Abstract
The water deer, although an internationally endangered species, is designated as a nuisance wild animal in South Korea and occupies a unique ecological niche. Studying the gut microbiome of this species is crucial for understanding its ecology. We amplified 16S rRNA DNA and compared the gut microbiomes of wild water deer from three regions with those of captive water deer from one region. Our results showed that the gut microbiome diversity of water deer did not differ significantly across regions in the wild but decreased significantly when raised in captivity. The similar microbiomes of water deer living in different regions are believed to be due to dietary diversity rather than dietary homogeneity. Furthermore, the monotony of the food supply appears to lead to significant variation in captive environments. From a conservation biology and biorestoration perspective, we suggest the importance of conserving the gut environments of animals conserved and restored outside their native habitats.
1. Introduction
The water deer (Hydropotes inermis) is classified as a Vulnerable species by the International Union for Conservation of Nature and Natural Resources (IUCN) []. The global population is small, except in South Korea [,], where it is so large that it is designated a nuisance wild animal []. This concentration of the global population of water deer almost entirely in South Korea is unique. With an area of approximately 10,026,600 ha, South Korea had, as of 2023, an average of 7.1 individuals per 100 ha according to the survey results of the South Korean Ministry of Environment, and they are distributed evenly throughout the country. Water deer have different ecological niches from roe deer, which mainly inhabit highland areas, and long-tailed goral, which mainly inhabit cliff areas; thus, it is necessary to study their differences from the perspective of conservation biology.
From a conservation biology perspective, habitat fragmentation is known to have a strong negative impact and is a major cause of population decline [,,,]. However, in South Korea, where many sections are cut off by highways and local roads, water deer do not seem to be greatly affected by habitat fragmentation. Despite extensive roadkill and ongoing hunting due to their designation as nuisance animals [,,,,,], with tens of thousands of them being hunted every year, the water deer population remains considerably large. Without hunting, the population would further increase, regardless of habitat fragmentation. At a time when many animal species are on the verge of extinction, it would be beneficial if research on the overpopulated water deer could be applied to the conservation of other animals to help maintain or increase their populations.
Research linking macroecological perspectives (the ecology of animals) and microecological perspectives (the ecology of their gut microbiomes) has recently become increasingly important in animal conservation. Previous studies have revealed co-evolution between wild animals and their gut microbiomes [], and numerous mammalian cases have revealed that the gut microbiome can serve as an indicator of animal well-being [].
In our previous study on the long-tailed goral, which has a very small population, we found that the ecology of intestinal microorganisms changed drastically when animals transitioned from living in the wild to being farmed by humans []. However, in the case of the water deer, which has a very large population, there is a lack of research on how the ecology of its intestinal microorganisms will change. In particular, since research on the gut microbiome of wild animals is rare in South Korea, conducting research to accumulate basic data is especially important.
In addition, since the water deer has no restrictions on its habitat and inhabits a range of environments in South Korea, including plains, watersides, mountains, and wetlands, it is easy to collect fecal samples. Therefore, by analyzing their gut microbiome, it is possible to study the regional differences in water deer, the changes when they are raised in captivity, and their conservation through comparison with other closely related species.
Our first hypothesis is that the regional differences in the gut microbiome ecology of South Korean water deer, which have excellent reproductive capacity and adaptability, will not be significant. The second hypothesis is that changes in the gut microbiome ecology will be less pronounced in hand-reared water deer compared to other animals. If water deer show regional differences in gut microbiome ecology like long-tailed gorals, they will not be able to adapt to the diverse environments of South Korea and will form regional groups. However, it is hypothesized that they are highly adaptable to extreme environmental changes, such as transitioning from living in the wild to being hand-reared.
Our study is important because there are very few studies that link gut microbiome ecology and mammalian ecology from a conservation biology perspective. Our research goals are to first elucidate how microecology and macroecology are connected, and then to explore how the factors we learn can be applied to the conservation of other endangered species.
