Evolutionary Insights from Dental Diversity in Afro-Asian Primates
Abstract
:1. Introduction
2. Material and Methods
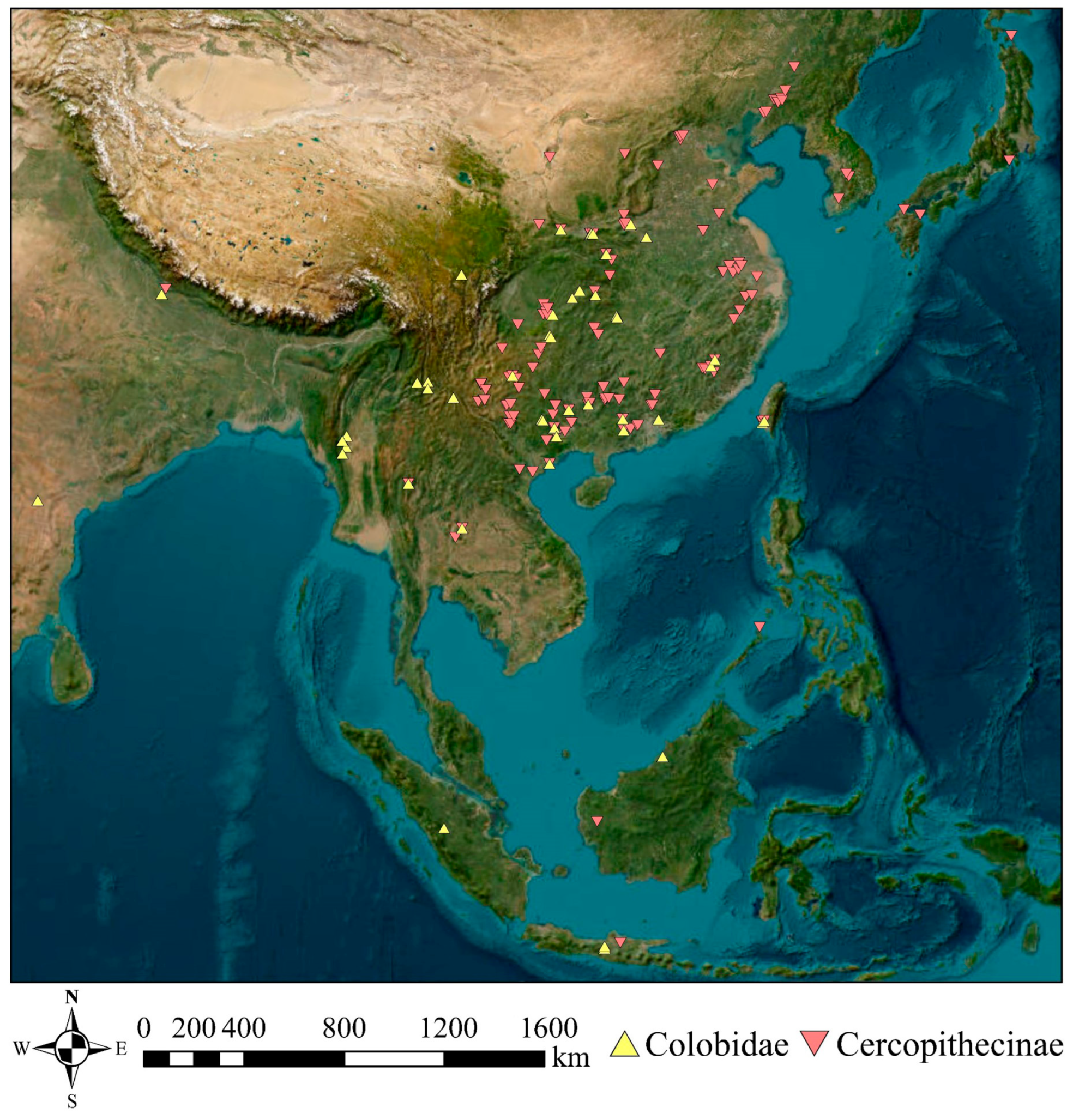
2.1. Material
2.2. Methods
- (1)
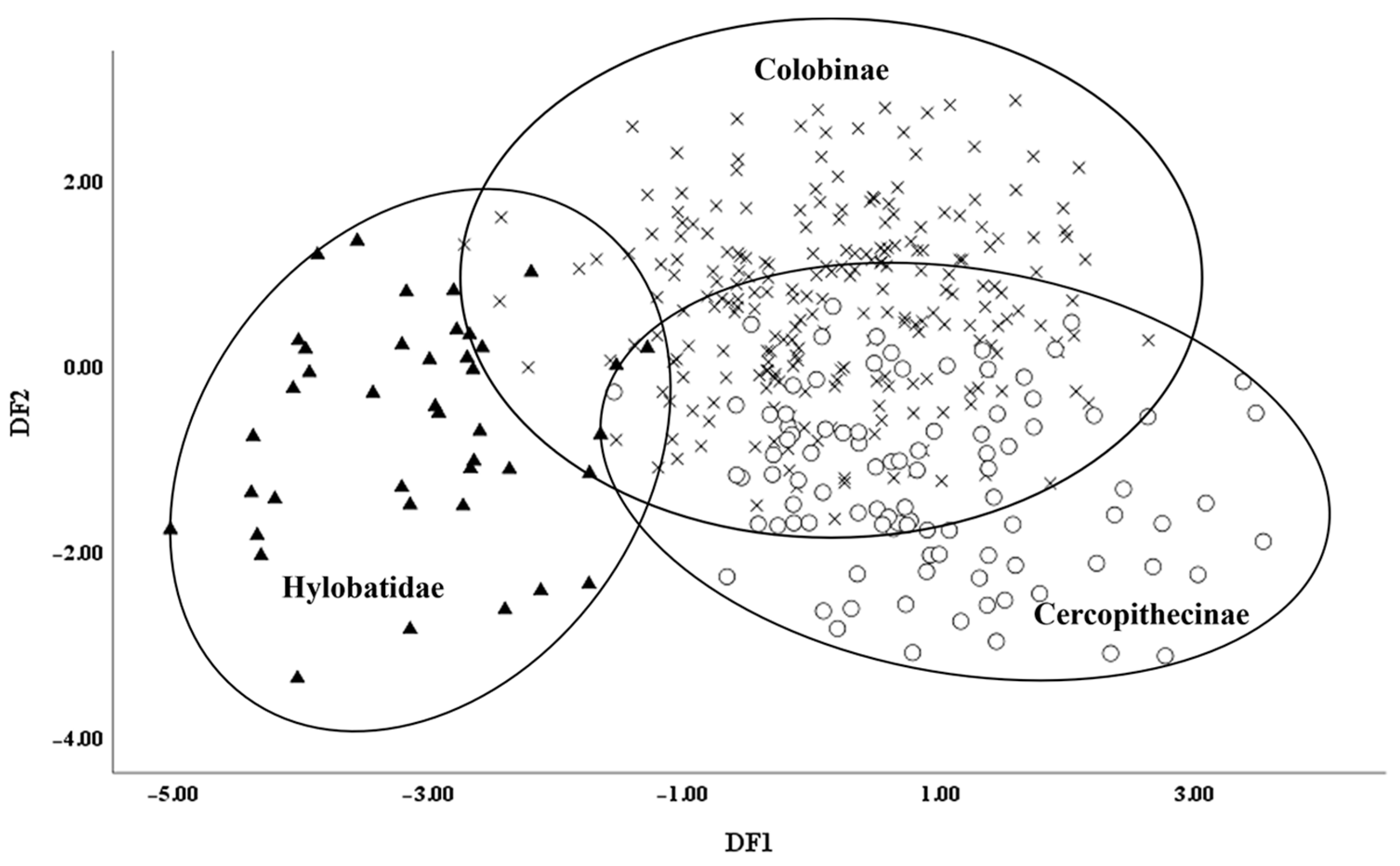
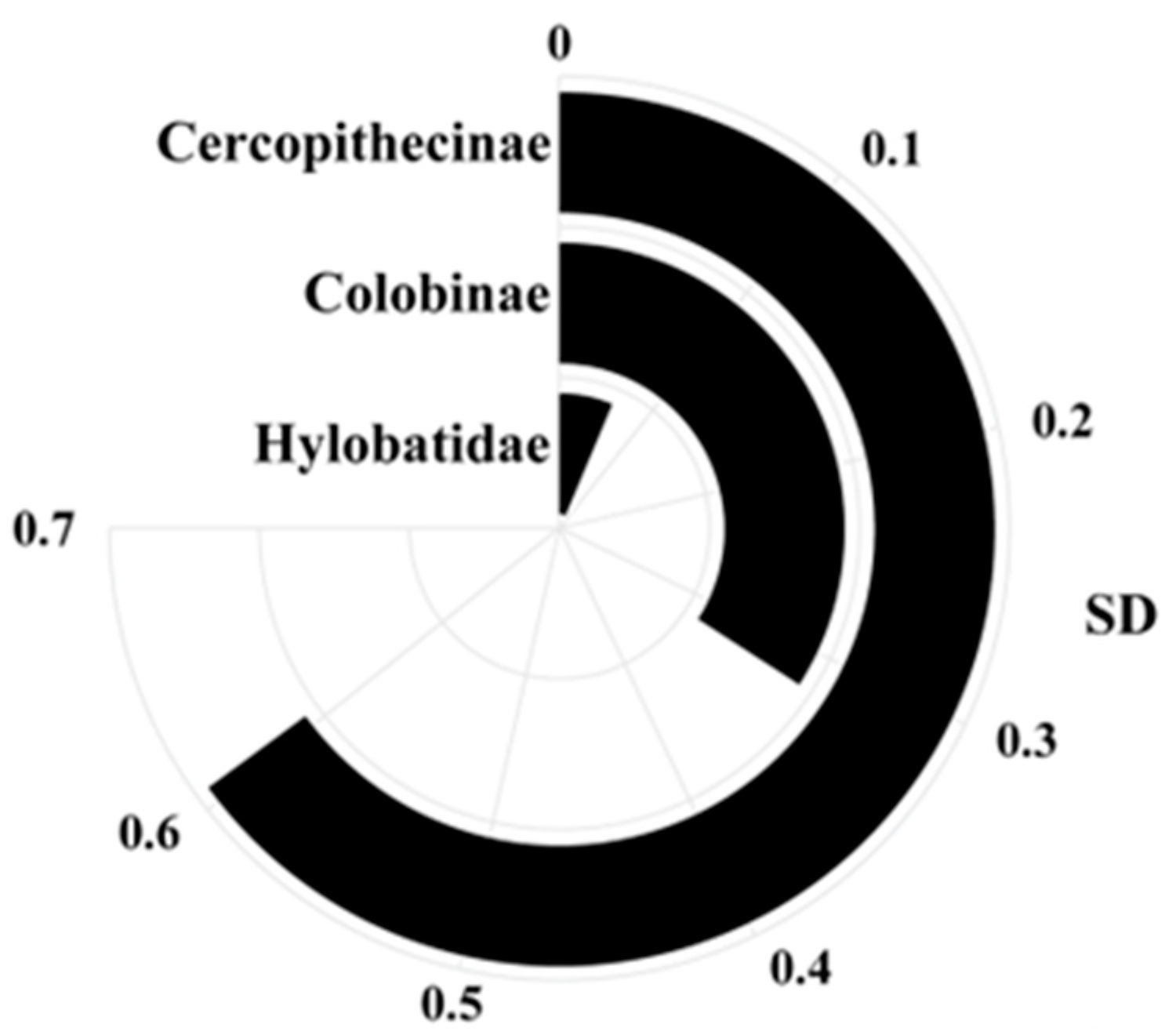
- Comparison between Cercopithecidae and Hylobatidae aims to explore the relationship between teeth and body size of catarrhines in Africa and Asia. As seen