 607–660. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Fordyce, R.E. Anatomy, feeding ecology, and ontogeny of a transitional baleen whale: A new genus and species of Eomysticetidae (Mammalia: Cetacea) from the Oligocene of New Zealand. PeerJ 2015, 3, e1129. [Google Scholar] [CrossRef]
- Fitzgerald, E.M.G. A bizarre new toothed mysticete (Cetacea) from Australia and the early evolution of baleen whales. Proc. R. Soc. B 2006, 273, 2955–2963. [Google Scholar] [CrossRef]
- Fitzgerald, E.M.G. The morphology and systematics of Mammalodon colliveri (Cetacea: Mysticeti), a toothed mysticete from the Oligocene of Australia. Zool. J. Linnaean Soc. 2010, 158, 367–476. [Google Scholar] [CrossRef]
- Geisler, J.H.; Sanders, A.E. Morphological evidence for the phylogeny of Cetacea. J. Mamm. Evol. 2003, 10, 23–129. [Google Scholar] [CrossRef]
- Lambert, O.; Martinez-Caceres, M.; Bianucci, G.; Di Celma, C.; Salas-Gismondi, R.; Steurbaut, E.; Urbina, M.; Muizon, C.d. Earliest mysticete from the late Eocene of Peru sheds new light on the origin of baleen whales. Curr. Biol. 2017, 27, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Caceres, M.; Lambert, O.; Muizon, C.d. The anatomy and phylogenetic affinities of Cynthiacetus peruvianus, a large Dorudon-like basilosaurid (Cetacea, Mammalia) from the late Eocene of Peru. Geodiversitas 2017, 39, 7–163. [Google Scholar] [CrossRef]
- Marx, F.G. The more the merrier? A large cladistic analysis of mysticetes, and comments on the transition from teeth to baleen. J. Mamm. Evol. 2011, 18, 77–100. [Google Scholar] [CrossRef]
- Marx, F.G.; Fordyce, R.E. Baleen boom and bust: A synthesis of mysticete phylogeny, diversity and disparity. R. Soc. Open Sci. 2015, 2, 140434. [Google Scholar] [CrossRef]
- Geisler, J.H.; Boessenecker, R.W.; Brown, K.M.; Beatty, B.L. The origin of filter feeding in whales. Curr. Biol. 2017, 27, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Boessenecker, R.W.; Beatty, B.L.; Geisler, J.H. New specimens and species of the Oligocene toothed baleen whale Coronodon from South Carolina and the origin of Neoceti. PeerJ 2023, 11, e14975. [Google Scholar] [CrossRef]
- Muizon, C.d.; Bianucci, G.; Martinez-Caceres, M.; Lambert, O. Mystacodon selenensis, the earliest known toothed mysticete (Cetacea, Mammalia) from the late Eocene of Peru: Anatomy, phylogeny, and feeding adaptations. Geodiversitas 2019, 41, 401–499. [Google Scholar] [CrossRef]
- Corrie, J.E.; Fordyce, R.E. A redescription and re-evaluation of Kekenodon onamata (Mammalia: Cetacea), a late-surviving archaeocete from the late Oligocene of New Zealand. Zool. J. Linnaean Soc. 2022. Online Early. [Google Scholar] [CrossRef]
- Fordyce, R.E. Evolution and zoogeography of cetaceans in Australia. In Vertebrate Zoogeography and Evolution in Australasia; Archer, M., Clayton, G., Eds.; Hesperian Press: Perth, Australia, 1984; pp. 929–948. [Google Scholar]
- Mitchell, E.D. A new cetacean from the late Eocene Meseta Formation, Seymour Island, Antarctic Peninsula. Can. J. Earth Sci. 1989, 46, 2219–2235. [Google Scholar] [CrossRef]
- Deméré, T.A.; McGowen, M.R.; Berta, A.; Gatesy, J. Morphological and molecular evidence for a stepwise evolutionary transition from teeth to baleen in mysticete whales. Syst. Biol. 2008, 57, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Ekdale, E.G.; Deméré, T.A. Neurovascular evidence for a co-occurrence of teeth and baleen in an Oligocene mysticete and the transition to filter-feeding in baleen whales. Zool. J. Linnaean Soc. 2022, 194, 395–415. [Google Scholar] [CrossRef]
- Ekdale, E.G.; Racicot, R.A. Anatomical evidence for low frequency sensitivity in an archaeocete whale: Comparison of the inner ear of Zygorhiza kochii with that of crown Mysticeti. J. Anat. 2015, 226, 22–39. [Google Scholar] [CrossRef]
- Gatesy, J.; Ekdale, E.G.; Deméré, T.A.; Lanzetti, A.; Randall, J.; Berta, A.; El Adli, J.J.; Springer, M.S.; McGowen, M.R. Anatomical, ontogenetic, and genomic homologies guide reconstructions of the teeth-to-baleen transition in mysticete whales. J. Mamm. Evol. 2022, 29, 891–930. [Google Scholar] [CrossRef]
- Peredo, C.M.; Pyenson, N.D.; Uhen, M.D. Lateral palatal foramina do not indicate baleen in fossil whales. Sci. Rep. 2022, 12, 11448. [Google Scholar] [CrossRef]
- Ekdale, E.G.; El Adli, J.J.; McGowen, M.R.; Deméré, T.A.; Lanzetti, A.; Berta, A.; Springer, M.S.; Boessenecker, R.W.; Gatesy, J. Lateral palatal foramina are not widespread in Artiodactyla and imply baleen in extinct mysticetes. Sci. Rep. 2024, 14, 10174. [Google Scholar] [CrossRef] [PubMed]
- Hocking, D.P.; Marx, F.G.; Fitzgerald, E.M.G.; Evans, A.R. Ancient whales did not filter feed with their teeth. Biol. Lett. 2017, 13, 20170348. [Google Scholar] [CrossRef]
- Marx, F.G.; Hocking, D.P.; Park, T.; Ziegler, T.; Evans, A.R.; Fitzgerald, E.M.G. Suction feeding preceded filtering in baleen whale evolution. Mem. Mus. Vic. 2016, 75, 71–82. [Google Scholar] [CrossRef]
- Peredo, C.M.; Pyenson, N.D.; Boersma, A.T. Decoupling tooth loss from the evolution of baleen in whales. Front. Mar. Sci. 2017, 4, 67. [Google Scholar] [CrossRef]