2. Materials and Methods
2.1. Fecal Sample Collection
Fecal samples from wild water deer were collected from Haepyeong Wetland in Gumi City, Dalseong Wetland in Daegu City, and Eulsukdo Wetland in Busan City (Table 1). We have added relevant information. Feces were collected from three wetlands along the Nakdong River, the longest river in Korea flowing from north to south, with elevation differences of less than 1 m. Fecal samples from captive water deer were collected from the National Institute of Ecology. Each wetland is known to harbor over 100 plant species, and wild deer have been observed consuming most of these, including sprouts, tender grasses, and dried grasses. Captive deer were provided with a limited variety of feed.

Table 1.
Information about fecal samples collected by region.
Because water deer tend to excrete dozens of bean-shaped feces, we collected the three samples needed for the experiment and discarded all remaining feces. To prevent duplication, each sample was randomly collected from very distant locations within the vast wetland.
Each sample was collected in early spring (March) between 6 and 8 a.m., the time of day when, in our experience, fresh deer feces are readily available. Only deer feces from the National Institute of Ecology were collected with the cooperation of the National Institute of Ecology.
Only fresh feces that were not contaminated with soil were collected and then placed in sterile Ziploc bags using sterile nitrile gloves. The Ziploc bags (S. C. Johnson & Son, Inc., Racine, WI, USA) were placed in chilled cooler bags and transported to the laboratory, where they were stored in a −80 °C deep freezer.
2.2. Bioinformatics Analysis
DNA extraction, library construction (V3-V4 region of 16S rRNA gene), sequencing, Quantitative Insights Into Microbial Ecology 2 (QIIME 2 version 2024.10), and R analysis (version 4.5.1) (relative abundance, alpha-diversity with Wilcoxon rank sum test, beta-diversity with Bray-Curtis and Aitchison distance, linear discriminant analysis effect size using log10 scale and threshold 5, and redundancy analysis) were conducted using the same process as in a previous study [].
3. Results
3.1. Relative Abundance
At the phylum level, the gut of wild1 water deer predominantly comprised five phyla: Firmicutes (~66.33%), Bacteroidota (~28.76%), Verrucomicrobiota (~2.65%), Actinobacteriota (~1.00%), Proteobacteria (~0.34%). The five phyla that dominated the gut of wild2 water deer were Firmicutes (~68.47%), Bacteroidota (~24.80%), Verrucomicrobiota (~2.38%), Euryarchaeota (~1.78%), Actinobacteriota (~1.32%). The five phyla that dominated the gut of wild3 water deer were Firmicutes (~71.12%), Bacteroidota (~25.74%), Verrucomicrobiota (~0.71%), Actinobacteriota (~0.39%), Planctomycetota (~0.38%). The five phyla that dominated the gut of captive water deer were Firmicutes (~68.00%), Bacteroidota (~19.53%), Spirochaetota (~5.46%), Proteobacteria (~1.88%), Verrucomicrobiota (~1.52%).