below, residuals were further analyzed using multivariate discriminant function analysis (DFA).
- (2)
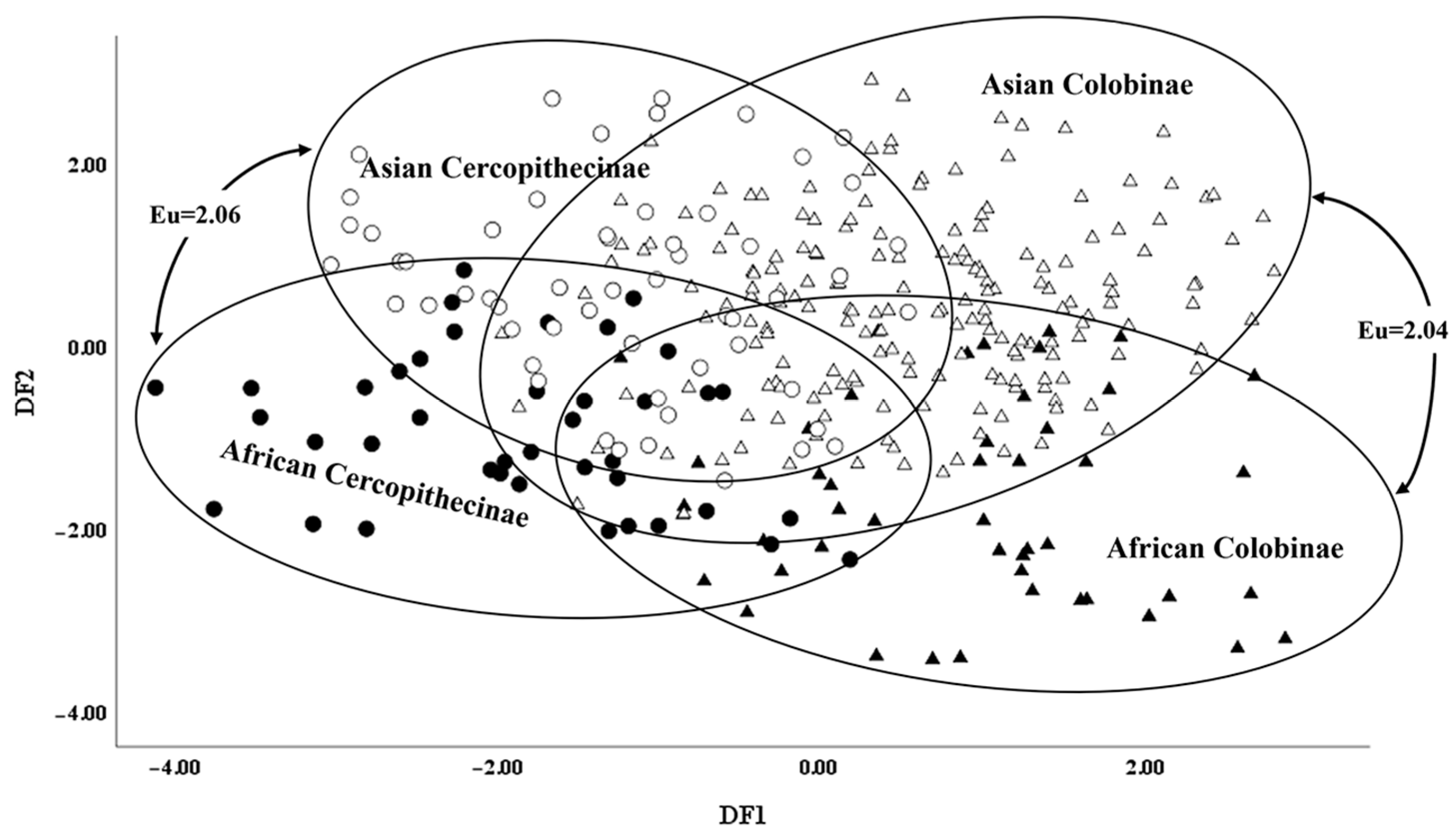
- Comparison within the Cercopithecidae aims to decipher differences between the representatives of Colobinae and Cercopithecinae in Africa and Asia. Residuals of the four groups (African Colobinae, African Cercopithecinae, Asian Colobinae, and Asian Cercopithecinae) were also analyzed using DFA.