- Fordyce, R.E.; Marx, F.G. Gigantism precedes filter feeding in baleen whale evolution. Curr. Biol. 2018, 28, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Marx, F.G.; Hocking, D.P.; Park, T.; Pollock, T.I.; Parker, W.M.G.; Rule, J.P.; Fitzgerald, E.M.G.; Evans, A.R. Suction causes novel tooth wear in marine mammals, with implications for feeding evolution in baleen whales. J. Mamm. Evol. 2023, 30, 493–505. [Google Scholar] [CrossRef]
- Bianucci, G.; Geisler, J.H.; Citron, S.; Collareta, A. The origins of the killer whale ecomorph. Curr. Biol. 2022, 32, 1843–1851. [Google Scholar] [CrossRef]
- Foote, A.D.; Newton, J.; Piertney, S.B.; Willerslev, E.; Gilbert, M.T.P. Ecological, morphological and genetic divergence of sympatric North Atlantic killer whale populations. Mol. Ecol. 2009, 18, 5207–5217. [Google Scholar] [CrossRef]
- Ford, J.K.B.; Ellis, G.M.; Matkin, C.O.; Wetklo, M.H.; Barrett-Lennard, L.G.; Withler, R.E. Shark predation and tooth wear in a population of northeastern Pacific killer whales. Aquat. Biol. 2011, 11, 213–224. [Google Scholar] [CrossRef]
- Fordyce, R.E. Cetacean fossil record. In Encyclopedia of Marine Mammals, 2nd ed.; Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: Burlington, MA, USA, 2009; pp. 207–215. [Google Scholar]
- Baird, R.W.; Abrams, P.A.; Dill, L.M. Possible indirect interactions between transient and resident killer whales: Implications for the evolution of foraging specializations in the genus Orcinus. Oecologia 1992, 89, 125–132. [Google Scholar] [CrossRef]
- Morton, A.B. A quantitative comparison of the behaviour of resident and transient forms of the killer whale off the central British Columbia coast. Rep. Int. Whal. Comm. Spec. Issue 1990, 12, 245–248. [Google Scholar]
- Morin, P.A.; Archer, F.I.; Foote, A.D.; Vilstrup, J.; Allen, E.; Wade, P.; Durban, J.; Parsons, K.; Pitman, R.; Li, L.; et al. Complete mitochondrial genome phylogeographic analysis of killer whales (Orcinus orca) indicates multiple species. Genome Res. 2010, 20, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Morin, P.A.; McCarthy, M.L.; Fung, C.W.; Durban, J.W.; Parsons, K.; Perrin, W.F.; Taylor, B.L.; Jefferson, T.A.; Archer, F.I. Revised taxonomy of eastern North Pacific killer whales (Orcinus orca): Bigg’s and resident ecotypes deserve species status. Proc. R. Soc. B 2024, 11, 231368. [Google Scholar] [CrossRef]
- Pitman, R.L.; Ensor, P. Three forms of killer whales (Orcinus orca) in Antarctic waters. J. Cetacean Res. Manag. 2003, 5, 131–139. [Google Scholar] [CrossRef]
- Hocking, D.P.; Evans, A.R.; Fitzgerald, E.M.G. Leopard seals (Hydrurga leptonyx) use suction and filter feeding when hunting small prey underwater. Polar Biol. 2013, 36, 211–222. [Google Scholar] [CrossRef]
- Losos, J.B. Convergence, adaptation, and constraint. Evolution 2011, 65, 1827–1840. [Google Scholar] [CrossRef]
- Bonaparte, J.F. Pterodaustro guinazui gen. et sp. nov. Pterosaurio de la Formación Lagarcito, Provincia de San Luis, Argentina y su significado en la geologia regional (Pterodactylidae). Acta Geol. Lilloana 1970, 10, 209–225. [Google Scholar]
- Compagno, L.J.V. Relationships of the megamouth shark, Megachasma pelagios (Lamniformes: Megachasmidae), with comments on its feeding habits. Natl. Ocean. Atmos. Adm. Technol. Rep. Natl. Mar. Fish. Serv. 1990, 90, 357–379. [Google Scholar]
- Friedman, M.; Shimada, K.; Martin, L.D.; Everhart, M.J.; Liston, J.; Maltese, A.; Triebold, M. 100-million-year dynasty of giant planktivorous bony fishes in the Mesozoic seas. Science 2010, 327, 990–993. [Google Scholar] [CrossRef]
- King, J.E. The feeding mechanism and jaws of the crabeaster seal (Lobodon carcinophagus). Mammalia 1961, 45, 462–466. [Google Scholar]
- O’Keefe, F.R.; Otero, R.A.; Acuña, S.S.; O’Gorman, J.P.; Chatterjee, S. Cranial anatomy of Mortuneria seymourensis from Antarctica, and the evolution of filter feeding in plesiosaurs of the Austral Late Cretaceous. J. Vertebr. Paleontol. 2017, 37, 31347570. [Google Scholar]
- Shimada, K.; Popov, E.V.; Siversson, M.; Welton, B.J.; Long, D.J. A new clade of putative plankton-feeding sharks from the Upper Cretaceous of Russia and the United States. J. Vertebr. Paleontol. 2015, 35, e981335. [Google Scholar] [CrossRef]
- Peredo, C.M.; Pyenson, N.D.; Marshall, C.D.; Uhen, M.D. Tooth loss precedes the origin of baleen in whales. Curr. Biol. 2018, 28, 3992–4000. [Google Scholar] [CrossRef] [PubMed]
- Ackermans, N.L. The history of mesowear: A review. PeerJ 2020, 8, e8519. [Google Scholar] [CrossRef]
- Corporation, I. IBM SPSS Statistics for Windows, Version 29.0.2.0; IBM Corporation: Armonk, NY, USA, 2023. [Google Scholar]
- Boessenecker, R.W.; Fordyce, R.E. A new eomysticetid from the Oligocene Kokoamu Greensand of New Zealand and a review of the Eomysticetidae (Mammalia, Cetacea). J. Syst. Palaeontol. 2017, 15, 429–469. [Google Scholar] [CrossRef]
- Fordyce, R.E.; Quilty, P.G.; Daniels, J. Australodelphis mirus, a bizarre new toothless ziphiid-like fossil dolphin (Cetacea: Delphinidae) from the Pliocene of Vestfold Hills, East Antarctica. Antarct. Sci. 2002, 14, 37–54. [Google Scholar] [CrossRef]
- Loch, C.; Simões-Lopes, P.C. Dental wear in dolphins (Cetacea: Delphinidae) from southern Brazil. Arch. Oral Biol. 2013, 58, 134–141. [Google Scholar] [CrossRef]