At the genus level, the gut of wild1 water deer predominantly comprised twenty genera: UCG-005 (~14.55%), UCG-010 (~11.32%), Bacteroides (~10.58%), Rikenellaceae RC9 gut group (~5.56%), Bacillus (~2.84%), [Eubacterium] coprostanoligenes group (~2.64%), Christensenellaceae R-7 group (~2.56%), Clostridia UCG-014 (~2.52%), Roseburia (~2.39%), Alistipes (~2.30%), Cerasicoccus (~1.50%), Ruminococcus (~1.38%), Akkermansia (~1.12%), Clostridia vadinBB60 group (~1.11%), Monoglobus (~0.86%), Phascolarctobacterium (~0.76%), NK4A214 group (~0.76%), Prevotellaceae UCG-004 (~0.64%), Arthrobacter (~0.63%), dgA-11 gut group (~0.59%). The twenty genera that dominated the gut of wild2 water deer were UCG-005 (~15.19%), UCG-010 (~8.57%), Bacteroides (~6.39%), Clostridia UCG-014 (~5.90%), Christensenellaceae R-7 group (~5.61%), Rikenellaceae RC9 gut group (~4.04%), Alistipes (~2.95%), [Eubacterium] coprostanoligenes group (~2.73%), Monoglobus (~2.65%), Akkermansia (~2.33%), NK4A214 group (~1.51%), Prevotellaceae UCG-004 (~1.49%), Methanobrevibacter (~1.31%), Dorea (~1.08%), Ruminococcus (~1.03%), [Eubacterium] brachy group (~0.92%), Ruminiclostridium (~0.75%), dgA-11 gut group (~0.75%), Roseburia (~0.66%), DNF00809 (~0.64%). The twenty genera that dominated the gut of wild3 water deer were UCG-005 (~15.80%), UCG-010 (~10.45%), Bacteroides (~10.10%), Rikenellaceae RC9 gut group (~4.82%), Christensenellaceae R-7 group (~3.50%), Clostridia UCG-014 (~3.28%), [Eubacterium] coprostanoligenes group (~3.07%), Alistipes (~2.28%), NK4A214 group (~1.87%), Monoglobus (~1.75%), Prevotellaceae UCG-004 (~1.60%), Ruminococcus (~1.47%), dgA-11 gut group (~1.41%), Roseburia (~0.94%), [Eubacterium] ruminantium group (~0.88%), Lysinibacillus (~0.87%), Clostridia vadinBB60 group (~0.80%), Phascolarctobacterium (~0.66%), Akkermansia (~0.55%), UCG-009 (~0.52%). The twenty genera that dominated the gut of captive water deer were Bacillus (~18.44%), Bacteroides (~6.93%), Lysinibacillus (~6.64%), UCG-005 (~5.71%), Treponema (~5.46%), Rikenellaceae RC9 gut group (~4.30%), UCG-010 (~4.01%), Clostridia UCG-014 (~3.73%), Ruminococcus (~3.39%), Alistipes (~2.63%), [Eubacterium] coprostanoligenes group (~2.44%), Christensenellaceae R-7 group (~2.36%), Methanobrevibacter (~1.33%), Prevotellaceae UCG-004 (~1.26%), Akkermansia (~1.10%), UCG-002 (~0.85%), Prevotella (~0.75%), Escherichia-Shigella (~0.74%), Phascolarctobacterium (~0.71%), dgA-11 gut group (~0.63%) (Figure 1).
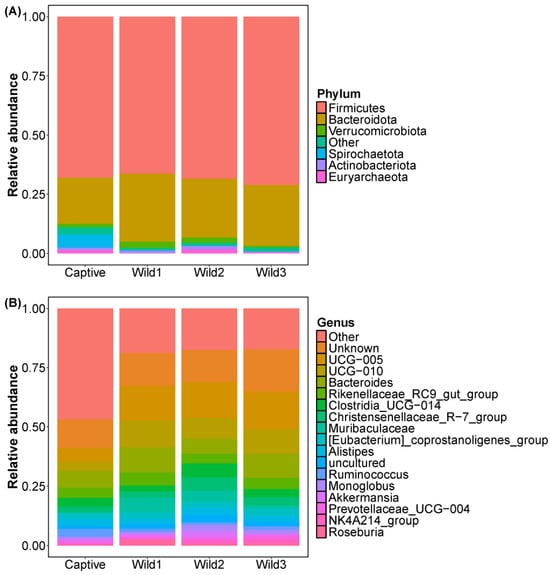
Figure 1.
Bar charts showing the relative abundance of total gut 16S rRNA gene sequences classified at the (A) phylum and (B) genus levels.
Wild water deer from the three different regions showed very similar community compositions, with no significant differences. The phylum Spirochaetota was more prevalent in the captive water deer group. At the genus level, UCG-005, UCG-010, and Bacteroides were less prevalent in the captive water deer group.