- (3)
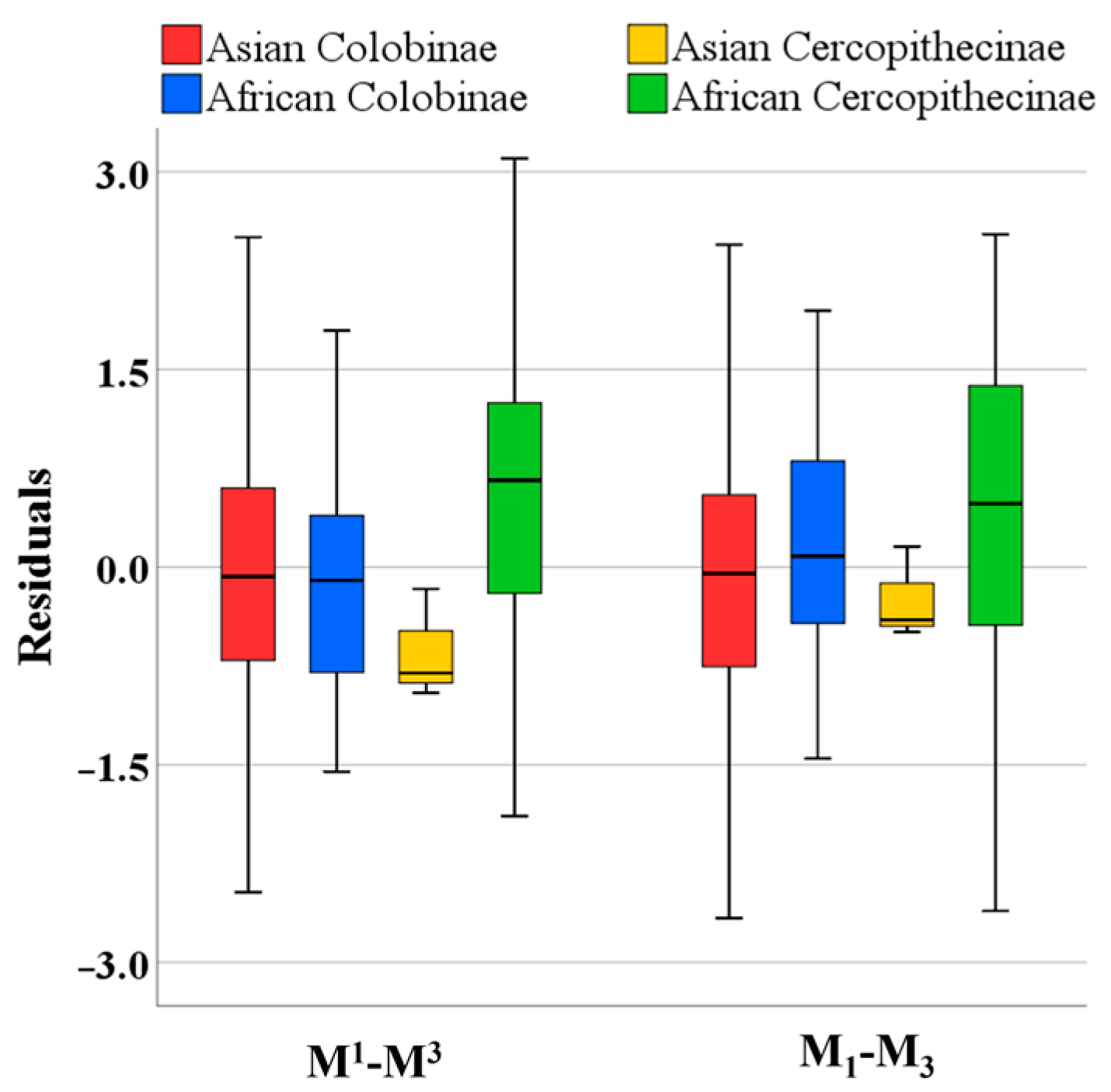
- Comparing the post-canine teeth size within the Cercopithecidae aims to determine the magnitude of dental variation for Colobinae and Cercopithecinae in Africa and Asia.