- Van Valkenburgh, B. Costs of carnivory: Tooth fracture in Pleistocene and recent carnivorans. Biol. J. Linn. Soc. 2009, 96, 68–81. [Google Scholar] [CrossRef]
- Lambert, O.; Bianucci, G. How to break a sperm whale’s teeth: Dental damage in a large Miocene physeteroid from the North Sea basin. J. Vertebr. Paleontol. 2019, 39, e1660987. [Google Scholar] [CrossRef]
- Caldwell, D.K.; Brown, D.H. Tooth wear as a correlate of described feeding behavior by the killer whale, with notes on a captive specimen. Bull. South. Calif. Acad. Sci. 1964, 63, 128–140. [Google Scholar]
- Lambert, O.; Muizon, C.d.; Bianucci, G. The most basal beaked whale Ninoziphius platyrostris Muizon, 1983: Clues on the evolutionary history of the family Ziphiidae (Cetacea: Odontoceti). Zool. J. Linn. Soc. 2013, 167, 569–598. [Google Scholar] [CrossRef]
- O’Leary, M.A.; Uhen, M.D. The time of origin of whales and the role of behavioral changes in the terrestrial-aquatic transition. Paleobiology 1999, 25, 534–556. [Google Scholar] [CrossRef]
- Uhen, M.D. Form, function, and anatomy of Dorudon atrox (Mammalia, Cetacea): An archaeocete from the middle to late Eocene of Egypt. Univ. Mich. Pap. Paleontol. 2004, 34, 1–222. [Google Scholar]
- Boessenecker, R.W.; Geisler, J.H. New skeletons of the ancient dolphin Xenorophus sloanii and Xenorophus simplicidens sp. nov. (Mammalia, Cetacea) from the Oligocene of South Carolina and the ontogeny, functional anatomy, asymmetry, pathology, and evolution of the earliest Odontoceti. Diversity 2023, 15, 1154. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Fraser, D.; Churchill, M.; Geisler, J.H. A toothless dwarf dolphin (Odontoceti: Xenorophidae) points to explosive feeding diversification of modern whales (Neoceti). Proc. R. Soc. B 2017, 284, 20170531. [Google Scholar] [CrossRef] [PubMed]
- Werth, A.J. Mandibular and dental variation and the evolution of suction feeding in Odontoceti. J. Mammal. 2006, 87, 579–588. [Google Scholar] [CrossRef]
- Johnston, C.; Berta, A. Comparative anatomy and evolutionary history of suction feeding in cetaceans. Mar. Mammal Sci. 2011, 27, 493–513. [Google Scholar] [CrossRef]
- Werth, A.J. Odontocete suction feeding: Experimental analysis of water flow and head shape. J. Morphol. 2006, 267, 1415–1428. [Google Scholar] [CrossRef]
- McCurry, M.R.; Evans, A.R.; Fitzgerald, E.M.G.; Adams, J.W.; Clausen, P.D.; McHenry, C.R. The remarkable convergence of skull shape in crocodilians and toothed whales. Proc. R. Soc. B 2017, 284, 20162348. [Google Scholar] [CrossRef]
- McCurry, M.R.; Pyenson, N.D. Hyper-longirostry and kinematic disparity in extinct toothed whales. Paleobiology 2018, 45, 21–29. [Google Scholar] [CrossRef]
- Gordon, K.R. Models of tongue movement in the walrus (Odobenus rosmarus). J. Morphol. 1984, 182, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Abler, W.L. The serrated teeth of tyrannosaurid dinosaurs, and biting structures in other animals. Paleobiology 1992, 18, 161–183. [Google Scholar] [CrossRef]
- Moyer, J.K.; Bemis, W.E. Shark teeth as edged weapons: Serrated teeth of three species of selachians. Zoology 2017, 120, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Fyler, C.A.; Reeder, T.W.; Berta, A.; Antonelis, G.; Aguilar, A.; Androulaki, A. Historical biogeography and phylogeny of monachine seals (Pinnipedia: Phocidae) based on mitochondrial and nuclear DNA data. J. Biogeogr. 2005, 32, 1267–1279. [Google Scholar] [CrossRef]
- Fulton, T.L.; Strobeck, C. Multiple markers and multiple individuals refine seal phylogeny and bring molecules and morphology back in line. Proc. R. Soc. B Biol. Sci. 2010, 277, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.S.; LaBarbera, M. Functional consequences of tooth design: Effects of blade shape on energetics of cutting. J. Exp. Biol. 2008, 211, 3619–3626. [Google Scholar] [CrossRef]
- Anderson, P.S. The effects of trapping and blade angle of notched dentitions on fracture of biological tissues. J. Exp. Biol. 2009, 212, 3627–3632. [Google Scholar] [CrossRef]
- Mellett, J.S. Mammalian carnassial function and the “every effect”. J. Mammal. 1981, 62, 164–166. [Google Scholar] [CrossRef]
- Berta, A.; Lanzetti, A. Feeding in marine mammals: An integration of evolution and ecology through time. Palaeontol. Electron. 2020, 23, 1–41. [Google Scholar] [CrossRef]
- Berta, A.; Lanzetti, A.; Ekdale, E.G.; Demére, T.A. From teeth to baleen and raptorial to bulk filter feeding in mysticete cetaceans: The role of paleontological, genetic, and geochemical data in feeding evolution and ecology. Integr. Comp. Biol. 2016, 56, 1271–1284. [Google Scholar] [CrossRef]
- Ishikawa, H.; Amasaki, H. Development and physiological degradation of tooth buds and development of rudiment of baleen plate in southern minke whale, Balaenoptera acutorostrata. J. Vet. Med. Sci. 1995, 57, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Lanzetti, A. Prenatal developmental sequence of the skull of minke whales and its implications for the evolution of mysticetes and the teeth-to-baleen transition. J. Anat. 2019, 235, 725–748. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, H. The origin of baleen whale—Comparative morphology of the toothed mysticetes and the minke whale fetuses. J. Foss. Res. 2008, 40, 120–130. [Google Scholar]