3.2. Alpha Diversity
A comparison of gut alpha diversity between captive and wild water deer was performed using four indices (Chao1, ACE, Shannon, and Simpson). In the Chao1 and ACE indices, there were significant differences between the three wild and captive water deer, with the difference being at the p < 0.05 level for wild1 and the p < 0.01 level for wild2 and wild3. In the Shannon and Simpson indices, the wild2 and wild3 water deer showed significantly higher diversity than the captive water deer, and all showed differences at the p < 0.01 level. There was no significant difference in alpha diversity among the three wild-type water deer in all four indices.
No differences in alpha diversity were observed between wild deer groups from different regions. Only the captive water deer group showed significant differences from the wild groups (Figure 2).
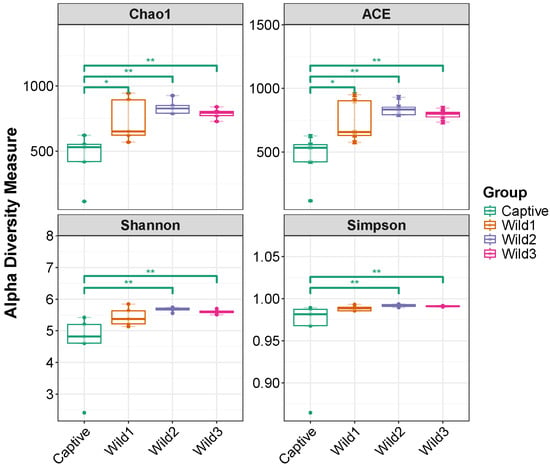
Figure 2.
Gut microbial alpha diversity of wild1 (Gumi Haepyeong Wetland), wild2 (Daegu Dalseong Wetland), wild3 (Busan Eulsukdo Wetland), and captive water deer. * p < 0.05, ** p < 0.01.
3.3. Beta Diversity
A comparison of gut beta diversity between captive and wild water deer was performed using representative multidimensional scaling methods: Bray–Curtis and Aitchison distances. The difference in the x-component between the most distant points was 21.4% for the Bray–Curtis method and 21.0% for the Aitchison method. The difference in the y-component between the most distant points was 11.2% for the Bray–Curtis method and 12.4% for the Aitchison method.
In the plot calculated using the two distances, the wild water deer group was closely clustered, showing great similarity, while captive water deer showed differences from wild types (Figure 3).
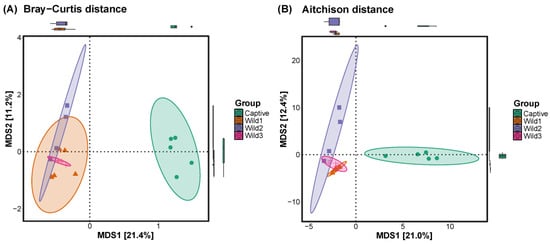
Figure 3.
Beta diversity plots were calculated using the (A) Bray–Curtis and (B) Aitchison distances to compare captive and wild water deer.
3.4. LEfSe
The LDA effect size (LefSe) was representative of taxa in the wild and captive groups. The class Clostridia and order Oscillospirales were the most represented in the wild3 group; the order Bacillales was the most represented in the captive group (Figure 4).
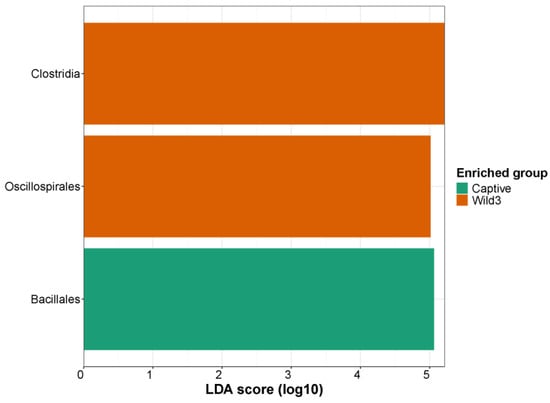
Figure 4.
Linear discriminant analysis effect size on a log10 scale illustrating differences in LDA scores between captive and wild water deer.