3. Results
4. Discussion
4.1. Evolutionary and Phylogenetic Diversity
4.2. Dental Structure and Dietary Selection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Lu, J.; Tang, S.; Huang, Z.; Cui, L.; Lan, D.; Wang, H.; Hou, R.; Xiao, W.; Guo, S.; et al. Southwest China, the Last Refuge of Continental Primates in East Asia. Biol. Conserv. 2022, 273, 109681. [Google Scholar] [CrossRef]
- Delson, E. Hominidae other than Ponginae in Eastern Asia: An Updated Survey. Asian Paleoprimatol. 2003, 3, 48–51. [Google Scholar]
- Wu, X.Z.; Poirier, F.E. Human Evolution of China. A Metric Description of the Fossils and a Review of the Sites; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Jablonski, N.G.; Whitfort, M.J. Environmental change during the Quaternary in East Asia and its consequences for mammals. Rec. West. Aust. Mus. 1999, 57, 307–315. [Google Scholar]
- Gilbert, C.C.; Ortiz, A.; Pugh, K.D.; Campisano, C.J.; Patel, B.A.; Singh, N.P.; Fleagle, J.G.; Patnaik, R. New Middle Miocene Ape (Primates: Hylobatidae) from Ramnagar, India Fills Major Gaps in the Hominoid Fossil Record. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201655. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.L. Probe on the Chinese Origin of Gibbons (Hylobates). Acta Theriol. Sin. 1979, 17, 13–23. [Google Scholar]
- Jablonski, N.G.; Chaplin, G. The Fossil Record of Gibbons. In The Gibbons. Developments in Primatology: Progress and Prospects; Whittaker, D.J., Lappan, S., Eds.; Springer: New York, NY, USA, 2009; pp. 111–130. [Google Scholar]
- Harrison, T. The Fossil Record and Evolutionary History of Hylobatids. In Evolution of Gibbons and Siamang. Developments in Primatology: Progress and Prospects; Reichard, U.H., Hirai, H., Barelli, C., Eds.; Springer: New York, NY, USA, 2016; pp. 91–110. [Google Scholar]
- Iwamoto, M.; Hasegawa, Y.; Koizumi, A. A Pliocene colobine from the Nakatsu Group, Kanagawa, Japan. Anthropol. Sci. 2005, 113, 123–127. [Google Scholar] [CrossRef]
- Harrison, T.; Jin, C.Z.; Zhang, Y.Q.; Wang, Y.; Zhu, M. Fossil Pongo from the Early Pleistocene Gigantopithecus Fauna of Chongzuo, Guangxi, Southern China. Quat. Int. 2014, 354, 59–67. [Google Scholar] [CrossRef]
- Delson, E. Fossil Macaques, Phyletic Relationships and a Scenario of Deployment. In The Macaques: Studies in Ecology, Behavior and Evolution; Lindburg, D., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1980; Volume 10, pp. 10–30. [Google Scholar]
- Rowley, D.B. Age of Initiation of Collision Between India and Asia: A Viewer of Stratigraphic Data. Earth Planet. Sci. Lett. 1996, 145, 1–13. [Google Scholar] [CrossRef]
- Bouilhol, P.; Jagoutz, O.; Hanchar, J.M.; Dudas, F.O. Dating the India–Eurasia collision through arc magmatic records. Earth Planet. Sci. Lett. 2013, 366, 163–175. [Google Scholar] [CrossRef]
- Chauhan, P.R. The Indian Subcontinent and ‘Out of Africa I’. In Out of Africa I: The First Hominin Colonization of Eurasia; Fleagle, J.G., Shea, J.J., Grine, F.E., Baden, A.L., Leakey, R.E., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 145–164. [Google Scholar]
- Patterson, E.W.; Johnson, K.R.; Griffiths, M.L.; Kinsley, C.W.; McGee, D.; Du, X.; Pico, T.; Wolf, A.; Ersek, V.; Mortlock, R.A.; et al. Glacial Changes in Sea Level Modulated Millennial-scale Variability of Southeast Asian Autumn Monsoon Rainfall. Proc. Natl. Acad. Sci. USA 2023, 120, e2219489120. [Google Scholar] [CrossRef]
- Burke, K.; Wilkinson, M.J. Landscape evolution in Africa during the Cenozoic and Quaternary—The legacy and limitations of Lester C. King. Can. J. Earth Sci. 2016, 53, 1089–1102. [Google Scholar] [CrossRef]
- Stokes, C.R.; Abram, N.J.; Bentley, M.J.; Edwards, T.L.; England, M.H.; Foppert, A.; Jamieson, S.S.R.; Jones, R.S.; King, M.A.; Lenaerts, J.T.M.; et al. Response of the East Antarctic Ice Sheet to Past and Future Climate Change. Nature 2022, 608, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Elbelrhiti, H. Initiation and early development of barchan dunes: A case study of the Moroccan Atlantic Sahara desert. Geomorphology 2012, 138, 181–188. [Google Scholar] [CrossRef]
- Bonnefille, R. Cenozoic vegetation, climate changes and hominid evolution in tropical Africa. Glob. Planet. Chang. 2010, 72, 390–411. [Google Scholar] [CrossRef]
- Kay, R.F. Allometry and Early Hominids. Science 1975, 189, 61–64. [Google Scholar]
- Kay, R.F. The functional adaptations of primate molar teeth. Am. J. Phys. Anthropol. 1975, 43, 195–216. [Google Scholar] [CrossRef]
- Pirie, P.L. Allometric scaling in the postcanine dentition with reference to primate diets. Primates 1978, 19, 583–591. [Google Scholar] [CrossRef]
- Scott, J.E. Folivory, frugivory, and postcanine size in the cercopithecoidea revisited. Am. J. Phys. Anthropol. 2011, 146, 20–27. [Google Scholar] [CrossRef]
- Pan, R.L. Dental morphometric variation between African and Asian colobines, with special reference to the other Old World monkeys. J. Morphol. 2006, 267, 1087–1098. [Google Scholar] [CrossRef]
- Roos, C.; Zinner, D.; Kubatko, L.S.; Schwarz, C.; Yang, M.Y.; Meyer, D.; Nash, S.D.; Xing, J.; Batzer, M.A.; Brameier, M.; et al. Nuclear Versus Mitochondrial DNA: Evidence for Hybridization in Colobine Monkeys. BMC Evol. Biol. 2011, 11, 77. [Google Scholar] [CrossRef]
- Roos, C.; Kothe, M.; Alba, D.M.; Delson, E.; Zinner, D. The Radiation of Macaques out of Africa: Evidence from Mitogenome Divergence Times and the Fossil Record. J. Hum. Evol. 2019, 133, 114–132. [Google Scholar] [CrossRef]
- Balolia, K.L.; Soligo, C.; Lockwood, C.A. Sexual Dimorphism and Facial Growth Beyond Dental Maturity in Great Apes and Gibbons. Int. J. Primatol. 2013, 34, 361–387. [Google Scholar] [CrossRef]