- Rice, D.W. Sperm whale, Physeter macrocephalus Linnaeus 1758. In Handbook of Marine Mammals 4: River Dolphins and the Larger Toothed Whales; Ridgway, S.H., Harrison, R.J., Eds.; Academic Press: Cambridge, MA, USA, 1989; pp. 177–233. [Google Scholar]
- Toledo, G.A.C.; Langguth, A. Maxillary teeth in sperm whales, Physeter macrocephalus (Cetacea: Physeteridae). J. Morphol. Sci. 2015, 32, 212–215. [Google Scholar] [CrossRef]
- Flower, W.H. Description of the skeleton of Inia geoffrensis and the skull of Pontoporia blainvillii, with remarks on the systematic position of these animals in the Order Cetacea. Trans. Zool. Soc. Lond. 1867, 6, 87–116. [Google Scholar] [CrossRef]
- Scapino, R. The third joint of the canine jaw. J. Morphol. 1965, 116, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Scapino, R. Morphological investigation into functions of the jaw symphysis in carnivorans. J. Morphol. 1981, 167, 339–375. [Google Scholar] [CrossRef]
- Scott, J.E.; Hogue, A.S.; Ravosa, M.J. The adaptive significance of mandibular symphyseal fusion in mammals. J. Evol. Biol. 2012, 25, 661–673. [Google Scholar] [CrossRef]
- Fordyce, R.E. Simocetus rayi (Odontoceti, Simocetidae, new family); a bizarre new archaic Oligocene dolphin from the eastern North Pacific. Smithson. Contrib. Paleobiol. 2002, 93, 185–222. [Google Scholar]
- Barnes, L.G.; Goedert, J.L.; Furusawa, H. The Earliest Known Echolocating Toothed Whales (Mammalia; Odontoceti): Preliminary Observations of Fossils from Washington State. Mesa Southwest Mus. Bull. 2001, 8, 91–100. [Google Scholar]
- Eschricht, D.F.; Reinhardt, J.T. On the Greenland right whale (Balaena mysticetus Linn.) with especial reference to its geographical distribution and migrations in times past and present, and to its external and internal characteristics. Ray Soc. Publ. 1866, 40, 3–150. [Google Scholar]
- Lambertsen, R.H.; Ulrich, N.; Straley, J. Frontomandibular stay of Balaenopteridae: A mechanism for momentum recapture during feeding. J. Mammal. 1995, 76, 877–899. [Google Scholar] [CrossRef]
- Johnston, C.; Deméré, T.A.; Berta, A.; Yonas, J.; St. Leger, J. Observations on the musculoskeletal anatomy of the head of a neonate gray whale (Eschrichtius robustus). Mar. Mammal Sci. 2010, 26, 186–194. [Google Scholar] [CrossRef]
- Pyenson, N.D.; Goldbogen, J.A.; Vogl, A.W.; Szathmary, G.; Drake, R.L.; Shadwick, R.E. Discovery of a sensory organ that coordinates lunge feeding in rorqual whales. Nature 2012, 485, 498–501. [Google Scholar] [CrossRef]
- Pivorunas, A. The fibrocartilage skeleton and related structures of the ventral pouch of balaenopterid whales. J. Morphol. 1977, 151, 299–314. [Google Scholar] [CrossRef]
- Werth, A.J.; Ito, H.; Ueda, K. Multiaxial movements at the minke whale temporomandibular joint. J. Morphol. 2020, 281, 402–412. [Google Scholar] [CrossRef]
- Goldbogen, J.A.; Pyenson, N.D.; Shadwick, R.E. Big gulps require high drag for fin whale lunge feeding. Mar. Ecol. Prog. Ser. 2007, 349, 289–301. [Google Scholar] [CrossRef]
- Pyenson, N.D.; Goldbogen, J.A.; Shadwick, R.E. Mandible allometry in extant and fossil Balaenopteridae (Cetacea: Mammalia): The largest vertebrate skeletal element and its role in rorqual lunge-feeding. Biol. J. Linn. Soc. 2013, 108, 586–599. [Google Scholar] [CrossRef]
- Huggenberger, S.; André, M.; Oelschläger, H.H. The nose of the sperm whale: Overviews of functional design, structural homologies and evolution. J. Mar. Biol. Assoc. UK 2016, 96, 783–806. [Google Scholar] [CrossRef]
- Goldbogen, J.A.; Cade, D.E.; Calambokidis, J.; Friedlaender, A.S.; Potvin, J.; Segre, P.S.; Werth, A.J. How baleen whales feed: The biomechanics of engulfment and filtration. Annu. Rev. Mar. Sci. 2017, 9, 367–386. [Google Scholar] [CrossRef]
- Geisler, J.H.; Theodor, J.M. Hippopotamus and whale phylogeny. Nature 2009, 458, E1–E4. [Google Scholar] [CrossRef] [PubMed]
- Geisler, J.H.; Theodor, J.M.; Uhen, M.D.; Foss, S.E. Phylogenetic relationships of cetaceans to terrestrial artiodactyls. In The Evolution of Artiodactyls; Prothero, D.R., Foss, S.E., Eds.; Johns Hopkins University Press: Baltimore, Maryland, 2007; pp. 19–31. [Google Scholar]
- Geisler, J.H.; Uhen, M.D. Phylogenetic relationships of extinct cetartiodactyls: Results of simultaneous analyses of molecular, morphological, and stratigraphic data. J. Mamm. Evol. 2005, 12, 145–160. [Google Scholar] [CrossRef]
- Thewissen, J.G.M.; Cooper, L.N.; Clementz, M.T.; Bajpai, S.; Tiwari, B.N. Whales originated from aquatic artiodactyls in the Eocene epoch of India. Nature 2007, 450, 1190–1194. [Google Scholar] [CrossRef]
- Thewissen, J.G.M.; Hieronymus, T.L.; George, J.C.; Suydam, R.; Stimmelmayr, R.; McBurney, D. Evolutionary aspects of the development of teeth and baleen in the bowhead whale. J. Anat. 2017, 230, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Thewissen, J.G.M. Phylogenetic aspects of cetacean origins: A morphological perspective. J. Mamm. Evol. 1994, 2, 157–184. [Google Scholar] [CrossRef]