3.5. Redundancy Analysis
The redundancy analysis was limited to the top ten bacterial genera and revealed the dominant bacterial genera in each group. The captive and wild groups were separated in relatively opposite directions. The three wild groups were densely clustered, whereas the captive group was widely distributed. The representative taxon of the wild groups was DNF00808. The representative taxa of the captive group were Romboutsia, Bacilli Class, Arthrobacter, Lysinibacillus, Planococcaceae Family, Bacillus, UCG-002, Cerasicoccus, and Treponema (Figure 5).
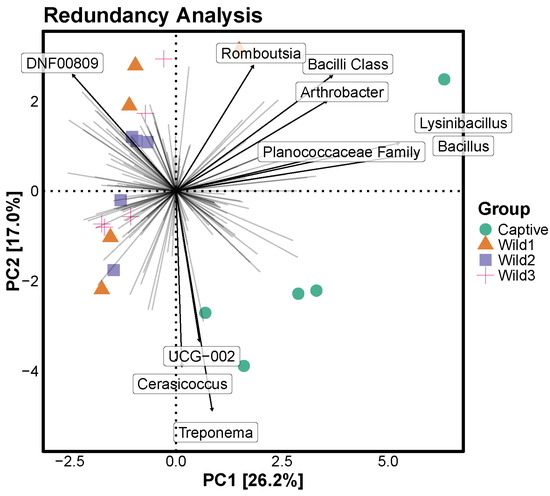
Figure 5.
RDA illustrating differences in abundance between captive and wild water deer at the genus level. The point where each line intersects represents a point where all genera are at 0, and each line indicates the direction in which a particular genera increases as it deviates from 0. For the analysis of the top 10 genera at the genus level, the bold arrows indicate the top 10 genera and the direction in which their abundance increases.
4. Discussion
4.1. The Importance of Diversity and Conservation of the Gut Microbiome
This study focused on analyzing the ecological characteristics of the gut microbiota of water deer, a species known for its remarkable environmental adaptability and population growth. Because it exhibits a distinct ecology from other endangered species, a thorough analysis of the water deer will contribute to the conservation of other endangered species.
Another wild ruminant studied in South Korea, the long-tailed goral, exhibits distinct regional differences in its gut microbiome, allowing for its analysis from a biogeographic perspective []. In contrast, wild water deer living in three wetland areas in South Korea showed no significant differences in alpha and beta diversity. This finding, despite the differences in environmental conditions across the three regions, suggests that the water deer’s gut microbiome is well established and largely unaffected by external factors. This finding supports the observation that the alpha diversity of the water deer’s gut microbiome decreases in captive environments, where food diversity is reduced, and that beta diversity significantly differs compared to wild conditions. The water deer living in the three regions consume a variety of different food sources, suggesting that the composition of the microbiome maintained by the species is independent of changes in food sources, and that food source diversity may be a key factor.
Long-tailed gorals, which have maintained the original form of ancient creatures, have a habit of establishing multiple shelter sites and accumulating feces in the same place, forming a living radius known as a home range around these shelters [,,,,,]. Although they are not confined solely to their home range, they are often active at relatively high altitudes in the mountains within their established home range, and the types of food they consume are also determined by their home range. On the other hand, water deer do not have fixed shelter sites and consume a wider variety of food from different environments, such as high mountains, plains, riversides, and coastal areas.
Various food sources can carry diverse microorganisms [,], which can be ingested directly through food intake []; alternatively, certain food sources can increase the number of microorganisms already present in the gut due to a preference for these sources [,,]. Under normal circumstances, when new microorganisms invade and proliferate in the gut [,], or when specific microorganisms already present in the gut proliferate, the composition of the entire microbial community within a limited space changes.
However, water deer and long-tailed gorals are not ordinary mammals: they are extraordinary ruminants, storing a significant amount of food in their rumen [,] and maintaining it at a constant level. They can maintain a stable microbial community through fermentation, regardless of diverse food sources or microbial invasion. This is where the differences between the two species arise. Long-tailed gorals, which prefer confined areas such as highlands and cliffs, face significant challenges in securing food sources during winter snowfall. This has led to the death of several dozen individuals from starvation in South Korea each year. This difficulty in finding food sources can cause gorals to empty their rumen each winter, increasing the likelihood of rumen invasion. In contrast, water deer, which inhabit a relatively wider area, have a remarkably high reproductive capacity. Despite being designated as a nuisance animal and being slaughtered in their tens of thousands each year, they have a remarkably low rate of starvation. This suggests that maintaining a fermentation environment in the rumen is important for maintaining a stable gut microbiota composition.