- Kelley, J. Sexual dimorphism in canine shape among extant great apes. Am. J. Phys. Anthropol. 1995, 96, 365–389. [Google Scholar] [CrossRef] [PubMed]
- Balolia, K. Craniodental Sexual Dimorphism Among Hylobatids. Int. J. Primatol. 2021, 42, 737–758. [Google Scholar] [CrossRef]
- Leutenegger, W.; Shell, B. Variability and sexual dimorphism in canine size of Australopithecus and extant hominoids. J. Hum. Evol. 1987, 16, 359–367. [Google Scholar] [CrossRef]
- Frisch, J.E. Sex-differences in the canines of the gibbon (Hylobates lar). Primates 1963, 4, 1–10. [Google Scholar] [CrossRef]
- Plavcan, J.M. Sexual Selection, Measures of Sexual Selection, and Sexual Dimorphism in Primates; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Plavcan, J.M.; van Schaik, C.P. Intrasexual competition and canine dimorphism in anthropoid primates. Am. J. Phys. Anthropol. 1992, 87, 461–477. [Google Scholar] [CrossRef]
- Pan, R.; Oxnard, C. Dental Variation among Asian Colobines (Nonhuman Primates): Phylogenetic Similarities or Functional Correspondence? Zool. Stud. 2003, 42, 93–105. [Google Scholar]
- Martin, R.D.; Genoud, M.; Hemelrijk, C.K. Problems of Allometric Scaling Analysis: Examples from Mammalian Reproductive Biology. J. Exp. Biol. 2005, 208 (Pt 9), 1731–1747. [Google Scholar] [CrossRef]
- Pan, R.; Oxnard, C. Metrical dental analysis on golden monkey (Rhinopithecus roxellana). Primates 2001, 42, 75–89. [Google Scholar] [CrossRef]
- Smith, R.J. On the Definition of Variables in Studies of Primate Dental Allometry. Am. J. Phys. Anthropol. 1981, 55, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.R. Dental Variation among Asian Colobines, with Specific Reference to the Macaques on the Same Continent. Zool. Res. 2007, 28, 569–579. [Google Scholar]
- Warton, D.I.; Wright, I.J.; Falster, D.S.; Westoby, M. Bivariate line-fitting methods for allometry. Biol. Rev. Camb. Philos. Soc. 2006, 81, 259–291. [Google Scholar] [CrossRef]
- Smith, R.J. Use and misuse of the reduced major axis for line-fitting. Am. J. Phys. Anthropol. 2009, 140, 476–486. [Google Scholar] [CrossRef]
- Brown, M.T.; Tinsley, H.E.A. Discriminant Analysis. J. Leis. Res. 2018, 15, 290–310. [Google Scholar] [CrossRef]
- Deutsch, A.R.; Dickinson, E.; Whichard, V.A.; Lagomarsino, G.R.; Perry, J.M.G.; Kupczik, K.; Hartstone-Rose, A. Primate body mass and dietary correlates of tooth root surface area. Am. J. Biol. Anthropol. 2022, 177, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Raaum, R.L.; Sterner, K.N.; Noviello, C.M.; Stewart, C.-B.; Disotell, T.R. Catarrhine Primate Divergence Dates Estimated from Complete Mitochondrial Genomes: Concordance with Fossil and Nuclear DNA Evidence. J. Hum. Evol. 2005, 48, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Brockelman, W.Y. Ecology and the Social System of Gibbons. In The Gibbons; Springer: New York, NY, USA, 2009; pp. 211–239. [Google Scholar]
- Reichard, U.H. Social Monogamy in Gibbons: The Male Perspective. In Monogamy; Reichard, U.H., Boesch, C., Eds.; Cambridge University: Cambridge, UK, 2003; pp. 190–213. [Google Scholar]
- Elton, S. Environmental Correlates of the Cercopithecoid Radiations. Folia Primatol. 2007, 78, 344–364. [Google Scholar] [CrossRef]
- Begun, D.R. Catarrhine Origins and Evolution. In A Companion to Biological Anthropology the Past and the Dead; Larsen, C.S., Ed.; Wiley Online Books: Hoboken, NJ, USA, 2023; pp. 365–640. [Google Scholar]
- Brandon-Jones, D.; Eudey, A.A.; Geissmann, T.; Groves, C.P.; Melnick, D.J.; Morales, J.C.; Shekelle, M.; Stewart, C.B. Asian Primate Classification. Int. J. Primatol. 2004, 25, 97–164. [Google Scholar] [CrossRef]
- Li, B.G.; He, G.; Guo, S.T.; Hou, R.; Huang, K.; Zhang, P.; Zhang, H.; Pan, R.L.; Chapman, C.A. Macaques in China: Evolutionary Dispersion and Subsequent Development. Am. J. Primatol. 2020, 82, e23142. [Google Scholar] [CrossRef]
- Maslin, M.A.; Christensen, B. Tectonics, Orbital Forcing, Global Climate Change, and Human Evolution in Africa: Introduction to the African Paleoclimate Special Volume. J. Hum. Evol. 2007, 53, 443–464. [Google Scholar] [CrossRef]
- Levin, N.E.; Quade, J.; Simpson, S.W.; Semaw, S.; Rogers, M. Isotopic evidence for Plio–Pleistocene environmental change at Gona, Ethiopia. Earth Planet. Sci. Lett. 2004, 219, 93–110. [Google Scholar] [CrossRef]
- deMenocal, P.B. Plio-Pleistocene African Climate. Science 1995, 270, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Williamson, P.G. Evidence for an early Plio-Pleistocene rainforest expansion in East Africa. Nature 1985, 315, 487–489. [Google Scholar] [CrossRef]
- Lewis, L.A.; Berry, L. African Environments and Resources; Routledge: London, UK; New York, NY, USA, 2011. [Google Scholar]
- Spicer, R.A.; Su, T.; Valdes, P.J.; Farnsworth, A.; Wu, F.X.; Shi, G.; Spicer, T.E.V.; Zhou, Z. Why ‘the Uplift of the Tibetan Plateau’ is A Myth. Natl. Sci. Rev. 2021, 8, nwaa091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.F.; Fengquan, L.; Jianmin, B. Eco-environmental Effects of the Qinghai-Tibet Plateau Uplift During the Quaternary in China. Environ. Geol. 2000, 39, 1352–1359. [Google Scholar] [CrossRef]
- Li, J.J. The Environmental Effects of the Uplift of the Qinghai-Xizang Plateau. Quat. Sci. Rev. 1991, 10, 479–483. [Google Scholar]
- Liu, X.D.; Dong, B.W. Influence of the Tibetan Plateau Uplift on the Asian Monsoon-arid Environment Evolution. Chin. Sci. Bull. 2013, 58, 4277–4291. [Google Scholar] [CrossRef]
- Shi, Z.G.; Sha, Y.Y.; Liu, X.D.; Xie, X.N.; Li, X.Z. Effect of Marginal Topography Around the Tibetan Plateau on the Evolution of Central Asian arid Climate: Yunnan–Guizhou and Mongolian Plateaux as Examples. Clim. Dyn. 2019, 53, 4433–4445. [Google Scholar] [CrossRef]