- Fahlke, J.M. Bite marks revisited—Evidence for middle-to-late Eocene Basilosaurus isis predation on Dorudon atrox (both Cetacea, Basilosauridae). Palaeontol. Electron. 2012, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Snively, E.; Fahlke, J.M.; Welsh, R.C. Bone-breaking bite force of Basilosaurus isis (Mammalia, Cetacea) from the Late Eocene of Egypt estimated by finite element analysis. PLoS ONE 2015, 10, 30118380. [Google Scholar] [CrossRef]
- Anderson, P.S. Making a point: Shared mechanics underlying the diversity of biological puncture. J. Exp. Biol. 2018, 221, jeb187294. [Google Scholar] [CrossRef]
- Fahlke, J.M.; Bastl, K.A.; Semprebon, G.M.; Gingerich, P.D. Paleoecology of archaeocete whales throughout the Eocene: Dietary adaptations revealed by microwear analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 386, 690–701. [Google Scholar] [CrossRef]
- Constantino, P.J.; Bush, M.B.; Barani, A.; Lawn, B.R. On the evolutionary advantage of multi-cusped teeth. J. R. Soc. Interface 2016, 13, 20160374. [Google Scholar] [CrossRef]
- Ishihara, U.; Miyazaki, N.; Yurkowski, D.J.; Watanabe, Y.Y. Multi-cusped postcanine teeth are associated with zooplankton feeding in phocid seals. Mar. Ecol. Prog. Ser. 2024, 729, 233–245. [Google Scholar] [CrossRef]
- Thewissen, J.G.M.; Hussain, S.T. Systematic review of Pakicetidae, early and middle Eocene Cetacea (Mammalia) from Pakistan and India. Bull. Carnegie Mus. Nat. Hist. 1998, 34, 220–238. [Google Scholar]
- Cooper, L.N.; Thewissen, J.G.M.; Hussain, S.T. New middle Eocene archaeocetes (Cetacea: Mammalia) from the Kuldana Formation of Northern Pakistan. J. Vertebr. Paleontol. 2009, 29, 1289–1299. [Google Scholar] [CrossRef]
- Churchill, M.; Clementz, M.T. Functional implications of variation in tooth spacing and crown size in Pinnipedimorpha (Mammalia: Carnivora). Anat. Rec. 2016, 298, 878–902. [Google Scholar] [CrossRef]
- Cappetta, H. Handbook of Paleoichthyology. Chondrichthyes (Mesozoic and Cenozoic Elasmobranchii: Teeth); Gustav Fisher: Stuttgart, Germany, 2012; Volume 3B, p. 512. [Google Scholar]
- Goloboff, P.A.; Farris, J.S.; Nixon, K.C. TNT, a free program for phylogenetic analysis. Cladistics 2008, 24, 774–786. [Google Scholar] [CrossRef]
- Marx, F.G.; Post, K.; Bosselaers, M.; Munsterman, D.K. A large late Miocene cetotheriid (Cetacea, Mysticeti) from The Netherlands clarifies the status of Tranatocetidae. PeerJ 2019, 7, e6426. [Google Scholar] [CrossRef]
- Bisconti, M.; Pellegrino, L.; Carnevale, G. The chronology of mysticete diversification (Mammalia, Cetacea, Mysticeti): Body size, morphological evolution and global change. Earth Sci. Rev. 2023, 239, 104373. [Google Scholar] [CrossRef]
- Deméré, T.A.; Berta, A.; McGowen, M.R. The taxonomic and evolutionary history of modern balaenopteroid mysticetes. J. Mamm. Evol. 2005, 12, 99–143. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis, Version 3.81. 2023. Available online: http://www.mesquiteproject.org/ (accessed on 6 June 2024).
- Bapst, D.W. Paleotree: An R package for paleontological and phylogenetic analyses of evolution. Methods Ecol. Evol. 2012, 3, 803–807. [Google Scholar] [CrossRef]
- Hale, F.A. Dental caries in the dog. Can. Vet. J. 2009, 50, 1301–1304. [Google Scholar] [CrossRef]
- Kane, E.A.; Marshall, C.D. Comparative feeding kinematics and performance of odontocetes: Belugas, Pacific white-sided dolphins and long-finned pilot whales. J. Exp. Biol. 2009, 212, 3939–3950. [Google Scholar] [CrossRef] [PubMed]
- Cundall, D.; Fernandez, E.; Irish, F. The suction mechanism of the pipid frog, Pipa pipa (Linnaeus, 1758). J. Morphol. 2017, 278, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Heyning, J.E.; Mead, J.G. Suction feeding in beaked whales: Morphological and observational evidence. Nat. Hist. Mus. Los. Angeles Cty. Contrib. Sci. 1996, 464, 1–12. [Google Scholar] [CrossRef]
- Emerson, S.B.; Radinsky, L. Functional analysis of sabertooth cranial morphology. Paleobiology 1980, 6, 295–312. [Google Scholar] [CrossRef]
- Joeckel, R.M. A functional interpretation of the masticatory system and paleoecology of entelodonts. Paleobiology 1990, 16, 459–482. [Google Scholar] [CrossRef]
- Werth, A.J.; Potvin, J.; Shadwick, R.E.; Jensen, M.M.; Cade, D.E.; Goldbogen, J.A. Filtration area scaling and evolution in mysticetes: Trophic niche partitioning and the curious cases of sei and pygmy right whales. Biol. J. Linn. Soc. 2018, 125, 264–279. [Google Scholar] [CrossRef]
- Brodie, P.F. The white whale Delphinapterus leucas (Pallas, 1776). In Handbook of Marine Mammals 4: River Dolphins and the Larger Toothed Whales; Ridgway, S.H., Harrison, R., Eds.; Academic Press: San Diego, CA, USA, 1989; pp. 119–144. [Google Scholar]
- Werth, A.J. A kinematic study of suction feeding and associated behavior in the long-finned pilot whale, Globicephala melas (Traill). Mar. Mammal. Sci. 2000, 16, 299–314. [Google Scholar] [CrossRef]
- Clementz, M.T.; Fordyce, R.E.; Peek, S.L.; Fox, D.L. Ancient marine isoscapes and isotopic evidence of bulk-feeding by Oligocene cetaceans. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 400, 28–40. [Google Scholar] [CrossRef]
- Hall-Aspland, S.A.; Hall, A.P.; Rogers, T.L. A new approach to the solution of the linear mixing model for a single isotope: Application to the case of an opportunistic predator. Oecologia 2005, 143, 143–147. [Google Scholar] [CrossRef]