4.2. Gut Microbiome for Animal Conservation
Ex situ conservation refers to the practice of capturing endangered species from the wild, propagating them, and maintaining their populations []. When wild individuals decline rapidly or become extinct, they are reintroduced to prevent complete extinction [,,]. This practice focuses on preserving individuals that can be reintroduced to the wild at any time. However, a comparison of wild and captive water deer reveals that their gut microbiomes have undergone significant changes due to prolonged exposure to a limited variety of food sources while being raised by humans. We must be cautious about whether this altered gut microbiome composition will immediately revert to its wild state upon reintroduction. When reintroduced animals that previously consumed large amounts of artificial food are forced to eat relatively small amounts at a time, constantly move to avoid predators, and consume a diverse diet, changes in their gut microbiomes are likely to be slow and relatively small. This raises the possibility of the emergence of new populations with artificial gut microbiomes. Considering the efforts to conserve and restore the environment as close to its original state as possible, this perspective should be considered in the ex situ conservation and reintroduction of living organisms. For animals being conserved and restored outside their native habitat, true conservation and restoration require not only preserving their lives but also maintaining their intestinal environment.
5. Conclusions
By comparing the gut microbiomes of wild and captive water deer, we present insights for their conservation and restoration at non-wild sites, such as zoos and research institutions. Furthermore, by comparing the microbiomes of water deer with those of previously studied long-tailed gorals, we offer deeper insight into their ecology. We hope future researchers will build on this work and accumulate data on the food sources and microbiomes of captive animals, enabling conservation and restoration efforts that maintain a more wild-like gut environment.
Author Contributions
Conceptualization, C.-E.P.; sample collection, C.-E.P. and H.-C.P.; experiment, C.-E.P.; data analysis, C.-E.P.; visualization, C.-E.P.; writing, C.-E.P.; review and editing, H.-C.P.; supervision, H.-C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data available on request due to restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Harris, R.B.; Duckworth, J.W. Hydropotes inermis. The IUCN Red List of Threatened Species 2015. 2015, e.T10329A22163569. Available online: https://www.iucnredlist.org/species/10329/22163569 (accessed on 11 August 2025).
- Schilling, A.M.; Rössner, G.E. The (sleeping) Beauty in the Beast—A review on the water deer, Hydropotes inermis. Hystrix 2017, 28, 121. [Google Scholar]
- Chen, Y.J.; Liu, K.H.; Chu, W.L. New record of water deer (Hydropotes inermis) from Iron Age archeological sites in central Taiwan. Collect. Res. 2016, 29, 31–39. [Google Scholar]
- Kim, B.; Chae, S.J.; Lee, Y.J.; Shin, H.; Kwak, S.; Jeong, H.; Lee, S.; Kwak, D.; Seo, M.G. Nationwide Geographical and Temporal Distribution of Tick-Borne Diseases in Korean Water Deer (Hydropotes inermis argyropus). Animals 2025, 15, 1499. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, R.J., Jr.; Reichert, B.E.; Holmes, K. The negative effects of habitat fragmentation operate at the scale of dispersal. Ecology 2018, 99, 2176–2186. [Google Scholar] [CrossRef]