- Li, X.R.; Jia, X.H.; Dong, G.R. Influence of desertification on vegetation pattern variations in the cold semi-arid grasslands of Qinghai-Tibet Plateau, North-west China. J. Arid. Environ. 2006, 64, 505–522. [Google Scholar] [CrossRef]
- Favre, A.; Packert, M.; Pauls, S.U.; Jahnig, S.C.; Uhl, D.; Michalak, I.; Muellner-Riehl, A.N. The Role of the Uplift of the Qinghai-Tibetan Plateau for the Evolution of Tibetan Biotas. Biol. Rev. Camb. Philos. Soc. 2015, 90, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Szalay, F.S.; Delson, E. Evolutionary History of the Primates; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Quade, J.; Cerling, T.E.; Bowman, J.R. Development of Asian monsoon revealed by marked ecological shift during the latest Miocene in northern Pakistan. Nature 1989, 342, 163–166. [Google Scholar] [CrossRef]
- Quade, J.; Cerling, T.E. Expansion of C4 grasses in the Late Miocene of Northern Pakistan: Evidence from stable isotopes in paleosols. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1995, 115, 91–116. [Google Scholar] [CrossRef]
- Singh, S.; Awasthi, A.K.; Parkash, B.; Kumar, S. Tectonics or climate: What drove the Miocene global expansion of C4 grasslands? Int. J. Earth Sci. 2013, 102, 2019–2031. [Google Scholar] [CrossRef]
- Kotla, S.S.; Patnaik, R.; Sehgal, R.K.; Kharya, A. Isotopic evidence for ecological and climate change in the richly fossiliferous Plio-Pleistocene Upper Siwalik deposits exposed around Chandigarh, India. J. Asian Earth Sci. 2018, 163, 32–42. [Google Scholar] [CrossRef]
- Corvinus, G. Homo erectus in East and Southeast Asia, and the questions of the age of the species and its association with stone artifacts, with special attention to handaxe-like tools. Quat. Int. 2004, 117, 141–151. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Li, X.Q.; Zhou, X.Y.; Zhao, K.L.; Yang, Q. Holocene Vegetation Succession and Response to Climate Change on the South Bank of the Heilongjiang-Amur River, Mohe County, Northeast China. Adv. Meteorol. 2016, 2016, 2450697. [Google Scholar] [CrossRef]
- Li, Z.-W.; Sun, L.; Li, B.-S.; Wang, F.-N.; Du, D.-D.; Song, Y.-G.; Zhang, H.-J.; Chen, L.-Q.; Xu, D. East Asian summer monsoon changes in subtropical China since late Pleistocene: Evidence from the Ailuropoda-Stegodon fauna. J. Mt. Sci. 2022, 19, 418–432. [Google Scholar] [CrossRef]
- Louys, J.; Turner, A. Environment, preferred habitats and potential refugia for Pleistocene Homo in Southeast Asia. Comptes Rendus Palevol 2012, 11, 203–211. [Google Scholar] [CrossRef]
- Bird, M.I.; Taylor, D.; Hunt, C. Palaeoenvironments of insular Southeast Asia during the Last Glacial Period: A savanna corridor in Sundaland? Quat. Sci. Rev. 2005, 24, 2228–2242. [Google Scholar] [CrossRef]
- Wurster, C.M.; Bird, M.I.; Bull, I.D.; Creed, F.; Bryant, C.; Dungait, J.A.; Paz, V. Forest Contraction in North Equatorial Southeast Asia During the Last Glacial Period. Proc. Natl. Acad. Sci. USA 2010, 107, 15508–15511. [Google Scholar] [CrossRef] [PubMed]
- Takai, M.; Soe, A.N.; Maung, M.; Tsubamoto, T.; Egi, N.; Nishimura, T.D.; Nishioka, Y. First Discovery of Colobine Fossils from the Late Miocene/Early Pliocene in Central Myanmar. J. Hum. Evol. 2015, 84, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Takai, M.; Ogino, S. First Discovery of Colobine Fossils from the Early to Middle Pleistocene of Southern Taiwan. J. Hum. Evol. 2012, 63, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Takai, M.; Nishioka, Y.; Thaung-Htike; Maung, M.; Khaing, K.; Zin-Maung-Maung-Thein; Tsubamoto, T.; Egi, N. Late Pliocene Semnopithecus Fossils from Central Myanmar: Rethinking of the Evolutionary History of Cercopithecid Monkeys in Southeast Asia. Hist. Biol. 2016, 28, 172–188. [Google Scholar] [CrossRef]
- Ogino, S.; Egi, N.; Takai, M. New Species of Agriotherium (Mammalia, Carnivora) from the Late Miocene to Early Pliocene of Central Myanmar. J. Asian Earth Sci. 2011, 42, 408–414. [Google Scholar] [CrossRef]
- Lopatin, A.; Maschenko, E.; Vislobokova, I.; Serdyuk, N.; Dac, L.X. Pleistocene Mammals from the Lang Trang Cave (Vietnam): New Data. In Doklady Biological Sciences; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Ito, T.; Lee, Y.-J.; Nishimura, T.D.; Tanaka, M.; Woo, J.-Y.; Takai, M. Phylogenetic Relationship of a Fossil Macaque (Macaca cf. robusta) from the Korean Peninsula to Extant Species of Macaques Based on Zygomaxillary Morphology. J. Hum. Evol. 2018, 119, 1–13. [Google Scholar] [CrossRef]
- Han, K.S.; So, K.S.; Choe, R.S.; Kang, J.C.; Kang, I.; Ri, C.U. First Record of Macaca mulatta (Cercopithecidae: Papionini) from the Taedong River Basin, the Democratic People’s Republic of Korea. Palaeoworld 2022, 32, 573–578. [Google Scholar] [CrossRef]
- Ingicco, T.; Amano, N.; Ochoa, J.; Détroit, F. An allometric study of Macaca fascicularis from the Late Pleistocene deposits at the Ille site (Philippines): A possible model for Southeast Asian Dwarf Hominins. Bull. Mémoires Société Dianthropologie Paris 2014. [Google Scholar] [CrossRef]
- Jouffroy, F. Un Crane Subfossile De Macaque Du Pléistocène Du Viet Nam. Bull. Mus Nat D’hist Nat. Paris 1959, 31, 309–316. [Google Scholar]
- Schillaci, M.A.; Klegarth, A.R.; Switzer, W.M.; Shattuck, M.R.; Lee, B.P.; Hollocher, H. Evolutionary Relationships of Macaca fascicularis fascicularis (Raffles 1821) (Primates: Cercopithecidae) from Singapore Revealed by Bayesian Analysis of Mitochondrial DNA Sequences. Raffles Bull. Zool. 2017, 65, 3–19. [Google Scholar]
- Ogino, S.; Otsuka, H.; Harunari, H. The Middle Pleistocene Matsugae Fauna, Northern Kyushu, West Japan. Paleontol. Res. 2009, 13, 367–384. [Google Scholar] [CrossRef]