- Carr, E.M.; Motta, P.J. Tooth length and occlusion in four species of piscivorous fishes: Getting a grip on prey. Environ. Biol. Fishes 2020, 103, 903–912. [Google Scholar] [CrossRef]
- Mihalitsis, M.; Bellwood, D. Functional implications of dentition-based morphotypes in piscivorous fishes. R. Soc. Open Sci. 2019, 6, 190040. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.; Antar, M.S.M.; Zalmout, I.S.; Gingerich, P.D. Stomach contents of the archaeocete Basilosaurus isis: Apex predator in oceans of the late Eocene. PLoS ONE 2019, 14, e0209021. [Google Scholar] [CrossRef] [PubMed]
- Lambert, O.; Bianucci, G.; Beatty, B.L. Bony outgrowths on the jaws of an extinct sperm whale support macroraptorial feeding in several stem physeteroids. Naturwissenschaften 2014, 101, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Popper, K. Logic of Scientific Discovery; Routledge Classics: New York, NY, USA, 2002; p. 513. [Google Scholar]
- Taylor, M.A. How tetrapods feed in water: A functional analysis by paradigm. Zool. J. Linn. Soc. 1987, 91, 171–195. [Google Scholar] [CrossRef]
- Kienle, S.S.; Law, C.J.; Costa, D.P.; Berta, A.; Mehta, R.S. Revisiting the behavioral framework of feeding in predatory aquatic mammals. Proc. R. Soc. B 2017, 284, 20171035. [Google Scholar] [CrossRef]
- Fordyce, R.E.; Muizon, C.d. Evolutionary history of cetaceans: A review. In Secondary Adaptations of Tetrapods to Life in Water; Mazin, J.M., Buffrenil, V.d., Eds.; Verlag Dr. Friedrich Pfeil: Munich, Germany, 2001; pp. 169–233. [Google Scholar]
- Grubich, J.R.; Rice, A.N.; Westneat, M.W. Functional morphology of bite mechanics in the great barracuda (Sphyraena barracuda). Zoology 2008, 111, 16–29. [Google Scholar] [CrossRef]
- Pedà, C.; Battaglia, P.; Scuderi, A.; Voliani, A.; Mancusi, C.; Andaloro, F.; Romeo, T. Cephalopod prey in the stomach contents of odontocete cetaceans stranded in the western Mediterranean Sea. Mar. Biol. Res. 2015, 11, 593–602. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Churchill, M.; Buchholtz, E.A.; Beatty, B.L.; Geisler, J.H. Convergent evolution of swimming adaptations in modern whales revealed by a large macrophagous dolphin from the Oligocene of South Carolina. Curr. Biol. 2020, 30, 3267–3273. [Google Scholar] [CrossRef]
- Weems, R.E.; Bybell, L.M.; Edwards, L.E.; Lewis, W.C.; Self-Trail, J.M.; Albright, L.B., III; Cicimurri, D.J.; Harris, W.B.; Osborne, J.E.; Sanders, A.E. Stratigraphic revision of the Cooper Group and Chandler Bridge and Edisto Formations in the coastal plain of South Carolina. South Carol. Geol. 2016, 49, 1–24. [Google Scholar]
- Katuna, M.P.; Geisler, J.H.; Colquhoun, D.J. Stratigraphic correlation of Oligocene marginal marine and fluvial deposits across the middle and lower coastal plain, South Carolina. Sediment. Geol. 1997, 108, 181–194. [Google Scholar] [CrossRef]
- Reeves, R.R.; Berger, J.; Clapham, P.J. Killer whales as predators of large baleen whales and sperm whales. In Whales, Whaling, and Ocean Ecosystems; Estes, J., Ed.; University of California Press: Berkeley, CA, USA, 2007; pp. 174–187. [Google Scholar]
- Marx, F.G.; Tsai, C.-H.; Fordyce, R.E. A new early Oligocene toothed ‘baleen’ whale (Mysticeti: Aetiocetidae) from western North America: One of the oldest and the smallest. R. Soc. Open Sci. 2015, 2, 150476. [Google Scholar] [CrossRef]
- Solounias, N.; Semprebon, H. Advances in the reconstruction of ungulate ecomorphology with application to early fossil equids. Am. Mus. Novit. 2002, 2002, 1–49. [Google Scholar] [CrossRef]
- Purnell, M.A.; Goodall, R.H.; Thomson, S.; Matthews, C.J. Tooth microwear texture in odontocete whales: Variation with tooth characteristics and implications for dietary analysis. Biosurface Biotribol. 2017, 3, 184–195. [Google Scholar] [CrossRef]
- Werth, A.J.; Beatty, B. Osteological correlates of evolutionary transitions in cetacean feeding and related oropharyngeal functions. Front. Ecol. Evol. 2023, 11, 1179804. [Google Scholar] [CrossRef]
- Werth, A.J.; Crompton, A.W. Cetacean tongue mobility and function: A comparative review. J. Anat. 2023, 243, 323–373. [Google Scholar] [CrossRef]



| Dependent/Independent Variables | N | Spearman Correlation | Pearson Chi-Square | Cramer’s V | Sig. |
|---|---|---|---|---|---|
| Apical Wear/Tooth Locus (UL) | 304 | −0.18 | NA | NA | <0.001 * |
| Apical Wear/Me, D, or P Cusp (UL) | 304 | NA | 38.993 | 0.253 | <0.001 *^ |
| Apical Wear/Me, D, or P Cusp (L) | 177 | NA | 43.273 | 0.35 | <0.001 *^ |
| Apical Wear/Me, D, or P Cusp (U) | 118 | NA | 14.602 | 0.249 | 0.006 *^ |
| Apical Wear/Cusp Height (UL) | 304 | −0.306 | NA | NA | <0.001 * |
| Apical Wear/Notch Wear (UL) | 250 | 0.374 | NA | NA | <0.001 * |
| Apical Wear/Species (UL) | 304 | NA | 23.089 | 0.276 | <0.001 * |
| Side Wear/Species (UL) | 71 | NA | 2.038 | 0.169 | 0.201 |
| Notch Wear/Species (UL) | 257 | NA | 12.8 | 0.223 | 0.002 * |
| Notch Wear/Occlusal Side (UL) | 257 | NA | 75.721 | 0.543 | <0.001 * |
| Side Wear/Occlusal Side (UL) | 30 | NA | 28.759 | 0.636 | <0.001 * |
| Notch Wear/Notch Height (UL) | 257 | −0.529 | NA | NA | <0.001 * |
| Analysis and Variable | 95% C.I. for EXP(B) | |||