- Fahrig, L.; Arroyo-Rodríguez, V.; Bennett, J.R.; Boucher-Lalonde, V.; Cazetta, E.; Currie, D.J.; Eigenbrod, F.; Ford, A.T.; Harrison, S.P.; Jaeger, J.A.G.; et al. Is habitat fragmentation bad for biodiversity? Biol. Conserv. 2019, 230, 179–186. [Google Scholar] [CrossRef]
- Saura, S. The Habitat Amount Hypothesis implies negative effects of habitat fragmentation on species richness. J. Biogeogr. 2021, 48, 11–22. [Google Scholar] [CrossRef]
- Fahrig, L. Ecological responses to habitat fragmentation per se. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Islam, O.; Matsuyama, R.; Min, K.D. Deforestation and predator species richness as potential environmental drivers for roadkill of wild water deer in South Korea. Front. Vet. Sci. 2025, 12, 1483563. [Google Scholar] [CrossRef]
- Kim, M.; Park, H.; Lee, S. Analysis of Roadkill on the Korean Expressways from 2004 to 2019. Int. J. Environ. Res. Public Health 2021, 18, 10252. [Google Scholar] [CrossRef]
- Choi, T.Y. Estimation of the water deer (Hydropotes inermis) roadkill frequency in South Korea. Ecol. Resilient Infrastruct. 2016, 3, 162–168. [Google Scholar] [CrossRef]
- Jang, W.; Kim, B.; Chung, O.S.; Lee, J.K. Analysis of water deer roadkills using point process modeling in Chungcheongnamdo, South Korea. Forests 2022, 13, 209. [Google Scholar] [CrossRef]
- Kim, W.; Hong, S.H. An empirical analysis on factors affecting water deer roadkills in Korea. KSCE J. Civ. Eng. 2021, 25, 3533–3539. [Google Scholar] [CrossRef]
- Kim, K.; Seo, H.; Woo, D.; Park, T.; Song, E. The water deer on a road: Road-kill characteristics of a nationally abundant but internationally threatened species. J. For. Environ. Sci. 2021, 37, 62–68. [Google Scholar]
- Wang, L.; Ding, J.; Yang, Z.; Chen, H.; Yao, R.; Dai, Q.; Zhu, L. Père David’s deer gut microbiome changes across captive and translocated populations: Implications for conservation. Evol. Appl. 2019, 12, 622–635. [Google Scholar] [CrossRef]
- De Jonge, N.; Carlsen, B.; Christensen, M.H.; Pertoldi, C.; Nielsen, J.L. The gut microbiome of 54 mammalian species. Front. Microbiol. 2022, 13, 886252. [Google Scholar] [CrossRef]
- Park, C.E.; Jo, Y.J.; Jung, D.R.; Park, H.C.; Shin, J.H. Comparative Analysis of Gut Microbiota between Captive and Wild Long-Tailed Gorals for Ex Situ Conservation. Microorganisms 2024, 12, 1419. [Google Scholar] [CrossRef] [PubMed]
- Park, C.E.; Cho, B.J.; Kim, M.J.; Park, H.C.; Shin, J.H. Geographical relationships between long-tailed goral (Naemorhedus caudatus) populations based on gut microbiome analysis. Microorganisms 2021, 9, 2002. [Google Scholar] [CrossRef]
- Lim, S.; Banjade, M.; Ahn, J.; Song, D.; Son, J.; Park, Y. Seasonal Variations and Sexual Differences in Home Range Sizes and Activity Patterns of Endangered Long-Tailed Gorals in South Korea. Animals 2024, 15, 27. [Google Scholar] [CrossRef]
- Yin, Y.; Tang, S.; Teng, Y.; Han, Z.; Wu, L.; Gao, F.; Bao, W. A pilot study on home range of female Chinese goral (Naemorhedus griseus): Exploring GPS tracking data in a cliff landscape. J. For. Res. 2024, 29, 123–129. [Google Scholar] [CrossRef]
- Park, H.B.; Hong, S. Habitat characteristics coincidence of dead and living long-tailed gorals (Naemorhedus caudatus) according to extreme snowfall. Animals 2021, 11, 997. [Google Scholar] [CrossRef]
- Lim, S.; Banjade, M.; Pandey, P.; Park, Y. Genetic Structure and Conservation Implications of Long-Tailed Gorals (Naemorhedus caudatus) in South Korea: Phylogenetic Analysis and Haplotype Networks. J. For. Environ. Sci. 2025, 41, 209–216. [Google Scholar]