- Filoux, A.; Wattanapituksakul, A.; Lespes, C.; Thongcharoenchaikit, C. A Pleistocene Mammal Assemblage Containing Ailuropoda and Pongo from Tham Prakai Phet Cave, Chaiyaphum Province, Thailand. Geobios 2015, 48, 341–349. [Google Scholar] [CrossRef]
- Bacon, A.-M.; Demeter, F.; Roussé, S.; Long, V.T.; Duringer, P.; Antoine, P.-O.; Thuy, N.K.; Mai, B.T.; Huong, N.T.M.; Dodo, Y.; et al. New Palaeontological Assemblage, Sedimentological and Chronological Data from the Pleistocene Ma U’Oi Cave (Northern Vietnam). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 230, 280–298. [Google Scholar] [CrossRef]
- Zeitoun, V.; Lenoble, A.; Laudet, F.; Thompson, J.; Rink, W.J.; Mallye, J.-B.; Chinnawut, W. The Cave of the Monk (Ban Fa Suai, Chiang Dao Wildlife Sanctuary, Northern Thailand). Quat. Int. 2010, 220, 160–173. [Google Scholar] [CrossRef]
- Pushkina, D.; Bocherens, H.; Chaimanee, Y.; Jaeger, J.-J. Stable Carbon Isotope Reconstructions of Diet and Paleoenvironment from the Late Middle Pleistocene Snake Cave in Northeastern Thailand. Naturwissenschaften 2010, 97, 299–309. [Google Scholar] [CrossRef]
- Guangxi-Museum. Museum. Museum Proceedings. In Guangxi Ethnic Museum; Guangxi-Museum: Guangxi, China, 2012. (In Chinese) [Google Scholar]
- Ito, T.; Nishimura, T.D.; Ebbestad, J.O.R.; Takai, M. Computed Tomography Examination of the Face of Macaca anderssoni (Early Pleistocene, Henan, northern China): Implications for the Biogeographic History of Asian Macaques. J. Hum. Evol. 2014, 72, 64–80. [Google Scholar] [CrossRef]
- Jablonski, N.G. Fossil Old World Monkeys: The Late Neogene Radiation. In Primate Fossil Record; Hartwig, W.C., Ed.; Cambridge University: Cambridge, UK, 2008; pp. 255–299. [Google Scholar]
- Rasmussen, D.T. Early Catarrhines of the African Eocene and Oligocene. In Primate Fossil Record; Hartwig, W.C., Ed.; Cambridge University: Cambridge, UK, 2002; pp. 255–299. [Google Scholar]
- Ji, X.P.; Youlatos, D.; Jablonski, N.G.; Pan, R.; Zhang, C.; Li, P.; Tang, M.; Yu, T.; Li, W.; Deng, C. Oldest Colobine Calcaneus from East Asia (Zhaotong, Yunnan, China). J. Hum. Evol. 2020, 147, 102866. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.P.; Jablonski, N.G.; Su, D.F.; Deng, C.L.; Flynn, L.J.; You, Y.S.; Kelley, J. Juvenile Hominoid Cranium from the Terminal Miocene of Yunnan, China. Chin. Sci. Bull. 2013, 58, 3771–3779. [Google Scholar] [CrossRef]
- Lucas, S.G. Chinese Fossil Vertebrates; Columbia University Press: New York, NY, USA, 2001; p. 320. [Google Scholar]
- Ortiz, A.; Pilbrow, V.; Villamil, C.I.; Korsgaard, J.G.; Bailey, S.E.; Harrison, T. The Taxonomic and Phylogenetic Affinities of Bunopithecus sericus, a Fossil Hylobatid from the Pleistocene of China. PLoS ONE 2015, 10, e0131206. [Google Scholar] [CrossRef]
- Pan, Y.; Peng, Y.; Zhang, X.; Pan, R. Cercopithecid Fossils Discovered in Yunnan and its Stratigraphical Significance. Acta Anthropol. Sin. 1992, 11, 303. [Google Scholar]
- Takai, M.; Maschenko, E.N. Parapresbytis Eohanuman: The Northernmost Colobine Monkey from the Pliocene of Transbaikalia. Asian Paleoprimatology 2009, 5, 1–14. [Google Scholar]
- Takai, M.; Zhang, Y.Q.; Kono, R.T.; Jin, C.Z. Changes in the Composition of the Pleistocene Primate Fauna in Southern China. Quat. Int. 2014, 354, 75–85. [Google Scholar] [CrossRef]
- Wu, X.Z. Fossil Humankind and other Anthropoid Primates of China. Int. J. Primatol. 2004, 25, 1093–1103. [Google Scholar] [CrossRef]
- Fa, J.E. The Genus Macaca: A Review of Taxonomy and Evolution. Mammal Rev. 1989, 19, 45–81. [Google Scholar] [CrossRef]
- Selig, K.R.; Silcox, M.T. Measuring Molarization: Change through Time in Premolar Function in an Extinct Stem Primate Lineage. J. Mamm. Evol. 2022, 29, 947–956. [Google Scholar] [CrossRef]
- Scott, J.E.; Campbell, R.M.; Baj, L.M.; Burns, M.C.; Price, M.S.; Sykes, J.D.; Vinyard, C.J. Dietary Signals in the Premolar Dentition of Primates. J. Hum. Evol. 2018, 121, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Hylander, W.L. The Functional Significance of Primate Mandibular Form. J. Morphol. 1979, 160, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.W. Dental Functional Morphology: How Teeth Work; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Napier, J.R.; Napier, P.H. A Handbook of Living Primates; Academic Press: London, UK, 1967. [Google Scholar]
- Smith, R.J. The Mandibular Corpus of Female Primates: Taxonomic, Dietary, and Allometric Correlates of Interspecific Variations in Size and Shape. Am. J. Phys. Anthropol. 1983, 61, 315–330. [Google Scholar] [CrossRef]
- Bunn, J.M.; Ungar, P.S. Dental topography and diets of four old world monkey species. Am. J. Primatol. 2009, 71, 466–477. [Google Scholar] [CrossRef]
- Chapman, C.A.; Lambert, J.E. Habitat alteration and the conservation of African primates: Case study of Kibale National Park, Uganda. Am. J. Primatol. 2000, 50, 169–185. [Google Scholar] [CrossRef]
- Albert, A.; McConkey, K.; Savini, T.; Huynen, M.-C. The value of disturbance-tolerant cercopithecine monkeys as seed dispersers in degraded habitats. Biol. Conserv. 2014, 170, 300–310. [Google Scholar] [CrossRef]
- Happel, R. Seed-eating by West African cercopithecines, with reference to the possible evolution of bilophodont molars. Am. J. Phys. Anthropol. 1988, 75, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Wrangham, R.W.; Conklin-Brittain, N.; Hunt, K.D. Dietary Response of Chimpanzees and Cercopithecines to Seasonal Variation in Fruit Abundance. I. Antifeedants. Int. J. Primatol. 1998, 19, 949–970. [Google Scholar] [CrossRef]
- Martin, F.; Plastiras, C.-A.; Merceron, G.; Souron, A.; Boisserie, J.-R. Dietary niches of terrestrial cercopithecines from the Plio-Pleistocene Shungura Formation, Ethiopia: Evidence from Dental Microwear Texture Analysis. Sci. Rep. 2018, 8, 14052. [Google Scholar] [CrossRef]