|---|---|---|---|---|
| Apical Wear, all Species (N = 295) | Exp(B) | Lower | Upper | Sig. |
| Species | 1.398 | 0.677 | 2.885 | 0.365 |
| Ordered Locus | 0.796 | 0.663 | 0.954 | 0.014 * |
| Upper/Lower | 8.112 | 4.039 | 16.296 | <0.001 * |
| Side | 1.461 | 0.768 | 2.781 | 0.248 |
| Mesial/Distal/Primary | - | - | - | <0.001 * |
| Mesial/Distal/Primary(1) | 6.483 | 3.28 | 12.814 | <0.001 * |
| Mesial/Distal/Primary(2) | 3.304 | 0.776 | 14.055 | 0.106 |
| Cusp count from Apex | 0.513 | 0.398 | 0.662 | <0.001 * |
| Apical Wear, C._havensteini (N = 198) | ||||
| Ordered Locus | 0.906 | 0.738 | 1.113 | 0.348 |
| Upper/Lower | 6.237 | 2.664 | 14.604 | <0.001 * |
| Side | 1.149 | 0.551 | 2.397 | 0.71 |
| Mesial/Distal/Primary | - | - | - | <0.001 * |
| Mesial/Distal/Primary(1) | 6.655 | 2.955 | 14.988 | <0.001 * |
| Mesial/Distal/Primary(2) | 5.386 | 0.591 | 49.078 | 0.135 |
| Cusp count from Apex | 0.458 | 0.335 | 0.625 | <0.001 * |
| Apical Wear, C. planifrons (N = 97) | ||||
| Ordered Locus | 0.515 | 0.316 | 0.84 | 0.008 * |
| Upper/Lower | 5.737 | 1.326 | 24.823 | 0.019 * |
| Side | 1.269 | 0.477 | 26.571 | 0.216 |
| Mesial/Distal/Primary | - | - | - | 0.028 * |
| Mesial/Distal/Primary(1) | 6.086 | 1.619 | 22.873 | 0.008 * |
| Mesial/Distal/Primary(2) | 2.571 | 0.225 | 29.316 | 0.447 |
| Cusp count from Apex | 0.638 | 0.397 | 1.024 | 0.62 |
| Notch Wear, all species (N = 250) | ||||
| Species | 6.468 | 2.481 | 16.863 | <0.001 * |
| Ordered Locus | 0.965 | 0.765 | 1.216 | 0.761 |
| Upper/Lower | 2.072 | 0.873 | 4.914 | 0.098 |
| Side | 1.285 | 0.562 | 2.938 | 0.552 |
| Mesial/Distal | 1.286 | 0.856 | 1.933 | 0.226 |
| Notch height | 0.255 | 0.163 | 0.397 | <0.001 * |
| Apical Wear | 6.903 | 2.459 | 19.374 | <0.001 * |
| Notch Wear, C._havensteini (N = 174) | ||||
| Ordered Locus | 1.245 | 0.942 | 1.645 | 0.124 |
| Upper/Lower | 3.664 | 1.141 | 11.77 | 0.029 * |
| Side | 1.413 | 0.532 | 3.753 | 0.488 |
| Mesial/Distal | 1.381 | 0.819 | 2.327 | 0.226 |
| Notch height | 0.226 | 0.118 | 0.433 | <0.001 * |
| Apical Wear | 5.81 | 1.542 | 21.89 | 0.009 * |
| Notch Wear, C. planifrons (N = 76) | ||||
| Ordered Locus | 0.386 | 0.183 | 0.814 | 0.012* |
| Upper/Lower | 2.726 | 0.335 | 22.199 | 0.349 |
| Side | 3.97 | 0.431 | 36.571 | 0.224 |
| Mesial/Distal | 1.667 | 0.683 | 4.071 | 0.262 |
| Notch height | 0.184 | 0.077 | 0.438 | <0.001 * |
| Apical Wear | 9.043 | 1.363 | 59.973 | 0.023 * |
| Side Wear, C. planifrons (N = 71) | ||||
| Species | 2.234 | 0.421 | 11.853 | 0.345 |
| Ordered Locus | 1.319 | 0.9 | 1.934 | 0.156 |
| Upper/Lower | 0.393 | 0.091 | 1.696 | 0.211 |
| Side | 1.318 | 0.339 | 5.115 | 0.69 |
| Occlusal/Non-occlusal side | 32.811 | 7.497 | 143.597 | <0.001 * |
| Side Wear, C._havensteini (N = 49) | ||||
| Ordered Locus | 1.808 | 0.997 | 3.277 | 0.51 |
| Upper/Lower | 0.12 | 0.1 | 1.467 | 0.097 |
| Side | 0.779 | 0.111 | 5.451 | 0.801 |
| Occlusal/Non-occlusal side | 0.005 | 0 | 0.086 | <0.001 * |
| Side Wear, C. planifrons (N = 22) | ||||
| Ordered Locus | 0.739 | 0.339 | 1.612 | 0.447 |
| Upper/Lower | 1.831 | 0.113 | 29.684 | 0.671 |
| Side | 1.691 | 0.137 | 20.812 | 0.682 |
| Occlusal/Non-occlusal side | 0.114 | 0.015 | 0.89 | 0.038 * |
| Taxon or Specimen | Raptorial Score | Dental Filtration Score | Baleen Filtration Score | Suction Score | Percent Complete |
|---|---|---|---|---|---|
| Dorudon atrox | 5.3 | −2.0 | −1.0 | 0.0 | 100% |
| Zygorhiza kochii | 5.3 | −2.0 | −1.0 | 0.0 | 100% |
| Kekenodon onamata | 4.8 to 4.1 | −1.3 to −1.0 | 0.0 | 0.3 to −0.5 | 64% |
| Basilosaurus spp. | 4.7 to 4.3 | −2.0 | −2.0 | 1.0 | 100% |
| ChM PV5720 | 3.3 | 1.0 | −1.0 | 0.0 | 100% |
| Agorophius spp. | 3.3 to 2.3 | 0.0 | 0.0 | 0.0 | 64% |
| Xenorophus sloanii | 3.0 | 0.0 | −1.0 | 0.0 | 93% |
| Janjucetus hunderi | 2.3 | 1.0 | −1.0 | 1.0 | 93% |
| Physeter macrocephalus | 2.3 | −1.0 | −0.5 | 0.0 | 79% |
| Coronodon planifrons | 2.3 to 2.1 | 2.0 to 1.8 | 0.0 | 0.3 to 0 | 79% |
| Coronodon havensteini | 2.3 to 1.0 | 2.0 | 0.0 | 0.0 | 100% |
| ZMT 62 | 2.0 to −2.0 | 1.0 to 0 | 2.0 to −1.0 | 0.0 | 43% |
| Borealodon osedax | 1.5 to 0.8 | 0.8 | 0.0 | 0.3 | 64% |
| Aetiocetus cotylalveus | 1.3 | 0.0 | 1.5 | 0.0 | 79% |
| Echovenator sandersi | 1.0 | −1.0 | 0.0 | 0.0 | 93% |
| Fucaia buelli | 1.0 | 1.0 | 0.0 | 0.0 | 57% |
| Mystacodon selenensis | 1.0 | −3.0 | −3.0 | 2.0 | 71% |
| Simocetus rayi | 1.0 | 0.0 | −1.0 | 0.0 | 93% |
| Waipatia maerewhenua | 0.3 | 0.0 | −1.0 | 0.0 | 86% |
| Ziphiidae | 0.3 | −0.8 | −0.3 | 1.8 | 57% |
| Llanocetus denticrenatus | 0.3 to −1.3 | −2.3 to −2.0 | 1.0 | 0.3 to 0 | 86% |
| Ashleycetus planicapitis | 0.0 | 0.0 | 0.0 | 0.0 | 7% |
| Atlanticetus patulus | 0.0 | 0.0 | 1.0 | 0.0 | 7% |
| Balaenella brachyrhynus | 0.0 | 0.0 | 1.0 | 0.0 | 14% |
| Coronodon newtonorum | 0.0 | 1.0 to 0 | −1.0 | 1.0 | 86% |
| Eubalaena shinshuensis | 0.0 | 0.0 | 1.0 to 0.5 | 0.0 | 7% |
| Fucaia goedertorum | 0.0 | 0.0 | 1.5 | 0.0 | 57% |
| Metasqualodon symmetricus | 0.0 | 1.0 | −1.0 | 1.0 | 71% |
| Miocaperea pulchra | 0.0 | 0.0 | 0.0 | 0.0 | 7% |
| Morawanocetus yabukii | 0.0 | 1.0 | 0.5 | 0.0 | 64% |
| Nehalaennia devossi | 0.0 | 0.0 | 1.0 to 0.5 | 0.0 | 7% |
| Norrisanima miocaena | 0.0 | 0.0 | 1.0 | 0.0 | 7% |