- Teng, Y.; Shupei, T.A.N.G.; Menghe, D.; Wu, L.; Zhiqing, H.A.N.; Yingying, H.A.N.; Weidong, B.A.O. A pilot study on home range and habitat use of Chinese goral (Naemorhedus griseus): Exploring GPS tracking data in cliff landscape by three estimation methods. Res. Sq. 2021, preprint. [Google Scholar]
- Kim, H.R.; Lee, J.B.; Jeong, S.M.; Kim, T.W.; Son, J.I.; Youn, J.J.; Han, S.H. Molecular evidence for occurrence of endangered long-tailed goral in Seoul metropolitan city, South Korea. J. Anim. Breed. Genom. 2023, 7, 85–93. [Google Scholar] [CrossRef]
- Choi, K.R.; Yu, H.E.; Lee, S.Y. Microbial food: Microorganisms repurposed for our food. Microb. Biotechnol. 2022, 15, 18–25. [Google Scholar] [CrossRef]
- Rocha, J.M.; Kovacevik, B.; Veličkovska, S.K.; Tamame, M.; Teixeira, J.A. Diversity of microorganisms and their metabolites in food. Microorganisms 2024, 12, 205. [Google Scholar] [CrossRef]
- Van Hul, M.; Cani, P.D. The gut microbiota in obesity and weight management: Microbes as friends or foe? Nat. Rev. Endocrinol. 2023, 19, 258–271. [Google Scholar] [CrossRef]
- de Wouters d’Oplinter, A.; Huwart, S.J.; Cani, P.D.; Everard, A. Gut microbes and food reward: From the gut to the brain. Front. Neurosci. 2022, 16, 947240. [Google Scholar] [CrossRef]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota revolution: How gut microbes regulate our lives. World J. Gastroenterol. 2023, 29, 4368. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Bulut, N.; Chambry, E.; Ruthes, A.; Iacomini, M.; Keshavarzian, A.; Johnson, T.A.; Hamaker, B.R. Dietary fiber hierarchical specificity: The missing link for predictable and strong shifts in gut bacterial communities. MBio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Tang, J. The interactions of Candida albicans with gut bacteria: A new strategy to prevent and treat invasive intestinal candidiasis. Gut Pathog. 2023, 15, 30. [Google Scholar] [CrossRef]
- Puschhof, J.; Pleguezuelos-Manzano, C.; Clevers, H. Organoids and organs-on-chips: Insights into human gut-microbe interactions. Cell Host Microbe 2021, 29, 867–878. [Google Scholar] [CrossRef]
- Singer, M.; Codron, D.; Lechner, I.; Rudnik, R.; Barboza, P.; Hummel, J.; Clauss, M. The effect of size and density on the mean retention time of particles in reindeer (Rangifer tarandus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2024, 292, 111621. [Google Scholar] [CrossRef] [PubMed]
- Hummel, J.; Clauss, M.; Zimmermann, W.; Johanson, K.; Nørgaard, C.; Pfeffer, E. Fluid and particle retention in captive okapi (Okapia johnstoni). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 140, 436–444. [Google Scholar] [CrossRef]
- Braverman, I. Conservation without nature: The trouble with in situ versus ex situ conservation. Geoforum 2014, 51, 47–57. [Google Scholar] [CrossRef]
- Mahanayak, B. Ex-situ and in-situ conservation of wild life. World J. Biol. Pharm. Health Sci. 2024, 18, 277–282. [Google Scholar] [CrossRef]
- Clark, F.E.; Greggor, A.L.; Montgomery, S.H.; Plotnik, J.M. The endangered brain: Actively preserving ex-situ animal behaviour and cognition will benefit in-situ conservation. R. Soc. Open Sci. 2023, 10, 230707. [Google Scholar] [CrossRef] [PubMed]
- Kasso, M.; Balakrishnan, M. Ex situ conservation of biodiversity with particular emphasis to Ethiopia. Int. Sch. Res. Not. 2013, 2013, 985037. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).