- Koyabu, D.B.; Endo, H. Craniofacial Variation and Dietary Adaptations of African Colobines. J. Hum. Evol. 2009, 56, 525–536. [Google Scholar] [CrossRef]
- Koyabu, D.B.; Endo, H. Craniodental Mechanics and Diet in Asian Colobines: Morphological Evidence of Mature Seed Predation and Sclerocarpy. Am. J. Phys. Anthropol. 2010, 142, 137–148. [Google Scholar] [CrossRef]
- Guatelli-Steinberg, D.; Schwartz, G.T.; O’Hara, M.C.; Gurian, K.; Rychel, J.; Dunham, N.; Cunneyworth, P.M.K.; Donaldson, A.; McGraw, W.S. Aspects of Molar form and Dietary Proclivities of African Colobines. J. Hum. Evol. 2023, 180, 103384. [Google Scholar] [CrossRef]
- Bouvier, M. A biomechanical analysis of mandibular scaling in old world monkeys. Am. J. Phys. Anthropol. 1986, 69, 473–482. [Google Scholar] [CrossRef]
- Ravosa, M.J. Functional Assessment of Subfamily Variation in Maxillomandibular Morphology Among Old World Monkeys. Am. J. Phys. Anthropol. 1990, 82, 199–212. [Google Scholar] [CrossRef]
- Davies, A.G.; Oates, J.F. Colobine Monkeys: Their Ecology, Behaviour, and Evolution; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Leutenegger, W.; Kelly, J.T. Relationship of Sexual Dimorphism in Canine Size and Body Size to Social, Behavioral, and Ecological Correlates in Anthropoid Primates. Primates 1977, 18, 117–136. [Google Scholar] [CrossRef]
- Petersdorf, M.; Weyher, A.H.; Kamilar, J.M.; Dubuc, C.; Higham, J.P. Sexual Selection in the Kinda Baboon. J. Hum. Evol. 2019, 135, 102635. [Google Scholar] [CrossRef] [PubMed]
- Dirks, W.; Lemmers, S.A.M.; Ngoubangoye, B.; Herbert, A.; Setchell, J.M. Odontochronologies in male and female mandrills (Mandrillus sphinx) and the development of dental sexual dimorphism. Am. J. Phys. Anthropol. 2020, 172, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.H.; Kavanagh, M.; Clutton-Brock, T.H. Sexual dimorphism in primate teeth. J. Zool. 2009, 186, 475–485. [Google Scholar] [CrossRef]
- Plavcan, J.M. Sexual dimorphism in primate evolution. Am. J. Phys. Anthropol. 2001, 116 (Suppl. S33), 25–53. [Google Scholar] [CrossRef] [PubMed]
| Continent | Family | Subfamily | Genus | Species (Sex) |
|---|---|---|---|---|
| Africa | Cercopithecidae | Colobinae | Cercocebus | torquatus (6F,10M) |
| Colobus | guereza (18F, 16M, 4U) | |||
| polykomos (2F, 1M, 1U) | ||||
| Piliocolobus | badius (18F, 16M, 2U) | |||
| Procolobus | verus (21F, 14M, 7U) | |||
| Cercopithecinae | Cercopithecus | mona (2F) | ||
| nictitans (3M, 1U) | ||||
| Mandrillus | leucophaeus (11F, 11M) | |||
| Papio | hamadryas (2F, 10M) | |||
| ursinus (7F, 11M) | ||||
| Macaca | sylvanus (10F, 11M) | |||
| Asia | Colobinae | Nasalis | larvatus (10F, 12M) | |
| Presbytis | chrysomelas (1F) | |||
| comata (3F, 4M) | ||||
| melalophos (4F, 5M) | ||||
| rubicunda (7F, 5M) | ||||
| Pygathrix | nemaeus (11F, 16M) | |||
| Rhinopithecus | avunculus (3F, 3M) | |||
| bieti (12F, 9M) | ||||
| brelichi (1F, 4M) | ||||
| roxellana (34F, 32M, 9U) | ||||
| Semnopithecus | entellus (5F, 5M) | |||
| Trachypithecus | cristatus (19F, 15M) | |||
| francoisi (8F, 10M) | ||||
| obscurus (10F, 10M) | ||||
| phayrei (26F, 10M) | ||||
| vetulus (5F, 5M) | ||||
| Simias | concolor (2F, 1M) | |||
| Cercopithecinae | Macaca | arctoides (3F, 3M) | ||
| assamensis (3F, 3M) | ||||
| fascicularis (3F, 3M) | ||||
| fuscata (4F, 3M) | ||||
| mulatta (4F, 3M) | ||||
| nemestrina (3F, 3M) | ||||
| nigra (3F, 3M) | ||||
| radiata (3F, 3M) | ||||
| silenus (3F, 3M) | ||||
| sinica (4F, 4M) | ||||
| thibetana (2F, 4M) | ||||
| Hylobatidae | Hylobates | agilis (10F, 10M, 2U) | ||
| lar (13F, 20M, 4U) | ||||
| muelleri (13U) | ||||
| pileatus (1F, 2M) | ||||
| Hoolock | hoolock (8F, 11M) |
| Three Groups | Four Groups | |||
|---|---|---|---|---|
| DF1 | DF2 | DF1 | DF2 | |
| Eigenvalue | 0.794 | 0.467 | 0.941 | 0.566 |
| Percentage | 63.0% | 37.0% | 53.0% | 31.8% |
| Cum. Per | 63.0% | 100.0% | 53.0% | 84.8% |
| Eigenvector | ||||
| UI1 | 0.16 | −0.45 | 0.14 | −0.48 |
| UI2 | 0.15 | 0.41 | 0.03 | 0.28 |
| UC | 0.32 | 0.29 | −0.46 | 0.38 |
| UP3 | −0.56 | 0.43 | 0.01 | 0.47 |
| UP4 | 0.50 | −0.21 | −0.70 | −0.25 |
| UM1 | −0.08 | 0.89 | 0.84 | 0.61 |
| UM2 | −0.27 | −1.12 | 0.23 | −1.14 |
| UM3 | 0.66 | 0.13 | 0.36 | 0.12 |
| LI1 | 0.49 | 0.06 | 0.19 | 0.19 |
| LI2 | −0.14 | 0.18 | −0.47 | 0.26 |
| LC | −0.61 | 0.10 | 0.35 | 0.10 |
| LP3 | 0.63 | 0.21 | 0.75 | 0.06 |
| LP4 | 0.27 | −0.68 | 0.30 | −0.68 |
| LM1 | −0.51 | −0.18 | −0.82 | −0.23 |
| LM2 | −0.39 | 0.36 | 0.03 | 0.47 |
| LM3 | 0.35 | 0.50 | −0.37 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Zhang, H.; Youlatos, D.; Wang, J.; He, G.; Guo, S.; Huang, K.; Hou, R.; Pan, R.; Fang, G.; et al. Evolutionary Insights from Dental Diversity in Afro-Asian Primates. Diversity 2024, 16, 565. https://doi.org/10.3390/d16090565
Pan H, Zhang H, Youlatos D, Wang J, He G, Guo S, Huang K, Hou R, Pan R, Fang G, et al. Evolutionary Insights from Dental Diversity in Afro-Asian Primates. Diversity. 2024; 16(9):565. https://doi.org/10.3390/d16090565
Chicago/Turabian StylePan, Hao, He Zhang, Dionisios Youlatos, Jing Wang, Gang He, Songtao Guo, Kang Huang, Rong Hou, Ruliang Pan, Gu Fang, and et al. 2024. "Evolutionary Insights from Dental Diversity in Afro-Asian Primates" Diversity 16, no. 9: 565. https://doi.org/10.3390/d16090565
APA StylePan, H., Zhang, H., Youlatos, D., Wang, J., He, G., Guo, S., Huang, K., Hou, R., Pan, R., Fang, G., Li, Y., Zhang, P., & Li, B. (2024). Evolutionary Insights from Dental Diversity in Afro-Asian Primates. Diversity, 16(9), 565. https://doi.org/10.3390/d16090565