| Olympicetus spp. | 0.0 | 1.0 | 0.0 | 0.0 | 36% |
| Tiucetus rosae | 0.0 | 0.0 | 1.0 to 0.5 | 0.0 | 7% |
| Aetiocetus polydentatus | −1.0 | 0.0 | 2.0 | 0.0 | 79% |
| Horopeta umarere | −1.0 | 1.0 | 1.0 | −1.0 | 7% |
| Mammalodon colliveri | −1.0 | 0.0 | −2.0 | 2.0 | 71% |
| Mammalodon hakataramea | −1.0 | −1.0 | −1.0 | 1.0 | 7% |
| Matapanui waihao | −1.8 | −0.8 | 1.7 | 0.8 | 14% |
| Aetiocetus weltoni | −2 to −0.7 | 0.0 | 2.5 | 0.0 | 71% |
| Eschrichtius akishimaensis | −2.0 | 0.0 | 2.0 | 0.0 | 14% |
| Herpetocetus sendaicus | −2.0 | 0.0 | 3.0 | 0.0 | 21% |
| Kurdalogonus mchedlidzei | −2.0 | 0.0 | 2.0 | 0.0 | 14% |
| Parietobalaena campiniana | −2.0 | 0.0 | 3.0 | 0.0 | 21% |
| Salishicetus meadi | −2.0 | 1.0 | 1.0 | 0.0 | 86% |
| Sitsqwayk cornishorum | −2.0 | 1.0 | 3.0 to 2.5 | −1.0 | 21% |
| Tokarahia kauaeroa | −2.0 | 1.0 | 2.0 | −1.0 | 21% |
| Tokarahia lophocephalus | −2.0 | 1.0 | 2.5 | −1.0 | 21% |
| Eomysticetus whitmorei | −2.8 | 0.3 | 2.7 | −0.3 | 29% |
| Maiabalaena nesbittae | −2.8 | 0.3 | 3.2 | −0.3 | 36% |
| Micromysticetus rothauseni | −2.8 | 0.3 | 3.2 | −0.3 | 36% |
| Waharoa ruwhenua | −2.8 | 0.3 | 3.2 | −0.3 | 36% |
| Yamatocetus canaliculatus | −2.8 | 0.3 | 3.2 | −0.3 | 36% |
| Aglaocetus moreni | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Antwerpibalaena liberatlas | −3.0 | 0.0 | 3.0 | 0.0 | 21% |
| Archaebalaenoptera castriarquati | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Archaeobalaena dosanko | −3.0 | 0.0 | 3.0 | 0.0 | 21% |
| Balaena mysticetus | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Balaena ricei | −3.0 | 0.0 | 4.0 | 0.0 | 29% |
| Balaenoptera acutorostrata | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Balaenoptera bonaerensis | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Balaenoptera borealis | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Balaenoptera edeni brydei | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Balaenoptera musculus | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Balaenoptera omurai | −3.0 | 0.0 | 4.0 | 0.0 | 29% |
| Balaenoptera physalus | −3.0 | 0.0 | 4.0 | 0.0 | 29% |
| Balaenoptera portisi | −3.0 | 0.0 | 3.0 | 0.0 | 21% |
| Balaenoptera siberi | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Balaenula astensis | −3.0 | 0.0 | 3.0 | 0.0 | 21% |
| Caperea marginata | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Cetotherium rathkii | −3.0 | 0.0 | 4.0 to 3.5 | 0.0 | 29% |
| Cetotherium riabinini | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Cophocetus oregonensis | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Diorocetus chichibuensis | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Diorocetus hiatus | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Eschrichtioides gastaldii | −3.0 | 0.0 | 4.0 | 0.0 | 29% |
| Eschrichtius robustus | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Eubalaena | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Herpetocetus bramblei | −3.0 | 0.0 | 4.0 | 0.0 | 29% |
| Herpetocetus morrowi | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Incakujira anillodefuego | −3.0 | 0.0 | 4.0 | 0.0 | 29% |
| Isanacetus laticephalus | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Joumocetus shimizui | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Mauicetus parki | −3.0 | 0.0 | 3.0 | 0.0 | 21% |
| Megaptera hubachi | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Megaptera novaeangliae | −3.0 | 0.0 | 4.0 | 0.0 | 29% |
| Niparajacetus | −3.0 | 0.0 | 2.0 | 0.0 | 71% |
| Otradnocetus spp. | −3.0 | 0.0 | 3.0 | 0.0 | 21% |
| Parabalaenoptera baulinensis | −3.0 | 0.0 | 3.0 | 0.0 | 29% |
| Parietobalaena palmeri | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Parietobalaena yamaokai | −3.0 | 0.0 | 3.0 | 0.0 | 29% |
| Pelocetus calvertensis | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Piscobalaena nana | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
| Plesiobalaenoptera quarantellii | −3.0 | 0.0 | 3.0 | 0.0 | 29% |
| Protororqualus cuvierii | −3.0 | 0.0 | 3.0 | 0.0 | 29% |
| Titanocetus sammarinensis | −3.0 | 0.0 | 4.0 | 0.0 | 29% |
| Uranocetus gramensis | −3.0 | 0.0 | 4.0 | 0.0 | 36% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geisler, J.H.; Beatty, B.L.; Boessenecker, R.W. New Evidence of the Feeding Behaviors of Coronodon and the Origin of Filter Feeding in Mysticetes (Mammalia: Cetacea) Revisited. Diversity 2024, 16, 549. https://doi.org/10.3390/d16090549
Geisler JH, Beatty BL, Boessenecker RW. New Evidence of the Feeding Behaviors of Coronodon and the Origin of Filter Feeding in Mysticetes (Mammalia: Cetacea) Revisited. Diversity. 2024; 16(9):549. https://doi.org/10.3390/d16090549
Chicago/Turabian StyleGeisler, Jonathan H., Brian L. Beatty, and Robert W. Boessenecker. 2024. "New Evidence of the Feeding Behaviors of Coronodon and the Origin of Filter Feeding in Mysticetes (Mammalia: Cetacea) Revisited" Diversity 16, no. 9: 549. https://doi.org/10.3390/d16090549
APA StyleGeisler, J. H., Beatty, B. L., & Boessenecker, R. W. (2024). New Evidence of the Feeding Behaviors of Coronodon and the Origin of Filter Feeding in Mysticetes (Mammalia: Cetacea) Revisited. Diversity, 16(9), 549. https://doi.org/10.3390/d16090549






